Abstract
The white shark (Carcharodon carcharias) is a globally distributed top predator. Due to its ecological importance and historical declining population trends, data contributing to conservation initiatives (e.g. habitat protections and resource management) pertaining to all life stages of this species are essential to facilitate population recovery. Of particular interest, the locations and discrete seasonality of C. carcharias parturition remain uncertain. Understanding C. carcharias parturition in relation to each population is relevant to population recovery since neonate to young-of-the-year (YOY) sharks are more vulnerable to predation and particularly threatened by and susceptible to commercial fishing pressure. Herein, this paper provides a synthesis from published literature across seven well-studied C. carcharias populations to identify common trends associated with parturition location, seasonality, and habitat characteristics. The data reviewed in this study are consistent with previous population-specific hypotheses, that C. carcharias parturition occurs during spring and summer for all populations. Further, this review also indicates that parturition likely occurs in insular shelf waters and water temperatures ranging from15.7 to 23.1 °C. Although discrete parturition sites were not identified, the compiled data are suggestive that C. carcharias parturition may occur over horizontal and vertical spatial scales that exceed the inshore, shallow water environments associated with nursery area habitat to perhaps minimize predation by conspecifics. Due to the vulnerability of C. carcharias, conducting non-lethal technological (e.g., baited remote underwater video systems—BRUVS), morphological (i.e., ontogenetic changes in dorsal fin shape), and reproductive (e.g., blood chemistry and ultrasonography) research that may help identify parturition location and seasonality are thus warranted.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The white shark (Carcharodon carcharias) is a top predator that has a global distribution in both temperate and tropical seas. C. carcharias is characterized by having a wide dietary preference, which ranges from vertebrates (e.g., marine mammals, teleosts, other elasmobranchs, chelonians) to invertebrates (e.g., cephalopods) (Casey and Pratt 1985; Cliff et al. 1989; Compagno 1984; Fergusson 1996; Klimley 1994; Martin et al. 2005; Tricas and McCosker 1984). This highly migratory species has low fecundity, producing 2–14 pups per litter (Francis 1996; Saïdi et al. 2005; Uchida et al. 1996), slow growth (Natanson and Skomal 2015; Wintner and Cliff 1999), and late sexual maturity (Natanson and Skomal 2015), which is estimated to occur at > 3.80 m total length (TL) for males and > 4.50 m TL for females (Francis 1996; Pratt 1996; Wintner and Cliff 1999). There are seven well-studied white shark populations: Southern-Western Australia (Bruce 2016; McAuley et al. 2017), Western North Atlantic (WNA, Franks et al. 2021; Skomal et al. 2017), Northeastern Pacific (NEP, Domeier and Nasby-Lucas 2013), Eastern Australian and New Zealand (Bruce et al. 2019), Mediterranean (Agostino Leone et al. 2020), South Africa (Kock et al. 2013), and Northwest Pacific (Tanaka et al. 2011; Fig. 1). Further, there are two understudied white shark populations: South American Atlantic (Cione and Barla 2008), and South American Pacific (Bustamante et al. 2014; Fig. 1). Due to its low rebound potential and current estimated stock status, C. carcharias is listed as vulnerable with a decreasing population trend on a global scale (Rigby et al. 2019) according to the International Union for the Conservation of Nature Red List. However, the Mediterranean population is listed as critically endangered due to a lack of effective management measures and extensive fishing pressure (Soldo et al. 2016a, b).
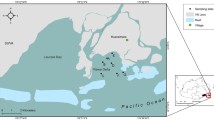
Map illustrating the relative locations of white shark (Carcharodon carcharias) populations (modified from Huveneers et al. 2018). The populations are: Northeastern Pacific (NEP), Western North Atlantic (WNA), Mediterranean (MED), South African (SA), Northwest Pacific (NWP), Southern-Western Australian (SWA), Eastern Australian and New Zealand (EA), South American Pacific (SAP), and South American Atlantic (SAA)
Due to their large size and substantial geographic ranges, considerable challenges exist in obtaining key life history (e.g., parturition and mating locations), behavioral, and ecological data on C. carcharias. Furthermore, the importance of sharks, especially top predators, to marine ecosystems (Burkholder et al. 2013; Ferretti et al. 2010) combined with their vulnerability to anthropogenic stressors (e.g., fishing gears; Benson et al. 2018) makes the identification of regions of critical life history stages, such as parturition and nursery areas, of increasing importance. Shark nursery areas are defined as critical regions where: (1) young-of-the-year (YOY)/juveniles are encountered more frequently in the area than in other areas; (2) YOY/juveniles remain in or return to the area over an extended period; and (3) there is repeated use of the area over several years by YOY/juvenile sharks (Heupel et al. 2007). Presently, the young life stage size classes for C. carcharias are as follows: neonate sharks range in size from 1.05 to 1.50 m in total length (TL; as described in Francis 1996; Pratt 1996), young-of-the-year sharks range from 1.50 to 1.75 m TL, and juveniles range from 1.75 to 3.00 m (as described in Bruce and Bradford 2012). To date, several nursery areas have been identified for white shark populations in the Northeastern Pacific (Anderson et al. 2021; Oñate-González et al. 2017; Tamburin et al. 2020), the western North Atlantic (WNA, Casey and Pratt 1985; O’Connell et al. 2021), and the South Pacific Ocean (Bruce and Bradford 2012; Spaet et al. 2020).
In addition to nursery areas, it is important to know the location of parturition or pupping habitat for C. carcharias. Recently pupped neonatal white sharks (i.e., 1.05–1.50 m TL) represent the most vulnerable of the species due to higher risks of predation (Benson et al. 2018) in addition to their susceptibility to capture in inshore fisheries (Lyons et al. 2013; Oñate-González et al. 2017). Presently, C. carcharias parturition grounds have not been identified.
Although C. carcharias populations are exhibiting signs of recovery in numerous regions (e.g., WNACurtis et al. 2014; Skomal et al. 2012), continued recovery is dependent upon the further identification and management of these critical habitats. Since a global synthesis of white shark parturition is lacking in the scientific literature, this review presents tagging, behavioral, and sightings data for the seven well-studied populations (i.e., excluding the lesser studied South American Atlantic and Pacific populations) with the objectives of: (1) identifying any abiotic (e.g., seasonality, water temperature, water depth) or biotic (e.g., prey availability) indicators of potential parturition sites; (2) determining any morphological characteristics indicative of recent parturition, thereby providing insight to parturition seasonality and location; and (3) providing future considerations for research.
White shark populations
Western North Atlantic region
In the Western North Atlantic (WNA), catch data in combination with extensive tagging research have provided a comprehensive understanding of C. carcharias migratory patterns (Curtis et al. 2018; Franks et al. 2021; Skomal et al. 2017). Sharks exhibit a large migratory range, extending from Newfoundland, Canada to the Gulf of Mexico, USA (Franks et al. 2021; Skomal et al. 2017; Fig. 1). Presently, one large and expansive nursery area has been identified in this region: the New York Bight (Casey and Pratt 1985; Curtis et al. 2018; O’Connell et al. 2021).
Key sightings and historical data
A collection of historical sightings records (i.e., fisheries-dependent data, media reports, scientific records) and field observations can provide a comprehensive understanding of various life-history characteristics of a species (e.g., migratory patterns, distribution; Casey and Pratt 1985; Curtis et al. 2014). One such WNA-based study compiled sightings/catches of 380 different C. carcharias (Casey and Pratt 1985), whereas a later study (Curtis et al. 2014) built upon that earlier work by incorporating historical data associated with an additional 269 (i.e., total analyzed was 649 sightings/catches) confirmed C. carcharias records from 1800 to 2010. Of these sightings, 124 were YOY and 310 were juvenile sharks. YOY sharks were found to be concentrated within continental shelf waters, with more frequent encounters occurring between New Jersey, USA and Massachusetts Bay, USA during the summer months, and exhibiting a southerly movement from November and December (Casey and Pratt 1985; Curtis et al. 2014). Neonate C. carcharias (n = 46) were documented more frequently within the region of Great Bay, NJ, USA to Shinnecock Inlet, NY, USA between June and October. Of these neonates, the smallest shark reported was 1.05 m TL, whereas the smallest shark examined by the authors was 1.22 m TL (Casey and Pratt 1985). While mature-sized female C. carcharias were reported during the summer months within this region, none of the few females examined were found to be gravid or port-partum and thus it remains uncertain as to the location of parturition. Beyond distribution, Curtis et al. (2014) also compiled data related to habitat use. The median depth and sea surface temperature (SST) of occurrence/capture was found to be 19.5 ± 1.9 °C (mean ± 1 SD) and 32 ± 19 m) for YOY and 18° ± 3.5 °C and 26 ± 74 m for juveniles, respectively.
More recently, between 2016 and 2021, a total of seven neonate to YOY C. carcharias were captured and tagged in Montauk, NY, USA (within the NY Bight; O’Connell et al. unpublished data). Captured using a rod-and-reel sampling technique, these five females and two males had a mean TL (± S.D.) of 1.33 m (± 0.18) (Table 1). Of note, two males (1.07 m TL and 1.22 m TL) had a conspicuous rounded apex on the first dorsal fin (Fig. 2). During these captures, sea surface temperature (SST) ranged from 19.2 to 21.2 °C and water depth ranged from 14.0 to 23.0 m.
Dorsal fin comparison between a neonate and adult white shark (Carcharodon carcharias). A. This image emphasizes the rounded apex on the first dorsal fin of a neonate white shark captured in Montauk, New York, USA (Western North Atlantic population). This male shark was 1.22 m total length (TL) and captured in August 2021 (O’Connell et al. Unpublished). B. This image emphasizes the more triangular shape and pointed apex on the first dorsal fin of an adult female white shark (5.0 m TL) sighted at Guadalupe Island, MX in December 2022
Selected tagging studies
Several white shark tagging studies have been conducted within the WNA that help shed light on both the fine-scale movements and distribution patterns on an inter-annual basis (Curtis et al. 2018; Franks et al. 2021; Skomal et al. 2017). Skomal et al. (2017) and Franks et al. (2021) focused on the movement ecology associated with 32 (2.4–5.2 m TL) and 48 C. carcharias (2.0–5.01 m TL), respectively. Consistent with the previously reported sightings data (e.g., Casey and Pratt 1985), these sharks exhibited an ontogenetic shift in movement patterns, with all sharks utilizing continental shelf waters, but sub-adult and adult sharks exhibiting a more expansive range that included pelagic habitat. The data collected by both studies suggested that adult females were more likely to utilize pelagic waters over males. Furthermore, Skomal et al. (2017) demonstrated that all tagged sharks remained in shelf waters during the summer months which may be suggestive of coastal parturition. However, Skomal et al. (2017) stated that they obtained no evidence of adult females aggregating within the New York Bight to give birth and suggested that this area may not be where parturition occurs, but rather a site that neonate and YOY C. carcharias migrate to forage on an abundance of prey (Casey and Pratt 1985; Sullivan 1991).
Most relevant to the present study, two recent tagging studies focused on neonates and juveniles tagged in the New York Bight (Curtis et al. 2018; Shaw et al. 2021). Curtis et al. (2018) analyzed data from 10 YOY sharks (1.38–1.66 m TL) whereas Shaw et al. (2021) built upon the aforementioned study by analyzing data from an additional 13 YOY sharks (1.38–1.66 m). Sharks were demonstrated to exhibit seasonal residency within the NY Bight from August to October, after which they moved southerly in late fall to North and South Carolina waters. While in the New York Bight, these sharks remained from 0.1 to 131.5 km from shore and selected for waters with depths of 20–30 m and SSTs ranging from 20.0 to 22.0 °C (Shaw et al. 2021). These authors suggested that the lack of large C. carcharias in this area makes the NY Bight an ideal habitat for young sharks due to minimal predation risk (Shaw et al. 2021).
