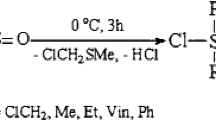Abstract
Substituted 4-arylamino-1,3-dioxanes were synthesized using a new synthetic protocol of the Prins reaction between aryl amines and acetaldehyde. This one-pot synthesis occurs under catalyst-free conditions in an aqueous medium. By employing condensation of excess acetaldehyde with an aromatic amine in water at 0–5 °C, the corresponding N-aryl-2,6-dimethyl-1,3-dioxan-4-amines are obtained in good yields. Consequently, this is an environmentally benign, simple, efficient, “green” procedure for the preparation of 1,3-dioxanes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Compounds with 1,3-dioxane units are widely used in organic synthesis both as intermediates and also as solvents [1]. Various biologically active molecules have been identified from libraries of natural product-like 1,3-dioxanes [2–4]. Some examples of compounds bearing 1,3-dioxane rings as structural moieties include (+)-dactylolide (a cytotoxic agent) [5] derivatives of 2-substituted-1,3-dioxanes such as cis-benzhydryl-N,N,N-trimethyl-1,3-dioxan-5-aminium iodide, cis-(2-benzhydryl-1,3-dioxan-5-yl)(dimethyl)sulfonium perchlorate (anti-muscarinic agents) [6] and (+)-SCH 351448 (a novel natural product isolated from Micromonospora microorganisms that act as an activator of low-density lipoprotein receptor promoters) [7]. 1,3-Dioxane derivatives [8, 9], especially 2,2-diphenyl-1,3-dioxanes, have been found to act as effective modulators for multidrug resistance in selected tumor cell lines [8] and other derivatives such as dexoxadrol and etoxadrol represent very important drug candidates and originally designed as anesthetics [9]. Over the years, many elegant methodologies have been described for the synthesis of these types of molecules by several research groups. Amongst such approaches is the Prins reaction, which is an acid-catalyzed condensation of olefins with aldehydes [10]. The major products of the Prins reaction are 1,3-dioxanes, tetrahydropyrans, 1,3-glycols, or unsaturated alcohols depending on the reaction conditions employed [11–15]. Since dioxanes have considerable potential as drug candidates per se, synthetic protocols for the preparation of this important scaffold are of interest. Generally, Lewis acids as well as Brønsted acids are used in either catalytic or stoichiometric amounts to synthesize the target 1,3-dioxanes [1, 16–25]. However, most acid catalysts described so far are highly corrosive and involve tedious work-up procedures as well as prolonged reaction times and/or high temperatures. As a result, the selectivity of products is poor to average, mainly due to polymerization of starting materials. Therefore, the development of convenient, efficient, inexpensive, and eco-friendly routes to employ readily with available reagents for the Prins cyclization reaction would extend the scope of the synthesis of 1,3-dioxanes. In addition, the excellent biological and pharmacological role of substances with 1,3-dioxanes units prompted us to develop new methodologies to synthesize this chemo type.
Results and discussion
In our initial experiments, an attempt was made to synthesize an amino-substituted β-keto ester by using a one-pot multi-component reaction of an aromatic amine aliphatic aldehyde, and β-keto ester and replacing the ethanol solvent with water [26, 27]. Unfortunately, this modification provided an epimeric mixture of 1,3-dioxane 5a (30 %) at the expense of the desired ethyl 2-acetyl-3-(phenylamino)butanoate 4 (60 %) (Scheme 1).
Since the 1,3-dioxane 5a was produced in reasonable yield, attention was focused on gaining an understanding of how this compound was formed. A proposed mechanism not involving the β-keto ester for the formation of 2,6-dimethyl-4-(arylamino)-1,3-dioxane is depicted in Fig. 1.
