Abstract
Weight-based (WB: 0.03 mg/kg) and fixed dose (FD: 1–1.5 mg) regimens of the glucagon stimulation test (GST) have been used to evaluate GH and cortisol secretion in children and adults, respectively. However, experience of the WB regimen in assessing GH and cortisol secretion in adults are limited. We describe a multicenter experience using WB and FD regimens in evaluating GH and cortisol secretion in adults suspected of GH deficiency and central adrenal insufficiency. Retrospective case series of GSTs (n = 515) performed at five tertiary centers. Peak and nadir glucose, and peak GH and peak cortisol responses occurred later with WB (mean dose: 2.77 mg) compared to FD (mean dose: 1.20 mg) regimens. Main side-effects were nausea and vomiting, particularly in younger females. Nausea was comparable but vomiting was more frequent in the WB regimen (WB: 10.0 % vs FD: 2.4 %; P < 0.05). Peak and nadir glucose, ΔGH, and peak and Δcortisol were higher in the WB regimen. In both regimens, age correlated negatively with peak cortisol levels, and body mass index (BMI), fasting, peak and nadir glucose correlated negatively with peak GH levels. WB and FD regimens can induce adult GH and cortisol secretion, but peak responses occur later in the WB regimen. Both regimens are relatively safe, and vomiting was more prevalent in the WB regimen. As age, BMI, and glucose tolerance negatively correlated with peak GH and cortisol levels, the WB regimen may be more effective than the FD regimen in older overweight glucose intolerant patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The insulin tolerance test (ITT) is considered the gold standard test in assessing GH reserve in adults, while the GHRH-arginine test has been endorsed by several consensus guidelines [1–3] as the main alternative when the ITT is contraindicated, with the exception of recent hypothalamic GH deficiency (GHD) [4]. Before the GHRH analogue (Geref®) was withdrawn in the United States in 2008, the glucagon stimulation test (GST) was infrequently used for diagnosing adult GHD [5]. The unavailability of Geref® has prompted increasing utilization of the GST in the United States as its alternative test for assessing GH reserve in adults [6].
The GST has been used to evaluate GH and cortisol secretion in both adults [7, 8] and children [9, 10]. The advantages of the GST are the availability of glucagon and possible efficacy in patients with hypothalamic GHD [10]; however the test has not been well-studied in large numbers of adults. Furthermore, the optimal duration to perform the GST and the influence of body mass index (BMI), fasting glucose and glycemic excursions on glucagon-induced GH and cortisol secretion are unclear.
Previous GST studies using the fixed dose (FD) regimen (1 mg for body weight ≤90 kg and 1.5 mg for body weight >90 kg) have demonstrated reliability in diagnosing adult GHD [7, 11, 12], but these studies enrolled patients who had known pre-established diagnosis of GHD [11–13] or hypothalamic-pituitary disease [7]. Conversely, the weight-based (WB) regimen has been validated with the ITT in assessing GH reserve [10] and hypothalamic–pituitary–adrenal (HPA) [9] axes in children. However, no WB dosing studies have yet been performed to assess the utility of the GST in adults suspected of GHD and/or central adrenal insufficiency (CAI).
The aims of this study were to: (1) describe the clinical characteristics of the WB and FD dosing regimens from a large number of GSTs in adults; (2) compare the WB to the FD regimens in the time frame of inducing GH and cortisol secretion; and (3) assess the safety of the two dosing regimens.
Patients and methods
Patients
Data from all the GSTs (n = 515) performed from September 2009 to August 2011 at 5 tertiary care centers [Oregon Health and Science University (OHSU), Massachusetts General Hospital (MGH), Stanford University Hospital (SUH), The Johns Hopkins University Hospital (JHUH), and Allegheny General Hospital (AGH)] were analyzed retrospectively. All patients were referred for testing of suspected GHD and in a subset of patients, possible CAI. Time points for peak GH, cortisol, and blood glucose levels, and the occurrence and timing of side effects were noted. The study was approved by the institutional review boards at all institutions.
Protocols for the GSTs
All subjects were tested between 0800 h and 1000 h after an overnight fast. Three centers (OHSU, MGH and SUH) performed FD GSTs over 4 h (4 h-FD); one center (JHUH) performed FD GSTs over 3 h (3 h-FD); and one center (AGH) performed WB (0.03 mg/kg) GSTs over 4 h.
