Abstract
Copper (Cu) is a bio-essential element and a potentially toxic pollutant in the plant–soil systems. Analysis of stable Cu isotopes can be a powerful tool for tracing the biogeochemical cycling of Cu in plant–soil systems. In this review, we examined the analysis method of stable Cu isotope ratios in plants and soils, and discussed the biogeochemical processes, including redox reactions, mineral dissolution, abiotic and biotic sorption, which fractionate Cu isotopes in plant–soil systems. We also reviewed the variability of the isotopic signature in different plants and plant tissues, as well as different soil types and profiles to discuss the relationship between the biogeochemical transformation of Cu and its isotope fractionation in plant–soil systems. The collected data show that δ65Cu values range from − 2.59 to + 1.73‰ in plant–soil systems, and ∆65Cu values range from − 1.00 to − 0.11‰ between the plant and soil. The variation in the ∆65Cu value between the plant and soil is mainly in response to the different uptake strategies during the acquisition of Cu from soils. Cu isotope analyses are proved to be a suitable technique during the biogeochemical transformation of Cu in plant–soil systems, especially during redox reactions. Ultimately, research challenges and future directions for Cu isotope techniques as a proxy for Cu biogeochemical cycles are also proposed. This review is beneficial for soil safety, food safety, and the sustainable development of agriculture and human health.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Copper (Cu) is a crucial micronutrient for the growth of plants, animals, and humans, primarily due to its significant role in enzyme activity (Hou et al. 2020; Zandi et al. 2020). The soil serves as a critical medium for the interaction of Cu within the environment and life systems, particularly in plant–soil systems. As part of the pedogenic process, rock weathering releases varying quantities of Cu into soils, but its concentration typically remains low (approximately 2 to 50 mg·kg−1) (Holmgren et al. 1993). Anthropogenic activities, such as mining, refining, the use of fungicides, and industrial processes, serve as major pathways leading to the accumulation of Cu in soils (Alloway 2013; Li et al. 2021). The excess presence of Cu in soils poses a threat to the environment and human health through the food chain, potentially causing severe health issues such as liver cirrhosis, brain damage, and kidney damage by disrupting Cu homeostasis in human bodies (Cornu et al. 2017; Hou et al. 2020; Shabbir et al. 2020; Zandi et al. 2020). Moreover, the depletion of Cu can harm the photosynthetic machinery, disrupting the corresponding electron transport chain in plants and resulting in necrotizing lesions (Gaetke et al. 2014; Shabbir et al. 2020; Wilson and Pyatt 2007). Therefore, it is crucial to investigate the transportation of Cu in plant–soil systems and establish a comprehensive model of the global Cu cycling.
Copper naturally exhibits two stable isotopes, 63Cu (abundance 69.2%) and 65Cu (30.8%) (Shields et al. 1964). The variation in Cu isotope composition primarily arises due to mass-dependent isotope fractionation. Slight differences in the efficiency of Cu isotopes participating in various chemical and physical processes, such as redox reactions, sorption, organic complexation, and mineral dissolution, are likely to lead to Cu isotope fractionation (Dotor-Almazan et al. 2017; Fekiacova et al. 2015; Kusonwiriyawong et al. 2017; Li et al. 2016). These differences are associated with subtle variations in equilibrium or kinetic isotope effects (Maréchal et al. 1999; Moynier et al. 2017; Zhu et al. 2000). As a result, Cu isotope compositions play a crucial role in identifying the mechanisms of uptake and accumulation of Cu within plants and the transport processes between plants and soils (Jouvin et al. 2012; Kribek et al. 2020; Li et al. 2016; Sillerova et al. 2017). Within plants, the variation in Cu isotope compositions between roots and aboveground tissues reflects different fractionation factors, depending on the translocation type (diffusion vs. convection) and plant height, except for the Cu tolerant plant E. splendens (Blotevogel et al. 2022, 2019; Li et al. 2016; Navarrete et al. 2011b). In comparison to soils, plants tend to preferentially absorb isotopically lighter Cu from the soil (Weinstein et al. 2011). Consequently, plant litter on the soil surface generally exhibits isotopically lighter characteristics. Additionally, aqueous Cu organic complexes formed during organic matter decomposition lead to the mobilization of heavier Cu isotopes in the soil (Kribek et al. 2020; Li et al. 2016; Mihaljevic et al. 2018; Weinstein et al. 2011). To date, the stable isotope technique has been successfully applied to study the biogeochemical processes of Cu in various environments, including agricultural soils (Babcsanyi et al. 2014; Blotevogel et al. 2018), river floodplain soils (Bigalke et al. 2013), Cu smelting, and mining-contaminated soils (Bigalke et al. 2010a; Dotor-Almazan et al. 2017; Mihaljevic et al. 2019; Roebbert et al. 2018), as well as paleosols (Little et al. 2019; Liu et al. 2014b).
Several reviews on environmental metal (including Cu) isotope geochemistry have been published (Albarede 2004; Eiler et al. 2014; Komárek et al. 2021; Wang et al. 2021), including the summary of Cu isotope geochemistry conducted by Moynier et al. (2017), Sullivan et al. (2022) and Wiggenhauser et al. (2022). However, in the last several years, significant progress has been made in understanding Cu isotope behavior within plant-soil systems (Burkhead et al. 2009; Kumar et al. 2021; Shabbir et al. 2020; Zandi et al. 2020). In this review, we consolidate various analytical methods for determining Cu isotope ratios in both plants and associated soils. Additionally, we present the latest findings on Cu isotope fractionation driven by both abiotic and biotic processes in plant–soil systems. Our examination includes a comparative analysis of the isotope signature variability in different plants, plant tissues, and various soil types characterized through soil profiles. Furthermore, we delve into the mechanisms of Cu isotope fractionation during soil–plant transfer. Finally, we address the challenges and prospects of utilizing Cu isotopes as a proxy for Cu biogeochemical cycles.
2 Methods to determine stable Cu isotope ratios
For isotope analyses, both plant and soil samples need to be digested and purified to enhance the analytical accuracy (Li et al. 2016; Little et al. 2019; Weinstein et al. 2011). Wet digestion methods are usually suitable for plant samples, but their effectiveness can be largely influenced by the binding forms of Cu (Ramanathan and Ting 2015; Sastre et al. 2002). Previous sequential extraction experiments have shown that Cu mainly exists as carbonate and organic fractions in plant–soil systems (Babcsanyi et al. 2016; Ramanathan and Ting 2015; Roebbert et al. 2018). Acids usually digest the carbonate fraction, while the usage of oxidizing agents results in organic matter oxidation during the dissolution of Cu phases (Ramanathan and Ting 2015). In addition, biogenic silica may also exist in plant–soil systems and HF has been validated to effectively dissolve silicates (Alsaleh et al. 2018; Sastre et al. 2002). Generally, pulverized plant and soil samples are dissolved using a mixture of concentrated acids, such as HCl–HNO3 or HCl–HNO3–HF–H2O2, while if any silicate-bound Cu is included in the sample, HF is indispensable (Jouvin et al. 2012; Kribek et al. 2020; Li et al. 2016; Weinstein et al. 2011). The repeatability (R.S.D. < 5%) and recoveries of Cu digestion by wet methods can ensure that the procedures are precise and accurate (Jouvin et al. 2012; Ryan et al. 2013; Weinstein et al. 2011). For Cu purification, the digest extracts were re-dissolved by using concentrated HCl, and the separation of the Cu fractionation from matrix components using ion-exchange chromatography was reported by Maréchal et al. (1999). It’s crucial to note that there is no standardization for the matrix reference material analysis within plant-soil systems (Sullivan et al. 2022). Soil reference materials such as ERM-CC141 (Loam soil), GSF-3 (Paddy soil), GSS-11 (Liaohe Plain soil), and GSS-13 (North China Plain soil) have been utilized in various applications and geographic regions based on specific requirements (Fig. S1) (Sullivan et al. 2022). However, the availability of well-characterized soil reference materials is notably limited, leading to a prevalent dependence on basalt references (Fig. S1) (e.g., BCR-1/2, BHVO-2, and BIR-1/1a) (Little et al. 2019; Zheng et al. 2023). This reliance is insufficient due to variations in copper concentrations and organic content, rendering this practice inadequate (Sullivan et al. 2022). Similarly, plant reference materials are also insufficient, with primary usage of BCR-414 (plankton) and SRM 1573a (tomato leaves) (Jeong et al. 2021; Ryan et al. 2013). In addition, various commercially available plant or soil materials, including NIST SRM 2709a (San Joaquin Soil), IAEA Soil-7, GBW 07602 (Bush branches and leaves), SRM 1515 (Apple leaves), SRM 1547 (Peach leaves), IAEA-336 (Lichen), BCR-402 (White clover), ERM-CD281 (Rye grass), SRM 1570a (Spinach leaves), SRM 1567b (Wheat flour), SRM 1568b (Rice flour), and ERM BC210 (Wheat flour), lack available δ65Cu values. Consequently, there is a need for determining the Cu isotope compositions of these plant or soil materials using MC–ICP–MS to gain insights into the transportation of Cu in plant-soil systems.
The earliest measurement of Cu isotope compositions was conducted by thermal ionization mass spectrometers (TIMS) and the analytical uncertainty was approximately 0.1‰ ~ 2‰ (Shields et al. 1964; Walker et al. 1959). With the development of multiple-collector inductively coupled plasma mass spectrometers (MC–ICP–MS), the accuracy of Cu isotope analysis was up to 0.04‰. Compared with TIMS, the MC–ICP–MS technique is more effective and less time-consuming (Maréchal et al. 1999). In addition, laser ablation–MC–ICP–MS (LA–MC–ICP–MS) and secondary ion mass spectrometry (SIMS) had also been used to determine Cu isotopes in Cu minerals. Although LA–MC–ICP–MS (0.08–0.14‰) and SIMS (0.5‰) have higher precision than TIMS, they are high-cost methods and harder implementation in most laboratories to achieve better precision than MC–ICP–MS. Moreover, for LA–MC–ICP/MS and for SIMS, it is difficult to determine the accuracy of a measurement, because of the lack of appropriate standards and because of a possible bias by the ablation/sputtering procedures that is difficult to correct (Lazarov and Horn 2015; Lv et al. 2020; Othmane et al. 2015). Therefore, MC–ICP–MS has a wider application in measuring the variation of δ65Cu values in terrestrial samples including plants and soils (Blotevogel et al. 2018; Weinstein et al. 2011). Common calibration methods include standard sample bracketing (SSB) (Yuan et al. 2017; Zhu et al. 2000), elemental doping (Zn, Ni, Ga) (Hou et al. 2016; Larner et al. 2011; Takano et al. 2017), and the combination of SSB and elemental doping. Furthermore, utilizing the combined SSB with an internal standard result in at least a twofold improvement in precision compared to the direct SSB (Hou et al. 2016; Liu et al. 2014a). In contrast to Zn and Ni doping, Ga isotopes, with fewer interferences and lower abundance, diminish contamination sources and recently appear more prevalent (Hou et al. 2016; Yang et al. 2023).
