Abstract
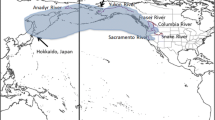
Pacific salmonids, cold-water fishes native to the northern hemisphere, span a massive geographic range (~ 33° latitude) and are exposed to a wide variety of environmental conditions regionally and temporally. California is home to the greatest concentration of at-risk anadromous salmonids and warming river temperatures pose both current and future threats to numerous populations. Thermal standards for management of California populations are currently based on guidelines for multiple salmonid species and from populations across the Pacific Coast. However, a growing body of literature suggests that salmonid populations exhibit population-specific thermal requirements. Furthermore, in California, salmonid populations regularly encounter temperatures that exceed current thermal standards based upon performance of outside populations. This review focuses on Chinook salmon (Oncorhynchus tshawytscha), providing evidence for interpopulation variation in thermal performance across life stages, and explores the drivers of variation. To describe the formation of interpopulation variation, we define fundamental and ecological thermal physiologies. Fundamental thermal physiology is the composite of intrinsic physiological traits and abiotic factors that define a species’ thermal window. Ecological and environmental interactions constrain this fundamental thermal physiology, yielding an ecological thermal physiology. Thermal physiology, viewed through this lens, provides researchers and managers avenues for salmonid research and conservation at the population scale. A more nuanced approach to west-coast salmonid conservation will be required to protect the most at-risk and vulnerable populations. Successful salmonid management must incorporate population-specific traits and present and future watershed conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Pacific salmonids (Oncorhynchus spp.) are native to northern latitudes and are broadly considered cold-water species. Increasing water temperatures are among a host of factors that have led to declining regional populations (Crossin et al. 2008; Moyle et al. 2017). Salmonids are strongly influenced by temperature via intrinsic physiology (e.g., metabolism) and extrinsic ecological interactions (e.g., predation, competition). Predicted increases in global temperature will undoubtedly alter these dynamics, leading to challenges in species management and conservation under a rapidly changing environment. Incorporating physiological thermal performance criteria into species management, especially in aquatic ecosystems, is widespread (U.S. Fish and Wildlife Service 1990, 1995, 2002a, b, c, 2015; U.S. Environmental Protection Agency 2003; National Marine Fisheries Service 2014). For instance, in the Pacific Northwest, the EPA Region 10 Guidance for Pacific Northwest State and Tribal Temperature Water Quality Standards (Region 10 Guidance) specifies thermal thresholds for different salmonid life-stages (egg incubation, juveniles, returning adults, etc.). Current management guidelines were developed by synthesizing data from multiple, often geographically disparate populations and species; by design this one-size-fits-all framework does not account for differences in thermal physiology between populations (U.S. Environmental Protection Agency 2003; U.S. Fish and Wildlife Service 2015; Gayeski et al. 2018). Recent evidence suggests that individual populations often differ in thermal physiology due to local and regional environmental variation (Fangue et al. 2006; Eliason et al. 2011; Chen et al. 2013; Stitt et al. 2014). This is particularly relevant for management of southern-edge populations, (e.g., California and Oregon populations) which are confronting the limits of their thermal capacity.
The greatest concentration of at-risk Pacific salmonid populations is in California. Moyle et al. (2017) identified 21 anadromous salmonid evolutionary significant units (ESU) in California, of which 14 are federally listed, and 11 are expected to be extinct within 50 years if present trends continue. Interactions between climate (e.g., increasing water temperature, drought severity, reduced snowpack) and anthropogenic effects (e.g., invasive species, pollutants, fisheries, hatcheries) have been identified as key factors driving many of these populations to the brink of extinction (Moyle et al. 2013, 2017; Katz et al. 2013). Air temperatures in California are expected to increase between 1.7 and 5.8 °C over the next century, causing increases in stream temperatures of 1.4–4.6 °C (Cayan et al. 2008; Null et al. 2013). River warming will be exacerbated during periods of low flow (Chang and Bonnette 2016), which are anticipated to increase in frequency and duration due to climate change impacts on snowmelt (Hamlet et al. 2005) and droughts (Diffenbaugh et al. 2015). Across California’s diverse landscape, these threats manifest themselves in different combinations and intensities posing a challenge to salmonid conservation and resource management. California is the southernmost range extent for six anadromous salmonid species including endangered endemic populations of Chinook salmon (O. tshawytscha) and steelhead trout (O. mykiss). Additionally, these populations are facing increasing urbanization and habitat modification leading to population declines. Broadly, conserving populations on the receding range edge is challenged by unusual and diverse phenotypes, not necessarily represented by the species as a whole (Hampe and Petit 2005). Ultimately, population-specific thermal guidelines may offer populations in California, and more broadly those across the Pacific Northwest, resiliency in a rapidly shifting climate.
Preserving salmonid populations has long been a stated goal of state and federal fisheries management agencies. However, existing temperature standards may be poorly suited for conserving salmonids in an era of climate change. The current, Region 10 Guidance provides thermal management criteria derived from thermal performance studies of more northern salmonid populations (U.S. Environmental Protection Agency 2003). However, ample evidence exists indicating that thermal performance among salmonid populations varies both interspecifically (Cech and Myrick 1999; Myrick and Cech 2001; Richter and Kolmes 2005; Verhille et al. 2016) and intraspecifically (Sauter et al. 2001; Stitt et al. 2014). For example, physiological performance traits of adult (Eliason et al. 2011) and alevin (Chen et al. 2013) sockeye salmon (O. nerka) from the Fraser River in British Columbia, demonstrated interpopulation variation among locally-relevant traits (e.g., migration difficulty, water temperature), supporting hypotheses of local adaptation to natal watersheds and migratory routes. Across their geographic range, anadromous Pacific salmonids may encounter annual temperature extremes ranging from 0 to18 °C in large, boreal rivers (Yang et al. 2014) and 7–25 °C in the Sacramento River (CA) watershed (Lowney 2000; Wagner et al. 2011) with variability occurring both temporally and spatially across habitats. Understanding the drivers of local thermal adaptation among salmonids and developing a mechanistic framework to predict population response to warming temperatures offers a solution to conserving salmonids in response to climate change.
We summarize the literature relevant to describing intraspecific variation of salmonid thermal performance and discuss these data in the context of design and application of temperature management criteria. More specifically, we synthesize research focused on the thermal performance, and variation therein, of Chinook salmon. Chinook salmon were chosen because they are relatively well-studied, wide-ranging, and include several at-risk populations currently confronting thermal stress, specifically in California (Yoshiyama et al. 2001; Moyle et al. 2017). This review then expands to explore the sources and drivers of intraspecific variation within Pacific salmonids. These drivers are organized by their influence on fundamental or ecological thermal physiology. We define fundamental thermal physiology as the collection of intrinsic physiological traits that delineate a species’ thermal capacity (Fry 1947; Pörtner and Farrell 2008). A species’ ecological thermal physiology is defined by the circumscription of the fundamental thermal physiology by environmental forces (Brett 1971) (Fig. 1). We argue that understanding the diversity of fundamental and ecological thermal physiologies and how they produce population-specific thermal performance is essential to developing management strategies for protecting Chinook salmon in California and salmonids more broadly. Finally, we also propose conservation strategies and research priorities that are fundamental to the conservation of salmonids in California and throughout the Pacific Northwest.
Conceptual diagram of fundamental and ecological thermal physiology. A fish’s intrinsic physiological traits dictate the size and shape of the fundamental thermal capacity (blue). Ecological factors such as competition for food resources or predation by warm water predators constrain the fundamental thermal physiology to smaller ecological thermal physiology (green). Sub-figures a and b are hypothetical fundamental thermal physiologies for cold-adapted or warm-adapted populations respectively. Star icons indicate the optimal temperature for ecological fitness of a given fundamental or ecological physiology. Tracking of the thermal optimum reveals how populations with the same fundamental thermal physiology (e.g., b, d, f, h) can have variable ecological thermal physiology dependent on ecological factors. Likewise, populations encountering the same ecological factors (e.g., c vs. d or g vs. h) will elicit different ecological thermal physiology, dependent upon their underlying fundamental thermal physiology
Chinook Salmon: life stages, development, and thermal limits
Myrick and Cech (2001, 2004) reviewed the literature for California Central Valley anadromous salmonids and presented knowledge gaps in our understanding of how temperature influences these species, seasonal runs, and populations. Subsequent reviews reported differences in thermal capacity among anadromous salmonid species, but did not highlight the capacity for intraspecific variation (e.g., Carter 2005; Richter and Kolmes 2005), nor the potential mechanisms contributing to such variation. Research since Myrick and Cech (2001, 2004) has exposed intraspecific variation in thermal performance within several salmonid species (e.g., Eliason et al. 2011; Chen et al. 2013; Stitt et al. 2014). Below, we review the literature on Chinook salmon regarding intraspecific variation in thermal performance across life stages. Chinook salmon exhibit several conserved life-history phenologies described as seasonal runs within which may exist one (e.g., winter-run), a few (e.g., spring-run), or many (e.g., fall-run) distinct populations. Literature on Chinook salmon is vast but controlled comparisons between populations are limited. Therefore, to facilitate comparisons across studies, this review focuses on available physiological trait datasets (e.g., growth rate, acute thermal tolerance) that are commonly quantified across populations using similar experimental conditions (e.g., ad libitum rations, stable temperatures).
Embryos and alevins
Chinook salmon embryos are laid in gravel redds where eggs incubate until hatching as alevin or yolk-sac fry. Myrick and Cech (2001) reviewed multiple studies and determined that Central Valley Chinook salmon embryos successfully developed at temperatures ranging from 1.7 to 16.7 °C, with mortality increasing dramatically toward thermal extremes. The upper thermal limits for prolonged embryo rearing of Central Valley fall-run Chinook salmon embryos are between 13.3 and13.9 °C for California winter-run Chinook salmon (USFWS 1999; Myrick and Cech 2001). Heming (1982) found that a fall-run Chinook salmon population from British Columbia had declining egg survivorship when reared at 12.0 °C. However, Jensen and Groot (1991) found that temperatures below 14.0 °C did not increase mortality of embryos from the Big Qualicum River (Canada). Upper thermal tolerance in Chinook salmon embryos among populations is relatively conserved, ranging from 12 to 14 °C. However, populations do appear to vary in their ontological response to temperature. Steel et al. (2012) reared Yakima River (WA) Chinook salmon eggs from eight families under eight variable thermal regimes designed to capture different absolute temperatures and amounts of thermal variability. They found that both thermal regime and family were significant factors in the ontogeny and phenology of Chinook salmon. Their work highlights two valuable results. First, that the commonly used management metric of ‘degree days’ to predict salmon development is insufficient under a changing landscape, and second, that variation in thermal physiological response was influenced by genetic traits.
