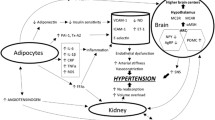
Abstract
Abdominal obesity and elevated blood pressure commonly occur in the same patient and are key components of the metabolic syndrome. However, the association between obesity and increased blood pressure is variable. We review mechanisms linking cardiovascular and metabolic disease in such patients including altered systemic and regional hemodynamic control, neurohumoral activation, and relative natriuretic peptide deficiency. Moreover, we discuss recent results using omics techniques providing insight in molecular pathways linking adiposity, metabolic disease, and arterial hypertension. Recognition of the mechanisms orchestrating the crosstalk between cardiovascular and metabolic regulation in individual patients may lead to better and more precise treatments. It is reassuring that recently developed cardiovascular and metabolic medications may in fact ameliorate, both, cardiovascular and metabolic risks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Clinical and epidemiological evidence
Abdominal adiposity and elevated blood pressure are diagnostic criteria for the metabolic syndrome. Both risk factors commonly occur in the same patient. In a survey among German primary care physicians, the prevalence of arterial hypertension was 34.3, 60.6, and >70 % in normal weight, overweight, and obese patients, respectively.[1] Conversely, approximately 75 % of the patients with arterial hypertension were overweight or obese.[1] In overweight participants of the Framingham Heart Study who were followed up to 44 years, age-adjusted relative risks for developing arterial hypertension were 1.75 in men and 1.8 in women.[2] Indeed, epidemiological surveys suggest that 60 to 70 % of arterial hypertension cases could be explained by excess adiposity.[3] Regression models correcting for age-related blood pressure changes showed an increase in systolic blood pressure of 1 mm Hg for a gain of 1.7 kg/m2 and 4.5 cm in men and 1.3 kg/m2 and 2.5 cm in women in body mass index or waist circumference, respectively.[4] In the Jackson Heart Study, which is a cohort study comprised of African Americans, the prevalence of arterial hypertension was 48.9, 59.6, and 68.7 % in normal weight, in overweight, and in obese participants, respectively.[5] Among male adolescents in this cohort, excess body weight was associated with an increase in blood pressure.[6]
Excess adiposity makes it more difficult controlling blood pressure in hypertensive patients and is a common cause of treatment resistant arterial hypertension.[7] In German primary care practices, odds ratios for controlling blood pressure to values <140/90 mm Hg in in patients with arterial hypertension were 0.8 in overweight patients, 0.6 in grade 1, 0.5 in grade 2, and 0.7 in grade 3 obese patients.[1] Obesity was associated with poor blood pressure control in multiple studies including the Framingham Heart Study [8], the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial [9], and the National Health and Nutrition Examination Surveys [10].
Overall, the evidence that excess adiposity can promote arterial hypertension is compelling. However, the relationship between adiposity and blood pressure is complex. For example, in Pima Indians, blood pressure appears to respond less to increased adiposity [11] and observations in large cohorts regarding the clustering of metabolic syndrome components support this idea. In the Framingham Offspring Cohort, three distinct while overlapping physiological domains of the insulin resistance syndrome were differentiated using factor analysis including a “central metabolic syndrome”, impaired glucose tolerance, and arterial hypertension.[12] Another analysis suggested that a metabolic factor comprising hyperinsulinemia/insulin resistance, dyslipidemia, and obesity may be linked to hypertension through shared correlation with hyperinsulinemia/insulin resistance.[13] In the Kuopio Ischemic Heart Disease Risk Factor Study, clustering of metabolic syndrome components using self-organizing maps revealed that hypertension may be a part of the insulin resistance syndrome.[14] The authors also concluded that a single physiological mechanism could not explain all the clusters. Whether or not hypertension is part of the metabolic syndrome has been debated. Perhaps, excess adiposity increases blood pressure only in susceptible persons. Indeed, we suggested earlier that blood pressure can be “fat sensitive” and “fat resistant” [15]
Mechanisms of obesity-associated arterial hypertension have been extensively discussed in recent reviews. We will review mechanisms that could explain the variable association between obesity and increased blood pressure. Moreover, we will discuss mechanisms orchestrating cardiovascular and metabolic crosstalk that could be therapeutically addressed.
2 Hemodynamic mechanisms in obesity and hypertension
Blood pressure is determined by cardiac output and systemic vascular resistance. Yet, whether adiposity-induced increases in blood pressure are secondary to increased cardiac output, increased vascular resistance, or both mechanisms combined is difficult to ascertain. In an earlier study applying dye dilution, cardiac output (but not index) was increased whereas vascular resistance was decreased in obese compared with normal weight individuals.[16] Obese individuals also exhibited an increase in intravascular volume.[16] Cardiac output determined by the Fick technique [17] or radionuclide ventriculography [18] was also increased in obese individuals. Increased cardiac output and reduced systemic vascular resistance has also been observed in dogs with diet-induced obesity.[19] Diet-induced obesity was also associated with sodium retention and increased extracellular fluid volume.[19]
In healthy persons, cardiac output is tuned to meet the metabolic demands of peripheral tissues. Obviously, more tissue requires more cardiac output. Commonly, cardiac index is calculated by adjusting cardiac output for calculated body surface area. Such an adjustment does not account for obesity-associated changes in adipose and lean tissue mass. At least in some studies, cardiac index was not increased in obese normotensive or hypertensive individuals.[16, 20] In a study comparing lean and obese patients with and without hypertension, forearm blood flow expressed per tissue volume was similar in all four groups. Unlike in lean hypertensives, forearm vascular resistance was not increased in obese hypertensives.[21] Compared with lean subjects, individuals with obesity or the metabolic syndrome showed increases in brachial artery diameter, forearm blood flow per unit tissue volume, and forearm vascular conductance.[22] In obese Zucker rats, cardiac output per unit body weight was decreased compared with lean control animals.[23] When blood flow was assessed in different tissues using the microsphere technique, obese animals showed unchanged or reduced blood flow per unit tissue weight.[23] Overall, adiposity-associated increases in cardiac output are at least in part explained by increases in tissue mass.
Unlike in lean hypertensive individuals, excess vascular resistance may be less prominent in obesity associated hypertension. Yet, there must be a mismatch between cardiac output and vascular resistance for blood pressure to increase. Remarkably, attenuated postprandial muscular vasodilation may contribute to impaired glucose metabolism in obesity.[24] Therefore, hemodynamic regulation at the systemic and organ level could provide a mechanistic link between elevated blood pressure and metabolic traits in patients with the metabolic syndrome. Vascular resistance is affected by vascular diameter, blood vessel length, and blood viscosity. The latter two mechanisms are usually ignored. Vascular length could conceivably change in obese individuals, particularly in adipose tissue. Earlier studies showed increases in blood viscosity, plasma protein, hematocrit, and red cell rigidity in hypertensive compared with normotensive men.[25, 26] A rather small study suggested that rheological properties of red cells may be worsened in severely obese individuals.[27] It has been suggested that increased blood viscosity could contribute to an increase in blood pressure in obesity.[28] It is tempting to speculate that all these mechanisms regulating vascular resistance could also affect peripheral insulin and nutrient supply. Indeed, microvascular dysfunction secondary to obesity has been proposed as a pathophysiological link between arterial hypertension and insulin resistance.[29]
3 Neurohumoral activation in obesity-associated hypertension
Neurohumoral mechanisms have been implicated in the pathogenesis of obesity-associated arterial hypertension. Both, renin angiotensin aldosterone system (RAAS) and sympathetic nervous system appear to be involved.