For all GSTs, an intravenous catheter was inserted and blood samples for baseline glucose, IGF-I, GH levels were collected. In a subset of patients in the 4 h-FD (n = 185) and in all patients in the WB regimen (n = 90), samples for measuring ACTH and cortisol levels were also requested by the attending physician. For FD regimens, 1 mg (body weight ≤90 kg) or 1.5 mg (body weight >90 kg) of glucagon was used. In all the tests, glucagon (GlucaGen™, Novo Nordisk, Bagsvaerd, Denmark) was administered intramuscularly. For the 4 h-FD and WB regimens, serum samples for GH, glucose and cortisol levels were obtained at 30, 60, 90, 120, 150, 180, 210 and 240 min, and for the 3 h-FD regimen, serum samples were obtained at 90, 120, 150 and 180 min. Patients on glucocorticoid replacement therapy withheld their morning dose, and patients with known diabetes withheld their anti-diabetic medications the morning of testing until test completion. Peak GH and peak cortisol responses of ≥3 μg/L [11, 12] and ≥9.1 μg/dL [14] were considered normal responses, respectively. All patients were asked to report side-effects associated with this test, and anti-emetics and rescue oral juice were administered at the discretion of the supervising physician at each center.
Protocol for the cosyntropin stimulation test
The cosyntropin stimulation test (CST) to assess for CAI was performed in 89 of the 185 patients in the 4 h-FD regimen that also had serum cortisol levels measured. After intravenous administration of 250 μg of cosyntropin, serum ACTH levels were measured at baseline and serum cortisol levels were measured at baseline, 30 and 60 min. Peak cortisol response ≥18 μg/dL was considered normal [15]. The CSTs were performed between 0800 h and 1000 h at least 7 days apart from the GST within one month.
Assays
All GSTs and CSTs were performed by physicians and nurses according to local clinical practices. Serum IGF-I levels were analyzed using methods previously described [16], and glucose levels were analyzed by a glucose analyzer (Beckman Glucose Analyzer; Fullerton, CA) at each participating center. At OHSU and MGH, serum GH levels were measured by a chemiluminescent immunoassay (Siemens Immulite 2000; Siemens Healthcare Diagnostics, Deerfield, IL) with the minimal detection limit of 0.05 μg/L. At SUH and AGH, serum GH levels were measured by chemiluminescent immunoassay (DPC Immulite 2000; Los Angeles, CA), and the minimal detection limits were 0.1 μg/L at SUH, and 0.05 μg/L at AGH. At JHUH, serum GH levels were measured by immunoenzymometric assay (Tosoh Bioscience; San Francisco, CA), and the minimal detection limit was 0.1 μg/L. Serum cortisol levels at OHSU were measured by a competitive binding immunoenzymatic assay (Beckman Coulter UniCel DxI 800, Brea, CA) with a total coefficient of variation of 5.1–7.6 %, and at MGH, by a chemiluminescent immunoassay (Abbott Diagnostics, Chicago, IL) with a total coefficient of variation of 2.5–7.7 %. Peak GH and peak cortisol values from all centers were combined for purposes of this analysis.
Statistical analyses
Statistical analyses were performed using SPSS for Windows (version 18.0, SPSS Inc., Chicago, IL). All data were expressed as mean ± SEM, except for the blood glucose data in Fig. 1 expressed as mean ± SD. Distributions of residuals were examined for normality by graphical methods. For continuous data, comparisons between the FD and WB regimens and between the 4 h-FD, 3 h-FD and WB regimens were performed using the Mann–Whitney U-test and two-way repeated measures ANOVA, respectively. For categorical data, Chi-squared or Fisher’s exact test were performed. Correlations between variables were determined by Pearson’s or Spearman’s correlation coefficients. P values <0.05 were considered statistically significant.
Results
Patient demographics (Table 1)
In total, 515 GSTs using two different regimens were performed. The three groups were well-matched with regard to BMI and gender distribution, but patients in the WB regimen were older than those in the 4 h-FD and 3-h FD regimens (ANOVA, P = 0.01). Approximately two-thirds of the patients received 1 mg glucagon in both FD regimens, whereas in the WB regimen, the mean glucagon dose administered was 2.77 mg (range 1.0–5.7 mg) (ANOVA, P < 0.001).