Geist et al. (2006) found that a population of Snake River Chinook salmon alevins from Washington survived rearing temperatures between 13.0 and 16.5 °C equally well, with survival declining precipitously at 17.0 °C. The authors suggest that this impressive tolerance may represent local adaption to historically warm river temperatures. Research by Garling and Masterson (1985) on Chinook salmon alevins from the Great Lakes (USA) showed reduced survival (74% vs. 98%) when alevins were reared at warmer temperatures (15.1 °C vs 11.4 °C). Fuhrman et al. (2018) compared emergence phenology and development among four hatchery populations of spring-run Chinook salmon across four thermal regimes. They observed population-specific significant variability in emergence traits (e.g., emergence date, size-at-emergence, etc.) likely reflecting local adaptations with important fitness consequences. Overall, research on embryonic and larval stages indicates that critical temperature thresholds are somewhat conserved across populations. However, interpopulation variation in ontogeny and phenology does appear to be temperature-dependent, reflecting local adaptation. These sub-lethal effects may have important consequences for how a population’s fundamental thermal physiology interacts with local environmental factors.
Juveniles
Once alevin absorb their yolk-sac and begin exogenous feeding they are considered juveniles. To compare populations of juvenile Chinook salmon we selected growth as a holistic physiological metric which integrates many physiological processes and stressors (Arendt 1997). Growth rate is temperature-dependent and widely assessed using agreed methodology, furthermore it is relevant to assessing ecological fitness and wildlife management. There have been several laboratory-based growth studies using juveniles from California Central Valley fall-run Chinook salmon populations. Optimal growth for juveniles from the Nimbus Hatchery (CA), fed at satiation rations under laboratory conditions, occurred at 19 °C (Cech and Myrick 1999) and growth was optimized between 17 and 20 °C for juveniles from the Coleman National Fish Hatchery (CA) (Marine and Cech 2004). This range of temperatures is broadly consistent with temperatures reported by Brett et al. (1982), who found that Chinook salmon juveniles from the Big Qualicum River (BC) hatchery and wild juveniles from the Nechako River (BC) grew optimally at 20.5 and 18.9 °C, respectively. More recently, Zillig et al. (2020) examined the thermal physiology of several populations of laboratory acclimated Chinook salmon from throughout the Pacific Northwest, revealing different responses to acclimation temperatures (11 °C, 16 °C or 20 °C) among populations. Growth rates among all populations were similar (~ 0.15 g/day) when fish were reared at 11 °C. Conversely, when different populations were reared at 20 °C, growth rates varied broadly between populations (e.g., Coleman hatchery fall-run population (CA), ~ 0.3 g/day; Trask hatchery fall-run population, OR; ~ 0.15 g/day). However, the capacity for laboratory conditions to influence thermal physiological performance cannot be ignored. Rich (1987) reared Nimbus Hatchery (CA) fall-run Chinook salmon using diverted river water and found that growth rates declined when fish were reared at temperatures exceeding 15.3 °C, a decrease of 3.7 °C as reported by Cech and Myrick (1999). This apparent discrepancy in growth rate could be attributed to the effects of disease or to differences in water chemistry between laboratory and field experiments (Myrick and Cech 2001) and highlights the importance of accounting for ecological factors when identifying management temperature targets. Optimizing growth rate is a common target for management and conservation and the studies summarized above indicate that populations of Chinook from across the West Coast may exhibit different temperature-dependent growth relationships. Understanding the drivers of these differences and managing for this variation is important in protecting at-risk populations.
There is a general lack of research comparing smoltification (i.e., the process of transitioning to saltwater and the transition from juvenile to sub-adult) physiology among Chinook salmon populations. However, it is well documented that this process is partially temperature sensitive (Folmar and Dickhoff 1980; Marine and Cech 2004). Sauter et al. (2001) compared the thermal preference of two seasonal Chinook salmon runs, spring- and fall-run, from Washington and found a significant change in thermal preference between runs during smoltification. Fall-run smolts shifted their thermal preference (from 17.7 to 11.2 °C) as they achieved maximal saltwater tolerance. Conversely, spring-run Chinook salmon smolts preferred 16.6 °C, with no observed change in thermal preference associated with smoltification. The authors interpreted differences between spring- and fall-run Chinook salmon to reflect differences in naturally occurring environmental conditions experienced by Chinook salmon during smoltification. Understanding how temperature influences smoltification phenology, and whether different populations or life-history strategies exhibit different temperature-dependent smoltification phenology is an important knowledge gap for future research.
Adults
Thermal physiology studies on adult Chinook salmon are relatively limited, especially when comparing populations. However, the temperature at which adult salmon are impeded during migration can serve as a coarse, comparable indicator of adult thermal performance. After examining several Chinook salmon populations from the Pacific Northwest, McCullough (1999) concluded that adults sought thermal refuge and migration ceased when water temperature reached 21 °C. Similarly, fall- and spring-run populations from the Columbia River (WA) limited upstream migration when temperatures reached 20 °C (Goniea et al. 2006; Mann and Snow 2018). Keefer et al. (2018) individually tagged, spring-, summer- and fall-run Chinook salmon migrating through the Columbia and Snake Rivers (WA). Migrating summer- and fall-run salmon experienced temperatures near upper thermal limits (20–22 °C) and would briefly (hours to days) halt migration and use thermal refuges when available. In California, Klamath River spring-run Chinook salmon halted migration when temperatures surpassed 23 °C (Strange 2012) and Hallock et al. (1970) reported that water temperature exceeding 19 °C inhibited migration of Chinook salmon in the San Joaquin River; however, in 2004, adults were observed migrating upstream in the San Joaquin River at temperatures exceeding 21 °C (Williams 2006). Attributing migration phenology to interpopulation variation is difficult because delays in migratory behavior may reflect intrinsic thermal physiological traits, ocean and river environmental factors (Keefer et al. 2008), state-dependent energetic limitations (Plumb 2018), or a combination of these variables. Therefore, understanding both the fundamental and ecological thermal physiology of returning adult Chinook salmon should help managers disentangle the drivers of adult migration behavior.
Extensive research on adult sockeye salmon from the Fraser River (BC), has documented thermal intraspecific variation between populations relevant to their migratory performance (Eliason et al. 2011; Anttila et al. 2019). This work, discussed in greater detail below, demonstrates local adaptation of nine populations to population-specific migration routes and spawning reaches. Given that Chinook salmon and sockeye salmon are congeners, share similar life history traits, and are sympatric throughout much of their ranges, the ability of adult Chinook salmon to show locally adapted thermal performance traits is not surprising.
Summary
Considering the traits reviewed here, Chinook salmon do exhibit interpopulation variation in thermal physiology. This variation appears greatest during the juvenile lifestage, with embryos and adults demonstrating less plasticity. However, this may be a result of study bias because juvenile salmon are easier to study in both the lab and field than ocean dwelling adults. Similarly, juveniles exhibit more measurable and comparable traits than developing embryos. There remain large knowledge gaps in the thermal physiology of spring-run Chinook salmon and late fall-run Chinook salmon. While current management guidance criteria (Table 1) are broadly protective, they may not protect unique at-risk populations (e.g., Sacramento River Winter-run Chinook salmon [CA]), or account for ecological differences (e.g., predators) between populations. Management goals should seek population specific thermal criteria, built upon an understanding of both a population’s fundamental thermal physiology and its ecological thermal physiology.
Fundamental thermal physiology
An organism’s thermal physiology is dictated by the interaction of environmental conditions, behavioral responses, and intrinsic physiological traits (Hochachka and Somero 2002). A large body of research has developed over the past two decades identifying sources of variation among fundamental thermal traits of salmonids. Some of the causes of variation are associated with genotypic differences between populations or species (Nichols et al. 2016; Chen et al. 2018b), while others are a result of phenotypic plasticity applied across diverse and dynamic environmental conditions (Narum et al. 2018). Below, we review the mechanisms by which variation in fundamental thermal physiology among populations is produced and maintained. We show that management actions can be tailored by understanding and incorporating these mechanisms to predict population-specific thermal performance under future conditions.
Acclimation and adaptation
The strategies by which organisms adjust to fluctuations in their thermal environment fall into two broad categories: (1) acclimation or physiological change over days to weeks, and (2) adaptation or genetic change across generations (Hochachka and Somero 1968; Hazel and Prosser 1974; Schulte et al. 2011; Schulte 2015). Management frameworks, however, often recommend static thermal thresholds to manage river temperatures (U.S. Environmental Protection Agency 2003). Ultimately, salmon thermal performance is dynamic, enabling responses to environmental conditions on short time-scales via acclimation and across generations via adaptation. The juxtaposition of static management strategies against biological dynamism may introduce and obscure pitfalls to effective management and conservation. Therefore, the role of acclimation and adaptation must be considered fundamental for the determination of thermal performance and definition of management strategies.
It is well documented that salmonids acclimate to local water temperatures. Acclimation to warmer water temperature has been shown to increase acute upper thermal tolerance in O. mykiss (Myrick and Cech 2000b, 2005), sockeye salmon (Chen et al. 2013) and Chinook salmon (Brett 1952; Zillig et al. 2020). Furthermore, comparisons among Chinook salmon from Northern California, the Oregon coast and Columbia River Basin demonstrated differences in acclimation capacity among populations (Zillig et al. 2020). Across these populations, Zillig et al. (2020) assessed acute thermal tolerance and growth rate of fish reared at three temperatures (11 °C, 16 °C and 20 °C). Acute thermal tolerance increased with acclimation temperature among all populations, but to differing extents, highlighting variation among populations in their acclimation capacity. Furthermore, growth rates also changed with acclimation temperature. Fall-run populations from California exhibited the greatest growth rate when reared at 20 °C, while the sympatric and critically endangered Sacramento River winter-run population grew at the slowest rate when acclimated to the same temperature. Differing capacity to acclimate to environmental change will alter how salmonids cope with changes across the thermal landscape. Populations with a limited thermal tolerance and reduced acclimation capacity will likely have the greatest difficulty adjusting to novel thermal environments under climate change and are therefore at the greatest risk of population decline and extinction.