Perhaps, the strongest evidence for involvement of the sympathetic nervous system in the pathogenesis of human obesity-associated hypertension stems from pharmacological studies. Combined treatment with alpha- and beta- adrenoreceptor antagonists was more effective in lowering blood pressure in obese compared with lean hypertensive patients.[30] Pharmacological blockade of ganglionic nicotinic acetylcholine receptors nearly completely interrupts sympathetic and parasympathetic traffic and can be utilized gauging autonomic contributions to blood pressure.[31] Using this approach, blood pressure was shown to me more dependent on autonomic nervous system activity in obese compared with lean individuals.[32] Direct efferent sympathetic nerve recordings using microneurography provided evidence for an increase in centrally generated sympathetic activity.[33, 34] Yet, kinetic studies assessing whole body and organ-specific norepinephrine spillover suggested that the obesity-associated sympathetic activation is not uniform. Obese normotensive subjects exhibit increased renal norepinephrine spillover, whereas cardiac norepinephrine spillover is reduced. Obese hypertensive patients on the other hand show increases in, both, renal and cardiac norepinephrine spillover.[35]
Several mechanisms may contribute to sympathetic activation in obese individuals and explain the large variability in “fat sensitivity”. In fact, sympathetic activity does not increase with increasing adiposity in Pima Indians who are also less likely to develop arterial hypertension.[11] The leptin melanocortin pathway appears to be particularly important in coupling adiposity to arterial hypertension as evidenced by carefully conducted pharmacological and physiological studies in genetic mouse models.[36, 37] In fact, blood pressure and urinary norepinephrine excretion are lower in obese individuals with genetic melanocortin 4 receptor deficiency compared with obese controls.[38] Moreover, human monogenic obesity due to leptin deficiency appears to be associated with reduced sympathetic activity.[39] However, leptin receptor deficient db/db mice showed increases in heart rate and blood pressure and responded more to sympathetic inhibition.[40] Thus, the leptin melanocortin pathway may not be the sole mechanisms increasing sympathetic activity in obesity. Baroreflex dysfunction, obstructive sleep apnea, and hyperinsulinemia may also contribute to sympathetic activation.[34, 41–43] Finally, obesity and metabolic syndrome further potentiate the sympathetic activation in heart failure patients.[44]
Weight loss attenuates sympathetic activation and blood pressure in obese individuals.[45, 46] The reduction in sympathetic activity appears to be more pronounced during the active weight loss phase compared with weight maintenance.[47]
Even though plasma volume and sodium retention are increased as discussed above, the systemic RAAS tends to be activated in obese individuals.[48] Adipose tissue also expresses all RAAS components and may contribute to systemic RAAS activity. Unlike the systemic RAAS, the adipose tissue RAAS does not respond to changes in sodium intake suggesting that it is regulated by different physiological pathways.[49] Moreover, adipose tissue secrets factors eliciting mineralocorticoid release.[50, 51] Weight loss ameliorates systemic and adipose tissue renin-angiotensin system activity.[48, 52] Yet, in the Third Generation Framingham Heart Study participants, plasma renin activity, serum aldosterone concentrations, and the aldosterone to renin ratio were not correlated with body mass index or regional adiposity measurements obtained by abdominal computed tomography.[53]
4 Neurohumoral mechanisms in cardiometabolic crosstalk
Muscular insulin sensitivity is determined by vascular glucose and insulin delivery to the muscular interstitial space, insulin mediated cellular glucose uptake, and intracellular glucose metabolism.[54] Given the central role of the renin angiotensin system and the sympathetic nervous system in regulating vascular tone, both could limit nutrient supply to peripheral tissues. Furthermore, both systems may directly affect glucose uptake and intracellular metabolism. For example, angiotensin may perturb insulin signaling through oxidative stress and NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells) activation.[55, 56]
Physiological and pharmacological studies suggest that the sympathetic nervous system may affect peripheral glucose metabolism. Sympathetic nerve activity was significantly increased in patients with the metabolic syndrome compared with control subjects even in the absence of hypertension. Moreover, sympathetic activity was correlated with the homeostasis model assessment (HOMA) index in this population.[57] Progression from metabolic syndrome to type 2 diabetes mellitus appears to associated with a further increase in central sympathetic drive and altered norepinephrine disposition [58] However, these associations cannot prove causality or direction of an association between sympathetic activity and glucose metabolism. In fact, insulin can elicit sympathetic activation.[59]
In obese hypertensive patients, chronic central sympathetic inhibition with moxonidine improved blood pressure and insulin sensitivity determined by hyperinsulinemic euglycemic clamp testing.[60] Near complete autonomic inhibition with the ganglionic blocker trimethaphan acutely improved insulin sensitivity in insulin resistant obese individuals.[61] Furthermore, the non-selective alpha-adrenoreceptor antagonist phentolamine improved forearm glucose uptake in patients with congestive heart failure [62], which is associated with sympathetic activation and insulin resistance. In contrast, forearm glucose uptake did not change when phentolamine was infused in healthy young subjects.[62] Axillary plexus blockade increased forearm blood flow but did not alter glucose uptake in healthy individuals.[63] Electrical carotid sinus stimulation elicits variable reductions in sympathetic activity and blood pressure in patients with resistant arterial hypertension.[64, 65] Yet, acute changes in baroreceptor stimulation did not elicit significant changes in muscular glucose delivery or whole body insulin sensitivity.[66] Epinephrine, but not norepinephrine, also increases hepatic glucose production while inhibiting insulin secretion.
Sympathetic mechanisms could also elicit beneficial metabolic responses. In fact, the system is important in regulating lipid mobilization from adipose tissue. Recent studies provide evidence for the adipokine leptin to promote catecholamine release from sympathetic nerve terminals specifically wrapped around adipocytes.[67] The system appears to regulate lipolysis locally. Moreover, catecholamines promote browning of white adipose tissue [68], which may augment energy expenditure through non-shivering thermogenesis as well as lipid and glucose homeostasis.[69] The mechanism could conceivably limit adiposity and insulin resistance in patients with the metabolic syndrome.[69] In human subjects, however, brown adipose tissue activity and body fat content are negatively correlated with each other [70, 71], likely due to whitening of classical brown adipose tissue depots [72]. Moreover, maneuvers promoting brown adipose tissue activation in human subjects, such as cold exposure, also increase arterial blood pressure.[73]
Overall, the exact contribution of the sympathetic nervous system to the different components of the metabolic syndrome in the setting of obesity deserves further study. Perhaps specific parts of the sympathetic nervous system could be targeted to make use of its beneficial effects while avoiding negative aspects on cardiovascular and metabolic control.
