Abstract
A biocidal level of hydrogen peroxide (H2O2), far beyond the natural level, is widely used to control bloom-forming cyanobacteria in freshwater. The extracellular polymeric substance of these cyanobacteria is a key factor in determining the applied H2O2 dosage. The exopolysaccharide (EPS) in the extracellular polymer shows H2O2 scavenging capability. However, the scavenging capabilities of EPSs from other cyanobacteria against biocidal levels of H2O2 as well as their protective roles against cyanobacterial cells are not well known. In this study, we used four nonbloom-forming cyanobacteria as target organisms, two with rich EPS envelopes (EPS-rich strains) and two with thin EPS envelopes (EPS-thin strains), to assess the roles of EPS. It was found that the two EPS-rich strains were much more tolerant to a high dose of exogenous H2O2 than the two EPS-thin strains. The EPSs extracted from the four strains exhibited similar but rapid H2O2 scavenging activity. Additionally, the EPSs from the EPS-rich strains could improve the tolerance of the EPS-thin strains to H2O2 stress, implying potentially nonselective protection against oxidative stress. In addition, all the cell lysates of the four strains showed H2O2 decomposition ability, with the efficiency being slightly different between the two types of strains. This study suggests that cyanobacterial EPS plays a generally crucial role against external strong oxidative stress and may provide a useful reference for the application of H2O2 in environmental management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrogen peroxide (H2O2) is the simplest peroxide and is also a powerful oxidizing agent. This molecule can occur naturally in aquatic environments from dissolved chromophoric organic materials exposed to sunlight (Clark et al. 2014). However, the major natural source of H2O2 is biologically generated by phytoplankton (Diaz and Plummer 2018). The natural H2O2 concentrations in ponds or lakes are very low (below 5.3 μM) (Ndungu et al. 2019; Weenink et al. 2021). Reactive oxygen species, including H2O2, at a low concentration usually serve as important signaling molecules (Neill et al. 2002). Cyanobacteria are a diverse group of prokaryotic microorganisms that perform oxygenic photosynthesis and widely exist in freshwater, marine, and terrestrial ecosystems. The application of exogenous H2O2 in the treatment of toxic cyanobacteria is environmentally friendly because of its decomposition to O2 and H2O. The H2O2 dosage applied is crucial for the compositional changes in phytoplankton and zooplankton communities (Weenink et al. 2015, 2021). High doses of exogenous H2O2 (e.g., 0.06 ~ 0.29 mM) have been reported to selectively eliminate toxic cyanobacteria such as Microcystis aeruginosa and Planktothrix agardhii, with a minimal impact on eukaryotic phytoplankton and zooplankton (Matthijs et al. 2012; Spoof et al. 2020; Santos et al. 2021). A biocidal level of H2O2 is supposed to trigger intracellular reactive oxygen species and result in severe oxidative stress and subsequent cell death (Drábková et al. 2007; Foo et al. 2020).
Many cyanobacteria are known to be able to synthesize extracellular polysaccharides or exopolysaccharides (EPSs) (Philippis and Vincenzini 1998). Although the functions of microbial EPS vary with the species, it serves as one of the primary mechanisms for cell survival in extreme habitats and defense against toxins, heavy metals, and antagonists (Rossi and De Philippis 2016; Bhatnagar and Bhatnagar 2019). Cyanobacterial EPS also exhibits antioxidant activity (Huang et al. 2017; Zhong et al. 2019; Yuan et al. 2023), and antioxidant activity is associated with multiple factors, such as molecular weight, monosaccharide composition, sulfate position and sulfate degree (Mohan and Thirupathi 2021). It has been reported that H2O2 levels above 0.27 mM are lethal to M. aeruginosa (Foo et al. 2020). In contrast, another study showed that Microcystis populations with a colony size below 25 μm collapsed under 0.15 mM H2O2, while a H2O2 level of 0.59 mM was needed to control larger Microcystis colonies (Liu et al. 2017a). The formation of cyanobacterial colonies is mainly facilitated by EPS (Limoli et al. 2015). The difference in the biocidal level of H2O2 may hint that it is crucial for cyanobacterial cells to have a high abundance of EPS to combat external H2O2 stress.
A previous study reported that the extracellular polymeric substance of M. aeruginosa, mainly composed of polysaccharides and proteins (approximately 3:1 ratio in weight), showed the capability to buffer H2O2 (Gao et al. 2015). To clarify the crucial role of the EPS itself in combating exogenous high-dose H2O2, we evaluated the H2O2 buffering or scavenging capability as well as the protective role of the EPSs from four nonbloom-forming cyanobacteria with different thicknesses of extracellular polymers. This study deepens our understanding of the crucial role of EPS in combating external oxidative stress and provides reference information for guiding better application of H2O2 in environmental management.
