Abstract
Simple Fe(NO3)2·9H2O was demonstrated to be able to catalyze the oxidation of monoterpenes by hydrogen peroxide in methyl alcohol solution. Compared with the previous iron-catalyzed methods, the present procedure avoids the use of stabilizing ligands, additives, and corrosive peroxide organic oxidants. A novel, simple and highly efficient catalyst system was developed for oxidizing monoterpenes into a valuable derivates using hydrogen peroxide, an environmentally friendly oxidant.
Graphical Abstract
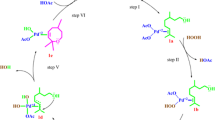
Possible steps involved in the Fe(III)-catalyzed oxidation of β-pinene by hydrogen peroxide into myrtenol methyl ether in methyl alcohol

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Oxidized monoterpenes are valuable raw materials for the preparation of commercially important products such as fragrances, perfumes and flavors; in addition, they are attractive molecules for the pharmaceutical, sanitary, cosmetic, agrochemical, and food industries [1]. Interest in these reactions is higher when environmentally benign oxidants, mainly dioxygen or hydrogen peroxide, are employed, because they produce only water as the by-product [2, 3]. Hydrogen peroxide is an inexpensive commercial product used in numerous industrial processes, because it is a non-flammable liquid oxidant, and compatible with a broad scope of catalysts [4]. Moreover, it presents a higher amount of active oxygen by mass unit and is lesser corrosive and easier to handle than organic peroxides [5].
Among transition metals, iron catalysts have significant advantages compared to the other noble metals; it is cheap, abundant, has low toxicity, being therefore an environmentally friendly metal catalyst [6]. Consequently, iron catalysts have been widely applied in plentiful organic transformations [7].
Peroxide oxidants have been used in olefin oxidations in the presence of iron–porphyrin complexes as catalysts [8]. However, porphyrin catalysts have some shortcoming such as the laborious synthesis, as well as the requirement of co-catalysts [9]. Alternatively, a nonheme iron complex was developed and successfully catalyzed the oxidation of bicyclic and tricyclic terpenoids with hydrogen peroxide [10]. Actually, the literature has described scarce examples where iron salts act as “solo” catalyst (i.e., without additives or radicalar initiators) in oxidation reactions. A rare instance of iron(III)-catalyzed reaction is the benzylic substrates oxidation to corresponding carbonyl compounds with tert-butyl hydroperoxide [11]. However, these FeCl3-catalyzed reactions take place at a long time (ca. 24 h); moreover, the major drawback is use pyridine as a solvent, which is economically and environmentally undesirable [11].
The development of monoterpene selective oxidation processes based on clean oxidants and active catalysts is a goal that has been pursued by our research group [12, 13]. Herein, we wish describe a simple and efficient Fe(III)-catalyzed monoterpene oxidation process with H2O2 in methyl alcohol solution, in the absence of bulker nitrogen stabilizing ligands, additives, or even co-catalysts. We paid special attention to study factors driving oxidation selectivity and to optimizing the main reaction parameters. Remarkably, after a short time reaction (1 h) the Fe(NO3)3-catalyzed oxidation reactions of α and β-pinene by H2O2 reached a very high conversion (i.e., 98 and 89 %, respectively), and combined selectivity for the two main products of ca. 73 %, a result superior to the described recently by us in the literature [14]. The maximum individual selectivity was of ca. 49 and 24 % for myrtenol methyl ether and α-terpineol, respectively. This result is lower than one reported in monoterpene oxidation reactions with H2O2 where epoxides are main products [15]. However, recent literature have described that oxidation of both α and β-pinene by H2O2 commonly result in the formation of a number allylic products, compromising the reaction selectivity [16–18]. To the best of our knowledge, this is the first report of monoterpene oxidation reactions with hydrogen peroxide catalyzed by Fe(III) cations in the absence of bulker nitrogen ligands, additives, or nitrogen solvent.
