Abstract
In this work, inexpensive and simple commercial transition metal salts were evaluated as catalysts in the acetalization of alkyl alcohols with β-citronellal, a renewable origin substrate. After an initial screening, FeCl3 was the most active and selective catalyst among the various transition metal salts evaluated toward the β-citronellal methyl acetal. The impacts of main reaction parameters such as time, temperature, catalyst load, and type of alcohol on conversion and selectivity of the reactions were investigated. Different iron salts were also investigated. It was demonstrated that both oxidation number and type of anion present in the salt play an essential role in this reaction. Notably, the dissolution of catalyst salts in solution triggered a decrease in the pH of the medium due to the hydrolysis (and or solvolysis) of the metal cation, impacting the conversion and reaction selectivity. The highest activity of FeCl3 was assigned to the greatest Lewis acidity strength, as demonstrated by the acidity measurements. This inexpensive, low-corrosive, and commercially affordable catalyst has advantages over traditional liquid mineral acid catalysts and provides an alternative route to synthesize alkyl terpene acetals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
β-Citronellal is a renewable raw material that plays a key role in various synthesis organic processes [1]. Through different condensation reactions, such as acetalization, ketalization, etherification, and esterification, aldehydes like β-citronellal are platform molecules easily converted to fine chemicals such as fragrance ingredients, pharmacies, building blocks in the synthesis of drugs, and agrochemicals [2,3,4].
Acetalization is one of the most used condensation reactions to protect carbonyl groups in aldehydes or ketones, which is an important step in several routes of organic synthesis. Liquid mineral acids like sulfuric, nitric, and hydrochloric, as well as organic acids such as trifluoracetic are effective catalysts in acetalization reactions, however, despite being cheap, those acids are corrosive and difficult to reuse, requiring still steps of neutralization steps that lead to a generation of waste and residues [5, 6]. Solid-supported catalysts can answer this demand, nonetheless, besides the laborious synthesis, they can be leached and deactivated during the processes [7,8,9]. Lewis’s acid catalysts have also been demonstrated to be an option, such as metal triflates, however, despite their efficiency, they are sensitive to water, which is a co-product of the reaction [10,11,12]. Noble metal complexes and titanium tetrachloride were also effective catalysts in acetalization or ketalization reactions [13,14,15]. Nonetheless, none of these catalysts was used in acetalization reactions of terpene aldehydes.
In previous works, we have evaluated the performance of simple and commercially available transition metal salts (i.e., chloride, sulfate, nitrate) in different monoterpene transformations, likewise esterification, oxidation, acetalization, and ketalization, which were very efficient homogeneous catalysts [16,17,18,19,20,21,22,23]. In almost all of these works, iron (III) salts were always the most active and selective catalysts. Similarly, tin(II) salts have also deserved a highlight in acid-catalyzed reactions [24,25,26,27]. Glycerol, monoterpenes, fatty acids, and benzoic acid were the main substrates and these reactions. Particularly, metal chloride salts are commercially available, inexpensive, and water-tolerant Lewis’s acids, which makes more attractive their use as catalysts in processes of valorization of renewable raw material such as β-citronellal.
In this work, the goal was to investigate the activity of different transition metal cations in alkyl alcohol acetalization reactions with β-citronellal. The effects of main reaction parameters such as time, temperature, catalyst load, and type of alcohol were assessed. Other iron salts with different anions were also evaluated and compared to iron(III) chloride.
Experimental section
Chemicals
All chemicals were acquired from commercial sources. β-citronellal and alkyl alcohols were all Sigma-Aldrich (99 wt%). The salts FeCl3·6H2O, Fe2(SO4)3·5H2O were Dinamica. FeSO4·7H2O and FeCl2·4H2O were Analítica. Fe(Acac)3 and alkyl alcohols were acquired from Sigma Aldrich. NiCl2·6 H2O and MnCl2·2H2O (Neon), CoCl2·2H2O (Vetec), CuCl2 (Dinamica), and ZnCl2 (Exode). All the salts with 97–99 wt% purity. Alkyl alcohols with 99 wt% purity.