Northeastern Pacific region
In the Northeastern Pacific (NEP), research has been conducted on various life-history aspects of C. carcharias (Domeier and Nasby-Lucas 2013; Weng et al. 2007). Some of this research has revealed unique offshore foraging and potential mating habitat (e.g., Domeier and Nasby-Lucas 2013; Jorgensen et al. 2012), nursery areas (Oñate-González et al. 2017; Weng et al. 2007), and varying coastal aggregation sites (e.g., Jorgensen et al. 2012; Weng et al. 2007; Domeier and Nasby-Lucas 2013). Presently, there are two nursery areas that have been identified in this region: the Southern California Bight (SCB; Anderson et al. 2021) and Baja California, Mexico (Oñate-González et al. 2017). However, recent captures of neonatal C. carcharias outside of this region may highlight that the nursery area could be more extensive than previously understood (Santana-Morales et al. 2020). Presently, this population ranges from Alaska (Martin 2004) to Mexico (e.g. Santana-Morales et al. 2012), with extensive offshore migrations (Domeier et al. 2012; Hoyos-Padilla et al. 2016; Fig. 1).
Key sightings and historical data
In the NEP, multiple studies reported on the capture of numerous neonate C. carcharias (Oñate-González et al. 2017; Santana-Morales et al. 2012, 2020). More specifically, Oñate-González et al., (2017) utilized incidental catch records from Bahía Sebastián Vizcaíno, Mexico. Data associated with the capture of 390 white sharks between 1999 and 2013 revealed that sharks were present in Bahia Sebastian Vizcaino consistently throughout the year; however, neonates were most frequently captured between May and September (Oñate-González et al. 2017). The authors suggested that, consistent with previously described nursery areas (e.g., Barton et al. 2012; Bruce and Bradford 2012), the abundance of prey within Bahia Sebastian Vizcaino was a driver of neonate-juvenile residency. Santana-Morales et al. (2012) analyzed the incidental captures (e.g. bycatch) of 111 YOY to juvenile C. carcharias from various fisheries along the Pacific coast of Baja California, Mexico. Of these reported captures, 79.8% were considered YOY sharks, whereas the remainder were juveniles. Captures occurred within continental shelf waters where SST ranged from 15.7 to 23.1 °C. Santana-Morales et al. (2020) provided a detailed report on an incidentally captured 1.07 m TL white shark from Baja California, MX. Although this specimen was dead, researchers noted that it had a rounded apex on the first dorsal (Fig. 3) and concluded that this was a free-living specimen because it had no embryonic teeth or dermal denticles in its stomach. Although parturition was not discussed, this specimen was taken between the two ENP nursery areas—Bahía Sebastián Vizcaíno, Mexico (Oñate-González et al. 2017) and the Southern California Bight (SCB, Weng et al. 2007) and was, therefore, indicative of potential extended trans-national nursery habitat (i.e., USA and Mexico), suggesting the need for a more comprehensive and multi-national management approach.
This image is from a 1.07 m total length (TL) white shark (Carcharodon carcharias) captured in June, 2018 in a bottom gillnet in Baja California, MX (Northeastern Pacific population; Modified from Santana-Morales et al. 2020) with permission from the American Society of Ichthyologists and Herpetologists. This specimen was dead, but researchers noted that the shark had a rounded apex on the first dorsal fin and was a free-swimming specimen
Selected tagging studies
Domeier and Nasby-Lucas (2013) conducted a multi-year satellite tagging study at Guadalupe Island, MX to assess the movements of adult female C. carcharias. Using these tags, four migratory phases were identified: (1) Offshore Gestation Phase (average duration = 15.5 months), (2) Parturition Phase along the Mexican coast between April and August; (3) Pre-Aggregation Phase during which females were in transition between the parturition phase and Guadalupe Island; and (4) Guadalupe Island Aggregation Phase when mature females arrive at Guadalupe Island between late September and early October (Domeier and Nasby-Lucas 2013). More specifically, the data demonstrated that this white shark population spent the greatest amount of time in the pelagic environment relative to any other habitat (e.g., coastal ecosystems). It was hypothesized that females utilize these offshore habitats as they provide warmer water temperatures facilitating optimal growth of developing embryos (Domeier and Nasby-Lucas 2013), after which they initiate the parturition phase within coastal ecosystems. Tagging data revealed that these large females remained within the coastal habitats for 52–77 days and, therefore, the exact location of parturition could not be determined; it was also uncertain if pups were born simultaneously or over a prolonged period (Domeier and Nasby-Lucas 2013).
Within the SCB nursery area, various studies utilized tagging to track the movements of YOY and juvenile C. carcharias that ranged in size from 147.0 to 250.0 cm TL (Weng et al. 2007) and 128.0–175.0 cm TL (Anderson et al. 2021). Weng et al. (2007) assessed the behavior of sharks of varying life classes (e.g., YOY vs. 3 years old) and showed that older sharks exhibited slightly deeper excursions into cooler water than YOY sharks. Furthermore, Weng et al. (2007) observed that YOY sharks moved into Mexican waters during the Autumn months in contrast to 3-yr old sharks, which exhibited a more northward trajectory during the winter months. It is suggested that this movement variation in relation to life-stage may be associated with niche expansion into a region that is physiologically restrictive to smaller, YOY sharks (Weng et al. 2007). Consistent with Weng et al. (2007), Anderson et al. (2021) demonstrated that sea temperature is a good predictor of shark presence, with generally lower YOY presence during temperature extremes (i.e., YOY white sharks have a narrower temperature range). Furthermore, this study revealed that the SCB is a broad region with suitable habitat resources that results in a spatiotemporally dynamic nursery area (Anderson et al. 2021). Although this study was the most comprehensive YOY study conducted in this region to date, it could not provide further insight as to where parturition occurs. Therefore, based on hypothesis presented in previous studies (e.g. Domeier 2012; Klimley 1994), Anderson et al. (2021) concluded that parturition is likely to occur offshore in deeper water after which neonates make their way inshore to ecologically rich and sheltered environments.
Although these studies detail the migratory patterns of YOY and juvenile white sharks, changing sea surface temperatures have influenced the range of these sharks (Tanaka et al. 2021). More recently, Tanaka et al. (2021) examined the effect of the North Pacific marine heatwave on YOY and juvenile C. carcharias within the SCB. In this study, data from field surveys and public observations in combination with satellite tags from 14 white sharks (1.4–2.0 m TL) were assessed. With a sea surface temperature that peaked approximately 6.2 °C above the historical average (Gentemann et al. 2017), these results demonstrated a significant, northerly shift in shark movement, or roughly 280 km straight line distance than what is typical for this population (Weng et al. 2007; White et al. 2019). Such a finding is relevant and similar to previous studies, which demonstrated that climate change is contributing to the redistribution of both marine and terrestrial species (Hammerschlag et al. 2022; Sanford et al 2019a, b). This redistribution warrants further research that identifies these critical regions (e.g., nursery and parturition areas) since continued environmental change may result in the shifting of these important life-history sites into a location with increased anthropogenic impacts and threats.
Eastern Australian-New Zealand region
In the Eastern Australian-New Zealand region, extensive research has been conducted on white shark migratory patterns (Bruce et al. 2019), population genetics (Blower et al. 2012), and nursery habitat (Bruce and Bradford 2012; Spaet et al. 2020). Although ongoing research suggests that Australian white sharks may be part of one large population, previous population genetics research suggested that Australia has two distinct C. carcharias populations that are seemingly reproductively divided by the Bass Strait: the southwestern Australian and eastern Australian populations (Blower et al. 2012). The Eastern Australian and New Zealand population ranges from Victoria to Central Queensland, Australia; however, this population has been demonstrated to carry out extensive migrations, from the Australian mainland to New Zealand (Bruce et al. 2019; Spaet et al. 2020; Fig. 1).
Key sightings and historical data
In November 1991, a 5.35 m TL gravid female C. carcharias was captured in a mesh fishing net in New Zealand (Francis 1996). While this animal was reported to have seven embryos, five were discarded and only two were recovered and measured. These embryos were 1.43 and 1.45 m TL, with both embryos exhibiting a rounded apex on the first dorsal fin (Francis 1996; Fig. 4A). Furthermore, there were two additional and notable neonate encounters within New Zealand waters. In March 2019, a dead specimen was found on Ninety Mile Beach that was 1.05 m TL and 7.8 kg (Auckland Museum, Unpublished Data; Fig. 4B). Similar to other embryonic C. carcharias, this neonatal specimen had a rounded apex on the first dorsal fin (Auckland Museum, Unpublished data). Lastly, a 1.59 m TL, 27.3 kg specimen was captured within a commercial fishing net in New Plymouth, New Zealand (Fig. 4C). This male shark exhibited similar characteristics to both the embryonic and neonate shark in that it had a rounded apex on the first dorsal fin and was further estimated to be 2 to 3 months old (NZ Dept of Conservation, Unpublished Data).
These images are of embryonic and free-swimming white sharks (Carcharodon carcharias) that were encountered in association with the Eastern Australian and New Zealand population. A. Image of a 1.45 m total length (TL) C. carcharias embryo that was one of seven full-term pups found within a 5.35 m TL gravid female C. carcharias (Francis 1996). This shark was captured in November of 1991at North Cape, New Zealand and was characterized by having a rounded apex on the first dorsal fin. B. Image of a 1.05 m TL C. carcharias that was found deceased in March 2019 on Ninety Mile Beach, New Zealand (©Auckland Museum). Similar to the embryonic shark, this shark was characterized by having a rounded apex on the first dorsal fin. C. This image is of a 1.59 m TL C. carcharias that was captured in a mesh net at New Plymouth, New Zealand in March 2017 (©New Zealand Department of Conservation). This shark had a rounded apex on the first dorsal fin
Selected tagging studies
There are two identified nursery areas associated with the eastern Australia population: Port Stephens, New South Wales and Corner Inlet/Ninety-Mile Beach, Victoria (Bruce and Bradford 2012). Within this nursery region, various studies were conducted on both YOY and juvenile sharks (Bruce and Bradford 2012; Bruce et al. 2019; Spaet et al. 2020). Bruce and Bradford (2012) assessed the movements of 22 juveniles ranging in size from 1.75 to 2.60 m TL and Bruce et al. (2019) built upon that previous study by assessing the movements of 43 YOY to juvenile sharks ranging from 1.70 to 3.20 m TL. A further study conducted by Spaet et al. (2020) analyzed data from 103 YOY and juvenile C. carcharias that ranged from 1.63 to 3.78 m TL. These studies showed that sharks exhibited seasonal movements within the respective nursery areas. They spent 45.9% of their time in water temperatures ranging from 18 to 20 °C; however, they occupied temperatures that ranged from 6–8 to 24–26 °C (Bruce and Bradford 2012). All studies demonstrated that these sharks exhibited expansive movements (e.g., Australian mainland to New Zealand Bruce et al. 2019; Spaet et al. 2020), both on the coast and the open ocean. Although these sharks did exhibit broad-scale movements (e.g., 8500 km in 536 days; Bruce et al. 2019), they also exhibited residency and inter-annual utilization of these nursery areas, which is consistent with the nursery area criteria provided by Heupel et al. (2007). Interestingly, there was connectivity between these nursery areas for individual sharks, but these movements were rapid and direct. Most notably and likely due to the large sample size, researchers were able to show connectivity between the southwestern Australian and eastern Australian populations (Spaet et al. 2020), although this was exhibited by only two sharks. However, ongoing research is aimed at determining if this mixing may be sufficient to facilitate adequate gene flow that would result in genetic similarities between the populations. Although these studies focused on YOY and juvenile sharks, little discussion was offered on shark parturition, where it may occur, and how large neonatal sharks are within this region.
Southern-Western Australian region
In association with the Southern-Western Australian population, research pertaining to various C. carcharias life-history characteristics has been conducted, with particular focus on well-known aggregation sites—the Neptune Islands (Bruce and Bradford 2015; Bradford et al. 2020; Watanabe et al. 2019), the Great Australian Bight (McAuley et al. 2017), and Recherche Archipelago (O’Connell et al. 2023; Werry 2017). The Southern-Western Australian population ranges from western Victoria to as far north as the Montebello Islands in the northwest of Western Australia (e.g., Bradford et al. 2020; Fig. 1); however, data identifying the location(s) of nursery or parturition areas within this region is insufficient (Bruce 2016; Werry 2017).