2 Experimental Procedures
2.1 Materials and Physical Methods
All of chemicals and solvents are commercially available and used without additional purification. The salts LiNO3 (99 %), Fe(NO3)3·9H2O (98 %), and Cu(NO3)2·3H2O (98 %) were purchased from Sigma-Aldrich. Camphene (95 %), β-pinene (99 %), α-pinene (99 %), and limonene (99 %) were also acquired from Sigma-Aldrich. 1H NMR and 13C NMR spectra were recorded on the Mercury-300 Varian Spectrometer 300 MHz for 1H instrument, with chemical shifts (ppm) reported relative to the internal standard tetramethylsilane. Major reaction products were purified by column chromatography using silica gel (60G). Gas chromatography analyses were carry out on a Varian 450 instrument with a FID detector and a CP-WAX capillary chromatographic column (25 m × 0.32 mm × 0.30 μm). GC–MS spectra were recorded on a Shimadzu 5050 gas chromatography–mass spectrometry instrument (i.e., 70 eV). The active oxygen content of the hydrogen peroxide oxidant (34 wt% aqueous solution; Vetec) was determined by permanganimetry prior to use.
2.2 Catalytic Oxidation of Monoterpenes
Catalytic runs were carried out under air in a glass reactor (50 mL) equipped with a magnetic stirrer and sampling septum. In a typical run, an adequate amount of Fe(III) catalyst and monoterpene was dissolved in methyl alcohol (15 mL), the reactor temperature was adjusted to 55 °C and then the reaction was initiated by the adding of hydrogen peroxide 34 wt%.
Reactions were monitored by analyzing aliquots taken at regular time intervals by gas chromatography (Varian 450 instrument, FID, Carbowax 20 M capillary column). Dodecane was the internal standard. Reaction conversions were estimated from the corresponding chromatographic peak areas comparing with the corresponding calibrating curve. The quantification of reaction products was made via co-injection in GC and response factors of product samples (pure or stander). The identification of products was done by GC–MS analyses operating at impact electronic mode (70 eV).
In order to determine concentrations of all oxidation products and decompose the intermediate hydroperoxides possibly present in the reaction solutions the aliquots were analyzed twice by mass spectroscopy (i.e., before and after their treatment with PPh3), as described in the literature [19].
3 Results and Discussion
3.1 General Aspects
Literature has described the use of iron(III) nitrate as reoxidant of the palladium catalysts in monoterpene oxidation reactions by dioxygen, where it also transfers oxygen atoms from the oxidant to olefin via intermediates organopalladium [20]. On the other hand, iron(III) complexes with bulk nitrogen ligands have been employed as catalysts in olefin oxidation reactions by both dioxygen and hydrogen peroxide [21, 22].
Herein, we assessed the Fe(NO3)3-catalyzed monoterpene oxidation reactions using hydrogen peroxide as stoichiometric oxidant, in bulky nitrogen ligand-free methyl alcohol solutions and in the absence of co-catalysts. The use of methanol as solvent in olefin oxidations reactions by H2O2 was previously described in the literature, and no formation of their oxidized derivatives was not reported [23, 24].
Under the reaction conditions, the methyl alcohol used as a solvent could be oxidized to volatile (i.e. formaldehyde, carbon oxides) or non-volatile products (i.e. formic acid), and compromise the β-pinene oxidation reaction because of extra consumption of H2O2. We checked this possibility carrying out reactions at the same conditions described in Table 1, but without the monoterpene presence, using different H2O2 load (i.e. 10, 20, 30 and 40 mmol of H2O2) and we find out that the methyl alcohol concentration remained almost constant, regardless of peroxide concentration used. Gas chromatography analyses not detected formic acid or methyl alcohol derivatives in the reaction solutions. Literature describes that the combination FeBr3/H2O2 was inefficient on primary alcohols [25]. Actually, methyl alcohol anol has lowest oxidation rate among all of alcohols [26].
In addition, monitoring these same reactions via titration against KMnO4 0.02 mol L−1 solution, we verified that hydrogen peroxide concentration quickly decreased within first reaction moments; instead of start 1.3 and 2.7 mol L−1 (i.e. initial concentration values), in the first aliquot titrated the concentration of remaining H2O2 was equal to 0.94 and 1.4 mol L−1, respectively (Fig. 1a, b). It is noteworthy that KMnO4 was normally used because it do not oxidize monoterpene in absence of acid catalysts.