Clinical characteristics and biochemical responses to the GSTs
Blood glucose levels (Fig. 1)
The majority of blood glucose levels peaked between 30 and 90 min (98.7 %) and achieved nadir levels between 150 and 240 min (86.9 %) in the 4 h-FD regimen, and peaked mainly at 90 min (95.8 %) and achieved nadir levels between 150 and 180 min (81.3 %) in the 3 h-FD regimen (blood samples were not collected at 30 and 60 min in the 3 h-FD regimen). In the WB regimen, the majority of blood glucose levels peaked between 60 and 150 min (88.1 %) and reached nadir levels between 210 and 240 min (89.5 %). The highest peak glucose levels were observed in patients with diabetes mellitus: 482 mg/dL at 90 min in the 4 h-FD regimen, 280 mg/dL at 150 min in the WB regimen, and 201 mg/dL at 90 min in the 3 h-FD regimen. The lowest nadir glucose level was 37 mg/dL at 150 min in the 4 h-FD regimen, 46 mg/dL at 210 min in the WB regimen, and 40 mg/dL at 180 min in the 3 h-FD regimen.
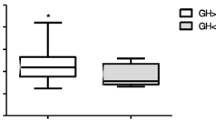
Serum GH and cortisol levels (Fig. 2)
The majority of peak GH levels in the 4 h-FD (74.9 %) and 3 h-FD (75.0 %) regimens occurred mainly between 120 and 180 min, whereas in the WB regimen, peak GH levels occurred mainly between 180 and 240 min (93.2 %). In the 4 h-FD regimen, peak cortisol levels occurred mainly between 150 and 180 min (51.3 %), whereas in the WB regimen, peak cortisol levels occurred mainly between 210 and 240 min (80.0 %).
A plot of individual peak cortisol responses to the GST against the CST in 89 patients. Empty circles represent peak cortisol levels in patients on oral estrogen replacement therapy. Dotted line indicates the arbitrary and accepted cortisol cutpoints for GST and CST respectively. To convert cortisol to nmol/L multiply by 27.59
Comparison of biochemical parameters between FD and WB regimens (Table 2)
Fasting (P < 0.001), peak (P < 0.01), and nadir (P < 0.001) glucose levels, but not Δglucose (peak-nadir glucose), were higher in the WB compared to the FD regimen. Basal GH levels were lower (P < 0.001), ΔGH (basal-peak GH) were higher (P < 0.01), and peak GH levels were similar in the WB compared to the FD regimen. Basal morning serum ACTH and cortisol levels were comparable with the two regimens, but peak (P < 0.01) and Δcortisol (basal-peak cortisol) (P < 0.001) were higher in the WB regimen. In the FD and WB regimens, 65 (20.8 %) and 29 (32.2 %) patients had impaired fasting glucose (IFG) levels (>100 mg/dL) respectively. Compared to patients with normal fasting glucose levels (≤100 mg/dL), patients with IFG in the FD (n = 65, 20.8 %) and WB (n = 29, 32.2 %) regimens achieved higher peak, nadir and Δglucose, and lower peak and ΔGH responses (data not shown). Compared to the FD regimen, patients with normal fasting glucose levels in the WB regimen achieved higher fasting, peak and nadir glucose, and lower Δglucose levels (data not shown). In contrast, no differences were observed in peak and Δcortisol responses in patients with normal compared to IFG in both regimens (data not shown).
Correlations (Table 3 and Fig. 3)
In the FD regimen, age correlated negatively with peak GH (all P < 0.01) and cortisol (P < 0.001) levels, whereas fasting, peak and nadir glucose, and BMI correlated negatively with peak GH (all P < 0.01) but not cortisol levels. In the WB regimen, fasting, peak and nadir glucose, and BMI (all P < 0.05) negatively correlated with peak GH levels, whereas age correlated negatively with peak cortisol levels (P < 0.001). However, peak cortisol levels did not correlate with fasting, peak, nadir and Δglucose, or BMI in either dose regimen. In the subset of patients that underwent the CST (n = 89), peak cortisol levels correlated positively to those induced by the FD regimen (r = 0.58; P < 0.0001).
Side-effects of the GSTs (Tables 4, 5)
The most common reported side-effects for both regimens were nausea and vomiting. Nausea rate was comparable in the WB (44.4 %) and the FD (37.2 %) regimens, and mainly occurred between 150 and 210 min in the WB (97.5 %), and between 60 and 150 min in the FD regimen (94.3 %). Vomiting rates were higher in the WB compared to the FD regimens (10.0 vs 2.4 %; P < 0.05). In the WB and FD regimens, 12 (30.8 %) and 21(13.3 %) patients received anti-emetics, and 4 (10.3 %) and 12 (7.6 %) patients received rescue oral juice for neuroglycopenic symptoms respectively.