Materials and methods
Cyanobacterial strains and culture conditions
The aquatic-living culture of Nostoc flagelliforme was previously prepared from a terrestrial natural sample in our laboratory (Cui et al. 2017). Its natural sample, presenting a filamentous colony form, consists of a large number of trichomes encased by dense EPS. Nostoc punctiforme PCC 73102 (hereafter referred to as N. punctiforme) was obtained from Pia Lindberg (Department of Chemistry-Ångström, Uppsala University), which can also produce a large amount of EPS (Soule et al. 2016). The N. flagelliforme and N. punctiforme strains were statically cultured in Blue Green-11 (BG-11) medium at 25 °C under constant white LED light of 40 µmol photons m−2 s−1. Two freshwater strains, Nostoc sp. PCC 7120 (hereafter referred to as Nostoc 7120) and Synechocystis sp. PCC 6803 (hereafter referred to as Synechocystis 6803), were statically cultured in BG-11 medium at 30℃ under the same light intensity. These cultures were gently shaken three times per day. Cell cultures at the exponential growth phase, with an optical density at 730 nm (OD730) of 0.2 ~ 0.3, were used for subsequent experiments.
Analysis of the H2O2 tolerance of four cyanobacteria
The H2O2 stock solution (30%, v/v; Damao®, Tianjin Damao Chemical Reagent Factory, China) was diluted to 100 mM with sterile water for use. Cell cultures of 20 mL were used to test the tolerance to exogenous H2O2 in 50 mL conical flasks. The cell cultures were first treated with 2 mM or 4 mM H2O2 (final concentration). For a rapid estimation of the physiological status of cells, the chlorophyll fluorescence parameter Fv'/Fm' was determined, which reflects the photochemical efficiency of open photosystem II centers under a given light acclimation status (Campbell et al. 1998). At different time points (0~24 h), 2 mL of cell culture was collected and subjected to detection using a portable Plant Efficiency Analyzer (AquaPen FP110, Czech Republic) according to the manufacturer’s instructions.
For the cell growth assay, cell cultures of 20 mL were treated with various concentrations of H2O2 (0 ~ 20 mM, final concentration). Then, the H2O2-treated cell cultures were cultivated for 4 days under the abovementioned conditions. Cell growth was monitored by measuring the concentration of chlorophyll a (Chl a). Chl a was extracted and quantified as previously described (Zhao et al. 2008). Briefly, 3 mL of cell suspension was concentrated by centrifugation at 6000 rpm for 5 min (TGL-16 M, Hunan Xiangyi Laboratory Instrument Inc., China) and then extracted with 3 mL 95% ethanol overnight at 4 °C. The absorbance was measured at 664.1 and 648.6 nm using an ultraviolet‒visible spectrophotometer (Shanghai Spectrum Instruments Inc., China). The concentration of Chl a was calculated using the following equation: Chl a (μg mL−1) = 13.36 × A664.1–5.19 × A648.6 (Lichtenthaler and Buschmann 2001).
Extraction and purification of EPS from cell cultures
The extraction of EPS was conducted with hot water as previously described (Huang et al. 1998) with slight modification. In brief, 10 mL of cell culture was extracted in 95 °C hot water for 2 h, and after centrifugation, the polysaccharide in the supernatant was precipitated overnight at 4 °C with ethanol (final concentration 80%, v/v). The crude polysaccharide was collected, dissolved in distilled water, and deproteinized with Sevag reagent (n-butanol:chloroform = 1:4, v/v) (Zheng et al. 2021). The purified EPS was freeze-dried, weighed, and dissolved in distilled water for use. The polysaccharide content was determined using the phenol‒sulfuric acid method (DuBois et al. 1956). To verify that there was no contamination of proteins, the polysaccharide solution (1 mg mL−1) was scanned by an ultraviolet‒visible spectrophotometer from 200 to 400 nm.
