Abstract
Kinetic resolution of 1-(4-(trifluoromethyl)phenyl)ethanol (TFMP) enantiomers was achieved through lipase-catalyzed transesterification in organic solvents. Lipase PS from Pseudomonas cepacia was selected as the best biological catalyst, and vinyl acetate was used as the acyl donor for the transesterification in isooctane. The effects of temperature, enzyme dosage, substrate ratio and time on the reaction were investigated. Response surface methodology was introduced as the tool for process optimization and the optimized conditions were obtained. The experimental results under the optimized conditions involving the temperature of 46 °C, substrate ratio of 1:12, enzyme dosage of 15 mg and time of 104 min, show that TFMP enantiomers were resolved with the enantiomeric excess of the remaining substrate (ees) higher than 99.0% and the conversion (c) of 50.3%, which indicates an efficient kinetic resolution process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The preparation of homochiral secondary alcohols is of significant importance in chemical, pharmaceutical and related fields, because the homochiral secondary alcohols provide building blocks for a wide range of biologically active compounds [1]. Methods to access homochiral secondary alcohols can be divided into three categories, synthesis starting from chiral pools, asymmetric synthesis and resolution of the racemate [2, 3]. Despite the remarkable progress in asymmetric synthesis, kinetic resolution of racemates is still the dominant method for production of homochiral secondary alcohols in industry, where the two enantiomers are resolved based on their different reaction kinetics in chiral entity [4]. The chiral entity is the key factor in kinetic resolution, it can be provided by a biocatalyst like enzyme and microorganism or a chemocatalyst like chiral acid, chiral base and chiral metal complex [5, 6]. Much attention has been paid to the chiral synthesis by chemocatalyst, however, biocatalyst has already gathered significant interest in kinetic resolution [7,8,9].
Use of a biocatalyst allows the process to be operated under mild conditions, and the inherent high regio- and enantioselectivity improves the atomic economy [10, 11]. Furthermore, the biocatalyst is biodegradable. Therefore, kinetic resolution with a biocatalyst, which better conforms to the concept of modern green chemical industry, is particularly advantageous [12, 13]. Among numerous biocatalysts, lipase has been found to be interesting in kinetic resolutions for production of optically pure compounds [14]. Lipases are hydrolases (E.C.3.1.1.3) and they can catalyze the hydrolysis of triglycerides into glycerol and fatty acids [15, 16]. Lipases with excellent catalytic properties have been well studied as catalysts because of their unique physicochemical behavior [17,18,19]. Enzymes are remarkably effective in the versatile reactions, such as hydrolysis [20,21,22], esterification [19, 23], transesterification [24], alcoholysis [25], and C–C bond formation [18]. Enzymes have been recently used as a potential biocatalyst in a large number of biotechnological sciences [26], more specifically, these include dairy products, detergents [18], pharmaceuticals [27, 28], chemicals [29, 30], agriculture products [18], and oil chemistry [30,31,32,33]. Enzymes act as a good catalyst, therefore, its production and utilization may be a better alternative of chemical catalysts.
The lipase-catalyzed kinetic resolutions are usually through a stereoselective reaction of nucleophiles with esters or their derivatives, such as a stereoselective transesterification reaction. Lipase has an active center of serine-histidine-aspartate catalytic triad [34], which is usually covered by a flexible region of the enzyme molecule, often called the lid. Interaction with a hydrophobic phase can cause opening of the lid to make the active site accessible [35]. Therefore, a lipase expresses higher catalytic activity at an organic–aqueous interface than in aqueous solution and the phenomenon is called interfacial activation [36]. The use of a low boiling point organic solvent in lipase-catalyzed transesterification can significantly increase the solubility of the substrates in reaction media and facilitate the recovery of the product with better overall yield [37, 38]. Furthermore, the use of lipase in an organic solvent offer several advantages, for example it minimizes the substrate or product inhibition, the possibility of denaturation and it also make the immobilization of enzymes not always required [39]. To promote the equilibrium of the acylation reaction shifting to the desired direction and inhibit the reverse reaction, the selected acyl donor is required, such as enol esters, whose leaving group is an enol that immediately suffer from a tautomeric reaction thereby promoting the reaction tends to be complete [40]. Thus, in the lipase-catalyzed kinetic resolution of racemic alcohols, the presence of a suitable lipase and acyl donor in an appropriate organic solvent, as well as the optimum temperature, enzyme dosage, substrate ratio and reaction time are all the important factors for biosynthesis of an optically pure alcohol product [41, 42]. The process optimization is therefore is very necessary in investigation of the above process. Response surface method (RSM) is a powerful tool that has been used to evaluate the interactions between multiple process factors and simulate the quantitative relation between the response values and the factors, which can further be used for process optimization [43].