Copper isotope compositions in plant and soil samples are commonly expressed as δ65Cu as the difference relative to the Cu isotope standard NIST-SRM-976 Eq. (1):
Most Cu isotope data published in the literature adopted NIST-SRM-976 as the reference standard. An issue is that the stock of NIST-SRM-976 is nearly exhausting and cannot be reproduced by the National Institute of Standards and Technology (NIST). Moeller et al. (2012) showed that the Cu isotope ratios of NIST-SRM-976 yielded δ65Cu − 0.01 ± 0.05‰ and − 0.21 ± 0.05‰ relative to ERM-AE633 and ERM-AE647 by the Institute for Reference Materials and Measurements (IRMM) (Moeller et al. 2012). This review uses 0.01‰ and 0.21‰ to convert δ65Cu of ERM-AE633 and ERM-AE647 to that of NIST-SRM-976.
To describe the extent of mass fractionation through a process, the fractionation factor Δ is usually used Eq. (2):
A or B can represent the product or the substrate in a kinetically controlled process or the two phases in an equilibrium process. Details on the laws of stable isotope fractionation have been reported by Moynier et al. (2017) and Wiederhold (2015).
3 Copper isotope fractionation affected by different biogeochemical processes
3.1 Redox reaction
The variation in δ65Cu values is usually apparent during the redox reaction. Under anoxic conditions, 65Cu would be enriched in the solution during the reduction process because 63Cu precipitates preferentially from the solution as stable sulfide-containing minerals (e.g., chalcocite, bornite, enargite) (Table S1) (Pękala et al. 2011). For example, Ehrlich et al. (2004) showed that the isotope signature of Cu(II)aq in solution was approximately 3.06‰, which was heavier than that of covellite (a Cu(I)S(-I) compound) at 20 °C, as a result of the reduction of Cu(II)aq to Cu(I)aq, followed by precipitation of Cu(I)aq–CuS. However, ∆65Cu ranged from + 1 to + 3‰ during the oxidation of Cu and/or Cu-Fe sulfides under laboratory conditions (Fernandez and Borrok 2009; Kimball et al. 2009; Mathur et al. 2005; Wall et al. 2011). These isotope fractionation factors are generally consistent with theoretical values predicted by ab initio modeling. For instance, the equilibrium isotope fractionation between chalcopyrite (CuFeS2) and Cu(II)aq was 3.1‰ at 25 °C (Sherman 2013). In addition, the effect of redox is recognized as a main factor that controls the isotope fractionation of Cu during the redox transformation of Cu–sulfide minerals (Fernandez and Borrok 2009; Maher et al. 2011; Rodriguez et al. 2013; Wall et al. 2011). For example, Fernandez and Borrok (2009) revealed that heavier Cu isotopes are preferentially dissolved during chalcopyrite dissolution under oxidizing conditions. Additionally, Cu isotope fractionation can be mainly observed in Cu(I)/Cu(II) redox reactions on the surface of sulfide minerals, and can be affected by the relative rates of surface oxidation and leaching in aqueous solution under different pH conditions (Fernandez and Borrok 2009). Furthermore, δ65Cu ranges from − 6.42 to + 19.73‰ in weathered shales and cherts, and the leaching of Cu from black shales and its isotope fractionation are extremely affected by the fluctuations in redox potential (Lv et al. 2016). In addition, the redox fluctuation enlarged the shift of δ65Cu compared with the initial value when Cu-rich sulfides were leached and refixed by Fe-sulfide during weathering (Lv et al. 2016; Mathur et al. 2009; Palacios et al. 2011).
In plant–soil systems, especially hydromorphic soils systems, redox fluctuations are generally driven by frequency and duration of waterlogging, such as flooded and drained processes (Fulda et al. 2013; Kusonwiriyawong et al. 2017). Vertical redox zonation in soils is affected by the amounts and reactivity of electron donors in sequence: O2, NO3−, Mn4+, Fe3+, and SO42− under the electron donor effect (i.e., organic carbon). Once soils are flooded, oxygen, nitrate, Mn(IV), Fe(III) (oxy)hydroxides, sulfate, and then CO2 in soils are sequentially reduced as electron acceptors (Borch et al. 2010; Kappler et al. 2021; Yu et al. 2021). Although Cu sorbed on Fe/Mn (oxy)hydroxides can be released to soil porewater during reductive dissolution of Fe/Mn (oxy)hydroxides, sulfides produced by microbial sulfate reduction can simultaneously fix released Cu as Cu-sulfides, which could decrease Cu solubility under anoxic conditions (Fulda et al. 2013; Pan et al. 2016; Xu et al. 2021). In a simulated flooding experiment, Kusonwiriyawong et al. (2016) reported the structural information of Cu in different chemical forms, and found that Cu in CuXS had a relatively long bond distance (2.27 ~ 2.30 Å) and low coordination number (1.5), compared with other chemical forms of Cu (e.g., Cu (H2O)52+, Cu (H2O)62+, CuCO3 and Cu–OM complex). They also characterized the changes in chemical partitioning with a five-step sequential chemical extraction (SCE) method, and found that different fractions initially showed a relatively low Cu isotope composition (0.83 ± 0.18‰) at the beginning of flooding, whereas the δ65Cu values elevated to 2.18 ± 0.17‰ after seven days of flooding. The fractionation of δ65Cu mainly occurred between F2 (NaOAc-extractable), F3 (NH4Ox-extractable), and F4 (hot H2O2/NH4OAc-extractable), where F2 and F3 were enriched in 65Cu. However, F4 had isotopically lighter Cu, due to the reduction of sulfate and rapid precipitation of CuXS under anoxic conditions. This study pointed out the importance of sulfate reducing bacteria (SRB) in Cu redistribution among soil fractions (Fig. 1). Upon drainage, the previously formed Cu-sulfides tend to be oxidized, releasing soluble Cu and in turn increasing Cu solubility (Kusonwiriyawong et al. 2017). Soluble Cu dominantly existed as Cu (II), which could form stable complexes with organic matter (OM) and be adsorbed by Fe/Mn (oxyhydr)oxides and clay minerals (Fig. 1). During oxidation, isotopically heavy Cu in the reduced fraction might be lost, leaving the residual Cu isotopically light (Kusonwiriyawong et al. 2017).
Moreover, redox processes induced by biological activities play an important role in Cu isotope fractionation in plant–soil systems (Navarrete et al. 2011a). Cu is a cofactor in proteins in biological systems (Gorman-Lewis et al. 2019). Both reduced Cu(I) and oxidized Cu(II) are bound to specific proteins and enzymes in biochemical reactions (Bertini et al. 2010; Burkhead et al. 2009; Navarrete et al. 2011a). Cu-containing proteins are actively involved in electron transport or function as enzymes that may catalyze redox reactions on the surface of the plasma membrane or when Cu is incorporated into cells (Burkhead et al. 2009; Ryan et al. 2013). These processes mediated by Cu-containing proteins have been shown to induce an enrichment of isotopically light Cu in bacteria and plants (Jouvin et al. 2012; Ryan et al. 2013; Weinstein et al. 2011; Zhu et al. 2002) (Table S1). Additionally, a recent study found that a mature phototrophic biofilm can result in a significant Cu isotope fractionation (Coutaud et al. 2018). Complexation with carboxylate ligands favors enrichment of heavy Cu isotopes, while reduction of CuII-O/N to CuI-sulfhydryl moieties enriches light Cu isotopes (Coutaud et al. 2018).
3.2 Sorption
Sorption processes control the solubility of Cu and dominate the solid–liquid partitioning of Cu in soils, but these processes are influenced by soil pH and organic matter content (Richards et al. 1998; Sauvé et al. 1997; Vialykh et al. 2019). In soil, with low pH and organic matter content, relatively high concentrations of mobile Cu(II) were found (Sauvé et al. 1997). Mobile Cu(II) can be transferred by diffusive and advective processes until it precipitates as Cu(II) sulfides with isotopically light Cu (Table S1) (Mathur et al. 2009; Pokrovsky et al. 2008). Cu isotope fractionation can occur during the adsorption on Fe/Mn (oxyhydr)oxides and clay minerals (Babcsanyi et al. 2016; Pokrovsky et al. 2008; Richards et al. 1998). Compared to aqueous ion, Cu adsorbed on the surface of Fe oxyhydroxides has shorter Cu–O bond lengths and a lower coordination number (Balistrieri et al. 2008; Moynier et al. 2017; Pokrovsky et al. 2008). For instance, Balistrieri et al. (2008) demonstrated that the Cu–O bond lengths of the ion in aqueous solution and Cu(II) adsorbed on the surface of amorphous Fe oxyhydroxides were 1.8–2.0 Å and 2.0–2.4 Å, respectively, and the obtained ∆65Cusorbed-solution value was 0.73‰. In addition, Pokrovsky et al. (2008) reported the ∆65Cusorbed-solution for Cu sorption on goethite (0.8‰) and gibbsite (1.0‰). Li et al. (2015) reported the ∆65Cusorbed-solution for Cu sorption on kaolinite (− 1.46‰ to − 0.27‰), and they found that the fractionations with no connection with pH and reactive temperature were correlated negatively with the ionic strength and positively with the initial Cu concentrations in the solutions. Recently, Ijichi et al. (2018) confirmed that isotopically light Cu was adsorbed on birnessite, and the isotope fractionation of Cu was 0.45 ± 0.18‰. Sherman and Little (2020) confirmed the presence of isotopically light Cu on birnessite and predicted equilibrium isotope fractionation (0.49‰) at 25 °C using the ab initio model. These simulated isotope fractionation values might be smaller than the values in the natural soils because edge-complexes did not form under either model or experimental conditions (Sherman and Little 2020).