In hypertensive patients, aldosterone plasma concentrations were positively correlated with fasting plasma insulin, C-peptides, and homeostasis model assessment (HOMA) index.[74] Patients with primary hyperaldosteronism also exhibit reductions in insulin sensitivity that improves following surgical tumor removal or pharmacological aldosterone receptor blockade.[75]
In some studies, animals genetically overexpressing renin angiotensin system components featured insulin resistance that responded to pharmacological renin angiotensin system inhibition.[76] Yet, in another study, mouse renin transgenic rats showed arterial hypertension while whole body, liver, skeletal muscle, and adipose tissue responded normally to insulin.[77] In human subjects, the insertion / deletion polymorphism of the angiotensin converting enzyme was associated with insulin sensitivity and glucose tolerance.[78]
Improvements in insulin resistance with renin-angiotensin system inhibition in human subjects were heterogeneous. In a small crossover study, angiotensin converting enzyme inhibition did not alter insulin sensitivity determined by hyperinsulinemic euglycemic clamp testing.[79] In another study using a similar design, angiotensin receptor blockade with valsartan ameliorated insulin sensitivity in impaired fasting glucose and/or impaired glucose tolerance.[80]
5 Relative natriuretic peptide deficiency in obesity and insulin resistance
Atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) are released from cardiac atria and ventricles, respectively. Both peptides are produced as preprohormone and are stored as prohomones in intracellular granules. Once released, natriuretic peptides elicit cardiovascular, renal, and metabolic responses primarily through guanylyl cyclase-coupled natriuretic peptide receptor (NPR) A. NPR-C, which is sometimes referred to as scavenger receptor, is devoid of guanylyl cyclase activity and facilitates cellular natriuretic peptide uptake and degradation.[81] NPR-C is highly expressed in human adipose tissue.[82] In addition, natriuretic peptides are enzymatically cleaved by neprilysin.[83]
Obesity, which is the prime risk factor for type 2 diabetes mellitus, is associated with reduced cardiac ANP gene expression in rats [84] and systemic natriuretic peptide deficiency in large epidemiological surveys.[85] Moreover, in obese individuals circulating ANP levels increased less with acute volume loading compared with lean control subjects.[86] Increased degradation of natriuretic peptides may be involved as well. Indeed, compared to non-obese, normotensive individuals, NPR-C expression is increased in adipose tissue of obese hypertensive patients.[87] Insulin induces NPRC mRNA expression in human adipocytes [88] and might, hence, link conditions associated with hyperinsulinemia, such as the metabolic syndrome and obesity to relative natriuretic peptide deficiency.
Natriuretic peptide deficiency in patients with components of the metabolic syndrome could predispose to arterial hypertension. In fact, natriuretic peptides elicit vasodilation and renal sodium excretion. Moreover, natriuretic peptides are physiological renin-angiotensin-aldosterone-system opponents and may attenuate sympathetic nerve activity.[89, 90] Obese hypertensive men while ingesting more sodium nevertheless exhibited reduced natriuretic peptide levels compared with a lean control group.[91] In obese hypertensive subjects, weight loss through hypocaloric dieting augmented renal and hemodynamic responses to ANP infusions.[92] The response may be explained in part by NPR-C down-regulation with weight loss.[93]
In addition to affecting blood pressure, natriuretic peptide deficiency may contribute to metabolic disease. Natriuretic peptides promote lipid mobilization from human adipose tissue in physiologically relevant concentrations.[94–96] Moreover, ANP acutely increases postprandial lipid oxidation in human subjects.[97] Both, in skeletal muscle cells and in adipocytes, natriuretic peptides enhance mitochondrial oxidative capacity and lipid oxidation.[98, 99] ANP infusion also raises circulating adiponectin concentrations.[100] Increasing circulating natriuretic peptide availability through genetic overexpression or pharmacological treatment improves blood glucose control and insulin sensitivity in mouse models.[101, 102] Finally, individuals with lower circulating midregional proANP concentrations were at higher risk for developing type 2 diabetes mellitus after adjustment for relevant risk factors including BMI.[103] Augmentation of cGMP through soluble guanylyl cyclase stimulation or phosphodiesterase 5 inhibition may also elicit metabolic improvements.[104, 105]
Common variants in the genes encoding ANP and BNP affect their circulating levels.[106] Recently, the micro RNA miR-425 was shown to negatively regulate ANP production and a common genetic variant makes ANP production resistant to miR-425.[107] Posttranslational proBNP modification appears to regulate BNP release.[108] Polymorphisms in the genes encoding ANP and BNP contribute to variability in blood pressure [106] and the risk for type 2 diabetes following adjustment for age, gender, and body mass index [109]. Together, these data suggest that natriuretic peptides might link obesity and the metabolic syndrome to arterial hypertension [110]
6 Molecular signatures linking adiposity and blood pressure
There has been much hype and some hope that genome-wide expression, metabolomics, and other methodologies in large cohorts could provide insight in the molecular underpinnings of any condition, including the link between adiposity and blood pressure. Metabolite profiling in participants of the Framingham Heart and Malmö Diet and Cancer studies revealed common metabolic signatures associated with metabolic syndrome components including adiposity and systolic blood pressure.[111] In particular, increased glutamine concentrations were associated with lower blood pressure and insulin levels. Moreover, glutamine supplementation improved glucose tolerance and blood pressure in mice.[111] However, the underlying mechanisms are poorly understood. Glutamine supplementation acutely increased circulating glucagon-like peptide 1 levels in human subjects [112] and at least in part rescued experimental endothelial dysfunction in mice [113].
The idea that common mechanisms could link metabolic and cardiovascular disease, particularly elevated blood pressure, is supported by a genome-wide association study in a very large sample. The authors identified common genetic variants predicting impaired glucose metabolism, elevated blood pressure, and relative increases in visceral fat. [114] Similarly, a pathway and network analysis suggested that there are genes with pleiotropic effects on cardiovascular and metabolic traits.[115] Yet, another analysis arrives at the conclusion that genes involved in lipid metabolism confer susceptibility to the metabolic syndrome with little evidence for a link with hypertension and glucose metabolism.[116] Thus far, none of these findings has made a difference in diagnosing or treating obese hypertensive patients. Except for the metabolomics data, the pathophysiological insight provided by these large-scale studies is limited.
7 Individualized therapies addressing several components of metabolic and vascular disease
The variety of therapeutic options to treat obesity associated hypertension, type 2 diabetes and obesity increased during the last decades. Treatment choices depend more and more on pleiotropic drug actions. Medications improving arterial blood pressure as well as other cardiometabolic conditions and vice versa are now becoming available.
There has been much interest in using RAAS inhibitors as cardiometabolic drugs. In fact, in addition to addressing metabolic dysfunction associated with RAAS activation, some drugs may have additional metabolic off-target effects. For example, some angiotensin II subtype 1 receptor antagonists also engaged peroxisome proliferator-activated receptors gamma.[117, 118] Renin inhibition with aliskiren was effective in lowering blood pressure in obese hypertensive patients.[119] The drug achieves therapeutically relevant concentrations in human metabolic target tissues including skeletal muscle and adipose tissue.[120] Because the drug substantially improved glucose metabolism in animal models [76], detailed metabolic studies in patients would make sense. Among patients with impaired glucose tolerance and cardiovascular disease or risk factors, angiotensin receptor blockade with valsartan for 5 years, along with lifestyle modification, led to a 14 % relative reduction in type 2 diabetes incidence.[121] However, in the Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication (DREAM) study, ramipril compared with placebo did not reduce the risk of new onset type 2 diabetes mellitus in patients with impaired fasting glucose levels.[122] In any case, RAAS inhibitors remain a good choice for hypertensive patients at increased metabolic risk. Yet, RAAS inhibition alone does not suffice to address obesity-associated metabolic disease.