Adaptation (i.e., changes in the fundamental thermal physiology) through mutation, genetic drift, and natural selection tunes organismal traits to increase biological fitness in response to environmental conditions (Narum et al. 2013). While operating across generations, adaptation can be important on management timescales and a critical part of effective conservation (Ashley et al. 2003). Muñoz et al. (2015) demonstrated that physiological adaptation to warmer temperatures was possible in Chinook salmon, provided adequate genetic variation existed. Given that the quantity of genetic diversity may vary between populations, it may be assumed that adaptation capacity varies intraspecifically as well. Therefore, defining acclimation and adaptation capacity is important to predicting population-specific responses to environmental change.
Watershed variation
Interpopulation variation is generated through a combination of environmental heterogeneity and salmonid life-history strategies that reduce gene flow (Hilborn et al. 2003). Pacific salmonids, and specifically Chinook salmon, span a broad latitudinal range, across which streams vary widely in environmental characteristics and habitat types. Life history plasticity enables salmon to adapt to most accessible river systems, while spawning site fidelity and adult homing behavior reduce regional gene-flow and permit genetic drift between geographically proximate populations (Taylor 1991; Dittman and Quinn 1995; Hilborn et al. 2003). Therefore, local watershed characteristics can strongly influence the thermal physiology of populations.
Eliason et al. (2011) demonstrated that multiple physiological traits (e.g., heart mass, aerobic scope, heart rate) correlated strongly with environmental conditions of migratory routes and spawning locations in Fraser River (BC) sockeye salmon. Researchers captured returning adults, genotyped them to identify different source populations, and collected data on a suite of physiological traits. They found that populations that migrated further and traversed challenging river features exhibited increased heart mass. Additionally, individuals belonging to populations native to warmer habitats exhibited improved aerobic scope and cardiac performance at warm temperatures when compared with populations associated with historically cooler thermal regimes. In an extension of this work, Chen et al. (2013) found that Fraser River sockeye salmon embryos and alevins exhibited population variation and local adaptation in upper thermal tolerance. Within the Central Valley of California, Tuolumne River steelhead trout juveniles exhibited `warm-adapted` phenotypes (Verhille et al. 2016) and Mokelumne River Chinook salmon juveniles revealed unusual temperature-independent metabolic performance (Poletto et al. 2017). The authors of both studies suggest that these results are evidence of local adaptation to elevated temperature regimes at the southern range boundary. Evidence of salmonid adaptation to local environmental conditions has also been observed in brook trout (Stitt et al. 2014), red band trout (Chen et al. 2018a, b), sockeye salmon (Anttila et al. 2019) and rainbow trout (Chen et al. 2015).
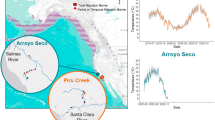
The capacity for anadromous salmonids to adapt to local watershed conditions is fundamental to advocating for population-specific management (Gayeski et al. 2018). Watersheds exhibit variation across numerous environmental gradients that influence the experienced temperatures of salmonids. For instance, Lisi et al. (2013) demonstrated that environmental characteristics such as watershed steepness, size, and the presence of lakes, accounted for variability in spawn timing of Alaska sockeye salmon, primarily through moderation of river temperature. Others have shown that water source (Nichols et al. 2014), discharge volume (Eliason et al. 2011; Anttila et al. 2019), riparian habitat (Moore et al. 2005), dissolved nutrients (Selbie et al. 2009; Ranalli and Macalady 2010), and turbidity (Thomas 1975) can co-vary with temperature and differentially between and within watersheds. Micheletti et al. (2018) explored relationships between environmental variables of migration routes and adaptive genetic variation among Columbia River steelhead. They found migration distance, migration slope, water temperature and precipitation correlated with changes in allelic frequencies among populations, further indicating that populations will genetically respond to local environmental conditions. Similarly, Spence and Dick (2014) modeled the role of photoperiod, temperature, flow and lunar phase in predicting coho salmon (O. kisutch) smolt out-migration across four geographically distant populations. Their results indicated that different combinations of environmental variables are capable of predicting the outmigration of different coho salmon populations. Environmental factors, even regional or watershed-specific, represent useful predictors in determining the fundamental thermal physiology of local salmonid populations. Metrics such as water temperature, flow regime, and migration distance are easily quantifiable and should be incorporated into conservation management actions (Fig. 2). Defining these population-specific watershed characteristics is useful when interpreting potential differences in fundamental thermal physiology (Fig. 1) between populations.
Determining population specific temperature criteria. First tier of experiments to assess and triage at-risk populations. Once threatened populations are prioritized, research focuses on quantifying population thermal performance traits and environmental risk-factors (e.g., low food abundance, lack of thermal refugia). Population-specific temperature criteria are produced that reflect the fundamental and ecological thermal physiology of the selected population and specific management goals (e.g., recruitment, growth, smoltification)
Ecological thermal physiology
Variation in fundamental physiology is a result of acclimation and adaptation applied to spatially and temporally heterogeneous environments, leading to differences in thermal physiology between populations. A population’s fundamental thermal physiology is then modified by secondary interactions to produce an ecological thermal physiology which further constrains, and potentially diversifies, a population’s thermal performance (Fig. 1). Here, we review some of the ecological factors that influence ecological thermal physiology.
Bioenergetics: growth, metabolism and asynchrony
Environmental temperature bounds the growth and survival of ectothermic organisms like most fishes, but some species have shown a capacity to compensate for increases in water temperature when food resources are abundant. Bioenergetic theory stipulates that ectotherm growth is a function of energy consumed versus energy expended or lost (Railsback and Rose 1999). Energy loss is generally dictated by metabolic activity which increases positively with temperature. As temperature increases, so do metabolic outputs such as egestion, excretion, and costs associated with digestion (Railsback and Rose 1999). Under such circumstances in the wild, salmonids must either seek out thermal refuges to reduce energy expenditure or compensate with increased food consumption (Lusardi et al. 2020). Otherwise, an energy deficit will occur, leading to reduced growth rates with potential consequences for fitness (see Beakes et al. 2010).
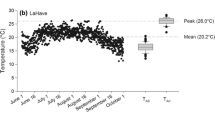
Most stream ecosystems are naturally oligotrophic (Allan and Castillo 2007), suggesting that behavioral thermoregulation and movement to thermal refuges is an effective strategy to deal with rising stream temperature (see Welsh et al. 2001). However, in productive ecosystems (e.g., spring-fed rivers, tailwaters below dams, floodplains, coastal lagoons) salmonids may be able to compensate for increases in stream temperature with increases in food consumption. The phenomenon has been shown to occur in numerous laboratory studies where salmonids are fed to satiation and exposed to warming temperatures. For instance, Foott et al. (2014) found that California juvenile coho salmon reared at 16.3 °C and 21.3 °C (mean temperatures) exhibited similar growth rates when fed to satiation. Empirical evidence for growth compensation in natural ecosystems has been less frequently observed. However, Bisson et al. (1988) found exceptionally high rates of juvenile coho salmon production in a Washington stream exhibiting daytime temperatures up to 29.5 °C and speculated that high food abundance was a causative mechanism supporting observed high rates of production. In a field experiment, Lusardi et al. (2020) reared juvenile coho salmon across a longitudinal gradient of temperature and food availability in a California spring-fed stream. They found food to be the proximate factor affecting juvenile coho salmon growth. Specifically, they found that juvenile coho salmon growth rates peaked at a maximum weekly maximum temperature of 21.1 °C and were sixfold greater than fish reared at a maximum weekly maximum temperature of 16 °C.
Modeling work has also supported the bioenergetic relationship between temperature and food availability in salmonids. Railsback and Rose (1999) found that food consumption was the primary determinant of O. mykiss growth during summer (as opposed to temperature) and Weber et al. (2014) used reach-specific food web data and bioenergetic models to accurately predict O. mykiss growth rates in several streams in the John Day River basin (OR). Work by McCarthy et al. (2009) on wild populations of steelhead in Trinity River tributaries found that high water temperatures and reduced feeding rate influenced growth, sometimes causing weight loss. Their models indicated that reduced growth may occur at temperatures as low as 15 °C and that increased food availability or quality would expand the window of viable temperatures. Their conclusions were extrapolated to indicate that under future warming conditions steelhead populations in food-limited systems will decline. Dodrill et al. (2016) modeled a similar response in rainbow trout, concluding that warmer temperatures resulted in reduced growth, unless accompanied by increases in prey availability and prey size. These studies suggest that river productivity, and subsequent prey availability, will strongly influence a population’s ability to survive under warming water temperatures. Food availability and productivity vary both across the landscape and through time; quantifying these environmental characteristics offers powerful predictors for understanding how the fundamental thermal physiology of salmonid populations may be energetically constrained into a population specific ecological thermal physiology (Fig. 1).
Annual changes in water temperature, habitat, and flow regime also alter the phenology, abundance and community composition of aquatic macroinvertebrate communities (Boulton et al. 1992; Bonada et al. 2007; Lusardi et al. 2016, 2018; Peterson et al. 2017) and phenological shifts in food availability have been shown to have population-level effects on predator species such as salmonids (Møller et al. 2008; Thackery et al. 2010). In extreme cases, termed ‘phenological mismatch’, shifts in the timing of species interactions can lead to population declines (Møller et al. 2008). Phenological mismatch is of greatest concern for species which are specialist predators or rely upon historically reliable but ephemeral food resources (Visser et al. 1998; Green 2010; Kudo and Ida 2013). Recent work by Campbell et al. (2019) explored temperature-dependent phenological traits among different populations of coho salmon from several southern Alaska rivers exhibiting diverse thermal profiles. All populations were physiologically tuned to local thermal conditions and exhibited synchrony in embryo hatching and development despite differences in temperature between rivers. Synchrony across such varied and population-specific thermal landscapes may unveil compensatory local adaptation to match resource timing (Campbell et al. 2019), but disturbance of historical temperature profiles may disrupt such synchrony. In summary, bioenergetic research highlights that even if fundamental salmonid thermal physiology among populations is conserved, differences between populations in food availability and quality could produce differences in population-specific ecological thermal physiology.