Key sightings and historical data
In a report by Malcolm et al. (2001), a 1.27 m TL, 14.5 kg C. carcharias was captured in a gillnet in March/April 1994 in South Australia. An additional small specimen was captured and considerable damage was done to the gillnet, which the authors suggested may have been due to a larger shark. Thus, the authors hypothesized that these two specimens may have been aborted pups since they had lower weights in comparison to reported neonatal weights of 26–32 kg (Francis 1996). In 2020, two additional captures by commercial gillnetters targeting gummy sharks (Mustelus antarcticus) were made in the insular shelf-associated waters (< 20 m) near Cocklebiddy, Western Australia (O’Connell et al. 2023). These female sharks were 1.40 m and 1.70 m TL, had no presence of scars suggestive that they had minimal biological (e.g., prey) and ecological (e.g., environmental) interactions, and most notably, the smaller shark had a rounded apex on the first dorsal fin; however, the larger shark did not (Fig. 5). Lastly, in February and March 2020–2021, researchers utilized stereo-photogrammetry and laser photogrammetry to accurately measure numerous YOY-juvenile C. carcharias at two known aggregation sites: Salisbury Island and Daw Island (Werry 2017). These sites ranged in depth from 9.0 to 17.0 m, with an SST of 18.0 to 20.5 °C. During these expeditions, researchers encountered a 1.58 m, 1.77 m, 1.83 m, and 1.95 m TL C. carcharias, with the latter three exhibiting multi-day site fidelity (n = 3 days) at Daw Island (O’Connell et al. 2023). It is important to note that the shark (1.58 m) encountered at Salisbury Island had a rounded apex on the first dorsal fin; however, the three sharks at Daw Island did not.
Examples of white shark (Carcharodon carcharias) bycatch associated with a commercial fishery operation in Western Australia (Southern-Western Australian population). A) A 1.40 m total length (TL) C. carcharias female captured in June 2020 and was characterized as having a rounded apex on the first dorsal (O’Connell et al. 2023). B) A 1.70 m TL C. carcharias female captured in November 2020 and did not have a rounded apex on the first dorsal (O’Connell et al. 2023)
Selected tagging and attractant studies
Passive and active acoustic telemetry (e.g., McAuley et al. 2017) and satellite tagging (e.g., Bruce 2016; Bradford et al. 2020) research has been conducted on the Southern-Western Australian C. carcharias population. Within this region, the Great Australian Bight and the Recherche Archipelago in Western Australia in combination with the Neptune Islands of South Australia are some of the best known aggregation sites (Bruce and Bradford 2015; Huveneers and Lloyd 2017; McAuley et al. 2017), coinciding with high densities of New Zealand fur seals (Arctocephalus forsteri) and Australian sea lions (Neophoca cinerea; Shaughnessy et al. 2007). To elucidate patterns of abundance and migration, as well as learn more about the life-history of this population, numerous studies have been conducted (Bruce 2016; Huveneers and Lloyd 2017; McAuley et al. 2017; Robbins 2007). McAuley et al. (2017) analyzed acoustic tag data collected from 89 sub-adult and adult C. carcharias in Western Australia between December 2008 and May 2016. Detections revealed that these sharks utilized the waters off the South and West coast of Western Australia throughout the year, however, abundance peaked between September and December. This seasonal peak in abundance was suggested to be prey-dependent, since it coincided with the seasonal spawning aggregations of snapper (Chrysophrys auratus; McAuley et al. 2017). Bruce (2016) tagged juvenile and adult C. carcharias with the intention of nursery habitat identification in association with this population. Sharks were tagged at the Neptune Islands (South Australia), Bremer Bay, Israelite Bay, and Cape Arid (Western Australia). Data associated with the adult sharks demonstrated that these sharks were highly migratory, utilizing shelf habitat within South Australia and the Great Australian Bight and made substantial offshore excursions, with one 4.60 m TL female traveling 1,800 km southwest of Western Australia. Furthermore, Bruce (2016) suggested that the nursery areas associated with this population are not discrete, but rather extend over broad regions within the continental shelf. Robbins (2007) used a chum-attractant as a means to determine if sexual segregation occurs at the Neptune Islands (South Australia) and what environmental (e.g., tidal height, cloud cover) variables may contribute to this segregation. Over the course of ~ 3 years, 92 male, 32 female and 2 unsexed C. carcharias were encountered. Data revealed temporal sexual segregation with females being more frequently encountered in April to June, a period coinciding with elevated sea surface temperatures and inexperienced New Zealand fur seal (A. forsteri) pups learning how to swim. Robbins (2007) attributed this sex-specific seasonality to prey availability and increased water temperature, which may maximize embryonic developmental growth rates. However, subsequent research at this location attributes female presence to variations in foraging strategies between males and females, rather than water temperature (Bruce and Bradford 2015). Notably, females were absent from the study site during spring and early summer, which Robbins (2007) attributed to mating or parturition as it coincides with previous hypothesized periods for these activities within this population (Bruce 1992; Francis 1996). Lastly, Bradford et al. (2020) deployed 43 satellite tags on juvenile and adult (1.90–5.70 m TL) white sharks to further understand the long-range spatial distribution and migratory patterns of this population. Data provided insight into differential migratory patterns based on sex, with females exhibiting wider-ranging dispersal that extended farther offshore than males (Bradford et al. 2020). However, the researchers concluded that insufficient evidence was gathered to identify parturition and/or nursery areas and thus recommended further research be conducted within this region (Bradford et al. 2020).
South African region
In South African waters, extensive white shark research has been conducted in relation to various biological and ecological characteristics (Cliff et al. 1989). Some of this research has provided a unique glimpse into the predator–prey relationship (Fallows et al. 2016; Towner et al. 2022), movement patterns (Bonfil et al. 2005; Kock et al. 2013), key aggregation sites and how these aggregation sites have changed with time (Towner et al. 2013a, b; Hammerschlag et al. 2019), population genetics (O’Leary et al. 2015), and population size (Irion et al. 2017; Towner et al. 2013a, b). Recent genetic research demonstrated that this population is genetically distinct from that in the NWA (O’Leary et al. 2015). Furthermore, even though transoceanic migrations associated with this population have been observed (South Africa to W. Australia and back to South Africa; Bonfil et al. 2005), the range of this population is from Namibia to Mozambique (Bonfil et al. 2005; Compagno 2001; Kock et al. 2013; Fig. 1). Within this genetically distinct population, minimal research has been conducted on nursery habitat; however, presently, it is hypothesized that Algoa Bay, South Africa may represent a key seasonal nursery area for these sharks (Dicken 2008; Dicken and Booth 2013).
Key sightings and historical data
Although there is no confirmed C. carcharias nursery in South Africa, there have been numerous observations of neonate to YOY sharks within Algoa Bay. As described in Smith (1951), a 1.40 m TL male was captured in in Algoa Bay at a depth of 37 m. Although only a drawing of the specimen was provided, the shark was described to have a rounded apex on the first dorsal fin (Smith 1951). Similarly, observations of multiple YOY-early-stage juvenile C. carcharias were made during October–November 2006 while opportunistically feeding on a 15 m TL humpback whale (Megaptera novaeangliae) carcass (Dicken 2008). The whale carcass was anchored in 20 m of water where the SST ranged from 18 to 20 °C. The smallest shark was 1.50 m TL, whereas several others measured 1.60 m and 1.80 m TL. These observations, in combination with Dicken (Pers. Obs) observing approximately 15 YOY and juvenile (< 2.50 m) C. carcharias being captured in Algoa Bay during a fishing competition in October 2002, led Dicken (2008) to suggest that Algoa Bay may serve as a nursery ground for this species.
Selected tagging and mark-recapture studies
Previous research in this region focused on fine- and broad-scale movement patterns through the use of active acoustic (e.g., Gennari et al. 2022), passive acoustic (Kock et al. 2013), satellite tagging (Bonfil et al. 2005), and mark-recapture approaches (e.g., photo identification; Hewitt et al. 2018). Gennari et al. (2022) compiled 877 h of movement data associated with 19 white sharks tagged in Mossel Bay, South Africa that ranged in size from 1.50 to 4.20 m TL. Although this study didn’t provide any direct evidence of parturition nor the movements of gravid females, fine-scale movement data yielded insight into the movement patterns of three YOY and early juveniles that were 1.50 m, 1.70 m, and 1.70 m TL. These smaller sharks exhibited increased diurnal foraging behavior in comparison to larger sharks, which exhibited more specific crepuscular hunting activity and a propensity to utilize water depths less than 20 m (Gennari et al. 2022).
In False Bay, South Africa, Hewitt et al. (2018) used photo identification associated with a total of 303 white sharks to assess temporal presence/absence and population structure. No YOY sharks and few sexually mature sharks were sighted, indicating that this site does not represent a parturition or nursery area, but rather was characterized as a seasonal foraging ground for sharks of differing life-stages (Hewitt et al. 2018). Lastly, Bonfil et al. (2005) deployed 25 pop-up archival satellite-transmitting (PAT) tags and 7 satellite tags on juvenile to sub-adult C. carcharias off the Western Cape, South Africa. These sharks were tagged between June 2002 and November 2003 and ranged in size from 2.50–4.20 m TL. They exhibited high site fidelity (up to 211 days), especially in known shark aggregation sites such as Mossel Bay and Gansbaai. Of the tagged sharks, one 3.80 m TL female C. carcharias made a transoceanic migration between Gansbaai, South Africa and northwestern Australia, a distance of approximately 11,100 km in the span of 99 days. Interestingly, the shark returned to the tagging site in August 2004. The researchers noted that this shark’s transoceanic migration occurred during the mating season (Francis 1996) and its eventual return to South Africa may be reflective of natal philopatry, which has been demonstrated in other shark species (Feldheim et al. 2014).
Mediterranean region
Unlike many other locations, C. carcharias is encountered infrequently within the Mediterranean Sea, likely due to its rare and critically endangered status (Moro et al. 2019). Furthermore, there are no known conventional aggregation sites (i.e., pinniped colonies) making it difficult to obtain biological and ecological data related to this population (Gubili et al. 2011; Schilds et al. 2019). Thus, few sharks have been successfully tagged causing uncertainty in the migratory patterns associated with this population; however, notable incidental captures (e.g. Bradai and Saïdi 2013; Kabasakal 2014; Kabasakal and Özgür Gedikoğlu 2008; Kabasakal et al. 2009) have occurred with this region that provide beneficial information pertaining to C. carcharias parturition and nursery grounds. Using mitochondrial DNA, this C. carcharias population was determined to have a greater evolutionary relationship with the Eastern Australian and NE Pacific populations, in comparison to both the South African and WNA populations (Agostino Leone et al. 2020). Capture records suggest that this population extends from France to Turkey, including parts of northwest Africa (Moro et al. 2019; Soldo et al. 2016a, b; Fig. 1).