Analysis of the H2O2 scavenging ability of the EPSs
The purified polysaccharide was added to a 5 mL H2O2 solution (2 mM) with final concentrations of 1 and 5 mg mL−1. The H2O2 solution without the polysaccharide addition (0 mg mL−1) was used for comparison. Then, the H2O2 concentration in the solution was determined at different time points (0~3 h) using the Hydrogen Peroxide Assay Kit (Beyotime Biotechnology Inc, Shanghai, China) according to the manufacturer’s instructions (Zang et al. 2018). The kit uses H2O2 to oxidize Fe2+ to Fe3+, leading to the formation of a purple color in a specific solution. Absorbance was read at 560 nm using a microplate reader (Infinite M Nano, Tecan, USA). The relative changes (%) in H2O2 concentration were calculated.
Analysis of EPS protection of cells against exogenous H2O2
The above purified polysaccharides from the N. flagelliforme and N. punctiforme cultures were added into the cell cultures (20 mL) of Synechocystis 6803 and Nostoc 7120, respectively, with a final concentration of 0.05 or 0.10 mg mL−1. The control cultures were not supplemented with the polysaccharides (0 mg mL−1). Then, the cultures were treated with 1 mM H2O2 (final concentration). Subsequently, the physiological response of the treated cells, in terms of the Fv'/Fm' value, was analyzed at different time points (0~6 h). In addition, cell growth was monitored by measuring the Chl a content at 0, 2, and 4 days.
Analysis of the H2O2 scavenging ability of cell lysates
The cell lysates of four cyanobacteria were prepared as previously described (Weenink et al. 2021). In brief, 5 mL of cell culture was centrifuged at 6000 rpm for 5 min, and the cell pellets were resuspended in 5 mL phosphate buffered solution (0.01 M, pH 7.4). The cells were further disrupted at 4 °C in a 10 mL glass homogenizer (tissue grinding tube) and were ground manually with a ground glass pestle in the tube for 10 min. The cell debris was removed by centrifugation at 4 °C for 5 min. The supernatant (cell lysate) was collected and subjected to protein detection by the Bradford method (Bradford 1976) and polysaccharide detection by the phenol‒sulfuric acid method (DuBois et al. 1956). The ratio of the polysaccharide to protein (mg mg−1) in cell lysates was calculated. Furthermore, the scavenging abilities of cell lysates against H2O2 were assayed in 5 mL H2O2 solution (1 mM) by supplementing the cell lysate containing 0.5 mg soluble proteins. The H2O2 concentration in the solution was measured, and the relative change (%) was calculated.
Statistical analysis
For Fv'/Fm' detection, six replicates were performed. Other experiments except the growth assay in response to a wide concentration range of H2O2 were performed in three replicates. For column diagrams, the statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparisons test (p < 0.05) (IBM SPSS Statistics 26).
Results
Different tolerances of four cyanobacteria to exogenous H2O2
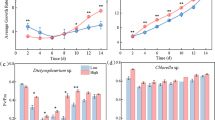
In this study, four cyanobacteria available in our laboratory were used for experiments. Three of them, Synechocystis 6803, Nostoc 7120, and N. punctiforme, are widely used model strains; the last strain, N. flagelliforme, is becoming a new model strain, particularly along with its genome being sequenced (Shang et al. 2019). Synechocystis 6803 and Nostoc 7120 strains with thin EPS envelopes are referred to here as EPS-thin strains; N. punctiforme and N. flagelliforme strains are usually present as visible particles or colonies in culture due to their abundant EPS and are referred to here as EPS-rich strains. The responses of their cells to exogenous H2O2 were first assessed in a short-term test (Fig. 1). Fv'/Fm' serves as a quick indicator of the photosynthetic performance of algae and plants under abiotic stresses (Singh and Raja Reddy 2011; She et al. 2022). In terms of Fv'/Fm', the physiological activities of the two EPS-thin strains were rapidly reduced to zero after either 2 or 4 mM H2O2 treatment. However, the physiological activities of the two EPS-rich strains were rapidly inhibited in the initial several hours and then gradually recovered; unlike the N. punctiforme strain, the activity of the N. flagelliforme strain did not recover to its original level under 4 mM H2O2 conditions.
Photophysiological activities (in terms of Fv'/Fm') of four cyanobacteria in response to exogenous H2O2. A, the treatment of cultures with 2 mM H2O2. B, the treatment of cultures with 4 mM H2O2. Data shown are the mean ± SD (n = 6). S. 6803, Synechocystis sp. PCC 6803; N. 7120, Nostoc sp. PCC 7120; N. pun, Nostoc punctiforme PCC 73102; N. fla, Nostoc flagelliforme
Furthermore, the cell growth of the four strains, in terms of Chl a content, was assessed in response to exogenous H2O2 (Fig. 2). In the relatively long-term treatment (4 days), an expanded concentration range of H2O2 was used. The Synechocystis 6803 and Nostoc 7120 strains showed similar tolerance to H2O2, and both strains were not tolerant to 2 mM H2O2. In contrast, N. punctiforme and N. flagelliforme strains were much more tolerant to H2O2. The N. punctiforme strain was not tolerant to H2O2 levels above 16 mM, while the N. flagelliforme strain was not tolerant to H2O2 levels above 4 mM. The appearances of these cultures in glass flasks after H2O2 treatment were consistent with the above observations (Supplementary Fig. S1).