Biotic interactions
Temperature also plays an important role in influencing biotic interactions (e.g., predation, competition, disease) of salmonids (Coutant 1973; Ward and Morton-Starner 2015). Biotic interactions can moderate a salmonid’s fundamental thermal physiology to produce observed thermal performance (Brett 1971). Different populations of salmonids confront different suites of biotic interactions and therefore, may exhibit different ecological thermal physiologies in response to different thermal regimes. These indirect, ecological drivers of salmonid thermal performance should be considered when evaluating thermal management guidelines of at-risk populations.
Competition
Competition may be amplified by the effects of warming water temperature and negatively affect salmonids (Bear et al. 2007; Myrvold and Kennedy 2017, 2018). Loss of cold-water habitats will increase fish density and competition for space in remaining cold-water refuges. As water temperature increases, salmonids experience increased metabolic demand (Fryer and Pilcher 1974), leading to enhanced demand for prey resources. Taken together, without corresponding increases in prey availability, habitat carrying capacity will decline. Reese and Harvey (2002) used artificial streams to test competitive dynamics between Sacramento pikeminnow (Ptychocheilus grandis) on the growth and behavior of juvenile steelhead from the Eel River (CA). Elevated temperatures (20–23 °C) coupled with competition by pikeminnow reduced juvenile steelhead growth by 50% (Reese and Harvey 2002). This growth reduction was not observed when fish were reared at lower water temperatures (15–18 °C) or without competitors, indicating a synergistic effect of temperature and competition stress. Similarly, Reeves et al. (1987) found that when redside shiner (Richardsonius balteatus) and steelhead trout were reared together at warm temperatures (19–22 °C), growth rate of steelhead declined by 54%. However, at cooler temperatures (12–15 °C) steelhead suffered no loss in production, instead redside shiner grew at reduced rates. Wenger et al. (2011) modeled the impact of future climate scenarios on four species of western trout and found that increases in temperature enhanced competitive interactions and reduced habitat carrying capacity. Differences in competitor assemblage, and therefore ecological thermal physiology (Fig. 1), between watersheds may lead to differential outcomes for salmonid populations managed under a shared thermal management paradigm.
Predation
Predation is considered to be a primary cause of juvenile salmonid mortality both directly and indirectly (Nehlsen et al. 1991; Lindley and Mohr 2003; Sabal et al. 2016; Erhardt et al. 2018). As ectotherms, the susceptibility of juvenile salmonids to predation is sensitive to environmental temperature. In the California Central Valley, Marine and Cech (2004) examined the influence of temperature on predation risk; they reared juvenile Chinook salmon from the Sacramento River at three temperature regimes (13–16 °C, 17–20 °C, and 21–24 °C) and exposed them to striped bass (Morone saxatilis). The authors found that fish reared at 21–24 °C were preferentially consumed. Petersen and Kitchell (2001) modeled the bioenergetics of three predators of juvenile salmonids (northern pikeminnow, Ptychocheilus oregonensis, smallmouth bass, Micropterus dolomieu, and walleye, Stizostedion vitreum) in the Columbia River (WA), and found that predation by all three increased during climatic warm periods. This is consistent with laboratory studies by Vigg and Burley (1991) who found that the rate of prey consumption of northern pikeminnow was temperature dependent and increased exponentially across a temperature gradient (8–21.5 °C). Temperature can also augment sub-lethal effects of predation. Kuehne et al. (2012) conducted semi-natural stream experiments observing changes in direct mortality, behavior and physiological traits of salmon exposed to predation by smallmouth bass at 15 °C and 20 °C. There were no observed differences in direct predation, although salmon occupying warmer water exhibited reduced growth relative to control treatments without smallmouth bass. Sub-lethal effects of temperature on predation risk for salmonids is poorly studied and warrants further research as such effects may represent a considerable portion of thermally influenced biotic interactions.
Predator assemblages (i.e., warm-water vs. cold-water predators) and predation risks also vary among watersheds with implications for salmonid ecological thermal physiologies (Fig. 1). Permutations of predator assemblage and thermal physiology may produce different predatory outcomes among salmonid populations experiencing the same temperature. For instance, the California Central Valley predator assemblage is highly invaded by non-native species (e.g., striped bass, black bass [Micropterus spp.], sunfish [Lepomis spp.]) which may present different, temperature-dependent, trophic pressures when compared to native or cold-water predator assemblages found elsewhere (e.g., pikeminnows [Ptychocheilus spp.], bull trout [Salvelinus confluentus], northern pike [Esox lucius]). Understanding the role of temperature in structuring trophic relationships and developing a mechanistic framework for ecological thermal physiology could improve temperature management guidelines that address the influences of temperature on salmonid predators.
Embracing population variation
Across the Pacific Coast, several factors have contributed to the loss of genetic and environmental variability among salmonid populations. Homogenization, both genetic and environmental, suggests that application of current, non-population-specific, thermal management frameworks may have some validity. However, the effects of homogenization on population-specific thermal performance is unknown. Furthermore, the erosion of the intrinsic diversity of salmonids is essential to population resilience via the portfolio effect (Hilborn et al. 2003; Schindler et al. 2010; Greene et al. 2010; Carlson and Satterthwaite 2011). The portfolio effect typically refers to life-history diversity but can be extended to diversity among physiological traits or even management actions (Sturrock et al. 2020). Contained within different life-history phenotypes are interpopulation differences in fundamental thermal physiology (Satterthwaite et al. 2017). A diverse portfolio of fundamental thermal physiological traits within and between populations can increase species resiliency to thermal stress and maintain variation for adaptive change. As populations become homogenized, selection pressures are reduced and locally adapted thermal traits may be lost. Furthermore, as genetic variation declines, the overall capacity of a population to physiologically adapt or acclimate to future environmental conditions becomes impaired (Carlson and Seamons 2008; McClure et al. 2008). Ongoing homogenization of genetic diversity and habitat heterogeneity erodes the portfolio effect and reduces population resilience to change (Moore et al. 2010; Carlson and Satterthwaite 2011; Satterthwaite and Carlson 2015; Dedrick and Baskett 2018).
Hatchery supplementation of wild salmonid stocks is an observed cause of widespread genetic homogenization in salmonids (Williamson and May 2005). Hatchery production of juvenile salmon has been shown to rapidly reduce the fitness of domesticated strains as well as hybrids in the wild (Araki et al. 2007, 2008) through amplification of hatchery-selected traits and outbreeding depression as these mal-adapted traits become incorporated into wild populations (Hindar et al. 1991; Araki et al. 2008; Lusardi et al. 2015). Williamson and May (2005) documented genetic homogenization among five hatchery populations and eight wild populations of Chinook salmon in California’s Central Valley and concluded that gene flow between wild and hatchery populations is due to the long history of hatchery production and out-of-basin release of juveniles. Similar research conducted on wild and hatchery populations of Chinook salmon elsewhere in the Pacific Northwest indicates that hatchery introgression and subsequent genetic homogenization is present but less widespread (Moore et al. 2010; Smith and Engle 2011; Matala et al. 2012; Van Doornik et al. 2013). Jasper et al. (2013) and McConnell et al. (2018) studied hatchery straying among wild and hatchery Alaskan chum salmon (O. keta) populations. Despite straying, populations maintained genetic and trait differences, revealing both local adaptation and local resistance to introgression among wild populations. Unfortunately, salmon populations in California have been strongly influenced by hatchery propagation for over a half-century (Sturrock et al. 2019) which may explain differences in homogenization observed between California populations and more northern populations. Despite improved understanding of the effect of hatchery fish on wild fish and the erosion of native genome, few studies have examined the direct consequences of this on thermal performance or distinctiveness between populations.
Land use and management may also erode environmental heterogeneity, reducing the environmental selection pressures that historically produced both genotypic and phenotypic diversity. Dams have eliminated access to historical habitat and altered ecological processes, river flows and thermal regimes, homogenizing the evolutionary experience of Chinook salmon and other anadromous fishes (e.g., Zarri et al. 2019). Numerous salmonid stocks in California can no longer access historical ranges (Lindley et al. 2006, Yoshiyama et al. 2011, Moyle et al. 2017), especially high elevation, cold-water habitats. (McClure et al. 2008). The loss of habitat diversity may increase homogenization of life-history strategies crucial to the portfolio effect. Finally, habitat loss reduces landscape carrying capacity and imposes greater sympatry amongst populations and seasonal runs, increasing risks of genetic introgression (Waples 1991; McClure et al. 2008).
Applying a uniform suite of temperature thresholds across populations, some of which are homogenized and others diverse, poses the same issues of mismatch caused by applying general thermal guidance to multiple unique populations. Successful management of salmon in a rapidly changing environment must embrace a portfolio of genetic and phenotypic diversity (Moore et al. 2010; Carlson and Satterthwaite 2011; Anderson et al. 2015; Moyle et al. 2017; Dedrick and Baskett 2018). Ultimately, maintaining remaining diversity (environmental or genetic) is fundamental to a population’s adaptive capacity and resilience to global change. Population-specific temperature guidelines of salmonids could protect remaining diversity in thermal performance traits that offer resilience against future global change.