Patients who experienced nausea in the FD regimens were younger, had higher BMIs and were more likely to be females; conversely patients in the WB regimen were also younger and were more likely to be females but had comparable BMIs. Patients in the WB regimen that experienced nausea and vomiting had higher peak GH, ΔGH, peak cortisol and Δcortisol (all P < 0.05). There were no differences between those that experienced nausea to those without nausea in fasting, peak, nadir and Δglucose, IGF-I, and basal morning ACTH and cortisol levels in the two regimens. The number of patients with nausea in the WB regimen that failed the GST for GH and cortisol responses were lower (all P < 0.05) compared to those without nausea.
Other reported side-effects included hunger, headaches, sleepiness, body chills, lightheadedness, and abdominal cramping that occurred mainly between 60 and 210 min (data not shown). Most of these events were rated as “mild” or “moderate” in severity, and all side-effects resolved by 240 min.
Discussion
To our knowledge, this is the largest case series evaluating the use of GSTs in inducing GH and cortisol secretion in adults suspected of GHD and CAI. Compared to the FD regimen, the WB regimen induced higher peak and nadir glucose levels, with peak GH and peak cortisol levels occurring later in the test. Vomiting was more prevalent in the WB regimen, and nausea and vomiting affected younger females more frequently. Both regimens were relatively safe and all side-effects resolved upon test completion. Age, BMI and glucose tolerance may impact glucagon-induced GH and cortisol secretion, and a positive correlation was observed between peak cortisol levels induced by the FD regimen and those with the CST. Overall, WB and FD regimens were effective in inducing GH and cortisol secretion in adults, but peak GH and peak cortisol levels occur at different time points in these two regimens.
Few studies have examined the differing glycemic excursions associated with the WB and FD regimens. In this study, we analyzed these regimens based on fasting glucose levels and noted that patients with IFG, particularly in the WB regimen, had higher peak, nadir and Δglucose levels than those with normal fasting glucose levels. A rise and fall in blood glucose levels can be expected due to glucagon-induced hepatic glucose output with subsequent insulin secretion [17], but such variability in blood glucose responses may reflect differing insulin responses to higher doses of glucagon, underlying hepatic insulin resistance, and the degree of fasting hyperglycemia. These results indicate that variations in timing and magnitude of blood glucose responses exist that are dependent upon the underlying glucose tolerance and glucagon doses used, and raises the question of whether interpreting the GST should take into account the patients’ fasting blood glucose levels.
Consistent with previous studies [9, 18, 19], the majority of peak GH levels in the WB regimen occurred later compared to the FD regimen. Not only peak GH and peak cortisol levels occurred later, but ΔGH and peak and Δcortisol were higher in the WB regimen; however firm conclusions cannot be made in this regard because different GH assays were used among centers. Patients in the WB regimen had comparable rates of nausea, but vomiting was reported more frequently than in the FD regimen. In both regimens, patients who experienced nausea and vomiting were younger and tended to be females. In addition, fasting, peak, nadir and Δglucose were similar in both regimens with nausea compared to those without nausea, suggesting that these symptoms are independent of the glucagon-induced glycemic fluctuations. The late rise of peak GH and peak cortisol levels and the greater magnitude of ΔGH and Δcortisol with the WB regimen may relate to the more severe “stress-like” symptoms of nausea and vomiting that preceded or coincided with the hormonal responses. Several investigators did not observe any association of glucagon-induced GH [19, 20] and cortisol [21] secretion with changes in blood glucose levels. Our study, thus, reinforces the likelihood that the mechanism of GH and cortisol stimulation in GSTs are more dependent on the glucagon doses used rather than glycemic fluctuations per se.
