Abstract
Ubiquitous presence of cyanobacteria even under extreme conditions since millions of years is due to their ease in adaptability to these environments through various cellular and molecular modifications. Formation of biofilms has been found to be one of the effective modes of survival under harsh environmental conditions, with exopolysaccharides (EPS) contributing to the formation of these biofilms. Cyanobacterial EPS are unique heteropolysaccharides and being anionic in nature, they have the capabilities for excellent heavy metal adsorption and thus, play a vital role in conferring tolerance against heavy metal toxicity. EPS biosynthesis occurs through multiple pathways involving a multitude of genes. A comprehensive overview on these genes in cyanobacteria using Nostoc sp. strain PCC7120 as the model organism has been discussed in this review. The review also emphasizes on the contribution of EPS to heavy metal remediation and biofilm formation. In the age of developing eco-friendly approaches for environmental clean-up, this information would help engineer cyanobacteria as a potential green technology tool for bioremediation and waste water management.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cyanobacteria are ancient organisms (Brock 1973), capable of important biological functions, such as nitrogen fixation and photosynthesis, that have contributed to the fixation of carbon and nitrogen, and oxygenation of earth (Schopf 2011; Zehr 2011). They are present in different morphological forms such as unicellular and filamentous, nitrogen-fixers and non-nitrogen fixers. Among them, the nitrogen-fixing cyanobacteria possess temporal or spatial separation of nitrogen-fixation and photosynthesis (Stewart 1980). The morphological adaptations of cyanobacteria are also important for survival upon exposure to extreme environmental conditions (Wynn-Williams 1990). One of the most important physical adaptation mechanisms adopted by cyanobacteria is the formation of biofilms, either intra/inter-species or in conjunction with other eubacterial species, which forms a shield against environmental stresses (Gorbushina and Broughton 2009).
Under natural conditions, majority of the substratum-associated microbiota comprises of cyanobacteria, algae and some marine microbes (Furey et al. 2019). Biofilms are comprised of multiple organisms like autotrophs, heterotrophs enclosed by a matrix of exopolymeric substances that are primarily composed of polysaccharides along with other components like proteins, lipids, nucleic acids, thereby forming heteropolymers like lipopolysaccharides, glycoproteins etc. (Decho 1990; Klock et al. 2007; Vu et al. 2009). Organic matter constitutes 50–90% of the biofilms and make up the basic framework for any microbial mat system that comprise of heterogeneous microorganisms including cyanobacteria (Decho 1990; Klock et al. 2007; Vu et al. 2009). Composition of EPS varies depending on the types of microorganisms, age of the biofilms and the environmental conditions (Vu et al. 2009). Synthesis and secretion of exopolysaccharides (EPS) has been observed to be involved in biofilm formation even in cyanobacteria (Rossi and De Philippis 2015). The utility of cyanobacterial biofilms or mats has also been demonstrated in heavy metal bioremediation (Bender et al. 1994a, b, c; Husien et al. 2020; Raghavan et al. 2020). In addition to being robust, use of biofilms for bioremediation cuts down the downstream processing costs (Strieth et al. 2018), thereby offering a considerable cost-advantage.
A direct link has also been observed between EPS synthesis and heavy metal exposure in cyanobacteria. Metals were shown to induce generation of EPS in microbial mats comprising of mixture of the cyanobacterium, Oscillatoria and other heterotrophic bacteria (Bender et al. 1994b). Algal biofilms recovered from mine tailings were found to be largely comprised of Phormidium and Chlorococcum species. These cells when treated with heavy metals exhibited 2–5.6-fold increase in EPS production after 5 days of subsequent exposure to heavy metals compared to control non-treated cells (Garcia-Meza et al. 2005). They also exhibited the ability to adsorb the heavy metals, with higher percentage of Cu adsorbed by the capsular fraction, while the loose fractions adsorbed more Zn (Garcia-Meza et al. 2005). Older biofilms having accumulated ~ 40% of their dry weight as EPS showed better tolerance to Cd and Zn toxicity than immature biofilms which possessed about 18% EPS per unit dry weight (Ivorra et al. 2000). In periphytic biofilms, cyanobacteria were used to alter the composition of the periphytic community which helped maintain high photosynthetic and metabolic rates, and the resulting EPS produced bound to the nanoparticles (NP) and thus decreased the toxicity observed with TiO2 NPs while retaining the Cu2+ removal ability (Liu et al. 2019). Increased synthesis of proteins and polysaccharides were observed in exopolymeric substance constituents of the cyanobacterium Phormidium autumnale monospecific biofilm in response to individual higher concentration of metal treatments of Cu and Zn respectively. However, in a combined challenge of high concentrations of Cu and Zn, only induction of protein was observed in the exopolymeric substances, while no induction of polysaccharide composition could be observed suggesting that in addition to quantitative increase, compositional variation of exopolymeric substances can also be seen in response to heavy metal challenges in cyanobacteria (Loustau et al. 2019). EPS is therefore a fundamental mechanism of heavy metal tolerance in cyanobacterial mat/biofilm systems.
However, the current understanding of cyanobacterial EPS and biofilms in terms of functional group chemistry involved in interaction with metals is limited and scattered. The present review consolidates the available information pertaining to cyanobacterial biofilm-metal interaction and remediation with specific emphasis on the role of EPS. This includes bioinformatics-based approaches for identifying the genes involved in biosynthesis of EPS and biophysical approaches which can be used for identifying the metal-interacting functional groups of EPS/biofilms. The review is aimed at understanding and tapping the potential of cyanobacterial EPS and biofilms for waste water management through removal of toxic heavy metals. The available information on the genome of the genetically amenable Nostoc PCC7120 can be utilized for identifying the putative genes involved in EPS biosynthesis, thereby opening the possibility of genetic manipulation of these genes to enhance the quality and quantity of EPS produced. This will open newer avenues for better understanding and development of eco-friendly cyanobacterial origin bioremediators.