According to this mechanism, an aryl amine reacts with an aliphatic aldehyde in two steps to afford an intermediate enamine en route to the 1,3-dioxane. Initially, the aromatic amine reacts with acetaldehyde to provide an enolizable imine that undergoes a 1,3-proton shift to an enamine form. In this step, an equilibrium is established between the enolizable imine and enamine. It is known that the enamine form is favored due to π-n-π conjugation [28]. Subsequently, the enamine reacts with two further equivalents of aldehyde and undergoes a cyclization to yield the desired 1,3-dioxane products. The second step constitutes the Prins reaction [10]. So, the mechanism proposed in this protocol is very much analogous to the Prins reaction but, proceeds via enamine formation, which is different when compared to the mechanism of conventional Prins reaction.
Considering the proposed mechanism, the 1,3-dioxanes could, in principle, be obtained simply by only reacting the amine and acetaldehyde. Consequently, this was tested by reacting 1 mmol of 1a and 3 mmol of 2. Gratifyingly, the expected 1,3-dioxane (5a) was obtained as the sole product in good yield (Scheme 2).
To optimize the reaction conditions, a mixture of aniline (1a, 1 mmol) and acetaldehyde (2, 3 mmol) was stirred neat or in various solvents/conditions (Table 1). When the reaction was carried out at 25 °C (Table 1, entries 2, 4, 6, and 8), irrespective of the solvents and stoichiometry, the desired product was undetectable, which may be due to the decomposition of imine before rearranging into enamine [29]. When the reaction was carried out at lower temperature (0–5 °C), the reaction provided a single product in good to average yields (Table 1, entries 1, 3, 5, and 7). In an effort to optimize yields, different solvents were screened under a variety of conditions. In general, the reactions proceeded smoothly at 0–5 °C in polar solvent including water, THF, ethanol, and also in solvent-free conditions to give the desired product (5a) in varying yields (Table 1). Notably, the choice of water as a solvent provided the desired compounds in excellent yield and proved to be the solvent of choice. In contrast, ethanol, THF, and solvent-free conditions proved a poor choice in achieving the desired outcome.
These results prompted us to widen the scope of the reaction by using various aryl amines (1a–m) with acetaldehyde. We successfully prepared a selection of substituted 1,3-dioxanes (5a–m) in good to excellent yields (Table 2) irrespective of the presence of electron-donating or electron-withdrawing groups. Notably, the presence of electron-donating groups enhances the nucleophilicity of amines, which favor the attack of amines on carbonyl carbon of acetaldehyde in the first step of the proposed mechanism. The aldimine formed is rearranged to the corresponding enamine, which is stabilized by the electron-withdrawing group of the amine. Consequently, electron-donating groups as well as electron-withdrawing groups favor the reaction.

All compounds (5a–l) were characterized by a combination of FTIR, 1H NMR, 13C NMR, and the compounds (5b–k) were characterized by either ESI or LC–MS. The structural elucidation of compound 5j by single-crystal X-ray diffraction studies (Fig. 2) allows an understanding of the conformational features present in these molecules, which may also prove useful for projected structure–activity investigations of these substances. Evidently, the dioxane ring adopts a chair conformation with the methyl substituents and the C–N bond in equatorial orientations [30]. According to X-ray diffraction studies of compound 5j, it is clear that all the compounds (5a–l) in this series are having (2R, 4S, 6S) configurations.
In summary, the protocol reported in this manuscript is a new alternative route for conventional Prins reaction, which is used for the synthesis of 1,3-dioxnes from alkenes and aliphatic aldehydes. Whereas, our protocol provides the new route for the synthesis of 1,3-dioxanes from aromatic amines and aliphatic aldehydes with good yields under catalyst-free conditions in water at 0–5 °C, via enamine formation. Consequently, this procedure is an environmentally benign, simple, efficient, green procedure for the synthesis of 1,3-dioxanes. Further investigations are underway to explore the scope of this procedure by using substituted aliphatic aldehydes. Further, there is considerable scope to obtain 1,3-diols upon cleavage of these 1,3-dioxanes and these 1,3-diols could be used as convenient synthons [31] for phosphorylation en route to bio-active molecules.