Identification of main reaction products
The reaction products were identified in a Shimadzu GC-2010 gas chromatographer coupled with a MS-QP 2010 mass spectrometer, operating at impact electronic mode (70 eV), within the m/z range of 50 to 450.
Catalytic tests
Catalytic runs were carried out in a three-necked glass reactor (ca. 25 mL), fitted with a reflux condenser and sampling septum. Typically, β-citronellal (1.00 mmol) was magnetically stirred, solved in CH3OH (10 mL), and heated to 298 K. The addition of the metal salt catalyst (5.0 mol%) started the reaction.
The progress reaction was followed for 2 h, periodically collecting aliquots and analyzing them in GC equipment (Shimadzu 2010, FID), fitted with a capillary column (Carbowax 20 M, 30 m length, 0.25 mm i.d., 0.25 mm film thickness). The temperature profile used in the GC analyses was as follows: 80 °C (3 min), heating rate (10 °C min−1) until 240 °C. The temperatures of the injector and detector were 250 °C and 280 °C, respectively. Equations 1 and 2 were used to calculate conversion and selectivity.
Here Ai = initial area of GC peak of β-citronellal, and Ar = remaining area of GC peak of β-citronellal.
Here Ap = product GC peak corrected area, Ai = initial area of GC peak of β-citronellal, and Ar = remaining area of GC peak of β-citronellal.
The difference between the consumed area of the GC peak of β-citronellal (Ai-Ar) and the sum of the GC peak corrected areas of the product (Ap) gave the oligomers selectivity (Eq. 3).
Results and discussion
Catalytic tests
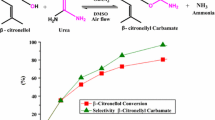
The catalytic activity of transition metal salts was evaluated in the condensation of methyl alcohol with β-citronellal, following conditions previously described by the literature [28] (Fig. 1). Notwithstanding the methyl alcohol excess, no acetal traces were detected (omitted by simplification).
Among all the metal chloride salts evaluated, only FeCl3 was an active catalyst to condense β-citronellal and methyl alcohol into β-citronellyl acetal (Fig. 1). In that reaction, only β-citronellyl acetal was selectively formed (Scheme 1).
The activity of metal salts in acid-catalyzed reactions has been associated with the ability of these metal cations to react with the water released during the reaction and or the alcohol solvent to release hydronium cations in solution, which may thyself catalyze the acetalization [29]. However, literature has also accepted that the metal cation itself may coordinate with the carbonyl group of the aldehyde in solution, polarizing its double bond and making the alcohol attack easier [30].
Herein, aiming to check this effect, pH measurements were carried out before and after the addition of salt to the alcohol solution (Table 1). Although it is not exactly a pH measurement since the solvent is not water but methyl alcohol, this measurement shows the amount of H+ ions released in the solution, whatever your origin (i.e., water or alcohol) (Eqs. 4 and 5).s
Table 1 shows the decisive role played by the H+ (or hydronium) cation, which consists in protonating the carbonyl group of β-citronellal favoring the nucleophilic attack of methyl alcohol, significantly increasing its conversion to acetal.
Effect of FeCl 3 concentration on the reactions of methyl alcohol with β-citronellal
The impact of the FeCl3∙6H2O catalyst concentration on the kinetic curves of β-citronellal condensation with methyl alcohol is displayed in Fig. 2.
An increase in catalyst concentration from 0.6 to 2.5 mol% led to higher conversions, which reached 98%, however, reactions carried out with loads greater than 2.5 mol% achieved almost the same conversion. The pH values were all checked in all the reactions. The pH values were progressively diminished when higher catalyst loads were used (Fig. 3). Conversely, the selectivity was minimally impacted, regardless of the catalyst concentration. In all the runs, a minority products mixture (i.e., nucleophilic addition products of water) was detected (Fig. 3).