2 Cyanobacterial exopolysaccharides: characteristics, biosynthesis and genome organization
2.1 General characteristics
The chemical structure of bacterial exopolysaccharides as homo- or hetero-polysaccharides has been extensively reviewed recently (Sun and Zhang 2021). Several cyanobacteria are known to produce polysaccharides having molecular mass in the range of 0.2–2 × 104 kDa, and these have been targeted for their potential use due to high water solubility as well as the ease of removal from liquid solutions (De Philippis and Vincenzini 1998; Pereira et al. 2009). The exopolysaccharides synthesized by cyanobacteria have two distinct characteristics which makes them unique compared to other bacterial polysaccharides. First is their anionic nature due to the presence of uronic acid and sulphate containing sugars (Pereira et al. 2009) which imparts in them the ability to form hydrated gels (Kehr and Dittmann 2015). Secondly, cyanobacterial exopolysaccharides are predominantly complex heteropolysaccharides with about 75% of them constituted of six or more different types of monosaccharide units (Pereira et al. 2009). Polysaccharides synthesised by bacteria, in general, are either intracellular, cell surface-linked capsular or extracellular (Pereira et al. 2009). Quite a few cyanobacteria also release a part of these extracellular materials into the surrounding medium, and such polysaccharides are referred to as released polysaccharides (De Philippis and Vincenzini 1998). The formation of dense aggregates of Microcystis aeruginosa, and its propagation to blooms has been reported to be due to both the tightly and the loosely bound extracellular polymeric substances (Xu et al 2013). In addition to carbohydrates, cyanobacterial EPS also contain polypeptides abundant in glycine, alanine, valine, leucine, isoleucine and phenylalanine amino acids (Flaibani et al. 1989; Marra et al. 1990). Few have a high percentage of aspartic and glutamic acids as well (Flaibani et al. 1989; Kawaguchi and Decho 2002).
Cyanobacterial EPS and the properties associated with them have a high potential for biotechnological applications (De Philippis et al. 2011). The presence of negative charge on the EPS gives an added advantage as effective chelators of metals from solutions (Tran et al 2016; De Phillips et al. 2011). Efforts aimed at exploitation of cyanobacterial EPS by redesigning them for multiple uses such as drug delivery, coatings or metal remediation is important and should be pursued (Pereira et al. 2019b). The EPS biosynthesis pathway and the genes involved in it have not been extensively explored in cyanobacteria, and have been dealt with in detail in the following subsections.
2.2 EPS biosynthesis pathways in cyanobacteria
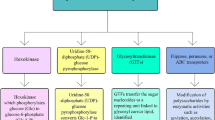
While it is evident that EPS synthesis and secretion is essential for the survival of cyanobacteria and cyanobacterial mat systems in a heavy metal rich environment, the EPS biosynthesis processes, genetic mechanisms and their regulation in cyanobacteria is a very complex process and very poorly understood. An overview of the processes involved in EPS biosynthesis is shown in Fig. 1. The genes encoding the enzymes participating in EPS biosynthesis are categorised into those involved in (1) Nucleotide sugar synthesis pathways, (2) Glycosyltransferases and (3) Processing of Oligo/polysaccharides (De Vuyst et al. 2001; Kleerebezem et al. 1999; Pereira et al. 2009; 2015; Sutherland 2001). Cyanobacteria are characterised by the presence of several genes encoding for similar proteins involved in EPS biosynthesis. Mutational analysis in the unicellular cyanobacterium Synechocystis PCC6803 for some of these genes revealed lack of any variation in their phenotype suggesting redundancy of function of these genes due to the presence of multiple copies of the same gene (Pereira et al. 2019a). However, mutation in genes coding for WzC and WzB influenced EPS production, with both RPS (released polysaccharides) and CPS (capsular polysaccharides) synthesis affected in the mutant strain lacking the WzC protein, while only that of RPS was affected in the mutant strain lacking the WzB protein, clearly indicating important functions for the two proteins WzB and WzC in exopolysaccahride biosynthesis in cyanobacteria (Pereira et al. 2019a). Another important aspect observed was the role of phosphorylation and dephosphorylation of tyrosine residues in EPS synthesis in cyanobacteria (Pereira et al. 2019a). Another gene found to play an important role in this process was kpsM. Mutation of kpsM in Synechocystis PCC6803 resulted in a significant decrease in RPS content, small reduction of CPS and an increased accumulation of polyhydroxybutyrate (PHB) in comparison to the wild type strain (Santos et al. 2021). Induction of expression of ExoD (alr2882) and increase in EPS synthesis was found to be concomitant in Nostoc 7120 hinting towards the possible involvement of ExoD in EPS biosynthesis (Singh et al. 2016; Kharwar et al. 2021). The unicellular fresh water cyanobacterium, Aphanothece sacrum was shown to possess the Wzx-Wzy-Wzz and Wza-Wzb-Wzc indicating the presence of WzY dependent pathway for EPS biosynthesis. However, the genes involved in those of capsular polysaccharides in other organisms such as kpsC and kpsS were missing, and a variation was observed in the copy numbers and extent of similarity in Wzx-Wzy-Wzz proteins in different species suggesting a unique mechanism for polymerisation of EPS (Ohki et al. 2019).
General Overview of the EPS synthesis and export in cyanobacteria. The different steps involved in synthesis of EPS along with the compartmentalisation in cyanobacterial cell have been graphically depicted. The first step of synthesis of monosaccharide  followed by conjugation with lipid carrier
followed by conjugation with lipid carrier  for transfer into the periplasmic space viz., the site of polymerization and finally extracellular release via various export pathways is schematically shown
for transfer into the periplasmic space viz., the site of polymerization and finally extracellular release via various export pathways is schematically shown
Several cyanobacterial genes speculated to be involved in the different EPS biosynthesis pathways have been listed earlier (Pereira et al. 2009, 2015), but is not a complete list as further genome-based bioinformatic studies revealed. A closer look at the genome sequence of the genetically amenable cyanobacterium, Nostoc sp. strain PCC7120 (https://www.genome.jp/kegg-bin/show_organism?org=ana) revealed the presence of several other genes with a potential role in EPS synthesis and these have been listed in Supplementary Tables S1 and S2. Analysis of the corresponding protein sequences bioinformatically for the presence of conserved domains using the NCBI-CDD software (Lu et al. 2020), helped identify the superfamily to which they belonged (Supplementary Tables S1 and S2).