1-[4-(Trifluoromethyl)phenyl] ethanol (TFMP) is an important intermediate for the synthesis of AIDS (acquired immune deficiency syndrome) drugs, and (R)-TFMP is an important intermediate for the synthesis of AD101 (SCH-350581), a chemokine CCR5 antagonist, that blocks the entry of HIV-1 into cells [44,45,46]. Chen et al. [44] achieved the chemical synthesis of (R)-TFMP by asymmetric reduction of 4-(trifluoromethyl)acetophenone over a chiral chemical catalyst of oxazaborolidine, however the expensive chiral catalyst and the potential risk of environmental pollution limit its application prospect. The method is improved through employing a recombinant whole cell to catalyze the asymmetric reduction, but the method is still restricted by the difficulty in process control [44]. Compared with the above, lipase-catalyzed kinetic resolution is an attractive method to prepare (R)-TFMP. Lipase-catalyzed kinetic resolution allows the resolution of TFMP enantiomers with high optical purity. The process can be operated under mild conditions and control of the process is much convenient.
Herein, we report the kinetic resolution of racemic TFMP through lipase-catalyzed enantioselective transesterification in the organic media. Lipase used this work is selected from the commercially available lipases and the effects of important process parameters such as temperature, substrate ratio, enzyme dosage and time on the resolution efficiency were investigated. RSM is further employed for process optimization to achieve a high efficiency of kinetic resolution.
Experimental section
Lipase and reagents
All lipases used in this work were commercially available and used in the experiments without further treatment. The origin of the lipases and other related information is shown in Table 1.
(R,S)-1-[4-(Trifluoromethyl)phenyl]ethanol (purity > 98% +) and vinyl acetate (purity > 99% +) was purchased from Adamas reagent Co., Ltd. (Shanghai, China). The n-hexane and isopropanol used for HPLC detection were chromatographic grade, while the other reagents were analytical grade and were purchased from different companies. All reagents were applied to the reaction without further treatment.
HPLC analysis
The quantification of TFMP enantiomers was performed by a Waters e2695 high performance liquid chromatography (HPLC) consisting of a Waters e2695 separation unit, a 2489 UV–visible detector. Daicel Chiralcel IG column (250 mm × 4.6 mm ID, Tokyo, Japan) was used. The mobile phase was composed of n-hexane/anhydrous ethanol 99:1 (v/v). The flow rate was maintained at 1 mL min−1. Injection volume of each sample was 10 μL; The column temperature was set at 25 °C. The wavelength is 210 nm. The enantiomer excess of the substrate (ees), the total conversion (c), the conversions of (R)-enantiomer (cR) and the conversions of (S)-enantiomer (cS) were calculated by Eqs. 1, 2, 3 and 4:
Here [R] and [S] are the concentrations of (S)-TFMP and (R)-TFMP in reaction mixture, respectively; [R]0, [S]0 is initial amount of (S)-TFMP and (R)-TFMP, respectively.
Lipase-catalyzed transesterification of TFMP
The experiments were performed in a 25 mL glass tube with a spiral seal. A typical experimental procedure was as follows: the reactants of TFMP racemate (5 mmol L−1) and vinyl acetate (30 mmol L−1) were dissolved in 25 mL of isooctane to form the raw material solution. Then, 2 mL of the raw material solution was added into the glass tube. The solution was stirred (500 rpm min−1) and heated by a thermostatic stirrer (IKA RCT Basic, Germany). After reaching the temperature of 45 °C, 15 mg of lipase was added. After the reaction was completed, the reaction mixture was poured out and filtered to obtain the sample HPLC analysis. Lipases, acyl donors and organic solvents were selected through screening experiments. Effects of conditions including temperature (25–65 °C), enzyme dosage (5–30 mg), molar ratio of TFMP to vinyl acetate (1:1–1:48) and reaction time (20–170 min) were investigated and optimized. The reaction is illustrated in Fig. 1. In this work, the enzyme dosage refers to the mass of various lipases including the powder of lyophilized enzyme, the solution of enzyme and the immobilized lipase (including the support), all of which are weighed and added into the reaction medium.
Kinetic study
Experiments on lipase-catalyzed transesterification between racemic TFMP and vinyl acetate were carried out at different TFMP concentrations and a constant concentration of vinyl acetate (90 mmol L−1), using lipase PS as the catalyst. The sample was drawn at the certain stages of reaction for analysis of the concentration of (R)-TFMP. The TFMP concentration–time curves were recorded (control the conversion of (R)-TFMP less than 20%) and the initial reaction rate V0 was determined by making the slope of the concentration–time curves in the initial reaction stage.
Experimental design and data analysis
Generally, lipase-catalyzed transesterification of TFMP is influenced by a series of factors, such as lipase, organic solvent, acyl donor, enzyme dosage, substrate molar ratio, temperature, and reaction time. On the basis of single factor investigation, Response surface methodology (RSM) method and Box-Behnken design were used to optimize the process.
