Abstract
Type 2 diabetes and its major risk factor, obesity, are an increasing worldwide health problem. The exact mechanisms that link obesity with insulin resistance, type 2 diabetes, hypertension, cardiovascular complications and renal diseases, are still not clarified sufficiently. Adipose tissue in general is an active endocrine and paracrine organ that may influence the development of these disorders. Excessive body fat in general obesity may also cause quantitative and functional alterations of specific adipose tissue compartments. Beside visceral and subcutaneous fat depots which exert systemic effects by the release of adipokines, cytokines and hormones, there are also locally acting fat depots such as peri- and epicardial fat, perivascular fat, and renal sinus fat. Perivascular adipose tissue is in close contact with the adventitia of large, medium and small diameter arteries, possesses unique features differing from other fat depots and may act also independently of general obesity. An increasing number of studies are dealing with the “good” or “bad” characteristics and functions of normally sized and dramatically increased perivascular fat mass in lean or heavily obese individuals. This review describes the origin of perivascular adipose tissue, its different locations, the dual role of a physiological and unphysiological fat mass and its impact on diabetes, cardiovascular and renal diseases. Clinical studies, new imaging methods, as well as basic research in cell culture experiments in the last decade helped to elucidate the various aspects of the unique fat compartment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Obesity, one of the major problems in humans of industrial countries is accompanied by an increased prevalence of metabolic diseases. The resistance of the skeletal muscle to insulin-mediated glucose uptake, as well as the resistance of other tissues to the metabolic and vascular actions of insulin are the consequence of an increased body fat mass. A still growing number of studies show that adipose tissue is an important source of adipokines. In addition, it is well accepted that adipose tissue secretes proinflammatory factors and that obesity is a state of low-grade inflammation [1]. As a consequence, circulating inflammatory factors contribute to the deposition of ectopic fat. The focus of interest in the last years is to study the exclusive role of perivascular adipose tissue which signals locally in a paracrine and vasocrine fashion to the vascular wall through outside-to-inside signalling. The classical description about the structure of the arterial blood vessel wall embraces the commonly known three layer morphology composed of the endothelium with endothelial cells, the media with smooth muscle cells and the adventitia containing fibroblasts and vasa vasorum. However, nearly all larger arterial blood vessels (except those in the brain) are enclosed by fat, the so-called perivascular adipose tissue, which borders directly at the adventitia without any barrier e.g. lamina. Most of the knowledge about perivascular adipose tissue in the last decade came from cardiological research because this specific fat depot is located adjacent to the arterial adventitia and plays an important role in normal vascular function as well as in the pathogenesis of vascular diseases. Not only the intima and media, but also the adventitia with the vasa vasorum, enabling the transport of factors via blood into the external regions of the vessel wall, was recognized as a highly active compartment of the arterial vessel wall contributing to both atherogenesis and restenosis [2]. The perivascular adipose tissue was originally stated to have only mechanical properties, serving as a kind of scaffold for blood vessels. However, the last years it became evident that it is a metabolically very active endocrine tissue which communicates not only with the adjacent vessel wall itself, but maybe also with other organs in the body. This review article tries to summarize all currently known aspects about the functional properties of perivascular adipose tissue dealing with its influence on other organs with the focus on the development and progression of type 2 diabetes and its complications.
1.1 Origin of perivascular fat tissue
In embryogenesis, blood vessel appearance and the first developed adipocytes are always found in parallel. On the basis of many earlier reports it seems to be undoubtable at present that there is proof for a close relationship between fat cell and blood vessel development [3]. Even if it’s not totally clarified whether capillarisation precedes adipocyte formation or vice versa or in parallel, to a very early time point in embryogenesis primitive organs develop containing mesenchymal cells and small lipid droplets. Arterioles are closely related to these primitive organs and are embedded in mesenchymal adventitial cells which proliferate also when vascular growth occurs. It was hypothesized that such mesenchymal cells in vascular areas may be progenitors for the development of fat cells [3, 4]. In the meanwhile, a variety of studies provided proof that mesenchymal stem cells from many different tissues are “perivascular cells” or “pericytes” [5].The first adipocytes were found to be located in the perivascular region. There are many reports dealing with theories or facts about the origin of adipocytes: they may develop out of endothelial cells, mature or immature macrophages, fibroblast-like or mesenchymal perivascular cells [6–8]. Another report about adipose stem cells even provided proof that adipose pluripotent stem cells originating from perivascular cells express α-SMA during differentiation. The authors concluded that perivascular cells are mainly composed of pericytes and the population of smooth muscle cells derived from pericytes [9]. A very interesting study of Planat-Benard et al. (2004) about the plasticity of adipose lineage cells [10] suggests that adipocytes and their closely located endothelial cells might share a common progenitor that could differentiate in both fat cells and vessel cells depending on the environment, as also reported by Cao et al. [6]. The first preadipocytes found show a fibroblast-like cell morphology which changes into a spherical shape when lipids accumulate in the cytoplasm. These mature adipocytes are not able to proliferate any more [3]. Even in the adult there is always a close relationship between angiogenesis and adipogenesis which was proposed to be a target for the regulation of fat cell development [11]. The review of Bays et al. (2011) illustrates that blood vessel cells and adipocytes share a common genetic lineage through the formation of mesenchymal stem cells [12]. The majority of fat tissue – including perivascular adipose tissue - cellular components are preadipocytes and fat-containing mature adipocytes which are surrounded by fibrous connective tissue, collagen, nerves, and blood vessels [13]. Other adipose tissue cell types, the so-called “stromal vascular fraction” cells, include mesenchymal cells, fibroblasts, endothelial precursor cells, smooth muscle cells, blood cells, and immune cells [12]. Adipose tissue-derived stem cells can differentiate into various cell types of both mesodermal and nonmesodermal origin. However, adipogenesis in the adult is not only responsible for the expansion of fat tissue e.g. in obesity but also for the maintenance of adipocyte numbers under normal conditions in healthy humans [5].