EPS production involves three major steps. The first step, catalysed by enzymes coded by the first group of genes (such as rfb) in the biosynthesis pathway takes place in the cytoplasm and is common to both polysaccharide and EPS synthesis. It involves synthesis of nucleotide sugars i.e. activated monosaccharides containing glycosyl groups (Pereira et al. 2009). The second step is catalysed by glycosyltransferases, categorised as the second group of enzymes, which catalyse the transfer of the sugar to specific acceptor molecules such as lipid carrier in the plasma membrane resulting in the addition of sugar moieties one by one (Reeves et al. 1996; Pereira et al. 2009). Compared to other bacteria, most cyanobacteria have numerous genes coding for glycosyltransferases. As per the KEGG database, Escherichia coli, Deinococcus radiodurans and Bacillus subtilis were found to have 14, 5 and 19 genes respectively coding for glycosyltrasferase as against at least 70 annotated genes in most cyanobacteria. A few exceptions to this include Synechocystis PCC6803, Synechococcus elongatus PCC7942, Thermosynechococcus elongatus, Gleobacter sp. and Prochlorococcus sp. which have 11, 19, 23, 33 and 14 genes respectively.
A search for glycosyltransferases in the genome of Nostoc PCC7120, revealed the presence of at least 81 genes coding for glycosyltransferases/probable glycosyltransferases and possessing the GT motif (Supplementary Table S1A). These are being reported for the first time and do not preclude finding several more. The significance of having multiple genes coding for glycosyltransferases in cyanobacteria is not known, but these being ancient organisms, they may have been involved in different processes of synthesis of polysaccharides for cell wall, biofilms or microbial mat formation, all adding as a means of self-protection under harsh environmental conditions present during the early days of evolution of earth. Phylogenetic analysis of these 81 sequences was carried out with desktop version of MegaX software (Kumar et al. 2018) using the maximum likelihood method and the JTT model (Jones et al. 1992). In brief, MegaX refers to Molecular Evolutionary Genetics Analysis, which involves several integrated programs helping in assembling the sequence alignments, developing the evolutionary trees, estimating genetic distances etc., and has been used with great success in biological fields. The different parameters used were Maximum likelihood as the statistical method, bootstrap with 500 replications and amino acid substitution using JTT method. The ML Heuristic method used for inference of tree was Nearest Neighbour Interchange (NNI). Two distinct clades were observed, with Clade I comprising of 46 glycosyltransferases and Clade II having 35 glycosyltransferases (Fig. 2). Each of these clade branches out to multiple glycosyltransferases, with some of them showing very little evolutionary distance. The pairwise matrix (Supplementary data sheet) reveals low nucleotide substitutions in these which was further confirmed with higher pairwise identity observed for these set of proteins through Clustal ω analysis (Supplementary Fig. S1). The substitutions per site among the glycosyltransferases varied from 0.409 to 2.883 in the pairwise analysis (Supplementary data sheet). This indicates multiple changes which may have taken place in the sequence probably for allowing the enzymes to have different substrate specificities as required under different environmental conditions. The implication of having plethora of genes coding for glycosyltransferases in the overall EPS production of cyanobacteria needs to be investigated.
Phylogenetic analyses of glycosyl transferases of Nostoc PCC7120. The different annotated and putative glycosyltransferases were analysed for phylogenetic lineage using the MegaX software. The 81 glycosyltransferases are represented by their gene annotation numbers as in KEGG database (https://www.genome.jp/kegg-bin/show_organism?org=ana). The software used for phylogenetic analysis is described in Kumar et al (2018). Multiple sequence alignment was carried out for all the sequences used for evolutionary analysis and the resulting sequence alignment in FASTA format was used as an input for MegaX. In order to have higher confidence in the evolutionary analysis, 500 replicates representing the evolutionary history were taken for the developing the bootstrap consensus tree
In the third step, which occurs in the perisplasmic side of the plasma membrane, the repeating units form a polymer and it is finally exported to the cell surface. The proteins involved in the third step can be further categorised broadly into proteins involved in the following pathways (1) the Wzx/Wzy-dependent pathway; (2) the ATP-binding cassette (ABC) transporter-dependent pathway; (3) the synthase-dependent pathway, and (4) the single sucrase protein based extracellular synthesis pathway (Pereira et al. 2009, 2015; Schmid et al. 2015) and also shown in Fig. 1. The genes in Nostoc PCC7120 identified to be coding for proteins homologous to the proteins known to be involved in these pathways are listed in Supplementary Table S2.
2.3 Organisation of EPS biosynthesis genes
In the cyanobacterial genomes, some of the annotated or putative genes involved in EPS production are clustered, while several others are scattered across various genomic locations (Pereira et al. 2009). In the cyanobacterium Nostoc PCC7120, a total of 8 clusters of five or more genes predominantly having glycosyltransferases genes were detected, varying in size from 4.87 kb to 22.1 kb (Fig. 3). These clusters probably consist of multiple contiguous operons instead of a single operon as bioinformatic analysis revealed presence of -10-like region upstream of several of the genes in these clusters (data not shown). The genes in the 8 clusters (Fig. 3) and involved in different steps of polysaccharide/EPS biosynthesis are listed in Supplementary Tables S1 and S2. Some of the clusters were interspersed with genes not belonging to these pathways and these are as follows: alr3061 and alr3074 coding respectively for acetyl transferase and UDP-3-O[3-hydroxymyristoyl] glucosamine N-acyl transferase involved in LPS synthesis in Cluster I, asr4421, all4427 and all4429 annotated as unknown, similar to phytanoyl-CoA hydroxylase and hlpA respectively in Cluster II, alr5236 coding for unknown protein in Cluster VI and alr2865 coding for unknown protein with methyltransferase domain in Cluster VIII (Fig. 3). The presence of the EPS biosynthesis genes in clusters may facilitate coordination of the entire process of EPS generation from synthesis to export. The understanding of the EPS biosynthesis pathways can help generating tailor made polysaccharides using various gene editing tools (Sun and Zhang 2021).