Experimental
All reagents and solvents were prepared following the procedure already reported in the literature [32] or were purchased from commercially available suppliers and used without further purification. Melting points were obtained from Elchem Microprocessor-based DT apparatus in open capillary tubes and are corrected relative to benzoic acid. Analytical thin-layer chromatography analysis was conducted on pre-coated silica gel plates (Merck, India). All compounds were characterized by IR, ESI, and LC–MS, 1H NMR, 13CNMR spectroscopy. The IR spectra were recorded on a Bruker Alpha-Eco ATR–FTIR (Attenuated total reflection–Fourier transform infrared) interferometer with single reflection sampling module equipped with ZnSe crystal. Mass spectra were recorded on a Shimadzu LCMS-8040 instrument by direct insertion, using EI mode (70 eV). The 1H NMR and 13C NMR spectra were recorded on a Bruker Avance 400-MHz spectrometer at 400 and 100 MHz, respectively, using CDCl3 as solvent and TMS as internal reference.
General procedure for the synthesis of the compounds 5a–l
In a 50-ml round-bottom flask charged with aryl amine (1 mmol) in water (5 ml) and kept at 0–5 °C was added acetaldehyde (3 mmol, 35 % in water). The resultant mixture was stirred until TLC analyses revealed the consumption of all starting materials. The reaction mixture was then washed with petroleum ether and extracted with diethyl ether (3 × 15 ml). The combined organic extracts were dried over magnesium sulfate and the solvent was evaporated under reduced pressure. The solid product was recrystallized using diethyl ether.
2,6-dimethyl-N-phenyl-1,3-dioxan-4-amine (5a)
Mp 65–67 °C. IR (cm−1): 1,122, 1,163, 1,289, 1,355,1,495, 1,596, 2,972, 3,320. 1H-NMR (400 MHz, CDCl3):δ = 1.29 (d, J = 6.0 Hz, 3H), 1.38 (d, J = 4.8 Hz, 3H), 1.45–1.55 (m, 1H), 1.82 (d, J = 12.4 Hz, 1H), 3.84–3.88 (m, 1H), 4.33 (bs, 1NH), 4.87 (q, J = 4.8 Hz, 1H), 4.97 (t, J = 10.0 Hz, 1H), 6.73 (d, J = 8.0 Hz, 2H), 6.80 (t, J = 7.6 Hz, 1H), 7.2 (t, J = 7.6 Hz, 2H). 13C-NMR (100 MHz, CDCl3):δ = 21.16, 21.96, 37.90, 72.06, 81.59, 96.67, 114.04, 118.92, 129.37, 145.07.
2,6-dimethyl-N-(o-tolyl)-1,3-dioxan-4-amine (5b)
Mp 95–97 °C. IR (cm−1): 1,092, 1,155, 1,261, 1,389, 1,448, 1,512, 1,590, 2,857, 2,978, 3,351.1H-NMR (400 MHz, CDCl3):δ = 1.31 (d, J = 6.0 Hz, 3H), 1.40 (d, J = 4.8 Hz, 3H), 1.49–1.54 (m, 1H), 1.87 (d, J = 12.0 Hz, 1H), 2.15 (s, 3H), 3.85–3.92 (m, 1H), 4.15 (bs, 1NH), 4.90 (q, J = 5.2 Hz, 1H), 4.99 (d, J = 10.0 Hz, 1H), 6.75 (t, J = 7.4 Hz, 1H), 6.84 (d, J = 8.0 Hz, 1H), 7.06 (d, J = 7.6 Hz, 1H), 7.12 (t, J = 7.8 Hz, 1H). 13C-NMR (100 MHz, CDCl3):δ = 17.54, 21.17, 21.59, 38.91, 72.10, 81.50, 96.66, 112.13, 118.99, 122.72, 127.15, 130.47, 143.14. EIMS: m/z = 221.60 (M+), 203.7 (M-H2O)+.
