Abstract
The hypothalamo-pituitary-adrenal (HPA) axis is one of the main components of the stress system. Maintenance of normal physiological events, which include stress responses to internal or external stimuli in the body, depends on appropriate HPA axis function. In the case of severe cortisol deficiency, especially when there is a triggering factor, the patient may develop a life-threatening adrenal crisis which may result in death unless early diagnosis and adequate treatment are carried out. The maintenance of normal physiology and survival depend upon a sufficient level of cortisol in the circulation. Life-long glucocorticoid replacement therapy, in most cases meeting but not exceeding the need of the patient, is essential for normal life expectancy and maintenance of the quality of life. To enable this, the initial step should be the correct diagnosis of adrenal insufficiency (AI) which requires careful evaluation of the HPA axis, a highly dynamic endocrine system. The diagnosis of AI in patients with frank manifestations is not challenging. These patients do not need dynamic tests, and basal cortisol is usually enough to give a correct diagnosis. However, most cases of secondary adrenal insufficiency (SAI) take place in a gray zone when clinical manifestations are mild. In this situation, more complicated methods that can simulate the response of the HPA axis to a major stress are required. Numerous studies in the assessment of HPA axis have been published in the world literature. In this review, the tests used in the diagnosis of secondary AI or in the investigation of suspected HPA axis insufficiency are discussed in detail, and in the light of this, various recommendations are made.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The hypothalamo-pituitary-adrenal (HPA) axis is one of the main components of the stress system. HPA axis dysfunction may be due to disorders involving the hypothalamus, pituitary or adrenals. As a result of cortisol deficiency, the body becomes unable to cope with stressful events which include infections, trauma, surgery or severe emotional stress. The diagnosis of adrenal insufficiency (AI) depends on a combination of a medical history, physical examination, and hormonal investigations including basal and dynamic tests. The diagnosis of secondary adrenal insufficiency (SAI) in patients with frank manifestations due to SAI is not challenging; these patients do not need dynamic tests and morning basal cortisol is usually enough to provide a correct diagnosis of SAI. Numerous studies in the assessment of HPA axis have been published in the literature since Thomas Addison’s classical report on AI in 1855 [1, 2].
2 Physiology of the HPA axis
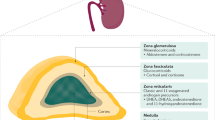
The neurons in the paraventricular nucleus (PVN) express corticotrophin-releasing hormone (CRH) and its co-secretagogue arginine vasopressin (AVP), and other neuropeptides that modulate the HPA axis [3]. The pituitary gland - which is designated as the ‘master gland of the endocrine system’ - is located under the hypothalamus and is connected to the hypothalamus via the pituitary stalk (infundibulum). Corticotroph cells in the anterior pituitary gland synthesise and secrete ACTH which stimulates the secretion of cortisol from the adrenal glands in response to stress, as well as increasing adrenal androgens [4]. In addition, plasma binding proteins, sex steroids, and the autonomic nervous system may all play some role in the regulation of the HPA axis.
Cortisol negatively regulates CRH, AVP and ACTH synthesis and secretion [5]. The negative feedback effect of cortisol occurs via mineralocorticoid receptors (MRs) and/or glucocorticoid receptors (GRs) located at multiple sites in the brain and in the pituitary, with rapid and slow both genomic and non-genomic effects [6].
3 Investigation of the HPA axis
3.1 Medical history and physical examination
Clinical judgment is still the most valuable tool in the initial suspicion of AI, but not enough on its own as a tool in the diagnosis of AI. Nevertheless, it is still important in spite of remarkable technical progress in the measurement of basal cortisol, ACTH and the other relevant hormones, together with a variety of hormonal stimulation tests. In mild to moderate cases, postural hypotension and tachycardia, fatigue, anorexia, weight loss, decreased libido, hypoglycaemia, and eosinophilia may be seen. Vascular collapse, which may be fatal, can be seen in patients in adrenal crisis since cortisol is essential for the maintenance of peripheral vascular tone. Unlike primary adrenal insufficiency (PAI), SAI demonstrates a less severe clinical presentation due to the sparing of aldosterone secretion, and additionally lacks the findings of hyperpigmentation and marked hyperkalaemia. AI is not an ‘all-or-none’ phenomenon [7], and thus the presence of pituitary or hypothalamic pathology requires investigation of the HPA axis, even if there are no obvious symptoms of AI. On the other hand, the diagnosis of SAI based on clinical findings alone is not sufficient in itself to start glucocorticoid replacement therapy (GRT) without hormonal confirmation, unless acute adrenal failure is apparently present when more detailed investigation may need to be delayed. SAI may be associated with the deficiency(s) of other anterior pituitary hormone(s) or may be isolated, the latter being less frequent except in hypophysitis.
3.2 Hormonal investigation
The final result of HPA axis insufficiency is the decreased secretion of cortisol from the adrenal glands, whatever the cause. All the tests used in the investigation of HPA axis depend upon the estimation of cortisol since measurement of ACTH levels after stimulation of the HPA axis have not been shown to have any additional benefit [8]. More or less, all the tests assess the integrity of the HPA axis as a whole, but none of them is able to identify the precise location of the abnormality responsible for reduced cortisol production.
When evaluating the HPA axis, the ‘sufficient’ cortisol response to dynamic tests is based on the cortisol responses given by healthy people when stressed. The study by Plumpton and Besser was probably the first one assessing the HPA axis as a response to major surgery [9]. Approximately 50 years later, a similar study using more modern highly specific cortisol assays revealed a positive correlation between the cortisol responses measured and severity of the operation [10]. The peak cortisol responses was 30%–38% lower than the previous study, presumably due to current highly-developed surgical and anaesthetic practices, as well changes in cortisol assays. A recent study also confirmed that serum cortisol levels during surgery are affected by the type of surgery, age, sex, surgical and anaesthetic technique [11]. It should therefore be emphasised that a normal response to a stimulation test may simply be a response within the normal range of a control population, but this does not necessarily equate with levels adequate for severe stressors such as surgery or trauma.
3.2.1 Methodological problems
The definition of the normal cortisol cut-off response to ACTH stimulation test may depend upon the method used for the measurement of cortisol [12]. In a recent study, the Elecsys Cortisol assay and Elecsys Cortisol II assay were compared in samples from dynamic tests. Cortisol values measured by new assay were found to be about 30% lower, and they suggested 374 nmol/L (13.5 μg/dl) as the revised cut-off in place of 500 nmol/L (18 μg/dl) for the insulin tolerance test [13]. Mean cortisol concentration measured by GC-MS was found to be significantly lower than cortisol measured by immunoassay in males for all five assays, and in females who were not on the oral contraceptive pill (OCP), for all but the Architect and Access assays. Post-ACTH cortisol levels measured by immunoassays were significantly higher in males and non-OCP females when compared to those in whom cortisol was measured by GC-MS [14]. Immunological tests including Immulite and Roche platforms were found to have similar results to LC-MS/MS despite higher median cortisol levels [15]. The assays used in the measurement of cortisol are of thus of major importance.
4 Basal hormone levels
4.1 Basal cortisol
Measurement of basal cortisol is generally the first and the easiest step to investigate the HPA axis. The best time for the measurement was suggested as before 09.00 h with a cut-off level of 375 nmol/L (13.5 μg/dl), as the median cortisol level decreases by approximately 30 nmol/L (1.08 μg/dl) per hour between 07.00 h and 12.00 h [16]. Various cut-off levels for basal serum cortisol have been suggested depending on the study population and reference test used. An upper basal cortisol cut-off level of 285 nmol/l (9.8 μg/dl) and a lower cut-off level of 98 nmol/l (3 μg/dl) were shown to reduce the number of subjects requiring stimulation tests [17]. Preoperative basal cortisol levels of <6 μg/dl and > 18 μg/dl could predict insufficient and sufficient cortisol responses respectively to an insulin tolerance test (ITT) in patients undergoing pituitary surgery. In the post-operative first month, a basal cortisol level < 7 μg/dl was able to predict an insufficient cortisol response to the ITT with great accuracy [18]. Basal cortisol was also tested for its ability to obviate the need for high-dose ACTH stimulation test (HDST) (with 250 μg Synacthen/Cosyntropin = ACTH (1, 24)) i.m. in patients with pituitary disorders. The authors accepted ˃550 nmol/l (20 μg/dl) as an adequate cortisol response to Synacthen and concluded that dynamic testing is not necessary if the basal cortisol is ˂100 nmol/l (3 μg/dl) or ˃330 nmol/l (12 μg/dl), while the HDST is required in patients with basal cortisol levels between 100 and 214 nmol/l (3–7.7 μg/dl) and suggested in patients with a basal cortisol between 214 and 330 nmol/l (7.7–12 μg/dl) when clinical risk factors including previous cranial radiotherapy or deficiencies of other anterior pituitary hormones are present [19]. Strong linear correlations were detected between basal serum cortisol levels and cortisol responses to the HDST at 30 min and 60 min during the test [20].
In a retrospective observational study including 346 patients, it was suggested that a basal morning serum cortisol value ≥400 nmol/l (14.4 μg/dl) could predict a normal cortisol response to the i.m. HDST [21]. Recently, in 416 patients, a basal cortisol level < 85 nmol/L (3 μg/dl) (specificity 99.7%) and > 350 nmol/L (12.6 μg/dl) (sensitivity 98.9%) were able to eliminate the need for 30% of low-dose Synacthen tests (LDST) with 1 μg i.v. synthetic ACTH [22]. The HDST could also have been avoided in a significant number of patients by utilising basal cortisol levels [23].
Assessment of the HPA axis is crucially important in the determination of recovery of adrenal function in patients with pituitary disease associated with SAI. Patients who had an initial basal cortisol level ˃175 nmol/L (6.3 μg/dl) were shown to have an almost 50% chance of recovery of HPA axis function [24]. A basal cortisol level of ≥300 nmol/l (10.8 μg/dl) measured on the post-operative second day after pituitary surgery was suggested to be a predictive marker of normal HPA axis according to the LDST 3 months after surgery in a study carried out in 83 patients. Peak cortisol level, either at 20 or 30 min, of ≥500 nmol/l (18 μg/dl) after the LDST, was accepted as an adequate cortisol response [25]. Glucocorticoid use is the most common cause of SAI due to a supressed HPA axis, and a timely decision on recovery of the HPA axis is essential to avoid unnecessary GC administration: in such patients; an early morning cortisol of ≥8.8 μg/dl was reported to be an independent predictor of adrenocortical recovery [26].
In general, then, basal cortisol is a good indicator of HPA axis in most of the patients with suspected AI, limiting the number of patients requiring dynamic tests, and is an appropriate first test in such patients.