The key to response surface optimization is the selection of experimental points. Therefore, the reasonable value range of the main influencing factors has been determined through single factor experiment. Then response surface analysis was used to optimize the lipase-catalyzed resolution conditions. Show in Table 2, the four independent variables in this work were temperature (A, 25–65 °C), substrate ratio (B, 1–24), enzyme dosage (C, 5–25 mg) and time (D, 60–160 min).
Here, c and ees represent conversion and enantiomeric excess of the remaining substrate, respectively; α0 and b0 are the constant coefficient; αi and bi, αij and bij, αii and bii are the linear coefficient, squared coefficient, and cross-product coefficient, respectively; k is the number of factors; xi and xj are the independent variable. All the coefficients in Eqs. 5 and 6 were calculated by analysis of variance (ANOVA). Design Expert (version 10.0.1) was employed to acquire all coefficient of model and optimize the multiple responses.
Results and discussion
Screening of lipase
Selection of a suitable lipase is very important for the lipase-catalyzed kinetic resolution. Nine commercially available lipases are tested and the results are shown in Table 3. It is found that different lipases exhibit large difference in catalytic activity (indicated by the substrate conversion, c) and selectivity (indicated by the enantiomeric excess of the remaining substrate, ees). Several lipases including lipase AK, lipase PS and Novocor ADL have good catalytic activity towards the substrate, the high conversion of 42.6% and 43.8% are achieved with lipase AK and lipase PS, among which lipase PS shows the best enantioselectivity with ees of 74.8% and c of 43.8%. Therefore, lipase PS was selected for the further investigation.
Screening of organic solvents
Lipase-catalysis in organic solvents broadens the use of biocatalyst, which is conventionally used in the aqueous media. It is also founded that the catalytic efficiency of the lipase is largely influenced by the solvent [47]. In order to select the most suitable solvent, the effect of organic solvents was investigated and the results are shown in Table 4. It is observed that the activity (indicated by c) was largely influenced by organic solvent and the influence largely depended on the polarity organic solvent [48,49,50,51]. LogP is used to evaluate the solvent polarity in general, and it would be increased with increasing the hydrophobicity of the solvent [52]. Lipase PS showed the highest activity and enantioselectivity when n-hexane and isooctane were used as solvents. The catalytic activity of lipase PS is reduced with the increase of the polarity of the solvent. The possible reason is that the hydration layer on the surface of the lipase molecules is necessary for the lipase to maintain its activated conformation [53]. Low polarity of the solvent well protects the hydration layer, but a polar solvent will enter and damage the hydration layer [54]. The highest c of 46.6% and ees of 81.8% was achieved with isooctane. Therefore, isooctane with logP of 4.3 was selected as organic solvent in this work.
Screening of acyl donors
In the lipase-catalyzed transesterification of TFMP racemate, the (R)-TFMP selectively reacts with the acyl donor to form the (R)-TFMP ester with high ee value and leaving (S)-TFMP as the enantiomerically pure unreacted enantiomer. The selection of acyl donor is therefore important. As is shown in Table 5, some acyl donors are tested. With the enol esters (vinyl acetate, vinyl propionate, isopropenyl acetate and vinyl butyrate), the resolution efficiency is significantly enhanced than that with other donors. This is because the leaving group of these donors undergoes a keto–enol tautomerization to yield the corresponding carbonyl compound (such as acetaldehyde), thereby preventing the reverse reaction and driving the reaction to completion. Based on the results in Table 5, vinyl acetate was selected.
Effect of temperature
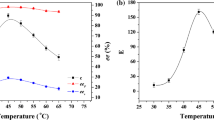
Since enzyme is a protein in chemical nature, temperature has a significant influence on the catalytic activity of the lipase [55, 56]. The effects of temperature on catalytic activity and enantioselectivity of lipase PS in the present lipase-catalyzed transesterification reaction system were studied in the range from 25 to 65 °C. As can be seen from Fig. 2, with the increase of temperature, the c and ees increase steadily. The conversion reaches its maximum at 45 °C and can maintain the maximum in the range from 45 to 60 °C, indicating that lipase PS has good thermal stability.
Effect of enzyme dosage
The enzyme dosage is also an important factor affecting the lipase-catalyzed resolution efficiency. As shown in Fig. 3, when the amount of enzyme increases from 5 to 15 mg, both c and ees gradually increase to the optimal value, where c of about 50.0% and ees higher than 99.0% are achieved. The powder of lipase PS was well dispersed in the reaction system. Furthermore, the substrates are well dissolved in the organic solvent reaction system. Therefore, diffusion limitations for the reaction system in this work is minimized. However, with the further increase of enzyme dosage, it can be observed that c and ees have been stable near the optimal value without change. This phenomenon can be explained by the fact that the dispersion of the powder of lipase PS becomes difficult and the contact between the enzyme and the substrate is not significantly increased when the enzyme dosage is high than 15 mg, thus the further increase in enzyme dosage don’t improve the conversion. The other possible reason is that the enzyme may experience protein aggregation and it made the catalytic center less accessible [57]
Effect of substrate ratio
Substrate ratio (molar ratio of vinyl acetate to TFMP) is also an important factor affecting the resolution efficiency. As is shown in Fig. 4, when the substrate ratio increased from 1:1 to 1:12, the c and ees increase rapidly to a high value. When the substrate ratio is further increase from 1:12, both c and ees reach a platform and remain basically unchanged. The possible reason is that when the addition of vinyl acetate is small, a large amount of catalytic active sites is not occupied and the increasing in addition of vinyl acetate can accelerate the reaction rate and promote a forward shift of the reaction equilibrium [58, 59]. Therefore, the c and ees are rapidly increased. When a large amount of vinyl acetate is added, the available catalytic active sites are saturated and the further increase in the addition of vinyl can’t improve the conversion.