2,6-dimethyl-N-(3-(trifluoromethyl)phenyl)-1,3-dioxan-4-amine (5c)
Mp 99–101 °C. IR (cm−1): 1,072, 1,125, 1,271, 1,364, 1,424, 1,525, 1,584, 2,862, 3,372. 1H-NMR (400 MHz, CDCl3):δ = 1.30 (d, J = 6.0 Hz, 3H), 1.39 (d, J = 4.8 Hz, 3H), 1.46 (q, J = 11.6 Hz, 1H), 1.84 (d, J = 12.8 Hz, 1H), 3.85-3.89 (m, 1H), 4.53 (s, NH), 4.89 (q, J = 4.4 Hz, 1H), 4.98 (t, J = 9.8 Hz, 1H), 6.86 (d, J = 8.0 Hz, 1H), 6.92 (s, 1H), 7.03 (d, J = 7.2 Hz, 2H), 7.25-7.29 (m, 1H).13C-NMR (100 MHz, CDCl3):δ = 21.09, 21.56, 38.54, 72.02, 81.15, 96.77, 110.67, 115.82, 117.37, 122.97, 125.68, 131.62, 145.39. LCMS: m/z = 258.16 (M-H2O)+.
N-(2,4-difluorophenyl)-2,6-dimethyl-1,3-dioxan-4-amine (5d)
Mp 88–90 °C. IR (cm−1): 848, 945, 1,092, 1,148, 1,335, 1,426, 1,515, 1,605, 2,876,2,983, 3,075, 3,344. 1H-NMR (400 MHz, CDCl3):δ = 1.28 (d, J = 6.0 Hz, 3H), 1.36 (d, J = 5.2 Hz, 3H), 1.47 (q, J = 11.2 Hz, 1H), 1.84 (d, J = 12.8 Hz, 1H), 3.82–3.84 (m, 1H), 4.38 (s, NH), 4.84–4.90 (m, 2H), 6.73–6.83 (m, 3H).13C-NMR (100 MHz, CDCl3):δ = 21.07, 21.51, 38.53, 72.02, 81.52, 96.69, 103.75, 110.87, 114.56, 130.02, 151.14.00, 155.16.LCMS: m/z = 244.14 (M + 1)+, 226.12 (M + 1-H2O)+.
N-(2-fluorophenyl)-2,6-dimethyl-1,3-dioxan-4-amine (5e)
Mp 71–73 °C. IR (cm−1): 851, 945, 1,095, 1,157, 1,328, 1,391, 1,454, 1,615, 2,865, 2,982, 3,044, 3,369. 1H-NMR (400 MHz, CDCl3):δ = 1.30 (d, J = 6.4 Hz, 3H), 1.38 (d, J = 5.2 Hz, 3H), 1.50 (q, J = 11.6 Hz, 1H), 1.86 (d, J = 12.8 Hz, 1H), 3.83–3.90 (m, 1H), 4.58 (bs, 1NH), 4.87 (q, J = 4.8 Hz, 1H), 4.95 (d, J = 9.6 Hz, 1H), 6.72 (q, J = 6.6 Hz, 1H), 6.89 (t, J = 8.4 Hz, 1H), 6.95–7.01 (m, 2H).13C-NMR (100 MHz, CDCl3):δ = 21.11, 21.55, 38.54, 72.04, 81.10, 96.69, 114.16, 114.97, 118.86, 124.61, 133.53, 151.66. EIMS: m/z = 207.6 (M-H2O)+.
2,6-dimethyl-N-(p-tolyl)-1,3-dioxan-4-amine (5f)
Mp 91–93 °C. IR (cm−1): 1,092, 1,155, 1,261, 1,389, 1,448, 1,512, 1,590, 2,857, 2,978, 3,351. 1H-NMR (400 MHz, CDCl3):δ = 1.287 (d, J = 5.6 Hz, 3H), 1.37 (d, J = 4.4 Hz, 3H), 1.42 (q, J = 11.2 Hz, 1H), 1.82 (d, J = 12.4 Hz, 1H), 2.24 (s, 3H), 3.80–3.88 (m, 1H), 4.2 (s, NH), 4.86 (q, J = 4.8 Hz, 1H), 4.94 (t, J = 9.8 Hz, 1H), 6.65 (d, J = 7.6 Hz, 2H), 7.00 (d, J = 8.0 Hz, 2H). 13C-NMR (100 MHz, CDCl3):δ = 20.60, 21.1, 21.79, 38.82, 72.08, 81.96, 96.69, 114.56, 128.56, 129.86, 142.72. LCMS: m/z = 222.14 (M + 1)+, 204.16 (M + 1-H2O)+.