Genome organisation of gene clusters involved in EPS synthesis and export in Nostoc PCC 7120. Eight clusters numbered Cluster I to Cluster VIII with gene numbers of Nostoc PCC7120 and the total cluster size are shown. The different coloured genes correspond to glycosyltransferases  , sugar transferases
, sugar transferases  , general polysaccharide synthesis genes
, general polysaccharide synthesis genes  , Wz pathway genes
, Wz pathway genes  , ABC transporter genes
, ABC transporter genes  , gene coding for protein involved in LPS biosynthesis
, gene coding for protein involved in LPS biosynthesis  , and genes with unknown functions or coding for proteins involved in other metabolic activities
, and genes with unknown functions or coding for proteins involved in other metabolic activities  . The organisation of genes shown in the clusters was obtained using the website: https://www.genome.jp/kegg-bin/show_genomemap?ORG=ana&ACCESSION=genenumber
. The organisation of genes shown in the clusters was obtained using the website: https://www.genome.jp/kegg-bin/show_genomemap?ORG=ana&ACCESSION=genenumber
3 Importance of cyanobacterial biofilms and EPS in heavy metal sequestration
3.1 Metal-EPS interaction
Metal and EPS-generating microbial mats have an interlinked relationship, and the microbial mats themselves are capable of influencing the biogeochemistry of the surrounding aqueous ecosystem (Lawrence et al. 1998). In the cyanobacterium, Microcystis aeruginosa the polysaccharide content of the loosely and tightly bound extracellular polymeric substances was found to increase in response to treatment with oxide nanoparticles of Ce, Cu and Zn (Hou et al. 2017), thus indicating that presence of metals could also induce EPS generation. The biofilm—heavy metal interaction is largely influenced by the nature of the heavy metals and the functional groups that are present on the surface of the biofilm. This interaction involves chemical processes at molecular scale such as electron transfer mechanisms, mineral nucleation etc. (Templeton and Knowles 2009). It is therefore important to understand the nature of heavy metals as well as kind of interactions they could have with EPS on biofilm surfaces, which depends on the class of heavy metal. The classical classification of metals as A, B and borderline, based on their inorganic properties of association with hard and soft acids is also valid for biological systems, with the difference being in the nature of ligands which is largely organic. The equilibrium constant associated with the metal–ligand interaction defining the preference of the ligands for the heavy metals, higher the equilibrium constant higher is the preference for interaction of the metal with the specific ligand (Niboer and Richardson 1980). The class A metals, such as Ca, Mg are oxygen seeking, and the order of preference of donor atoms in ligands is O > N > S. Some of the preferred ligands for class A metals in biological systems are ROR, ROH, RCOO−, keto, PO4−, SO42−, NO3−, OH−, O2−, H2O to name a few (Niboer and Richardson 1980). The class B metals such as Hg, Pd, on the other hand, do not prefer oxygen containing ligand and seek out S and N-containing ligands, such as S2−, RS−, R−, CN−, I− (Niboer and Richardson 1980). The borderline metals exhibit and reveal ambivalent nature and have a broad affinity to the various ligands in biological systems (Niboer and Richardson 1980). Class A metals seem quite separate from borderline metals, however, this sort of separation between borderline and class B metals is not very definite (Niboer and Richardson 1980). The interaction of the functional groups resulting in sorption could be either electrostatic bonding for alkaline earth and alkali metal elements or covalent coordinate bonding in case of transition metal (Folsom et al. 1986; Lawrence et al. 1998), and the extent of interaction results in the observed preferences of sorption of different classes of metals by the cell surface (Tien and Chen 2013).
The interaction of the metal with EPS depends on the negatively charged functional group of EPS such as carboxylate, phosphates and sulphate to name a few (Garcia-Meza et al. 2005; Kiran and Kaushik 2008; Pereira et al. 2011; Thapa et al. 2017; Cui et al. 2021). The importance of functional groups such as that of carboxyl, amino groups have been individually studied and established, as seen by a few examples stated here. Methylation of amine groups and esterification of carboxyl group was found to reduce the metal adsorption capability in the fungus Aspergillus niger (Kapoor and Viraraghavan 1997). More recently studies have shown that esterification of carboxyl and phosphate groups have resulted in hindering the uptake of Cr(III) by Spirulina (Chojnacka et al. 2005), suggesting the importance of functional groups such as carboxyl groups in metal interaction. In cyanobacteria, the carboxyl rich functional groups such as uronic acids present in the exopolysaccharides largely contribute to the heavy metal sequestration (De Philipis et al. 2011). In cyanobacteria, the anionic nature of EPS largely is the essential contributor to the heavy metal sequestration (De Philipis et al. 2011). The presence of negatively charged components such as, glucuronic acid and galacturonic acid, in the cyanobacterial bioflocculants aids in metal sorption (Bender et al. 1994b). In the Aphanothece sacrum strains FPU1 and FPU3, the EPS was shown to contain carboxylate and sulphate groups in addition to the typical functional groups of sugars (Ohki et al. 2019). While functional groups containing carboxylic, phosphate and amino groups enhance the binding of cations with EPS, the opposite interaction with oxyanions such as HCrO4− and Cr2O72− have also been found to occur at lower pH in certain cyanobacteria species due to the presence of protonation of certain functional groups like amino groups on the EPS (Anjana et al. 2007; De Philipis et al. 2011). Hence it is evident that in addition to the types of functional groups that might exist, environmental conditions such as pH induced changes in the nature of these functional groups could play a key role in the nature of metal interaction with the EPS.
3.2 Approaches for understanding different facets of EPS-metal interaction and chemistry
Several approaches utilizing advanced biophysical techniques have thrown light on the functional groups involved in interactions with heavy metals along with concomitant cell surface changes and respective chemistry thereof. These mostly involve biophysical techniques such as those based on X-ray spectroscopy and Fourier transformed infrared spectroscopy (FTIR), and microscopic techniques such as Atomic force Microscopy (AFM) and Scanning Electron Microscopy SEM have thrown light on the functional groups involved in interactions with heavy metals along with concomitant cell surface changes and respective chemistry thereof. Understanding the nature of functional groups available for binding to heavy metals is very important for designing appropriate systems for bioremediation.