Key sightings and historical data
Presently, there are no known C. carcharias discrete nursery areas within the Mediterranean, however, scientists have suggested potential locations due to numerous captures of (1) neonates and juveniles (e.g., Sicilian Channel—Fergusson 2002; Edremit Bay—Kabasakal 2020; Gulf of Gabès – Bradai and Saïdi 2013; Fergusson 1996; Saïdi et al. 2005) or (2) gravid females with near-term embryos (Saïdi et al. 2005). In a report by Saïdi et al. (2005), an opportunistic analysis was conducted on a 5.87 m TL C. carcharias that was captured in a purse seine off the Tunisian coast (i.e., Gulf of Gabès) in February 2004. Four developing embryos were found, ranging in size from 1.32 to 1.35 m TL. These embryos were considered still developing (yolk mass of 43–45%) and all had a rounded apex on the first dorsal fin (Fig. 6A), with three embryos having a distended abdomen from yolk mass. Bradai and Saïdi (2013) compiled data collected between 1953 and 2012 from 59 incidentally captured white sharks from along the Tunisian coast. Of those captured, 47.78% were neonate to juveniles (< 200 cm TL) and concentrated in the Gulf of Gabès, and interestingly, 2 gravid females were also captured within this region. The presence of neonate to juveniles in combination with gravid C. carcharias is suggestive that the Gulf of Gabès is a nursery area and parturition may occur nearby (Bradai and Saïdi 2013). Similarly, notable neonate captures have occurred within Turkish waters (Aegean Sea; Kabasakal and Özgür Gedikoğlu 2008; Kabasakal 2014). Between the years of 2008 and 2018, six YOY C. carcharias were captured in various fisheries (gillnet and trammel net; Kabasakal and Özgür Gedikoğlu 2008; Kabasakal 2014). These sharks ranged in size from 0.85 to 1.45 m TL and were captured during the summer months (June-July). An 0.85 m TL shark was captured within a trammel net in July 2011 and released alive (Kabasakal 2014; Fig. 6B). Similarly, three specimens ranging in size from 0.80 to 1.0 m TL were captured within a gill- or trammel-net in late June/early July 2010 (Kabasakal 2014). The 1.26 m and 1.45 m TL neonate C. carcharias were also captured in gillnets within the Aegean Sea in July 2008 (Kabasakal and Özgür Gedikoğlu 2008). Both the 1.26 m and 1.45 m TL specimens exhibited the presence of an umbilical scar, suggesting these sharks were recently birthed; however, an image detailing the shape of the dorsal fin is unavailable. While photographs of the other three YOY white sharks (i.e., 0.8–1.0 m TL) are unavailable, the 0.85 m TL shark exhibited a rounded apex on the first dorsal fin (Fig. 6B), which is consistent with the embryonic sharks detailed in Saïdi et al. (2005). This 0.85 m TL C. carcharias represents the smallest free-swimming and living white shark ever recorded. Since this shark was captured in July and three other neonates (0.80–1.0 m TL) were captured in late June/early July, it is suggestive that C. carcharias parturition within the Mediterranean may occur in early summer, which is consistent with the late-spring to summer parturition postulation proposed by Francis (1996).
Examples of white shark (Carcharodon carcharias) captures in association with the Mediterranean population. A. This image is one of four embryos that were between 1.32–1.35 m total length (TL) found within a 5.87 m TL C. carcharias. This shark was captured in February 2004 in the Gulf of Gabès within a purse seine. The embryos were characterized by having a rounded apex on the first dorsal and had approximately 43–45% of their yolk mass remaining, thus illustrating that these embryos were still developing (Saïdi et al. 2005). B. This image is the world’s smallest free-swimming C. carcharias (The Photograph was taken from video collected by Cenk Balkan). The 0.85 m TL animal was captured in July 2011 in a mesh net in Edremit Bay (Aegean Sea) and released (Kabasacal, 2014). C. This image is an umbilical scar from one neonate specimen (1.26 m TL) illustrating that this shark was recently birthed. This shark was captured in the Aegean Sea in July 2008 within a gillnet (Kabasakal and Özgür Gedikoğlu 2008)
Northwest Pacific region
In the Northwest Pacific Ocean, C. carcharias data have been collected through opportunistic fishing encounters (e.g., Christiansen et al. 2014; Uchida et al. 1996). Sharks within this population have been captured in the waters around Russia, Korea, Japan, Vietnam, Taiwan, and the Philippines (Christiansen et al. 2014; Fig. 1).
Key sightings and historical data
Presently, there are no identified discrete nursery areas in association with this population; however, of the 240 opportunistic encounters of C. carcharias between 1951 to 2012, numerous neonates and gravid females were captured that help shed light on the unique characteristics, size at and seasonality of parturition (Christiansen et al. 2014; Uchida et al. 1996). Uchida et al. (1996) reported the capture of multiple gravid female C. carcharias. Notably, three gravid females (4.70 m TL – April 1986; 4.80 m TL – May 1992; and 5.15 m TL—May 1992) were captured in the waters of Japan. The 4.70 m TL shark was captured in Taiji, Japan and contained 7 embryos that were estimated to be 1.0–1.1 m TL. All sharks had a rounded apex on the first dorsal fin with ruptured yolk stomachs, indicating that they were not fully developed (Uchida et al. 1996; Fig. 7A). The 4.80 m TL shark contained 5 embryos that were approximately 1.30 m TL, and each shark had a rounded apex on the first dorsal fin and mildly distended yolk stomachs (Uchida et al. 1996; Fig. 7B). Lastly, the 5.15 m TL C. carcharias contained ten embryos, eight of which were measured and ranged in size from 1.35–1.51 m TL (Uchida et al. 1996; Fig. 7C). These sharks each had a rounded apex on the first dorsal and based on various reported sizes of free-swimming sharks (e.g., 122 cm TL—Casey and Pratt 1985), the researchers concluded that the embryos from both the 4.8 m and 5.15 m TL C. carcharias were of maximum length. Both of these specimens were captured in May and thus is consistent with the proposed parturition period (late-spring to summer; Francis 1996). Although unpublished in the scientific literature, another gravid female (4.70 m TL) was captured in Taiwan in March 2019 that contained 14 embryos (Hickok 2019). The sizes of the embryos were not reported; however, images illustrate a rounded apex on the first dorsal fin and a distended stomach, suggesting that these embryos were still developing (Fig. 7E). Of particular importance, this 4.70 m TL specimen contained the largest C. carcharias litter ever reported. In addition to these embryos, five neonates were captured (as noted in Christiansen et al. 2014). However, data and associated photos are unavailable for several of these captures, including a 1.3 m TL white shark captured in Korea in 2010, two 1.5 m TL white sharks captured in Japan (one in July 1996 in a bottom trawl and the other is undisclosed), and a 1.60 m TL white shark captured in a set net in Vietnam in June 2011 (Christiansen et al. 2014). Lastly, a 1.26 m TL white shark was captured in a gillnet in the Sea of Japan in September 2011, where SST ranged from 17–20 °C; this shark had a rounded apex on the first dorsal fin (Dolganov 2012; Fig. 7D).
Examples of both embryonic and free-swimming white sharks (Carcharodon carcharias) captured in association with the Northwest Pacific population. A. A gravid 4.7 m total length (TL) C. carcharias contained 7 embryos that ranged in size from 1.0–1.1 m TL. This shark was captured in Japan in April 1986 and embryos had a rounded apex on their first dorsal fin; however, they still had substantial yolk mass and thus were not considered near term (Uchida et al. 1996). B. Another 4.8 m TL gravid C. carcharias was captured in May 1992 in Japan and contained 5 embryos. The embryos were 1.3 m TL, had a rounded apex on the first dorsal (Uchida et al. 1996). C. A 5.15 m TL gravid C. carcharias was captured in May 1992. The shark had ten embryos, eight of which were measured and ranged in size from 1.35–1.51 m TL, with each embryo being characterized of having a rounded apex on the first dorsal fin (Uchida et al. 1996). D. Image of a 1.26 m TL C. carcharias that was captured in Peter the Great Bay (Sea of Japan) in September 2011. The shark was captured in a gillnet and was characterized by having a rounded apex on the first dorsal fin (Dolganov 2012). E. Image of 14 embryonic C. carcharias collected from a 4.7 m TL gravid female captured in Taiwan in March 2019 (©Chen Sanfa). It is uncertain as to the embryo’s TL; however, these embryos are characterized by having a rounded apex on the first dorsal and a distended stomach, suggesting that these sharks were still developing
Discussion
The current body of research provides insight related to a unique morphological characteristic (dorsal fin shape), broad-scale habitat data, and seasonality related to gravid (Saïdi et al. 2005), embryonic (Uchida et al. 1996), neonate (Kabasakal 2014) and YOY (Dicken 2008) C. carcharias. However, encounters with these specimens, especially gravid and neonate white sharks (e.g. Kabasakal 2014; Saïdi et al. 2005; Uchida et al. 1996), are infrequent on a global scale and, thus, determining the spatial and temporal scales of parturition remains difficult without visible evidence. Although sightings and capture data are insufficient, the reviewed studies and additional unpublished data indicate the season of C. carcharias parturition is late spring to summer, which is consistent with that proposed in previous studies (Bruce 1992; Francis 1996). However, localized capture of both gravid and neonate C. carcharias (Bradai and Saïdi 2013; Kabasakal and Özgür Gedikoğlu 2008; Kabasakal 2014) from the critically endangered Mediterranean population (i.e., Gulf of Gabès and Aegean Sea) over a short temporal scale provides a more concise estimate for parturition in June and July.
Characteristics
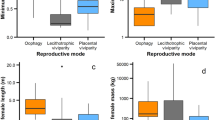
Extensive research has been conducted on morphometrics and length–weight characteristics of C. carcharias (Christiansen et al. 2016; Logan et al. 2018; Natanson and Skomal 2015). Such analyses can allow researchers to predict the age of captured specimens (Christiansen et al. 2016; Natanson and Skomal 2015) or allow for inter-population comparisons based on morphological characteristics and how changes in these characteristics may influence the ecology of a species (Fu and Irschick 2016; Logan et al. 2018). However, the present study demonstrates a key morphological feature that is consistent among embryonic and neonate white sharks: the rounded apex of the first dorsal fin (e.g. Dolganov 2012; Uchida et al. 1996). As observed with tiger sharks (Galeocerdo cuvier), caudal fin and head shape have been demonstrated to change with ontogeny (Fu and Irschick 2016). These changes are hypothesized to aid in key life history and ecological characteristics of the species to maximize survivability (Fu and Irschick 2016). Furthermore, ontogenetic changes in dentition have been found in C. carcharias (French et al. 2017). While dentition was found to change with sex and ontogeny (French et al. 2017), these changes were demonstrated to benefit foraging success (Estrada et al. 2006). Therefore, it is suggested that future research involves detailed morphological analyses on C. carcharias dorsal fin shape in relation to early life-stages (embryo, neonate, YOY) as a way to non-invasively determine the age of captured specimens (time at large post-birth). Although the data in the present review are limited, the few reports of a rounded apex of the first dorsal fin do not occur after 1.61–1.7 m TL (Fig. 8). Therefore, it appears dorsal fin shape does change with ontogeny, but it is unknown whether this is a progressive change that can be used as a reliable and non-invasive predictor of shark age. Such findings may allow scientists to non-invasively assess time at large after parturition and allow for more precise estimation of parturition grounds.
Graphical representation pertaining to white shark (Carcharodon carcharias) captures in relation to shark size (e.g., embryos to 200 cm total length (TL)) from all populations. While sample size is small, this graph illustrates how many of the captured sharks exhibited a rounded apex on the first dorsal. Data are compiled from Table 1 in combination with embryos from reported pregnant females captures (e.g., Francis 1996; Uchida et al. 1996; Saïdi et al. 2005; Taiwan, Unpublished Data)
Habitat
Depth and water temperature
Sharks exhibit seasonal distribution patterns based on a variety of biotic and abiotic factors (e.g. Hammerschlag et al. 2022; Kessel et al. 2014; Weng et al. 2007). Two notable characteristics examined in this study were depth and water temperature. Based on all the compiled sightings, catches, and reports of neonate-YOY C. carcharias in the present study, water temperature seems to be an important factor influencing their presence. In all associated regions (e.g. Mediterranean, WNA), neonate-YOY C. carcharias were more commonly found in waters ranging from 15.7 °C (Santana-Morales et al. 2012) to 23.1 °C (Santana-Morales et al. 2012). While in some instances, satellite tagging data demonstrate that these neonate-YOY sharks moved vertically into waters outside of this range (7.9–26.2 °C; Shaw et al. 2021), the narrow temperature range (15.7–23.1 °C) remained fairly consistent for all reported interactions. It is likely that smaller sharks exhibit a preference for a narrower temperature range, since neonate-YOY C. carcharias have a larger surface area to body volume ratio in comparison to larger animals (Schmidt-Nielsen, 1984). Thus, smaller animals may exhibit an increased propensity for heat loss due to the greater heat emitting surface in comparison to decreased heat conserving body volume (Bernvi 2016; Block and Finnerty 1994; Carey et al. 1971, 1982). Although adults exhibit the ability to tolerate a wide water temperature range (1.6–30.4 °C; Skomal et al. 2017), since neonate-YOY sharks more commonly utilize waters within a narrow temperature range, it is likely that parturition would occur in regions exhibiting this narrow temperature range to maximize the survivability of the newborn sharks.