2,6-dimethyl-N-(3-nitrophenyl)-1,3-dioxan-4-amine (5 g)
Mp 122–124 °C. IR (cm−1): 1,093, 1,152, 1,332, 1,520, 1,618, 2,868, 2,980, 3,093, 3,398. 1H-NMR (400 MHz, CDCl3):δ = 1.27 (d, J = 6.0 Hz, 3H), 1.37 (d, J = 5.2 Hz, 3H), 1.46–1.56 (m, 1H), 1.83 (d, J = 12.8 Hz, 1H), 3.82–3.90 (m, 1H), 4.04-4.1 (bs, 1NH), 4.90 (q, J = 5.2 Hz, 1H), 5.00 (d, J = 4.8 Hz, 1H), 6.94 (d, J = 8.4 Hz, 1H), 7.21 (t, J = 8.2 Hz, 1H), 7.46 (s, 1H), 7.52 (d, J = 8.8 Hz, 1H).13C-NMR (100 MHz, CDCl3):δ = 20.97, 21.43, 38.13, 71.96, 80.94, 96.75, 108.27, 113.65, 120.15, 129.77, 146.13, 149.11. EIMS: m/z = 234.60 (M-H2O)+.
N-(4-bromophenyl)-2,6-dimethyl-1,3-dioxan-4-amine (5 h)
Mp 124–126 °C. IR (cm−1): 587, 836, 1,089, 1,160, 1,380, 1,497, 1,589, 2,868, 2,979, 3,068, 3,357. 1H-NMR (400 MHz, CDCl3):δ = 1.21 (d, J = 6.0 Hz, 3H), 1.30 (d, J = 5.2 Hz, 3H), 1.37 (q, J = 10.9 Hz, 1H), 1.75 (d, J = 12.8 Hz, 1H), 3.73–3.81 (m, 1H), 4.37 (s, NH), 4.79 (q, J = 5.0 Hz, 1H), 4.90 (dd, J 1 = 2.0 Hz, J 2 = 2.0 Hz, 1H), 6.65 (d, J = 8.4 Hz, 2H), 7.13 (d, J = 8.4 Hz, 2H). 13C-NMR (100 MHz, CDCl3):δ = 21.09, 21.47, 38.62, 71.19, 81.64, 96.73, 111.20, 116.03, 132.15, 144.20. EIMS: m/z = 267.50 (M-H2O)+.
N-(4-chlorophenyl)-2,6-dimethyl-1,3-dioxan-4-amine (5i)
Mp 119–121 °C. IR (cm−1): 595, 833, 1,088, 1,150, 1,380, 1,430, 1,498, 1,595, 2,868, 2,980, 3,356. 1H-NMR (400 MHz, CDCl3):δ = 1.30 (d, J = 6.0 Hz, 3H), 1.29 (d, J = 5.2 Hz, 3H), 1.43 (q, J = 10.8 Hz, 1H), 1.82 (d, J = 12.4 1H), 3.80–3.87 (m, 1H), 4.37 (s, NH), 4.78 (q, J = 5.2 Hz, 1H), 4.90 (dd, J 1 = 2.4 Hz, J 2 = 2.4 Hz, 1H), 6.55 (d, J = 8.4 Hz, 2H), 7.13 (d, J = 8.4 Hz, 2H). 13C-NMR (100 MHz, CDCl3):δ = 21.11, 21.57, 40.51, 72.05, 81.64, 96.75, 115.58, 124.05, 129.24, 143.75. EIMS: m/z = 223.60 (M-H2O)+.