1.2 Characteristics of perivascular adipose tissue in the adult
In the grown-up organism nearly all systemic arteries (except arteries of the brain) and also small vessels in organs were surrounded by perivascular adipose tissue. There is no mechanical barrier, maybe composed of elastic or fibrous layers, which might separate the fat cells and the adventitial cells, as the external elastic lamina. Similarly to the adventitia, perivascular adipose tissue was considered only as a passive mechanical support for the vasculature, and it was routinely removed e.g. during heart or kidney transplantation. Compared to other fat depots in the body, such as subcutaneous or visceral fat, perivascular fat is different because of its direct contact to blood vessels. However, perivascular fat represents only up to 3 % of the total body fat mass whereas subcutaneous adipose tissue (82–97 % of total fat) and VAT (10–15 % of total fat) represent the main fat portion [14, 15]. Due to this special anatomical proximity, perivascular adipose tissue was found to have a stronger influence on the cardiovascular system and highly vascularized organs (e.g. the kidney). In small vessels and microvessels adipocytes are an integral part of the vascular wall, constituting the adventitial layer [16]. Thus, as the adventitia, perivascular fat is a kind of highly vascularized connective tissue. Besides cells (mainly adipocytes, but also endothelial cells, fibroblasts and mononuclear cells), it contains collagen and elastic fibres, nerves and vasa vasorum [17]. Adventitial vasa vasorum neovascularization occurs during vascular injury and inflammation, providing a direct way to transmit substances released by perivascular fat to the inner vasculature [18, 19]. However a variety of studies in the last decade demonstrated that not only the structure and composition but also functional properties of perivascular adipose tissue vary in different vascular beds [20–23]. The presence of perivascular fat is normal and necessary in the human organism. In obesity, the mass of visceral fat increases and this is accompanied with increases in some ectopic fat depots. However, there are also studies providing evidences that the mass of some perivascular fat locations changes independently of general obesity or visceral fat augmentation [24–26]. As a consequence also the functional properties are altered which is also described in detail in this review. These functional changes of perivascular fat are not characterized sufficiently in either hypertension, kidney diseases or diabetes, but there are a growing number of experimental studies published the last years. The following sections describe different perivascular fat locations and their impact for the (patho)physiology of adjacent tissues or organs.
1.3 Perivascular fat and vascular (patho)physiology
The role of perivascular adipose tissue in the regulation of vascular function resulting in cardiovascular diseases is obvious because of its exclusive location surrounding arterial vessels, such as coronary arteries, large arteries (aorta), medium-sized arteries (mesenteric), and small arteries (coronary arteries). However, its function is dependent on the anatomic site. Nevertheless, the term “perivascular fat” is used independently of the different vascular location in the body until now.
Obesity which is accompanied by the augmentation of perivascular fat mass around blood vessels is one of the major causes for the development of vascular diseases. Thus, altered fat mass or dysfunctional properties of this fat compartment in obese individuals may be an important risk factor for both atherosclerosis and hypertension.
Several studies provided proof that perivascular fat exerts anti-contractile or vasodilatative effects in different vascular locations. On the basis of functional studies in animals the presence of perivascular fat-derived relaxant factors was postulated. Soltis and Cassis showed already in 1991 that perivascular adipose tissue significantly influences vascular responsiveness in an in vitro setting [27]. Many years later, these observations initiated several other studies about the presence of the so-called adipocyte-derived relaxing factor (ADRF) [28–31]. In 2005, Barandier et al. described that ADRF influences the vasocontrictive effects of some humoral factors [32] but until now it is still unclear if it represents an already known factor or a still unidentified new one [33]. The following known factors were discussed to be candidates because of their anti-contractile effects: adiponectin ([34], H2S [35], NO [36], prostacyclin or angiotensin (Ang) 1–7 [37] and palmitic acid methyl esters [38]. The balance of relaxing or contractile factors (e.g. ADRF) was found to be altered in obesity [39] and hypertension [21, 39, 40]. This may be due to perivascular dysfunction. When the fat mass was reduced in very obese subjects, not only reduced weight, blood pressure, inflammation, and metabolic alterations could be achieved but also an improvement of the anticontractile function of their perivascular adipose tissue [41]. The authors stated that this reversal is due to an improved adiponectin and nitric oxide bioavailability. In a further study it was proposed that prostacyclin is not only secreted by the endothelium but also by perivascular adipose tissue and may act as the perivascular relaxing factor which protects against endothelial dysfunction [42]. Furthermore, a potential role of NO as a perivascular-derived relaxing factor was studied in experiments where rat aorta or lipoatrophic mice aorta with or without perivascular adipose tissue reacted differently concerning their contractile responses to phenylephrine and 5- HT, as well as their anti-contractile effects to Ang1–7 [37, 40, 43].
The theory that perivascular adipose tissue may play a dual role was discussed in a very interesting review of Gao et al. [36]. Perivascular fat promotes not only vasodilatation by ADRF etc. and protects against hypertension and vascular diseases but enhances also vasconstriction by ADRF. The balance of such counterplayers is disturbed when the amount of perivascular adipose tissue increases e.g. in obesity which results in functional changes. The production of constrictive factors is enhanced and the release of relaxing factors is decreased.
Furthermore, cytokines and adipokines released from perivascular adipose tissue around small coronary arteries, aorta and systemic arteries can regulate vascular smooth muscle and endothelial cell function [44]. The adjacent adventitia is nerved by vasa vasorum which enables the transport of vasoregulatory factors, cytokines and adipokines produced by perivascular adipose tissue into the vascular vessel wall. Since atherosclerotic changes and plaque formation are accompanied by an enhanced neovascularization, the transport of many substances - including (pro)inflammatory factors - into the vessel wall and towards the vessel lumen is improved [45].
1.4 Perivascular adipose tissue surrounding coronary arteries
Coronary arteries are in close contact with pericoronary fat, but also in direct proximity of the pericardial fat - which is located between the external surface of the parietal pericardium and the internal border of the mediastinum - and the epicardial fat which is located within the pericardial sac [46]. Pericardial fat has direct effects on the development of coronary atherosclerosis by the release of paracrine factors. In the Framingham Heart Study, pericardial fat was associated with vascular calcification of more than one thousand participants, which suggests that these fat depots may exert local toxic effects on the vasculature [47]. Pericardial fat volumes were well correlated with cardiovascular risk factors and the associations with pericardial fat were independent of BMI, waist circumference, age, smoking, and alcohol consumption. Thoracic periaortic fat was also found to be associated with measures of adiposity, metabolic risk factors, and coronary calcification in a Framingham Offspring Study cohort [48].