Further, depth data from the present study demonstrate that YOY-neonate C. carcharias are more commonly found at shallower depths (e.g., Dicken 2008; O’Connell et al. 2021). More specifically, the reported interactions exhibit that these small sharks are more commonly encountered in a depth range from 9 to 37 m (O’Connell et al. 2023; Smith 1951). It is possible that these sharks utilize these depths and habitats due to a variety of reasons, including enriched foraging opportunities (e.g., Bruce and Bradford 2012) and as an anti-predation strategy (e.g., Hoyos-Padilla et al. 2016). Therefore, to maximize survivability, it is hypothesized that C. carcharias parturition occurs within insular shelf waters.
Lastly, it is important to note that climate change or other climatic events (e.g., El Niño) can influence the water temperature and, consequently, the geographic range and movements of top predators, including C. carcharias (e.g., Hazen et al. 2013; Sanford et al. 2019a, b; Tanaka et al. 2021; White et al. 2019). Along the Californian coast, El Niño can warm the SST to more than 6ºC above average (Hayward 1993; Gentemann et al. 2017). Within the NEP, juvenile C. carcharias exhibited a northly expansion in response to an abnormal warm water mass deemed the Pacific Warm Anomaly in 2014 and the 2015 El Niño event (White et al. 2019; Tanaka et al. 2021). More specifically, although the Monterey Bay ecosystem is considered approximately 2.5ºN in latitude above what is normally considered within the traditional range for YOY-juvenile C. carcharias, these smaller sharks were observed more frequently at this latitude during these climatic events (Tanaka et al. 2021; White et al. 2019). Therefore, although larger sharks may exhibit a wider water temperature tolerance (e.g., Hoyos-Padilla et al. 2016; Nasby-Lucas et al. 2009), these climate-based water temperature variations may not solely influence C. carcharias movements and/or range. In fact, similar to potential seasonal shifts in nursery area locations (e.g., White 2016), white shark parturition sites may also vary thus complicating parturition site identification.
Prey availability
Sharks have been demonstrated to aggregate around sites of known prey productivity (Mourier et al. 2016; Schilds et al. 2019). For C. carcharias, adults have been known to aggregate around pinniped colonies (Johnson et al. 2009; Schilds et al. 2019) and neonate to subadults have been known to aggregate in areas with an abundance of bottom dwelling fishes and elasmobranchs (White et al. 2019; Grainger et al. 2020). Similar to C. Carcharias nursery areas that have been characterized as having abundant prey resources (e.g., Oñate-González et al. 2017; Santana-Morales et al. 2012) and as a result of their high metabolic rates (Semmens et al. 2013), it can be inferred that parturition sites should exhibit high prey availability to maximize survival. For example, lemon shark (Negaprion brevirostris) parturition has been demonstrated to occur in shallow water areas characterized by reduced predatory influence and high prey availability (Chapman et al. 2009; Freitas et al. 2006). Therefore, it is suggestive that successful C. carcharias parturition location that would maximize offspring survival may be correlated with high prey density.
While sites of high prey productivity in combination with the presence of neonate or gravid C. carcharias may be ideal for parturition and thus warrant further investigation, these sites are also usually heavily impacted by commercial fisheries (Boldrocchi et al. 2017; Oñate-González et al. 2017). Therefore, continued research aimed at identifying the importance of these areas of high productivity (e.g., Aegean Sea, Mediterranean population; Bahía Sebastián Vizcaíno, Mexico, Northeastern Pacific population) in relation to parturition may help contribute to further management measures relating to reduced fishing effort or different fishing strategies that minimize shark bycatch during peak seasonality and presence of neonate and gravid C. carcharias. Such efforts may not only result in the identification of key parturition grounds but may also help to facilitate population recovery, especially in association with the critically endangered Mediterranean population.
Proximity to shore
Beyond depth (Dicken 2008; Smith 1951), SST (Bruce and Bradford 2012; Cliff et al. 1989; Werry et al. 2012; Weng et al. 2007), and prey availability (White et al. 2019; Grainger et al. 2020) associated with C. carcharias nursery areas, shoreline proximity may impact parturition location. Satellite telemetry data and other YOY encounters (e.g., captures) illustrate the repeated utilization of shallow habitats within coastal areas (e.g., Domeier 2012; Shaw et al. 2021). For example, the movements of 13 YOY in the WNA population exhibited a coastal preference, with movements averaging 12.7 ± 0.2 km from shore (Shaw et al. 2021). Similarly, in the NEP population, YOY and juveniles are encountered more frequently within < 5.5 km where they spend the majority of their time in coastal habitats (Dewar et al. 2004; Weng et al. 2007; Lowe et al. 2012; Lyons et al. 2013). While the repeated movements of these YOY and juvenile sharks are more closely related with shark nursery areas (Heupel et al. 2007), it is important to note that in relation to parturition, the smallest free-swimming C. carcharias (i.e., 0.85–1.07 m TL) were all encountered within several kilometers from shore (Kabasakal and Özgür Gedikoğlu 2008; Kabasakal 2014; O’Connell et al. unpublished data; Santana-Morales et al. 2020). In relation to the NWA population, a 1.07 m TL C. carcharias was captured approximately 2.0 km off Montauk, New York, USA in August 2021 (O’Connell et al. Unpublished) and represents the smallest free-swimming C. carcharias tagged and released in association with this population. Furthermore, this shark exhibited characteristics of a newly pupped specimen (e.g., size, seasonality of capture, and rounded apex on the first dorsal fin; O’Connell et al. Unpublished). However, regardless of shark size, it may be premature to conclude that parturition occurs at the site of capture as research in the WNA did not find any evidence that parturition occurs within coastal regions of the New York Bight (Skomal et al. 2017). With satellite tracking data demonstrating that large female C. carcharias can utilize shelf or pelagic waters (Franks et al. 2021; Skomal et al. 2017), this has led to the general notion or hypothesis that parturition occurs offshore or outside of the Bight and neonates travel inshore to areas that offer more protection to maximize survivability and provide favorable biological (e.g., prey) and ecological (e.g., water temperature) conditions (as suggested in Skomal et al. 2017; Anderson et al. 2021; Oñate-González et al. 2017). Furthermore, C. carcharias is a highly migratory species (e.g., Bonfil et al. 2005). As an example, a 2.8 m TL juvenile covered a distance of > 8500 km over the course of 536 days (Bruce et al. 2019). Similarly, Weng et al. (2007) provided data pertaining to a YOY 1.56 m TL C. carcharias that migrated 700 km away from its nursery habitat in less than one month. Therefore, the limited data associated with the 1.07 m TL C. carcharias from the WNA population in combination with the other small specimens (Kabasakal and Özgür Gedikoğlu 2008; Kabasakal 2014; Santana-Morales et al. 2020) are insufficient to provide a firm conclusion about parturition location and illustrates that a more comprehensive data set is required to determine parturition locality (e.g., presence of multiple neonates, presence of gravid females, visual evidence of parturition).
Predators
Within their range, one key predator of YOY-juvenile C. carcharias could be larger conspecifics. However, due to their small size at birth (1.05–1.50 m TL; Francis 1996; Pratt 1996), it is possible other large shark species may be considered viable predators. Within the inshore reaches of nursery habitats, satellite telemetry demonstrates that there typically is not an initial presence of large females (Bruce 2016; Skomal et al. 2017). One would hypothesize that large females would occasionally be encountered at these nursery area sites, whether through incidental captures or sightings, if parturition occurred within the nursery area. Therefore, and based on present satellite telemetry data (e.g., Bruce 2016; Franks et al. 2021; Skomal et al. 2017), it is hypothesized that although parturition strategies may vary based on population and/or in relation to various biological and environmental variables, parturition may occur in insular shelf waters that spatially exceed (i.e., horizontal and vertical scales) the inshore, shallow water environments associated with nursery area habitat to minimize predation by conspecifics. While there are conditions that would limit the viability of this hypothesis (i.e., SST limitations, prey availability), it is possible that having an increased area on both horizontal and vertical spatial scales, may decrease the predatory success by other large female conspecifics that may be utilizing that region. Should this hypothesis be correct, it would be suggestive that C. carcharias parturition occurs in insular shelf-associated waters as suggested in previous studies (Bruce 2016; Skomal et al. 2017; Domeier and Nasby-Lucas 2013), but not directly within the nursery areas.
Seasonality
Seasonality in relation to shark behavior (e.g., migration, foraging, and parturition) is well documented in the scientific literature (Domeier and Nasby-Lucas 2013; Kock et al. 2013; Ulrich et al. 2007). Furthermore, regions with extensive satellite tagging effort and/or bycatch data from commercial and recreational fishermen have demonstrated seasonal nursery habitat utilization for C. carcharias (Bruce and Bradford 2012). For example, in the Eastern Australian population, there are two key C. carcharias nursery areas—Port Stephens in New South Wales and Corner Inlet in eastern Victoria (Bruce and Bradford 2012; Harasti et al. 2017). White sharks utilize the Port Stephens nursery from late winter to mid-summer, after which they migrate southward to the Corner Inlet nursery area (Bruce and Bradford 2012).
Based on the opportunistic capture of both gravid females and neonates, parturition is likely to occur in late spring to summer on a global scale (Francis 1996; Bruce 1992). However, within both the Mediterranean and Northwest Pacific populations, data exist to define more precisely the parturition season. In the Mediterranean, the details from one gravid C. carcharias captured in February 2004 indicates that the embryos were still in development (i.e., yolk mass was 43–45%). In addition to this capture, numerous localized captures of neonates were reported. Specifically, the world’s smallest free-swimming C. carcharias (0.85 m TL) was captured in July 2011 and five additional specimens that ranged from 0.80 to 1.45 m TL were captured during the months of June and July. Similar to that proposed by Kabasakal (2014), while these only represent a few notable captures, this indicates a discrete parturition seasonality of late June and early July and a potential location for parturition in the Northern Aegean Sea, Turkey. With the current population status of C. carcharias within the Mediterranean, it is recommended that more substantial management initiatives are implemented within this region during this time period and locality to maximize population recruitment and survivability.
In the Northwest Pacific population, numerous gravid C. carcharias were captured that contained embryos of different stages of development that helps facilitate a more precise estimate of parturition season. Specifically, two gravid females were captured in April 1986 and March 2019, both of which contained embryos with either distended stomachs (indicating high yolk mass) or ruptured yolk stomachs (Uchida et al. 1996; Taiwan, Unpublished). However, two additional gravid females were captured in May and contained either 1.3 m TL embryos with mildly distended stomachs or 1.35–1.51 m TL embryos that appeared near term. While it is possible that the date of capture may be several months after parturition (i.e., suggesting late-spring parturition), when combining the encounters with gravid females and the captures of a 1.6 m TL neonate in June 2011 (Vietnam), a 1.5 m TL neonate in July 1996 (Japan) and a 1.26 m TL in September 2011 (Sea of Japan), it can be proposed that the parturition period within this region may occur in the summer, which is a more defined parturition seasonality than proposed in previous studies (i.e., late-spring to summer; Francis 1996; Bruce 1992).
Conclusions and future directions
This review provides a global assessment of potential C. carcharias parturition and nursery areas based on relevant captures and tagging of gravid females, neonates, and/or juveniles (e.g., Kabasakal 2014; McAuley et al. 2017) as well as the migratory patterns of adult females (e.g., Skomal et al. 2017). Continued research is required on a global scale, since neonate to juvenile C. carcharias utilize shallow coastal waters (e.g., Curtis et al. 2018; White et al. 2019) and within these waters, anthropogenic threats are persistent and come in the form of commercial and recreational fishing (Lyons et al. 2013; Santana-Morales et al. 2012), pollutants (e.g., Gelsleichter and Walker 2010; Lipej et al. 2022), and coastal development that can directly transform ideal coastal habitats (e.g., Stump 2013). However, management initiatives may be ineffective if they are focused on discrete areas rather than regional scales, as neonate and juvenile sharks are capable of long-distance migrations that extend outside their nursery regions (Weng et al. 2007; Bruce and Bradford 2012).