In addition, soil organic matter (SOM) possesses the maximum adsorption affinity for Cu relative to Fe-Mn oxides and other clay minerals, and thus mainly responsible for retaining adsorbed Cu (Vialykh et al. 2019). Some molecular structure studies showed that the Cu adsorbed on SOM was primarily present in the form of the bidentate complex. In the first coordination sphere, Cu(II) was displayed in a 6-coordinate structure with four equatorial O or N atoms and two axial O atoms at distances of 1.94 Å and 2.29 Å, respectively (Strawn and Baker 2009). Karlsson et al. (2006) suggested that four O or N atoms were present in the equatorial plane of the Jahn-Teller distorted octahedron. However, Peacock and Sherman (2004) indicated that Cu(II) adsorbed on the surface of Fe (oxyhydr)oxides had a structure with four equatorial O atoms in the first-shell coordination environment at a distance of 1.85–2.05 Å. Additionally, some studies reported that the adsorbed Cu(II) on the surface of iron (oxyhydr)oxide presented as (CuO4Hn)n-6 and (Cu2O6Hn)n-8 complexes at a distance of 2.9 Å (Moon and Peacock 2012; Sauvé et al. 1997). In general, heavy Cu isotopes are preferentially combined with lower coordination numbers and shorter, stronger bonds (Ryan et al. 2014; Sherman and Little 2020). Thus, the organo-complexation of Cu can affect the isotope fractionation of Cu in soils due to the relatively strong bond strength between SOM and 65Cu (Bigalke et al. 2010a; Pokrovsky et al. 2008). Ryan et al. (2014) performed an experiment to investigate the isotope fractionation between Cu(II)aq and Cu bound to insoluble humic acid at pH 2–7, and found that organo-complexed Cu was isotopically heavier relative to the solution (∆65CuIHA-solution = 0.26±0.11‰). Ryan et al. (2014) used Donnan membranes to assess the isotope fractionation of Cu during complexation with humic acid, and they found that the ∆65Cucomplex-free values ranged, from + 0.14‰ to + 0.84‰. They also verified that the magnitude of isotope fractionation has a strongly positive correlation with the logarithms of the stability constants (log K) of organic Cu complexes. Ilina et al. (2013) reported that no isotope fractionation occurred between Cu-organic complexes of different sizes (1 kDa-0.22 μm) and dissolved pools (<1 kDa), because Cu-organic complexes of different sizes had similar structures and stabilities.
Moreover, the adsorption of Cu on bacteria surfaces is considered an important process affecting the fractionation of Cu in plant–soil systems (Mathur and Fantle 2015), and the magnitude of the fractionation of Cu isotopes caused by adsorption is smaller relative to active metabolic processes (Navarrete et al. 2011a). Navarrete et al. (2011a) observed that heavy Cu isotopes were preferentially adsorbed on heat-killed bacteria. The ∆65Cusoild-solution values were 0.69 ± 0.25‰ at pH = 4.1 and 0.49 ± 0.02‰ at pH = 5.1. Nevertheless, there was no obvious fractionation at pH = 3.4–4.0, and heavier Cu isotopes were preferentially sequestered in the bacteria at pH = 2.7. The authors accounted for this sequestered for cell lysis. However, Pokrovsky et al. (2008) reported that no isotope fractionation was observed between the cell and solution (∆65Cusolution-soild = 0.0 ± 0.4‰) when Cu was adsorbed on bacteria at pH 4.0–6.5, whereas light Cu isotopes were adsorbed on the cell surface of soil P.aureofaciens bacterium at pH 1.8–3.5 (∆65Cusoild-solution = − 1.2 ± 0.5‰).
3.3 Mineral dissolution
Dissolution is a surface chemical reaction at the water–mineral interface and is controlled by the effect of protons and organic ligands (Murphy et al. 1989). The dissolution of both primary and secondary Cu minerals can release Cu ions into soil solutions. Cu isotope fractionation can be observed during the oxidative weathering of host minerals, including Cu-bearing sulfides, carbonates, and hydroxide minerals (Kimball et al. 2009; Mathur et al. 2005; Wall et al. 2011). Many studies have reported that heavier Cu isotopes could be released into the soil solution, whereas lighter Cu isotopes are preferentially retained during the oxidative weathering of Cu sulfide minerals, such as chalcopyrite, chalcocite, and bornite, since Cu (II) is more soluble than Cu (I) (Fekiacova et al. 2015; Fernandez and Borrok 2009; Kimball et al. 2009; Vance et al. 2016). In addition, isotopically heavy Cu may be released into soil solution when ligand-promoted dissolution enhances with the presence of organic ligands. The existence of bacteria can also result in Cu isotope fractionation during the dissolution of sulfide minerals. However, the order of magnitude of Cu isotope fractionation caused by biotic dissolution is less than that caused by abiotic dissolution (Kimball et al. 2009; Rodriguez et al. 2013).
4 Copper isotope fractionation in plants
4.1 Copper isotope fractionation in different plant species
Copper isotope data of 12 different plant species, including lichens and vascular plants such as trees, grasses, and grain crops were collected, and the δ65Cu values in these plants ranged from − 2.59 to + 0.64‰ (Fig. 2). Plants tend to enrich lighter Cu isotopes than soils, and the majority of studies focus on vascular plants, which constitute the main base of the plant–soil ecosystem and food chain (Jouvin et al. 2012; Li et al. 2016; Mihaljevic et al. 2018; Sillerova et al. 2017). The vascular plants can be divided into the strategy I and strategy II plants, according to the acquisition of Cu from the soil by plant roots (Jouvin et al. 2012; Ryan 2014). Strategy I is a sequential acidification-reduction-transport strategy: (i) the acidification of the rhizosphere excretes H+ by a plasma membrane H+ ATPase (AHA); (ii) nicotinamide adenine dinucleotide phosphate (NADPH)-dependent ferric reductase oxidase 4 and 5 (FRO4/5) reduces Cu(II) to Cu(I) which is then available to plants; and (iii) Cu can be transported through plant roots by the Cu transporter COPT/Ctr-like protein family (Fig. 3) (Bernal et al. 2012; Chai and Schachtman 2021; Santi and Schmidt 2009). Strategy II is the chelation-based strategy, which is basically dependent on the complexation of Cu (Hell and Stephan 2003). Cu(II) can be transported in the form of Cu(II)- phytosiderophores complexes in the rhizosphere, and then can be absorbed by plants via two transporters of the ZIP family (zinc iron regulated protein transporters: ZIP 2 and ZIP 4) (Fig. 3) (Bigalke et al. 2010a; Kavitha et al. 2015; Wintz et al. 2003).
Cu stable isotopes in different plant species: a strategy I vascular plants, b strategy II vascular plants, and c nonvascular plants (Blotevogel et al. 2018; Jouvin et al. 2012; Kribek et al. 2020; Li et al. 2016; Mihaljevic et al. 2018; Rodriguez et al. 2013; Ryan et al. 2013; Sillerova et al. 2017; Vance et al. 2016; Weinstein et al. 2011)
Schematic layout of Cu isotope variation within plants during Cu uptake and translocation for both strategy plants at the plasma membrane level. It is modified by Jouvin et al. (2012), Wu et al. (2019) and Zandi et al. (2020). a the green circle represents phytoavailable elements, and the blue circle represents mineral elements in soil or nutrient solution
In this review, we have collected 181 δ65Cu value data in different vascular plants, including eight species of strategy I plants and three species of strategy II plants. To compare the δ65Cu values of strategy I and strategy II plants, the whole-plant δ65Cuwhole-plant (‰) value is calculated using Eq. (3)
where Fi is the fraction of Cu in a given tissue sample i and δ65Cui (‰) is the Cu isotope compositions in the tissue samples (Li et al. 2016). The range of δ65Cu values in aboveground tissues is shown in Fig. 2, including five species of strategy I plants that have been reported (e.g., lentil (Lens culinaris), lettuce (Latuca sativa L.), tomato (Lycopersicon esculentum L. or Solanum lycopersicum), Elsholtzia splendens, and Prosopis pubescens) and 3 species of strategy II plants (e.g., oat (Avena sativa), durum wheat (Triticum turgidum L. cv. Acalou and Elymus virginicus), and rice (Oryza, sativa L. cv. IR 64), and the strategy I plants are generally enriched 63Cu with a median δ65Cu of -0.61‰ for the whole plants, while strategy II plants fractionate less towards light isotopes with a median δ65Cu of -0.39‰ for the whole plants.
4.2 Copper isotope fractionation in different plant tissues
As a micronutrient element, Cu is actively taken up by vascular plants and transported to plant tissues (Jouvin et al. 2012; Li et al. 2016; Ryan et al. 2013). We complied with the δ65Cu values of 5 different plant tissues, including roots, stems, leaves, flowers and seeds, from the published literature (Fig. 4). It seems that 65Cu tends to be slightly enriched in the aboveground tissues (stems, leaves, and flowers), with a mean δ65Cu of − 0.45‰ ± 0.41‰, compared to the roots (δ65Cu = − 0.58‰ ± 0.39‰) (Fig. 4). In addition, the δ65Cu values of seeds (− 0.53‰ ± 0.13‰) also display the enrichment of 65Cu, compared with the roots (Fig. 4). It is assumed that aboveground tissues may be enriched in heavier Cu isotopes through the xylem due to complexation and adsorption, relative to roots (Li et al. 2016). In addition, Cu translocation in plants mainly involves three stages: (i) Cu transport in stems and branches; (ii) Cu translocation from stems and branches to leaves; and (iii) Cu translocation from leaves to flowers through branches (Li et al. 2016) (Fig. 3). Therefore, we calculate the isotope fractionation between different tissues, and the results show that ∆65Custem-leaf and ∆65Culeaf-flower in strategy I plants are 0.14‰ and − 0.42‰, respectively, and ∆65Custem-leaf is 0.37‰ in strategy II plants.
The variation in δ65Cu values of different tissues in plants with different uptake strategy (Jouvin et al. 2012; Li et al. 2016; Ryan et al. 2013; Weinstein et al. 2011). The red circles represent that the δ65Cu values of strategy I plant, and the blue diamond represent that the δ65Cu values of strategy II plant. The δ65Cu value distribution of all plants are displayed in the black boxplots
5 Copper isotope fractionation in soils
Soil is a biogeochemically altered material that lies at the interface between the lithosphere and the atmosphere (Han et al. 2020; Ronald 2003; Song et al. 2018; Xia et al. 2019). For Cu cycling in soils, the external inputs and outputs of Cu have different isotope signatures. Weathering-derived Cu originates from parent materials, and decay of Cu from plants and deposition from atmospheric aerosols are thought to be the external inputs of Cu in soils. The outputs of terrestrial Cu can eventually be transported into oceans by soil weathering and leaching (Moynier et al. 2017). We compiled δ65Cu values from previous studies and suggested that rocks and dissolved Cu in rivers had heavier isotope compositions and that plants were isotopically lighter than the related soils. Spatial heterogeneity for δ65Cu values was also displayed due to different effects of weathering and subsequent pedogenic processes in soils (Bigalke et al. 2010a; Kusonwiriyawong et al. 2017; Liu et al. 2014b; Wu et al. 2019).
5.1 Copper isotope fractionation in different soil horizon
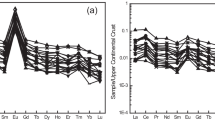
The δ65Cu values in different types of soils range from − 1.09 ~ 1.73‰, and spatial heterogeneity exists in different soil horizons and types (Fig. 5). In general, δ65Cu values in the C horizon are similar to those in bedrocks and have a relatively limited variation, ranging from − 0.39 ~ 0.18‰ (Fig. 5). Meanwhile, there were three main characteristic Cu isotope signatures from C to A horizon: (i) δ65Cu values increased or did not change from C to B horizon, while Cu concentrations decreased and δ65Cu values increased from B to A horizon, and the minimum δ65Cu values were found in the B horizon (Andosols, Albeluvisols, Luvisols), (ii) δ65Cu values decreased from B to E horizon, while Cu concentrations and δ65Cu values increased from E to A horizon, since the minimum δ65Cu values were found in E horizon (Pozols) and δ65Cu values in E horizon merge into A horizon in Fig. 5, and (iii) no apparent variation of δ65Cu values with soil depth (Chernozem, Cambisols). Furthermore, δ65Cu values ranged from − 0.45 ~ 0.92‰ in O horizon where δ65Cu values were highly dependent on the influence of atmospheric inputs, anthropogenic activities and plant activities.