Figure 1 suggests that H2O2 undergo thermal decomposition, because the kinetic curves obtained with or without β-pinene are close or coincident; only using lower H2O2 load was possible perceive a slightly higher consumption of oxidant due to the presence of β-pinene.
3.2 β-Pinene Oxidation by Hydrogen Peroxide in the Presence of Different Salts
The activity of M(NO3) n (M = Li(I), Cu(II), or Fe(III); n = 1, 2 or 3) nitrate salts was evaluated in the β-pinene oxidation reactions by H2O2 in CH3OH solutions and the main results are summarized in Table 1.
Even though an oxidant excess have been used (i.e., β-pinene: H2O2 molar ratio equal to 1: 6), only a poor conversion was obtained in the reaction-blanck (Run 1, Table 1). On the other hand, the ineffectiveness of LiNO3 discarded a possible stoichiometric oxidation of β-pinene by the nitrate anion (Run 2, Table 1). Conversely, in the presence of transition metal cations, such as Cu(II) and more remarkably Fe(III), the oxidation reactions became truly catalytic. Notably, the Fe(NO3)3 catalyst was the most efficient and promoted the selective oxidation of β-pinene (1) to myrtenol methyl ether (1a) and α-terpineol (1b), achieving highest conversion (ca. 98 %) with selectivity of 50 and 18 % for these products, respectively (Fig. 2). Although Cu(NO3)2 was also effective, it was lesser selective and active than Fe(NO3)3 catalyst.
It is important to note that α-terpineol normally corresponds the skeletal rearrangement product of β-pinene followed by the water addition [27]. Nevertheless, herein its formation only occurred in presence of the hydrogen peroxide oxidant and of the catalyst Fe(NO3)3, then it will be named “oxidized product”.
Recently, we have reported the oxidation of β-pinene by hydrogen peroxide into the same product 1a, in PdCl2-catalyzed reactions [14]. However, the palladium is an expensive catalyst, and in those reactions, the ether 1a was obtained with other three allylic products. Whereas, the Fe(NO3)3-catalyzed reactions has low cost and the good combined selectivity to two main products, making this oxidative process much more attractive. Moreover, α-terpineol 1b is a valuable commercially product because it is used as fragrance and flavor ingredient [28].
No epoxide product was detected herein. These data are in agreement with the literature, which reports a highly selective formation of allylic products from β-pinene and no epoxide formation in oxidation reactions with H2O2 [12, 14].
A crucial point in alkane or olefin oxidation reactions with H2O2 is the probable hydroperoxides formation, which can be decomposed into ketone in GC injector, masking the selectivity results [29]. Hydroperoxide amounts can be determined via a double injection, with and without reduction of the reaction mixture by trimethylphosphine. From the obtained increase in alcohol content, the corresponding hydroperoxide content can be determined [30]. We addressed this issue and summarized the main results in Fig. 3. Only the chromatograms of the aliquots collected after 30 min were shown by simplification.
We find out that although triphenylphosphine oxide have been formed no significant variation was observed in the area of the GC–MS peaks (Fig. 2). We monitored the reactions during the two first hours (i.e. aliquots were analyzed at 30 min intervals), and verified that only the amounts of triphenylphosphine oxide and triphenylphosphine were appreciably modified. Initially, all PPh3 was converted to oxide, however, with an increase of reaction time, the OPPh3 quantity formed was lowering. As showed in Fig. 1a, this behavior can be attributed to the decrease of H2O2 concentration that remained in solution.
3.3 Fe(NO3)3-Catalyzed Oxidation of β-Pinene by Hydrogen Peroxide in CH3OH: Effect of Catalyst Concentration
The effect of catalyst concentration was investigated in the range of 0.02–0.21 mmol and the results are summarized in Table 2.
Different from PdCl2-catalyzed β-pinene oxidation, in all runs catalyzed by Fe(III), the high initial reaction rate allows maximum conversion within of first hour reaction, remaining almost constant from this point forward [12, 14]. It can be seen that a decreasing on catalyst concentration did not result in any significant change to the conversion or reaction selectivity (Table 2; Fig. 4). Regardless of catalyst concentration, the combined selectivity of products 1a and 1b remained in the range of 68–73 %, with myrtenol methyl ether (1a) as the major product.