In conclusion, more research is needed on a global scale to further understand various aspects of C. carcharias parturition. However, this research should not solely focus on pups, but should also pursue various non-lethal research techniques (e.g., baited remote underwater video systems [BRUVS; Harasti et al. 2017; O’Connell et al. 2021], long-term autonomous underwater vehicles [as used in Packard et al. 2013], blood chemistry analysis [reproductive hormone analysis; Verkamp et al. 2021], ultrasonography [Sulikowski et al. 2016], and the novel intrauterine satellite tag (Sulikowski and Hammerschlag 2023)) as a way to: (1) identify the routine presence or absence of adult female C. carcharias within a study location; (2) collect video evidence of the birthing process in C. carcharias to obtain tangible evidence of both the spatial and temporal scales of the activity; or (3) identify unique morphological characteristics, reproductive hormones, or direct observations of gravid females and neonates to further narrow down parturition sites and patterns of when the behavior routinely occurs. Continued research may yield pertinent information associated with potential differences and similarities between parturition and nursery area designations for this species. Therefore, increased biological and ecological research on these populations is warranted on a global scale, especially on neonate sharks as these sharks are most vulnerable due to higher risk of predation (Benson et al. 2018) as well as being susceptible to capture in fisheries (Lyons et al. 2013; Santana-Morales et al. 2012).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Anderson JM, Clevenstine AJ, Stirling BS et al (2021) Non-random co-occurrence of juvenile white sharks (Carcharodon carcharias) at seasonal aggregation sites in Southern California. Front Mar Sci 8:688505. https://doi.org/10.3389/fmars.2021.688505
Barton J, Howe S, Pope A (2012) Marine natural values study. Vol 2: Marine Protected Areas of the Victorian Embayments Bioregion, Part 2 Western Bay & Corner Inlet Parks Victoria Technical Series. Parks Victoria, Melbourne.
Benson JF, Jorgenson SJ, O’Sullivan JB et al (2018) Juvenile survival, competing risks, and spatial variation in mortality risk of a marine apex predator. J Appl Ecol 55(6):2888–2897. https://doi.org/10.1111/1365-2664.13158
Bernvi D (2016) Ontogenetic Influences on Endothermy in the Great White Shark (Carcharodon carcharias). Stockholm University. https://doi.org/10.13140/RG.2.1.2888.5367
Block BA, Finnerty JR (1994) Endothermy in fishes: a phylogenetic analysis of constraints, predispositions, and selection pressures. Environ Biol Fish 40:283–302. https://doi.org/10.1007/BF00002518
Blower DC, Pandolfi JM, Bruce BD et al (2012) Population genetics of Australian white sharks reveals fine-scale spatial structure, transoceanic dispersal events and low effective population sizes. Mar Ecol Prog Ser 455:229–244. https://doi.org/10.3354/meps09659
Boldrocchi G, Kiszka J, Purkis S, Storai T, Zinzula L, Burkholder D (2017) Distribution, ecology, and status of the white shark, Carcharodon carcharias, in the Mediterranean Sea. Rev Fish Biol Fisheries 27:515–534. https://doi.org/10.1007/s11160-017-9470-5
Bonfil R, Meÿer M, Scholl MC, Johnson R, O’Brien S, Oosthuizen H, Swanson S, Kotze D, Paterson M (2005) Transoceanic migration, spatial dynamics, and population linkages of white sharks. Science 5745:100–103. https://doi.org/10.1126/science.1114898
Bradai MN, Saïdi B (2013) On the occurrence of the great white shark (Carcharodon carcharias) in Tunisian coasts. Rapports Commission Internationale Mer Méditerranée 40:489
Bradford RW, Patterson TA, Rogers PJ, McAuley R, Mountford S, Huveneers C, Robbins R, Fox A, Bruce BD (2020) Evidence of diverse movement strategies and habitat use by white sharks, Carcharodon carcharias, off southern Australia. Mar Biol 167(96):1–12. https://doi.org/10.1007/s00227-020-03712-y
Bruce BD (1992) Preliminary observations on the biology of the white shark, Carcharodon carcharias, in south Australian waters. Mar Freshw Res 43(1):1–11. https://doi.org/10.1071/MF9920001
Bruce BD, Bradford RW (2012) Habitat use and spatial dynamics of juvenile white sharks, Carcharodon carcharias, in eastern Australia. In: Domeier ML (ed) Global perspectives on the biology and life history of the white shark. CRC Press, Boca Raton, pp 225–254
Bruce BD, Bradford RW (2015) Segregation or aggregation? Sex-specific patterns in the seasonal occurrence of white sharks Carcharodon carcharias at the Neptune Islands, South Australia. J Fish Biol 87(6):1355–1370. https://doi.org/10.1111/jfb.12827
Bruce BD, Harasti D, Lee K, Gallen C, Bradford R (2019) Broad-scale movements of juvenile white sharks Carcharodon carcharias in eastern Australia from acoustic and satellite telemetry. Mar Ecol Prog Ser 619:1–15. https://doi.org/10.3354/meps12969
Bruce BD (2016) Determining the size and trend of the West Coast white shark population. Marine Biodiversity Hub, National Environmental Science Program report. https://www.nespmarine.edu.au/document/determining-size-and-trend-west-coast-white-shark-population
Burkholder DA, Heithaus MR, Fourqurean JW et al (2013) Patterns of top-down control in a seagrass ecosystem: could a roving apex predator induce a behavior-mediated trophic cascade? J Anim Ecol 82(6):1192–1202. https://doi.org/10.1111/1365-2656.12097
Bustamante C, Vargas-Caro C, Bennett MB (2014) Not all fish are equal: functional biodiversity of cartilaginous fishes (Elasmobranchii and Holocephali) in Chile. J Fish Biol 85(5):1617–1633. https://doi.org/10.1111/jfb.12517
Carey FG, Teal JM, Kanwisher JW, Lawson KD, Beckett JS (1971) Warm-bodied fish. Am Zool 11(1):137–143. https://doi.org/10.1086/423743
Carey FG, Kanwisher JW, Brazier O, Gabrielson G, Casey JG, Pratt HL Jr (1982) Temperature and activities of a white shark, Carcharodon carcharias. Copeia 28:254–260. https://doi.org/10.2307/1444603
Casey JG, Pratt HL (1985) Distribution of the white shark, Carcharodon carcharias, in the western North Atlantic. Mem South Calif Acad Sci 9:2–14
Chapman DD, Babcock EA, Gruber SH, Dibattista JD, Franks BR, Kessel SA, Guttridge T, Pikitch EK, Feldheim KA (2009) Long-term natal site-fidelity by immature lemon sharks (Negaprion brevirostris) at a subtropical island. Mol Ecol 18(16):3500–3507. https://doi.org/10.1111/j.1365-294X.2009.04289.x
Christiansen HM, Lin V, Tanaka S, Velikanov A, Mollet HF, Wintner SP, Fordham SV, Fisk AT, Hussey NE (2014) The last frontier: catch records of white sharks (Carcharodon carcharias) in the Northwest Pacific Ocean. PLoS ONE. https://doi.org/10.1371/journal.pone.0094407
Christiansen HM, Campana SE, Fisk AT, Cliff G, Wintner SP, Dudley SFJ, Kerr LA, Hussey NE (2016) Using bomb radiocarbon to estimate age and growth of the white shark, Carcharodon carcharias, from the southwestern Indian Ocean. Marine Biol 163(144):1–13. https://doi.org/10.1007/s00227-016-2916-9
Cione AL, Barla MJ (2008) Causes and contrasts in current and past distribution of the white shark (Lamniformes: Carcharodon carcharias) off southeastern South America. Revista Del Museo Argentino De Ciencias Naturales 10:175–184. https://doi.org/10.22179/REVMACN.10.275
Cliff G, Dudley SFJ, Davis B (1989) Sharks caught in the protective gill nets off Natal, South Africa 2. The great white shark Carcharodon carcharias (Linnaeus). South Afric J Mar Sci 8(1):131–144. https://doi.org/10.2989/02577618909504556
Compagno LV (1984) FAO species catalogue, vol. 4, parts 1 & 2: sharks of the world. An annotated and illustrated catalogue of the shark species known to date. FAO Fish Synop, pp 125–655.
Compagno LJ (2001) Sharks of the world: an annotated and illustrated catalogue of shark species known to date (Vol. 2). Food & Agriculture Org
Curtis TH, McCandless CT, Carlson JK et al (2014) Seasonal distribution and historic trends in abundance of white sharks, Carcharodon carcharias, in the Western North Atlantic Ocean. PLoS ONE 9(6):e99240. https://doi.org/10.1371/journal.pone.0099240
Curtis TH, Metzger G, Fischer C, McBride B, McCallister M, Winn LJ, Quinlan J, Ajemian MJ (2018) First insights into the movements of young-of-the-year white sharks (Carcharodon carcharias) in the western North Atlantic Ocean. Sci Rep 1(8):1–8. https://doi.org/10.1038/s41598-018-29180-5
Dewar H, Domeier M, Nasby-Lucas N (2004) Insights into young of the year white shark, Carcharodon carcharias, behavior in the Southern California Bight. Environ Biol Fishes 70:133–143. https://doi.org/10.1023/B:EBFI.0000029343.54027.6a
Dicken ML (2008) First observations of young of the year and juvenile great white sharks (Carcharodon carcharias) scavenging from a whale carcass. Mar Freshw Res 7(59):596–602. https://doi.org/10.1071/MF07223
Dicken ML, Booth AJ (2013) Surveys of white sharks (Carcharodon carcharias) off bathing beaches in Algoa Bay. South Afric Marine Freshw Res 64(6):530–539. https://doi.org/10.1071/MF12336
Dolganov VN (2012) The capture of a great white shark Carcharodon carcharias Linnaeus, 1758 (Carcharodontidae) in Peter the Great Bay (Sea of Japan). Russ J Mar Biol 38:88–90. https://doi.org/10.1134/S106307401201004X
Domeier ML (2012) Global perspectives on the biology and life history of the white shark. CRC Press, Boca Raton
Domeier ML, Nasby-Lucas N (2013) Two-year migration of adult female white sharks (Carcharodon carcharias) reveals widely separated nursery areas and conservation concerns. Anim Biotelem. https://doi.org/10.1186/2050-3385-1-2
Domeier ML, Nasby-Lucas N, Palacios DM (2012) The Northeastern Pacific white shark shared offshore foraging area (SOFA). A first examination and description from ship observations and remote sensing. In: Domeier ML (ed) Global perspectives on the biology and life history of the white shark. CRC Press, Boca Raton, pp 147–158
Estrada JA, Rice AN, Natanson LJ, Skomal GB (2006) Use of isotopic analysis of vertebrae in reconstructing ontogenetic feeding ecology in white sharks. Ecology 87(4):829–834. https://doi.org/10.1890/0012-9658(2006)87[829:UOIAOV]2.0.CO;2
Fallows C, Fallows M, Hammerschlag N (2016) Effects of lunar phase on predator-prey interactions between white shark (Carcharodon carcharias) and Cape fur seals (Arctocephalus pusillus pusillus). Environ Biol Fish 99:805–812. https://doi.org/10.1007/s10641-016-0515-8
Feldheim KA, Gruber SH, Dibattista JD, Babcock EA, Kessel ST, Hendry AP, Pikitch EK, Ashley MV, Chapman DD (2014) Two decades of genetic profiling yields first evidence of natal philopatry and long-term fidelity to parturition sites in sharks. Mol Ecol 23(1):110–117. https://doi.org/10.1111/mec.12583
Fergusson IK (1996) Distribution and autecology of the white shark in the eastern North Atlantic Ocean and the Mediterranean Sea. In: Great White Sharks. Academic Press, San Diego, pp 321–345
Fergusson IK (2002) Occurrence and biology of the great white shark, Carcharodon carcharias, in the Central Mediterranean Sea: A review. In: Vacchi M, La Mesa G, Serena F, Seret B (eds) Proceedings of the 4th European Elasmo- branch Association Meeting, Livorno (Italy), 2000. ICRAM, ARPAT & SFI pp 7–30. https://doi.org/10.3750/AIP2013.43.4.11
Ferretti F, Worm B, Britten GL et al (2010) Patterns and ecosystem consequences of shark declines in the ocean. Ecol Lett 13(8):1055–1071. https://doi.org/10.1111/j.1461-0248.2010.01489.x
Francis MP (1996) Observations on a Pregnant white shark with a review in reproduction biology. In: Klimley AP, Ainley DG (eds) Great white sharks: the biology of Carcharodon carcharias. Academic Press, San Diego, pp 157–172
Franks BR, Tyminski JP, Hussey NE et al (2021) Spatio-temporal variability in white shark (Carcharodon carcharias) movement ecology during residency and migration phases in the Western North Atlantic. Front Mar Sci 8:744202. https://doi.org/10.3389/fmars.2021.744202
Freitas RH, Rosa RS, Gruber SH, Wetherbee BM (2006) Early growth and juvenile population structure of lemon sharks Negaprion brevirostris in the Atol das Rocas Biological Reserve, off north-east Brazil. J Fish Biol 68(5):1319–1332. https://doi.org/10.1111/j.0022-1112.2006.00999.x
French GCA, Stürup M, Rizzuto S, Van Wyk JH, Edwards D, Dolan RW, Wintner SP, Towner AV, Hughes WOH (2017) The tooth, the whole tooth and nothing but the tooth: tooth shape and ontogenetic shift dynamics in the white shark Carcharodon carcharias. J Fish Biol 91(4):1032–1047. https://doi.org/10.1111/jfb.13396
Fu CY, Irschick DJ (2016) Ontogeny of head and caudal fin shape of an apex marine predator: The tiger shark (Galeocerdo cuvier). J Morphol 277(5):556–564. https://doi.org/10.1002/jmor.20515
Gelsleichter J, Walker CJ (2010) Pollutant exposure and effects in sharks and their relatives. In. Sharks and their relatives II. CRC Press pp 507–554.