The cell growth (in terms of Chl a content) of four cyanobacteria after treatment with exogenous H2O2. A and B, the growth of Synechocystis 6803 and Nostoc 7120, respectively. Cells were treated with 0 ~ 2.0 mM H2O2. C and D, the growth of N. punctiforme and N. flagelliforme, respectively. Cells were treated with 0 ~ 20 mM H2O2
The scavenging ability of the EPSs against H2O2
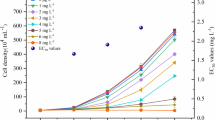
The EPS encases the cyanobacterial cell and can also facilitate the formation of the colony; thus, its protective function by scavenging H2O2 should be important. The H2O2 scavenging abilities of the EPSs from four cyanobacteria were assayed (Fig. 3). The EPSs were extracted from the four cell cultures with proteins removed. A slow natural decomposition of H2O2 was observed, but all the EPSs could greatly accelerate the decomposition rate. At 3 h, 31.1% and 63.0% of the initial H2O2 was scavenged by 1 and 5 mg mL−1 Synechocystis 6803 EPS, respectively; the scavenging rates were 34.0% and 61.3% for 1 and 5 mg mL−1 Nostoc 7120 EPS, 43.8% and 72.1% for 1 and 5 mg mL−1 N. punctiforme EPS, and 35.0% and 70.7% for 1 and 5 mg mL−1 N. flagelliforme EPS, respectively. It was also noted that rapid H2O2 decomposition by these EPSs was observed within 1 h.
The scavenging ability of four EPSs against H2O2. A, the Synechocystis 6803 EPS. B, the Nostoc 7120 EPS. C, the N. punctiforme EPS. D, the N. flagelliforme EPS. The polysaccharide concentrations in 2 mM H2O2 solution were 0, 1.0, and 5.0 mg mL.−1. The relative changes (%) in H2O2 concentration in the solutions were calculated. Data shown are the mean ± SD (n = 3)
Protection of the EPSs against exogenous H2O2 in cyanobacteria
As shown in Figs. 1 and 2, Synechocystis 6803 and Nostoc 7120 were more sensitive to H2O2. Thus, their cultures were chosen for physiological activity and cell growth assays upon H2O2 treatment after supplementation with additional EPSs. The physiological activity (in terms of Fv'/Fm') changes of Synechocystis 6803 and Nostoc 7120 cells against H2O2 were first assayed (Fig. 4). In the absence of additional EPSs (as the control), the physiological activities of the H2O2-treated Synechocystis 6803 and Nostoc 7120 cells rapidly decreased to zero or near zero within 3 h. In the presence of additional EPSs, the physiological activities of Synechocystis 6803 cells were stimulated within 1 h, slightly reduced at 3 h, and then recovered to a steady level at 6 h, while the physiological activities of Nostoc 7120 cells were rapidly reduced within 1 h and then recovered to some extent at 3 or 6 h. Furthermore, the cell growth (in terms of Chl a content) of Synechocystis 6803 and Nostoc 7120 after H2O2 treatment was assayed (Fig. 5). Cells were cultivated for 4 days following H2O2 treatment. With supplementation of additional EPSs, both Synechocystis 6803 and Nostoc 7120 cells showed better growth than their corresponding controls without EPS supplementation.