4.2 Free cortisol
Serum cortisol levels may be affected by the levels of cortisol binding globulin (CBG). Free cortisol measurement may be of value in case of conditions affecting CBG levels. Peechakara et al. compared the serum total and free cortisol responses to different doses of Synacthen (LDST i.v., medium-dose ACTH stimulation test (MDST i.m. 25 μg) and the HDST i.m. in 10 patients with hypothalamo-pituitary disease and in 12 healthy control subjects. It was found that a serum free cortisol cut-off of 0.9 μg/dl at 30 min could be used as pass criterion during the LDST i.v., MDST and HDST i.m. and 1.3 μg/dl at 60 min during HDST [27]. However, it would be more useful if all the tests would have been performed in a more standardised pattern in that study. In another study, a cut-off level of 0.9 μg/dl for a peak serum free cortisol response to the HDST was suggested to be used to make a differential diagnosis between patients with AI and healthy subjects [28]. The free cortisol levels during the ACTH stimulation test can be especially helpful in females on oestrogen therapy such as the OCP, in patients who present with apparent clinical manifestations of AI but have normal total cortisol levels, or in patients who are suspected to have CBG abnormalities such as serious illness [29]. However, currently the routine measurement of serum free cortisol level is not practical and very limited due to its complicated analysis [30].
4.3 Basal ACTH
Determination of plasma ACTH is important for the differential diagnosis of AI. A plasma ACTH level > 300 ng/L (66 pmol/L) stimulates cortisol synthesis to its maximum [31]. A cortisol level < 140 nmol/L (5 μg/dL) with an elevated ACTH in a patient is highly predictive of PAI [31,32,33,34]. The elevation in ACTH generally precedes hypocortisolaemia in PAI. However, it is not easy to set a specific cut-off level for ACTH because of the analytical bias in ACTH assays [35, 36]. The Endocrine Society recommends two-times the upper limit of the plasma ACTH reference interval as a cut-off for the diagnosis of PAI [37]. Clinical findings, and the use of steroid and non-steroid medications, should be taken into account when interpreting the levels of ACTH [38]. Another problem with the measurement of ACTH is related to its sampling procedure, as the sample needs to be taken into a chilled tube and placed on ice immediately; if not, degradation of this peptide hormone can occur and lead to inappropriately low ACTH levels [39, 40]. Thus, plasma ACTH levels are important for the differential diagnosis of AI, but the accurate estimation of ACTH can be difficult.
4.4 Basal DHEAS
The secretion of DHEAS from the adrenal gland, which is the most abundant steroid hormone in the circulation, is controlled by ACTH. Almost all DHEAS in the circulation is secreted by the adrenal glands, with only a slight contribution from the testes in men. DHEAS has a long half-life and lacks diurnal variation, making the measurement possible at any time of the day with widely-available assays [41]. However, age and gender specific ranges for DHEAS are essential for a proper evaluation, since levels decrease with age and are lower in women [42].
Some authors have suggested the use of DHEAS as a marker for the assesment of HPA axis integrity in patients with a pituitary tumour [43, 44]. Patients with SAI were found to have lower basal and ACTH-stimulated cortisol, DHEA, and DHEAS levels, and a higher baseline cortisol to DHEA molar ratio which increased further after the LDST [44]. Adrenal androgen secretion is impaired before loss of cortisol secretion in patients with impaired HPA axis function [45, 46]. This may be due to stimulation of DHEA secretion with intra-adrenal cortisol in a dose-dependent fashion, presumably via inhibition of 3β-hydroxysteroid dehydrogenase type II activity [47]. As a result, a small reduction of intra-adrenal cortisol concentration in early AI may lead to decreased DHEA secretion from the adrenal glands. Teleologically, this may be regarded as a means whereby DHEAS is sacrificed to the more significant cortisol.
In patients younger than 30 years of age, a Z-score of (calculated using age- and gender-specific references) less than −2.0 for DHEAS showed 100% sensitivity and specificity in terms of estimating HPA axis dysfunction, but was less useful in older patients [48]. Normal age- and gender-specific DHEAS levels predicted a sufficient cortisol response to the LDST with a sensitivity of 87.1% and a specificity of 86.7%. The authors suggested a DHEAS ratio (DHEAS divided by the lower limit of respective reference range of the substrates) of more than 1.78 as a minimum in order to identify intact HPA integrity [49]. Thus, DHEAS levels can be used only as an additional or adjunctive tool in the assessment of HPA axis function.
5 Dynamic tests
5.1 Insulin tolerance test (Table 1)
The use of the ITT as a test in the investigation of pituitary disorders goes back 80 years ago. The studies using the ITT are summarized in Table 1 [8, 9, 17, 18, 50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76]. The ITT had been found valuable in the differential diagnosis of panhypopopituitarism and anorexia nervosa or primary hypothyroidism by demonstrating unresponsiveness to hypoglycaemia [77]. Although 0.15 U/kg of insulin was used in earlier studies [50], an insulin dose of 0.1 U/kg is usually sufficient to achieve adequate hypoglycaemia (glucose level ˂40 mg/dl, 2.2 mmol/L, in venous blood) during the ITT. If the fasting blood glucose is ≥100 mg/dl, then a second dose of insulin is usually needed [78]. An optimised calculation method for insulin dosage in place of conventional method in the ITT has also been proposed [79]. Recently, we have shown that symptomatic hypoglycaemia, even without a decrease of glucose to <40 mg/dl during the ITT, is also sufficient to stimulate the HPA and GH axes in suspected patients [76]. The other causes of inadequate hypoglycaemia during the ITT can be active or persistent acromegaly or Cushing’s disease as both are associated with insulin resistance, and with such diagnoses higher doses of insulin have been recommended (0.2–0.3 U/kg).
The reproducibility of the ITT has not been extensively investigated in healthy subjects despite its general acceptance as a gold-standard test in the investigation of the HPA axis as well as the GH axis for many years. Although less variable than GH responses, peak cortisol responses to the ITT do vary even within the same subject, and the reproducibility of the ITT in the assessment of the HPA axis is not perfect [55].
Normally, serum cortisol responses to the ITT are measured basally and at 30, 60, 90 and 120 min after achievement of hypoglycaemia. A significant positive correlation has also been detected between the peak ACTH and the peak cortisol concentrations during the ITT. However, as noted above, it is not possible to define an acceptable cut-off value for the peak plasma ACTH responses to ITT because of wide variations in levels [8].
On the other hand, the ITT is not free of side-effects, some of which may be serious. The test itself can be unpleasant for some patients, is time-consuming, and needs to be performed in a specialised centre by experienced medical staff [80]. The contraindications to the ITT consist of coronary artery disease, ischaemic cerebral disease, and seizure disorders. In one large study including 220 patients who had an ITT, 2% had adverse events; one of the patients developed chest pain and finally had coronary artery by-pass surgery, while four patients developed blackouts. The depth of hypoglycaemia was lower than the targeted level for a prolonged period in a significant number of the patients. Nevertheless, overall the adverse events were few and they were not related to the depth of hypoglycaemia [81]. Glucose infusion may shorten the duration of hypoglycaemia without changing the stimulated cortisol levels. In one study a glucose infusion was shown to reduce plasma adrenaline/epinephrine levels and ameliorate patient discomfort after hypoglycaemia, but this procedure of ‘reversal’ is not in uniform use [82]. In general, the ITT remains the gold-standard test which is usually well tolerated and provides accurate information on both cortisol and GH levels, but should always be carried out by experienced personnel.
The cut-offs for a sufficient peak cortisol responses to ITT are summarised in Table 1. The rise in plasma cortisol including either 7 μg/dl above the baseline or doubling the basal cortisol concentration was associated with very high false positive and negative rates, and changes in cortisol are not recommended as the criterion to predict HPA axis integrity [56]. The evolution of other dynamic tests after the ITT has led to many attempts to ascertain normative values for the ITT in order to compare to newer dynamic test procedures.
Although the ITT has been accepted as the gold-standard test to investigate the HPA axis for decades, and has the advantage of also evaluating the GH axis, the unpleasant effects of hypoglycaemia, some contraindications, uncertain reproducibility and the requirement of experienced medical staff, limit the use of ITT in daily clinical practice. On the other hand, new cut-off levels for the peak cortisol responses to ITT are also needed with newer assays (see above).
5.2 ACTH stimulation test (Table 2)
Synthetic ACTH stimulates the adrenal gland directly and give rise to the synthesis of both glucocorticoids and sex steroids. The theory on which the test is based is that in PAI there will be little or no response in a damaged adrenal, while a lack of hypothalamo-pituitary function will lead over time to adrenal atrophy. A decreased cortisol response to ACTH cannot discriminate primary or secondary AI due to resultant atrophy of the adrenal glands in SAI. The ACTH stimulation test (also called the short Synacthen test (SST), Cosyntropin stimulation test, rapid ACTH stimulation test) has been commonly used as an alternative test to the ITT [83]. In the early days purified i.m. ACTH was used and uric acid/creatinine ratio in urine and eosinophil count as a response to ACTH were measured instead of cortisol, but cortisol is now measured directly. The ACTH stimulation test may be performed as an HDST or LDST. Long-acting i.m. depot Synacthen, which is commonly available worldwide, has been suggested as a reliable and safe test in the investigation HPA axis insufficiency where short-acting Synacthen is unavailable as the responses over the first 60 min are identical [84]. The studies using the ACTH stimulation test are summarized in Table 2 [12, 15, 20, 31, 85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102].
5.2.1 High dose ACTH stimulation test (HDST)
The high dose ACTH stimulation test has been used in the diagnosis of AI for more than 50 years around the world. In one of the first studies, the HDST i.v. was investigated in healthy subjects, patients with Addison’s disease and SAI. In all control subjects, peak cortisol values were found to be equal or higher than 18 μg/dl [103]. The peak cortisol response to the HDST was found to be significantly correlated with the peak cortisol response to the ITT in the studies that followed [88], although another study revealed a clear discrepancy between ITT and HDST in terms of cortisol responses [54].
The optimal time for measurement of cortisol response to an HDST is generally at 30 min. However, a recent study has shown that both 30 and 60 min cortisol responses have an adequate index of consistency, but the same is not true in terms of absolute agreement, particularly when a SAI is suspected: 10% of patients with a subnormal response at 30 min had a normal response in 60 min in cases of SAI [20]. Except in a few early studies which used the i.m. route, HDST is usually performed by i.v injection [103] (Table 2).
When the HDST was compared with the ITT, the necessity for the presence of both criteria for the hypoglycaemia: i) a glucose level of <2.2 mmol/L and ii) neuroglycopenic symptoms such as sweating were thought to be the reason for similar results obtained from both tests [88]. The ITT and i.m. HDST were compared in 166 consecutive patients with suspected SAI compared to the results obtained from healthy volunteers. The HDST 30 min cortisol response of 600 nmol/l (21.6 μg/dl) could be used in place of the ITT to rule out the possibility of HPA axis insufficiency in 94.4% of the patients [53].
In a retrospective study including 399 patients, the cortisol level was measured at baseline, 30 and 60 min in the HDST. A peak cortisol level ≥ 20 μg/dl was suggested as a sufficient single criterion for normal adrenocortical function [104]. The peak cortisol response in the ITT has also been accepted the criterion for a normal ACTH stimulation test in most of the studies published so far in the literature. However, recent data suggest that each test should have its own cut-off value [68, 69, 105]. Cho et al. proposed cut-off values of 15, 18, 20 μg/dl for the ITT, LDST and HDST respectively in normal subjects and 16 μg/dl for the LDST, 17 or 18 μg/dl for HDST in patients with pituitary disorders, in order to determine HPA axis sufficiency [68]. The HDST can also be used to determine adrenocortical recovery with increased diagnostic accuracy when combined with a subsequent random morning cortisol [101].