Inhibition of neprilysin, the neutral endopeptidase responsible for cleaving natriuretic peptides among other substrates, is another potential “cardiometabolic” treatment approach. The combined angiotensin receptor and neprilysin inhibitor LCZ696 (sacubitril/valsartan) has recently been introduced for the treatment of heart failure. In the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial, LCZ696 was superior to enalapril in reducing the risks of death and heart failure hospitalizations.[123]. LCZ696 was also effective in treating arterial hypertension.[124] Studies testing metabolic responses to LCZ696 are ongoing.
Similarly, new medications improving glucose metabolism in type 2 diabetes also affect blood pressure and body weight.[125, 126] Sodium glucose co-transporter 2 (SGLT2) is a low-affinity, high-capacity member of a large family of co-transporters that is highly expressed in the proximal renal tubule where it is responsible for the reabsorption of ~90 % of filtered glucose.[127] Genetic SGLT2 deletion increases urinary glucose excretion thereby protecting mice from diet-induced obesity while preserving glucose metabolism.[128] Rare SGLT2 mutations are a cause of familial glucosuria.[129] Meanwhile, several selective SGLT2 inhibitors have been developed and approved for the treatment of type 2 diabetes. These drugs improve fasting and postprandial glycaemia by enhancing urinary glucose excretion. The osmotic diuresis secondary to increased glucose excretion may contribute to 3–5 mmHg systolic and 1–3 mmHg diastolic blood pressure reduction.[130] Moreover, the negative energy balance through glucose loss is associated with body weight reduction. In the EMPA-REG outcome trial, SGLT2 inhibition with empagliflozin compared to standard therapy plus placebo resulted in superiority in regards to the primary composite cardiovascular endpoint, hospitalization for heart failure, cardiovascular mortality and all-cause mortality [131].
The gut hormone glucagon-like peptide-1 (GLP-1) leads to a glucose dependent and rapid release of insulin. GLP-1 analogs are in clinical use for the treatment of type 2 diabetes. Since some of them also lead to substantial weight loss, specific GLP-1 receptor agonists (GLP-1 RA), such as liraglutide, are approved for the treatment of obesity.[130] Interestingly, GLP-1 analogs have been shown to modestly lower blood pressure.[125, 132, 133] The mechanism through which GLP-1 agonism lowers blood pressure is incompletely understood. Remarkably, the reduction in blood pressure is observed prior to significant weight loss, although weight loss likely contributes to the long-term maintenance of arterial blood pressure reduction. In animal models, GLP-1 agonism induced ANP release from the heart.[134] However, in hypertensive patients with type 2 diabetes, treatment with the GLP-1 agonist liraglutide did not increase plasma ANP concentrations.[135] In contrast to SGLT2 inhibitors, GLP-1 agonists produce variable increases in heart rate. Central nervous GLP-1 receptor stimulation may raise sympathetic activity.[136] However, recent findings suggest that GLP-1 mediated increases in heart rate may result from direct GLP-1 receptor stimulation in cardiac atria rather than changes in cardiac sympathetic control.[137, 138] Overall, several therapeutic approaches that have been specifically developed for cardiovascular or therapeutic indications may have utility in addressing a broader spectrum of cardiometabolic risks.
8 Conclusion
A single underlying mechanism cannot explain all metabolic syndrome components in all patients. Nevertheless, its metabolic traits and arterial hypertension share common underlying mechanisms. Recognition of the mechanisms orchestrating the crosstalk between cardiovascular and metabolic regulation in individual patients may lead to better and more precise treatments. It is reassuring that recently developed cardiovascular and metabolic medications may in fact ameliorate both, cardiovascular and metabolic risks. One potential risk is that current clinical development paths are somewhat narrowly focused on either metabolic or cardiovascular conditions and do not account for the individual underlying pathophysiology. Time will tell whether or not “precision medicine” [139], which has the goal to target treatments to the individual disease mechanism, will in fact result in better treatments for the metabolic syndrome patient in front of us.
References
Bramlage P, Pittrow D, Wittchen HU, Kirch W, Boehler S, Lehnert H, et al. Hypertension in overweight and obese primary care patients is highly prevalent and poorly controlled. Am J Hypertens. 2004;17(10):904–10.
Wilson PW, D’Agostino RB, Sullivan L, Parise H, Kannel WB. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med. 2002;162(16):1867–72.
Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282(16):1523–9.
Doll S, Paccaud F, Bovet P, Burnier M, Wietlisbach V. Body mass index, abdominal adiposity and blood pressure: consistency of their association across developing and developed countries. Int J Obes Relat Metab Disord. 2002;26(1):48–57.
Wyatt SB, Akylbekova EL, Wofford MR, Coady SA, Walker ER, Andrew ME, et al. Prevalence, awareness, treatment, and control of hypertension in the Jackson Heart Study. Hypertension. 2008;51(3):650–6.
Bruce MA, Beech BM, Griffith DM, Thorpe Jr RJ. Weight status and blood pressure among adolescent African American males: the Jackson Heart KIDS Pilot Study. Ethn Dis. 2015;25(3):305–12.
Jordan J, Yumuk V, Schlaich M, Nilsson PM, Zahorska-Markiewicz B, Grassi G, et al. Joint statement of the European Association for the Study of Obesity and the European Society of Hypertension: obesity and difficult to treat arterial hypertension. J Hypertens. 2012;30(6):1047–55.
Lloyd-Jones DM, Evans JC, Larson MG, O’donnell CJ, Roccella EJ, Levy D. Differential control of systolic and diastolic blood pressure : factors associated with lack of blood pressure control in the community. Hypertension. 2000;36(4):594–9.
Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens (Greenwich). 2002;4(6):393–404.
Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation. 2011;124:1046–58.
Weyer C, Pratley RE, Snitker S, Spraul M, Ravussin E, Tataranni PA. Ethnic differences in insulinemia and sympathetic tone as links between obesity and blood pressure. Hypertension. 2000;36(4):531–7.
Meigs JB, D’Agostino Sr RB, Wilson PW, Cupples LA, Nathan DM, Singer DE. Risk variable clustering in the insulin resistance syndrome. the Framingham Offspring Study. Diabetes. 1997;46(10):1594–600.
Chen W, Srinivasan SR, Elkasabany A, Berenson GS. Cardiovascular risk factors clustering features of insulin resistance syndrome (Syndrome X) in a biracial (Black-White) population of children, adolescents, and young adults: the Bogalusa Heart Study. Am J Epidemiol. 1999;150(7):667–74.
Valkonen VP, Kolehmainen M, Lakka HM, Salonen JT. Insulin resistance syndrome revisited: application of self-organizing maps. Int J Epidemiol. 2002;31(4):864–71.