The accuracy of GH and cortisol dynamic tests has been questioned because of the influence of age, BMI, insulin resistance, and central adiposity [8, 13, 14, 22, 23] on GH and cortisol responses. We found that age correlated negatively with peak GH and peak cortisol levels in the FD regimen and with peak cortisol levels in the WB regimen, whereas fasting, peak and nadir glucose and BMI correlated negatively with peak GH in both regimens. As there is a known inverse relationship between peak GH response to GHRH-arginine stimulation and glucose levels [22] and central adiposity [13], the impact of glucose intolerance and BMI on peak GH and peak cortisol responses to glucagon stimulation observed in this study is of clinical relevance. Using the receiver operator characteristic curve analysis, two studies reported that the GST GH cutpoint level of 3 μg/L reliably differentiated patients with GHD from healthy adults [11, 12] with no association between BMI and peak GH levels [12]. These studies, however, were relatively small and included patients who had a pre-established diagnosis of adult GHD and lower BMIs. Our study was not designed to determine specific GH cutpoint levels for GSTs based on age, BMI and glucose tolerance, but does highlight the need for additional prospective studies involving gold standard tests such as the ITT and a control group to provide definitive evidence in establishing specific cutpoint levels.
The diagnosis of CAI can be difficult particularly in patients with recent pituitary surgery or brain irradiation when the adrenal cortex may still be responsive to stress but the hypothalamic-pituitary function is compromised. To address this notion, a number of studies have investigated the utility of GST in evaluating the HPA axis [7, 18, 24]. Our findings in the FD regimen are in line with previous studies demonstrating that peak cortisol responses occurred mainly between 150 and 180 min [7, 25]. Interestingly, there were 28 (15.1 %) and 6 (6.6 %) patients in the FD and WB regimens respectively whose peak cortisol levels were observed at baseline. This observation has been reported before [7, 19], and presumably reflects the stress associated with the testing procedure itself. More recently, di Iorgi et al. [9] reported the accuracy of the GST in diagnosing CAI in children with GHD. In line with that study, we found a positive correlation between peak cortisol levels of the GST and CST, demonstrating the potential utility of the GST in assessing adult HPA axis. Notably, all patients in our study who failed the GST with a cortisol cutpoint of 9.1 μg/dL also failed the CST. Although different cortisol assays were used, our results imply that the cortisol cutpoint for the GST might be lower than that validated for the CST.
Prior GST studies have reported that the rates of side-effects ranged from 15 to 50 % [7, 19], with nausea being the commonest. In our study, nausea ranged from 37.2 to 43.3 % in both regimens, occurring mainly between 90 and 150 min in the FD regimen and between 120 and 210 min in the WB regimen. Other side-effects such as vomiting and headaches have also been reported in 10–34 % of patients [7, 20] that are compatible with our findings. Importantly, all side-effects resolved by 240 mins of the test.
Previous studies have proposed shortening the FD GSTs to 3 h [26, 27], and evaluating GH and cortisol levels between 3 and 5 time points (0, 90, 120, 150 and 180 min) as the majority of GH and cortisol peaks occur between 120 and 180 min [7, 18]. Our data indicate that 3-h GSTs will miss 6.6 % of GH peaks and 15.1 % of cortisol peaks with the FD regimen, and 71.9 % of GH peaks and 80 % of cortisol peaks with the WB regimen. Hence, prolonging the FD and WB regimens to 4 h would seem reasonable to avoid misclassifying adults with GHD and CAI.
Limitations while conducting a multi-center retrospective study are acknowledged. First, there may be ascertainment bias at different institutions, and pooling data from different patient subsets. Second, referral for testing of patients suspected of GHD and/or CAI reflects local clinical practice and may vary among centers. Third, different GH assays were used between the centers. While these limitations are inherent to such a multi-center study [28], our study does provide data on large subject numbers that are difficult to attain in single-center studies. Fourth, oral estrogen replacement in females may yield falsely elevated serum total cortisol levels due to increased cortisol binding globulin levels [29] and GH levels due to its action in impairing GH-induced hepatic IGF-I generation [30]. As only about 9 % of females in this study were taking oral estrogen and all but only one patient on oral estrogen passed and failed both the GST and CST, we do not feel that patients on oral estrogen substantially impacted the cortisol and GH data. Finally, our study lacked a healthy control group to determine the sensitivity and specificity of GH and cortisol cutpoints. Despite these limitations, our study shows for the first time the efficacy of the WB regimen in stimulating GH and cortisol secretion in adults.