Effect of anion present and oxidation number of the iron catalysts
The impact of the anion present in the iron salt was also investigated, as well as the effect of oxidation number. The kinetic curves are presented in Fig. 4.
All the catalysts were used at the same iron load. The iron oxidation number plays a key role in the catalytic activity of metal cations, once it affects their Lewis acidity strength [16, 22]. This explains why Fe2(SO4)3·5H2O was a catalyst much more active than FeSO4, notwithstanding both FeSO4·7H2O salt being soluble in the reaction medium and Fe2(SO4)3·5H2O being insoluble.
On the other hand, the nature of the anion was also important for the efficiency of the catalyst. Interestingly, the difference in activity between Fe2+ and Fe3+ chlorides was much lower than the sulfates. Although FeCl3 6 H2O has continued to be the most active (98% conversion), the conversion achieved in the FeCl2-catalyzed reaction was 90%. Conversely, while FeSO4 was basically inactive the Fe2(SO4)3-catalyzed reaction achieved 80% of conversion.
The Fe(Acac)3 salt was soluble, however, it was also an inactive catalyst. It is a consequence of acetylacetonate anion being a strong ligand, hampering the coordination of Fe(III) cation to the carbonyl group of β-citronellal. A similar effect was observed when palladium or tin catalysts containing different ligands were used in acetalization furfural reactions [29, 31].
The pH values of the iron salt solutions were also differently impacted and can be useful to explain the reached. Table 2 summarizes the results.
The lower conversions were accomplished in the solutions with lower acidity (i.e., higher pH values, and FeSO4 and Fe(acac)3). Conversely, the most active catalysts were those that triggered the higher decrease in pH value (i.e., Fe2(SO4)3, FeCl2, and FeCl3, respectively). The difference between pH values of Fe2+ salts (chloride and sulfate) can be assigned to the probable hydrolysis of sulfate anion, which consumes protons or hydronium cations.
Insights on the reaction mechanism
Commonly, the acetalization reactions have been carried out using Brønsted acids. In these cases, H+ ions are responsible for carbonyl activation of aldehyde through the protonation step, favoring thus its nucleophilic attack by the hydroxyl group of alcohol.
Herein, the catalyst used was the Fe3+ cations, which can act either polarizing the carbonyl of β-citronellal or generating H+ ions that act as catalysts too. In Scheme 2, the mechanism proposal involving these two catalytic species is depicted.
In the first step, the carbonyl group of β-citronellal (1) is activated by the Fe3+ or H+ cations (intermediate 1a), which favor the nucleophilic attack by the methyl alcohol. The hydroxyl group of hemiacetal formed in step II (intermediate 1b), interacts with Fe3+ or H+ cations (intermediate 1c; step III), promoting the exchanges of a proton with a methyl group of another molecule of methyl alcohol, releasing water the dimethyl acetal β-citronellal (2) (step IV).
Influence of temperature on the FeCl3-catalyzed acetalization reactions of β-citronellal with methyl alcohol
To investigate the impact of temperature, FeCl3·6H2O was selected and the reactions were carried out using a low catalyst load to make more visible this effect (Fig. 5).
An increase in temperature accelerated the reactions, probably due to a greater number of effective collisions between the reactant molecules, resulting in a higher conversion. In addition, the acetal selectivity was higher in the reactions at temperatures greater than 298 K (Fig. 6).
Influence of alcohol on the FeCl 3 -catalyzed acetalization reactions of β-citronellal
To verify how the steric hindrance effects may affect the conversion and selectivity of the acetalization reactions of β-citronellal, primary and secondary alcohols were evaluated. Figure 7 displays the kinetic curves obtained after 2 h of reaction.