Rethinking thermal management
To combat the effects of warming river temperatures associated with anthropogenic and environmental change, management agencies have established thermal criteria intended to constrain river warming and protect salmonid populations throughout the Pacific Northwest. These temperature criteria allow for rapid determination of thermal risks to fish and can trigger release of cold-water reserves from reservoirs. The largest of these management frameworks is the Region 10 Guidance (U.S. Environmental Protection Agency 2003) implemented throughout the Pacific Northwest and considered for application to California. The Region 10 document was heavily researched, combining thermal performance data (e.g., mortality and growth) across populations and species to provide temperature thresholds for the protection of native salmonids (Table 1). The Region 10 temperature guidelines appear to be broadly protective of California salmon. However, these thresholds do not account for interpopulation variation or the observable diversity in thermal physiology and ecological parameters known to influence population thermal performance. Furthermore, these criteria use a rolling seven-day average of daily maximums (7DADM) as a metric for river temperature. While intuitive and easily calculated, the 7DADM metric captures neither the absolute maximum nor the duration of exposure, crucial aspects to a fish’s thermal experience. Successful salmonid conservation requires protecting inherent thermal diversity among populations. While broad management regulations (e.g., Region 10 Guidelines) may serve as a starting point or backstop, conserving diversity requires population-specific management strategies. Population-specific thermal regulations may be considered overly burdensome to common regulatory frameworks; however, managing at the population level amplifies diversity and leverages the portfolio effect to protect species regionally. Managing to conserve diverse thermal physiologies will increase the resilience of populations and their ability to withstand stochastic events and adapt to environmental change.
Accounting for thermal eco-physiology in salmonids
California contains the southern range boundary for several native migratory salmonid species, including endemic and critically endangered populations (Moyle et al. 2017). In the future, these populations will confront increasingly severe and frequent drought conditions (Diffenbaugh et al. 2015). For instance, the recent prolonged and severe California drought (2012–2016) led to the collapse of Sacramento River winter-run Chinook salmon population. This population is reliant upon cold-water releases from Shasta Dam in the Sacramento River (ICF International et al. 2016). Despite having population-specific thermal criteria (13.3 °C 7DADM for rearing embryos, USFWS 1999) the extended drought conditions exhausted the cold-water pool in Shasta Reservoir and water temperatures exceeded 15.5 °C, leading to extremely low embryo and larval survival (Moyle et al. 2017; Durand et al. 2020). Continued persistence of this population is aided by a conservation hatchery and reintroductions into Battle Creek in Northern California (ICF International et al. 2016).
As addressed above, salmonid populations vary in their ecological thermal physiology, dependent upon how their fundamental thermal physiology interacts with ecological factors. A population’s specific combination of factors, (e.g., prey-availability, acclimation capacity, life-history strategy) can aid management in defining critical thermal thresholds (e.g., upper physiological limits) to prevent mortality as well as optimal temperature targets (e.g., fastest growth, smoltification success, maximum juvenile recruitment) to improve population performance. Quantifying population-specific, ecologically linked thermal criteria is necessary to manage at-risk salmonids under warming climatic conditions, where meeting rigid temperature thresholds in California’s Mediterranean climate and highly-modified hydroscape will become increasingly difficult. More broadly, this approach can be used to assess and identify vulnerable populations throughout the Pacific Northwest.
We pose a series of research questions to improve assessment of population-specific thermal vulnerability and to offer insights actionable for management.
-
1.
Do the seasonal runs of Chinook salmon exhibit differences in thermal physiology and performance?
-
2.
Do temperature tolerances or acclimation capacities differ between wild and hatchery salmonid populations?
-
3.
How does temperature influence smoltification success; does the relationship vary between populations or seasonal runs?
-
4.
How does energetic state (e.g., satiated vs. starved) influence a fish’s thermal performance?
-
5.
How does temperature influence salmonid prey and predator species and their effects on juvenile salmon populations?
-
6.
How do interspecific and intraspecific competition influence thermal physiology?
Optimizing the thermal landscape requires data addressing both ecological and physiological traits of different populations. We outline a research framework to assess ecological factors pertinent to fish thermal performance and to develop population-specific datasets for California salmonids (Fig. 2). We recommend two tiers of data collection. First, a comprehensive collection of a few, rapidly sampled environmental characteristics meant to identify populations with declining environmental or ecological conditions (e.g., lack of thermal refugia, poor water quality, limited rearing habitat). For instance, populations with limited thermal refugia may have greater difficulty responding to environmental warming. Understanding these environmental characteristics would allow for identification of at-risk populations for which more thorough thermal assessments are warranted. Once at-risk populations are identified, a second tier of data collection should focus on important physiological and ecological parameters necessary to determine both fundamental and ecological thermal physiologies of different populations. Defining these physiologies will provide managers with metrics useful for establishing thermal thresholds [e.g., growth rates (Marine and Cech 2004; Lugert et al. 2016), critical thermal maximums (Becker and Genoway 1979), temperature dependent metabolism (Farrell et al. 2008; Clark et al. 2013)]. Defining fundamental and ecological thermal physiology of at-risk populations will also help identify strategies for improving population robustness and resiliency (e.g., predator removal, reduced hatchery supplementation, genetic rescue).
We recommend prioritizing research on early migrating populations (e.g., Sacramento River winter-run, Columbia River and Klamath Basin spring-run populations) or populations that exhibit an over-summering component to their freshwater life history (e.g., coho salmon) because many are at risk of extinction within 50 years (Moyle et al. 2017). Indeed, many of these populations are already listed as federally threatened or endangered. Furthermore, Chinook populations arising from the San Joaquin River watershed and steelhead trout from southern California coastal streams warrant prioritization because they represent the southernmost populations of their species. Understanding the effect of hatchery supplementation and genetic homogenization on populations which support commercial fisheries will be important in predicting the response of these economically and culturally valuable resources.
Conclusion
Pacific salmonids are a collection of wide-spread and differing populations. Across their ranges, diverse environmental factors have produced variation in both their fundamental and ecological thermal physiologies. Understanding how variation in thermal physiology yields population-specific thermal performance of populations is crucial from a management perspective. For each population, thermal performance is challenged by rapidly changing environmental conditions. The inherent complexity of interactions between changing ecosystems and organismal thermal physiology challenges the application of broad thermal management criteria. Simple static temperature criteria can be improved by incorporating local data on salmonid fundamental physiology and on ecological conditions to produce population-specific thermal management strategies.
References
Allan JD, Castillo MM (2007) Stream ecology: structure and function of running waters, 2nd edn. Springer, Dordrecht, The Netherlands
Anderson SC, Moore JW, McClure MM et al (2015) Portfolio conservation of metapopulations under climate change. Ecol Appl 25:559–572. https://doi.org/10.1890/14-0266.1
Anttila K, Farrell AP, Patterson DA et al (2019) Cardiac SERCA activity in sockeye salmon populations: an adaptive response to migration conditions. Can J Fish Aquat Sci 76:1–5. https://doi.org/10.1139/cjfas-2018-0334
Araki H, Berejikian BA, Ford MJ, Blouin MS (2008) Fitness of hatchery-reared salmonids in the wild: fitness of hatchery fish. Evol Appl 1:342–355. https://doi.org/10.1111/j.1752-4571.2008.00026.x
Araki H, Cooper B, Blouin MS (2007) Genetic effects of captive breeding cause a rapid, cumulative fitness decline in the wild. Science 318:100–103. https://doi.org/10.1126/science.1145621
Arendt JD (1997) Adaptive intrinsic growth rates: an integration across taxa. Q Rev Biol 72:149–177. https://doi.org/10.1086/419764
Ashley MV, Willson MF, Pergams ORW et al (2003) Evolutionarily enlightened management. Biol Cons 111:115–123. https://doi.org/10.1016/S0006-3207(02)00279-3
Bear EA, McMahon TE, Zale AV (2007) Comparative thermal requirements of westslope cutthroat trout and rainbow trout: implications for species interactions and development of thermal protection standards. Trans Am Fish Soc 136:1113–1121. https://doi.org/10.1577/T06-072.1
Becker CD, Genoway RG (1979) Evaluation of the critical thermal maximum for determining thermal tolerance of freshwater fish. Environ Biol Fishes 4:245–256. https://doi.org/10.1007/BF00005481