Photophysiological activities (in terms of Fv'/Fm') of two EPS-thin cyanobacteria after H2O2 treatment in the presence of additional EPSs. The concentrations of additional EPSs in the cultures were 0, 0.05, or 0.10 mg mL.−1, and then the cultures were treated with 1.0 mM H2O2. A and B, Synechocystis 6803 cells protected by N. punctiforme EPS and N. flagelliforme EPS, respectively. C and D, Nostoc 7120 cells protected by N. punctiforme EPS and N. flagelliforme EPS, respectively. Data shown are the mean ± SD (n = 6)
The cell growth (in terms of Chl a content) of two EPS-thin cyanobacteria after H2O2 treatment in the presence of additional EPSs. The concentrations of additional EPSs in the cultures were 0, 0.05, or 0.10 mg mL.−1, and the cultures were treated with 1.0 mM H2O2. A and B, Synechocystis 6803 cells protected by N. punctiforme EPS and N. flagelliforme EPS, respectively. C and D, Nostoc 7120 cells protected by N. punctiforme EPS and N. flagelliforme EPS, respectively. The letters (a, b) in each group of data suggest significant differences (P < 0.05, Tukey’s test). Data shown are the mean ± SD (n = 3)
The H2O2 scavenging ability of cell lysates from four cyanobacteria
When the H2O2 dosage exceeds the scavenging capacity of the total polysaccharide, protection from intracellular antioxidant substances should also be important. Here, we evaluated the H2O2 scavenging ability of cell lysates from four cyanobacteria (Fig. 6). The cell lysates were characterized by detecting the protein and polysaccharide contents. The content ratios of polysaccharides to proteins were evaluated (Fig. 6A). The EPS/protein ratios of N. punctiforme and N. flagelliforme cells were 2.6- and 3.0-fold higher than those (0.31) of Synechocystis 6803 and Nostoc 7120 cells, respectively. The H2O2 scavenging abilities of the cell lysates were further assayed (Fig. 6B). Overall, all the cell lysates could efficiently scavenge H2O2. Despite having a much higher EPS/protein ratio, the cell lysates of the N. punctiforme and N. flagelliforme strains only showed slightly higher scavenging activities (below 5%) than those of the Synechocystis 6803 and Nostoc 7120 strains.
The scavenging ability of cell lysates from four cyanobacteria against exogenous H2O2. A, the relative contents of EPS to protein in cell lysates. The letters (a, b, c) on the columns suggest significant differences (P < 0.05, Tukey’s test). B, the H2O2 scavenging ability of cell lysates. Each cell lysate with an equal protein level (0.5 mg) was added to the H2O2 solution. The relative change (%) in H2O2 content in the solution was calculated. Data shown are the mean ± SD (n = 3)
Discussion
Although a biocidal level of H2O2 has been widely applied in controlling boom-forming cyanobacteria, the resilience of other cyanobacteria to cope with a high dose of exogenous H2O2 is not well known. Extracellular polymeric substances have been reported to be crucial for combating various environmental stresses (Gao et al. 2015, 2019; Tamaru et al. 2005; Liu et al. 2017b). In the extracellular polymeric substance of M. aeruginosa, the protein content varied in a large range, with the EPS/protein ratio being 0.24 ~ 2.88:1 (Gao et al. 2015; Ni et al. 2017). Both polysaccharides and proteins function in H2O2 decomposition (Gao et al. 2015). However, studies of terrestrial cyanobacteria have shown that extracellular polymeric substances contain only a minor proportion of exoproteins (Stuart et al. 2016; Shirkey et al. 2000). Aquatic toxic cyanobacteria seem to have a relatively larger amount of exoproteins in their extracellular polymers. Taking the four nonbloom-forming cyanobacteria with emphasis on the polysaccharide itself, we further clarified the importance of EPS in combating exogenous high-dose H2O2.
The EPS-rich cyanobacteria were much more tolerant to exogenous H2O2 than the EPS-thin cyanobacteria (Figs. 1 and 2). Further investigation showed that the EPSs of four cyanobacteria exhibited efficient H2O2 scavenging capability (Fig. 3) and seemed to provide rapid and nonselective protection for cells (Figs. 4 and 5). However, the physiological responses of Synechocystis 6803 and Nostoc 7120 were somewhat different (Fig. 4), implying a species/strain-dependent difference in combating H2O2 stress. The H2O2 decomposition rate was positively associated with the concentration of EPS (Fig. 3), implying that the polysaccharide amount plays a critical role. The H2O2 scavenging ability of the cell lysate was also observed (Fig. 6), which might help rescue the damaged cells to recover vitality. In previous studies, the activities of polysaccharides were mostly biochemically evaluated with the sensitive substrates 2,2-diphenyl-1-picrylhydrazyl, 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid, and hydroxyl radical (Zhong et al. 2019). With N. flagelliforme as an example, cultural conditions and environmental treatment could also affect the antioxidant activity of the produced EPS (Shen et al. 2018; Yuan et al. 2023). In contrast, this study demonstrated a similar capability of four EPSs in combating a high dose of H2O2. Due to its phycosphere distribution, the amount of EPS should play a crucial role in cells against exogenous strong oxidative stress.