The cost of Synacthen shows great variation across countries, and is not universally available. It has also varied over time as some commercial operations have taken up the licences for its manufacture and massively increased these costs without any clear clinical reason. Ben-Shlomo et al. implemented an electronic medical record (EMR) system protocol, and by using this system they reduced the number of wasted tests and maximised staff time and resources [106]. Long-acting porcine sequence ACTH known as Acton Prolongatum® was suggested to be used instead of Cosyntropin in the diagnosis of adrenal insufficiency when Cosyntropin is unavailable [107]. Very recently, the nasal administration of tetracosactide was shown to generate similar serum cortisol response, while measurement of salivary cortisol or cortisone provided a non-invasive test [108]. However, where the drug is available, the HDST is a simple and relatively accurate way to assess HPA function, and as long as it is not used in acute situations (as immediately post-pituitary surgery as the adrenal requires time to atrophy), it will remain in extensive use.
5.2.2 Low dose ACTH stimulation test (LDST)
The lower sensitivity of the HDST in detecting especially mild SAI resulted in seeking for different doses of ACTH stimulation test. Dickstein et al. reported similar cortisol responses to HDST in both healthy volunteers and in patients on long-term steroid treatment for 2–4 yr. In contrast, the cortisol response to the LDST in patients was significantly lower than in normal subjects at 30 min [90]. The unique advantage of the LDST is its capability to reveal a mild SAI overlooked by more powerful tests such as the ITT and HDST. The sensitivity and specifity of the LDST is 71% and 93% respectively when compared to the ITT [57]. It is also safe and inexpensive [57, 58]. Abdu et al. suggested that the LDST could not only replace the HDST, but also the ITT, for the initial investigation of the HPA axis in patients with pituitary disease [59]. The LDST was found to be more concordant with the ITT than the HDST in the investigation of the HPA axis immediately after pituitary surgery [110]. However, performing any test straight after surgery in clinical practice is debatable [111].
The LDST with a 30 min sampling time point may be used instead of the HDST in the diagnosis of especially mild SAI since cortisol measured at 30 min during the LDST is similar to that obtained in the HDST in healthy subjects [112].
The optimal time points of sampling for cortisol were suggested to be at baseline and 30 and 40 min after the i.v. ACTH administration [113]. Recently, 103 patients with suspected PAI and SAI were evaluated with the LDST and a cut-off value of 402 nmol/L (14.5 μg/dl) was suggested with 100% sensitivity and 93.9% specifity in predicting normal HPA axis function, according to clinical follow-up and HDST in suspicious cases [99]. However, they did not perform a confirmatory test in all patients.
In healthy adults with an age range of 25–69 years, and a normal cortisol response to the HDST, the lowest peak cortisol response obtained after the LDST was found to be 12.5 μg/dl [94]. Gonzalbez et al. reported similar serum cortisol responses to the HDST, LDST and the ITT in healthy volunteers [60]. Although the LDST and the HDST resulted in statistically similar cortisol responses in suspected AI, the levels were slightly higher in the HDST [93]. Nevertheless, this study did not show any clear advantage of the LDST over the HDST.
The widespread use of salivary cortisol (SC) in recent years has led to studies on their use during the LDST. Peak SC and cortisone to LDST which were measured by LC-MS/MS have been suggested to perform well in the diagnosis of AI with similar accuracy [97]. However, since the data are scanty, it is too early to recommend SC measurement after ACTH stimulation test instead of serum cortisol, but the concept is promising [114].
One of the controversial issues regarding the LDST is related to the plastic intravenous line which may be responsible for subnormal cortisol response because of Cosyntropin adherence to the tube and insufficient delivery of the dosage [115, 116]. In healthy adults, a 2.5 cm plastic tube does not alter delivered Cosyntropin dosage or cortisol stimulation compared to direct i.v. Cosyntropin administration [117]. Another concern with the LDST is the stability of Cosyntropin after dilution. However, it has been reported that 1 μg ACTH (1–24 ACTH) was stable when refrigerated at 4C in saline in plastic tubes for 4 months [90].
In conclusion, the LDST with appropriate cut-off levels may also be used in the diagnosis of SAI particularly when a mild or recent-onset SAI is suspected in place of the HDST.
5.3 Glucagon stimulation test (GST) (Table 3)
Studies using the GST are reviewed in Table 3 [66, 69, 94, 105, 118,119,120,121,122,123,124,125,126,127,128,129]. Glucagon is able to stimulate both GH and HPA axes when administered either subcutaneously or intramuscularly [130]. Although the cortisol response to glucagon s.c. was assumed to be ACTH-dependent, it is not known for certain whether glucagon stimulates the synthesis and/or secretion of ACTH directly from the pituitary. The GST is characterised by significantly increased copeptin, which correlates well with ACTH levels [127]. Glucagon was not able to stimulate ACTH and cortisol secretion when it was given i.v. On the other hand, i.m. glucagon was shown to be as effective as i.v hCRH and more effective than vasopressin on ACTH and cortisol secretion in the same study. It seems that the stimulatory effect of i.m. glucagon on ACTH secretion from corticotroph cells is not selectively mediated by endogenous hCRH or AVP, which have only additive effects on ACTH secretion [131, 132]. Ghrelin has also been assessed as to whether it was associated with the stimulatory effect of glucagon or hypoglycaemia on HPA and GH axes. However, it was shown that ghrelin does not mediate the ACTH or GH responses to either the GST or the ITT [133]. The fall in blood glucose level was also found not to be responsible for cortisol release either [122]. While some have suggested that it is the nausea associated with glucagon injection that is a non-specific stressor, the possible underlying mechanisms regarding the stimulatory effects of glucagon on cortisol and GH curently remain unclear.
Forty five years ago, a prospective study including normal subjects and patients with pituitary tumours, showed a good agreement between the ITT and the subcutaneous glucagon (1 mg) test in terms of GH and cortisol responses [118]. The GST was shown to be reproducible and suggested as the test preferable to ITT as a screening procedure because of its safety, reliability and ease of use [120]. Because of the great variability in cortisol responses and increased unpleasant side effects, i.m. GST was recommended instead of an s.c. test [130], but the s.c. test remains in most use.
The GST is a long test which lasts 4 h with 7 cortisol levels are obtained at baseline, 90th, 120th, 150th, 180th, 210th and 240th minutes. The peak cortisol response was reported to be obtained at 180th min by 1 mg sc glucagon [118]. The peak cortisol responses to GST are obtained at 150–180 min in majority of volunteers [94, 105, 122]. The duration of the GST was shown to be reliably shortened to 3 h by including fixed-dose and weight-based regimens.
The cortisol response to glucagon was demonstrated to be lower in men than in women, but not affected by age or BMI. A high basal cortisol level was associated with lower cortisol responses to glucagon [120, 128, 129]. Fixed-dose (1 or 1.5 mg in patients ˃90 kg) glucagon was shown to be associated with lower cortisol responses when compared to a weight-based dosing (WB:0.03 mg/kg) [128]. Thus, the potential influencers of cortisol response to GST are gender, baseline cortisol (in terms of rise) and dose of glucagon used.
An important point in evaluating the HPA axis during the GST is the diagnostic cut-off points used. The mean peak cortisol response to the GST was found to be significantly lower than that obtained during the HDST, but similar to that obtained during LDST in healthy adults. The lowest peak cortisol response achieved during the GST was found to be 9.1 μg/dl in healthy adults [94]. Hamrahian et al. suggested that the GST may be an acceptable alternative to the ITT in the investigation of the HPA axis with cortisol cut-off points of 9 μg/dl for fixed dose-GST and 11 μg/dl for weight-based GST as appropriate criteria to diagnose SAI [128]. Another study showed a good concordance between the ITT and GST, but ROC analysis revealed a cut-off for a peak cortisol response to the GST as 16.7 μg/dl for HPA axis sufficiency [105] which was higher than in the previously mentioned studies. In a study including 129 patients, a peak cortisol response of ˂298 mmol/l (10.7 μg/dl) to the ITT was found to be 97% sensitive and 75% specific for determining SAI according to the confirmed concordant results of LDST and GST with local cut-off levels. The reproducibilities of the tests were reported as 88%, 83%, and 79% for the GST, LDST and ITT, respectively [69]. Any of three tests can be used in the investigation of the HPA axis, but it would be appropriate to individualise cut-off levels used for the diagnosis of AI.
Recently, we compared the GST, LDST and a new combination test of the LDST and GST in 41 adult patients with pituitary disorders and 20 healthy subjects. The combination test was performed by injection of 1 μg ACTH i.v. at the 180th minute of a standard GST, with blood samples for cortisol measurement obtained at 210 and 240 min: 3 patients with discordant results during the LDST and GST had normal cortisol responses to the combination test and were clinically HPA axis sufficient. It is possible that this test may provide additional information in patients with unequivocal results in the GST and ACTH stimulation tests [134].
The GST can be associated with side effects including occasional nausea with vomiting, mild flushing, sweating, and headache in ˂10% [66]. However, more recent studies reported side effects in 21.4% of the patients including severe symptomatic hypotension, dizziness and sweating [129]. The most common side effects related to glucagon were reported as nausea (24%) and vomiting (22.16%); symptomatic hypoglycaemia was not reported [105].
In conclusion, the GST is a good alternative in patients with contra-indications to the ITT who require assessment of both HPA and GH axes, and is in more frequent use in the very young and the elderly. However, the test results should be carefully interpreted keeping in mind that glucagon is a weaker stimulant of the HPA axis than the ITT or the HDST, that the dose of glucagon used may influence the results when the tested patient is over-weight or obese, and that patients may suffer nausea and/or vomiting.
5.4 Metyrapone test (Table 4)
Metyrapone is an inhibitor of adrenal 11-β-hydroxylase enzyme which is responsible for conversion of 11-deoxycortisol to cortisol (Fig. 1). Metyrapone-induced reduction in cortisol levels in the circulation leads to stimulation of the HPA axis and increases 11-deoxycortisol proximal to the blocked enzyme: 11-deoxycortisol is not able to effectively supress ACTH secretion from the pituitary. When compared to the stimulation tests such as the ITT and GST, the mechanism by which metyrapone affects the HPA axis is totally different and it is characterised by a negative feedback stimulus instead of direct stimulation of the hypothalamus and/or pitutary.