The reaction selectivity was mostly independent on catalyst concentration; myrtenol methyl ether and α-terpineol were always the main products. Similar results were described in both homolytic or heterolytic β-pinene oxidation previously assessed by us [12, 31] Probably, the selectivity is governed by stability of intermediate alkyl hydroperoxides, which are promptly decomposed by Fe(III) cations and therefore was not affected by their concentration.
The kinetic curves analysis shows that, regardless of catalyst concentration, high initial reaction rates were achieved and maximum conversion was reached within the first three hours of reaction (Fig. 4). This fact, in addition to the TON obtained, comprises positive aspects of these reactions. Figure 4 supports the hypothesis that iron (III) catalyst promote the intermediate alkyl hydroperoxides decomposition into oxidation products at beginning of the reaction; consequently the hydroperoxides were not detected via reduction tests with triphenylphosphine.
3.4 Effect of Hydrogen Peroxide Concentration on the Fe(NO3)3-Catalyzed β-Pinene Oxidation Reactions in CH3OH
Normally, in homolyitic oxidation reactions catalyzed by transition metal type Mn+/Mn+1, the presence of a large amount of hydroperoxo radicals may reduce the reaction selectivity favoring concurrent reactions, such as oligomerization, isomerization, as well as promoting the formation of manifold oxidized products. These features are great drawbacks to be overcome when monoterpenes, which reactivity is generally hardly controllable, are the substrates to oxidize. This effect was investigated by varying the oxidant concentration in the range of 5–30 mmol (i.e., H2O2 34 wt% aqueous solution), and the major results are displayed in Table 3.
It was observed that an increase of H2O2 amount until 20 mmol (ca. substrate/oxidant molar ratio equal to 1:4) did not compromise oxidation selectivity (Runs 1–3, Table 3). However, when amounts higher than 30 mmol of oxidant were employed other products were formed, resulting in a decrease of oxidation selectivity (Run 4, Table 3) (Fig. 5).
Notably, reaction rates were highly dependent of the hydrogen peroxide concentration employed. The maximum initial reaction rate was achieved with 20 mmol of oxidant that resulted in the highest conversion and selectivity of β-pinene oxidation. On the other hand, the greater amount of oxidant (ca. 30 mmol) was apparently less efficient because it lowered not only the reaction rate (Fig. 3), but also the oxidation selectivity (Run 4, Table 3).
3.5 Fe(III)-Catalyzed β-Pinene Oxidation Reactions by H2O2 in CH3OH: Insights into the Role of Fe(III) Cations
Homolytic oxidations involve intermediate free radicals being frequently catalyzed by first-row transition metals characterized by one-electron redox steps (e.g., Co(II)/Co(III), Mn(II)/Mn(III), and Cu(I)/Cu(II) [32]. In these reactions, the substrate is generally not coordinated to the metal and it is oxidized outside the coordination sphere via a radical chain mechanism.
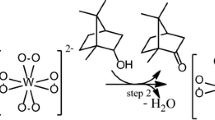
The main role of the metal catalyst is generally decompose intermediate hydroperoxides, which are spontaneously formed or through the action of an initiator agent generating radicals to sustain the reaction. This behavior is also known as the Haber–Weiss mechanism (Fig. 6) [33]. A significant difference herein is that iron catalyst has the highest oxidation number, which excludes the first reaction proposed in Fig. 6.
Although the literature describes the formation of Fe(IV) species in iron-catalyzed reactions with peroxo oxidants, in general they require nitrogen ligands to stabilize these intermediates [34]. Thus, we suggest that a similar mechanism (type Waber-Weiss) based on the initial generation of cations H+ could be involved herein. From this point, it is reasonable to assume that the three species present in the solution could react with Fe(III) cations to provide H+ cations (Fig. 7).