Although the cyanobacteria used in this study were not bloom-forming strains, they should still shed light on the understanding of environmental management. As implied in Fig. 3, the scavenging ability of the EPSs for exogenous H2O2 was rapid but also limited. In other words, the EPS amount plays a pivotal role. H2O2 sensitivity has been reported to sometimes be very different in the same strains (Liu et al. 2017a; Foo et al. 2020). The H2O2 tolerance concentrations of the N. punctiforme and Nostoc 7120 strains shown in this study were also different from those reported by Samanta et al. (Samanta et al. 2022). The reasons might be largely attributed to the EPS content per cell, colony size (still relevant to the EPS content), or ambient polysaccharide amount. In addition, the H2O2 sensitivity of cyanobacterial cells also depends on other factors, such as nutrient availability, metal ions, light intensity, heterotrophic bacteria, and eukaryotic algae (Drábková et al. 2007; Shen et al. 2011; Xu et al. 2016; Sandrini et al. 2020; Weenink et al. 2021; Moreno-Andrés et al. 2022), which increases the difficulty in precisely assessing the H2O2 dosage for environmental use. Considering the first-line role of EPS in H2O2 decomposition, the total polysaccharide amount in freshwater may be a primary consideration in determining H2O2 dosage for application.
In summary, this study suggests that cyanobacterial EPSs play a general role against exogenous high-dose H2O2 and also the polysaccharide amount plays a crucial role in protecting cells against external strong oxidative stress. This study provides a useful reference for the application of H2O2 in environmental management.
Data availability
All data supporting the findings of this study are included in the main article and its supplementary files.
References
Bhatnagar M, Bhatnagar A (2019) Diversity of polysaccharides in cyanobacteria. In: Satyanarayana T, Johri B, Das S (eds) Microbial diversity in ecosystem sustainability and biotechnological applications. Springer, Singapore, pp 447–496
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Campbell D, Hurry V, Clarke AK, Gustafsson P, Oquist G (1998) Chlorophyll fluorescence analysis of cyanobacterial photosynthesis and acclimation. Microbiol Mol Biol Rev 62:667–683
Clark CD, de Bruyn W, Jones JG (2014) Photoproduction of hydrogen peroxide in aqueous solution from model compounds for chromophoric dissolved organic matter (CDOM). Mar Pollut Bull 79:54–60
Cui L, Xu H, Zhu Z, Gao X (2017) The effects of the exopolysaccharide and growth rate on the morphogenesis of the terrestrial filamentous cyanobacterium Nostoc flagelliforme. Biol Open 6:1329–1335
Diaz JM, Plummer S (2018) Production of extracellular reactive oxygen species by phytoplankton: past and future directions. J Plankton Res 40:655–666
Drábková M, Matthijs HCP, Admiraal W, Maršálek B (2007) Selective effects of H2O2 on cyanobacterial photosynthesis. Photosynthetica 45:363–369
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28:350–356
Foo SC, Chapman IJ, Hartnell DM, Turner AD, Franklin DJ (2020) Effects of H2O2 on growth, metabolic activity and membrane integrity in three strains of Microcystis aeruginosa. Environ Sci Pollut Res 27:38916–38927
Gao L, Pan X, Zhang D, Mu S, Lee DJ, Halik U (2015) Extracellular polymeric substances buffer against the biocidal effect of H2O2 on the bloom-forming cyanobacterium Microcystis aeruginosa. Water Res 69:51–58
Gao X, Liu LT, Liu B (2019) Dryland cyanobacterial exopolysaccharides show protection against acid deposition damage. Environ Sci Pollut Res 26:24300–24304
Huang ZB, Liu YD, Paulsen BS, Klaveness D (1998) Studies on polysaccharides from three edible species of Nostoc (Cyanobacteria) with different colony morphologies: comparison of monosaccharide compositions and viscosities of polysaccharides from field colonies and suspension cultures. J Phycol 34:962–968
Huang G, Mei X, Hu J (2017) The antioxidant activities of natural polysaccharides. Curr Drug Targets 18:1296–1300