The metyrapone test has been used in order to investigate the HPA axis since 1950s [135]. It can be used when the ACTH stimulation test results in normal adrenal function but fails to exclude SAI. The studies using metyrapone test are summarized in Table 4 [136,137,138,139,140,141,142,143,144,145,146]. The single-dose metyrapone test has been shown to be a simple and reliable test as the standard metyrapone test (750 mg every four hours, six times) [139]. Overnight single-dose metyrapone (at 11 p.m. metyrapone 2 g for subjects ˂60 kg, 3 g for ˃60 kg) was found to be more sensitive than the ITT and HDST in detecting subtle degrees of HPA axis insufficiency [147]. However, a similar study later showed that metyrapone test was not better than an early morning cortisol level in the prediction of glucocorticoid need 6 months after pituitary surgery [146]. The short overnight metyrapone test was able to reveal more patients with ACTH deficiency after pituitary surgery when compared to the ITT, but it was not clear whether the patients who failed the metyrapone test but not the ITT required GRT [61]. However, in another study, the overnight metyrapone test and the ITT were found to produce very similar results [148].
A peak serum 11-deoxycortisol ˃7 μg/dl and simultaneous serum cortisol ˂10 μg/dl were accepted as the cut-off values for the metyrapone test [147]. The plasma ACTH level may stil not be sufficient enough despite a normal 11-deoxycortisol response in the setting of mild SAI [149]. The sum of 11-deoxycortisol and cortisol responses with a cut-off level of 450 nmol/l after single dose of 2.0 g metyrapone given at midnight may lead a better diagnostic accuracy when compared to 11-deoxycortisol measurement alone. On the other hand, an ACTH level > 150 ng/l after single dose of metyrapone was found to be valuable in detecting a safe pituitary response [145] and may increase the sensitivity of the test. Half of the patients with a subnormal response to metyrapone had a normal cortisol (>414 nmol/l, 15 μg/dl) response to the LDST [91].
Metyrapone was demonstrated to cause some side effects such as unusual limb sensations, nausea and vomiting, dizziness without postural hypotension, and nightmares in the 576 tests evaluated. Worsening of adrenal function was not reported in that study [148].
So, the metyrapone test seems to be very sensitive in the detection of SAI but does not have clear superiority over other tests, and the significance of such minor chages is unclear. Theoretically, as it explores feedback at a pituitary level, it may not be sensitive to changes in hypothalamic function. The requirement of both cortisol and 11-deoxycortisol estimation and its lack of universal availability limits its use in routine clinical practice.
5.5 Corticotrophin-releasing hormone test
During CRH stimulation test 100 μg of corticoliberin (human CRH) is administered i.v., and cortisol and ACTH levels are measured at baseline and 30, 45, and 60 min. The results of the CRH test have been reported to correlate with those of the ITT in patients with HPA axis suppression with GCs [150]. The mean and minimum peak cortisol response after hCRH in healthy controls were found to be 594.8 ± 21.7 nmol/l (21.4 ± 0.8 μg/dl) and 400 nmol/l (14.4 μg/dl), respectively. A peak cortisol level < 377 nmol/l (13.6 μg/dl) after the hCRH test was suggested to be optimal for the diagnosis of AI but the sensitivity of the test was low [17].
The cortisol response to CRH was demonstrated to show a poor correlation with that of the ITT, except in patients with overt AI, in another study. Although the CRH test was shown to provide better results in accuracy than the LDST and HDST taking the ITT as the reference [151], the cortisol response to CRH was highly variable in normal subjects. In the studies carried out later, the early post-operative CRH-test was reported to be insufficient to reliably predict adrenal function after pituitary surgery in all patients, and retesting became essential [152]. Recently, the sensitivity and specificity of CRH test was found to be 78% and 90%, respectively and its diagnostic performance was shown to be worse than a single basal cortisol measurement [153]. CRH is not widely available and it is expensive. The current data suggest that CRH stimulation test is unhelpful and unlikely to replace the other traditional tests in the investigation of the HPA axis. GH secretagogues stimulate GH secretion by binding to a GHS-R1 for which ghrelin is a natural ligand [154, 155]. Ghrelin stimulates the HPA axis through both CRH, and particularly AVP release from the hypothalamus [156]. GHRP2 stimulates HPA axis via the GH secretagogue in the hypothalamus [157] and ACTH directly from the pituitary [158]. However, neither GHRP nor ghrelin seem to have additional benefits to the traditional tests [154, 157,158,159,160].
6 Assessment of the HPA axis in patients with pituitary tumours undergoing pituitary surgery
Assessment of the HPA axis after pituitary surgery is crucially important not only to identify the patients who developed HPA axis insufficiency and require GRT, but also to avoid unnecessary glucocorticoid supplementation. Different doses of glucocorticoids are still given to patients undergoing pituitary surgery on the day of surgery and post-operatively because of local traditions, fear of post-surgical hypopituitarism, and uncertainty about the definition of subclinical HPA axis insufficiency [161].
Non-functioning pituitary adenomas (NFPAs) may cause SAI due to mass effects or due to surgery performed to relieve compressive signs. Although decompression may improve hypocortisolism, it was shown to result in de novo hypocortisolism in 10.3% of patients with normal adrenal function before surgery. Only patients with a basal cortisol level of ˂8 μg/dl pre-operatively were suggested to be given GRT [162]. A cortisol level ≥ 15 μg/dl measured on the morning of the first post-operative day and a cortisol peak of ≥18 μg/dl to the MDST (25 μg) at post-operative 4–6 weeks were shown to be associated with normal HPA axis function [163]. Cortisol levels may be very low during the first part of surgery presumably due to anaesthetics, followed by a remarkable increase after intrasellar manipulation [164]. Recently, a retrospective study including 149 patients who underwent TSS for pituitary tumours assessed the place of recovery-room (RR) cortisol and found it to be the most accurate method when compared to day 1, 2 and 3 post-surgical basal cortisol. The RR cortisol threshold of 757.5 nmol/L (27.5 μg/dl) had 100% sensitivity and 70% specificity in the determination of necessity for long-term GRT [165]. The early post-operative basal cortisol was recommended to be a safe and simple measurement to guide (dis)continuation of GRT [153].
Pre-operative MDST and immediate post-operative MDST were found to have the highest sensitivity, accuracy, and positive predictive value (PPV) for a normal post-operative HPA axis, but pre-operative testing was found to be more cost effective (including costs of tests and hydrocortisone treatment) [166]. Neither the LDST nor the HDST were found to be reliable in determining the integrity of the HPA axis a week after pituitary surgery [167]. Although the LDST has been found to be more closely correlated with the ITT than the HDST immediately after pituitary surgery, none of them can completely correctly estimate the status of the HPA axis 3 months post-operatively [110]. Normalisation of HPA axis function can be seen in the first months after surgery. A recent study reported that a pre-operative SST 30-min cortisol cut-off level of 350 nmol/L (12.7 μg/dL) as the best predictor of HPA axis status [101]. However periodic testing is important post-operatively since recovery of the HPA axis can be seen in the postoperative 9–12 months [102].
The GST was proposed as a potential alternative to the ITT 3 months post-operatively for the assessment of GH reserve, but a poor test for ACTH reserve. A peak cortisol level < 500 nmol/l (18 μg/dl) was accepted as the criterion for the diagnosis of AI, but different criteria were not investigated for the ITT and GST, and the study did not contain a healthy population [66]. The metyrapone test was also not better than an early morning cortisol level in the prediction of glucocorticoid need 6 months after surgery [146].
In a study which aimed to limit GC exposure in patients undergoing TSS, patients with a normal HPA axis before TSS were not given peri-operative GC coverage and followed up by daily cortisol measurements. Of these patients, 45% received GC treatment for the following reasons: serum cortisol ˂5 μg/dl, cortisol between 5 and 12 μg/dl accompanied by manifestations of AI, moderate to severe post-operative hyponatraemia and severe headache, nausea and vomiting, fatigue or anorexia with cortisol ˃12 μg/dl. Only 14% of the patients were on GRT at 12 weeks. GRT was not found to be essential in most of the patients undergoing TSS [168]. The administration of corticosteroids peri-operatively in patients with an intact HPA axis is not only unnecessary, but also interferes with the assessment of the HPA axis after surgery. So, it was usggested that perioperative corticosteroid administration coud be safely witheld in these patients [169].
The definition of hypocortisolism as being a basal cortisol <8 μg/dL on 3rd post-operative day was suggested to be the single most significant predictor of hypocortisolism (a peak cortisol response to LDST <16 μg/dL) 12 weeks following surgery. The post-operative 3rd day basal cortisol was found to correctly predict eucortisolism 12 weeks after surgery with a sensitivity of 73% and specificity of 79% [100].
In a very recent prospective study including 92 patients without pre-operative AI and not receiving GRT, the 2nd postoperative day basal cortisol levels ≤3.2 μg/dL (89 nmol/L) and > 14 μg/dL (386 nmol/L) have been found to be diagnostic of SAI and normal function, respectively [170].
In conclusion, measurement of basal cortisol or an SST, if required, may be good alternatives for the preoperative evaluation of patients undergoing pituitary surgery with a cut-off value depending on the preferred dose of ACTH used. If the HPA axis is intact pre-operatively, then GRT can be avoided according to the clinical findings of the patient. Post-operatively, the best method for the evaluation of HPA axis is clinical follow-up with measurement of basal cortisol. Considering that the level of serum cortisol is highest immediately after surgery and decreases gradually, clinical findings of AI should be carefully evaluated dynamically. If clinical findings of SAI are present, GRT should be given until the next evaluation, probably at the first post-operative month. If clinical findings of AI are not present, then the basal serum cortisol level, depending on the post-operative day of measurement, will aid the decision of GRT. In the early post-operative period the HDST can lead to false negative results. So, when basal cortisol is inconclusive in the first month then an ITT, GST or LDST may be used. However, it should be kept in mind that the HPA axis may normalise during the post-operative 3 months. The assessment should be repeated 3 months after surgery.
7 The effects of age, sex and body mass indices on the HPA axis or on the cortisol response to dynamic stimulation tests
Current data in the literature suggest good maintenance of cortisol secretion with aging but a clear impairment of secretion of androgens in elderly subjects. A significant increase in the cortisol/DHEAS molar ratio occurs as a result of both physiological and pathological aging [171]. The cortisol responses to the HDST, LDST, ITT and GST in healthy volunteers did not show differences in terms of age and sex [15, 60, 94, 128, 172], but in a study evaluating the cortisol response to GST in elderly patients, the cortisol peak was found to be significantly different between subjects stratified by age ˂ or ˃80 years (22.4 and 18.5 μg/dl, respectively) [129].
Studies regarding the effects of sex have also shown conflicting results. Mean daily cortisol levels were found to be lower in premenopausal women than men in subjects <50 years of age, but this effects of gender were not sustained after 50y [173]. Although basal and 1 μg ACTH-stimulated cortisol responses were not found to differ between older and younger individuals, older men had significantly lower cortisol responsiveness than older women [95]. There may be a complex effect of age, sex and menopausal status. In contrast, the HDST i.m.was found to be associated with higher peak cortisol levels and incremental responses in females than in males independent of age. The authors revealed the need of sex-specific cut-off levels for cortisol responses [12].
On the other hand, the peak calculated free cortisol and free cortisol index after LDST and HDST were found to be lower in women due to higher CBG levels despite similar serum cortisol responses [172]. Oral contraceptives (OCP) may also affect total cortisol levels, so premenopausal women on OCP (or pregnant women, where deemed appropriate) may need a separate reference limit for cortisol [14].