Effect of time
Time is also an important factor for the lipase-catalyzed kinetic resolution. It is proposed to terminate the reaction at an appropriate time, where the fast-reaction enantiomer is just completely transformed, while the slow-reaction enantiomer has only the minimum amount of transformation. As can be seen from Fig. 5, ees and c increase gradually with the time first and then the growth becomes very slow after 80 min of reaction. When the reaction time was 110 min, ees and c reached the maximum and keep nearly unchanged with the further increase of time.
Kinetic study
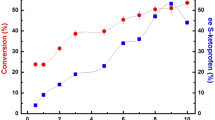
Kinetic study was carried out through the method mentioned above. A constant concentration of vinyl acetate was set (much higher than TFMP) and the reaction was terminated at the initial stage of the reaction to evaluate the initial reaction rate, therefore, it can be approximately considered that the concentration of vinyl acetate remained constant during the reaction and the reaction was a single-substrate reaction. Fig. 6 shows the plots of V0 versus [(R)-TFMP]0, in which the experimental results (presented as scattered symbols) are compared graphically with the calculated values (presented as solid line). The experimental data was fitted to Michaelis–Menten equation through the non-linear least squares fitting method [60]. It is found that the calculated values agree well with the experimental results. The kinetic parameters Km and Vmax were estimated (Table 6) through the non-linear regression analysis with a R2 value of 0.98. The parameters Km and Vmax are comparable with the results in the literature [61, 62].
Regression model and statistical analysis
The single-factor experiments show that four factors, namely temperature, substrate ratio, enzyme dosage and time, largely influence the resolution efficiency. However, it is hard to obtain the optimized conditions on the basis of the single-factor experiments. To further optimized the conditions, Box–Behnken experiment was designed with enantiomer excess and conversion as response values. Design-Expert software was used to conduct response surface regression analysis on the experimental data (Table 7), and the quadratic multinomial regression model of enantiomer excess and conversion and all investigated variables was obtained as follows:
The ANOVA for the model is shown in Tables 8. It is found that F of ees regression model is 81.1 with P < 0.0001, F of c regression model is 46.74 with P < 0.0001, indicating that the significance of the two models is very high. At the same time, correlation coefficient R2 of ees equation is evaluated as 0.9878, indicating that this model can be used to explain 98.78% of the change in response values, and correlation coefficient R2 of c equation is evaluated as 0.9791, indicating that this model can be used to explain 97.91% of the change in response values, indicating that the two test models are in good fit and the model has high reliability.
In addition, the P-value can be used to check the significance of each coefficient. By comparing the P values of each factor. In this case, A, B, C, D, AC, AD, BC, CD, A2, B2, C2 were significance for model ees. A, B, C, D, AC, AD, BC, B2, C2 were significant for model c, and the order of significance was B > A = C > D. The following discusses the multi-factors interactions of the important factors based on the regression model.
Influence of substrate ratio and temperature
Fig. 7 shows the cross-impact of substrate ratio and temperature. From Fig. 7a, at a fixed substrate ratio, ees gradually increases with increasing temperature, however the increasing is not very significant. At lower substrate ratios (< 1:12), the effect of temperature on ees is smaller, e.g., when the substrate ratio is 1:1, ees changes from 12.4% to about 40% with increasing temperature. However, with the substrate ratio increases, the ees increases significantly and then decreases slowly. From Fig. 7b, the adjustment of substrate ratio can lead to an obvious change in c. It is observed that the enhanced ees and c can be achieved when the temperature is about 45 °C and substrate ratio is about 1:12.5.
Influence of time and substrate ratio
Fig. 8 shows the interaction of time and substrate ratio on the performance of lipase-catalyzed resolution at a fixed temperature of 45 °C. When the time increases from 60 to 160 min, c and ees show a slight increase, showing an upward trend, while when the substrate ratio is increase from 1:1 to 1:24, c and ees increase significantly at first and then decrease slightly, the maximum value is reached around the substrate ratio of about 1:12.5. It shows that the increase of the substrate ratio has a strong effect on c and ees. The time of 110 min is proposed to achieve the optimized c and ees.