There is also evidence that pericardial fat, perivascular fat, pericoronary fat, and myocardial fat exert their effects on the heart and blood vessels not only by direct lipotoxicity but by indirect cytokine secretion [49, 50]. In addition, Kralova et al. described a close relationship between the ratio of macrophages in the arterial wall and adjacent perivascular adipose tissue in patients with coronary heart disease [51]. It has been recently reported that human coronary artery perivascular adipocytes are responsible for regulating vascular morphology, inflammation, and haemostasis [52]. Insulin resistance and the progression of atherosclerosis seem to be triggered by factors released from fat depots around the heart and coronary arteries via outside-to-inside signalling [53]. A role of the adventitia for the development and progression of atherosclerosis beside the classical way on the luminal side of the vessel after endothelial injury was already described in the 80th by Prescott et al. [54]. Predominantly the cytokines IL-8, Il-1 and the chemoattractant MCP-1 were found to be secreted from perivascular adipose tissue. These factors are mainly responsible for the inflammation and invasion of immune cells into the vessel wall and contribute to atherosclerosis and plaque vulnerability [55].
Another fat compartment relevant for cardiovascular and heart diseases is the epicardial fat tissue which is also located along the coronary arteries [56]. However, it is a kind of visceral thoracic fat depot and no perivascular adipose tissue which directly surrounds the coronary arteries [57]. Epicardial fat tissue has an important mechanical buffering function but plays also a role in the fatty acids homeostasis [58]. Epicardial fat releases paracrine factors such as leptin, resistin, tumor necrosis factor-α, interleukin-6, visfatin, and chemerin [49]. However, a study comparing adipokines expressed by epicardial adipose tissue in postmortem samples indicate that coronary perivascular adipose tissue seems to be more metabolically active than epicardial adipose tissue [59]. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. However, the description of the detailed function of this very specific fat compartment is beyond the scope of this review.
1.5 Perivascular adipose tissue surrounding peripheral arteries
The question arises if perivascular adipose tissue of other arterial sites, e.g. conduit arteries is identical or differs from fat depots of the heart. Our group examined the mRNA expression and protein secretion of perivascular fat cells in vitro which were isolated from fat depots located around human arm arteries [20]. This in vitro study demonstrated that perivascular (pre-)adipocytes differ substantially from subcutaneous and visceral (pre-)adipocytes regarding the mRNA and protein expression of inflammatory, metabolic and angiogenic factors. We found that perivascular fat cells in vitro have a much higher capacity to secrete angiogenic factors than other fat cell types. Perivascular preadipocytes expressed significantly higher levels of the angiogenic proteins HGF-1, FGF-acidic, VEGF, serpin-E1, TSP-1 and IGFBP-3 when compared to visceral and subcutaneous preadipocytes. Thus, the vessel-near location of perivascular fat cells and its higher angiogenic potential underscore the hypothesis that this specific fat depot influences the arterial vessel wall physiology and pathophysiology significantly and contributes to important features of atherosclerosis, as plaque growth, vulnerability and differentiation.
Furthermore, in a coculture model the secretory behaviour of perivascular preadipocytes and mature adipocytes was found to be influenced by human arterial endothelial cells and vice versa. As already alluded to above for coronary arteries, arterial endothelial cell activation and angiogenesis via perivascular fat might be important processes because intraplaque neovascularization contributes to the progression of atherosclerosis, induces also changes in smooth muscle cell function and supports instable plaque formation [60]. Vulnerable atherosclerotic plaques prone to rupture contain an increased number of vasa vasorum and intraplaque haemorrhage. Furthermore, the diversity of several in vitro studies about the morphology, size and lipid content of perivascular fat cells indicates also that perivascular fat cells from different blood vessels might be inhomogeneous and differ in function. Whereas Chatterjee et al. (2009) described that perivascular adipocytes isolated from fat around human coronary arteries are irregularly shaped, smaller in size and less lipid-filled when compared to subcutaneous and visceral adipocytes in vitro [61], our group found that perivascular cells around radial arteries are larger and fulfilled with lipid droplets. In a swine model Owen et al. has shown that perivascular fat may act differently according to the anatomic location [62] and Kirkland et al. (1996) showed in isolated rat cells a variability in physiological characteristics among different adipose depots [63]. These data suggest that perivascular fat depots differ depending on their location and that the paracrine factors released may affect vascular functions differentially.
1.6 Perivascular adipose tissue surrounding arteries of the renal sinus
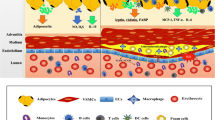
Since it is a very rare event allowing to harvest arteries together with perivascular fat from humans beside bypass surgery procedures, a limited number of studies are published until now to characterize the so-called renal sinus fat compartment. Our group had the opportunity to gain samples of renal sinus fat from kidney transplantations which enable us to isolate and cultivate renal sinus fat cells and to analyse resections of the human renal sinus (Fig. 1). Furthermore, we did cocultures experiments with endothelial cells and podocytes because the interactions between renal sinus fat and glomerular cells from human origin via paracrine factors has not been reported yet. In accordance to some other reports about the release of the “positive” relaxing factor ADRF and the “negative” proinflammatory factors and adipokines by perivascular fat, we found also a dual role of renal sinus fat. This renal sinus fat compartment per se was found to reduce the release of pro(inflammatory) factors by endothelial cells and podocytes indicating a benign effect on the kidney. However, functional or mechanical influences alter the benign effect of the renal sinus fat. One very important trigger for the alteration of renal sinus fat from an antiinflammatory to a proinflammatory status is the interplay with the fatty liver derived hepatokine fetuin-A. As we already described previously for perivascular fat around arm arteries, fetuin-A in combination with palmitate causes a huge increase in the expression of (pro)inflammatory factors in both perivascular and renal sinus fat cells which potentiates the (pro)inflammatory influences on the kidney (unpublished data).