X-ray spectroscopy-based approaches have revealed specific intricacies of metal EPS interactions, such as the functional groups involved, the speciation of the metals adsorbed, its oxidation number and the localization of the metals on the cell surface and isotopic content. A key feature of utilizing X-ray based spectroscopic techniques is that there is a correlation between the energy values of elements of a particular atomic number and their X-ray absorption spectra edges which helps in identifying the elements as well as its state and degree of oxidation (Minkina et al. 2016). With better chemical sensitivity, element specificity and the ability to investigate heterogeneous elements i.e. with both mineralogical and biological constituents, X-ray spectroscopy also allows direct study of interfacial chemistry in these microenvironments (Templeton and Knowles 2009). The use of these techniques for cyanobacterial biofilms/mats has been successful. Earlier studies on cyanobacterial biofilms using X-ray fluorescence helped estimate the amount of sequestered Zn and Mn by cyanobacterial mats, however, the speciation pattern could not be deciphered (Bender et al. 1994a). Energy dispersive X-ray spectroscopy combined with electron microscopy helped identify Fe, Al- silicate complex with traces of K in epilithic biofilms consisting of bacterial and cyanobacterial constituents collected from different sites of Brahmani River in India (Konhauser et al. 1998). Using soft X-ray Scanning Transmission X-ray Microscopy (STXM), two species of Fe, i.e. Fe(II) and Fe(III), Mn and Ni were mapped on the river water biofilm comprising of bacteria and alga consortium, which were found to be associated with polysaccharides (Dynes et al. 2006). In recent times, X-ray spectroscopy of biofilms comprised of cyanobacteria (Lyngbya sp. and Leptolyngbya sp.) with a minor component of diatom Achnanthes exigua revealed preferential binding of Cu to O and N and a small fraction to S (Coutaud et al. 2018). Another interesting aspect revealed by this study was the isotopic fractionation of Cu isotopes depending on the type of sorption, with heavier isotopic fractionation favoured during the adsorption phase predominantly involving the carboxylate ligands and lighter isotope fractionation observed more when Cu was incorporated inside the biofilm (Coutaud et al., 2018). The adsorption of the heavier isotope was earlier shown to be influenced by covalent bond formation with surface moieties of the biofilm (Coutaud et al. 2014). X-ray Photoelectron Spectroscopy (XPS) of Nostoc muscorum biofilm exposed to cadmium revealed the presence of Cd in + 2 oxidation state during interaction with the biofilm (Raghavan et al. 2020).
Scanning electron microscopy (SEM) along with energy dispersive spectroscopy (EDS) allows for targeted analysis of surface of materials and can be used to identify the different metals present on the surface and have been extensively and successfully used to analyse biological cell surfaces as well. SEM–EDS has been used to understand the metal distribution on biofilms as has been reported earlier for Cd(II) (Raghavan et al. 2020) and for multiple metals simultaneously (Fig. 4a–c). SEM–EDX analysis revealed binding of metals to RPS isolated from Cyanothece in addition to other elements such as Ca, Mg, Na and Cl (Mota et al 2016). Upon contact with metal ions, the concentration of C was found to increase while that of O, Mg, S and Ca were found to decrease on RPS surface (Mota et al 2016).
Microscopic analysis of Nostoc muscorum biofilms challenged with multiple heavy metals. (a–c) SEM–EDS of N. muscorum biofilm after challenge with Pb(II),Cd(II) and Ni(II) simultaneously showing (a) the biofilm surface, (b) EDS spectrum showing presence of different metals including Pb, Cd and Ni and (c) mapping distribution of Pb(II), Cd(II) and Ni(II) on the biofilm cell surface. (d and e) AFM image of N. muscorum biofilm (d) before and (e) after exposure to Pb(II)
Fourier-transform Infrared Spectroscopy (FTIR) helps identify different functional groups based on the absorption peaks which are specific for a functional group irrespective of the molecule in which the functional group is present. FTIR based studies have shown mechanisms of Cr(IV) uptake in algal mats by identification of the changes in the functional groups, namely complexation of carboxylate group with reduced chromium, involvement of C–O group of polysaccharides in chromium biosorption and appearance of a chromium (VI)-O bond specific signal was shown on the algal consortium mat surface after binding to the heavy metal Cr through FTIR analysis (Shukla et al. 2012). Similarly, the binding of Cd to N. muscorum biofilm as Cd–O and C–N linkages was also confirmed by FTIR in combination with XPS (Raghavan et al. 2020). FTIR analysis of Cyanothece RPS treated with deionized water, acid or alkaline exposed to mono metals [Cu(II), Cd(II), Pb(II)] showed distinct features observed in the shifts under the three conditions, with a marked shift in the absorbance peak of the O–H stretching in RPS treated with base compared to acid and water (Mota et al. 2016). Thus, the information generated helped identify the changes in functional groups contributing the observed differences in the remediation of mono metals under basic conditions (Mota et al. 2016). Similar studies with exopolymeric substances helped identify the oxygen of the carboxyl group in the constituent proteins as the key site for EPS-Cd complexation (Xie et al. 2020).
The topographical changes occurring on the cell surface due to metal challenges are on a microscopic scale of few nano meters, thus Atomic Force Microscope (AFM) is useful in measuring these changes. AFM based approaches have enhanced our understanding on such aspects of metal-Biofilm-EPS interaction. The different parameters which can be evaluated using AFM are changes in (1) cells size (Tekaya et al. 2013; Raghavan et al. 2020), (2) cellular morphology or cell lysis as shown for Arthospira platensis biofilm when exposed to heavy metals (Tekaya et al. 2013), and (3) roughness of cell surface as shown for N. muscorum biofilm upon exposure to cadmium (Raghavan et al. 2020) and Pb (Fig. 4d, e).
3.3 Utilization of cyanobacterial EPS/RPS mats and biofilms in bioremediation
Being photosynthetic, cyanobacteria along with green algae form the upper layer of the naturally occurring microbial mats comprising of heterotrophic bacteria and algae (Bender et al. 1994c) and act as one of the important nature’s way of dealing with heavy metal sequestration in soil and aqueous environments. Microbial mats can also be designed by incorporating the preferred microbial strains during early microbial mat forming stages (Bender et al. 1989). The naturally forming mats as well as tailor made/multi species microbial mats exhibited the ability to decrease the concentration of heavy metals in waste water and aqueous solutions (Shukla et al. 2012; Miranda et al. 2017). The efficiency of biosorption in microbial mats, which depends on the sorptive sites, varies proportionally with the increase in biomass and rate of flow of water current (Hill et al. 2000). There are several reports on successful use of cyanobacterial biofilms for heavy metal sequestration which has been summarized below.