Bisson PA, Nielsen JL, Ward JW (1988) Summer production of Coho salmon stocked in Mount St. helens streams 3–6 years after the 1980 eruption. Trans Am Fish Soc 117:322–335. https://doi.org/10.1577/1548-8659(1988)117%3c0322:SPOCSS%3e2.3.CO;2
Bonada N, Rieradevall M, Prat N (2007) Macroinvertebrate community structure and biological traits related to flow permanence in a Mediterranean river network. Hydrobiologia 589:91–106. https://doi.org/10.1007/s10750-007-0723-5
Boulton AJ, Peterson CG, Grimm NB, Fisher SG (1992) Stability of an aquatic macroinvertebrate community in a multiyear hydrologic disturbance regime. Ecology 73:2192–2207. https://doi.org/10.2307/1941467
Brett JR (1952) Temperature tolerance in young Pacific salmon, genus Oncorhynchus. J Fish Res Board Can 9:265–323. https://doi.org/10.1139/f52-016
Brett JR (1971) Energetic responses of salmon to temperature. A study of some thermal relations in the physiology and freshwater ecology of sockeye salmon (Oncorhynchus nerka). Am Zool 11:99–113. https://doi.org/10.1093/icb/11.1.99
Brett JR, Clarke WC, Shelbourn JE (1982) Experiments on thermal requirements for growth and food conversion efficiency of juvenile Chinook salmon Oncorhynchus tshawytscha. Can Tech Rep Fish Aquat Sci 1127:35
Campbell EY, Dunham JB, Reeves GH, Wondzell SM (2019) Phenology of hatching, emergence, and end-of-season body size in young-of-year Coho salmon in thermally contrasting streams draining the Copper River Delta, Alaska. Can J Fish Aquat Sci 76:185–191. https://doi.org/10.1139/cjfas-2018-0003
Carlson SM, Satterthwaite WH (2011) Weakened portfolio effect in a collapsed salmon population complex. Can J Fish Aquat Sci 68:1579–1589. https://doi.org/10.1139/f2011-084
Carlson SM, Seamons TR (2008) A review of quantitative genetic components of fitness in salmonids: implications for adaptation to future change. Evol Appl 1:222–238. https://doi.org/10.1111/j.1752-4571.2008.00025.x
Carter K (2005) The effects of temperature on steelhead trout, coho salmon, and Chinook salmon biology and function by life stage. California Regional Water Quality Control Board, California
Cayan DR, Maurer EP, Dettinger MD et al (2008) Climate change scenarios for the California region. Clim Chang 87:21–42. https://doi.org/10.1007/s10584-007-9377-6
Cech JJ, Myrick CA (1999) Steelhead and Chinook salmon bioenergetics: temperature, ration, and genetic effects. University of California Water Resources Center, California
Chang H, Bonnette MR (2016) Climate change and water-related ecosystem services: impacts of drought in California, USA. Ecosyst Health Sustain 2:1–19. https://doi.org/10.1002/ehs2.1254
Chen Z, Anttila K, Wu J et al (2013) Optimum and maximum temperatures of sockeye salmon (Oncorhynchus nerka) populations hatched at different temperatures. Can J Zool 91:265–274. https://doi.org/10.1139/cjz-2012-0300
Chen Z, Farrell AP, Matala A et al (2018a) Physiological and genomic signatures of evolutionary thermal adaptation in redband trout from extreme climates. Evol Appl 11:1686–1699. https://doi.org/10.1111/eva.12672
Chen Z, Farrell AP, Matala A, Narum SR (2018b) Mechanisms of thermal adaptation and evolutionary potential of conspecific populations to changing environments. Mol Ecol 27:659–674. https://doi.org/10.1111/mec.14475
Chen Z, Snow M, Lawrence CS et al (2015) Selection for upper thermal tolerance in rainbow trout (Oncorhynchus mykiss Walbaum). J Exp Biol 218:803–812. https://doi.org/10.1242/jeb.113993
Clark TD, Sandblom E, Jutfelt F (2013) Aerobic scope measurements of fishes in an era of climate change: respirometry, relevance and recommendations. J Exp Biol 216:2771–2782. https://doi.org/10.1242/jeb.084251
Coutant CC (1973) Effect of thermal shock on vulnerability of juvenile salmonids to predation. J Fish Res Board Can 30:965–973. https://doi.org/10.1139/f73-157
Crossin GT, Hinch SG, Cooke SJ et al (2008) Exposure to high temperature influences the behaviour, physiology, and survival of sockeye salmon during spawning migration. Can J Zool 86:127–140. https://doi.org/10.1139/Z07-122
Dedrick AG, Baskett ML (2018) Integrating genetic and demographic effects of connectivity on population stability: the case of hatchery trucking in salmon. Am Nat 192:E62–E80. https://doi.org/10.1086/697581
Diffenbaugh NS, Swain DL, Touma D (2015) Anthropogenic warming has increased drought risk in California. Proc Natl Acad Sci 112:3931–3936. https://doi.org/10.1073/pnas.1422385112
Dittman AH, Quinn TP (1995) Homing in Pacific salmon: mechanisms and ecological basis. J Exp Biol 199:83–91. https://doi.org/10.1007/978-1-4613-2763-9_21
Dodrill MJ, Yackulic CB, Kennedy TA, Hayes JW (2016) Prey size and availability limits maximum size of rainbow trout in a large tailwater: insights from a drift-foraging bioenergetics model. Can J Fish Aquat Sci 73:759–772. https://doi.org/10.1139/cjfas-2015-0268
Durand J, Bombardelli F, Fleenor W et al (2020) Drought and the Sacramento-San Joaquin Delta 2012–2016: environmental review and lessons. San Franc Estuary Watershed 18:2. https://doi.org/10.15447/sfews.2020v18iss2art2
Eliason EJ, Clark TD, Hague MJ et al (2011) Differences in thermal tolerance among sockeye salmon populations. Science 332:109–112. https://doi.org/10.1126/science.1199158
Erhardt JM, Tiffan KF, Connor WP (2018) Juvenile Chinook salmon mortality in a Snake River Reservoir: smallmouth bass predation revisited. Trans Am Fish Soc 147:316–328. https://doi.org/10.1002/tafs.10026
Fangue NA, Hofmeister M, Schulte PM (2006) Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish, Fundulus heteroclitus. J Exp Biol 209:2859–2872. https://doi.org/10.1242/jeb.02260
Farrell AP, Hinch SG, Cooke SJ et al (2008) Pacific salmon in hot water: applying aerobic scope models and biotelemetry to predict the success of spawning migrations. Physiol Biochem Zool 81:697–709. https://doi.org/10.1086/592057
Folmar LC, Dickhoff WW (1980) The parr-smolt transformation (smoltification) and seawater adaptation in salmonids. Aquaculture 21:1–37. https://doi.org/10.1016/0044-8486(80)90123-4
Foott JS, Harmon R, Stone R (2014) Effect of summer water temperatures on growth and bioenergetics in juvenile Klamath River coho salmon (Oncorhynchus kisutch). U.S. Fish and Wildlife Service California-Nevada Fish Health Center, Anderson, CA
Fry FEJ (1947) Effects of the environment on animal activity. Pub Ontario Fish Res Lab 68:1–52
Fryer J, Pilcher K (1974) Effects of temperature on diseases of salmonid fishes. Office of Research and Development U.S, Environmental Protection Agency, Washington, D.C.
Fuhrman AE, Larsen DA, Steel EA et al (2018) Chinook salmon emergence phenotypes: describing the relationships between temperature, emergence timing and condition factor in a reaction norm framework. Ecol Freshw Fish 27:350–362. https://doi.org/10.1111/eff.12351
Garling DL, Masterson M (1985) Survival of Lake Michigan Chinook salmon eggs and fry incubated at three temperatures. Prog Fish-Culturist 47:63–66. https://doi.org/10.1577/1548-8640
Gayeski NJ, Stanford JA, Montgomery DR et al (2018) The failure of wild salmon management: need for a place-based conceptual foundation. Fisheries 43:303–309. https://doi.org/10.1002/fsh.10062
Geist DR, Abernethy CS, Hand KD et al (2006) Survival, development, and growth of fall Chinook salmon embryos, alevins, and fry exposed to variable thermal and dissolved oxygen regimes. Trans Am Fish Soc 135:1462–1477. https://doi.org/10.1577/T05-294.1
Goniea TM, Keefer ML, Bjornn TC et al (2006) Behavioral thermoregulation and slowed migration by adult fall Chinook salmon in response to high Columbia River water temperatures. Trans Am Fish Soc 135:408–419. https://doi.org/10.1577/T04-113.1
Green K (2010) Alpine taxa exhibit differing responses to climate warming in the Snowy Mountains of Australia. J Mt Sci 7:167–175. https://doi.org/10.1007/s11629-010-1115-2
Greene CM, Hall JE, Guilbault KR, Quinn TP (2010) Improved viability of populations with diverse life-history portfolios. Biol Lett 6:382–386. https://doi.org/10.1098/rsbl.2009.0780
Hallock RJ, Elwell RF, Fry DH (1970) Migrations of adult king salmon Oncorhynchus tshawytscha in the San Joaquin delta as demonstrated by the use of sonic tags. University of California, San Diego, Scripps Institution of Oceanography
Hamlet AF, Mote PW, Clark MP, Lettenmaier DP (2005) Effects of temperature and precipitation variability on snowpack trends in the western United States. J Clim 18:4545–4561. https://doi.org/10.1175/JCLI3538.1
Hampe A, Petit RJ (2005) Conserving biodiversity under climate change: the rear edge matters. Ecol Lett 8:461–467. https://doi.org/10.1111/j.1461-0248.2005.00739.x
Hazel JR, Prosser CL (1974) Molecular mechanisms of temperature compensation in poikilotherms. Physiol Rev 54:620–677. https://doi.org/10.1152/physrev.1974.54.3.620
Heming TA (1982) Effects of temperature on utilization of yolk by Chinook salmon (Oncorhynchus tshawytscha) eggs and alevins. Can J Fish Aquat Sci 39:184–190. https://doi.org/10.1139/f82-021
Hilborn R, Quinn TP, Schindler DE, Rogers DE (2003) Biocomplexity and fisheries sustainability. Proc Natl Acad Sci 100:6564–6568. https://doi.org/10.1073/pnas.1037274100
Hindar K, Ryman N, Utter F (1991) Genetic effects of cultured fish on natural fish populations. Can J Fish Aquat Sci 48:945–957. https://doi.org/10.1139/f91-111
Hochachka PW, Somero GN (2002) Biochemical adaptation: mechanisms and process in physiological evolution. Oxford University Press, New York
Hochachka PW, Somero GN (1968) The adaptation of enzymes to temperature. Comp Biochem Physiol 27:659–668. https://doi.org/10.1016/0010-406X(68)90605-1
ICF International, McConnaha WE, Blair G, Lecky J (2016) Battle creek winter-run Chinook salmon reintroduction plan. California Department of Fish and Wildlife, Sacramento, CA
Jasper JR, Habicht C, Moffitt S et al (2013) Source-sink estimates of genetic introgression show influence of hatchery strays on wild chum salmon populations in Prince William Sound, Alaska. PLoS One San Franc 8:e81916. https://doi.org/10.1371/journal.pone.0081916
Jensen JOT, Groot EP (1991) The effect of moist air incubation conditions and temperature on Chinook salmon egg survival. In: Colt J, White RJ (eds) Fisheries bioengineering symposium: American fisheries society symposium 10. American Fisheries Society, Bethesda, MD, pp 529–538