Gennari E, Irion DT, Cowley PD (2022) Active acoustic telemetry reveals ontogenetic habitat-related variations in the coastal movement ecology of the white shark. Anim Biotelem. https://doi.org/10.1186/s40317-022-00295-x
Gentemann CL, Fewings MR, García-Reyes M (2017) Satellite sea surface temperatures along the West Coast of the United States during the 2014–2016 northeast Pacific marine heat wave. Geophys Res Lett 44:312–319. https://doi.org/10.1002/2016GL071039
Grainger R, Peddemors VM, Raubenheimer D, Machovsky-Capuska GE (2020) Diet composition and nutritional niche breadth variability in juvenile white sharks (Carcharodon carcharias). Front Mar Sci. https://doi.org/10.3389/fmars.2020.00422
Gubili C, Bilgin R, Kalkan E et al (2011) Antipodean white sharks on a Mediterranean walkabout? Historical dispersal leads to genetic discontinuity and an endangered anomalous population. Biology. https://doi.org/10.1098/rspb.2010.1856
Hammerschlag N, Williams L, Fallows M, Fallows C (2019) Disappearance of white sharks leads to the novel emergence of an allopatric apex predator, the sevengill shark. Sci Rep 9(1):1–6. https://doi.org/10.1038/s41598-018-37576-6
Hammerschlag N, McDonnell LH, Rider MJ et al (2022) Ocean warming alters the distributional range, migratory timing, and spatial protections of an apex predator, the tiger shark (Galeocerdo cuvier). Glob Change Biol 28(6):1990–2005
Harasti D, Lee K, Bruce B, Gallen C, Bradford R (2017) Juvenile white sharks Carcharodon carcharias use estuarine environments in south-eastern Australia. Mar Biol 164(3):58. https://doi.org/10.1007/s00227-017-3087-z
Hayward TL (1993) Preliminary observations of the 1991–1992 El Niño in the California Current. Calif Cooperat Ocean Fish Investig Rep 34:21–29
Hazen EL, Jorgenson S, Rykaczewski RR et al (2013) Predicted habitat shifts of Pacific top predators in a changing climate. Nat Clim Chang 3:234–238. https://doi.org/10.1038/nclimate1686
Heupel MR, Carlson JK, Simpfendorfer CA (2007) Shark nursery areas: concepts, definition, characterization, and assumptions. Mar Ecol Prog Ser 337:287–297. https://doi.org/10.3354/meps337287
Hewitt AM, Kock AA, Booth AJ, Griffiths CL (2018) Trends in sightings and population structure of white sharks, Carcharodon carcharias, at Seal Island, False Bay, South Africa, and the emigration of subadult female sharks approaching maturity. Environ Biol Fishes 101:39–54. https://doi.org/10.1007/s10641-017-0679-x
Hickok, K (2019) Enormous great white shark pregnant with record 14 pups was caught and sold in Taiwan. Live Sci, https://www.livescience.com/65058-big-mamma-shark-caught-sold.html
Hoyos-Padilla EM, Klimley AP, Galván-Magaña F, Antoniou A (2016) Contrasts in the movements and habitat use of juvenile and adult white sharks (Carcharodon carcharias) at Guadalupe Island, Mexico. Anim Biotelem 4:1–4. https://doi.org/10.1186/s40317-016-0106-7
Huveneers C, Apps K, Becerril-García EE, Bruce B, Butcher PA, Carlisle AB, Chapple TK, Christiansen HM, Cliff G, Curtis TH, Daly-Engel TS (2018) Future research directions on the “elusive” white shark. Front Mar Sci 17:455
Huveneers C, Lloyd M (2017) Residency of white sharks, Carcharodon carcharias, at the Neptune Islands Group Marine Park (2016–17). Report to the Department of the Environment, Water and Natural Resources. Adelaide, South Australia, Flinders University
Irion DT, Noble LR, Kock AA, Gennari E, Dicken ML, Hewitt AM, Towe AV, Booth AJ, Smale MJ, Cliff G (2017) Pessimistic assessment of white shark population status in South Africa: comment on Andreotti et al. (2006). Marine Ecol Progress Ser 577:51–255. https://doi.org/10.3354/meps12283
Johnson R, Bester MN, Dudley SF, Oosthuizen WH, Meÿer M, Hancke L, Gennari E (2009) Coastal swimming patterns of white sharks (Carcharodon carcharias) at Mossel Bay, South Africa. Environ Biol Fishes 85:189–200. https://doi.org/10.1007/s10641-009-9477-4
Jorgensen SJ, Arnoldi NS, Estess EE, Chapple TK, Rückert M et al (2012) Eating or meeting? Cluster analysis reveals intricacies of white shark (Carcharodon carcharias) migration and offshore behavior. PLoS ONE 7(10):e47819. https://doi.org/10.1371/journal.pone.0047819
Kabasakal H (2014) The Status of the great white shark (Carcharodon carcharias) in Turkey’s waters. Marine Biodiv Records. https://doi.org/10.1017/S1755267214000980
Kabasakal H (2020) Exploring a possible nursery ground of white shark (Carcharodon carcharias) in Edremit Bay (northeastern Aegean Sea, Turkey). Ichthyol Res Soc 26(2):176–189
Kabasakal H, Özgür Gedikoğlu S (2008) Two new-born great white sharks, Carcharodon carcharias (Linnaeus, 1758) (Lamniformes; Lamnidae) from Turkish waters of the north Aegean Sea. Acta Adriat 49(2):125–135
Kabasakal H, Yarmaz A, Gedikoglu SO (2009) Two juvenile white sharks, Carcharodon carcharias (Linnaeus, 1758) (Chondrichthyes; Lamnidae), caught in northern Aegean sea. Annales Naturalis 2:127–134
Kessel ST, Chapman DD, Franks BR, Gedamke T, Gruber SH, Newman JM, White ER, Perkins RG (2014) Predictable temperature-regulated residency, movement and migration in a large, highly mobile marine predator (Negaprion brevirostris). Mar Ecol Prog Ser 514:175–190
Klimley AP (1994) The Predatory Behavior of the White Shark. Am Sci 82(2):122–133
Kock A, O’Riain MJ, Mauff K, Meÿer M, Kotze D, Griffiths C (2013) Residency, habitat use and sexual segregation of white sharks, Carcharodon carcharias in False Bay. South Africa Plos One 8(1):55048. https://doi.org/10.1371/journal.pone.0055048
Leone A, Puncher GN, Ferretti F, Sperone E, Tripepi S, Micarelli P, Gambarelli A, Sarà M, Arculeo M, Doria G, Garibaldi F, Bressi N, Dall’Asta A, Minelli D, Ccilli E, Vanni S, Serena F, Díaz-Jaimes P, Baele G, Cariani A, Tinti F (2020) Pliocene colonization of the Mediterranean by Great White Shark inferred from fossil records, historical jaws, phylogeographic and divergence time analyses. J Biogeograph 47(5):1119–1129. https://doi.org/10.1111/jbi.13794
Lipej L, Cumani F, Acquavita A, Bettoso N (2022) Plastic impact on sharks and rays. In Plastic Pollution and Marine Conservation. Academic Press, pp 153–185. https://doi.org/10.1016/B978-0-12-822471-7.00005-5
Logan RK, White CF, Winkler C, Jorgensen SJ, O’Sullivan JB, Lowe CG, Lyons K (2018) An evaluation of body condition and morphometric relationships within southern California juvenile white sharks Carcharodon carcharias. J Fish Biol 93(5):842–849. https://doi.org/10.1111/jfb.13785
Lowe CG, Blasius ME, Jarvis ET, Mason TJ, Goodmanlowe GD, O’Sullivan JB (2012) Historic fishery interactions with white sharks in the Southern California Bight. Global perspectives on the biology and life history of the white shark. Taylor & Francis, Boca Raton, pp 169–186
Lyons K, Jarvis ET, Jorgensen SJ, Weng K, O’Sullivan J, Winkler C, Lowe CG (2013) The degree and result of gillnet fishery interactions with juvenile white sharks in southern California assessed by fishery-independent and-dependent methods. Fish Res 147:370–380. https://doi.org/10.1016/j.fishres.2013.07.009
Malcolm H, Bruce BD, Stevens JD (2001) A review of the biology and status of white sharks in Australian waters In: Report to environment Australia, Marine Protection Program. CSIRO Div. of Marine Research. Holbart, Tasmania pp 113.
Martin R, Hammerschlag N, Collier R, Fallows C (2005) Predatory behaviour of white sharks (Carcharodon carcharias) at Seal island, South Africa. J Mar Biol Assoc UK 85(5):1121–1135. https://doi.org/10.1017/S002531540501218X
Martin RA (2004) Northerly distribution of white sharks, Carcharodon carcharias, in the eastern Pacific and relation to ENSO events. Marine Fish Rev 66(1).