The heterogenous microbial mat ecosystem consisting of Anabaena species NB-19-Pb and other bacteria was used to successfully remove Pb (Bender et al. 1989). Similar heterogeneous systems utilizing bacteria and cyanobacterial population from contaminated sediments were employed for removal of selenium (Bender et al. 1991) from aqueous solutions. The microbial mix of cyanobacteria and heterotrophic bacteria pre-adapted to high heavy metal concentrations exhibited high sequestration ability for Mn and Zn as immobilized algal mats (Bender et al. 1994c) as well as immobilized cell system (Bender et al. 1994a). The metal in turn enhanced the concentration of the heterogenous EPS bioflocculant, a subpopulation of which was found to have polyanionic character (Bender et al. 1994b). The sequestration ability was also found to be dependent on the cyanobacterial species dominating the microbial mat composition. Biofilms dominated by Phormidium species exhibited high sequestration (~ 99%) for Cu when challenged with 20 mg/dm3 of Cu2+ solution, while those dominated by Nostoc commune could sequester only 87% Cu, and axenic N. linckia only 50% (Fokina et al. 2017). It has been observed that cyanobacterial biofilms show good heavy metal tolerance (Barranguet et al. 2000) suggesting cyanobacterial biofilms hold a good promise for heavy metal bioremediation. The cyanobacterium Cyanothece sp. CCY0110 has been successfully used for removal of Cu, Cd and Pb individually and in combination from water bodies. Released polysaccharides (RPS) was found to be most efficient in removal of the heavy metals (Mota et al. 2016). The RPS could also bind multiple metal ions when exposed to them sequentially also. Comparison of binding of two heavy metals Cu(II) and Cd(II) added sequentially showed that while Cu(II) could displace bound Cd(II), displacement of bound Cu(II) by Cd(II) was not significant, in fact initial binding of Cd(II) resulted in higher binding of Cu (II) (Mota et al. 2016). Among the three mono metals, Cu(II), Pb(II) and Cd(II), removal of Cu(II) was primarily found to be through biosorption by both RPS and cell surface, while Pb(II) was mostly adsorbed by RPS and Cd(II) completely by RPS (Mota et al. 2016).
Addition of support base for the formation of biofilms also allowed the retention of heavy metal sequestration capabilities of the cyanobacterial biofilms. Biofilms generated from axenic cultures of Nostoc muscorum using glass as a support base efficiently sequestered Cd(II) from aqueous solutions in the pH range of 5–9 and concentration range of 1–100 ppm Cd(II) and also from waste water (Raghavan et al. 2020). Heavy metal adsorption by cyanobacterial biofilms follow Langmuir adsorption isotherm as shown for N. muscorum biofilm for Cd(II) (Raghavan et al. 2020) and Nostoc sp. biofilm for Cr (Husien et al. 2020). It is noteworthy that in all the above studies the heavy metal interaction with the microbial mats and biofilms are mostly mediated via functional groups present on the EPS on the cell surface. This ability of cyanobacterial biofilms to sequester different heavy metals can be harnessed to provide a green technology to the ever-increasing heavy metal pollution in soil and water due to the influx of various industries and agricultural practices. While the above-mentioned approaches are important to our understanding of metal-EPS interaction in algal mats/biofilms, knowing the natural complex chemical composition of the exopolysaccharides in algal mats and biofilms can also be beneficial for the utilization of only EPS without the live or dead cells for bioremediating heavy metals and employ them as eco-friendly technologies.
4 Conclusions
Cyanobacteria through its multiple networks of EPS biosynthesis pathways including the presence of multiple genes coding for proteins with similar function weaves a web of different types of EPS which is highly beneficial and serves as a protective mechanism against extreme environmental stresses. One of the major advantages arising out of it is the ability to sequester heavy metals from both soil and water and thus helping the environment. The understanding of EPS biosynthesis pathways and the identification of the participating functional groups in the biofilms through use of various biophysical techniques will help in designing and developing better cyanobacterial biofilms for remediation of heavy metals. Thus, a combined approach at understanding the interrelationship between cyanobacterial EPS and biofilms would pave the way for optimal use of cyanobacterial biofilms as a central point for green technology-based strategies for environmental clean-up.
Change history
19 August 2021
A Correction to this paper has been published: https://doi.org/10.1007/s11157-021-09590-0
References
Anjana K, Kaushik A, Kiran B, Nisha R (2007) Biosorption of Cr(VI) by immobilized biomass of two indigenous strains of cyanobacteria isolated from metal contaminated soil. J Hazard Mater 148:383–386
Barranguet C, Charantoni E, Plans M, Admiraal W (2000) Short-term response of monospecific and natural algal biofilms to copper exposure. Eur J Phycol 35:397–406
Bender J, Gould JP, Vatcharapijarn Y, Young JS, Phillips P (1994a) Removal of zinc and manganese from contaminated water with cyanobacteria mats. Water Environ Res 66:679–683
Bender J, Rodriguez-Eaton S, Ekanemesang UM, Phillips P (1994b) Characterization of metal-binding bioflocculants produced by the cyanobacterial component of mixed microbial mats. Appl Environ Microbiol 60:2311–2315
Bender J, Gould JP, Vatcharapijarn Y, Saha G (1991) Uptake, transformation and fixation of Se(VI) by a mixed selenium-tolerant ecosystem. Water Air Soil Pollut 59:359–367
Bender J, Washington JR, Graves B, Phillips P, Abotsi G (1994c) Deposit of zinc and manganese in an aqueous environment mediated by microbial mats. Water Air Soil Pollut 75:195–204