Influence of enzyme dosage and substrate ratio
As is shown in Fig. 9, with the increase of substrate ratio, ees and c show a trend of increase first and then decreases a little. The results are consistent with that observed in Fig. 9. On the other hand, ees and c are slightly enhanced by the increase of enzyme dosage. When the enzyme dosage is higher than 15 mg, the increase of c and ees with the enzyme dosage is slight. This indicates that continuing to increase the enzyme dosage has less effect on increasing c and ees values, so the amount of enzyme is kept at about 15 mg.
Influence of time and enzyme dosage
Fig. 10 shows the interaction of enzyme dosage and time on the resolution efficiency at a fixed temperature of 45 °C and substrate ratio of 12.5:1. It is observed that when the time is between 60 and 160 min and the enzyme dosage is between 5 and 25 mg, c and ees showed a trend of gradual increase with the increase of time and enzyme dosage. The optimal c and ees can be achieved in region where time is higher than 110 min and the enzyme dosage is higher than 15 mg.
Application and validation of the model
Based on the regression model, the optimal conditions for kinetic resolution of TFMP enantiomers by lipase-catalyzed transesterification are obtained as follows: temperature of 46 °C, substrate ratio of 1:12, enzyme dosage of 15 mg, time of 104 min. Under these conditions, the ees higher than 99.0% and c of about 50.0% are expected to be obtained. The chromatograms of TFMP are shown in Fig. 11a, b respectively. As shown in Table 9, three parallel verification experiments were carried out under the optimal conditions, and the measured ees higher than 99.5% and c of 50.3% are achieved (reported with the average value), which proves the validity of the model.
Although good results for the kinetic resolution of TFMP enantiomers with lipase PS are achieved, the powder of lipase PS is hardly able to be reused, because the powder is only partially recovered from the reaction system and the expressed activity of the recycled powder is considerably reduced. In order to further improve the industrial application ability of lipase PS, more attention should be paid to the research of enzyme immobilization.
Conclusion
Kinetic resolution of TFMP enantiomers through lipase-catalyzed transesterification in an organic solvent was performed. Lipase PS was selected as the biocatalyst and isooctane was selected as the organic solvent. Single-factor experiments were performed to identify the important factors that influence the resolution efficiency. The results showed that ees and c mainly depended on substrate ratio, temperature, enzyme dosage and time. the RSM with Box-Behnken experimental designation was employed for further investigation and optimization of the reaction conditions. Results showed that the regression models for ees and c are significant and the optimized conditions were obtained. Under the optimized conditions including temperature of 46 °C, substrate ratio of 1:12, enzyme dosage of 15 mg and time of 104 min, TFMP enantiomers were efficiently resolved with ees higher than 99.0% and c of 50.3%. The resolution system has good industrial application potential.
References
Liu H, Duan WD, De Souza FZR, Liu L, Chen BS (2018) Asymmetric ketone reduction by immobilized Rhodotorula mucilaginosa. Catalysts 8:165. https://doi.org/10.3390/catal8040165
Harwood LA, Wong LL, Robertson J (2020) Enzymatic kinetic resolution by addition of oxygen. Angew Chem Int Ed 60:4434–4447. https://doi.org/10.1002/anie.202011468
Lorenz H, Seidel-Morgenstern A (2014) Processes to separate enantiomers. Angew Chem Int Ed 53:1218–1250. https://doi.org/10.1002/anie.201302823
Berkessel A, Sebastian-Ibarz M, Müller T (2006) Lipase/aluminum-catalyzed dynamic kinetic resolution of secondary alcohols. Angew Chem Int Ed 45:6567–6570. https://doi.org/10.1002/anie.200600379
Jiang W, Fang BS (2020) Synthesizing chiral drug intermediates by biocatalysis. Appl Biochem Biotechnol 192:146–179. https://doi.org/10.1007/s12010-020-03272-3
Hönig M, Sondermann P, Turner NJ, Carreira EM (2017) Enantioselective chemo- and biocatalysis: partners in retrosynthesis. Angew Chem Int Ed 56:8942–8973. https://doi.org/10.1002/anie.201612462