N-(4-fluorophenyl)-2,6-dimethyl-1,3-dioxan-4-amine (5j)
Mp 102–104 °C. IR (cm−1): 816, 917, 1,095, 1,150, 1,421, 1,508, 1,610, 2,866, 2,976, 3,060, 3,400. 1H-NMR (400 MHz, CDCl3):δ = 1.27 (d, J = 5.6 Hz, 3H), 1.37 (d, J = 5.2 Hz, 3H), 1.41–1.47 (m, 1H), 1.80 (d, J = 12.8 Hz, 1H), 3.79–3.85 (m, 1H), 4.33 (bs, 1NH), 4.82–4.89 (m, 2H), 6.64–6.66 (m, 2H), 6.85–6.89 (m, 2H).13C-NMR (100 MHz, CDCl3):δ = 21.05, 21.48, 38.56, 72.03, 82.13, 96.62, 115.32, 115.70, 141.33, 156.76. EIMS: m/z = 207.6 (M-H2O)+.
2,6-dimethyl-N-(4-nitrophenyl)-1,3-dioxan-4-amine (5 k)
Mp 116–118 °C. IR (cm−1): 1,093, 1,152, 1,332, 1,520, 1,618, 2,868, 2,980, 3,093, 3,398. 1H-NMR (400 MHz, CDCl3):δ = 1.30 (d, J = 6.4 Hz, 3H), 1.38 (d, J = 4.8 Hz, 3H), 1.42–1.50 (m, 1H), 1.86–1.9 (m, 1H), 3.79–3.92 (m, 1H), 4.46 (bs, 1NH), 4.88–4.92 (m, 1H), 5.01–5.08 (m, 1H), 6.61 (d, J = 8.8 Hz, 2H), 8.05 (d, J = 9.2 Hz, 2H).13C-NMR (100 MHz, CDCl3): δ = 21.00, 21.48, 38.15, 71.88, 80.49, 96.86, 113.47,126.48, 139.13, 152.71. EIMS: m/z = 234.60 (M-H2O)+.
N-(4-methoxyphenyl)-2,6-dimethyl-1,3-dioxan-4-amine (5 l)
Mp 105–107 °C. IR (cm−1): 1,092, 1,155, 1,261, 1,389, 1,448, 1,512, 1,590, 2,857, 2,978, 3,351. 1H-NMR (400 MHz, CDCl3):δ = 1.21 (d, J = 6.0 Hz, 3H), 1.30 (d, J = 4.8 Hz, 3H), 1.42 (q, J = 11.2 Hz, 1H), 1.82 (d, J = 12.4 Hz, 1H), 3.632 (s, 3H), 3.67-3.69 (m, 1H), 4.1 (s, NH), 4.75–4.82 (m,2H), 6.63 (d, J = 9.2 Hz, 2H), 6.71 (d, J = 8.8 Hz, 2H).13C-NMR (100 MHz, CDCl3):δ = 21.0, 21.39, 38.80, 55.78, 71.99, 82.54, 96.59, 114.85, 115.43, 138.94, 153.27.