Summary data of δ65Cu values in different soil horizons and types, according to IUSS Working Group WRB, 2014, the common master (upper case, such as organic layer (O horizon), topsoil (A horizon), subsoil (B horizon), parent material (C horizon)) is used. (Babcsanyi et al. 2016; Bigalke et al. 2013, 2010a, 2011; Blotevogel et al. 2018; Dotor-Almazan et al. 2017; Fekiacova et al. 2015; Kusonwiriyawong et al. 2017; Mihaljevic et al. 2019; Vance et al. 2016)
The patterns of δ65Cu values in Andosols, Albeluvisols, Luvisols and Retisols were similar. Taking Luvisols as an example, it is characterized by the different behaviors of clay minerals in the entire soil profile, with eluviation in surface horizons (E horizons) and transport and accumulation in deeper horizons (Bt horizons) (Kusonwiriyawong et al. 2017). Cu concentrations and isotope compositions in Luvisols change in different horizons (Fig. 5). In most profiles, Cu concentrations increased and δ65Cu values decreased with the increasing depth until Bt horizon, since the minimum δ65Cu values were found in Bt horizon. δ65Cu values increased or did not change with soil depth (Fekiacova et al. 2015; Kusonwiriyawong et al. 2017). The patterns of δ65Cu values might be due to the following processes: (i) isotopically lighter Cu is enriched in soil solutions and heavier Cu is enriched in soil particles during the weathering and leaching processes of parent materials or bedrocks, and isotopically lighter Cu in soil solutions move downwards in the form of uncharged mobile Cu complexes; (ii) the mobilized Cu in soil solutions could be adsorbed by Fe–Mn oxides in soil particles and isotopically heavier Cu could be enriched in deeper horizons (BC horizons) (Mihaljevic et al. 2019; Pokrovsky et al. 2008). Weathering intensity and redox conditions affect the δ65Cu values in the soil profiles (Liu et al. 2014b; Vance et al. 2016). Limited δ65Cu values were found in weakly weathered samples, but as the weathering intensity increased, the soil profiles generally showed that Cu in the O horizon was isotopically heavier than that in the A/B horizon (Bigalke et al. 2011; Vance et al. 2016). In addition, δ65Cu values in soil profiles changed with the redox conditions. The anoxic conditions could be enhanced with increasing annual precipitation, and 65Cu was lost together with a reduction in minerals from the soil profiles (Vance et al. 2016). For example, lower δ65Cu values (− 1.09‰) were found in the B horizon at site 2 of Maui because heavy Cu moved out of the soil and then complexed to aqueous organic species, and light Cu was retained in soils that were sorbed on Fe oxide phases. However, when the loss of Cu becomes extreme, the δ65Cu values of the residual pool in the soil profiles shift to heavier isotopes due to the reduction of Fe oxides in the residual pool. Because of the relative light Cu loss during the reduction of Fe oxides, which was sorbed on Fe oxide phases and then lost, the δ65Cu values returned towards the protolith with the loss of Cu (Vance et al. 2016).
However, the patterns of δ65Cu values in podzols were mainly due to the high affinity of Cu with organic matter, and organic complexation might dominate the vertical differentiation of Cu isotopes during pedogenetic processes in developed podzols. The complexation between Cu and SOM and then illuviation might be the reason for the elevated δ65Cu values in the underlying B horizons of podzols (Bigalke et al. 2011), while the δ65Cu values are different between the A and E horizons, which is ascribed to biogeochemical cycling and anthropogenic influence, but the main contributors are obscured. For example, Vance et al. (2016) demonstrated that biolifting and fractionation by vegetation could explain the δ65Cu values in the surface horizons (AEh horizons) at the podzols of Glen Feshie.
5.2 Integrated weathering profiles and isotope fractionation during weathering
The net retention and fraction of Cu during weathering and pedogenetic processes is controlled by the sorption or coprecipitation of aluminosilicate and Fe/Mn oxides in clay minerals and organic matter in soils (Al-Sid-Cheikh et al. 2015; Bigalke et al. 2011; Lotfi-Kalahroodi et al. 2019; Vance et al. 2016). The tau parameter (τ) can be used to quantify the net loss or gain of an element relative to the parent material (Chadwick et al. 1990) Eq. (4):
where C is the measured concentration, i is the element of interest (i.e., Cu in this review), j is an immobile reference element (Nb, Th, Ti or Zr), h is the weathering product, and p is the unaltered parent lithology. Figure 6a shows the relationship between τ and δ65Cu values for the data from the literature (Little et al. 2019; Liu et al. 2014b; Lv et al. 2016; Vance et al. 2016). There are two tendencies between τ and δ65Cu values: (i) δ65Cu values decreased with increased Cu loss because 65Cu adsorbed on Fe oxides and then lost during weathering, while relatively light Cu was retained in soils; (ii) δ65Cu values increased with increased Cu loss when Cu was lost together with clay minerals during extreme weathering, and δ65Cu values returned towards the protolith with the loss of Cu (Vance et al. 2016). When Cu is retained through binding to soil colloids, the values of ∆65Curetained-solution are higher than 0‰, such that Cu can be adsorbed on the surface of Fe oxides and can be complexed by organic matter (Bigalke et al. 2010b; Contin et al. 2007; Seda et al. 2016). However, Cu sorption may be limited to multicomponent systems (Henneberry et al. 2012; Witzgall et al. 2021). The reaction between inorganic cations and DOM can inhibit Cu sorption and thus sorption-induced Cu isotope fractionation (Kappler et al. 2021; Martinez-Villegas and Martinez 2008; Seda et al. 2016; Wu et al. 2019).
Soil weathering plays an important role in matter cycling on the Earth's surface from a large-scale perspective (Li et al. 2022; Liu et al. 2020; Moynier et al. 2017). The integrated τ and ∆65Cu values for entire soil profiles can quantify the contribution of the soil system to matter cycling on the Earth's surface. The integrated τ is expressed as follows Eq. (5):
where i is the element of interest (i.e., Cu), h refers to each horizon and t refers to the entire profile, p is the density for individual horizons and z represents the thickness for individual horizons (Vance et al. 2016). Figure 6b plots the τ data from the literature versus integrated soil ∆65Cusoil-parent material. These results show that Cu is lost from the soil profile during weathering under natural conditions. The Cu that is lost shows an enrichment in 65Cu when compared to the parent material and residual soils. However, both ∆65Cusoil-parent material and \({\tau }_{i}^{int}\) may have positive values when perturbed by human activities (Fig. 6b).
6 Copper isotope fractionation in plant–soil systems
6.1 Copper isotope fractionation during root uptake
The acquisition of Cu from soils by roots has two efficient strategies, including strategy I and strategy II (Printz et al. 2016). Cu isotope fractionation exists for both uptake strategies in plant–soil systems, and this review suggests that strategy I plants (mean δ65Cuwhole-plant = − 0.61‰) take up 63Cu, while strategy II plants (mean δ65Cuwhole-plant = − 0.39‰) fractionate less towards light isotopes. The mean δ65Cuwhole-plant values of strategy I and strategy II plants are similar to the research of Jouvin et al. (2012), who compared the isotope fractionation of Cu in tomato (strategy I plants) and oat (strategy II plants). They observed that light Cu isotopes were enriched in both plant species, but strategy I plants had relatively large degrees of Cu isotope fractionation (∆65Cuwhole plant-solution≈−1‰) between the plant and related nutrient solution compared with strategy II plants (− 0.20‰ < ∆65Cuwhole plant-solution < − 0.11‰). The authors ascribed this to higher levels of soluble Cu observed in the rhizosphere of strategy I plants, compared to strategy II plants. Cu in the rhizosphere of strategy I plants could be acidified through proton excretion via plasmalemma H+-ATPase and then exhibited a reduction process in strategy I plants, while Strategy II could be dependent on the complexation of Cu. However, Cu in the rhizosphere of strategy II plants relied on chelation. X-ray absorption spectroscopy (XAS) provides a possibility to assess Cu speciation in situ (Kupper et al. 2009; Mijovilovich et al. 2009). Ryan et al. (2013) concurrently used isotope fractionation and XAS to examine the uptake of Cu in plants and found that ∆65Curoot-solution was − 1.00‰ in strategy I plants, while ∆65Curoot-solution ranged from − 0.48 to − 0.11‰ in strategy II plants. They pointed out that the light Cu isotope enrichment in both plant species could be attributed to the reduction of Cu (II) at the surfaces of the root cell membranes. Via combining the variation in ∆65Cu values with the results of Extended X-Ray Absorption Fine Structure (EXAFS), they found that the difference was mainly caused by a redox-selective transport during translocation within strategy I plants, while strategy II plants showed no sign of dominant redox selection. Pękala et al. (2011) collected evidence suggesting that the reduction process could result in a solution enriched in 65Cu, while 63Cu from the solution would reprecipitate as sulfide-phase minerals (e.g., chalcocite, bornite, enargite). Cu isotope signature of these processes was approximately + 3.06‰, and Lv et al. (2016) pointed out that the isotope signature of Cu was more evident during redox oscillations. Additionally, Ryan et al. (2014) found that the organo-complexation of Cu was preferentially enriched isotopically heavy Cu in laboratory experiments compared to aqueous ions, and ∆65Cucomplex-free values ranged from + 0.14 to + 0.84‰.
6.2 Copper isotope fractionation during translocation
The acquisition of Cu in plants is similar to Fe-acquisition mechanisms via epidermal root cells, parenchyma and then endodermis and xylem (Kumar et al. 2021). Cu isotope fractionation associated with plant root uptake was first reported by Weinstein et al. (2011), who observed that lighter Cu isotopes were similarly enriched in the shoots (stems, leaves and seeds), and δ65Cu values decreased from soils to shoots in both strategy I and strategy II plants (− 0.94‰ < ∆65Cushoots-soil < − 0.33‰). In this review, the isotope fractionation between different tissues shows that ∆65Custem-leaf and ∆65Culeaf-flower for strategy I plants are 0.14‰ and -0.42‰, respectively, and ∆65Custem-leaf is 0.37‰ in strategy II plants. Diffusion and convection have been assumed to be the two main mechanisms of Cu transport in stems and branches (Fig. 5) (Weinstein et al. 2011). Li et al. (2016) found that Cu transport in stems and branches may be controlled by convective transpiration flows. However, there was no isotope fractionation of Cu during convection. Active transport plays a crucial role in Cu translocation from stems and branches to leaves and leads to heavy Cu enrichment in leaves (Burkhead et al. 2009; Curie et al. 2009; Li et al. 2016). Cu isotope fractionation was considered to be in existence when Cu was transported from leaves to branches, then to flowers remobilization influence (Li et al. 2016). Cu was remobilized from old leaves to flowers by transport proteins, such as yellow stripe-like (ysl) oligopeptide transporter proteins (Burkhead et al. 2009; Curie et al. 2009; Li et al. 2016). Lighter Cu isotopes were enriched in leaves, whereas heavier Cu isotopes were enriched in flowers, and the Cu isotope compositions varied in leaves and flowers in a complementary manner (Li et al. 2016). Moreover, δ65Cu values in flowers and leaves vary with the height of plants. Flowers in the lower height position were enriched in heavier Cu than those in the upper height position (Fig. 7).