Lian XZ, Fang Y, Joseph E, Wang Q, Li JL, Banerjee S, Lollar C, Wang X, Zhou HC (2017) Enzyme-MOF (metal-organic framework) composites. Chem Soc Rev 46:3386–3401. https://doi.org/10.1039/c7cs00058h
Green AP, Turner NJ (2016) Biocatalytic retrosynthesis: redesigning synthetic routes to high-value chemicals. Perspect Sci 9:42–48. https://doi.org/10.1016/j.pisc.2016.04.106
Turner NJ, O’Reilly E (2013) Biocatalytic retrosynthesis. Nat Chem Biol 9:285–288. https://doi.org/10.1038/nchembio.1235
Karadeniz F, Bayraktar E, Mehmetoglu U (2010) Kinetic resolution of racemic 1-phenyl-1-propanol by lipase catalyzed enantioselective esterification reaction. Artif Cells Blood Substit Immobil Biotechnol 38:288–293. https://doi.org/10.3109/10731199.2010.494579
Chandrasekaran SM, Bhartiya S, Wangikar PP (2006) Substrate specificity of lipases in alkoxycarbonylation reaction: QSAR model development and experimental validation. Biotechnol Bioeng 94:554–564. https://doi.org/10.1002/bit.20879
Kim H, Choi YK, Lee J, Lee EY, Park J, Kim MJ (2011) Ionic-surfactant-coated burkholderia cepacia lipase as a highly active and enantioselective catalyst for the dynamic kinetic resolution of secondary alcohols. Angew Chem 123:11136–11140. https://doi.org/10.1002/ange.201104141
Klibanov AM (2001) Improving enzymes by using them in organic solvents. Nature 409:241–246. https://doi.org/10.1002/chin.200114287
de Miranda AS, Miranda LSM, de Souza ROMA (2015) Lipases: valuable catalysts for dynamic kinetic resolutions. Biotechnol Adv 33(5):372–393. https://doi.org/10.1016/j.biotechadv.2015.02.015
Moreira K, de Oliveira ALB, de Moura Júnior LS et al (2022) Taguchi design-assisted co-immobilization of lipase A and B from Candida antarctica onto chitosan: characterization, kinetic resolution application, and docking studies. Chem Eng Res Des 177:223–244. https://doi.org/10.1016/j.cherd.2021.10.033
Garcia-Galan C, Barbosa O, Hernandez K, Santos J, Rodrigues R, Fernandez-Lafuente R (2014) Evaluation of styrene-divinylbenzene beads as a support to immobilize lipases. Molecules 19(6):7629–7645. https://doi.org/10.3390/molecules19067629
Fernandez-Lopez L, Bartolome-Cabrero R, Rodriguez MD, Dos Santos CS, Rueda N, Fernandez-Lafuente R (2015) Stabilizing effects of cations on lipases depend on the immobilization protocol. RSC Adv 5(102):83868–83875. https://doi.org/10.1039/C5RA18344H
Valério RBR, Cavalcante ALG, Mota GF (2022) Understanding the biocatalytic potential of lipase from rhizopus chinensis. Biointerface Res Appl Chem 12(3):4230–4260. https://doi.org/10.33263/BRIAC123.42304260
da Fonseca AM, de Freitas ÍB, Soares NB et al (2022) Synthesis, biological activity, and in silico study of bioesters derived from bixin by the CALB enzyme. Biointerface Res Appl Chem 12(5):5901–5917. https://doi.org/10.3263/BRIAC125.59015917
Lima GV, da Silva MR, de Sousa FT et al (2017) Chemoenzymatic synthesis of (S)-Pindolol using lipases. Appl Catal A Gen 546:7–14. https://doi.org/10.1016/j.apcata.2017.08.003
Monteiro RRC, de Oliveira ALB, de Menezes FL et al (2022) Improvement of enzymatic activity and stability of lipase A from Candida antartica onto halloysite nanotubes with Taguchi method for optimized immobilization. Appl Clay Sci 228:106634. https://doi.org/10.1016/j.clay.2022.106634
Pinheiro MP, Rios NS, Fonseca TDS et al (2018) Kinetic resolution of drug intermediates catalyzed by lipase B from Candida antarctica immobilized on immobead-350. Biotechnol Progr 34(4):878–889. https://doi.org/10.1002/btpr.2630
Bezerra RM, Monteiro RRC, Neto DMA et al (2020) A new heterofunctional support for enzyme immobilization: PEI functionalized Fe3O4 MNPs activated with divinyl sulfone application in the immobilization of lipase from Thermomyces lanuginosus. Enzyme Microb Technol 138:109560. https://doi.org/10.1016/j.enzmictec.2020.109560
Galvão WS, Pinheiro BB, Golçalves LRB et al (2018) Novel nanohybrid biocatalyst: application in the kinetic resolution of secondary alcohols. J Mater Science 53(20):14121–14137. https://doi.org/10.1007/s10853-018-2641-5
Verdasco-Martín CM, Villalba M, dos Santos JCS et al (2016) Effect of chemical modification of Novozym 435 on its performance in the alcoholysis of camelina oil. Bioch Eng 111:75–86. https://doi.org/10.1016/j.bej.2016.03.004