Because myrtenol methyl ether was always the main oxidation product, it can be concluded that participation of the solvent is obligatory. We suggested that, in the presence of hydrogen peroxide and Fe(III) cations, the methyl alcohol is converted into highly active methoxy radical, which reacts with β-pinene radical, oxidizing it into myrtenol methyl ether (Fig. 7). We suppose that regenerating Fe(III) cations occur via reaction of Fe(II) and H+ cations and perhydroxyl radical, resulting in water and another active specie (i.e., hydroxyl radical). We know that other radicalar steps and other intermediates should be involved [35, 36]. However, the steps described here are adequate to explain formation of main product, as well as the participation of Fe(III) cations.
3.6 Effect of Temperature on the Fe(NO3)3-Catalyzed β-Pinene Oxidation Reactions by H2O2 in CH3OH
The changes on reaction temperature drastically affected the efficiency of Fe(III)-catalyzed reactions conversion. At room temperature, the system becomes biphasic; however, after solvent extraction, it was verified that only a poor conversion of β-pinene was obtained (ca. 5 %). Even when the reaction temperature was increased to 35 or 45 °C, both maximum conversion and oxidation selectivity were lower than those obtained at 55 °C. In general, radicalar reactions are highly sensitive to temperature increases, which also promote the decomposition of intermediate hydroperoxides.
3.7 Fe(NO3)3-Catalyzed Oxidation Reactions with Hydrogen Peroxide in CH3OH: Effect of Substrate Nature
The main factors that affect the monoterpenes reactivity are the level of substitution of the olefinic double bonds, the type of carbon skeletal, and the accessibility of yours vinylic and allylic hydrogens. All of substrates with allylic hydrogens easily replaceable promptly reacted in Fe(NO3)3/H2O2/CH3OH system (Table 4). Contrarily, the camphene that have only one allylic hydrogen that occupies the bridgehead position, and thus is consequently not easily abstractable was the less reactive monoterpene. In Table 5, we summarized the data of conversion and selectivity obtained in these reactions.
It was observed that α-pinene has a similar reactivity to the β-pinene, being mostly oxidized into myrtenol methyl ether and pinocarveol, with high conversion (ca. 89 %) and oxidation selectivity (ca. 65 %). However, in the oxidation reactions of camphene the undesirable formation of oligomers occurred. These products were not detectable by GC analysis; however, their formation was confirmed by monitoring the mass balance, and by the precipitation of a white solid, formed after reaction aliquots cooling to temperatures lower than room temperature.
4 Conclusion
A Fe(III)-catalyzed natural olefin oxidation process based on an inexpensive and commercially available catalyst and an environmentally benign oxidant (i.e., hydrogen peroxide) was developed. In these reactions, α and β-pinene were preferentially oxidized into an allylic ether (i.e., myrtenol methyl ether). The high TONs achieved revealed that the Fe(NO3)3 was more effective catalyst than bioinspired (i.e., porphyrins) or noble catalysts (i.e., palladium) described in the literature. β and α-pinene were mostly oxidized within a 1–2 h reaction, with conversion close to 90 %. Compared with the previous iron-catalyzed method, the present procedure avoids the use of stabilizing ligands, additives, and corrosive peroxide organic oxidants. Although the oxidation reactions homolytically proceeded, only two products were obtained with combined selectivity of 73 % (i.e. myrtenol methyl ether and α-terpineol), always with conversions of 91–98 %, regardless of Fe(NO3)3 catalyst concentration employed.