Thus, the entry of Cu into plant cells is mediated by transporter or complexes via various chemical reactions, which have an influence on the isotope fractionation of Cu (Caldelas and Weiss 2017; Palmer and Guerinot 2009; Ryan 2014) as demonstrated in Fig. 7 and summarized below: (1) desorption of Cu from the solid phase (shown here as organic matter), with 65Cu accumulating in the complexes; (2) Cu binding to various ligands in the xylem, the cytosol, or the vacuole, with 65Cu accumulating in the complexes; (3) adsorption of Cu2+/Cu+ on to the xylem walls, resulting in xylem sap with 63Cu; (4) bulk flowed driven by transpiration, in favor of 63Cu; (5) xylem unloading into the leaf symplast by Cu transporters at the plasma membrane, probably in favor of 63Cu; (6) diffusion of Cu cations in the symplast, which would be faster for 63Cu; (7) activity of vacuolar transporters regulating Cu concentration in the cytosol, Cu available for transport, and Cu storage during grain filling; δ65Cu values of the vacuolar Cu pool is probably determined by the vacuolar transporters and Cu speciation in the vacuole; (8) phloem loading, mediated by transporters at the plasma membrane, favors 63Cu; (9) reduction of Cu2+ to Cu+ in the xylem and subsequent uptake of the reduced species into the phloem, in favor of 63Cu; (10) phloem unloading of Cu, mediated by transporters at the plasma membrane, in favor of 63Cu; (11) xylem- to phloem direct transfer, in favor of 63Cu.
7 Summary and outlook
This study summarized information on Cu isotope compositions and fractionation in plant–soil systems, compiling numerous Cu biogeochemistry cycle information and research in one study. The major conclusions are as follows: (1) the main characteristic Cu isotope signature that plants tend to be rich in 63Cu relative to soils, whereas sorption/desorption or coprecipitation/re-released of aluminosilicate and Fe/Mn oxides in clay minerals and organic matter in soils and biolifting are thought to control the magnitude of isotope fractionation in different soil horizons and types; (2) strategy I plants fractionate more towards light isotopes, due to the reduction of Cu(II) at the surfaces of the root cell membranes; (3) aboveground tissues may be enriched in 65Cu relative to root tissues, while various chemical reactions (such as redox, diffusion, complexation and adsorption) influence the translocation of Cu and δ65Cu; and (4) compared with parent materials and residue soils, the Cu lost during soil weathering is isotopically heavier during soil weathering, except the perturbation of human activities.
Copper isotope fractionation in plant–soil systems can record metabolism and exchange during environmental evolution. However, the current research is limited. Here we highlight some of the future directions for Cu isotope techniques in plant–soil systems as follows.
Firstly, the partitioning of Cu is controlled by many competing processes in plant–soil systems. Fe/Mn oxides and/or organic matter are the main absorbates that retain Cu in the solid phase. However, organic carbon (OC) has the potential to inhibit structure changes in Fe oxides and then alter the binding or retention of Cu. Thus, future research is needed to examine how does the coupled interactions between Fe, OM and Cu during the alteration of redox conditions affect Cu retention or release under different environmental conditions (Henneberry et al. 2012; Mejia et al. 2018; Seda et al. 2016). In particular, it is crucial to distinguish the variation in the δ65Cu value between Cu sorption and incorporation, which strongly influences Cu reactivity during redox changes (Little et al. 2019; Vance et al. 2016). Combining Cu isotope analysis with advanced imaging and spectroscopic techniques, we can benefit gaining fundamental knowledge at the micro- and molecular-scales. Such information will further help us to predict how Cu availability will respond under submerged conditions. This is crucial for paddy soils and wetlands, which frequently alter redox cycling conditions and directly influence human health.
Secondly, phytoremediation tends to be a more sustainable approach to coping with meta-contaminated soils than traditional thermal or physico-chemical techniques (Hou et al. 2020). Cu and its isotopes may therefore be an interesting marker for Cu migration and accumulation during the growth of plants, especially for hyper-accumulating plants. Future research is needed to combine Cu isotope techniques with genomics so as to decode the password of Cu accumulation in plants at the molecular scale. This is crucial for food safety and sustainability.
Thirdly, adequate prediction of pollutant distribution cannot be separated from the identification of pollution sources, which will be beneficial to global soil mapping (Hou et al. 2020). However, the effect of using Cu isotope techniques to identify pollution sources is limited. Source identification and apportionment can be better constrained by combining the isotopes of Cu and other elements as well as receptor modeling. Future research is needed on a cross view between Cu isotope techniques and other academic disciplines, for example, probability and statistical and artificial intelligence.
References
Albarede F (2004) The stable isotope geochemistry of copper and zinc. In: Johnson CM, Beard BL and Albarede F (eds) Geochemistry of non-traditional stable isotopes. Reviews in Mineralogy & Geochemistry, pp. 409–427. https://doi.org/10.2138/gsrmg.55.1.409
Alloway, B.J., 2013. Sources of heavy metals and metalloids in soils. In: Alloway BJ (ed), Heavy metals in soils: trace metals and metalloids in soils and their bioavailability. Springer Netherlands, Dordrecht, pp. 11–50. https://doi.org/10.1007/978-94-007-4470-7_2
Alsaleh KAM, Meuser H, Usman ARA, Al-Wabel MI, Al-Farraj AS (2018) A comparison of two digestion methods for assessing heavy metals level in urban soils influenced by mining and industrial activities. J Environ Manage 206:731–739. https://doi.org/10.1016/j.jenvman.2017.11.026
Al-Sid-Cheikh M, Pedrot M, Dia A, Guenet H, Vantelon D, Davranche M, Gruau G, Delhaye T (2015) Interactions between natural organic matter, sulfur, arsenic and iron oxides in re-oxidation compounds within riparian wetlands: NanoSIMS and X-ray adsorption spectroscopy evidences. ScTEn 515:118–128. https://doi.org/10.1016/j.scitotenv.2015.02.047
Babcsanyi I, Imfeld G, Granet M, Chabaux F (2014) Copper stable isotopes to trace copper behavior in wetland systems. Environ Sci Technol 48(10):5520–5529. https://doi.org/10.1021/es405688v
Babcsanyi I, Chabaux F, Granet M, Meite F, Payraudeau S, Duplay J, Imfeld G (2016) Copper in soil fractions and runoff in a vineyard catchment: Insights from copper stable isotopes. ScTEn 557–558:154–162. https://doi.org/10.1016/j.scitotenv.2016.03.037
Balistrieri LS, Borrok DM, Wanty RB, Ridley WI (2008) Fractionation of Cu and Zn isotopes during adsorption onto amorphous Fe(III) oxyhydroxide: experimental mixing of acid rock drainage and ambient river water. Geochim Cosmochim Acta 72(2):311–328. https://doi.org/10.1016/j.gca.2007.11.013
Bernal M, Casero D, Singh V, Wilson GT, Grande A, Yang H, Dodani SC, Pellegrini M, Huijser P, Connolly EL, Merchant SS, Kraemer U (2012) Transcriptome sequencing identifies SPL7-regulated copper acquisition genes FRO4/FRO5 and the copper dependence of iron homeostasis in Arabidopsis. Plant Cell 24(2):738–761. https://doi.org/10.1105/tpc.111.090431
Bertini I, Cavallaro G, Mcgreevy KS (2010) Cellular copper management—a draft user’s guide. Coord Chem Rev 254(5–6):506–524. https://doi.org/10.1016/j.ccr.2009.07.024
Bigalke M, Weyer S, Kobza J, Wilcke W (2010a) Stable Cu and Zn isotope ratios as tracers of sources and transport of Cu and Zn in contaminated soil. Geochim Cosmochim Acta 74(23):6801–6813. https://doi.org/10.1016/j.gca.2010.08.044
Bigalke M, Weyer S, Wilcke W (2010b) Copper isotope fractionation during complexation with insolubilized humic acid. Environ Sci Technol 44(14):5496–5502. https://doi.org/10.1021/es1017653
Bigalke M, Weyer S, Wilcke W (2011) Stable Cu isotope fractionation in soils during oxic weathering and podzolization. Geochim Cosmochim Acta 75(11):3119–3134. https://doi.org/10.1016/j.gca.2011.03.005
Bigalke M, Kersten M, Weyer S, Wilcke W (2013) Isotopes trace biogeochemistry and sources of Cu and Zn in an intertidal soil. SSSAJ 77(2):680–691. https://doi.org/10.2136/sssaj2012.0225
Blotevogel S, Oliva P, Sobanska S, Viers J, Vezin H, Audry S, Prunier J, Darrozes J, Orgogozo L, Courjault-Rade P, Schreck E (2018) The fate of Cu pesticides in vineyard soils: a case study using δ65Cu isotope ratios and EPR analysis. ChGeo 477:35–46. https://doi.org/10.1016/j.chemgeo.2017.11.032
Blotevogel S, Schreck E, Audry S, Saldi GD, Viers J, Courjault-Rade P, Darrozes J, Orgogozo L, Oliva P (2019) Contribution of soil elemental contents and Cu and Sr isotope ratios to the understanding of pedogenetic processes and mechanisms involved in the soil-to-grape transfer (Soave vineyard, Italy). Geoderma 343:72–85. https://doi.org/10.1016/j.geoderma.2019.02.015
Blotevogel S, Oliva P, Denaix L, Audry S, Viers J, Schreck E (2022) Stable Cu isotope ratios show changes in Cu uptake and transport mechanisms in Vitis vinifera due to high Cu exposure. Front Plant Sci 12:755944. https://doi.org/10.3389/fpls.2021.755944