1.7 Perivascular adipose tissue and clinical outcomes in patients
On the basis of these in vitro findings the question remains if there are clinical correlates to these in vitro findings. In a clinical study at prediabetic patients, our group determined previously whether perivascular fat around the brachial artery influences NO signalling - reflected in flow-mediated dilation - or insulin sensitivity in humans. We found a close relationship between perivascular adipose tissue and insulin sensitivity. Using high-resolution magnetic resonance imaging the mass of perivascular adipose tissue was found to be negatively correlated with insulin sensitivity and the post-ischemic increase in blood flow [64]. On the basis of in vitro studies (which will described in detail in 1.8) we determined also the HGF serum levels in patients with low and high amounts of perivascular, visceral and subcutaneous fat. 95 individuals underwent whole body MRT imaging to measure fat distribution. In our univariate analysis, PVAT was the only fat compartment which was correlated with serum HGF levels. In a stepwise regression analysis including perivascular, visceral and subcutaneous fat, age and gender, only age and perivascular fat turned out to be determining factors for HGF serum levels [20]. According to our in vitro data, this in vivo correlation suggests an enhanced secretion of angiogenic factors, like HGF, in patients with a higher perivascular fat mass which might be of pathophysiological importance. Regarding a potential relevance of renal sinus fat on kidney function, also some clinical data were published previously. In a subcohort of the Framingham study (n = 2923), the renal sinus fat area was associated with both hypertension and chronic kidney disease independent of generalized or abdominal adiposity [65]. Furthermore, our group demonstrated that the renal sinus fat mass is associated with elevated microalbuminuria under exercise conditions [25].
1.8 The role of perivascular adipose tissue as an endocrine organ
In the meanwhile it is widely proven that perivascular adipose tissue of each vascular location does not only serve as a mechanical support in the vascular bed. It has characteristic secretory properties which differ from other fat depots. The absence of a separating lamina, as the lamina elastic interna or externa, enables and promotes the paracrine exchange of many factors through outside-to-inside signalling between perivascular fat cells and vascular cells, as adventitial fibroblasts, medial smooth muscle cells and intimal endothelial cells [1]. Furthermore, perivascular fat cells which infiltrate the adventitia are in close contact to vasa vasorum where secreted factors are able to diffuse into the blood stream. In general, all fat tissues release a great variety of hormones and factors which exert autocrine, paracrine and endocrine effects. Beside energy storage, fat tissue represents a kind of endocrine and immune organ. However, the highly differentiated adipose tissue in adults changes in mass and distribution throughout life [66]. Depending on age, several diseases or drug treatments, its functions alter or a lipodystrophy occurs followed by a redistribution of subcutaneous and visceral as well as ectopic fat depots [67]. Furthermore, the composition of the adipose tissue is not uniform and stable but undergoes renewal during life. Fat tissue consists of many cell types, including, preadipocytes, fibroblasts, endothelial cells, and immune cells such as macrophages and T cells and mainly differentiated adipocytes [68]. However, because of common lineages (e.g. mesenchymal stem cells) it is rather difficult to count the number of each cell type in adipose tissue e.g. by staining with specific antibodies. It was estimated that preadipocytes account for 15–50 % of cells in fat tissue which serve as the direct precursors developing into new adipocytes [66]. Thus, it is difficult to estimate or measure the contribution of each cell type on the expression and secretion of factors released by the different adipose tissues. Furthermore, Wei et al. (2013) showed that mature adipocytes possess the ability to dedifferentiate into a population of proliferation-competent progeny cells known as adipofibroblasts or dedifferentiated fat cells [4]. Adipocytes are fully differentiated cells that cannot divide anymore. In contrast, studies in aging rats demonstrated that preadipocytes possess a lifelong potential for making new fat cells. Furthermore, the number of fat cells changes upon stem cell recruitment and increases also with age [69]. Preadipocytes are present throughout the life span [70]. Functional analyses provided proof that the potency of preadipocytes and adipocytes to express and secrete many factors differs. To study these differences and their specific contribution to the secretory profile of different fat tissues, cell isolation and cultivation of preadipocytes and their differentiation to mature adipocytes in vitro have been shown to be valuable methods. Some preadipocyte cell lines were used [71], but the sources of human fat tissue, especially perivascular adipose tissue, for the isolation of primary cells are very limited. Whereas human subcutaneous or visceral fat cells are easier to get, there is still insufficient number of studies dealing with primary human perivascular fat cells from arm arteries, the aorta or coronary arteries or newly studied perivascular fat cells, as renal sinus fat cells.
We and others could previously show that the amount of proinflammatory factors and adipokines varies depending on the fat depot as well as between preadiopcytes and adipocytes [20, 63]. Our group provided proof that both perivascular pre-adipocytes and mature adipocytes exhibit a different expression profile and amount of angiogenic, growth stimulatory, inflammatory and metabolic factors compared with cells from other fat stores, e.g. subcutaneous or visceral fat. All fat cell types secreted a great variety of proinflammatory factors, e.g. IL-8, Il-6 and MCP-1, as well as the angiogenic factors HGF, IGFBP-2, IGFBP-3, aFGF, pentraxin-3, serpin-E1, VEGF, IGFBP-1, IGFBP-2, IGFBP-3, TSP-1, serpin-F1 and TIMP-4. However, we found that perivascular fat cells in vitro and in vivo have a much higher capacity to secrete angiogenic factors, predominantly HGF, than the other fat cell types. Furthermore cocultures with arterial endothelial cells demonstrated that vessel cells influence the secretory behaviour of pre-adipocytes and mature adipocytes. Therefore, HGF levels in 95 individuals were measured and the tissue mass of different fat compartments was determined by MRI. The data of this in vivo study suggests enhanced secretion of angiogenic factors, such as HGF, in patients with a greater perivascular fat mass, which may be of pathophysiological relevance [20].