Katz J, Moyle PB, Quiñones RM et al (2013) Impending extinction of salmon, steelhead, and trout (Salmonidae) in California. Environ Biol Fishes 96:1169–1186. https://doi.org/10.1007/s10641-012-9974-8
Keefer ML, Peery CA, Caudill CC (2008) Migration timing of Columbia River spring Chinook salmon: effects of temperature, river discharge, and ocean environment. Trans Am Fish Soc 137:1120–1133. https://doi.org/10.1577/T07-008.1
Keefer ML, Clabough TS, Jepson MA et al (2018) Thermal exposure of adult Chinook salmon and steelhead: diverse behavioral strategies in a large and warming river system. PLoS ONE 13(9):e0204274. https://doi.org/10.1371/journal.pone.020474
Kudo G, Ida TY (2013) Early onset of spring increases the phenological mismatch between plants and pollinators. Ecology 94:2311–2320. https://doi.org/10.1890/12-2003.1
Kuehne LM, Olden JD, Duda JJ (2012) Costs of living for juvenile Chinook salmon (Oncorhynchus tshawytscha) in an increasingly warming and invaded world. Can J Fish Aquat Sci 69:1621–1630. https://doi.org/10.1139/f2012-094
Lindley ST, Mohr MS (2003) Modeling the effect of striped bass (Morone saxatilis) on the population viability of Sacramento River winter-run Chinook salmon (Oncorhynchus tshawytscha). Fish Bull 101:321–331
Lindley ST, Schick RS, Agrawal A et al (2006) Historical population structure of Central Valley steelhead and its alteration by dams. San Franc Estuary Watershed Sci. https://doi.org/10.15447/sfews.2006v4iss1art3
Lisi PJ, Schindler DE, Bentley KT, Pess GR (2013) Association between geomorphic attributes of watersheds, water temperature, and salmon spawn timing in Alaskan streams. Geomorphology 185:78–86. https://doi.org/10.1016/j.geomorph.2012.12.013
Lowney CL (2000) Stream temperature variation in regulated rivers: evidence for a spatial pattern in daily minimum and maximum magnitudes. Water Resour Res 36:2947–2955. https://doi.org/10.1029/2000WR900142
Lugert V, Thaller G, Tetens J et al (2016) A review on fish growth calculation: multiple functions in fish production and their specific application. Rev Aquacult 8:30–42. https://doi.org/10.1111/raq.12071
Lusardi RA, Bogan MT, Moyle PB, Dahlgren RA (2016) Environment shapes invertebrate assemblage structure differences between volcanic springfed and runoff rivers in northern California. Freshw Sci 35:1010–1022. https://doi.org/10.1086/687114
Lusardi RA, Hammock BG, Jeffres CA et al (2020) Oversummer growth and survival of juvenile coho salmon (Oncorhynchus kisutch) across a natural gradient of stream water temperature and prey availability: an in situ enclosure experiment. Can J Fish Aquat Sci 77:413–424. https://doi.org/10.1139/cjfas-2018-0484
Lusardi RA, Jeffres CA, Moyle PB (2018) Stream macrophytes increase invertebrate production and fish habitat utilization in a California stream. River Res Appl 34(8):1003–1012. https://doi.org/10.1002/rra.3331
Lusardi RA, Stephens MR, Moyle PB et al (2015) Threat evolution: negative feedbacks between management action and species recovery in threatened trout (Salmonidae). Rev Fish Biol Fish 25:521–535. https://doi.org/10.1007/s11160-015-9394-x
Mann RD, Snow CG (2018) Population-specific migration patterns of wild adult summer-run Chinook salmon passing Wells Dam, Washington. N Am J Fish Manag 38:377–392. https://doi.org/10.1002/nafm.10042
Marine KR, Cech JJ (2004) Effects of high water temperature on growth, smoltification, and predator avoidance in juvenile Sacramento River Chinook salmon. Nor Am J Fish Manag 24:198–210. https://doi.org/10.1577/M02-142
Matala AP, Narum SR, Young W, Vogel JL (2012) Influences of hatchery supplementation, spawner distribution, and habitat on genetic structure of Chinook salmon in the South Fork Salmon River, Idaho. N Am J Fish Manag 32:346–359. https://doi.org/10.1080/02755947.2012.678961
McCarthy SG, Duda JJ, Emlen JM et al (2009) Linking habitat quality with trophic performance of steelhead along forest gradients in the South Fork Trinity River watershed, California. Trans Am Fish Soc 138:506–521. https://doi.org/10.1577/T08-053.1
McClure MM, Carlson SM, Beechie TJ et al (2008) Evolutionary consequences of habitat loss for Pacific anadromous salmonids: salmonid habitat loss and evolution. Evol Appl 1:300–318. https://doi.org/10.1111/j.1752-4571.2008.00030.x
McConnell C, Westley P, McPhee M (2018) Differences in fitness-associated traits between hatchery and wild chum salmon despite long-term immigration by strays. Aquac Environ Interact 10:99–113. https://doi.org/10.3354/aei00261
McCullough DA (1999) A review and synthesis of effects of alterations to the water temperature regime on freshwater life stages of salmonids with special reference to Chinook salmon. U S Environmental Protection Agency, Region 10, Seattle, Washington
Micheletti SJ, Matala AR, Matala AP, Narum SR (2018) Landscape features along migratory routes influence adaptive genomic variation in anadromous steelhead (Oncorhynchus mykiss). Mol Ecol 27:128–145. https://doi.org/10.1111/mec.14407
Møller AP, Rubolini D, Lehikoinen E (2008) Populations of migratory bird species that did not show a phenological response to climate change are declining. Proc Natl Acad Sci 105:16195–16200. https://doi.org/10.1073/pnas.0803825105
Moore JW, McClure M, Rogers LA, Schindler DE (2010) Synchronization and portfolio performance of threatened salmon. Conserv Lett 3:340–348. https://doi.org/10.1111/j.1755-263X.2010.00119.x
Moore RD, Spittlehouse DL, Story A (2005) Riparian microclimate and stream temperature response to forest harvesting: a review. J Am Water Resour Assoc 41:813–834
Moyle PB, Kiernan JD, Crain PK, Quiñones RM (2013) Climate change vulnerability of native and alien freshwater fishes of California: a systematic assessment approach. PLoS ONE 8:e63883. https://doi.org/10.1371/journal.pone.0063883
Moyle PB, Lusardi RA, Samuel PJ, Katz JVE (2017) State of salmonids: status of California’s emblematic fishes. Center for Watershed Sci, University of California, Davis and California Trout, San Francisco, CA
Muñoz NJ, Farrell AP, Heath JW, Neff BD (2015) Adaptive potential of a Pacific salmon challenged by climate change. Nat Clim Chang 5:163–166. https://doi.org/10.1038/nclimate2473
Myrick CA, Cech JJ (2001) Temperature effects on Chinook salmon and steelhead: a review focusing on California’s Central Valley populations. Bay Delta Modeling Forum, California
Myrick CA, Cech JJ (2004) Temperature effects on juvenile anadromous salmonids in California’s Central Valley: what don’t we know? Rev Fish Biol Fish 14:113–123. https://doi.org/10.1007/s11160-004-2739-5
Myrvold KM, Kennedy BP (2017) Size and growth relationships in juvenile steelhead: the advantage of large relative size diminishes with increasing water temperatures. Environ Biol Fishes 100:1373–1382. https://doi.org/10.1007/s10641-017-0649-3
Myrvold KM, Kennedy BP (2018) Increasing water temperatures exacerbate the potential for density dependence in juvenile steelhead. Can J Fish Aquat Sci 75:897–907. https://doi.org/10.1139/cjfas-2016-0497
Narum SR, Campbell NR, Meyer KA et al (2013) Thermal adaptation and acclimation of ectotherms from differing aquatic climates. Mol Ecol 22:3090–3097. https://doi.org/10.1111/mec.12240
Narum SR, Di Genova A, Micheletti SJ, Maass A (2018) Genomic variation underlying complex life-history traits revealed by genome sequencing in Chinook salmon. Proc R Soc B 285:20180935. https://doi.org/10.1098/rspb.2018.0935
Nehlsen W, Williams JE, Lichatowich JA (1991) Pacific salmon at the crossroads: stocks at risk from California, Oregon, Idaho, and Washington. Fisheries 16:4–21. https://doi.org/10.1577/1548-8446(1991)016%3c0004:PSATCS%3e2.0.CO;2
Nichols AL, Willis AD, Jeffres CA, Deas ML (2014) Water temperature patterns below large groundwater springs: Management implications for coho salmon in the Shasta River, California. River Res Appl 30:442–455. https://doi.org/10.1002/rra.2655
Nichols KM, Kozfkay CC, Narum SR (2016) Genomic signatures among Oncorhynchus nerka ecotypes to inform conservation and management of endangered sockeye salmon. Evol Appl 9:1285–1300. https://doi.org/10.1111/eva.12412
Null SE, Viers JH, Deas ML et al (2013) Stream temperature sensitivity to climate warming in California’s Sierra Nevada: impacts to coldwater habitat. Climatic Change 116:149–170. https://doi.org/10.1007/s10584-012-0459-8
Petersen JH, Kitchell JF (2001) Climate regimes and water temperature changes in the Columbia River: bioenergetic implications for predators of juvenile salmon. Can J Fish Aquat Sci 58:1831–1841. https://doi.org/10.1139/cjfas-58-8-1831
Peterson MG, Lunde KB, Chiu M-C, Resh VH (2017) Seasonal progression of aquatic organisms in a temporary wetland in Northern California. West N Am Nat 77:176–188. https://doi.org/10.3398/064.077.0205
Plumb JM (2018) A bioenergetics evaluation of temperature-dependent selection for the spawning phenology by Snake River fall Chinook salmon. Ecol Evol 8:9633–9645. https://doi.org/10.1002/ece3.4353
Poletto JB, Cocherell DE, Baird SE et al (2017) Unusual aerobic performance at high temperatures in juvenile Chinook salmon, Oncorhynchus tshawytscha. Conserv Physiol 5:cow067. https://doi.org/10.1093/conphys/cow067
Pörtner HO, Farrell AP (2008) Physiology and climate change. Science 322:690–692. https://doi.org/10.1126/science.1163156
Railsback SF, Rose KA (1999) Bioenergetics modeling of stream trout growth: temperature and food consumption effects. Trans Am Fish Soc 128:241–256. https://doi.org/10.1577/1548-8659
Ranalli AJ, Macalady DL (2010) The importance of the riparian zone and in-stream processes in nitrate attenuation in undisturbed and agricultural watersheds—a review of the scientific literature. J Hydrol 389:406–415. https://doi.org/10.1016/j.jhydrol.2010.05.045
Reese CD, Harvey BC (2002) Temperature-dependent interactions between juvenile steelhead and Sacramento pikeminnow in laboratory streams. Trans Am Fish Soc 131:599–606. https://doi.org/10.1577/1548-8659