Borch T, Kretzschmar R, Kappler A, Van Cappellen P, Ginder-Vogel M, Voegelin A, Campbell K (2010) Biogeochemical redox processes and their impact on contaminant dynamics. Environ Sci Technol 44(1):15–23. https://doi.org/10.1021/es9026248
Burkhead J, Reynolds K, Abdel-Ghany S, Cohu C (2009) Copper homeostasis. New Phytol 182(4):799–816. https://doi.org/10.1111/j.1469-8137.2009.02846.x
Caldelas C, Weiss DJ (2017) Zinc Homeostasis and isotopic fractionation in plants: a review. Plant Soil 411(1–2):17–46. https://doi.org/10.1007/s11104-016-3146-0
Chadwick OA, Brimhall GH, Hendricks DM (1990) From a black to a gray box—A mass balance interpretation of pedogenesis. Geomo 3(3):369–390. https://doi.org/10.1016/0169-555X(90)90012-F
Chai YN, Schachtman DP (2021) Root exudates impact plant performance under abiotic stress. Trends Plant Sci 27(1):80–91. https://doi.org/10.1016/j.tplants.2021.08.003
Contin M, Mondini C, Leita L, De Nobili M (2007) Enhanced soil toxic metal fixation in iron (hydr)oxides by redox cycles. Geoderma 140(1–2):164–175. https://doi.org/10.1016/j.geoderma.2007.03.017
Cornu J-Y, Huguenot D, Jézéquel K, Lollier M, Lebeau T (2017) Bioremediation of copper-contaminated soils by bacteria. World J Microbiol Biotechnol 33(2):26. https://doi.org/10.1007/s11274-016-2191-4
Coutaud M, Méheut M, Glatzel P, Pokrovski GS, Viers J, Rols J-L, Pokrovsky OS (2018) Small changes in Cu redox state and speciation generate large isotope fractionation during adsorption and incorporation of Cu by a phototrophic biofilm. Geochim Cosmochim Acta 220:1–18. https://doi.org/10.1016/j.gca.2017.09.018
Curie C, Cassin G, Couch D, Divol F, Higuchi K, Le Jean M, Misson J, Schikora A, Czernic P, Mari S (2009) Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann Bot 103(1):1–11. https://doi.org/10.1093/aob/mcn207
Dotor-Almazan A, Aurora Armienta-Hernandez M, Talavera-Mendoza O, Ruiz J (2017) Geochemical behavior of Cu and sulfur isotopes in the tropical mining region of Taxco, Guerrero (southern Mexico). ChGeo 471:1–12. https://doi.org/10.1016/j.chemgeo.2017.09.005
Ehrlich S, Butler I, Halicz L, Rickard D, Oldroyd A, Matthews A (2004) Experimental study of the copper isotope fractionation between aqueous Cu(II) and covellite. CuS Chgeo 209(3–4):259–269. https://doi.org/10.1016/j.chemgeo.2004.06.010
Eiler JM, Bergquist B, Bourg I, Cartigny P, Farquhar J, Gagnon A, Guo W, Halevy I, Hofmann A, Larson TE, Levin N, Schauble EA, Stolper D (2014) Frontiers of stable isotope geoscience. Chgeo 372:119–143. https://doi.org/10.1016/j.chemgeo.2014.02.006
Fekiacova Z, Cornu S, Pichat S (2015) Tracing contamination sources in soils with Cu and Zn isotopic ratios. ScTEn 517:96–105. https://doi.org/10.1016/j.scitotenv.2015.02.046
Fernandez A, Borrok DM (2009) Fractionation of Cu, Fe, and Zn isotopes during the oxidative weathering of sulfide-rich rocks. ChGeo 264(1–4):1–12. https://doi.org/10.1016/j.chemgeo.2009.01.024
Fulda B, Voegelin A, Ehlert K, Kretzschmar R (2013) Redox transformation, solid phase speciation and solution dynamics of copper during soil reduction and reoxidation as affected by sulfate availability. Geochim Cosmochim Acta 123:385–402. https://doi.org/10.1016/j.gca.2013.07.017
Gaetke LM, Chow-Johnson HS, Chow CK (2014) Copper: toxicological relevance and mechanisms. Arch Toxicol 88(11):1929–1938. https://doi.org/10.1007/s00204-014-1355-y
Gorman-Lewis D, Martens-Habbena W, Stahl DA (2019) Cu(II) adsorption onto ammonia-oxidizing bacteria and archaea. Geochim Cosmochim Acta 255:127–143. https://doi.org/10.1016/j.gca.2019.04.011
Han G, Tang Y, Liu M, Van Zwieten L, Yang X, Yu C, Wang H, Song Z (2020) Carbon-nitrogen isotope coupling of soil organic matter in a karst region under land use change. Southwest China Agric Ecosyst Environ 301:107027. https://doi.org/10.1016/j.agee.2020.107027
Hell R, Stephan UW (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216(4):541–551. https://doi.org/10.1007/s00425-002-0920-4
Henneberry YK, Kraus TEC, Nico PS, Horwath WR (2012) Structural stability of coprecipitated natural organic matter and ferric iron under reducing conditions. OrGeo 48:81–89. https://doi.org/10.1016/j.orggeochem.2012.04.005
Holmgren G, Meyer MW, Chaney RL, Daniels RB (1993) Cadmium, Lead, Zinc, Copper, and Nickel in agricultural soils of the united states of America. J Environ Qual 22(2):335–348. https://doi.org/10.2134/jeq1993.00472425002200020015x
Hou Q, Zhou L, Gao S, Zhang T, Feng L, Yang L (2016) Use of Ga for mass bias correction for the accurate determination of copper isotope ratio in the NIST SRM 3114 Cu standard and geological samples by MC-ICP-MS. JAAS 31(1):280–287. https://doi.org/10.1039/c4ja00488d
Hou D, O’Connor D, Igalavithana AD, Alessi DS, Luo J, Tsang DCW, Sparks DL, Yamauchi Y, Rinklebe J, Ok YS (2020) Metal contamination and bioremediation of agricultural soils for food safety and sustainability. Nat Rev Earth Environ 1(7):366–381. https://doi.org/10.1038/s43017-020-0061-y
Ijichi Y, Ohno T, Sakata S (2018) Copper isotopic fractionation during adsorption on manganese oxide: Effects of pH and desorption. Geochem J 52(2):e1–e6. https://doi.org/10.2343/geochemj.2.0516
Ilina SM, Viers JM, Lapitsky SA, Mialle S, Mavromatis V, Chmeleff JM, Brunet P, Alekhin YV, Isnard H, Pokrovsky OS (2013) Stable (Cu, Mg) and radiogenic (Sr, Nd) isotope fractionation in colloids of boreal organic-rich waters. ChGeo 342:63–75. https://doi.org/10.1016/j.chemgeo.2013.01.019
Jeong H, Ra K, Choi JY (2021) Copper, zinc and lead isotopic delta values and isotope ratios of various geological and biological reference materials. Geostand Geoanal Res 45(3):551–563. https://doi.org/10.1111/ggr.12379
Jouvin D, Weiss DJ, Mason TFM, Bravin MN, Louvat P, Zhao F, Ferec F, Hinsinger P, Benedetti MF (2012) Stable isotopes of Cu and Zn in higher plants : evidence for Cu reduction at the root surface and two conceptual models for isotopic fractionation processes. Environ Sci Technol 46(5):2652–2660. https://doi.org/10.1021/es202587m
Kappler A, Bryce C, Mansor M, Lueder U, Byrne JM, Swanner ED (2021) An evolving view on biogeochemical cycling of iron. Nat Rev Microbiol 19(6):360–374. https://doi.org/10.1038/s41579-020-00502-7
Karlsson T, Persson P, Skyllberg U (2006) Complexation of copper(II) in organic soils and in dissolved organic matter-EXAFS evidence for chelate ring structures. Environ Sci Technol 40(8):2623–2628. https://doi.org/10.1021/es052211f
Kavitha PG, Kuruvilla S, Mathew MK (2015) Functional characterization of a transition metal ion transporter, OsZIP6 from rice (Oryza sativa L.). Plant Physiol Biochem 97:165–174. https://doi.org/10.1016/j.plaphy.2015.10.005
Kimball BE, Mathur R, Dohnalkova AC, Wall AJ, Runkel RL, Brantley SL (2009) Copper isotope fractionation in acid mine drainage. Geochim Cosmochim Acta 73(5):1247–1263. https://doi.org/10.1016/j.gca.2008.11.035
Komárek M, Ratié G, Vaňková Z, Šípková A, Chrastný V (2021) Metal isotope complexation with environmentally relevant surfaces: Opening the isotope fractionation black box. Crit Rev Environ Sci Technol (in Press). https://doi.org/10.1080/10643389.2021.1955601
Kribek B, Mikova J, Knesl I, Mihaljevic M, Sykorova I (2020) Uptake of trace elements and isotope fractionation of Cu and Zn by birch (Betula pendula) growing on mineralized coal waste pile. Appl Geochem 122(104741):1–14. https://doi.org/10.1016/j.apgeochem.2020.104741
Kumar V, Pandita S, Sidhu GPS, Sharma A, Khanna K, Kaur P, Bali AS, Setia R (2021) Copper bioavailability, uptake, toxicity and tolerance in plants: a comprehensive review. Chemosphere 262(127810):1–25. https://doi.org/10.1016/j.chemosphere.2020.127810
Kupper H, Gotz B, Mijovilovich A, Kupper FC, Meyer-Klaucke W (2009) Complexation and toxicity of copper in higher plants I. Characterization of copper accumulation, speciation, and toxicity in Crassula helmsii as a new copper accumulator. Plant Physiol 151(2):702–714. https://doi.org/10.1104/pp.109.139717
Kusonwiriyawong C, Bigalke M, Abgottspon F, Lazarov M, Wilcke W (2016) Response of Cu partitioning to flooding: a δ65Cu approach in a carbonatic alluvial soil. ChGeo 420:69–76. https://doi.org/10.1016/j.chemgeo.2015.11.005
Kusonwiriyawong C, Bigalke M, Cornu S, Montagne D, Fekiacova Z, Lazarov M, Wilcke W (2017) Response of copper concentrations and stable isotope ratios to artificial drainage in a French Retisol. Geoderma 300:44–54. https://doi.org/10.1016/j.geoderma.2017.04.003
Larner F, Rehkaemper M, Coles BJ, Kreissig K, Weiss DJ, Sampson B, Unsworth C, Strekopytov S (2011) A new separation procedure for Cu prior to stable isotope analysis by MC-ICP-MS. JAAS 26(8):1627–1632. https://doi.org/10.1039/c1ja10067j
Lazarov M, Horn I (2015) Matrix and energy effects during in-situ determination of Cu isotope ratios by ultraviolet-femtosecond laser ablation multicollector inductively coupled plasma mass spectrometry. Spectrochimica Acta Part B-Atomic Spectrosc 111:64–73. https://doi.org/10.1016/j.sab.2015.06.013
Li D, Liu SA, Li S (2015) Copper isotope fractionation during adsorption onto kaolinite: experimental approach and applications. ChGeo 396:74–82. https://doi.org/10.1016/j.chemgeo.2014.12.020
Li SZ, Zhu XK, Wu LH, Luo YM (2016) Cu isotopic compositions in Elsholtzia splendens: influence of soil condition and growth period on Cu isotopic fractionation in plant tissue. ChGeo 444:49–58. https://doi.org/10.1016/j.chemgeo.2016.09.036