Furthermore, we proved the hypothesis that differentiation of preadipocytes to mature adipocytes may alter the release of angiogenic factors and adipokines. Among all tested angiogenic proteins, the secretion of HGF, IGFBP-1, IL-8, MMP-9 and TSP-1 was significantly lower after differentiation. In contrast, levels of serpin-E1, serpin-F1 and pentraxin-3 increased after differentiation. In regard to the classical adipogenic factors, predominantly serpin-E1 and visfatin, but also lower levels of leptin, resistin, fetuin-A and apelin could also be detected. After differentiation to adipocytes, the mRNA expression of all adipogenic proteins decreased markedly despite adiponectin, which is only expressed significantly by differentiated adipocytes. Thus, preadipocytes and adipocytes differ in regard to the mRNA expression profile and amount of secreted proteins [20]. This was also described by others: Mack et al. (2009) could show in primary human preadipocytes from the SGB cell strain that preadipocytes have a greater potency compared with adipocytes as endothelial cell activators under normoxia, hypoxia, and TNF-α exposure [72]. An interesting study of Skruk et al. (2007) also described that adipocytes per se are an important production site for many adipokines and cytokines, such as leptin, IL-6, IL-8, TNF-α and MCP-1. However, when adipocytes were fractioned according to their cell size, the hypertrophic, very large adipocytes showed a markedly impaired adipokine and cytokine secretion, which promotes inflammation in human adipose tissue. The authors concluded that the adipocyte size and a dysregulation of hypertrophic cells are important determinants of a proinflammatory adipokine release [73].
Yudkin et al. (2005) described a vascocrine signalling where perivascular fat cells secrete adipocytokines which regulate the insulin-mediated balance between NO and endothelin-1. In obesity, an increased adipocytokine release induces the inhibition of the PI3-K pathway of insulin signalling. The vasoregulatory properties of periarteriolar fat are altered and result in a reduced insulin-induced vasodilatation or vasoconstriction by vascular medial smooth muscle cells. Furthermore, high concentrations of TNFα access the vascular lumen and inhibit this endothelial PI3-K dependent insulin signalling in downstream vessels. The authors conclude that the reduced insulin-mediated enhancement of muscle nutritive blood flow will contribute to insulin resistance [53].
As already alluded to, perivascular fat has an impact on vascular smooth muscle vasodilation and contraction. However, perivascular adipose tissue contributes also to smooth muscle cell proliferation and migration [74]. Several adipokines were proposed to be an adipocyte-derived growth factor, such as visfatin, leptin or resistin, but also NEFA [32]. In contrast, adiponectin was found to decrease smooth muscle cell proliferation [75]. As already well know, the classical adipocytokines released by adipose tissue exert pro-inflammatory (leptin, resistin and visfatin) or anti-inflammatory (adiponectin) effects. These observations have impact on vascular remodelling because the endocrine and paracrine actions of adipocytes are especially important for the cardiovascular system and link obesity and cardiovascular diseases [50].
One of the major findings of fat research is that inflammatory cells – macrophages and T cells – are found in adipose tissue which may be present at any time beside adipocytes and endothelial cells or were invaded from outside. Predominantly adipocytokines (leptin and resistin) and chemoattractants (MCP-1) are able to attract inflammatory cells. In respect of the exclusive location of perivascular adipose tissue, the attraction of macrophages into this fat compartment and the adjacent vessel wall is of major importance for cardiovascular and renal diseases [76].
1.9 Perivascular adipose tissue crosstalk with the liver
Fetuin-A or alpha-2-HS-glycoprotein, a major non-phosphorylated sialoprotein in plasma, is exclusively expressed in the liver (except for the tongue and the placenta) [77]. It is a natural inhibitor of the insulin-stimulated insulin receptor tyrosine kinase. Fetuin-A may serve as a mediator of signals between the fatty liver and insulin resistance. We and others found that high plasma levels of the hepatokine fetuin-A are associated with insulin resistance in humans. The EPIC-Potsdam study supports that high circulating fetuin-A is associated with diabetes risk in middle-aged individuals [78]. However, the last years it became evident that fetuin-A may have also a crucial role in the pathology of atherosclerosis [24, 79]. Clinical studies showed that there is a link between high plasma fetuin-A levels and an increased risk for atherosclerotic complications, such as myocardial infarction and ischemic stroke [78, 80]. The fatty liver which produces high fetuin-A levels contributes also to subclinical inflammation including the cardiovascular system [81]. In a case-cohort study based on the EPIC-study significantly increased risks of myocardial infarction and ischemic stroke were also found in subjects with higher fetuin-A levels in plasma [78]. Since perivascular adipose tissue mass and function are correlated with insulin resistence and cardiovascular diseases, a potential crosstalk with the fatty liver was postulated. Previously, we provided proof in an in vitro study that fetuin-A specifically affects the function of human perivascular fat cells. Fetuin-A inhibited cell growth and increased the mRNA expression and secretion of the proinflammatory proteins IL-8, IL-6, MCP-1 and PAI-1. Furthermore, fetuin-A changed the expression of angiogenic factors [82]. As already mentioned above we found also that perivascular fat cells possess a higher angiogenic potential than visceral and subcutaneous fat cells. Fetuin-A upregulated VEGF, PDGF, bFGF and PlGF whereas HGF, the major angiogenic protein of perivascular adipose tissue, was significantly downregulated [82]. Thus, fetuin-A seems to have an important impact on perivascular fat cell function. Our data indicate that fetuin-A influences both the secretion of proinflammatory and angiogenic factors from vascular cells and perivascular fat cells via different signalling pathways. Thus, this crosstalk with fetuin-A, delivered from the (fatty) liver, may be at least involved in the generation of cardiovascular and renal diseases by influencing perivascular fat function.
2 Summary
Until the first publication in 1991 [27] there was nearly no interest in studying the role of perivascular adipose tissue, despite the existence of several reports about the secretory function and role of visceral and subcutaneous fat depots. However, since cardiologists accepted that the arterial vessel wall do not consist of a 3-layer but a 4-layer structure, perivascular adipose tissue which is directly connected to the adventitia, was recognized as a functionally very active compartment which differs from other fat locations. The following studies were first focused on the influence of perivascular adipose tissue on cardiovascular diseases. On the basis of studies about the inflammatory behaviour of visceral fat, it was assumed that the release of adipokines and inflammatory factors are the cause of arterial vessel wall alterations leading to atherosclerosis. In the meanwhile, perivascular adipose tissue is recognized as a dual-faced compartment that is capable to release both contractile and anticontractile, as well as inflammatory and antiinflammatory factors. The positive or negative impact on the cardiometabolic syndrome is dependent on changes in mass and function of this fat tissue.