In conclusion, the WB and FD regimens can induce adult GH and cortisol secretion, but peak GH and peak cortisol responses were observed later with higher rates of vomiting, particularly in younger females, in the WB regimen. Both WB and FD regimens were relatively safe. To ensure that delayed peak GH and peak cortisol responses are not missed, we recommend performing the FD and WB regimens of GSTs over 4 h. As age, BMI, and glucose tolerance may influence GH and cortisol responses to glucagon stimulation, the FD regimen may be more appropriate for younger normoglycemic patients whose body weight is less than 90 kg, whereas the WB regimen may be more appropriate for older overweight patients with glucose intolerance. Thus, further refinements to the GH and cortisol cutpoints for the GST are required to improve the diagnostic accuracy of the WB and FD regimens in reliably assessing GH and cortisol secretion in adults.
References
Cook DM, Yuen KC, Biller BM, Kemp SF, Vance ML (2009) American Association of clinical endocrinologists medical guidelines for clinical practice for growth hormone use in growth hormone-deficient adults and transition patients—2009 update. Endocr Pract 15(suppl 2):1–29
Ho KK (2007) Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: a statement of the GH Research Society in association with the European Society for Pediatric Endocrinology, Lawson Wilkins Society, European Society of Endocrinology, Japan Endocrine Society, and Endocrine Society of Australia. Eur J Endocrinol 157:695–700
Molitch ME, Clemmons DR, Malozowski S, Merriam GR, Vance ML (2011) Evaluation and treatment of adult growth hormone deficiency: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 96:1587–1609
Darzy KH, Aimaretti G, Wieringa G, Gattamaneni HR, Ghigo E, Shalet SM (2003) The usefulness of the combined growth hormone (GH)-releasing hormone and arginine stimulation test in the diagnosis of radiation-induced GH deficiency is dependent on the post-irradiation time interval. J Clin Endocrinol Metab 88:95–102
Brabant G, Poll EM, Jonsson P, Polydorou D, Kreitschmann-Andermahr I (2009) Etiology, baseline characteristics, and biochemical diagnosis of GH deficiency in the adult: are there regional variations? Eur J Endocrinol 161(Suppl 1):S25–S31
Yuen KC, Biller BM, Molitch ME, Cook DM (2009) Clinical review: is lack of recombinant growth hormone (GH)-releasing hormone in the United States a setback or time to consider glucagon testing for adult GH deficiency? J Clin Endocrinol Metab 94:2702–2707
Leong KS, Walker AB, Martin I, Wile D, Wilding J, MacFarlane IA (2001) An audit of 500 subcutaneous glucagon stimulation tests to assess growth hormone and ACTH secretion in patients with hypothalamic-pituitary disease. Clin Endocrinol (Oxf) 54:463–468
Micmacher E, Assumpcao RP, Redorat RG, Spina LD, Cruz IC, Silva CA, Vaisman M, Conceicao FL (2009) Growth hormone secretion in response to glucagon stimulation test in healthy middle-aged men. Arq Bras Endocrinol Metabol 53:853–858
di Iorgi N, Napoli F, Allegri A, Secco A, Calandra E, Calcagno A, Frassinetti C, Ghezzi M, Ambrosini L, Parodi S, Gastaldi R, Loche S, Maghnie M (2010) The accuracy of the glucagon test compared to the insulin tolerance test in the diagnosis of adrenal insufficiency in young children with growth hormone deficiency. J Clin Endocrinol Metab 95:2132–2139
Secco A, di Iorgi N, Napoli F, Calandra E, Ghezzi M, Frassinetti C, Parodi S, Casini MR, Lorini R, Loche S, Maghnie M (2009) The glucagon test in the diagnosis of growth hormone deficiency in children with short stature younger than 6 years. J Clin Endocrinol Metab 94:4251–4257
Conceicao FL, e Silva A, Leal Costa AJ, Vaisman M (2003) Glucagon stimulation test for the diagnosis of GH deficiency in adults. J Endocrinol Invest 26:1065–1070
Gomez JM, Espadero RM, Escobar-Jimenez F, Hawkins F, Pico A, Herrera-Pombo JL, Vilardell E, Duran A, Mesa J, Faure E, Sanmarti A (2002) Growth hormone release after glucagon as a reliable test of growth hormone assessment in adults. Clin Endocrinol (Oxf) 56:329–334