The BMI does not also seem to affect the cortisol response to dynamic stimulation tests including the LDST, HDST and GST [18] and daily cortisol secretion [173]. The effects of age, sex and BMI on the tests evaluating the HPA axis seem to be, at least clinically, negligible according to present data except in very elderly patients and OCP using or pregnant women. However, further detailed studies would help to better understand the effects of age, sex and BMI on HPA axis.
8 Conclusions
There is no gold standard test in the investigation of SAI meeting all the criteria of being safe, cheap, practical, easy, sensitive, specific and reproducible. Measurement of basal cortisol eliminates the need of dynamic test in majority of the patients when clinical findings are concordant. However, many patients with unequivocal basal cortisol levels may also require a dynamic test for a correct diagnosis. An ACTH-stimulation test or an ITT may be the first options for the evaluation of the HPA axis as dynamic tests, with 250 μg ACTH given i.m. or i.v., and serum cortisol measured at 30 mins, being most validated in clinical use, although the LDST may offer some advantages. The GST may be a good alternative to the ITT when this is contraindicated and GH axis evaluation is also required. Each test, for each time point and for each method used, requires its own minimum threshold of normality to assess the HPA axis and to determine the requirement of GRT. There may still be gray zones for cut-off levels used, then clinical judgement is essential.
Search Strategy: References in this review were identified through searches of PubMed articles by using the following terms: Insulin tolerance test, low dose test, short synacthen test, high dose synacthen test, insulin hypoglycemia test, insulin stress test, HPA axis, secondary AI, secondary adrenal failure, hypopituitarism.
*1 μg/dl was multiplied by 27.59 for conversion to nmol/L.
References
Thomas A. On the constitutional and local effects of disease of the supra-renal capsules. In: London: Samuel Highley of 32 Fleet Street London. First ed; 1855.
Kline GA, Holmes DT. De-evolution of diagnostic testing for adrenal insufficiency. Lancet Diabetes & Endocrinol. 2017;5(2):88–90.
De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19(3):269–301.
Seoane L.M.T.S, Dieguez C. Hypothalamic-pituitary diseases endocrinology. Casanueva F. GE, editor: Springer, Cham; 2018.
Gjerstad JK, Lightman SL, Spiga F. Role of glucocorticoid negative feedback in the regulation of HPA axis pulsatility. Stress. 2018;21(5):403–16.
Keller-Wood M. Hypothalamic-pituitary–adrenal Axis-feedback control. Compr Physi. 2015;5(3):1161–82.
Drury PL, Besser GM. Adrenal cortex. In: Reginald BGM, editor. Fundamentals of clinical endocrinology. Fourth ed. New York: Churchill Livingstone; 1989.
Borm K, Slawik M, Seiler L, Flohr F, Petrick M, Honegger J, et al. Is the plasma ACTH concentration a reliable parameter in the insulin tolerance test? Eur J Endocrinol. 2003;149(6):535–41.
Plumpton FS, Besser GM. The adrenocortical response to surgery and insulin-induced hypoglycaemia in corticosteroid-treated and normal subjects. Br J Surg. 1969;56(3):216–9.
Khoo B, Boshier PR, Freethy A, Tharakan G, Saeed S, Hill N, et al. Redefining the stress cortisol response to surgery. Clin Endocrinol. 2017;87(5):451–8.
Prete A, Yan Q. The cortisol stress response induced by surgery: A systematic review and meta-analysis. Clin Endocrinol. 2018;89(5):554–67.
Clark PM, Neylon I, Raggatt PR, Sheppard MC, Stewart PM. Defining the normal cortisol response to the short Synacthen test: implications for the investigation of hypothalamic-pituitary disorders. Clin Endocrinol. 1998;49(3):287–92.
Raverot V, Richet C, Morel Y, Raverot G, Borson-Chazot F. Establishment of revised diagnostic cut-offs for adrenal laboratory investigation using the new Roche diagnostics Elecsys(®) cortisol II assay. Ann Endocrinol. 2016;77(5):620–2.
El-Farhan N, Pickett A, Ducroq D, Bailey C, Mitchem K, Morgan N, et al. Method-specific serum cortisol responses to the adrenocorticotrophin test: comparison of gas chromatography-mass spectrometry and five automated immunoassays. Clin Endocrinol. 2013;78(5):673–80.
Ueland GA, Methlie P, Oksnes M, Thordarson HB, Sagen J, Kellmann R, et al. The short Cosyntropin test revisited: new Normal reference range using LC-MS/MS. J Clin Endocrinol Metab. 2018;103(4):1696–703.
Brown S, Hadlow N, Badshah I, Henley D. A time-adjusted cortisol cut-off can reduce referral rate for Synacthen stimulation test whilst maintaining diagnostic performance. Clin Endocrinol. 2017;87(5):418–424.
Schmidt IL, Lahner H, Mann K, Petersenn S. Diagnosis of adrenal insufficiency: evaluation of the corticotropin-releasing hormone test and basal serum cortisol in comparison to the insulin tolerance test in patients with hypothalamic-pituitary-adrenal disease. Clin Endocrinol. 2003;88(9):4193–8.
Karaca Z, Tanriverdi F, Atmaca H, Gokce C, Elbuken G, Selcuklu A, et al. Can basal cortisol measurement be an alternative to the insulin tolerance test in the assessment of the hypothalamicpituitary-adrenal axis before and after pituitary surgery? Eur J Endocrinol. 2010;163(3):377–82.
Yo WS, Toh LM, Brown SJ, Howe WD, Henley DE, Lim EM. How good is a morning cortisol in predicting an adequate response to intramuscular synacthen stimulation? Clin Endocrinol. 2014;81(1):19–24.
Ortiz-Flores AE, Santacruz E, Jimenez-Mendiguchia L, Garcia-Cano A, Nattero-Chavez L, Escobar-Morreale HF, et al. Role of sampling times and serum cortisol cut-off concentrations on the routine assessment of adrenal function using the standard cosyntropin test in an academic hospital from Spain: a retrospective chart review. BMJ Open. 2018;8(5):e019273.
Varadhan L, Nayak AU, Mukherjee A, Jose B, Varughese GI. Can a baseline morning cortisol predict outcome of short Synacthen test in an endocrine unit in an outpatient setting. Clin Endocrinol. 2015;82(2):309–11.
Manosroi W, Phimphilai M, Khorana J, Atthakomol P. Diagnostic performance of basal cortisol level at 0900-1300h in adrenal insufficiency. PLoS One. 2019;14(11):e0225255.
Sbardella E, Isidori AM, Woods CP, Argese N, Tomlinson JW, Shine B, et al. Baseline morning cortisol level as a predictor of pituitary-adrenal reserve: a comparison across three assays. Clin Endocrinol. 2017;86(2):177–84.
Munro V, Tugwell B, Doucette S, Clarke DB, Lacroix A, Imran SA. Recovery of adrenal function after chronic secondary adrenal insufficiency in patients with hypopituitarism. Clin Endocrinol. 2016;85(2):216–22.
Polovina TS, Kraljevic I, Solak M, Balasko A, Haxhiu A, Haxhiu A, et al. Early basal cortisol level as a predictor of Hypothalamic-Pituitary-Adrenal (HPA) axis function after pituitary tumor surgery. Exp Clin Endocrinol Diabetes. 2019;128(11):709–14.
Leong SH, Shander S, Ratnasingam J. Predicting recovery of the hypothalamic-pituitary-adrenal axis after prolonged glucocorticoid use. Endocrine. 2018;24(1):14–20.
Peechakara S, Bena J, Clarke NJ, McPhaul MJ, Reitz RE, Weil RJ, et al. Total and free cortisol levels during 1 µg, 25 µg, and 250 µg cosyntropin stimulation tests compared to insulin tolerance test: results of a randomized, prospective, pilot study. Endocrine. 2017;57(3):388–93.
Rauschecker M, Abraham SB, Abel BS, Wesley R, Saverino E, Trivedi A, et al. Cosyntropin-stimulated serum free cortisol in healthy, Adrenally insufficient, and mildly cirrhotic populations. J Clin Endocrinol Metab. 2016;101(3):1075–81.
Bancos I, Erickson D, Bryant S, Hines J, Nippoldt TB, Natt N, et al. Performance of free versus total cortisol following COSYNTROPIN stimulation testing in an outpatient setting. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2015;21(12):1353–63.
Turpeinen U, Hamalainen E. Determination of cortisol in serum, saliva and urine. Best Pract Res Clin Endocrinol Metab. 2013;27(6):795–801.
Oelkers W, Boelke T, Bahr V. Dose-response relationships between plasma adrenocorticotropin (ACTH), cortisol, aldosterone, and 18-hydroxycorticosterone after injection of ACTH-(1-39) or human corticotropin-releasing hormone in man. J Clin Endocrinol Metab. 1988;66(1):181–6.
Hagg E, Asplund K, Lithner F. Value of basal plasma cortisol assays in the assessment of pituitary-adrenal insufficiency. Clin Endocrinol. 1987;26(2):221–6.
Jenkins D, Forsham PH, Laidlaw JC, Reddy WJ, Thorn GW. Use of ACTH in the diagnosis of adrenal cortical insufficiency. Am J Med. 1955;18(1):3–14.
Lee MK, Vasikaran S, Doery JC, Wijeratne N, Prentice D. Cortisol: ACTH ratio to test for primary hypoadrenalism: a pilot study. Postgrad Med J. 2013;89(1057):617–20.
Pecori Giraldi F, Saccani A, Cavagnini F. Assessment of ACTH assay variability: a multicenter study. Eur J Endocrinol. 2011;164(4):505–12.
Van Rijn JL, Van Landeghem BA, Haima P, Goldschmidt HM. Evaluation of ACTH immunoradiometric assays. Clin Biochem. 1996;29(1):93–5.
Bornstein SR, Allolio B, Arlt W, Barthel A, Don-Wauchope A, Hammer GD, et al. Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364–89.
Abraham SB, Abel BS, Sinaii N, Saverino E, Wade M, Nieman LK. Primary vs secondary adrenal insufficiency: ACTHstimulated aldosterone diagnostic cut-off values by tandem mass spectrometry. Clin Endocrinol. 2015;83(3):308–14.
Shi J, Dhaliwal P, Zi Zheng Y, Wong T, Straseski JA, Cervinski MA, et al. An intact ACTH LC-MS/MS assay as an arbiter of clinically discordant immunoassay results. Clin Chem. 2019;65(11):1397–404.
Nieman LK. Dynamic evaluation of adrenal hypofunction. J Endocrinol Invest. 2003;26(7 Suppl):74–82.
A V. In: GR MV, editor. Androgen secretion by adrenals and gonads. Boston: John Wright–PS; 1983.
Orentreich N, Brind JL, Rizer RL, Vogelman JH. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J Clin Endocrinol Metab. 1984;59(3):551–5.
Nasrallah MP, Arafah BM. The value of dehydroepiandrosterone sulfate measurements in the assessment of adrenal function. J Clin Endocrinol Metab. 2003;88(11):5293–8.