Moreira KDS, de Oliveira ALB, Júnior LSDM et al (2020) Lipase from Rhizomucor miehei immobilized on magnetic nanoparticles: performance in fatty acid ethyl ester (FAEE) optimized production by the taguchi method. Front Bioeng Biotech 8:693. https://doi.org/10.3389/fbioe.2020.00693
Liu DM, Dong C (2020) Recent advances in nano-carrier immobilized enzymes and their applications. Process Biochem 92:464–475. https://doi.org/10.1016/j.procbio.2020.02.005
Zahirinejad S, Hemmati R, Homaei A, Dinari A, Hosseinkhani S, Mohammadi S, Vianello F (2021) Nano-organic supports for enzyme immobilization: scopes and perspectives. Colloid Surface B 204:111774. https://doi.org/10.1016/j.colsurfb.202
Silva ARM, Alexandre JYNH, Souza JES (2022) The Chemistry and applications of metal-organic frameworks (MOFs) as industrial enzyme immobilization systems. Molecules 27:4529. https://doi.org/10.3390/molecules27144529
Lima PJM, da Silva RM, Neto CACG et al (2021) An overview on the conversion of glycerol to value-added industrial products via chemical and biochemical routes. Biotechnol Appl Bioc 68:2098. https://doi.org/10.1002/bab.2098
Mota GF, de Sousa IG, de Oliveira ALB et al (2022) Biodiesel production from microalgae using lipase-based catalysts: current challenges and prospects. Algal Res 62:102616. https://doi.org/10.1016/j.algal.2021.102616
Cavalcante FTT, Neto FS, de Aguiar Falcão IR et al (2020) Opportunities for improving biodiesel production via lipase catalysis. Fuel 288:119577. https://doi.org/10.1016/j.fuel.2020.119577
Cavalcante FTT, da Fonseca AM, Alexandre JYNH, dos Santos JCS (2022) A stepwise docking and molecular dynamics approach for enzymatic biolubricant production using Lipase Eversa® transform as a biocatalyst. Ind Crop Prod 187:115450. https://doi.org/10.1016/j.indcrop.2022.115450
Dheeman DS, Frias JM, Henehan GTM (2010) Influence of cultivation conditions on the production of a thermostable extracellular lipase from Amycolatopsis mediterranei DSM 43304. J Ind Microbiol Biotechnol 37:1–17. https://doi.org/10.1007/s10295-009-0643-7
Tokunaga M, Larrow JF, Kakiuchi F (1997) Asymmetric catalysis with water: efficient kinetic resolution of terminal epoxides by means of catalytic hydrolysis. Science 277:936–938. https://doi.org/10.2307/2892907
Jaeger KE, Eggert T (2002) Lipases for biotechnology. Curr Opin Biotech 13:390–397. https://doi.org/10.4028/www.scientific.net/MSF.636-637.1194
Singh AK, Mukhopadhyay M (2016) Lipase-catalyzed glycerolysis of olive oil in organic solvent medium: optimization using response surface methodology. Korean J Chem Eng 33:1247–1254. https://doi.org/10.1007/s11814-015-0272-y
Cai ZQ, Cai JY, Li SS, Zhang WJ, Shi S, Zhu XL (2014) Biosynthesis of myristyl serinate by immobilized Candida antarctica lipase in two-phase system. J Mol Catal B: Enzym 108:118–122. https://doi.org/10.1016/j.molcatb.2014.07.008
Ghanem A, Aboul-Enein HY (2004) Lipase-mediated chiral resolution of racemates in organic solvents. Tetrahedron Asymmetry 15(21):3331–3351. https://doi.org/10.1002/chin.200508294
Palmeira DJ, Abreu JC, Andrade LH (2011) Lipase-catalyzed kinetic resolution of aryltrimethylsilyl chiral alcohols. Molecules 16:9697–9713. https://doi.org/10.3390/molecules16119697
Yuan X, Liu GY, Zhang PL, Xu WF, Tang KW (2019) Lipase-catalyzed production of (S)-carprofen enhanced by hydroxyethyl-β-cyclodextrins: experiment and optimization. Org Process Res Dev 23:891–899. https://doi.org/10.1021/acs.oprd.9b00009
Nishihara T, Shiomi A, Kadotani S, Nokami T, Iton T (2017) Remarkable improved stability and enhanced activity of a burkholderia cepacia lipase by coating with a triazolium alkyl-PEG sulfate ionic liquid. Green Chem 19:5250–5256. https://doi.org/10.1039/C7GC02319G
Shahedi M, Yousefi M, Habibi Z, Mohammadi M (2019) Co-immobilization of rhizomucor miehei lipase and candida antarctica lipase b and optimization of biocatalytic biodiesel production from palm oil using response surface methodology. Renewable Energy 141:847–857. https://doi.org/10.1016/j.renene.2019.04.042
Chen Y, Xia NN, Liu YW, Wang P (2019) Efficient biocatalytic preparation of optically pure (R)-1-[4-(trifluoromethyl)phenyl]ethanol by recombinant whole-cell-mediated reduction. Catalysts 9:391. https://doi.org/10.3390/catal9040391