Makimura H, Stanley T, Mun D, You SM, Grinspoon S (2008) The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J Clin Endocrinol Metab 93:4254–4260
Karaca Z, Lale A, Tanriverdi F, Kula M, Unluhizarci K, Kelestimur F (2011) The comparison of low and standard dose ACTH and glucagon stimulation tests in the evaluation of hypothalamo-pituitary-adrenal axis in healthy adults. Pituitary 14:134–140
Neary N, Nieman L (2010) Adrenal insufficiency: etiology, diagnosis and treatment. Curr Opin Endocrinol Diabetes Obes 17:217–223
Biller BM, Samuels MH, Zagar A, Cook DM, Arafah BM, Bonert V, Stavrou S, Kleinberg DL, Chipman JJ, Hartman ML (2002) Sensitivity and specificity of six tests for the diagnosis of adult GH deficiency. J Clin Endocrinol Metab 87:2067–2079
Huypens P, Ling Z, Pipeleers D, Schuit F (2000) Glucagon receptors on human islet cells contribute to glucose competence of insulin release. Diabetologia 43:1012–1019
Orme SM, Price A, Weetman AP, Ross RJ (1998) Comparison of the diagnostic utility of the simplified and standard i.m. glucagon stimulation test (IMGST). Clin Endocrinol (Oxf) 49:773–778
Rao RH, Spathis GS (1987) Intramuscular glucagon as a provocative stimulus for the assessment of pituitary function: growth hormone and cortisol responses. Metabolism 36:658–663
Giuffrida FM, Berger K, Monte L, Oliveira CH, Hoff AO, Maciel RM, Vieira JG (2009) Relationship between GH response and glycemic fluctuations in the glucagon stimulation test. Growth Horm IGF Res 19:77–81
Waldhausl W, Haydl H, Nowotny P (1976) ACTH and cortisol responses to glucagon stimulation. J Clin Endocrinol Metab 43:675–678
Carmichael JD, Danoff A, Milani D, Roubenoff R, Lesser ML, Livote E, Reitz RE, Ferris S, Kleinberg DL (2006) GH peak response to GHRH-arginine: relationship to insulin resistance and other cardiovascular risk factors in a population of adults aged 50–90. Clin Endocrinol (Oxf) 65:169–177
Colao A, Di Somma C, Savastano S, Rota F, Savanelli MC, Aimaretti G, Lombardi G (2009) A reappraisal of diagnosing GH deficiency in adults: role of gender, age, waist circumference, and body mass index. J Clin Endocrinol Metab 94:4414–4422
Arvat E, Maccagno B, Ramunni J, Maccario M, Giordano R, Broglio F, Camanni F, Ghigo E (2000) Interaction between glucagon and human corticotropin-releasing hormone or vasopressin on ACTH and cortisol secretion in humans. Eur J Endocrinol 143:99–104
Littley MD, Gibson S, White A, Shalet SM (1989) Comparison of the ACTH and cortisol responses to provocative testing with glucagon and insulin hypoglycaemia in normal subjects. Clin Endocrinol (Oxf) 31:527–533
Cain JP, Williams GH, Dluhy RG (1972) Glucagon-initiated human growth hormone release: a comparative study. Can Med Assoc J 107:617–622
Mitchell ML, Byrne MJ, Sanchez Y, Sawin CT (1970) Detection of growth-hormone deficiency: the glucagon stimulation test. N Engl J Med 282:539–541
Hazem A, Elamin M, Malaga G, Bancos I, Prevost Y, Zeballos-Palacios C, Velasquez ER, Erwin PJ, Natt N, Montori VM, Murad M (2011) The accuracy of diagnostic tests for growth hormone deficiency in adults: a systematic review and meta-analysis. Eur J Endocrinol 165:841–849
Meulenberg PM, Ross HA, Swinkels LM, Benraad TJ (1987) The effect of oral contraceptives on plasma-free and salivary cortisol and cortisone. Clin Chim Acta 165:379–385
Leung KC, Johannsson G, Leong GM, Ho KK (2004) Estrogen regulation of growth hormone action. Endocr Rev 25:693–721
Acknowledgments
KCJY, BMKB and MBG have received research grants and consulting honoraria from Novo Nordisk. RS and DMC have received consulting honoraria from Novo Nordisk. This research did not receive any specific grant from any funding agency in the public, commercial or non-profit sector.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yuen, K.C.J., Biller, B.M.K., Katznelson, L. et al. Clinical characteristics, timing of peak responses and safety aspects of two dosing regimens of the glucagon stimulation test in evaluating growth hormone and cortisol secretion in adults. Pituitary 16, 220–230 (2013). https://doi.org/10.1007/s11102-012-0407-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-012-0407-7