Sayyed Kassem L, El Sibai K, Chaiban J, Abdelmannan D, Arafah BM. Measurements of serum DHEA and DHEA sulphate levels improve the accuracy of the low-dose cosyntropin test in the diagnosis of central adrenal insufficiency. J Clin Endocrinol Metab. 2012;97(10):3655–62.
Laureti S, Arvat E, Candeloro P, Di Vito L, Ghigo E, Santeusanio F, et al. Low dose (1 microg) ACTH test in the evaluation of adrenal dysfunction in pre-clinical Addison's disease. Clin Endocrinol. 2000;53(1):107–15.
Laureti S, Candeloro P, Aglietti MC, Giordano R, Arvat E, Ghigo E, et al. Dehydroepiandrosterone, 17alpha-hydroxyprogesterone and aldosterone responses to the low-dose (1 micro g) ACTH test in subjects with preclinical adrenal autoimmunity. Clin Endocrinol. 2002;57(5):677–83.
Topor LS, Asai M, Dunn J, Majzoub JA. Cortisol stimulates secretion of dehydroepiandrosterone in human adrenocortical cells through inhibition of 3 beta-HSD2. J Clin Endocrinol Metab. 2011;96(1):E31–9.
Fischli S, Jenni S, Allemann S, Zwahlen M, Diem P, Christ ER, et al. Dehydroepiandrosterone sulfate in the assessment of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 2008;93(2):539–42.
Charoensri S, Chailurkit L, Muntham D, Bunnag P. Serum dehydroepiandrosterone sulfate in assessing the integrity of the hypothalamic-pituitary-adrenal axis. J Clin Transl Endocrinol. 2017;7:42–6.
Greenwood FC, Landon J, Stamp TC. The plasma sugar, free fatty acid, cortisol, and growth hormone response to insulin. I. in control subjects. J Clin Invest. 1966;45(4):429–36.
Landon J, Greenwood FC, Stamp TC, Wynn V. The plasma sugar, free fatty acid, cortisol, and growth hormone response to insulin, and the comparison of this procedure with other tests of pituitary and adrenal function. II. In patients with hypothalamic or pituitary dysfunction or anorexia nervosa. J Clin Invest. 1966;45(4):437–49.
Jones SL, Trainer PJ, Perry L, Wass JA, Bessser GM, Grossman A. An audit of the insulin tolerance test in adult subjects in an acute investigation unit over one year. Clin Endocrinol. 1994;41(1):123–8.
Hurel SJ, Thompson CJ, Watson MJ, Harris MM, Baylis PH, Kendall-Taylor P. The short Synacthen and insulin stress tests in the assessment of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol. 1996;44(2):141–6.
Ammari F, Issa BG, Millward E, Scanion MF. A comparison between short ACTH and insulin stress tests for assessing hypothalamo-pituitary-adrenal function. Clin Endocrinol. 1996;44(4):473–6.
Vestergaard P, Hoeck HC, Jakobsen PE, Laurberg P. Reproducibility of growth hormone and cortisol responses to the insulin tolerance test and the short ACTH test in normal adults. Horm Metab Res. 1997;29(3):106–10.
Erturk E, Jaffe CA, Barkan AL. Evaluation of the integrity of the hypothalamic-pituitary-adrenal axis by insulin hypoglycemia test. J Clin Endocrinol Metab. 1998;83(7):2350–4.
Ambrosi B, Barbetta L, Re T, Passini E, Faglia G. The one microgram adrenocorticotropin test in the assessment of hypothalamic-pituitary-adrenal function. Eur J Endocrinol. 1998;139(6):575–9.
Dullaart RP, Riemens SC, Scheek LM, Van Tol A. Insulin decreases plasma cholesteryl ester transfer but not cholesterol esterification in healthy subjects as well as in normotriglyceridaemic patients with type 2 diabetes. Eur J Clin Investig. 1999;29(8):663–71.
Abdu TA, Elhadd TA, Neary R, Clayton RN. Comparison of the low dose short synacthen test (1 microg), the conventional dose short synacthen test (250 microg), and the insulin tolerance test for assessment of the hypothalamo-pituitary-adrenal axis in patients with pituitary disease. J Clin Endocrinol Metab. 1999;84(3):838–43.
Gonzalbez J, Villabona C, Ramon J, Navarro MA, Gimenez O, Ricart W, et al. Establishment of reference values for standard dose short synacthen test (250 microgram), low dose short synacthen test (1 microgram) and insulin tolerance test for assessment of the hypothalamo-pituitary-adrenal axis in normal subjects. Clin Endocrinol. 2000;53(2):199–204.
Courtney CH, McAllister AS, McCance DR, Hadden DR, Leslie H, Sheridan B, et al. The insulin hypoglycaemia and overnight metyrapone tests in the assessment of the hypothalamic-pituitary-adrenal axis following pituitary surgery. Clin Endocrinol. 2000a;53(3):309–12.
Lange M, Svendsen OL, Skakkebaek NE, Müller J, Juul A, Schmiegelow M, et al. An audit of the insulin-tolerance test in 255 patients with pituitary disease. Eur J Endocrinol. 2002;147(1):41–7.
Finucane FM, Liew A, Thornton E, Rogers B, Tormey W, Agha A. Clinical insights into the safety and utility of the insulin tolerance test (ITT) in the assessment of the hypothalamo-pituitary-adrenal axis. Clin Endocrinol. 2008;69(4):603–7.
Giordano R, Picu A, Bonelli L, Balbo M, Berardelli R, Marinazzo E, et al. Hypothalamus-pituitary-adrenal axis evaluation in patients with hypothalamo-pituitary disorders: comparison of different provocative tests. Clin Endocrinol. 2008;68(6):935–41.
Deutschbein T, Unger N, Mann K, Petersenn S. Diagnosis of secondary adrenal insufficiency: unstimulated early morning cortisol in saliva and serum in comparison with the insulin tolerance test. Horm Metab Res. 2009;41(11):834–9.
Berg C, Meinel T, Lahner H, Yuece A, Mann K, Petersenn S. Diagnostic utility of the glucagon stimulation test in comparison to the insulin tolerance test in patients following pituitary surgery. Eur J Endocrinol. 2010;162(3):477–82.
Ferrante E, Morelli V, Giavoli C, Mantovani G, Verrua E, Sala E, et al. Is the 250 μg ACTH test a useful tool for the diagnosis of central hypoadrenalism in adult patients with pituitary disorders? Hormones. 2012;11(4):428–35.
Cho HY, Kim JH, Kim SW, Shin CS, Park KS, Kim SW, et al. Different cut-off values of the insulin tolerance test, the high-dose short Synacthen test (250 μg) and the low-dose short Synacthen test (1 μg) in assessing central adrenal insufficiency. Clin Endocrinol. 2014;81(1):77–84.
Simsek Y, Karaca Z, Tanriverdi F, Unluhizarci K, Selcuklu A, Kelestimur F. A comparison of low-dose ACTH, glucagon stimulation and insulin tolerance test in patients with pituitary disorders. Clin Endocrinol. 2015;82(1):45–52.
Simunkova K, Duskova M, Kosak M, Krsek M, Hana V, Hill M, et al. Response of cortisol metabolites in the insulin tolerance test and Synacthen tests. Physiol Res. 2015;64(Suppl 2):S237–46.
Kacheva S, Kolk K, Morgenthaler NG, Brabant G, Karges W. Gender-specific co-activation of arginine vasopressin and the hypothalamic-pituitary-adrenal axis during stress. Clin Endocrinol. 2015;82(4):570–6.
Cerina V, Kruljac I, Radosevic JM, Kirigin LS, Stipic D, Pecina HI, et al. Diagnostic accuracy of perioperative measurement of basal anterior pituitary and target gland hormones in predicting adrenal insufficiency after pituitary surgery. Medicine. 2016;95(9):e2898.
Kosak M, Duskova M, Starka L, Jandikova H, Pospisilova H, Sramkova M, et al. Can the gold standard be beaten? How reliable are various modifications of the Synacthen test compared to the insulin tolerance test. Physiol Res. 2017;66(Suppl 3):S387–s95.
Cadegiani FA, Kater CE. Hypothalamic-pituitary-adrenal (HPA) Axis functioning in overtraining syndrome: findings from endocrine and metabolic responses on overtraining syndrome (EROS)-EROS-HPA Axis. Sports Med Open. 2017;3(1):45.
Taieb A, Asma BA, Yosra H, Amel M, Maha K, Molka C, et al. Assessing the adrenal axis by the glucagon stimulation test in children with idiopathic growth hormone deficiency. Pediatr Endocrinol Diabetes Metab. 2018;24(4):161–6.
Simsek Y, Karaca Z, Diri H, Tanriverdi F, Unluhizarci K, Kelestemur F. Is biochemical hypoglycemia necessary during an insulin tolerance test? Arc Endocrinol Metab. 2020;64(1):82–8.
Fraser RAF, Smith PH. The value of the glucose tolerance test, the insulin tolerance test and the glucose insulin tolerance test in the diagnosis of endocrinologic disorders of glucose metabolism. J Clin Endocrinol Metab. 1941;1:297–306.
Lee P, Greenfield JR, Ho KK. Factors determining inadequate hypoglycaemia during insulin tolerance testing (ITT) after pituitary surgery. Clin Endocrinol. 2009;71(1):82–5.
Zhang Y, Sun S, Jia H, Qi Y, Zhang J, Lin L, et al. The optimized calculation method for insulin dosage in an insulin tolerance test (ITT): a randomized parallel control study. Front Endocrinol. 2020;11:202.
Dickstein G. The assessment of the hypothalamo-pituitary-adrenal axis in pituitary disease: are there short cuts? J Endocrinol Investig. 2003;26(7 Suppl):25–30.
Ajala O, Lockett H, Twine G, Flanagan DE. Depth and duration of hypoglycaemia achieved during the insulin tolerance test. Eur J Endocrinol. 2012;167(1):59–65.
Borm K, Slawik M, Beuschlein F, Seiler L, Flohr F, Berg A, et al. Low-dose glucose infusion after achieving critical hypoglycemia during insulin tolerance testing: effects on time of hypoglycemia, neuroendocrine stress response and patient's discomfort in a pilot study. Eur J Endocrinol. 2005;153(4):521–6.
Thorn GW, Forsham PH. A test for adrenal cortical insufficiency; the response to pituitary andrenocorticotropic hormone. J Am Med Assoc. 1948;137(12):1005–9.
Kelestimur F, Akgun A, Gunay O. A comparison between short synacthen test and depot synacthen test in the evaluation of cortisol reserve of adrenal gland in normal subjects. J Endocrinol Invest. 1995;18(11):823–6.
Landon J, Wynn V, James VH, Wood JB. Adrenal response to infused corticotropin in subjects receiving glucocorticoids. J Clin Endocrinol Metab. 1965;25:602–11.
Wood JB, Frankland AW, James VH, Landon J. A rapid test of adrenocortical function. Lancet. 1965;1(7379):243–5.