Li J, Du J, Zhong S, Ci E, Wei C (2021) Changes in the profile properties and chemical weathering characteristics of cultivated soils affected by anthropic activities. Sci Rep 11(1):20822–20822. https://doi.org/10.1038/s41598-021-00302-w
Li X, Han G, Liu M, Liu J, Zhang Q, Qu R (2022) Potassium and its isotope behaviour during chemical weathering in a tropical catchment affected by evaporite dissolution. Geochim Cosmochim Acta 316:105–121. https://doi.org/10.1016/j.gca.2021.10.009
Little SH, Munson S, Prytulak J, Coles BJ, Hammond SJ, Widdowson M (2019) Cu and Zn isotope fractionation during extreme chemical weathering. Geochim Cosmochim Acta 263:85–107. https://doi.org/10.1016/j.gca.2019.07.057
Liu SA, Li DD, Li SG, Teng FZ, Ke S, He YS, Lu YH (2014a) High-precision copper and iron isotope analysis of igneous rock standards by MC-ICP-MS. JAAS 29(1):122–133. https://doi.org/10.1039/c3ja50232e
Liu SA, Teng FZ, Li SG, Wei G, Ma JL, Li DD (2014b) Copper and iron isotope fractionation during weathering and pedogenesis: Insights from saprolite profiles. Geochim Cosmochim Acta 146:59–75. https://doi.org/10.1016/j.gca.2014.09.040
Liu M, Han G, Zhang Q (2020) Effects of agricultural abandonment on soil aggregation, soil organic carbon storage and stabilization: results from observation in a small karst catchment, Southwest China. Agr Ecosyst Environ 288:106719. https://doi.org/10.1016/j.agee.2019.106719
Lotfi-Kalahroodi E, Pierson-Wickmann A-C, Guenet H, Rouxel O, Ponzevera E, Bouhnik-Le Coz M, Vantelon D, Dia A, Davranche M (2019) Iron isotope fractionation in iron-organic matter associations: experimental evidence using filtration and ultrafiltration. Geochim Cosmochim Acta 250:98–116. https://doi.org/10.1016/j.gca.2019.01.036
Lv Y, Liu SA, Zhu JM, Li SG (2016) Copper and zinc isotope fractionation during deposition and weathering of highly metalliferous black shales in central China. ChGeo 445:24–35. https://doi.org/10.1016/j.chemgeo.2016.01.016
Lv N, Bao Z, Chen L, Chen K, Zhang Y, Yuan H (2020) Accurate determination of Cu isotope compositions in Cu-bearing minerals using microdrilling and MC-ICP-MS. Int J Mass Spectrom 457(116414):1–7. https://doi.org/10.1016/j.ijms.2020.116414
Maher KC, Jackson S, Mountain B (2011) Experimental evaluation of the fluid-mineral fractionation of Cu isotopes at 250 degrees C and 300 degrees C. ChGeo 286(3–4):229–239. https://doi.org/10.1016/j.chemgeo.2011.05.008
Maréchal CN, Télouk P, Albarède F (1999) Precise analysis of copper and zinc isotopic compositions by plasma-source mass spectrometry. ChGeo 156(1–4):251–273. https://doi.org/10.1016/s0009-2541(98)00191-0
Martinez-Villegas N, Martinez CE (2008) Solid- and solution-phase organics dictate copper distribution and speciation in multicomponent systems containing ferrihydrite, organic matter, and montmorillonite. Environ Sci Technol 42(8):2833–2838. https://doi.org/10.1021/es072012r
Mathur R, Fantle MS (2015) Copper isotopic perspectives on supergene processes: implications for the global Cu cycle. Elements 11(5):323–329. https://doi.org/10.2113/gselements.11.5.323
Mathur R, Ruiz J, Titley S, Liermann L, Buss H, Brantley S (2005) Cu isotopic fractionation in the supergene environment with and without bacteria. Geochim Cosmochim Acta 69(22):5233–5246. https://doi.org/10.1016/j.gca.2005.06.022
Mathur R, Titley S, Barra F, Brantley S, Wilson M, Phillips A, Munizaga F, Maksaev V, Vervoort J, Hart G (2009) Exploration potential of Cu isotope fractionation in porphyry copper deposits. J Geochem Explor 102(1):1–6. https://doi.org/10.1016/j.gexplo.2008.09.004
Mejia J, He S, Yang Y, Ginder-Vogel M, Roden EE (2018) Stability of ferrihydrite-humic acid coprecipitates under iron-reducing conditions. Environ Sci Technol 52(22):13174–13183. https://doi.org/10.1021/acs.est.8b03615
Mihaljevic M, Jarosikova A, Ettler V, Vanek A, Penizek V, Kribek B, Chrastny V, Sracek O, Trubac J, Svoboda M, Nyambe I (2018) Copper isotopic record in soils and tree rings near a copper smelter, Copperbelt, Zambia. ScTEn 621:9–17. https://doi.org/10.1016/j.scitotenv.2017.11.114
Mihaljevic M, Baieta R, Ettler V, Vanek A, Kribek B, Penizek V, Drahota P, Trubac J, Sracek O, Chrastny V, Mapani BS (2019) Tracing the metal dynamics in semi-arid soils near mine tailings using stable Cu and Pb isotopes. ChGeo 515:61–76. https://doi.org/10.1016/j.chemgeo.2019.03.026
Mijovilovich A, Leitenmaier B, Meyer-Klaucke W, Kroneck PMH, Goetz B, Kuepper H (2009) Complexation and toxicity of copper in higher plants II. Different mechanisms for copper versus cadmium detoxification in the copper-sensitive Cadmium/Zinc hyperaccumulator Thlaspi caerulescens (Ganges Ecotype). Plant Physiol 151(2):715–731. https://doi.org/10.1104/pp.109.144675
Moeller K, Schoenberg R, Pedersen RB, Weiss D, Dong S (2012) Calibration of the new certified reference materials ERM-AE633 and ERM-AE647 for copper and IRMM-3702 for zinc isotope amount ratio determinations. Geostand Geoanal Res 36(2):177–199. https://doi.org/10.1111/j.1751-908X.2011.00153.x
Moon EM, Peacock CL (2012) Adsorption of Cu(II) to ferrihydrite and ferrihydrite–bacteria composites: Importance of the carboxyl group for Cu mobility in natural environments. Geochim Cosmochim Acta 92:203–219. https://doi.org/10.1016/j.gca.2012.06.012
Moynier F, Vance D, Fujii T, Savage P (2017) The isotope geochemistry of zinc and copper. Rev Mineral Geochem 82:543–600. https://doi.org/10.2138/rmg.2017.82.13
Murphy WM, Oelkers EH, Lichtner PC (1989) Surface reaction versus diffusion control of mineral dissolution and growth rates in geochemical processes. Chem Geol 78(3):357–380. https://doi.org/10.1016/0009-2541(89)90069-7
Navarrete JU, Borrok DM, Viveros M, Ellzey JT (2011a) Copper isotope fractionation during surface adsorption and intracellular incorporation by bacteria. Geochim Cosmochim Acta 75(3):784–799. https://doi.org/10.1016/j.gca.2010.11.011
Navarrete JU, Viveros M, Ellzey JT, Borrok DM (2011b) Copper isotope fractionation by desert shrubs. Appl Geochem 26:S319–S321. https://doi.org/10.1016/j.apgeochem.2011.04.002
Othmane G, Hull S, Fayek M, Rouxel O, Geagea ML, Kyser TK (2015) Hydrogen and copper isotope analysis of turquoise by SIMS: calibration and matrix effects. ChGeo 395:41–49. https://doi.org/10.1016/j.chemgeo.2014.11.024
Palacios C, Rouxel O, Reich M, Cameron EM, Leybourne MI (2011) Pleistocene recycling of copper at a porphyry system, Atacama Desert, Chile: Cu isotope evidence. MinDe 46(1):1–7. https://doi.org/10.1007/s00126-010-0315-6
Palmer CM, Guerinot ML (2009) Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat Chem Biol 5(5):333–340. https://doi.org/10.1038/nchembio.166
Pan Y, Bonten LTC, Koopmans GF, Song J, Luo Y, Temminghoff EJM, Comans RNJ (2016) Solubility of trace metals in two contaminated paddy soils exposed to alternating flooding and drainage. Geoderma 261:59–69. https://doi.org/10.1016/j.geoderma.2015.07.011
Peacock CL, Sherman DM (2004) Copper(II) sorption onto goethite, hematite and lepidocrocite: a surface complexation model based on ab initio molecular geometries and EXAFS spectroscopy. Geochim Cosmochim Acta 68(12):2623–2637. https://doi.org/10.1016/j.gca.2003.11.030
Pękala M, Asael D, Butler IB, Matthews A, Rickard D (2011) Experimental study of Cu isotope fractionation during the reaction of aqueous Cu(II) with Fe(II) sulphides at temperatures between 40 and 200 degrees C. ChGeo 289(1–2):31–38. https://doi.org/10.1016/j.chemgeo.2011.07.004
Pokrovsky OS, Viers J, Emnova EE, Kompantseva EI, Freydier R (2008) Copper isotope fractionation during its interaction with soil and aquatic microorganisms and metal oxy(hydr)oxides: Possible structural control. Geochim Cosmochim Acta 72(7):1742–1757. https://doi.org/10.1016/j.gca.2008.01.018
Printz B, Lutts S, Hausman JF, Sergeants K (2016) Copper trafficking in plants and its implication on cell wall dynamics. Front Plant Sci 7(601):1–16. https://doi.org/10.3389/fpls.2016.00601
Ramanathan T, Ting YP (2015) Selection of wet digestion methods for metal quantification in hazardous solid wastes. J Environ Chem Eng 3(3):1459–1467. https://doi.org/10.1016/j.jece.2015.05.006
Richards BK, Steenhuis TS, Peverly JH, Mcbride MB (1998) Metal mobility at an old, heavily loaded sludge application site. Environ Pollut 99(3):365–377. https://doi.org/10.1016/S0269-7491(98)00011-6
Rodriguez NP, Engstrom E, Rodushkin I, Nason P, Alakangas L, Ohlander B (2013) Copper and iron isotope fractionation in mine tailings at the Laver and Kristineberg mines, northern Sweden. Appl Geochem 32:204–215. https://doi.org/10.1016/j.apgeochem.2012.10.012
Roebbert Y, Rabe K, Lazarov M, Schuth S, Schippers A, Dold B, Weyer S (2018) Fractionation of Fe and Cu isotopes in acid mine tailings: modification and application of a sequential extraction method. ChGeo 493:67–79. https://doi.org/10.1016/j.chemgeo.2018.05.026
Ronald A (2003) Soil Formation. In: Holland HD and Turekian KK (eds), Treatise on geochemistry. Surface and groundwater, Weathering and Soils. Elsevier Science, pp. 1–26. https://doi.org/10.1016/B0-08-043751-6/05088-2
Ryan BM, Kirby JK, Degryse F, Harris H, Mclaughlin MJ, Scheiderich K (2013) Copper speciation and isotopic fractionation in plants: uptake and translocation mechanisms. New Phytol 199(2):367–378. https://doi.org/10.1111/nph.12276