McAuley RB, Bruce BD, Keay IS, Mountford S, Pinnell T, Whoriskey FG (2017) Broad-scale coastal movements of white sharks off Western Australia described by passive acoustic telemetry data. Mar Freshw Res 68:1518–1531. https://doi.org/10.1071/MF16222
Moro S, Jona-Lasinio G, Block B, Micheli F, De Leo G, Serena F, Massimilano B, Scacco U, Ferretti F (2019) Abundance and distribution of the white shark in the Mediterranean Sea. Fish Fish 21(2):338–349. https://doi.org/10.1111/faf.12432
Mourier J, Maynard J, Parravicini V, Ballesta L, Clua E, Domeier ML, Planes S (2016) Extreme inverted trophic pyramid of reef sharks supported by spawning groupers. Curr Biol 26(15):2011–2016. https://doi.org/10.1016/j.cub.2016.05.058
Nasby-Lucas N, Dewar H, Lam CH, Goldman KJ, Domeier ML (2009) White shark offshore habitat: a behavioral and environmental characterization of the eastern Pacific shared offshore foraging area. PLoS ONE 4(12):8163. https://doi.org/10.1371/journal.pone.0008163
Natanson LJ, Skomal GB (2015) Age and growth of the white shark, Carcharodon carcharias, in the western North Atlantic Ocean. Mar Freshw Res 66(5):387–398. https://doi.org/10.1071/MF14127
O’Connell CP, Dayan D, Healy C, He P (2021) The Use of Advanced Baited Remote Underwater Video Systems (BRUVS) and Novel Shark Cameras to Non-Invasively Characterize a Potential White Shark (Carcharodon carcharias) Nursery off Eastern Long Island New York. Marine Technol Soc J 55(1):30–37
O’Connell CP, Payne M, Payne S et al (2023) Observations of Multiple Young-of-the-Year to Juvenile White Sharks (Carcharodon carcharias) within South-West Australian Waters and Its Implications for a Potential Nursery Area(s). J Marine Sci Eng 11(3):563
O’Leary SJ, Feldheim KA, Fields AT, Natanson LJ, Wintner S, Hussey N, Shivji MS, Chapman DD (2015) Genetic diversity of white sharks, Carcharodon carcharias, in the Northwest Atlantic and southern Africa. J Hered 106(3):258–265. https://doi.org/10.1093/jhered/esv001
Oñate-González E, Sosa-Nishizaki O, Herzka SZ et al (2017) Importance of Bahia Sebastian Vizcaino as a nursery area for white sharks (Carcharodon carcharias) in the Northeastern Pacific: A fishery dependent analysis. Fish Res 188:125–137. https://doi.org/10.1016/j.fishres.2016.12.014
Packard GE, Kukulya A, Austin T, Dennett M, Littlefield R, Packard G, Purcell, M, Stokey R, Skomal G (2013) Continuous autonomous tracking and imaging of white sharks and basking sharks using a REMUS-100 AUV. OCEANS-San Diego pp 1–5 https://doi.org/10.23919/OCEANS.2013.6741213
Pratt HL (1996) Reproduction in the male white shark. In: Klimley AP, Ainley DG (eds) Great white sharks: the biology of Carcharodon carcharias. Academic Press, San Diego, pp 131–138
Rigby CL, Barreto R, Carlson J, Fernando D, Fordham S, Francis MP, Jabado RW, Liu KM, Marshall A, Pacoureau N, Romanov E, Sherley RB, Winker H (2019) White Shark (Carchardon carcharias). IUCN Red List of Threatened Species.
Robbins, (2007) Environmental variable affecting the sexual segregation of great white sharks Carchardon carcharias at the Neptune Islands South Australia. J Fish Biol 70(5):1350–1364. https://doi.org/10.1111/j.1095-8649.2007.01414.x
Saïdi B, Bradaï MN, Bouaïn A et al (2005) Capture of a pregnant female white shark, Carchardon carcharias (Lamnidae) in the gulf of Gabès (southern Tunisia, central Mediterranean) with comments on oophagy in sharks. Cybium 29(3):303–307
Sanford M, Painter J, Yasseri T, Lorimer J (2019a) Controversy around climate change reports: a case study of Twitter responses to the 2019 IPCC report on land. Sci Rev 167(59):1–25. https://doi.org/10.1007/s10584-021-03182-1
Sanford E, Sones JL, García-Reyes M, Goddard JH, Largier JL (2019b) Widespread shifts in the coastal biota of northern California during the 2014–2016 marine heatwaves. Sci Rep 9:1–14. https://doi.org/10.1038/s41598-019-40784-3
Santana-Morales O, Sosa-Nishizaki O, Escobedo-Olvera M, Oñate-González E, Osullivan J, Cartamil D (2012) Incidental Catch and Ecological Observations of Juvenile White Sharks, Carcharodon carcharias, in Western Baja California, Mexico. In: Domeier ML (ed) Global perspectives on the biology and life history of the white shark. CRC Press, Boca Raton, pp 187–198
Santana-Morales O, Abadía-Cardoso A, Hoyos-Padilla M, Naylor GJP, Corrigan S, Malpica-Cruz L, Aquino-Baleytó M, Beas-Luna R, Sepúlveda CA, Castillo-Géniz JL (2020) The smallest known free-living white shark carcharodon carcharias (Lamniformes: Lamnidae): ecological and management implications. Copeia 108(1):39–46. https://doi.org/10.1643/OT-19-233
Schilds A, Mourier J, Huveneers C, Nazimi L, Fox A, Leu ST (2019) Evidence for non-random co-occurrences in a white shark aggregation. Behav Ecol Sociobiol 73(138):1–12. https://doi.org/10.1007/s00265-019-2745-1
Schmidt-Nielsen K (1984) Scaling why is animal size so important? Cambridge University Press, Cambridge
Semmens JM, Payne NL, Huveneers C, Sims DW, Bruce BD (2013) Feeding requirements of white sharks may be higher than originally thought. Sci Rep 3(1):1471. https://doi.org/10.1038/srep01471
Shaughnessy PD, Berris M, Dennis TE (2007) Predation on Australian sea lions Neophoca cinerea by white sharks Carcharodon carcharias in South Australia. Aust Mammal 29:69–75. https://doi.org/10.1071/AM07008
Shaw R, Curtis TH, Metzger G et al (2021) Three-dimensional movements and habitat selection of young white sharks (Carchardon carcharias) across a temperate continental shelf ecosystem. Front Mar Sci 8(643831):1–15. https://doi.org/10.3389/fmars.2021.643831
Skomal G, Chisholm J, Corriea S (2012) Implications of increasing pinniped populations on the diet and abundance of white sharks off the coast of Massachusetts. In: Domeier M (ed) Global perspectives on the biology and life history of the white shark. CRC Press, Florida, pp 405–419
Skomal GB, Braun CD, Chisholm JH, Thorrold SR (2017) Movements of the white shark Carcharodon carcharias in the North Atlantic Ocean. Mar Ecol Prog Ser 580:1–16
Smith JLB (1951) LXIX—A juvenile of the man-eater, Carcharodon carcharias Linn. Ann Magaz Nat History 4(44):729–736. https://doi.org/10.1080/00222935108654201
Soldo V, Borovìc S, Lepoša L, Boban L (2016b) Comparison of different methods for ground thermal properties determination in a clastic sedimentary environment. Geotherm 61:1–11. https://doi.org/10.1016/j.geothermics.2015.12.010
Soldo A, Bradai MN, Walls RHL (2016) Carcharodon carcharias. The IUCN Red List of Threatened Species 2016:eT3855A16527829
Spaet JLY, Manica A, Brand CP, Gallen C, Butcher PA (2020) Environmental conditions are poor predictors of immature white shark Carcharodon carcharias occurrences on coastal beaches of eastern Australia. Mar Ecol Prog Ser 653:167–179. https://doi.org/10.3354/meps13488
Stump KL (2013) The effects of nursery habitat loss on juvenile lemon sharks, Negaprion brevirostris. University of Miami.
Sulikowski JA, Hammerschlag N (2023) A novel intrauterine satellite transmitter to identify parturition in large sharks. Sci Adv 9(9):eadd6340. https://doi.org/10.1126/sciadv.add6340
Sulikowski JA, Wheeler CR, Gallagher AJ, Prohaska BK, Langan JA, Hammerschlag N (2016) Seasonal and life-stage variation in the reproductive ecology of a marine apex predator, the tiger shark Galeocerdo cuvier, at a protected female-dominated site. Aquat Biol 24(3):175–184. https://doi.org/10.3354/ab00648
Sullivan JK (1991) Fish and wildlife populations and habitat stats and trends in the New York bight. Dynamac Corporation, pp 1–120.
Tamburin E, Elorriaga-Verplancken FR, Estupiñan-Montaño C et al (2020) New insights into the trophic ecology of young white sharks (Carchardon carcharias) in waters off the Baja California Peninsula. Mexico Mar Biol 167(55):1–15. https://doi.org/10.1007/s00227-020-3660-8
Tanaka S, Kitamura T, Mochizuki T, Kofuji K (2011) Age, growth and genetic status of the white shark (Carcharodon carcharias) from Kashima-nada, Japan. Marine Freshwater Res 62(6):548–556. https://doi.org/10.1071/MF10130
Tanaka KR, Van Houtan KS, Mailander E, Dias BS, Galginaitis C, O’Sullivan J, Lowe CG, Jorgensen SJ (2021) North Pacific warming shifts the juvenile range of a marine apex predator. Sci Rep 11(1):3373. https://doi.org/10.1038/s41598-021-82424-9
Towner AV, Underhill LG, Jewell OJ, Smale MJ (2013a) Environmental influences on the abundance and sexual composition of white sharks Carcharodon carcharias in Gansbaai. South Africa Plos ONE 8(8):e71197. https://doi.org/10.1371/journal.pone.0071197
Towner AV, Wcisel MA, Reisinger RR, Edwards D, Jewell OJ (2013b) Gauging the threat: the first population estimate for white sharks in South Africa using photo identification and automated software. PLoS ONE 8(6):66035. https://doi.org/10.1371/journal.pone.0066035
Towner AV, Watson RGA, Kock AA, Papastamatiou Y, Sturup M, Gennari E, Baker K, Booth T, Dicken M, Chivell W, Elwen S, Kaschke T, Edwards D, Smale MJ (2022) Fear at the top: killer whale predation drives white shark absence at South Africa’s largest aggregation site. Afr J Mar Sci 44(2):139–152. https://doi.org/10.2989/1814232X.2022.2066723
Tricas TC, McCosker JE (1984) Predatory behavior of th white shark, Carcharodon carcharias, with notes on its biology. Proc Calif Acad Sci 14:221–238
Uchida S, Toda M, Teshima K, Yano K (1996) Pregnant white sharks and full-term embryos from Japan. In: Klimley AP, Ainley DG (eds) Great white sharks: the biology of Carcharodon carcharias. Academic Press, San Diego, pp 139–155
Ulrich GF, Jones CM, Driggers WB, Drymon JM, Oakley D, Riley C (2007) Habitat utilization, relative abundance, and seasonality of sharks in the estuarine and nearshore waters of South Carolina. In: American Fisheries Society Symposium vol 50, pp 125. American Fisheries Society
Verkamp HJ, Skomal G, Winton M, Sulikowski JA (2021) Using reproductive hormone concentrations from the muscle of white sharks Carcharodon carcharias to evaluate reproductive status in the Northwest Atlantic Ocean. Endang Spec Res 44:231–236. https://doi.org/10.3354/esr01109
Watanabe YY, Payne NL, Semmens JM, Fox A, Huveneers C (2019) Hunting behaviour of white sharks recorded by animal-borne accelerometers and cameras. Marine Ecol Progress Ser 621:221–227. https://doi.org/10.3354/meps12981
Weng KC, O’Sullivan JB, Lowe CG, Winkler CE, Dewar H, Block BA (2007) Movements, behavior and habitat preferences of juvenile white sharks Carcharodon carcharias in the eastern Pacific. Marine Ecol Progress Ser 338:211–224. https://doi.org/10.3354/meps338211
Werry JM, Bruce BD, Sumpton W, Reid D, Mayer DG (2012) Beach areas used by juvenile white sharks, Carcharodon carcharias, in eastern Australia. Global Perspectives on the Biology and Life History of the White Shark. Taylor & Francis, Boca Raton. https://doi.org/10.1201/b11532
Werry JM (2017) Investigation of fine-scale white shark movement with potential for identification of white shark pupping grounds in South-West Australia. A report for the Western Australian Government for Exemption Permit 2887.
White CF, Lyons K, Jorgensen SJ, O’Sullivan J, Winkler C, Weng KC, Lowe CG (2019) Quantifying habitat selection and variability in habitat suitability for juvenile white sharks. PLoS ONE 14(5):e0214642. https://doi.org/10.1371/journal.pone.0214642
White CF (2016) Quantifying the habitat selection of juvenile white sharks, Carcharodon carcharias, and predicting seasonal shifts in nursery habitat use. California State University, Long Beach. https://doi.org/10.1371/journal.pone.0214642
Wintner SP, Cliff G (1999) Age and growth determination of the white shark, Carcharodon carcharias, from the east coast of South Africa. Fish Bull 97:153–169
Acknowledgements
We would like to thank the board of O’Seas Conservation Foundation, Inc. and the staff associated at the Atlantic Shark Institute that helped provide the resources to make the baseline data (e.g., Northwest Atlantic capture data) available.
Funding
The authors did not receive support from any organization for the submitted work. The authors declare they have no financial interests.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
O’Connell, C.P., Dodd, J.F., Crews, J. et al. A global review of white shark (Carcharodon carcharias) parturition. Rev Fish Biol Fisheries 34, 869–893 (2024). https://doi.org/10.1007/s11160-024-09856-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-024-09856-0