Kehlet H, Binder C. Value of an ACTH test in assessing hypothalamic-pituitary-adrenocortical function in glucocorticoid-treated patients. Br Med J. 1973;2(5859):147–9.
Kehlet H, Blichert-Toft M, Lindholm J, Rasmussen P. Short ACTH test in assessing hypothalamic-pituitary-adrenocortical function. Br Med J. 1976;1(6004):249–51.
Cunningham SK, Moore A, McKenna TJ. Normal cortisol response to corticotropin in patients with secondary adrenal failure. Arch Intern Med. 1983;143(12):2276–9.
Dickstein G, Shechner C, Nicholson WE, Rosner I, Shen-Orr Z, Adawi F, et al. Adrenocorticotropin stimulation test: effects of basal cortisol level, time of day, and suggested new sensitive low dose test. J Clin Endocrinol Metab. 1991;72(4):773–8.
Soule S, Van Zyl SC, Parolis G, Attenborough S, Peter D, Kinvig S, et al. The low dose ACTH stimulation test is less sensitive than the overnight metyrapone test for the diagnosis of secondary hypoadrenalism. Clin Endocrinol. 2000;53(2):221–7.
Agha A, Tomlinson JW, Clark PM, Holder G, Stewart PM. The long-term predictive accuracy of the short synacthen (corticotropin) stimulation test for assessment of the hypothalamic-pituitary-adrenal axis. J Clin Endocrinol Metab. 2006;91(1):43–7.
Dekkers OM, Timmermans JM, Smit JW, Romijn JA, Pereira AM. Comparison of the cortisol responses to testing with two doses of ACTH in patients with suspected adrenal insufficiency. Eur J Endocrinol. 2011;164(1):83–7.
Karaca Z, Lale A, Tanriverdi F, Kula M, Unluhizarci K, Kelestimur F. The comparison of low and standard dose ACTH and glucagon stimulation tests in the evaluation of hypothalamo-pituitary-adrenal axis in healthy adults. Pituitary. 2011;14(2):134–40.
Lekkakou L, Tzanela M, Lymberi M, Consoulas C, Tsagarakis S, Koutsilieris M. Effects of gender and age on hypothalamicpituitary-adrenal reactivity after pharmacological challenge with low-dose 1-μg ACTH test: a prospective study in healthy adults. Clin Endocrinol. 2013;79(5):683–8.
Chitale A, Musonda P, McGregor AM, Dhatariya KK. Determining the utility of the 60 min cortisol measurement in the short synacthen test. Clin Endocrinol. 2013;79(1):14–9.
Mak IYF, Au Yeung BYT, Ng YW, Choi CH, Iu HYP, Shek CC, et al. Salivary cortisol and cortisone after low-dose Corticotropin stimulation in the diagnosis of adrenal insufficiency. J Endocr Soc. 2017;1(2):96–108.
Struja T, Briner L, Meier A, Kutz A, Mundwiler E, Huber A, et al. Diagnostic accuracy of basal cortisol level to predict adrenal insufficiency in cosyntropin testing: results from an observational cohort study with 804 patients. Endocr Pract. 2017;23(8):949–61.
Mongioi LM, Condorelli RA. Accuracy of the low-dose ACTH stimulation test for adrenal insufficiency diagnosis: a re-assessment of the cut-off value. J Clin Med. 2019;8(6):806.
Garg A, Mishra SK, Dubey S, Singh VP, Kuchay MS, Mithal A. Low-dose ACTH test for evaluation of hypothalamus-pituitaryadrenal axis preoperatively and 3-month follow-up in nonfunctioning pituitary adenomas. J Endocrinol Invest. 2020; 43(12):1769–1777.
Pofi R, Feliciano C, Sbardella E, Argese N, Woods CP, Grossman AB, et al. The short Synacthen (Corticotropin) test can be used to predict recovery of Hypothalamo-pituitary-adrenal Axis function. J Clin Endocrinol Metab. 2018;103(8):3050–9.
Pofi R, Gunatilake S, Macgregor V, Shine B, Joseph R, Grossman AB, et al. Recovery of the hypothalamo-pituitary-adrenal axis after transsphenoidal adenomectomy for non-ACTH-secreting macroadenomas. J Clin Endocrinol Metab. 2019;104(11):5316–24.
Speckart PF, Nicoloff JT, Bethune JE. Screening for adrenocortical insufficiency with cosyntropin (synthetic ACTH). Arch Intern Med. 1971;128(5):761–3.
May ME, Carey RM. Rapid adrenocorticotropic hormone test in practice. Retrospective review. Am J Med. 1985;79(6):679–84.
Ach T, Yosra H, Jihen M, Abdelkarim Asma B, Maha K, Molka C, et al. Cortisol cut-points for the glucagon stimulation test in the evaluation of hypothalamic pituitary adrenal axis. Endocr J. 2018;65(9):935–42.
Ben-Shlomo A, Guzman J, Mirocha J. Enhanced cosyntropin stimulation test performance enabled by electronic medical record. Pituitary. 2016;19(5):503–6.
Inder WJ. Long-acting porcine sequence ACTH in the diagnosis of adrenal insufficiency: a cost-effective alternative to the ACTH1-24 test. Eur J Endocrinol. 2020;182(2):C5–c7.
Elder CJ, Vilela R, Johnson TN, Taylor RN, Kemp EH, Keevil BG, et al. Pharmacodynamic studies of nasal tetracosactide with salivary glucocorticoids for a noninvasive Short Synacthen Test. J Clin Endocrinol Metab. 2020;105(8):dgaa323.
Oelkers W. Dose-response aspects in the clinical assessment of the hypothalamo-pituitary-adrenal axis, and the low-dose adrenocorticotropin test. Eur J Endocrinol. 1996;135(1):27–33.
Dokmetas HS, Colak R, Kelestimur F, Selcuklu A, Unluhizarci K, Bayram F. A comparison between the 1-microg adrenocorticotropin (ACTH) test, the short ACTH (250 microg) test, and the insülin tolerance test in the assessment of hypothalamo-pituitaryadrenal axis immediately after pituitary surgery. J Clin Endocrinol Metab. 2000;85(10):3713–9.
Mukherjee JJ, de Castro JJ, Kaltsas G, Afshar F, Grossman AB, Wass JA, et al. A comparison of the insulin tolerance/glucagon test with the short ACTH stimulation test in the assessment of the hypothalamo-pituitary-adrenal axis in the early post-operative period after hypophysectomy. Clin Endocrinol. 1997;47(1):51–60.
Tordjman K, Jaffe A, Grazas N, Apter C, Stern N. The role of the low dose (1 microgram) adrenocorticotropin test in the evaluation of patients with pituitary diseases. J Clin Endocrinol Metab. 1995;80(4):1301–5.
Rasmuson S, Olsson T, Hagg E. A low dose ACTH test to assess the function of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol. 1996;44(2):151–156.
Dennedy MC. Salivary cortisone and cortisol following synacthen, a future replacement for serum cortisol? Commentary to: use of salivary cortisol and cortisone in the high and low dose synacthen test. Clin Endocrinol. 2018;88(6):770–1.
Murphy H, Livesey J, Espiner EA, Donald RA. The low döşe ACTH test–a further word of caution. J Clin Endocrinol Metab. 1998;83(2):712–3.
Wade M, Baid S, Calis K, Raff H, Sinaii N, Nieman L. Technical details influence the diagnostic accuracy of the 1 microg ACTH stimulation test. Eur J Endocrinol. 2010;162(1):109–13.
Saiegh L, Abu-Ahmad A, Sheikh-Ahmad M, Reut M, Chen-Konak L, Jiries N, et al. Performance of low-dose cosyntropin stimulation test handled via plastic tube. Endocrine. 2017;57(1):46–50.
Spathis GS, BloomSR JWJ, Millar JG, Kurtz A, Pyasena MR, et al. Subcutaneous glucagon as a test of the ability of the pituitary to secrete GH and ACTH. Clin Endocrinol. 1974;3(2):175–86.
Waldhäusl W, Haydl H, Nowotny P. ACTH and cortisol responses to glucagon stimulation. J Clin Endocrinol Metab. 1976;43(3):675–8.
Rao RH, Spathis GS. Intramuscular glucagon as a provocative stimulus for the assessment of pituitary function: growth hormone and cortisol responses. Metab Clin Exp. 1987;36(7):658–63.
Littley MD, Gibson S, White A, Shalet SM. Comparison of the ACTH and cortisol responses to provocative testing with glucagon and insulin hypoglycaemia in normal subjects. Clin Endocrinol. 1989;31(5):527–33.
Leong KS, Walker AB, Martin I, Wile D, Wilding J, MacFarlane IA. An audit of 500 subcutaneous glucagon stimulation tests to assess growth hormone and ACTH secretion in patients with hypothalamic-pituitary disease. Clin Endocrinol. 2001;54(4):463–8.
Agha A, Rogers B, Sherlock M, O'Kelly P, TormeyW PJ, et al. Anterior pituitary dysfunction in survivors of traumatic brain injury. J Clin Endocrinol Metab. 2004;89(10):4929–36.
Tanriverdi F, Dagli AT, Karaca Z, Unluhizarci K, Selcuklu A, Casanueva FF, et al. High risk of pituitary dysfunction due to aneurysmal subarachnoid haemorrhage: a prospective investigation of anterior pituitary function in the acute phase and 12months after the event. Clin Endocrinol. 2007;67(6):931–7.
Cegla J, Jones B, Seyani L, Papadoulou D, Wynne K, Martin NM, et al. Comparison of the overnight metyrapone and glucagon stimulation tests in the assessment of secondary hypoadrenalism. Clin Endocrinol. 2013;78(5):738–42.
Yuen KC, Biller BM, Katznelson L, Rhoads SA, Gurel MH, Chu O, et al. Clinical characteristics, timing of peak responses and safety aspects of two dosing regimens of the glucagon stimulation test in evaluating growth hormone and cortisol secretion in adults. Pituitary. 2013;16(2):220–30.
Lewandowski KC, Lewinski A, Skowronska-Jozwiak E, Stasiak M, Horzelski W, Brabant G. Copeptin under glucagon stimulation. Endocrine. 2016;52(2):344–51.
Hamrahian AH, Yuen KC, Gordon MB, Pulaski-Liebert KJ, Bena J, Biller BM. Revised GH and cortisol cut-points for the glucagon stimulation test in the evaluation of GH and hypothalamic pituitary-adrenal axes in adults: results from a prospective randomized multicenter study. Pituitary. 2016;19(3):332–41.
Tavares ABW, Seixas-da-Silva IA, Silvestre DHS, Pinheiro MFC, Vaisman M, Conceicao FL. Growth hormone and cortisol secretion in the elderly evaluated using the glucagon stimulation test. Endocrine. 2017;56(2):317–24.
Salata RA, Jarrett DB, Verbalis JG, Robinson AG. Vasopressin stimulation of adrenocorticotropin hormone (ACTH) in humans. In vivo bioassay of corticotropin-releasing factor (CRF) which provides evidence for CRF mediation of the diurnal rhythm of ACTH. J Clin Invest. 1988;81(3):766–74.