Adipose tissue in general, but also perivascular fat, may also influence insulin sensitivity by the secretion of adipokines from (pre)adipocytes themselves, or proinflammatory factors from both adipocytes and infiltrating immune cells, a phenomenon that is increased in obesity. Furthermore, renal sinus fat comes into the focus of research because renal function heavily depends on blood flow. Preliminary in vitro-data demonstrated that pro-inflammatory factors released from functionally altered renal sinus fat (e.g. by fatty liver-derived fetuin-A) may influence glomerular cell function which is relevant for the development of chronic kidney disease associated with insulin resistance. Great improvements of imaging methods for the assessment of adipose tissue compartments with magnetic resonance (MR) imaging and MR spectroscopy allows now to quantify and characterize also smaller fat depots, such as renal sinus fat or pancreatic fat, more precisely. However, there is much need for further in vivo and in vitro-studies in animals but more importantly with human primary cells which resemble the human situation much better.
References
Lee DE, Kehlenbrink S, Lee H, Hawkins M, Yudkin JS. Getting the message across: mechanisms of physiological cross talk by adipose tissue. Am J Physiol Endocrinol Metab. 2009;296:E1210–29.
Majesky MW. Adventitia and perivascular cells. Arterioscler Thromb Vasc Biol. 2015;35:e31–5.
Hausman GJ, Campion DR, Martin RJ. Search for the adipocyte precursor cell and factors that promote its differentiation. J Lipid Res. 1980;21:657–70.
Wei S, Duarte MS, Zan L, Du M, Jiang Z, Guan L, Chen J, Hausman GJ, Dodson MV. Cellular and molecular implications of mature adipocyte dedifferentiation. J Genomics. 2013;1:5–12.
Cawthorn WP, Scheller EL, MacDougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53:227–46.
Cao Y. Angiogenesis modulates adipogenesis and obesity. J Clin Invest. 2007;117:2362–8.
Sanchez-Gurmaches J, Guertin DA. Adipocyte lineages: tracing back the origins of fat. Biochim Biophys Acta. 2014;1842:340–51.
Majka SM, Barak Y, Klemm DJ. Concise review: adipocyte origins: weighing the possibilities. Stem Cells. 2011;29:1034–40.
Cai X, Lin Y, Hauschka PV, Grottkau BE. Adipose stem cells originate from perivascular cells. Biol Cell. 2011;103:435–47.
Planat-Benard V, Silvestre JS, Cousin B, Andre M, Nibbelink M, Tamarat R, Clergue M, Manneville C, Saillan-Barreau C, Duriez M, Tedgui A, Levy B, Penicaud L, Casteilla L. Plasticity of human adipose lineage cells toward endothelial cells: physiological and therapeutic perspectives. Circulation. 2004;109:656–63.
Hausman GJ, Richardson RL. Adipose tissue angiogenesis. J Anim Sci. 2004;82:925–34.
Bays HE. Adiposopathy is “sick fat” a cardiovascular disease? J Am Coll Cardiol. 2011;57:2461–73.
Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918.
Gastaldelli A, Basta G. Ectopic fat and cardiovascular disease: what is the link? Nutr Metab Cardiovasc Dis. 2010;20:481–90.
Lee HY, Despres JP, Koh KK. Perivascular adipose tissue in the pathogenesis of cardiovascular disease. Atherosclerosis. 2013;230:177–84.
Hausman GJ, Richardson RL. Cellular and vascular development in immature rat adipose tissue. J Lipid Res. 1983;24:522–32.
Lebona GT. The presence of paraganglia in the human ascending aortic fold: histological and ultrastructural studies. J Anat. 1993;183:35–41.
Gossl M, Herrmann J, Tang H, Versari D, Galili O, Mannheim D, Rajkumar SV, Lerman LO, Lerman A. Prevention of vasa vasorum neovascularization attenuates early neointima formation in experimental hypercholesterolemia. Basic Res Cardiol. 2009;104:695–706.
Miao CY, Li ZY. The role of perivascular adipose tissue in vascular smooth muscle cell growth. Br J Pharmacol. 2012;165:643–58.
Rittig K, Dolderer JH, Balletshofer B, Machann J, Schick F, Meile T, Kuper M, Stock UA, Staiger H, Machicao F, Schaller HE, Konigsrainer A, Haring HU, Siegel-Axel DI. The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia. 2012;55:1514–25.
Galvez-Prieto B, Somoza B, Gil-Ortega M, Garcia-Prieto CF, Las Heras AI, Gonzalez MC, Arribas S, Aranguez I, Bolbrinker J, Kreutz R, Ruiz-Gayo M, Fernandez-Alfonso MS. Anticontractile effect of perivascular adipose tissue and leptin are reduced in hypertension. Front Pharmacol. 2012;3:103.
Villacorta L, Chang L. The role of perivascular adipose tissue in vasoconstriction, arterial stiffness, and aneurysm. Horm Mol Biol Clin Investig. 2015;21:137–47.
Gil-Ortega M, Somoza B, Huang Y, Gollasch M, Fernandez-Alfonso MS. Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol Metab. 2015;26:367–75.
Rittig K, Thamer C, Haupt A, Machann J, Peter A, Balletshofer B, Fritsche A, Haring HU, Stefan N. High plasma fetuin-A is associated with increased carotid intima-media thickness in a middle-aged population. Atherosclerosis. 2009;207:341–2.
Wagner R, Machann J, Lehmann R, Rittig K, Schick F, Lenhart J, Artunc F, Linder K, Claussen CD, Schleicher E, Fritsche A, Haring HU, Weyrich P. Exercise-induced albuminuria is associated with perivascular renal sinus fat in individuals at increased risk of type 2 diabetes. Diabetologia. 2012;55:2054–8.