Bender JA, Archibold ER, Ibeanusi V, Gould JP (1989) Lead removal from contaminated water by a mixed microbial ecosystem. Water Sci Technol 21:1661–1664
Brock TD (1973) Lower pH limit for the existence of blue-green algae: evolutionary and ecological implications. Science 179:480–483
Chojnacka K, Chojnacki A, Górecka H (2005) Biosorption of Cr3+, Cd2+ and Cu+ ions by blue-green algae Spirulina sp.: kinetics, equilibrium and the mechanism of the process. Chemosphere 59(1):75–84
Coutaud A, Meheut M, Viers J et al (2014) Zn isotope fractionation during interaction with phototrophic biofilm. Chem Geol 390:46–60
Coutaud M, Meheut M, Glatzel P et al (2018) Small changes in Cu redox state and speciation generate large isotope fractionation during adsorption and incorporation of Cu by a phototrophic biofilm. Geochim Cosmochim Acta 220:1–18
Cui J, Xie Y, Sun T, Chen L, Zhang W (2021) Deciphering and engineering photosynthetic cyanobacteria for heavy metal bioremediation. Sci Total Environ 761:144111
De Philippis R, Vincenzini M (1998) Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol Rev 22:151–175
De Philippis R, Colica G, Micheletti E (2011) Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: Molecular basis and practical applicability of the biosorption process. Applied Microbiol Biotechnol 92:697–708
Decho AW (1990) Microbial exopolymer secretions in ocean environments: their role(s) in food webs and marine processes. Oceanogr Mar Biol Annu Rev 28:73–153
De Vuyst L, De Vin F, Vaningelgem F, Degeest B (2001) Recent developments in the biosynthesis and applications of heteropolysaccharides from lactic acid bacteria. Int Dairy J 11:687–707
Dynes JJ, Tyliszczak T, Araki T et al (2006) Speciation and quantitative mapping of metal species in microbial biofilms using scanning transmission X-ray microscopy. Environ Sci Technol 40:1556–1565
Flaibani A, Olsen Y, Painter TJ (1989) Polysaccharides in desert reclamation: compositions of exocellular proteoglycan complexes produced by filamentous blue-green and unicellular green edaphic algae. Carbohydr Res 190:235–248
Fokina AI, Ogorodnikova SY, Domracheva LI et al (2017) Cyanobacteria as test organisms and biosorbents. Eurasian Soil Sci 50:70–77
Folsom B, Popescu NA, Wood JM (1986) Comparative study of aluminum and copper transport and toxicity in an acid-tolerant freshwater green alga. Environ Sci Technol 20:616–620
Furey PC, Liess A, Lee S (2019) Substratum-associated microbiota. Water Environ Res 91:1326–1341
Garcia-Meza JV, Barrangue C, Admiraal W (2005) Biofilm formation by algae as a mechanism for surviving on mine tailings. Environ Toxicol Chem 24:573–581
Gorbushina AA, Broughton WJ (2009) Microbiology of the atmosphere-rock interface: how biological interactions and physical stresses modulate a sophisticated microbial ecosystem. Annu Rev Microbiol 63:431–450
Hill WR, Bednarek AT, Larsen IL (2000) Cadmium sorption and toxicity in autotrophic biofilms. Can J Fish Aquat Sci 57:530–537
Hou J, Yang Y, Wang P et al (2017) Effects of CeO2, CuO, and ZnO nanoparticles on physiological features of Microcystis aeruginosa and the production and composition of extracellular polymeric substances. Environ Sci Pollut Res Int 24:226–235
Husien S, Labena A, El-Belely E et al (2020) Application of Nostoc sp. for hexavalent chromium [Cr(VI)] removal: planktonic and biofilm. Int J Environ Anal Chem. https://doi.org/10.1080/03067319.2020.1773454
Ivorra N, Bremer S, Guasch H et al (2000) Differences in the sensitivity of benthic microalgae to ZN and CD regarding biofilm development and exposure history. Environ Toxicol Chem 19:1332–1339
Jones DT, Taylor WR, Thronton JM (1992) The rapid generation of mutation data matrices from protein sequences. Bioinformatics 8:275–282
Kawaguchi T, Decho AW (2002) A laboratory investigation of cyanobacterial extracellular polymeric secretions (EPS) in influencing CaCO3 polymorphism. J Cryst Growth 240:230–235
Kapoor TA, Viraraghavan T (1997) Heavy metal biosorption sites in Aspergillus niger. Biores Tech 61:221–227
Kehr J-C, Dittmann E (2015) Biosynthesis and function of extracellular glycans in cyanobacteria. Life 5:164–180
Kharwar S, Bhattacharjee S, Mishra AK (2021) Disentangling the impact of sulfur limitation on exopolysaccharide and functionality of Alr2882 by In Silico approaches in Anabaena sp. PCC 7120. Appl Biochem Biotechnol 193:1447–1468
Kiran B, Kaushik A (2008) Chromium binding capacity of Lyngbya putealis exopolysaccharides. Biochem Eng J 38:47–54
Kleerebezem M, van Kranenburg R, Tuinier R et al (1999) Exopolysaccharides produced by Lactococcus lactis: from genetic engineering to improved rheological properties? Antonie Van Leeuwenhoek 76:357–365
Klock J, Wieland A, Seifert R, Michaelis W (2007) Extracellular polymeric substances (EPS) from cyanobacterial mats: characterisation and isolation method optimisation. Marine Biol 152:1077–1085
Konhauser KO, Fisher QJ, Fyfe WS et al (1998) Authigenic mineralization and detrital clay binding by freshwater biofilms: the Brahmani river, India. Geomicrobiol J 15:209–222
Kumar S, Stecher G, Li M et al (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol Bio Evo 35:1547–1549
Lawrence JR, Swerhone GDW, Kwong YTJ (1998) Natural attenuation of aqueous metal contamination by an algal mat. Can J Microbiol 44:825–832
Liu J, Tang J, Wan J et al (2019) Functional sustainability of periphytic biofilms in organic matter and Cu(2+) removal during prolonged exposure to TiO(2) nanoparticles. Hazard Mater 370:4–12
Loustau E, Ferriol J, Koteiche S et al (2019) Physiological responses of three mono-species phototrophic biofilms exposed to copper and zinc. Environ Sci Pollut Res Int 26:35107–35120
Lu S, Wang J, Chitsaz F et al (2020) CDD/SPARCLE: the conserved domain database in 2020. Nucleic Acids Res 48(D1):D265–D268
Marra M, Palmeri A, Ballio A, Segre A, Slodki ME (1990) Structural characterisation of the exocellular polysaccharide from Cyanospira capsulate. Carbohydr Res 197:338–344