Noticeably, methyl alcohol was the most reactive substrate. Conversely, ethyl and propyl alcohols reacted at the same rate, achieving the same conversion after a 2 h reaction. Possibly, this different behavior can be attributed to the donating electron effect of the methyl group exercised on the hydroxyl group of methyl alcohol. This should enhance its nucleophilicity, favoring their attack on the carbonyl group of β-citronellal, after it has been protonated (i.e., in the presence of H+ ions), or polarized (i.e., in the presence of Fe(III) cations). An increase in the carbon chain size reduces this electronic effect, making ethyl and propyl alcohols less reactive. Consequently, the butyl alcohol had the lowest reactivity.
The steric hindrance is also an effect that drastically reduces the alcohol reactivity. It can be verified in the reaction with isopropyl alcohol, whose acetalization reached the lowest conversion. The reaction selectivity and final conversion after 2 h of reaction are depicted in Fig. 8.
Regardless of the type of alcohol, the alkyl β-citronellyl acetal was always the main product. In alcohols with higher polarity, the oligomerization of β-citronellal was favoured, as well as the formation of minority products. The impact of the addition of FeCl3·6H2O to the alcohol solution was checked (Table 3).
Results in Table 3 show that in some cases, there was a competitive effect between pH, electronic and steric effects, which are decisive to explain the alcohol's reactivity. First of all, compared to other alcohols, the greatest decrease in pH value was observed in the methyl alcohol solution, making him the most reactive substrate. Moreover, higher conversions were achieved in the more acidic solutions (methyl and isobutyl alcohol). However, even with a low value of pH, the isopropyl alcohol was less reactive, due to steric hindrance.
Although butyl and isobutyl alcohols are primary alcohols with the same number of carbon atoms, they had different reactivity; the acetalization of β-citronellal was more effective with isobutyl than butyl alcohol. It can be assigned to the greater decrease in pH value achieved after the addition of the FeCl3 catalyst (Table 3).
Conclusions
The iron-catalyzed acetalization reactions of β-citronellal with alkyl alcohols were assessed. Initially, a series of transition metal chlorides were evaluated in reactions of methyl alcohol with β-citronellal, among them, only FeCl3 6 H2O was an effective catalyst. It was ascribed to the higher decrease in pH value triggered by the addition of the FeCl3 catalyst. The activity of FeCl3 was compared to the other Fe3+ or Fe2+ salts containing different anions. The higher oxidation number favored the activity of the catalyst, regardless of the anion present in the salt. On the other hand, for the oxidation number, chloride salts were more reactive than sulfate ones. Once more it could be correlated to the pH values of alcohol solutions of catalysts. An increase in temperature or catalyst load enhances the conversion of reactions. Different alcohols were acetalized with β-citronellal. The carbon chain sizes, besides the electronic and hysteric effects, affected the reactivity of alcohols, being methyl alcohol the most reactive. Surprisingly, isobutyl alcohol was more reactive than shorter-chain alcohols like ethyl and propyl alcohols. Measurement of the pH of its FeCl3·6H2O solution was compatible with this result showing that it reacts strongly with FeCl3. The mild reaction conditions, and the use of a non-corrosive, inexpensive, and commercially affordable catalyst are positive aspects of this process.