Jordan J, Engeli S, Redon J, Sharma AM, Luft FC, Narkiewicz K, et al. European Society of Hypertension Working Group on Obesity: background, aims and perspectives. J Hypertens. 2007;25(4):897–900.
Messerli FH, Christie B, DeCarvalho JG, Aristimuno GG, Suarez DH, Dreslinski GR, et al. Obesity and essential hypertension. hemodynamics, intravascular volume, sodium excretion, and plasma renin activity. Arch Intern Med. 1981;141(1):81–5.
de Divitiis O, Fazio S, Petitto M, Maddalena G, Contaldo F, Mancini M. Obesity and cardiac function. Circulation. 1981;64(3):477–82.
Licata G, Scaglione R, Barbagallo M, Parrinello G, Capuana G, Lipari R, et al. Effect of obesity on left ventricular function studied by radionuclide angiocardiography. Int J Obes. 1991;15(4):295–302.
Hall JE, Brands MW, Dixon WN, Smith Jr MJ. Obesity-induced hypertension. renal function and systemic hemodynamics. Hypertension. 1993;22(3):292–9.
Mujais SK, Tarazi RC, Dustan HP, Fouad FM, Bravo EL. Hypertension in obese patients: hemodynamic and volume studies. Hypertension. 1982;4(1):84–92.
Raison JM, Safar ME, Cambien FA, London GM. Forearm haemodynamics in obese normotensive and hypertensive subjects. J Hypertens. 1988;6(4):299–303.
Limberg JK, Kellawan JM, Harrell JW, Johansson RE, Eldridge MW, Proctor LT, et al. Exercise-mediated vasodilation in human obesity and metabolic syndrome: effect of acute ascorbic acid infusion. Am J Physiol Heart Circ Physiol. 2014;307(6):H840–7.
Lucas PD, Foy JM. Effects of experimental diabetes and genetic obesity on regional blood flow in the rat. Diabetes. 1977;26(8):786–92.
Baron AD, Laakso M, Brechtel G, Hoit B, Watt C, Edelman SV. Reduced postprandial skeletal muscle blood flow contributes to glucose intolerance in human obesity. J Clin Endocrinol Metab. 1990;70(6):1525–33.
Tibblin G, Bergentz SE, Bjure J, Wilhelmsen L. Hematocrit, plasma protein, plasma volume, and viscosity in early hypertensive disease. Am Heart J. 1966;72(2):165–76.
Sandhagen B, Frithz G, Waern U, Ronquist G. Increased whole blood viscosity combined with decreased erythrocyte fluidity in untreated patients with essential hypertension. J Intern Med. 1990;228(6):623–6.
Levy Y, Elias N, Cogan U, Yeshurun D. Abnormal erythrocyte rheology in patients with morbid obesity. Angiology. 1993;44(9):713–7.
Messerli FH. Cardiovascular effects of obesity and hypertension. Lancet. 1982;1(8282):1165–8.
Karaca U, Schram MT, Houben AJ, Muris DM, Stehouwer CD. Microvascular dysfunction as a link between obesity, insulin resistance and hypertension. Diabetes Res Clin Pract. 2014;103(3):382–7.
Wofford MR, Anderson Jr DC, Brown CA, Jones DW, Miller ME, Hall JE. Antihypertensive effect of alpha- and beta-adrenergic blockade in obese and lean hypertensive subjects. Am J Hypertens. 2001;14(7 Pt 1):694–8.
Shannon JR, Jordan J, Diedrich A, Pohar B, Black BK, Robertson D, et al. Sympathetically mediated hypertension in autonomic failure. Circulation. 2000;101:2710–5.
Shibao C, Gamboa A, Diedrich A, Ertl AC, Chen KY, Byrne DW, et al. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49(1):27–33.
Grassi G, Seravalle G, Cattaneo BM, Bolla GB, Lanfranchi A, Colombo M, et al. Sympathetic activation in obese normotensive subjects. Hypertension. 1995;25(4 Pt 1):560–3.
Grassi G, Seravalle G, Dell’Oro R, Turri C, Bolla GB, Mancia G. Adrenergic and reflex abnormalities in obesity-related hypertension. Hypertension. 2000;36(4):538–42.
Rumantir MS, Vaz M, Jennings GL, Collier G, Kaye DM, Seals DR, et al. Neural mechanisms in human obesity-related hypertension. J Hypertens. 1999;17(8):1125–33.
Rahmouni K, Haynes WG, Mark AL. Cardiovascular and sympathetic effects of leptin. Curr Hypertens Rep. 2002;4(2):119–25.
Hall JE, Da Silva AA, do Carmo JM, Dubinion J, Hamza S, Munusamy S. Obesity-induced hypertension: role of sympathetic nervous system, leptin, and melanocortins. J Biol Chem. 2010;285(23):17271–6.
Greenfield JR, Miller JW, Keogh JM, Henning E, Satterwhite JH, Cameron GS, et al. Modulation of blood pressure by central melanocortinergic pathways. N Engl J Med. 2009;360(1):44–52.
Ozata M, Ozdemir IC, Licinio J. Human leptin deficiency caused by a missense mutation: multiple endocrine defects, decreased sympathetic tone, and immune system dysfunction indicate new targets for leptin action, greater central than peripheral resistance to the effects of leptin, and spontaneous correction of leptin-mediated defects. J Clin Endocrinol Metab. 1999;84(10):3686–95.
Goncalves AC, Tank J, Diedrich A, Hilzendeger A, Plehm R, Bader M, et al. Diabetic hypertensive leptin receptor-deficient db/db mice develop cardioregulatory autonomic dysfunction. Hypertension. 2009;53(2):387–92.
Grassi G, Facchini A, Trevano FQ, Dell’Oro R, Arenare F, Tana F, et al. Obstructive sleep apnea-dependent and -independent adrenergic activation in obesity. Hypertension. 2005;46(2):321–5.
Narkiewicz K, van de Borne PJH, Cooley RL, Dyken ME, Somers VK. Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation. 1998;98(8):772–6.
Jamerson KA, Julius S, Gudbrandsson T, Andersson O, Brant DO. Reflex sympathetic activation induces acute insulin resistance in the human forearm. Hypertension. 1993;21(5):618–23.
Grassi G, Seravalle G, Quarti-Trevano F, Scopelliti F, Dell’Oro R, Bolla G, et al. Excessive sympathetic activation in heart failure with obesity and metabolic syndrome: characteristics and mechanisms. Hypertension. 2007;49(3):535–41.
Seravalle G, Colombo M, Perego P, Giardini V, Volpe M, Dell’Oro R, et al. Long-term sympathoinhibitory effects of surgically induced weight loss in severe obese patients. Hypertension. 2014;64(2):431–7.
Straznicky NE, Lambert EA, Grima MT, Eikelis N, Richards K, Nestel PJ, et al. The effects of dietary weight loss on indices of norepinephrine turnover: modulatory influence of hyperinsulinemia. Obesity (Silver Spring). 2014;22(3):652–62.
Straznicky NE, Grima MT, Eikelis N, Nestel PJ, Dawood T, Schlaich MP, et al. The effects of weight loss versus weight loss maintenance on sympathetic nervous system activity and metabolic syndrome components. J Clin Endocrinol Metab. 2011;96(3):E503–8.