References
T. Bach, J. Lobel, Synthesis 2521 (2002)
S. Shang, D.S. Tan, Curr. Opin. Chem. Biol. 9, 248 (2005)
J.C. Wong, S.M. Sternson, J.B. Louca, R. Hong, S.L. Schreiber, Chem. Biol. 11, 1279 (2004)
C. Pinilla, J.R. Appel, E. Borras, R.A. Houghten, Nat. Med. 9, 118 (2003)
D.L. Aubele, S. Wan, P.E. Floreancig, Angew. Chem. Int. Ed. 44, 3485 (2005)
G. Marucci, A. Piero, L. Brasili, M. Buccioni, D. Giardina, U. Gulini, A. Piergentili, G. Sagratini, Med. Chem. Res. 14, 274 (2005)
K. Chan, Y.H. Ling, T. Loh, Chem. Commun. 939 (2007)
M. Schmidt, J. Ungvari, J. Glode, B. Dobner, A. Langner, Bioorg. Med. Chem. 15, 2283 (2007)
M. Sax, B. Wünsch, Current topics. Med. Chem. 6, 723 (2006)
N.C. Yang, D.H. Yang, C.B. Ross, J. Am. Chem. Soc. 81, 133 (1959)
R.C. Fuson, W.E. Ross, C.H. McKeever, J. Am. Chem. Soc. 60, 2935 (1938)
W.C. Lumma, O.H. Ma, J. Org. Chem. 35, 2391 (1970)
R. Jasti, S.D. Rychnovsky, J. Am. Chem. Soc. 128, 13640 (2006)
J. Barluenga, A. Dieguez, A. Fernandez, F. Rodriguez, F.J. Fananas, Angew. Chem. Int. Ed. 45, 2091 (2006)
Y.S. Cho, K. Karupaiyan, H.J. Kang, A.N. Pae, J.H. Cha, H.Y. Koh, M.H. Chang, Chem. Commun. 2346 (2003)
H. Prins, J. Chem. Weekbl. 16, 1072 (1919)
D.R. Adams, S.P. Bhatnagar, Synthesis 661 (1977)
E. Arundale, L.A. Mikeska, Chem. Rev. 51, 505 (1952)
Y. Gu, A. Karam, F. Jerome, Barrault. J. Org. Lett. 9, 3145 (2007)
E.A. Kobzar, D.V. Korchagina, N.F. Salakhutdinov, K.G. Ione, V.A. Barkhash, J. Org. Chem. USSR (Engl. Transl.) 28, 1030 (1992)
J. Tateiwa, K. Hashimoto, T. Yamauchi, S. Uemura, Bull. Chem. Soc. Jpn 69, 2361 (1996)
S. Chandrasekhar, B.V.S. Reddy, Synlett 851 (1998)
B.B. Snider, The Prins and carbonyl-ene reactions, in Comprehensive Organic Synthesis, 4th edn., ed. by B.M. Trost (Pergamon Press, Oxford, 1991)
M. Delmas, A. Gaset, Synthesis 871 (1980)
R.E. Gharbi, M. Delmas, A. Gaset, Synthesis 361 (1981)
K. Rajesh, B. Palakshi Reddy, V. Vijayakumar, Res. Chem. Intermediat. 38, 1629 (2012)
S.R. Jebas, H.-K. Fun, C.K. Quah, C.W. Ooi, G. Rambabu, V. Vijayakumar, S. Sarveswari, J. Chem. Crystallogr. 43, 523 (2013)
A. Savignac, M. Bon, A. Lattes, Bull. Soc. Chim. Fr. 3167 (1972)
Y. Wu, D. Li, J. Am. Chem. Soc. 134, 14334 (2012)
G. Rambabu, Z. Fatima, B. Palakshi Reddy, V. Vijayakumar, D. Velmurugan, Acta. Cryst. 69, 1602 (2013)
Y.B. Kiran, C.D. Reddy, D. Gunasekar, C.S. Reddy, A. Leon, L.C.A. Barbosa, Eur. J. Med. Chem. 43, 885 (2008)
D.D. Perrin, W.L.F. Armarego, Purification of laboratory chemicals, 4th edn. (Butterworth-Heinemann, Oxford, 2003)
Acknowledgments
The authors are thankful to the management of Sree Vidyanikethan Engineering College for their generous support and facilities and also to the management of VIT University, Vellore, for their support. We thank SIF-Chemistry, VIT University, for providing NMR facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rambabu, G., Palakshi Reddy, B., Kiran, Y.B. et al. Simple and efficient synthesis of 4-arylamino-1,3-dioxanes in aqueous medium: a new route for the Prins reaction. Res Chem Intermed 41, 8441–8450 (2015). https://doi.org/10.1007/s11164-014-1902-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-014-1902-4