Minkina TM, Soldatov AV, Nevidomskaya DG et al (2016) New approaches to studying heavy metals in soils by X-ray absorption spectroscopy (XANES) and extractive fractionation. Geochem Int 54:197–204
Miranda A, Ramkumar N, Andriotis C et al (2017) Applications of microalgal biofilms for wastewater treatment and bioenergy production. Biotechnol Biofuels. https://doi.org/10.1186/s13068-017-0798-9
Mota R, Rossi F, Andrenelli L et al (2016) Released polysaccharides (RPS) from Cyanothece sp. CCY 0110 as biosorbent for heavy metals bioremediation: interactions between metals and RPS binding sites. Appl Microbiol Biotechnol 100:7765–7775
Nieboer E, Richardson DHS (1980) The replacement of the nondescript term ‘heavy metals’ by a biologically and chemically significant classification of metal ions. Environ Pollut B 1:3–26
Ohki K, Kanesaki Y, Suzuki N et al (2019) Physiological properties and genetic analysis related to exopolysaccharide (EPS) production in the fresh-water unicellular cyanobacterium Aphanothece sacrum (Suizenji Nori). Gen Appl Microbiol 65:39–46
Pereira S, Mota R, Vieira C, Vieira J, Tamagnini P (2015) Phylum-wide analysis of genes/proteins related to the last steps of assembly and export of extracellular polymeric substances (EPS) in cyanobacteria. Sci Rep 5:14835. https://doi.org/10.1038/srep14835
Pereira SB, Santos M, Leite JP et al (2019) The role of the tyrosine kinase Wzc (Sll0923) and the phosphatase Wzb (Slr0328) in the production of extracellular polymeric substances (EPS) by Synechocystis PCC 6803. Microbiol open 8(6):e00753
Pereira S, Zille A, Micheletti E et al (2009) Complexity of cyanobacterial exopolysaccharides: composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol Rev 33:917–941
Pereira S, Micheletti E, Zille A et al (2011) Using extracellular polymeric substances (EPS)-producing cyanobacteria for the bioremediation of heavy metals: do cations compete for the EPS functional groups and also accumulate inside the cell? Microbiol 157:451–458
Pereira SB, Sousa A, Santos M et al (2019b) Strategies to obtain designer polymers based on cyanobacterial extracellular polymeric substances (EPS). Int J Mol Sci 20:5693. https://doi.org/10.3390/ijms20225693
Raghavan PS, Potnis AA, Bhattacharyya K et al (2020) Axenic cyanobacterial (Nostoc muscorum) biofilm as a platform for Cd(II) sequestration from aqueous solutions. Algal Res 46:101778. https://doi.org/10.1016/j.algal.2019.101778
Reeves PR, Hobbs M, Valvano MA et al (1996) Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol 4:495–503
Rossi F, De Philippis R (2015) Role of cyanobacterial exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life (basel) 5:1218–1238
Santos M, Pereira SB, Flores C et al (2021) Absence of KpsM (Slr0977) impairs the secretion of extracellular polymeric substances (EPS) and impacts carbon fluxes in synechocystis sp PCC 6803. mSphere 6(1):e00003-21
Schmid J, Sieber V, Rehm B (2015) Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front Microbiol 26:496
Schopf WJ (2011) The paleobiological record of photosynthesis. Photosynth Res 107:87–101
Shukla D, Vankar PS, Srivastava SK (2012) Bioremediation of hexavalent chromium by a cyanobacterial mat. Appl Water Sci 2:245–251
Singh S, Verma E, Niveshika et al (2016) Exopolysaccharide production in Anabaena sp. PCC 7120 under different CaCl 2 regimes. Physiol Mol Biol Plants 22:557–566
Stewart WD (1980) Some aspects of structure and function in N2-fixing cyanobacteria. Annu Rev Microbio 34:497–536
Strieth D, Ulber R, Muffler K (2018) Application of phototrophic biofilms: from fundamentals to processes. Bioprocess Biosyst Eng 41:295–312
Sun X, Zhang J (2021) Bacterial exoploysaccharides: chemical structures, gene clusters and genetic engineering. Int J Biol Macromol 173:481–490
Sutherland IW (2001) Microbial polysaccharides from gram-negative bacteria. Int Dairy J 11:663–674
Tekaya N, Gammoudi I, Braiek M et al (2013) Acoustic, electrochemical and microscopic characterization of interaction of Arthrospira platensis biofilm and heavy metal ions. J Environ Chem Eng 1:609–619
Templeton A, Knowles E (2009) Microbial transformations of minerals and metals: recent advances in geomicrobiology derived from synchrotron-based X-Ray spectroscopy and X-Ray microscopy. Annu Rev Earth Planet Sci 37:367–391
Thapa S, Bharti A, Prasanna R (2017) Algal biofilms and their biotechnological significance. In: Algal green chemistry recent progress in biotechnology. pp. 285–303
Tien C-J, Chen CS (2013) Patterns of metal accumulation by natural river biofilms during their growth and seasonal succession. Arch Environ Contam Toxicol 64:605–616
Tran HT, Vu ND, Matsukawa M et al (2016) Heavy metal biosorption from aqueous solutions by algae inhabiting rice paddies in Vietnam. J Environ Chem Eng 4:2529–2535
Vu B, Chen M, Crawford RJ, Ivanova EP (2009) Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 14:2535–2554
Wynn-Williams DD (1990) Ecological aspects of antarctic microbiology. Adv Microbial Ecol 11:71–146
Xie Q, Liu N, Lin D et al (2020) The complexation with proteins in extracellular polymeric substances alleviates the toxicity of Cd (II) to Chlorella vulgaris. Environ Pollut 263((Pt A)):114102
Xu H, Yu G, Jiang H (2013) Investigation on extracellular polymeric substances from mucilaginous cyanobacterial blooms in eutrophic freshwater lakes. Chemosphere 93:75–81
Zehr JP (2011) Nitrogen fixation by marine cyanobacteria. Trends Microbiol 19:162–173
Acknowledgements
The authors thank Dr. Bhaskar Paul and Dr. J. K. Sonber, MPD, BARC for SEM-EDS analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Potnis, A.A., Raghavan, P.S. & Rajaram, H. Overview on cyanobacterial exopolysaccharides and biofilms: role in bioremediation. Rev Environ Sci Biotechnol 20, 781–794 (2021). https://doi.org/10.1007/s11157-021-09586-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-021-09586-w