Tuck ML, Sowers J, Dornfeld L, Kledzik G, Maxwell M. The effect of weight reduction on blood pressure, plasma renin activity, and plasma aldosterone levels in obese patients. N Engl J Med. 1981;304(16):930–3.
Engeli S, Boschmann M, Frings P, Beck L, Janke J, Titze J, et al. Influence of salt intake on renin-angiotensin and natriuretic peptide system genes in human adipose tissue. Hypertension. 2006;48(6):1103–8.
Ehrhart-Bornstein M, Lamounier-Zepter V, Schraven A, Langenbach J, Willenberg HS, Barthel A, et al. Human adipocytes secrete mineralocorticoid-releasing factors. Proc Natl Acad Sci U S A. 2003;100(24):14211–6.
Vleugels K, Schinner S, Krause D, Morawietz H, Bornstein SR, Ehrhart-Bornstein M, et al. ERK1/2 MAPKs and Wnt signaling pathways are independently involved in adipocytokine-mediated aldosterone secretion. Exp Clin Endocrinol Diabetes. 2011;119(10):644–8.
Engeli S, Bohnke J, Gorzelniak K, Janke J, Schling P, Bader M, et al. Weight loss and the renin-angiotensin-aldosterone system. Hypertension. 2005;45(3):356–62.
O’Seaghdha CM, Hwang SJ, Vasan RS, Larson MG, Hoffmann U, Wang TJ, et al. Correlation of renin angiotensin and aldosterone system activity with subcutaneous and visceral adiposity: the framingham heart study. BMC Endocr Disord. 2012;12:3.
Wasserman DH. Four grams of glucose. Am J Physiol Endocrinol Metab. 2009;296(1):E11–21.
Zhou MS, Schulman IH, Raij L. Role of angiotensin II and oxidative stress in vascular insulin resistance linked to hypertension. Am J Physiol Heart Circ Physiol. 2009;296(3):H833–9.
Zhou MS, Liu C, Tian R, Nishiyama A, Raij L. Skeletal muscle insulin resistance in salt-sensitive hypertension: role of angiotensin II activation of NFkappaB. Cardiovasc Diabetol. 2015;14:45.
Grassi G, Dell’Oro R, Quarti-Trevano F, Scopelliti F, Seravalle G, Paleari F, et al. Neuroadrenergic and reflex abnormalities in patients with metabolic syndrome. Diabetologia. 2005;48(7):1359–65.
Straznicky NE, Grima MT, Sari CI, Eikelis N, Lambert EA, Nestel PJ, et al. Neuroadrenergic dysfunction along the diabetes continuum: a comparative study in obese metabolic syndrome subjects. Diabetes. 2012;61(10):2506–16.
Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest. 1991;87(6):2246–52.
Haenni A, Lithell H. Moxonidine improves insulin sensitivity in insulin-resistant hypertensives. J Hypertens Suppl. 1999;17(3):S29–35.
Gamboa A, Okamoto LE, Arnold AC, Figueroa RA, Diedrich A, Raj SR, et al. Autonomic blockade improves insulin sensitivity in obese subjects. Hypertension. 2014;64(4):867–74.
Gomes ME, Mulder A, Bellersen L, Verheugt FW, Smits P, Tack CJ. Alpha-receptor blockade improves muscle perfusion and glucose uptake in heart failure. Eur J Heart Fail. 2010;12(10):1061–6.
Lind L, Eriksson M. Influence of nervous blockade on insulin-mediated glucose uptake in the human forearm. Metabolism. 2003;52(4):413–7.
Heusser K, Tank J, Engeli S, Diedrich A, Menne J, Eckert S, et al. Carotid baroreceptor stimulation, sympathetic activity, baroreflex function, and blood pressure in hypertensive patients. Hypertension. 2010;55(3):619–26.
Heusser K, Tank J, Brinkmann J, Menne J, Kaufeld J, Linnenweber-Held S, et al. Acute response to unilateral unipolar electrical carotid sinus stimulation in patients with resistant arterial hypertension. Hypertension. 2016.
May M, Ahrens J, Menne J, Haller H, Beige J, Eckert S, et al. Limited acute influences of electrical baroreceptor activation on insulin sensitivity and glucose delivery: a randomized, double-blind, cross-over clinical study. Diabetes. 2014.
Zeng W, Pirzgalska RM, Pereira MM, Kubasova N, Barateiro A, Seixas E, et al. Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell. 2015;163(1):84–94.
Laurila PP, Soronen J, Kooijman S, Forsstrom S, Boon MR, Surakka I, et al. USF1 deficiency activates brown adipose tissue and improves cardiometabolic health. Sci Transl Med. 2016;8(323):323ra13.
Hanssen MJ, van der Lans AA, Brans B, Hoeks J, Jardon KM, Schaart G, et al. Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes. 2015.
Vijgen GH, Bouvy ND, Teule GJ, Brans B, Schrauwen P, van Marken Lichtenbelt WD. Brown adipose tissue in morbidly obese subjects. PLoS One. 2011;6(2):e17247.
Yoneshiro T, Aita S, Matsushita M, Okamatsu-Ogura Y, Kameya T, Kawai Y, et al. Age-related decrease in cold-activated brown adipose tissue and accumulation of body fat in healthy humans. Obesity (Silver Spring). 2011;19(9):1755–60.
Blondin DP, Labbe SM, Noll C, Kunach M, Phoenix S, Guerin B, et al. Selective impairment of glucose but not fatty acid or oxidative metabolism in brown adipose tissue of subjects with type 2 diabetes. Diabetes. 2015;64(7):2388–97.
Kingma BR, Frijns AJ, Saris WH, van Steenhoven AA, Lichtenbelt WD. Increased systolic blood pressure after mild cold and rewarming: relation to cold-induced thermogenesis and age. Acta Physiol (Oxf). 2011;203(4):419–27.
Colussi G, Catena C, Lapenna R, Nadalini E, Chiuch A, Sechi LA. Insulin resistance and hyperinsulinemia are related to plasma aldosterone levels in hypertensive patients. Diabetes Care. 2007;30(9):2349–54.
Catena C, Lapenna R, Baroselli S, Nadalini E, Colussi G, Novello M, et al. Insulin sensitivity in patients with primary aldosteronism: a follow-up study. J Clin Endocrinol Metab. 2006;91(9):3457–63.
Habibi J, Whaley-Connell A, Hayden MR, DeMarco VG, Schneider R, Sowers SD, et al. Renin inhibition attenuates insulin resistance, oxidative stress, and pancreatic remodeling in the transgenic Ren2 rat. Endocrinology. 2008;149(11):5643–53.
Vettor R, Cusin I, Ganten D, Rohner-Jeanrenaud F, Ferrannini E, Jeanrenaud B. Insulin resistance and hypertension: studies in transgenic hypertensive TGR(mREN-2)27 rats. Am J Physiol. 1994;267(6 Pt 2):R1503–9.
Bonnet F, Patel S, Laville M, Balkau B, Favuzzi A, Monti LD, et al. Influence of the ACE gene insertion/deletion polymorphism on insulin sensitivity and impaired glucose tolerance in healthy subjects. Diabetes Care. 2008;31(4):789–94.