Reeves GH, Everest FH, Hall JD (1987) Interactions between the redside shiner (Richardsonius balteatus) and the steelhead trout (Salmo gairdneri) in Western Oregon: the influence of water temperature. Can J Fish Aquat Sci 44:1603–1613. https://doi.org/10.1139/f87-194
Rich A (1987) Report on studies conducted by Sacramento County to determine the temperatures which optimize growth and survival in juvenile Chinook salmon (Oncorhynchus tshawytscha). McDonough, Holland and Allen, 555 Capitol Mall Sacramento, Sacramento
Richter A, Kolmes SA (2005) Maximum temperature limits for Chinook, coho, and chum salmon, and steelhead trout in the Pacific Northwest. Rev Fish Sci 13:23–49. https://doi.org/10.1080/10641260590885861
Sabal M, Hayes S, Merz J, Setka J (2016) Habitat alterations and a nonnative predator, the striped bass, increase native Chinook salmon mortality in the Central Valley, California. North Am J Fish Manag 36:309–320. https://doi.org/10.1080/02755947.2015.1121938
Satterthwaite WH, Carlson SM (2015) Weakening portfolio effect strength in a hatchery-supplemented Chinook salmon population complex. Can J Fish Aquat Sci 72:1860–1875. https://doi.org/10.1139/cjfas-2015-0169
Satterthwaite WH, Carlson SM, Criss A (2017) Ocean size and corresponding life history diversity among the four run timings of California Central Valley Chinook salmon. Trans Am Fish Soc 146:594–610. https://doi.org/10.1080/00028487.2017.1293562
Sauter ST, Crawshaw LI, Maule AG (2001) Behavioral thermoregulation by juvenile spring and fall Chinook salmon, Oncorhynchus tshawytscha, during smoltification. Environ Biol Fishes 61:295–304
Schindler DE, Hilborn R, Chasco B et al (2010) Population diversity and the portfolio effect in an exploited species. Nature 465:609–612. https://doi.org/10.1038/nature09060
Schulte PM (2015) The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to a changing environment. J Exp Biol 218:1856–1866. https://doi.org/10.1242/jeb.118851
Schulte PM, Healy TM, Fangue NA (2011) Thermal performance curves, phenotypic plasticity, and the time scales of temperature exposure. Integr Comp Biol 51:691–702. https://doi.org/10.1093/icb/icr097
Selbie DT, Finney BP, Barto D et al (2009) Ecological, landscape, and climatic regulation of sediment geochemistry in North American sockeye salmon nursery lakes: insights for paleoecological salmon investigations. Limnol Oceanogr 54:1733–1745. https://doi.org/10.4319/lo.2009.54.5.1733
Smith CT, Engle R (2011) Persistent reproductive isolation between sympatric lineages of fall Chinook salmon in White Salmon River, Washington. Trans Am Fish Soc 140:699–715. https://doi.org/10.1080/00028487.2011.584490
Spence BC, Dick EJ (2014) Geographic variation in environmental factors regulating outmigration timing of coho salmon (Oncorhynchus kisutch) smolts. Can J Fish Aquat Sci 71:56–69. https://doi.org/10.1139/cjfas-2012-0479
Steel EA, Tillotson A, Larsen DA et al (2012) Beyond the mean: The role of variability in predicting ecological effects of stream temperature on salmon. Ecosphere. https://doi.org/10.1890/ES12-00255.1
Stitt BC, Burness G, Burgomaster KA et al (2014) Intraspecific variation in thermal tolerance and acclimation capacity in brook trout (Salvelinus fontinalis): Physiological implications for climate change. Physiol Biochem Zool 87:15–29. https://doi.org/10.1086/675259
Strange JS (2012) Migration strategies of adult Chinook salmon runs in response to diverse environmental conditions in the Klamath River Basin. Trans Am Fish Soc 141:1622–1636. https://doi.org/10.1080/00028487.2012.716010
Sturrock AM, Carlson SM, Wikert JD et al (2020) Unnatural selection of salmon life histories in a modified riverscape. Glob Change Biol 26:1235–1247. https://doi.org/10.1111/gcb.14896
Sturrock AM, Satterthwaite WH, Cervantes-Yoshida KM et al (2019) Eight decades of hatchery salmon releases in the California Central Valley: factors influencing straying and resilience. Fisheries 44:433–444. https://doi.org/10.1002/fsh.10267
Taylor EB (1991) A review of local adaptation in Salmonidae with particular reference to Pacific and Atlantic salmon. Aquaculture 98:185–207. https://doi.org/10.1016/0044-8486(91)90383-i
Thackery SJ, Sparks TH, Frederiksen M et al (2010) Trophic level asynchrony in rates of phenological change for marine, freshwater and terrestrial environments. Glob Change Biol 16:3304–3313. https://doi.org/10.1111/j.1365-2486.2010.02165.x
Thomas AE (1975) Migration of Chinook salmon fry from simulated incubation channels in relation to water temperature, flow and turbidity. Progress Fish-Culturist 37:219–223. https://doi.org/10.1577/1548-8659
US Environmental Protection Agency (2003) EPA region 10 guidance for Pacific Northwest state and tribal temperature water quality standards. EPA 910-B-03-002, Region 10 Office of Water, Seattle, WA
U. S. Fish and Wildlife Service, USFWS (1999) Effect of temperature on early-life survival of Sacramento river fall- and winter-run Chinook salmon. Northern Central Valley Fish and Wildlife Office, Red Bluff, CA
Van Doornik DM, Eddy DL, Waples RS et al (2013) Genetic monitoring of threatened Chinook salmon populations: estimating introgression of nonnative hatchery stocks and temporal genetic changes. North Am J Fish Manag 33:693–706. https://doi.org/10.1080/02755947.2013.790861
Verhille CE, English KK, Cocherell DE et al (2016) High thermal tolerance of a rainbow trout population near its southern range limit suggests local thermal adjustment. Conserv Physiol. https://doi.org/10.1093/conphys/cow057
Vigg S, Burley CC (1991) Temperature-dependent maximum daily consumption of juvenile salmonids by Northern squawfish (Ptychocheilus oregonensis) from the Columbia River. Can J Fish Aquat Sci 48:2491–2498. https://doi.org/10.1139/f91-290
Visser ME, van Noordwijk AJ, Tinbergen JM, Lessells CM (1998) Warmer springs lead to mistimed reproduction in great tits (Parus major). Proc R Soc Lond B 265:1867–1870. https://doi.org/10.1098/rspb.1998.0514
Wagner RW, Stacey M, Brown LR, Dettinger M (2011) Statistical models of temperature in the Sacramento-San Joaquin Delta under climate-change scenarios and ecological implications. Estuaries Coast 34:544–556. https://doi.org/10.1007/s12237-010-9369-z
Waples RS (1991) Genetic interactions between hatchery and wild salmonids: lessons from the Pacific Northwest. Can J Fish Aquat Sci 48:124–133. https://doi.org/10.1139/f91-311
Ward DL, Morton-Starner R (2015) Effects of water temperature and fish size on predation vulnerability of juvenile humpback chub to rainbow trout and brown trout. Trans Am Fish Soc 144:1184–1191. https://doi.org/10.1080/00028487.2015.1077160
Weber N, Bouwes N, Jordan CE (2014) Estimation of salmonid habitat growth potential through measurements of invertebrate food abundance and temperature. Can J Fish Aquat Sci 71:1158–1170. https://doi.org/10.1139/cjfas-2013-0390
Welsh HH, Hodgson GR, Harvey BC, Roche MF (2001) Distribution of juvenile coho salmon in relation to water temperatures in tributaries of the Mattole River, California. North Am J Fish Manag 21:464–470. https://doi.org/10.1577/1548-8675
Wenger SJ, Isaak DJ, Luce CH et al (2011) Flow regime, temperature, and biotic interactions drive differential declines of trout species under climate change. Proc Natl Acad Sci 108:14175–14180. https://doi.org/10.1073/pnas.1103097108
Williams JG (2006) Central valley salmon: a perspective on chinook and steelhead in the central Valley of California. San Franc Estuary Watershed Sci. https://doi.org/10.15447/sfews.2006v4iss3art2
Williamson KS, May B (2005) Homogenization of fall-run Chinook salmon gene pools in the Central Valley of California, USA. North Am J Fish Manag 25:993–1009. https://doi.org/10.1577/M04-136.1
Yang D, Marsh P, Ge S (2014) Heat flux calculations for Mackenzie and Yukon Rivers. Polar Sci 8:232–241. https://doi.org/10.1016/j.polar.2014.05.001
Yoshiyama RM, Gerstung ER, Fisher FW, Moyle PB (2001) Historical and present distribution of Chinook salmon in the Central Valley drainage of California. Fish Bulletin 179. California Department of Fish and Game, Sacramento (CA), pp 71–176
Zarri LJ, Danner EM, Daniels ME, Palkovacs EP (2019) Managing hydropower dam releases for water users and imperiled fishes with contrasting thermal habitat requirements. J Appl Ecol 56:2423–2430. https://doi.org/10.1111/1365-2664.13478
Zillig KW, Cocherell DE, Fangue NA (2020) Interpopulation variation among juvenile Chinook salmon from California and Oregon. The United States Environmental Protection Agency Region 9—Pacific Southwest Region, San Francisco, CA. https://www.epa.gov/sfbay-delta/2020-chinook-salmon-interpopulation-thermal-tolerance-investigation
Acknowledgements
Betty Yee and the California State Water Resources Control Board for commissioned and funded this review paper (UC Davis Cooperative Agreement #D16-15001 awarded to N.A.F). Bryan McFadin, Daniel Worth, Matthew Holland, Monica Gutierrez, Melanie Okoro, William Anderson, Mike Grill, Brian Thompson, John Wikert, Kelly Souza, Dan Kratville, Amber Villalobos, Stephen Louie, Rob Titus, Jonathan Nelson, Alyssa FitzGerald, Benjamin Martin, Stephen Maurano, Valentina Cabrera-Stagno, and Joe Cech provided comments and information during the process. Two anonymous reviewers provided constructive feedback.
Funding
Funding for this project was provided by the California State Water Resources Control Board and from the U.S. Environmental Protection Agency. The contents of this document do not necessarily reflect the views and policies of the State Water Resources Control Board or the U.S. Environmental Protection Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
Author information
Authors and Affiliations
Contributions
KZ and RL both contributed to idea generation, literature searching, drafting of the manuscript and editing. PM assisted with critical editing and advising. NF contributed to idea generation, critical editing and advising.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zillig, K.W., Lusardi, R.A., Moyle, P.B. et al. One size does not fit all: variation in thermal eco-physiology among Pacific salmonids. Rev Fish Biol Fisheries 31, 95–114 (2021). https://doi.org/10.1007/s11160-020-09632-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11160-020-09632-w