Lichtenthaler HK, Buschmann C (2001) Chlorophylls and carotenoids: Measurement and characterization by UV-vis spectroscopy. In Lichtenthaler HK (ed) Current Protocols in Food Analytical Chemistry (Suppl. 1). New York, Wiley
Limoli DH, Jones CJ, Wozniak DJ (2015) Bacterial extracellular polysaccharides in biofilm formation and function. Microbiol Spectr 3:MB-0011–2014
Liu M, Shi X, Chen C, Yu L, Sun C (2017a) Responses of Microcystis colonies of different sizes to hydrogen peroxide stress. Toxins 9:306
Liu W, Cui L, Xu H, Zhu Z, Gao X (2017b) Flexibility-rigidity coordination of the dense exopolysaccharide matrix in terrestrial cyanobacteria acclimated to periodic desiccation. Appl Environ Microbiol 83:e01619-e1717
Matthijs HC, Visser PM, Reeze B, Meeuse J, Slot PC, Wijn G, Talens R, Huisman J (2012) Selective suppression of harmful cyanobacteria in an entire lake with hydrogen peroxide. Water Res 46:1460–1472
Mohan SC, Thirupathi A (2021) Antioxidant and antibacterial activities of polysaccharides. In: Oliveira J, Radhouani H, Reis RL (eds) Polysaccharides of microbial origin. Springer, Cham, pp 1–27
Moreno-Andrés J, Rivas-Zaballos I, Acevedo-Merino A, Nebot E (2022) On the efficacy of H2O2 or S2O82− at promoting the inactivation of a consortium of cyanobacteria and bacteria in Algae-Laden Water. Microorganisms 10:735
Ndungu LK, Steele JH, Hancock TL, Bartleson RD, Milbrandt EC, Parsons ML, Urakawa H (2019) Hydrogen peroxide measurements in subtropical aquatic systems and their implications for cyanobacterial blooms. Ecol Eng 138:444–453
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5:388–395
Ni L, Li D, Rong S, Su L, Zhou W, Wang P, Wang C, Li S, Acharya K (2017) Characterization of extracellular polymeric substance (EPS) fractions produced by Microcystis aeruginosa under the stress of linoleic acid sustained-release microspheres. Environ Sci Pollut Res 24:21091–21102
Philippis RD, Vincenzini M (1998) Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol Rev 22:151–175
Rossi F, De Philippis R (2016) Exocellular polysaccharides in microalgae and cyanobacteria: chemical features, role and enzymes and genes involved in their biosynthesis. In: Borowitzka M, Beardall J, Raven J (eds) The physiology of microalgae. Developments in applied phycology. Springer, Cham, pp 565–590
Samanta L, Stensjö K, Lindblad P, Bhattacharya J (2022) Differential catalase activity and tolerance to hydrogen peroxide in the filamentous cyanobacteria Nostoc punctiforme ATCC 29133 and Anabaena sp. PCC 7120. Arch Microbiol 204:121
Sandrini G, Piel T, Xu T, White E, Qin H, Slot PC, Huisman J, Visser PM (2020) Sensitivity to hydrogen peroxide of the bloom-forming cyanobacterium Microcystis PCC 7806 depends on nutrient availability. Harmful Algae 99:101916
Santos AA, Guedes DO, Barros MUG, Oliveira S, Pacheco ABF, Azevedo SMFO, Magalhães VF, Pestana CJ, Edwards C, Lawton LA, Capelo-Neto J (2021) Effect of hydrogen peroxide on natural phytoplankton and bacterioplankton in a drinking water reservoir: Mesocosm-scale study. Water Res 197:117069
Shang JL, Chen M, Hou S, Li T, Yang YW, Li Q, Jiang HB, Dai GZ, Zhang ZC, Hess WR, Qiu BS (2019) Genomic and transcriptomic insights into the survival of the subaerial cyanobacterium Nostoc flagelliforme in arid and exposed habitats. Environ Microbiol 21:845–863
She Y, Gao X, Jing X, Wang J, Dong Y, Cui J, Xue H, Li Z, Zhu D (2022) Effects of nitrogen source and NaCl stress on oil production in Vischeria sp. WL1 (Eustigmatophyceae) isolated from dryland biological soil crusts in China. J Appl Phycol 34:1281–1291
Shen H, Niu YA, Xie P, Tao M, Yang X (2011) Morphological and physiological changes in Microcystis aeruginosa as a result of interactions with heterotrophic bacteria. Freshwater Biol 56:1065–1080
Shen S, Jia S, Wu Y, Yan R, Lin Y, Zhao D, Han P (2018) Effect of culture conditions on the physicochemical properties and antioxidant activities of polysaccharides from Nostoc flagelliforme. Carbohydr Polym 198:426–433
Shirkey B, Kovarcik DP, Wright DJ, Wilmoth G, Prickett TF, Helm RF, Gregory EM, Potts M (2000) Active Fe-containing superoxide dismutase and abundant sodF mRNA in Nostoc commune (Cyanobacteria) after years of desiccation. J Bacteriol 182:189–197