Petrie JR, Morris AD, Ueda S, Small M, Donnelly R, Connell JM, et al. Trandolapril does not improve insulin sensitivity in patients with hypertension and type 2 diabetes: a double-blind, placebo-controlled crossover trial. J Clin Endocrinol Metab. 2000;85(5):1882–9.
van der Zijl NJ, Moors CC, Goossens GH, Hermans MM, Blaak EE, Diamant M. Valsartan improves {beta}-cell function and insulin sensitivity in subjects with impaired glucose metabolism: a randomized controlled trial. Diabetes Care. 2011;34(4):845–51.
Maack T, Suzuki M, Almeida FA, Nussenzveig D, Scarborough RM, McEnroe GA, et al. Physiological role of silent receptors of atrial natriuretic factor. Science. 1987;238(4827):675–8.
Sarzani R, Dessi-Fulgheri P, Paci VM, Espinosa E, Rappelli A. Expression of natriuretic peptide receptors in human adipose and other tissues. J Endocrinol Invest. 1996;19(9):581–5.
Kenny AJ, Bourne A, Ingram J. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11. Biochem J. 1993;291(Pt 1):83–8.
Morabito D, Vallotton MB, Lang U. Obesity is associated with impaired ventricular protein kinase C-MAP kinase signaling and altered ANP mRNA expression in the heart of adult Zucker rats. J Investig Med. 2001;49(4):310–8.
Khan AM, Cheng S, Magnusson M, Larson MG, Newton-Cheh C, McCabe EL, et al. Cardiac natriuretic peptides, obesity, and insulin resistance: evidence from two community-based studies. J Clin Endocrinol Metab. 2011;96(10):3242–9.
Licata G, Volpe M, Scaglione R, Rubattu S. Salt-regulating hormones in young normotensive obese subjects. Effects of saline load. Hypertension. 1994;23(1 Suppl):I20–I4.
Dessi-Fulgheri P, Sarzani R, Tamburrini P, Moraca A, Espinosa E, Cola G, et al. Plasma atrial natriuretic peptide and natriuretic peptide receptor gene expression in adipose tissue of normotensive and hypertensive obese patients. J Hypertens. 1997;15(12 Pt 2):1695–9.
Nakatsuji H, Maeda N, Hibuse T, Hiuge A, Hirata A, Kuroda Y, et al. Reciprocal regulation of natriuretic peptide receptors by insulin in adipose cells. Biochem Biophys Res Commun. 2010;392(1):100–5.
Floras JS. Sympathoinhibitory effects of atrial natriuretic factor in normal humans. Circulation. 1990;81(6):1860–73.
Brunner-La Rocca HP, Kaye DM, Woods RL, Hastings J, Esler MD. Effects of intravenous brain natriuretic peptide on regional sympathetic activity in patients with chronic heart failure as compared with healthy control subjects. J Am Coll Cardiol. 2001;37(5):1221–7.
Asferg CL, Nielsen SJ, Andersen UB, Linneberg A, Moller DV, Hedley PL, et al. Relative atrial natriuretic peptide deficiency and inadequate renin and angiotensin II suppression in obese hypertensive men. Hypertension. 2013;62(1):147–53.
Dessi-Fulgheri P, Sarzani R, Serenelli M, Tamburrini P, Spagnolo D, Giantomassi L, et al. Low calorie diet enhances renal, hemodynamic, and humoral effects of exogenous atrial natriuretic peptide in obese hypertensives. Hypertension. 1999;33(2):658–62.
Haufe S, Kaminski J, Utz W, Haas V, Mahler A, Daniels MA, et al. Differential response of the natriuretic peptide system to weight loss and exercise in overweight or obese patients. J Hypertens. 2015;33(7):1458–64.
Lafontan M, Moro C, Berlan M, Crampes F, Sengenes C, Galitzky J. Control of lipolysis by natriuretic peptides and cyclic GMP. Trends Endocrinol Metab. 2008;19(4):130–7.
Sengenes C, Bouloumie A, Hauner H, Berlan M, Busse R, Lafontan M, et al. Involvement of a cGMP-dependent pathway in the natriuretic peptide-mediated hormone-sensitive lipase phosphorylation in human adipocytes. J Biol Chem. 2003;278(49):48617–26.
Birkenfeld AL, Boschmann M, Moro C, Adams F, Heusser K, Franke G, et al. lipid mobilization with physiological atrial natriuretic peptide concentrations in humans. J Clin Endocrinol Metab. 2005;90:3622–8.
Birkenfeld AL, Budziarek P, Boschmann M, Moro C, Adams F, Franke G, et al. Atrial natriuretic peptide induces postprandial lipid oxidation in humans. Diabetes. 2008;57(12):3199–204.
Engeli S, Birkenfeld AL, Badin PM, Bourlier V, Louche K, Viguerie N, et al. Natriuretic peptides enhance the oxidative capacity of human skeletal muscle. J Clin Invest. 2012;122(12):4675–9.
Bordicchia M, Liu D, Amri EZ, Ailhaud G, ssi-Fulgheri P, Zhang C. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J Clin Invest. 2012;122(3):1022–36.
Birkenfeld AL, Boschmann M, Engeli S, Moro C, Arafat AM, Luft FC, et al. Atrial natriuretic peptide and adiponectin interactions in man. PLoS One. 2012;7(8):e43238.
Miyashita K, Itoh H, Tsujimoto H, Tamura N, Fukunaga Y, Sone M, et al. Natriuretic peptides/cGMP/cGMP-dependent protein kinase cascades promote muscle mitochondrial biogenesis and prevent obesity. Diabetes. 2009;58(12):2880–92.
Coue M, Badin PM, Vila IK, Laurens C, Louche K, Marques MA, et al. Defective natriuretic peptide receptor signaling in skeletal muscle links obesity to type 2 diabetes. Diabetes. 2015.
Magnusson M, Jujic A, Hedblad B, Engstrom G, Persson M, Struck J, et al. Low plasma level of atrial natriuretic peptide predicts development of diabetes: the prospective Malmo Diet and Cancer study. J Clin Endocrinol Metab. 2012;97(2):638–45.
Hoffmann LS, Etzrodt J, Willkomm L, Sanyal A, Scheja L, Fischer AW, et al. Stimulation of soluble guanylyl cyclase protects against obesity by recruiting brown adipose tissue. Nat Commun. 2015;6:7235.
Nyberg M, Piil P, Egelund J, Sprague RS, Mortensen SP, Hellsten Y et al. Potentiation of cGMP signaling increases oxygen delivery and oxidative metabolism in contracting skeletal muscle of older but not young humans. Physiol Rep. 2015; 3 (8).
Newton-Cheh C, Larson MG, Vasan RS, Levy D, Bloch KD, Surti A, et al. Association of common variants in NPPA and NPPB with circulating natriuretic peptides and blood pressure. Nat Genet. 2009;41(3):348–53.
Arora P, Wu C, Khan AM, Bloch DB, vis-Dusenbery BN, Ghorbani A. Atrial natriuretic peptide is negatively regulated by microRNA-425. J Clin Invest. 2013;123(8):3378–82.