Lamacchia O, Nicastro V, Camarchio D, Valente U, Grisorio R, Gesualdo L, Cignarelli M. Para- and perirenal fat thickness is an independent predictor of chronic kidney disease, increased renal resistance index and hyperuricaemia in type-2 diabetic patients. Nephrol Dial Transplant. 2011;26:892–8.
Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13:277–96.
Dubrovska G, Verlohren S, Luft FC, Gollasch M. Mechanisms of ADRF release from rat aortic adventitial adipose tissue. Am J Physiol Heart Circ Physiol. 2004;286:H1107–13.
Gollasch M. Vasodilator signals from perivascular adipose tissue. Br J Pharmacol. 2012;165:633–42.
Zavaritskaya O, Zhuravleva N, Schleifenbaum J, Gloe T, Devermann L, Kluge R, Mladenov M, Frey M, Gagov H, Fesus G, Gollasch M, Schubert R. Role of KCNQ channels in skeletal muscle arteries and periadventitial vascular dysfunction. Hypertension. 2013;61:151–9.
Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB J. 2002;16:1057–63.
Barandier C, Montani JP, Yang Z. Mature adipocytes and perivascular adipose tissue stimulate vascular smooth muscle cell proliferation: effects of aging and obesity. Am J Physiol Heart Circ Physiol. 2005;289:H1807–13.
Tano JY, Schleifenbaum J, Gollasch M. Perivascular adipose tissue, potassium channels, and vascular dysfunction. Arterioscler Thromb Vasc Biol. 2014;34:1827–30.
Fesus G, Dubrovska G, Gorzelniak K, Kluge R, Huang Y, Luft FC, Gollasch M. Adiponectin is a novel humoral vasodilator. Cardiovasc Res. 2007;75:719–27.
Wojcicka G, Jamroz-Wisniewska A, Atanasova P, Chaldakov GN, Chylinska-Kula B, Beltowski J. Differential effects of statins on endogenous H2S formation in perivascular adipose tissue. Pharmacol Res. 2011;63:68–76.
Gao YJ. Dual modulation of vascular function by perivascular adipose tissue and its potential correlation with adiposity/lipoatrophy-related vascular dysfunction. Curr Pharm Des. 2007;13:2185–92.
Lee RM, Lu C, Su LY, Gao YJ. Endothelium-dependent relaxation factor released by perivascular adipose tissue. J Hypertens. 2009;27:782–90.
Lee YC, Chang HH, Chiang CL, Liu CH, Yeh JI, Chen MF, Chen PY, Kuo JS, Lee TJ. Role of perivascular adipose tissue-derived methyl palmitate in vascular tone regulation and pathogenesis of hypertension. Circulation. 2011;124:1160–71.
Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, Tune JD. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase C-beta pathway. Arterioscler Thromb Vasc Biol. 2010;30:1711–7.
Lu C, Su LY, Lee RM, Gao YJ. Alterations in perivascular adipose tissue structure and function in hypertension. Eur J Pharmacol. 2011;656:68–73.
Aghamohammadzadeh R, Greenstein AS, Yadav R, Jeziorska M, Hama S, Soltani F, Pemberton PW, Ammori B, Malik RA, Soran H, Heagerty AM. Effects of bariatric surgery on human small artery function: evidence for reduction in perivascular adipocyte inflammation, and the restoration of normal anticontractile activity despite persistent obesity. J Am Coll Cardiol. 2013;62:128–35.
Chang L, Milton H, Eitzman DT, Chen YE. Paradoxical roles of perivascular adipose tissue in atherosclerosis and hypertension. Circ J. 2013;77:11–8.
Takemori K, Gao YJ, Ding L, Lu C, Su LY, An WS, Vinson C, Lee RM. Elevated blood pressure in transgenic lipoatrophic mice and altered vascular function. Hypertension. 2007;49:365–72.
Szasz T, Bomfim GF, Webb RC. The influence of perivascular adipose tissue on vascular homeostasis. Vasc Health Risk Manag. 2013;9:105–16.
van Hinsbergh VW, Eringa EC, Daemen MJ. Neovascularization of the atherosclerotic plaque: interplay between atherosclerotic lesion, adventitia-derived microvessels and perivascular fat. Curr Opin Lipidol. 2015;26:405–11.
Lim S, Meigs JB. Ectopic fat and cardiometabolic and vascular risk. Int J Cardiol. 2013;169:166–76.
Rosito GA, Massaro JM, Hoffmann U, Ruberg FL, Mahabadi AA, Vasan RS, O'Donnell CJ, Fox CS. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–13.
Lehman SJ, Massaro JM, Schlett CL, O'Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: the Framingham Heart Study. Atherosclerosis. 2010;210:656–61.
Lim S, Meigs JB. Links between ectopic fat and vascular disease in humans. Arterioscler Thromb Vasc Biol. 2014;34:1820–6.
Engeli S. Is there a pathophysiological role for perivascular adipocytes? Am J Physiol Heart Circ Physiol. 2005;289:H1794–5.
Kralova LI, Tonar Z, Malek I, Maluskova J, Nedorost L, Pirk J, Pitha J, Lanska V, Poledne R. Is the amount of coronary perivascular fat related to atherosclerosis? Physiol Res. 2015;64(Suppl 3):S435–43.
Chatterjee TK, Aronow BJ, Tong WS, Manka D, Tang Y, Bogdanov VY, Unruh D, Blomkalns AL, Piegore Jr MG, Weintraub DS, Rudich SM, Kuhel DG, Hui DY, Weintraub NL. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiol Genomics. 2013;45:697–709.
Yudkin JS, Eringa E, Stehouwer CD: “Vasocrine” signalling from perivascular fat: a mechanism linking insulin resistance to vascular disease. Lancet 2005 365:1817–1820.
Prescott MF, McBride CK, Court M. Development of intimal lesions after leukocyte migration into the vascular wall. Am J Pathol. 1989;135:835–46.
Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10.
Keegan J, Gatehouse PD, Yang GZ, Firmin DN. Spiral phase velocity mapping of left and right coronary artery blood flow: correction for through-plane motion using selective fat-only excitation. J Magn Reson Imaging. 2004;20:953–60.