Singh SK, Raja Reddy K (2011) Regulation of photosynthesis, fluorescence, stomatal conductance and water-use efficiency of cowpea (Vigna unguiculata [L.] Walp.) under drought. J Photochem Photobiol B 105:40–50
Soule T, Shipe D, Lothamer J (2016) Extracellular polysaccharide production in a scytonemin-deficient mutant of Nostoc punctiforme under UVA and oxidative stress. Curr Microbiol 73:455–462
Spoof L, Jaakkola S, Važić T, Häggqvist K, Kirkkala T, Ventelä AM, Kirkkala T, Svirčev Z, Meriluoto J (2020) Elimination of cyanobacteria and microcystins in irrigation water-effects of hydrogen peroxide treatment. Environ Sci Pollut Res 27:8638–8652
Stuart RK, Mayali X, Lee JZ, Craig Everroad R, Hwang M, Bebout BM, Weber PK, Pett-Ridge J, Thelen MP (2016) Cyanobacterial reuse of extracellular organic carbon in microbial mats. ISME J 10:1240–1251
Tamaru Y, Takani Y, Yoshida T, Sakamoto T (2005) Crucial role of extracellular polysaccharides in desiccation and freezing tolerance in the terrestrial cyanobacterium Nostoc commune. Appl Environ Microbiol 71:7327–7333
Weenink EFJ, Luimstra VM, Schuurmans JM, Van Herk MJ, Visser PM, Matthijs HCP (2015) Combatting cyanobacteria with hydrogen peroxide: a laboratory study on the consequences for phytoplankton community and diversity. Front Microbiol 6:714
Weenink EFJ, Matthijs HCP, Schuurmans JM, Piel T, van Herk MJ, Sigon CAM, Visser PM, Huisman J (2021) Interspecific protection against oxidative stress: green algae protect harmful cyanobacteria against hydrogen peroxide. Environ Microbiol 23:2404–2419
Xu F, Zhu W, Xiao M, Li M (2016) Interspecific variation in extracellular polysaccharide content and colony formation of Microcystis spp. cultured under different light intensities and temperatures. J Appl Phycol 28:1533–1541
Yuan XL, Gao X, Zheng T, Wang J, Dong YB, Xue HD (2023) Carbon nanomaterial-treated cell cultures of Nostoc flagelliforme produce exopolysaccharides with ameliorative physio-chemical properties. Int J Biol Macromol 227:726–735
Zang Y, Liu J, Tang XX, Zhou B (2018) Description of a Zostera marina catalase gene involved in responses to temperature stress. PeerJ 6:e4532
Zhao XM, Bi YH, Chen L, Hu S, Hu ZY (2008) Responses of photosynthetic activity in the drought-tolerant cyanobacterium, Nostoc flagelliforme to rehydration at different temperature. J Arid Environ 72:370–377
Zheng L, Ma Y, Zhang Y, Meng Q, Yang J, Wang B, Liu Q, Cai L, Gong W, Yang Y, Shi J (2021) Increased antioxidant activity and improved structural characterization of sulfuric acid-treated stepwise degraded polysaccharides from Pholiota nameko PN-01. Int J Biol Macromol 166:1220–11229
Zhong Q, Wei B, Wang S, Ke S, Chen J, Zhang H, Wang H (2019) The antioxidant activity of polysaccharides derived from marine organisms: an overview. Mar Drugs 17:674
Funding
This work has been supported by the Natural Science Basic Research Program of Shaanxi Province, China (No. 2020JZ-51) and the Shaanxi Province Qin Chuangyuan “Scientist + Engineer” Team Construction Project (No. 2023KXJ-206).
Author information
Authors and Affiliations
Contributions
Xiang Gao: Conceptualization, Supervision, Data curation, Writing-original draft and review & editing. Tao Zheng: Investigation, Methodology, Visualization, Writing-original draft. Xiaolong Yuan: Methodology, Investigation, Validation. Yibei Dong and Chang Liu: Investigation.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publish
Not applicable.
Competing interests
The authors declare no conflict of interest.
Additional information
Responsible Editor: Diane Purchase
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gao, X., Zheng, T., Yuan, X. et al. Biocidal H2O2 treatment emphasizes the crucial role of cyanobacterial extracellular polysaccharides against external strong oxidative stress. Environ Sci Pollut Res 30, 60654–60662 (2023). https://doi.org/10.1007/s11356-023-26840-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-023-26840-6