Ryan BM, Kirby JK, Degryse F, Scheiderich K, Mclaughlin MJ (2014) Copper isotope fractionation during equilibration with natural and synthetic ligands. Environ Sci Technol 48(15):8620–8626. https://doi.org/10.1021/es500764x
Ryan BM (2014) The isotopic discrimination of copper in soil-plant systems: examining sources, uptake and translocation pathways
Santi S, Schmidt W (2009) Dissecting iron deficiency-induced proton extrusion in Arabidopsis roots. New Phytol 183(4):1072–1084. https://doi.org/10.1111/j.1469-8137.2009.02908.x
Sastre J, Sahuquillo A, Vidal M, Rauret G (2002) Determination of Cd, Cu, Pb and Zn in environmental samples: microwave-assisted total digestion versus aqua regia and nitric acid extraction. Anal Chim Acta 462(1):59–72. https://doi.org/10.1016/s0003-2670(02)00307-0
Sauvé S, Mcbride MB, Norvell WA, Hendershot WH (1997) Copper solubility and speciation of in situ contaminated soils: effects of copper level, pH and organic matter. Water Air Soil Pollut 100(1–2):133–149. https://doi.org/10.1023/A:1018312109677
Seda NN, Koenigsmark F, Vadas TM (2016) Sorption and coprecipitation of copper to ferrihydrite and humic acid organomineral complexes and controls on copper availability. Chemosphere 147:272–278. https://doi.org/10.1016/j.chemosphere.2015.12.106
Shabbir Z, Sardar A, Shabbir A, Abbas G, Shamshad S, Khalid S, Natasha Murtaza G, Dumat C, Shahid M (2020) Copper uptake, essentiality, toxicity, detoxification and risk assessment in soil-plant environment. Chemosphere 259(127436):1–28. https://doi.org/10.1016/j.chemosphere.2020.127436
Sherman DM (2013) Equilibrium isotopic fractionation of copper during oxidation/reduction, aqueous complexation and ore-forming processes: predictions from hybrid density functional theory. Geochim Cosmochim Acta 118:85–97. https://doi.org/10.1016/j.gca.2013.04.030
Sherman DM, Little SH (2020) Isotopic disequilibrium of Cu in marine ferromanganese crusts: evidence from ab initio predictions of Cu isotope fractionation on sorption to birnessite. Earth Planet Sci Lett 549(116540):1–7. https://doi.org/10.1016/j.epsl.2020.116540
Shields WR, Garner EL, Murphy TJ (1964) Absolute isotopic abundance ratio and the atomic weight of a reference sample of copper. J Res Nat Bur Stand Sect Phys Chem A 68(6):589–592. https://doi.org/10.6028/jres.068A.056
Sillerova H, Chrastny V, Vitkova M, Francova A, Jehlicka J, Gutsch MR, Kocourkova J, Aspholm PE, Nilsson LO, Berglen TF, Jensen HKB, Komarek M (2017) Stable isotope tracing of Ni and Cu pollution in North-East Norway: potentials and drawbacks. Environ Pollut 228:149–157. https://doi.org/10.1016/j.envpol.2017.05.030
Song Z, Liu C, Muller K, Yang X, Wu Y, Wang H (2018) Silicon regulation of soil organic carbon stabilization and its potential to mitigate climate change. Earth-Sci Rev 185:463–475. https://doi.org/10.1016/j.earscirev.2018.06.020
Strawn DG, Baker LL (2009) Molecular characterization of copper in soils using X-ray absorption spectroscopy. Environ Pollut 157(10):2813–2821. https://doi.org/10.1016/j.envpol.2009.04.018
Sullivan KV, Kidder JA, Junqueira TP, Vanhaecke F, Leybourne MI (2022) Emerging applications of high-precision Cu isotopic analysis by MC-ICP-MS. ScTEn 838:156084. https://doi.org/10.1016/j.scitotenv.2022.156084
Takano S, Tanimizu M, Hirata T, Shin K-C, Fukami Y, Suzuki K, Sohrin Y (2017) A simple and rapid method for isotopic analysis of nickel, copper, and zinc in seawater using chelating extraction and anion exchange. Anal Chim Acta 967:1–11. https://doi.org/10.1016/j.aca.2017.09.001
Vance D, Matthews A, Keech A, Archer C, Hudson G, Pett-Ridge J, Chadwick OA (2016) The behaviour of Cu and Zn isotopes during soil development: controls on the dissolved load of rivers. ChGeo 445:36–53. https://doi.org/10.1016/j.chemgeo.2016.06.002
Vialykh EA, Salahub DR, Achari G (2019) Metal ion binding by humic substances as emergent functions of labile supramolecular assemblies. Environ Chem 17(3):252–265. https://doi.org/10.1071/EN19198
Walker EC, Cuttitta F, Senftle FE (1959) Some natural variations in the relative abundance of copper isotopes. Geochim Cosmochim Acta 15(3):183–194. https://doi.org/10.1016/0016-7037(58)90056-5
Wall AJ, Mathur R, Post JE, Heaney PJ (2011) Cu isotope fractionation during bornite dissolution: an in situ x-ray diffraction analysis. Ore Geol Rev 42(1):62–70. https://doi.org/10.1016/j.oregeorev.2011.01.001
Wang L, Jin Y, Weiss DJ, Schleicher NJ, Wilcke W, Wu L, Guo Q, Chen J, O’Connor D, Hou D (2021) Possible application of stable isotope compositions for the identification of metal sources in soil. J Hazard Mater 407(124812):1–17. https://doi.org/10.1016/j.jhazmat.2020.124812
Weinstein C, Moynier F, Wang K, Paniello R, Foriel J, Catalano J, Pichat S (2011) Isotopic fractionation of Cu in plants. ChGeo 286(3–4):266–271. https://doi.org/10.1016/j.chemgeo.2011.05.010
Wiederhold JG (2015) Metal stable isotope signatures as tracers in environmental geochemistry. Environ Sci Technol 49(5):2606–2624. https://doi.org/10.1021/es504683e
Wiggenhauser M, Moore R, Wang P, Bienert G, Laursen K, Blotevogel S (2022) Stable isotope fractionation of metals and metalloids in plants: a review. Front Plant Sci 13:840941. https://doi.org/10.3389/fpls.2022.840941
Wilson B, Pyatt FB (2007) Heavy metal dispersion, persistance, and bioccumulation around an ancient copper mine situated in Anglesey. UK Ecotoxicol Environ Saf 66(2):224–231. https://doi.org/10.1016/j.ecoenv.2006.02.015
Wintz H, Fox T, Wu YY, Feng V, Chen WQ, Chang HS, Zhu T, Vulpe C (2003) Expression profiles of Arabidopsis thaliana in mineral deficiencies reveal novel transporters involved in metal homeostasis. J Biol Chem 278(48):47644–47653. https://doi.org/10.1074/jbc.M309338200
Witzgall K, Vidal A, Schubert DI, Hoeschen C, Schweizer SA, Buegger F, Pouteau V, Chenu C, Mueller CW (2021) Particulate organic matter as a functional soil component for persistent soil organic carbon. Nat Commun 12:4115. https://doi.org/10.1038/s41467-021-24192-8
Wu B, Amelung W, Xing Y, Bol R, Berns AE (2019) Iron cycling and isotope fractionation in terrestrial ecosystems. Earth-Sci Rev 190:323–352. https://doi.org/10.1016/j.earscirev.2018.12.012
Xia S, Song Z, Jeyakumar P, Shaheen SM, Rinklebe J, Ok YS, Bolan N, Wang H (2019) A critical review on bioremediation technologies for Cr(VI)-contaminated soils and wastewater. Crit Rev Environ Sci Technol 49(12):1027–1078. https://doi.org/10.1080/10643389.2018.1564526
Xu H, Xia B, He E, Qiu R, Peijnenburg WJGM, Qiu H, Zhao L, Xu X, Cao X (2021) Dynamic release and transformation of metallic copper colloids in flooded paddy soil: role of soil reducible sulfate and temperature. J Hazard Mater 402(123462):1–9. https://doi.org/10.1016/j.jhazmat.2020.123462
Yang W, Zhang W, Hu Z, Zhou L, Liu S-A, Qiu X, Tong X (2023) Development of three chalcopyrites and one copper metal as potential reference materials for copper isotopic analysis by LA-MC-ICP-MS. Rapid Commun Mass Spectrom 37(15):e9538. https://doi.org/10.1002/rcm.9538
Yu C, Xie S, Song Z, Xia S, Astrom ME (2021) Biogeochemical cycling of iron (hydr-)oxides and its impact on organic carbon turnover in coastal wetlands: a global synthesis and perspective. Earth-Sci Rev 218:103658. https://doi.org/10.1016/j.earscirev.2021.103658
Yuan H, Yuan W, Bao Z, Chen K, Huang F, Liu S (2017) Development of two new copper isotope standard solutions and their copper isotopic compositions. Geostand Geoanal Res 41(1):77–84. https://doi.org/10.1111/ggr.12127
Zandi P, Yang J, Mozdzen K, Barabasz-Krasny B (2020) A review of copper speciation and transformation in plant and soil/wetland systems. In: Sparks DL (ed), Advances in Agronomy, Vol 160. Advances in Agronomy, pp. 249–293. https://doi.org/10.1016/bs.agron.2019.11.001
Zheng X, Han G, Zhang Q, Liang B, Liu M, Yu C, Liu L, Zhao Y, Song Z (2023) Extreme copper isotope fractionation driven by redox oscillation during gleysols weathering in Mun River Basin, Northeast Thailand. J Geophys Res Earth Surf 128(3):e2022JF007025. https://doi.org/10.1029/2022JF007025
Zhu XK, O’Nions RK, Guo Y, Belshaw NS, Rickard D (2000) Determination of natural Cu-isotope variation by plasma-source mass spectrometry: implications for use as geochemical tracers. ChGeo 163(1–4):139–149. https://doi.org/10.1016/s0009-2541(99)00076-5
Zhu XK, Guo Y, Williams RJP, O’Nions RK, Matthews A, Belshaw NS, Canters GW, de Waal EC, Weser U, Burgess BK, Salvato B (2002) Mass fractionation processes of transition metal isotopes. Earth Planet Sci Lett 200(1–2):47–62. https://doi.org/10.1016/s0012-821x(02)00615-5
Acknowledgements
This work was supported by the “Deep-time Digital Earth” Science and Technology Leading Talents Team Funds for the Central Universities for the Frontiers Science Center for Deep-time Digital Earth, China University of Geosciences (Beijing) (Grant No. 2652023001), and the National Natural Science Foundation of China (Grant No. 41661144029).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no know competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zheng, X., Han, G., Song, Z. et al. Biogeochemical cycle and isotope fractionation of copper in plant–soil systems: a review. Rev Environ Sci Biotechnol 23, 21–41 (2024). https://doi.org/10.1007/s11157-024-09681-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-024-09681-8