Vodovar N, Seronde MF, Laribi S, Gayat E, Lassus J, Boukef R, et al. Post-translational modifications enhance NT-proBNP and BNP production in acute decompensated heart failure. Eur Heart J. 2014;35(48):3434–41.
Jujic A, Nilsson PM, Engstrom G, Hedblad B, Melander O, Magnusson M. Atrial natriuretic peptide and type 2 diabetes development--biomarker and genotype association study. PLoS One. 2014;9(2):e89201.
Schlueter N, De SA, Willmes DM, Spranger J, Jordan J, Birkenfeld AL. Metabolic actions of natriuretic peptides and therapeutic potential in the metabolic syndrome. Pharmacol Ther. 2014;144(1):12–27.
Cheng S, Rhee EP, Larson MG, Lewis GD, McCabe EL, Shen D, et al. Metabolite profiling identifies pathways associated with metabolic risk in humans. Circulation. 2012;125(18):2222–31.
Greenfield JR, Farooqi IS, Keogh JM, Henning E, Habib AM, Blackwood A, et al. Oral glutamine increases circulating glucagon-like peptide 1, glucagon, and insulin concentrations in lean, obese, and type 2 diabetic subjects. Am J Clin Nutr. 2009;89(1):106–13.
Addabbo F, Chen Q, Patel DP, Rabadi M, Ratliff B, Zhang F, et al. Glutamine supplementation alleviates vasculopathy and corrects metabolic profile in an in vivo model of endothelial cell dysfunction. PLoS One. 2013;8(6):e65458.
Yaghootkar H, Scott RA, White CC, Zhang W, Speliotes E, Munroe PB, et al. Genetic evidence for a normal-weight “metabolically obese” phenotype linking insulin resistance, hypertension, coronary artery disease, and type 2 diabetes. Diabetes. 2014;63(12):4369–77.
Rankinen T, Sarzynski MA, Ghosh S, Bouchard C. Are there genetic paths common to obesity, cardiovascular disease outcomes, and cardiovascular risk factors? Circ Res. 2015;116(5):909–22.
Kristiansson K, Perola M, Tikkanen E, Kettunen J, Surakka I, Havulinna AS, et al. Genome-wide screen for metabolic syndrome susceptibility Loci reveals strong lipid gene contribution but no evidence for common genetic basis for clustering of metabolic syndrome traits. Circ Cardiovasc Genet. 2012;5(2):242–9.
Schupp M, Janke J, Clasen R, Unger T, Kintscher U. Angiotensin type 1 receptor blockers induce peroxisome proliferator-activated receptor-gamma activity. Circulation. 2004;109(17):2054–7.
Janke J, Schupp M, Engeli S, Gorzelniak K, Boschmann M, Sauma L, et al. Angiotensin type 1 receptor antagonists induce human in-vitro adipogenesis through peroxisome proliferator-activated receptor-gamma activation. J Hypertens. 2006;24(9):1809–16.
Jordan J, Engeli S, Boye SW, Le Breton S, Keefe DL. Direct Renin inhibition with aliskiren in obese patients with arterial hypertension. Hypertension. 2007;49(5):1047–55.
Boschmann M, Nussberger J, Engeli S, Danser AH, Yeh CM, Prescott MF, et al. Aliskiren penetrates adipose and skeletal muscle tissue and reduces renin-angiotensin system activity in obese hypertensive patients. J Hypertens. 2012;30(3):561–6.
McMurray JJ, Holman RR, Haffner SM, Bethel MA, Holzhauer B, Hua TA, et al. Effect of valsartan on the incidence of diabetes and cardiovascular events. N Engl J Med. 2010;362(16):1477–90.
Bosch J, Yusuf S, Gerstein HC, Pogue J, Sheridan P, Dagenais G, et al. Effect of ramipril on the incidence of diabetes. N Engl J Med. 2006;355(15):1551–62.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993–1004.
Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375(9722):1255–66.
Engeli S, Jordan J. Blood pressure effects of glucagon-like peptide 1 analogues and sodium glucose transporter 2 inhibitors. Curr Opin Nephrol Hypertens. 2014;23(5):468–72.
Goodwill AG, Mather KJ, Conteh AM, Sassoon DJ, Noblet JN, Tune JD. Cardiovascular and hemodynamic effects of glucagon-like peptide-1. Rev Endocr Metab Disord. 2014;15(3):209–17.
Kanai Y, Lee WS, You G, Brown D, Hediger MA. The human kidney low affinity Na+/glucose cotransporter SGLT2. delineation of the major renal reabsorptive mechanism for D-glucose. J Clin Invest. 1994;93(1):397–404.
Jurczak MJ, Lee HY, Birkenfeld AL, Jornayvaz FR, Frederick DW, Pongratz RL, et al. SGLT2 deletion improves glucose homeostasis and preserves pancreatic beta-cell function. Diabetes. 2011;60(3):890–8.
Santer R, Kinner M, Lassen CL, Schneppenheim R, Eggert P, Bald M, et al. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol. 2003;14(11):2873–82.
Ferrannini E, DeFronzo RA. Impact of glucose-lowering drugs on cardiovascular disease in type 2 diabetes. Eur Heart J. 2015;36(34):2288–96.
Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–28.
Robinson LE, Holt TA, Rees K, Randeva HS, O’Hare JP. Effects of exenatide and liraglutide on heart rate, blood pressure and body weight: systematic review and meta-analysis. BMJ Open. 2013;3:e001986.
Katout M, Zhu H, Rutsky J, Shah P, Brook RD, Zhong J, et al. Effect of GLP-1 mimetics on blood pressure and relationship to weight loss and glycemia lowering: results of a systematic meta-analysis and meta-regression. Am J Hypertens. 2014;27(1):130–9.
Kim M, Platt MJ, Shibasaki T, Quaggin SE, Backx PH, Seino S, et al. GLP-1 receptor activation and Epac2 link atrial natriuretic peptide secretion to control of blood pressure. Nat Med. 2013;19(5):567–75.
Lovshin JA, Barnie A, DeAlmeida A, Logan A, Zinman B, Drucker DJ. Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes. Diabetes Care. 2015;38(1):132–9.
Yamamoto H, Lee CE, Marcus JN, Williams TD, Overton JM, Lopez ME, et al. Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. J Clin Invest. 2002;110(1):43–52.
Ussher JR, Drucker DJ. Cardiovascular actions of incretin-based therapies. Circ Res. 2014;114(11):1788–803.
Pyke C, Heller RS, Kirk RK, Orskov C, Reedtz-Runge S, Kaastrup P, et al. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology. 2014;155(4):1280–90.
Collins FS, Varmus H. A new initiative on precision medicine. N Engl J Med. 2015;372(9):793–5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
JJ served as advisor for Boehringer-Ingelheim, Janssen-Cilag, Novartis, Orexigen, Vivus. ALB has received honoraria for preparing scientific CME events from Boehringer-Ingelheim, AstraZeneca, MSD, Sanofi, and Novo and served as advisor for MSD and Novo.
Rights and permissions
About this article
Cite this article
Jordan, J., Birkenfeld, A.L. Cardiometabolic crosstalk in obesity-associated arterial hypertension. Rev Endocr Metab Disord 17, 19–28 (2016). https://doi.org/10.1007/s11154-016-9348-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-016-9348-1