Ouwens DM, Sell H, Greulich S, Eckel J. The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J Cell Mol Med. 2010;14:2223–34.
Marchington JM, Pond CM. Site-specific properties of pericardial and epicardial adipose tissue: the effects of insulin and high-fat feeding on lipogenesis and the incorporation of fatty acids in vitro. Int J Obes. 1990;14:1013–22.
Spiroglou SG, Kostopoulos CG, Varakis JN, Papadaki HH. Adipokines in periaortic and epicardial adipose tissue: differential expression and relation to atherosclerosis. J Atheroscler Thromb. 2010;17:115–30.
Di Stefano R, Felice F, Balbarini A. Angiogenesis as risk factor for plaque vulnerability. Curr Pharm Des. 2009;15:1095–106.
Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ Res. 2009;104:541–9.
Owen MK, Witzmann FA, McKenney ML, Lai X, Berwick ZC, Moberly SP, Alloosh M, Sturek M, Tune JD. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: influence of obesity. Circulation. 2013;128:9–18.
Kirkland JL, Hollenberg CH, Gillon WS. Effects of fat depot site on differentiation-dependent gene expression in rat preadipocytes. Int J Obes Relat Metab Disord. 1996;20(Suppl 3):S102–7.
Rittig K, Staib K, Machann J, Bottcher M, Peter A, Schick F, Claussen C, Stefan N, Fritsche A, Haring HU, Balletshofer B. Perivascular fatty tissue at the brachial artery is linked to insulin resistance but not to local endothelial dysfunction. Diabetologia. 2008;51:2093–9.
Foster MC, Hwang SJ, Porter SA, Massaro JM, Hoffmann U, Fox CS. Fatty kidney, hypertension, and chronic kidney disease: the Framingham Heart Study. Hypertension. 2011;58:784–90.
Sepe A, Tchkonia T, Thomou T, Zamboni M, Kirkland JL. Aging and regional differences in fat cell progenitors - a mini-review. Gerontology. 2011;57:66–75.
van de Voorde J, Boydens C, Pauwels B, Decaluwe K. Perivascular adipose tissue, inflammation and vascular dysfunction in obesity. Curr Vasc Pharmacol. 2014;12:403–11.
Suganami T, Tanaka M, Ogawa Y. Adipose tissue inflammation and ectopic lipid accumulation. Endocr J. 2012;59:849–57.
Cartwright MJ, Schlauch K, Lenburg ME, Tchkonia T, Pirtskhalava T, Cartwright A, Thomou T, Kirkland JL. Aging, depot origin, and preadipocyte gene expression. J Gerontol A Biol Sci Med Sci. 2010;65:242–51.
Kras KM, Hausman DB, Hausman GJ, Martin RJ. Adipocyte development is dependent upon stem cell recruitment and proliferation of preadipocytes. Obes Res. 1999;7:491–7.
Gao D, Trayhurn P, Bing C. 1,25-Dihydroxyvitamin D3 inhibits the cytokine-induced secretion of MCP-1 and reduces monocyte recruitment by human preadipocytes. Int J Obes. 2013;37:357–65.
Mack I, BelAiba RS, Djordjevic T, Gorlach A, Hauner H, Bader BL. Functional analyses reveal the greater potency of preadipocytes compared with adipocytes as endothelial cell activator under normoxia, hypoxia, and TNFalpha exposure. Am J Physiol Endocrinol Metab. 2009;297:E735–48.
Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023–33.
Szasz T, Webb RC. Perivascular adipose tissue: more than just structural support. Clin Sci (Lond). 2012;122:1–12.
Lamers D, Schlich R, Greulich S, Sasson S, Sell H, Eckel J. Oleic acid and adipokines synergize in inducing proliferation and inflammatory signalling in human vascular smooth muscle cells. J Cell Mol Med. 2011;15:1177–88.
Guzik TJ, Marvar PJ, Czesnikiewicz-Guzik M, Korbut R. Perivascular adipose tissue as a messenger of the brain-vessel axis: role in vascular inflammation and dysfunction. J Physiol Pharmacol. 2007;58:591–610.
Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Krober SM, Machicao F, Fritsche A, Haring HU. Alpha2-Heremans-Schmid glycoprotein/fetuin-A is associated with insulin resistance and fat accumulation in the liver in humans. Diabetes Care. 2006;29:853–7.
Weikert C, Stefan N, Schulze MB, Pischon T, Berger K, Joost HG, Haring HU, Boeing H, Fritsche A. Plasma fetuin-a levels and the risk of myocardial infarction and ischemic stroke. Circulation. 2008;118:2555–62.
Mori K, Ikari Y, Jono S, Emoto M, Shioi A, Koyama H, Shoji T, Ishimura E, Inaba M, Hara K, Nishizawa Y. Fetuin-A is associated with calcified coronary artery disease. Coron Artery Dis. 2010;21:281–5.
Jensen MK, Bartz TM, Mukamal KJ, Djousse L, Kizer J, Tracy RP, Zieman SJ, Rimm EB, Siscovick DS, Shlipak M, Ix JH. Fetuin-A, Type 2 Diabetes, and Risk of Cardiovascular Disease in Older Adults: The Cardiovascular Health Study. Diabetes Care. 2013;36(5):1222–8.
Heinrichsdorff J, Olefsky JM. Fetuin-A: the missing link in lipid-induced inflammation. Nat Med. 2012;18:1182–3.
Siegel-Axel DI, Ullrich S, Stefan N, Rittig K, Gerst F, Klingler C, Schmidt U, Schreiner B, Randrianarisoa E, Schaller HE, Stock UA, Weigert C, Konigsrainer A, Haring HU. Fetuin-A influences vascular cell growth and production of proinflammatory and angiogenic proteins by human perivascular fat cells. Diabetologia. 2014;57:1057–66.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest Statement
There is no conflict of interest relating to this manuscript.
Rights and permissions
About this article
Cite this article
Siegel-Axel, D.I., Häring, H.U. Perivascular adipose tissue: An unique fat compartment relevant for the cardiometabolic syndrome. Rev Endocr Metab Disord 17, 51–60 (2016). https://doi.org/10.1007/s11154-016-9346-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11154-016-9346-3