References
Lenardão EJ, Botteselle GV, De Azambuja F, Perin G, Jaco RG (2007) Citronellal as a key compound in organic synthesis. Tetrahedron 63:6671–6712. https://doi.org/10.1016/j.tet.2007.03.159
Tsolakis N, Bam W, Srai JS, Kumar M (2019) Renewable chemical feedstock supply network design: The case of terpenes. J Clean Prod 222:802–822. https://doi.org/10.1016/j.jclepro.2019.02.108
Wu L, Moteki T, Gokhale AA, Flaherty DW, Toste FD (2016) Production of fuels and chemicals from biomass: condensation reactions and beyond. Chem 1:32–58. https://doi.org/10.1016/j.chempr.2016.05.002
Sanchez LM, Thomas HJ, Climent MJ et al (2016) Heteropolycompounds as catalysts for biomass product transformations. Catal Rev 58:497–586. https://doi.org/10.1080/01614940.2016.1248721
Hamada N, Kazahaya K, Shimizu H, Sato T (2004) An efficient and versatile procedure for the synthesis of acetals from aldehydes and ketones catalyzed by lithium tetrafluoroborate. Synlett. https://doi.org/10.1055/s-2004-820038
Dong J-L, Yu L-S-H, Xie J-W (2018) A Simple and versatile method for the formation of Acetals/Ketals Using trace conventional acids. ACS Omega 3:4974–4985. https://doi.org/10.1021/acsomega.8b00159
Corma A, García H (2003) Lewis acids: from conventional homogeneous to green homogeneous and heterogeneous catalysis. Chem Rev 103:4307–4366. https://doi.org/10.1021/cr030680z
Wegenhart BL, Liu S, Thom M, Stanley D, Abu-Omar MM (2012) Solvent-free methods for making acetals derived from glycerol and furfural and their use as a biodiesel fuel component. ACS Catal 2:2524–2530. https://doi.org/10.1021/cs300562e
Umbarkar SB, Kotbagi TV, Biradar AV, Pasricha R, Chanale J, Dongare MK, Mamede A-S, Lancelot C, Payen E (2009) Acetalization of glycerol using mesoporous MoO3/SiO2 solid acid catalyst. J Mol Catal A 310:150–158. https://doi.org/10.1016/j.molcata.2009.06.010
Leonard NM, Oswald MC, Freiberg DA, Nattier BA, Smith RC, Mohan RS (2002) A simple and versatile method for the synthesis of Acetals from aldehydes and ketones using bismuth triflate. J Org Chem 67:5202–5207. https://doi.org/10.1021/jo0258249
Smith BM, Graham AE (2011) Indium triflate mediated tandem acetalisation-acetal exchange reactions under solvent-free conditions. Tetrahedron Lett 52:6281–6283. https://doi.org/10.1016/j.tetlet.2011.09.087
Smith BM, Kubczyk TM, Graham AE (2012) Indium(III) triflate catalysed transacetalisation reactions of diols and triols under solvent-free conditions. Tetrahedron 68:7775–7781. https://doi.org/10.1016/j.tet.2012.07.048
Cooks RG, Chen H, Eberlin MN, Zheng X, Tao WA (2006) Polar acetalization and transacetalization in the gas phase: the Eberlin reaction. Chem Rev 106:188–211. https://doi.org/10.1021/cr0400921
Zhu Z, Espenson JH (1997) Organometallic catalysis: formation of 1,3-dioxolanes and their analogs catalyzed by methylrhenium trioxide (MTO). Organometallics 16:3658–3663. https://doi.org/10.1021/om970225r
Clerici A, Pastori N, Porta O (2001) Mild acetalisation of mono and dicarbonyl compounds catalysed by titanium tetrachloride. Facile synthesis of β-keto enol ethers. Tetrahedron 57:217–225. https://doi.org/10.1016/S0040-4020(00)01001-2
da Silva MJ, de Oliveira CM (2021) Metal nitrate-catalyzed one-pot oxidative esterification of benzaldehyde with hydrogen peroxide in alcoholic solutions at room temperature. New J Chem 45:3683–3691. https://doi.org/10.1039/D0NJ05671E
da Silva MJ, Ayala DAM (2016) Unravelling transition metal-catalyzed terpenic alcohol esterification: a straightforward process for the synthesis of fragrances. Catal Sci Technol 6:3197–3207. https://doi.org/10.1039/C5CY01538C
Martins FP, Rodrigues FA, da Silva MJ (2018) Fe2(SO4)3-catalyzed Levulinic acid esterification: production of fuel bioadditives. Energies 11:1263. https://doi.org/10.3390/en11051263