Arvat E, Maccagno B, Ramunni J, Giordano R, DiVito L, Broglio F, et al. Glucagon is an ACTH secretagogue as effective as hCRH after intramuscolar administration while it is ineffective when given intravenously in normal subjects. Pituitary. 2000a;3(3):169–73.
Arvat E, Maccagno B, Ramunni J, Maccario M, Giordano R, Broglio F, et al. Interaction between glucagon and human corticotropin-releasing hormone or vasopressin on ACTH and cortisol secretion in humans. Eur J Endocrinol. 2000b;143(1):99–104.
Broglio F, Prodam F, Gottero C, Destefanis S, Me E, Riganti F, et al. Ghrelin does not mediate the somatotroph and corticotroph responses to the stimulatory effect of glucagon or insulin-induced hypoglycaemia in humans. Clin Endocrinol. 2004;60(6):699–704.
Unluhizarci K, Kokoglu EO, Hacioglu A, Karaca Z, Kelestimur F. Comparison of a combination test (1 μg ACTH test plus glucagon test) versus 1 μg ACTH test and glucagon test in the evaluation of the hypothalamic-pituitary-adrenal axis in patients with pituitary disorders. Arc Endocrinol Metab. 2020.
Liddle GW, Estep HL, Kendall JW Jr, Williams WC Jr, Townes AW. Clinical application of a new test of pituitary reserve. J Clin Endocrinol Metab. 1959;19:875–94.
Buus O, Binder C, Petersen F. Metopirone dosage in pituitary function test. Lancet. 1962;1(7238):1040–1.
Metcalf MG, Beaven DW. The metopirone test of pituitary corticotrophin release. Evaluation of 101 tests. Am J Med. 1968;45(2):176–86.
Strott CA, West CD, Nakagawa K, Kondo T, Tyler FH. Plasma 11-deoxycorticosteroid and ACTH response to metyrapone (plasma metyrapone test). J Clin Endocrinol Metab. 1969;29(1):6–11.
Jubiz W, Meikle AW, West CD, Tyler FH. Single-dose metyrapone test. Arch Intern Med. 1970;125(3):472–4.
Meikle AW, West SC, Weed JA, Tyler FH. Single dose metyrapone test: 11 beta-hydroxylase inhibition by metyrapone and reduced metyrapone assayed by radioimmunoassay. J Clin Endocrinol Metab. 1975;40(2):290–5.
Spiger M, Jubiz W, Meikle AW, West CD, Tylor FH. Single-dose metyrapone test: review of a four-year experience. Arch Intern Med. 1975;135(5):698–700.
Staub JJ, Noelpp B, Girard J, Baumann JB, Graf S, Ratcliffe JG. The short metyrapone test: comparison of the plasma ACTH response to metyrapone and insulin-induced hypoglycaemia. Clin Endocrinol. 1979;10(6):595–601.
Dolman LI, Nolan G, Jubiz W. Metyrapone test with adrenocorticotrophic levels. Separating primary from secondary adrenal insufficiency. JAMA. 1979;241(12):1251–3.
Feek CM, Bevan JS, Ratcliffe JG, Gray CE, Blundell G. The short metyrapone test: comparison of the plasma ACTH response to metyrapone with the cortisol response to insulin-induced hypoglycaemia in patients with pituitary disease. Clin Endocrinol. 1981;15(1):75–80.
Berneis K, Staub JJ, Gessler A, Meier C, Girard J, Muller B. Combined stimulation of adrenocorticotropin and compound-S by single dosemetyrapone test as an outpatient procedure to assess hypothalamic-pituitary-adrenal function. J Clin Endocrinol Metab. 2002;87(12):5470–5.
English K, Inder WJ. Prospective evaluation of a week one overnight metyrapone test with subsequent dynamic assessments of hypothalamic-pituitary-adrenal axis function after pituitary surgery. Clin Endocrinol. 2017;87(1):35–43.
Hartzband PI, Van Herle AJ, Sorger L, Cope D. Assessment of hypothalamic-pituitary-adrenal (HPA) axis dysfunction: comparison of ACTH stimulation, insulin-hypoglycemia and metyrapone. J Endocrinol Invest. 1988;11(11):769–76.
Fiad TM, Kirby JM, Cunningham SK, McKenna TJ. The overnight single-dose metyrapone test is a simple and reliable index of the hypothalamic-pituitary-adrenal axis. Clin Endocrinol. 1994;40(5):603–9.
Steiner H, Bahr V, Exner P, Oelkers PW. Pituitary function tests: comparison of ACTH and 11-deoxy-cortisol responses in the metyrapone test and with the insulin hypoglycemia test. Exp Clin Endocrinol. 1994;102(1):33–8.
Schlaghecke R, Kornely E, Santen RT, Ridderskamp P. The effect of long-term glucocorticoid therapy on pituitary-adrenal responses to exogenous corticotropin-releasing hormone. N Engl J Med. 1992;326(4):226–30.
Maghnie M, Uga E, Temporini F, Di Iorgi N, Secco A, Tinelli C, et al. Evaluation of adrenal function in patients with growth hormone deficiency and hypothalamic-pituitary disorders: comparison between insulin-induced hypoglycemia, low-dose ACTH, standard ACTH and CRH stimulation tests. Eur J Endocrinol. 2005;152(5):735–41.
Kokshoorn NE, Romijn JA, Roelfsema F, Rambach AH, Smit JW, Biermasz NR, et al. The use of an early postoperative CRH test to assess adrenal function after transsphenoidal surgery for pituitary adenomas. Pituitary. 2012;15(3):436–44.
de Vries F, Lobatto DJ. Early postoperative HPA-axis testing after pituitary tumor surgery: reliability and safety of basal cortisol and CRH test. Endocrine. 2020;67(1):161–71.
Kano T, Sugihara H, Sudo M, Nagao M, Harada T, Ishizaki A, et al. Comparison of pituitary-adrenal responsiveness between insulin tolerance test and growth hormone-releasing peptide-2 test: a pilot study. Peptides. 2010;31(4):657–61.
Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60.
Mozid AM, Tringali G, Forsling ML, Hendricks MS, Ajodha S, Edwards R, et al. Ghrelin is released from rat hypothalamic explants and stimulates corticotrophin-releasing hormone and arginine-vasopressin. Horm Metab Res. 2003;35(8):455–9.
Hayakawa T, Kitamura T, Tamada D, Mukai K, Hayashi R, Takahara M, et al. Evaluation of hypothalamic-pituitary-adrenal axis by the GHRP2 test: comparison with the insulin tolerance test. J Endocr Soc. 2018;2(8):860–9.
Arimura H, Hashiguchi H, Yamamoto K, Shinnakasu A, Arimura A, Kikuchi A, et al. Investigation of the clinical significance of the growth hormone-releasing peptide-2 test for the diagnosis of secondary adrenal failure. Endocr J. 2016;63(6):533–44.
Gasco V, Berton A, Caprino MP, Karamouzis I, Maccario M, Ghigo E, et al. Acylated ghrelin as provocative test for the diagnosis of ACTH deficiency in patients with hypothalamus-pituitary disease. Endocrine. 2015;50(2):474–82.
Grossman A. Assessment of the HPA Axis: another new test? Endocrine. 2015;50(2):268–9.
Fridman-Bengtsson O, Hoybye C, Porthen L, Stjarne P, Hulting AL, Sunnergren O. Evaluation of different hydrocortisone treatment strategies in transsphenoidal pituitary surgery. Acta Neurochir. 2019;161(8):1715–21.
Cozzi R, Lasio G, Cardia A, Felisati G, Montini M, Attanasio R. Perioperative cortisol can predict hypothalamus-pituitary-adrenal status in clinically non-functioning pituitary adenomas. J Endocrinol Invest. 2009;32(5):460–4.
Marko NF, Hamrahian AH, Weil RJ. Immediate postoperative cortisol levels accurately predict postoperative hypothalamic-pituitary-adrenal axis function after transsphenoidal surgery for pituitary tumors. Pituitary. 2010;13(3):249–55.
Borg H, Siesjö P, Kahlon B, Fjalldal S, Erfurth EM. Perioperative serum cortisol levels in ACTH sufficient and ACTH deficient patients during transsphenoidal surgery of pituitary adenoma. Endocrine. 2018;62(1):83–9.
Qaddoura A, Shalung TN, Meier MP, Goguen J, Jing R, Zhang S, et al. Recovery room cortisol predicts long-term glucocorticoid need after Transsphenoidal surgery for pituitary tumors. Neurosurgery. 2019;84(3):616–23.
Marko NF, Weil RJ. A comparative effectiveness analysis of alternative strategies to assess hypothalamic-pituitary-adrenal axis function after microsurgical resection of pituitary tumors. Neurosurgery. 2011;68(6):1576–84.
Courtney CH, McAllister AS, McCance DR, Bell PM, Hadden DR, Leslie H, et al. Comparison of one week 0900 h serum cortisol, low and standard dose synacthen tests with a 4 to 6 week insulin hypoglycaemia test after pituitary surgery in assessing HPA axis. Clin Endocrinol. 2000b;53(4):431–6.
Jia X, Pendharkar AV, Loftus P, Dodd RL, Chu O, Fraenkel M, et al. Utility of a glucocorticoid sparing strategy in the management of patients following transsphenoidal surgery. Endocr Pract. 2016;22(9):1033–9.
Sterl K, Thompson B, GossCW DRG, Rich KM, Zipfel GJ, et al. Withholding perioperative steroids in patients undergoing Transsphenoidal resection for pituitary disease: randomized prospective clinical trial to assess safety. Neurosurgery. 2019;85(2):E226–e32.
Prencipe N, Parasiliti-Caprino M, Gatti F, Penner F, Berton AM, Bona C, et al. Second day morning cortisol levels after transsphenoidal surgery are accurate predictors of secondary adrenal insufficiency with diagnostic cut-offs similar to non-stressed conditions. Neuroendocrinology. 2020.
Ferrari E, Cravello L, Muzzoni B, Casarotti D, Paltro M, Solerte SB, et al. Age-related changes of the hypothalamic-pituitaryadrenal axis: pathophysiological correlates. Eur J Endocrinol. 2001;144(4):319–29.
Elbuken G, Tanriverdi F, Karaca Z, Kula M, Gokahmetoglu S, Unluhizarci K, et al. Comparison of salivary and calculated free cortisol levels during low and standard dose of ACTH stimulation tests in healthy volunteers. Endocrine. 2015;48(2):439–43.
Roelfsema F, van Heemst D, IranmaneshA TP, Yang R, Veldhuis JD. Impact of age, sex and body mass index on cortisol secretion in 143 healthy adults. Endocr Connect. 2017;6(7):500–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest of the authors.
Ethical approval
There is no ethical approval or informed consent since the article is a review article, not a study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Karaca, Z., Grossman, A. & Kelestimur, F. Investigation of the Hypothalamo-pituitary-adrenal (HPA) axis: a contemporary synthesis. Rev Endocr Metab Disord 22, 179–204 (2021). https://doi.org/10.1007/s11154-020-09611-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-020-09611-3