References
Chapuis C, Jacoby D (2001) Appl Catal A 221:93–117
Gallezot P (2007) Catal Today 121:76–91
Narog D, Szczepanik A, Sobkowiak A (2008) Catal Lett 120:320–325
Anastas PT, Bartlett LB, Kirchhoff MM, Williamson TC (2000) Catal Today 55:11–22
Campos-Martin JM, Blanco-Brieva G, Fierro JLG (2006) Angew Chem Int Ed 45:6962–6984
Shejwalkar P, Rath NP, Bauer EB (2011) Dalton Trans 40:7617–7631
Mayer AC, Bolm C (2008) In: Plietker B (ed) Iron catalysis in organic chemistry. Wiley-VCH, Weinheim, pp 73–123
Que L Jr, Tolman WB (2008) Nature 455:333–340
Martins RRL, Graça M, Neves PMS, Silvestre AJD, Simões MMQ, Silva AMS, Tomé AC, Cavaleiro JAS, Tagliatesta P, Crestini C (2001) J Mol Catal A 172:3–342
Clemente-Tejeda D, Lopez-Moreno A, Bermejo FA (2013) Tetrahedron 69:2977–2986
Nakanishi M, Bolm C (2007) Adv Synth Catal 349:861–864
de Oliveira AA, da Silva ML, da Silva MJ (2009) Catal Lett 130:424–431
da Silva MJ, Carari DM, Teixeira RR (2009) J Organomet Chem 694:3254–3261
da Silva MJ, Vieira LMM, Oliveira AA, Ribeiro MC (2013) Monasth Chem 144:321–326
Balula SS, Santos ICMS, Silva LC, Carvalho AP, Pires J, Freire C, Cavaleiro JAS, Castro B, Cavaleiro AMV (2013) Catal Today 203:95–102
Timofeeva MN, Panchenko VN, Hasan Zubair, Jhung Sung Hwa (2013) Appl Catal A 455:71–85
Canepa AL, Chanquia CM, Eimera GA, Casuscelli SG (2013) Appl Catal A 462–463:8–14
da Silva MJ, Coelho JV, Oliveira LCA, Moura FCC, de Souza PP, Silva CA, Batista KB (2012) Appl Catal A 419–420:215–220
G. B. Shul’pin, Org Chem 6 (2009) 95–104
da Silva MJ, Gusevskaya EV (2001) J Mol Catal A 176:23–27
Selke M, Sisemore MF, Ho RYN, Wertz DL, Valentine JS (1997) J Mol Catal A 117:71–82
Stephenson NA, Bell AT (2007) J Mol Catal A 275:54–62
Corma A, Esteve P, Martinez A, Valencia S (1995) J Catal 152:18–24
Ahuja G, Kumar R, Mathu P (2012) J Mol Struct 1011:166–171
Martın SE, Garrone A (2003) Tetrahedron Lett 44:549–552
Maspero F, Romano U (1994) J Catal 146:476–482
Lana EJL, Rocha KAS, Kozhevnikov IV, Gusevskaya EV (2006) J Mol Catal A 259:99–102
Yuasa Y, Yuasa Y (2006) Org Process Res Dev 10:1231–1232
Shul’pin GB, Kozlov YN, Shul’pina LS, Kudinov AR, Mandelli D (2009) Inorg Chem 48:10480–10482
Shul’pin GB, Kozlov YN, Shul’pina LS, Petrovskiy PV (2010) Appl Organomet Chem 24:464–472
da Silva MJ, Robles-Dutenhefner PA, Menini L, Gusevskaya EV (2003) J Mol Catal A 201:71–77
Sheldon RA, Kochi JK (1981) Metal-catalysed oxidation of organic compounds. Academic Press, New York (Chapter 3)
Kozlov YN, Nadezhdin AD, Purmal AP (1974) Int J Chem Kinet 6(6):383–394
Pariyar A, Bose S, Biswas AN, Das P, Bandyopadhyay P (2013) Catal Commun 32:23–27
Neuenschwander U, Meier E, Hermans I (2011) ChemSusChem 4:1613–1621
Shul’pin GB, Kozlov YN, Nizova GV, Süss-Fink G, Stanislas S, Kitaygorodskiy A, Kulikova VS (2001) J Chem Soc Perkin Trans 2:1351–1371
Acknowledgments
The authors are grateful for the financial support from CAPES, CNPq, FAPEMIG, and FUNARBE (Brazil). They also wish to thank Prof. L.C. Barbosa and A. L. Cardoso for the GC–MS analyses.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Carari, D.M., da Silva, M.J. Fe(NO3)3-Catalyzed Monoterpene Oxidation by Hydrogen Peroxide: An Inexpensive and Environmentally Benign Oxidative Process. Catal Lett 144, 615–622 (2014). https://doi.org/10.1007/s10562-013-1189-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10562-013-1189-x