da Silva MJ, Julio AA, Ayala DAM, de Miranda LMP (2018) Fe2(SO4)3-catalyzed synthesis of terpenic alcohols esters: a simple and bifunctional reusable solid catalyst. ChemSelect 3:5742–5748. https://doi.org/10.1002/slct.201800643
da Silva MJ, Carari DM, da Silva AM (2015) Fe(III)-catalyzed α-terpinyl derivatives synthesis from β-pinene via reactions with hydrogen peroxide in alcoholic solutions. RSC Adv 5:10529–10536. https://doi.org/10.1039/C4RA13112F
Carari DM, da Silva MJ (2014) Fe(NO3)3-catalyzed monoterpene oxidation by hydrogen peroxide: an inexpensive and environmentally benign oxidative process. Catal Lett 144:615–622. https://doi.org/10.1007/s10562-013-1189-x
da Silva MJ, Teixeira MG (2018) Assessment on the double role of the transition metal salts on the acetalization of furfural: Lewis and Brønsted acid catalysts. Mol Catal 461:40–47. https://doi.org/10.1016/j.mcat.2018.10.002
da Silva M, Silva G, Sampaio V et al (2020) One-pot synthesis of benzaldehyde derivatives in PdCl2-catalyzed reactions with H2O2 in alcoholic solutions. Chem Pap. https://doi.org/10.1007/s11696-020-01408-7
da Silva MJ, de Ávila RF, Júlio AA (2017) SnF2-catalyzed glycerol ketalization: a friendly environmentally process to synthesize solketal at room temperature over on solid and reusable Lewis acid. Chem Engin J 307:828–835. https://doi.org/10.1016/j.cej.2016.09.002
Cardoso AL, Neves SCG, da Silva MJ (2009) Kinetic study of alcoholysis of the fatty acids catalyzed by tin chloride(II): an alternative catalyst for biodiesel production. Energ Fuels 23:1718–1722. https://doi.org/10.1021/ef800639h
Karamé I, Alamé M, Kanj A, Baydoun GN, Hazimeh H, el Masri M, Christ L (2011) Mild and efficient protection of diol and carbonyls as cyclic acetals catalysed by iron (III) chloride. C R Chim 14:525–529. https://doi.org/10.1016/j.crci.2010.12.001
Zaher S, Christ L, Abd El Rahim M, Kanj A, Karamé I (2017) Green acetalization of glycerol and carbonyl catalyzed by FeCl3·6H2O. Mol Catal 438:204–213. https://doi.org/10.1016/j.mcat.2017.06.006
da Silva MJ, Ribeiro CJA, Vilanculo CB (2023) How the content of protons and vanadium affects the activity of H3+nPMo12-nVnO40 (n = 0, 1, 2, or 3) catalysts on the oxidative esterification of benzaldehyde with hydrogen peroxide. Catal Lett 153:2045–2056. https://doi.org/10.1007/s10562-022-04132-x
da Silva MJ, Teixeira MG, Natalino R (2019) Highly selective synthesis under benign reaction conditions of furfural dialkyl acetal using SnCl2 as a recyclable catalyst. New J Chem 43:8606–8612. https://doi.org/10.1039/C9NJ01284B
Krompiec S, Penkala M, Szczubiałka K, Kowalska E (2012) Transition metal compounds and complexes as catalysts in the synthesis of acetals and orthoesters: theoretical, mechanistic and practical aspects. Coordin Chem Rev 256:2057–2095. https://doi.org/10.1016/j.ccr.2012.05.006
Teixeira MG, Natalino R, da Silva MJ (2020) A kinetic study of heteropolyacid-catalyzed furfural acetalization with methanol at room temperature via ultraviolet spectroscopy. Catal Today 344:143–149. https://doi.org/10.1016/j.cattod.2018.11.071
Acknowledgements
The authors are grateful for the financial support from CNPq and FAPEMIG (Brazil). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Funding
Coordenação de Aperfeiçoamento de Pessoal de Nível Superior,001,Marcio Silva
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Venâncio, A.N., Ribeiro, C.J.A., Júlio, A.A. et al. Assessments on the transition metal salt-catalyzed β-citronellal condensation reactions with alkyl alcohols. Reac Kinet Mech Cat 137, 149–161 (2024). https://doi.org/10.1007/s11144-023-02528-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-023-02528-3