Tsamis F, Gavrilov S, Kajumo F, Seibert C, Kuhmann S, Ketas T, Trkola A, Palani A, Clader JW, Tagat JR, McCombie S, Baroudy B, Moore JP, Sakmar TP, Dragic T (2003) Analysis of the mechanism by which the small-molecule CCR5 antagonists SCH-351125 and SCH-350581 Inhibit human immunodeficiency virus type 1 entry. Virol J 77:5201–5208. https://doi.org/10.1128/JVI.77.9.5201-5208.2003
Tagat JR, Steensma RW, McCombie SW, Nazareno DV, Lin SI, Neustadt BR, Cox K, Xu S, Wojcik L, Murray MG, Vantuno N, Baroudy BM, Strizki JM (2001) Piperazine-based CCR5 antagonists as HIV-1 inhibitors. II. discovery of 1-[(2,4-dimethyl-3-pyridinyl)carbonyl]-4-methyl-4-[3(S)-methyl-4-[1(S)-[4-(trifluoromethyl)phenyl]ethyl]-1-piperazinyl]-piperidine N1-oxide (Sch-350634), an orally bioavailable, potent CCR5 antagonist. J Med Chem 44:3343–3346. https://doi.org/10.1021/jm0155401
Kumar A, Dhar K, Singh S, Arora PK (2016) Lipase catalysis in organic solvents: advantages and applications. Biol Proced Online 18:2. https://doi.org/10.1186/s12575-016-0033-2
Laane C, Boeren S, Vos K, Veeger C (2009) Rules for optimization of biocatalysis in organic solvents. Biotechnol Bioeng 102:1–8. https://doi.org/10.1002/bit.22209
Kharrat N, Ali YB, Marzouk S, Gargouri YT, Karra-Châabouni M (2011) Immobilization of rhizopus oryzae lipase on silica aerogels by adsorption: comparison with the free enzyme. Process Biochem 46:1083–1089. https://doi.org/10.1016/j.procbio.2011.01.029
Kaewthong W, Kittikun AH (2004) Glycerolysis of palm olein by immobilized lipase PS in organic solvents. Enzyme Microb Technol 35:218–222. https://doi.org/10.1016/j.enzmictec.2004.04.011
Li X, Xu L, Wang G, Zhang H, Yan Y (2013) Conformation studies on Burkholderia cenocepacia lipase via resolution of racemic 1-phenylethanol in non-aqueous medium and its process optimization. Process Biochem 48:1905–1913. https://doi.org/10.1016/j.procbio.2013.09.001
Hazarika S, Goswami P, Dutta NN (2002) Ethyl oleate synthesis by Porcine pancreatic lipase in organic solvents. Chem Eng J 85:61–68. https://doi.org/10.1016/s1385-8947(01)00144-9
Kovalenko G, Perminova L, Beklemishev A (2020) Heterogeneous biocatalytical esterification by recombinant Thermomyces lanuginosus lipase immobilized on macroporous carbon aerogel. Catal Today 379:36–41. https://doi.org/10.1016/j.cattod.2020.11.018
Stepankova V, Bidmanova S, Koudelakova T, Prokop Z, Chaloupkova R, Damborsky J (2013) Strategies for stabilization of enzymes in organic solvents. Acs Catal 3:2823–2836. https://doi.org/10.1021/cs400684x
Phillips RS (1996) Temperature modulation of the stereochemistry of enzymatic catalysis: prospects for exploitation. Trends Biotechnol 14:13–16. https://doi.org/10.1016/0167-7799(96)80908-5
Gupta SM, Kamble MP, Yadav GD (2017) Insight into microwave assisted enzyme catalysis in process intensification of reaction and selectivity: kinetic resolution of (R, S)-flurbiprofen with alcohols. Mol Catal 440:50–53. https://doi.org/10.1016/j.mcat.2017.06.020
Sanchez A, Cruz J, Rueda N, dos Santos JCS et al (2016) Inactivation of immobilized trypsin under dissimilar conditions produces trypsin molecules with different structures. RSC Adv 6(33):27329–27334. https://doi.org/10.1039/c6ra03627a
Yuan X, Ou J, Zhang PL, Xu WF, Jiang BH, Tang KW (2020) PEG-modified lipase immobilized onto NH2-MIL-53 MOF for efficient resolution of 4-fluoromandelic acid enantiomers. Int J Biol Macromol 165:1793–1802. https://doi.org/10.1016/j.ijbiomac.2020.10.076
Bakkali-Hassani C, Poutrel QA, Langenbach J, Chappuis S, Blaker JJ, Gresil M, Tournilhac F (2021) Lipase-catalyzed epoxy-acid addition and Transesterification: from model molecule studies to network build-up. Biomacromol 22:4544–4551. https://doi.org/10.1021/acs.biomac.1c00820
Lente G (2015) Deterministic kinetics in chemistry and systems biology the dynamics of complex reaction networks. Springer, Cham. https://doi.org/10.1007/978-3-319-15482-4
Fu JY, Wang ZY, Luo W, Xing SY, Lv PM, Wang ZM, Yuan ZH (2018) A novel sanger’s reagent-like styrene polymer for the immobilization of Burkholderia cepacia lipase. ACS Appl Mater Interfaces 10(37):30973–30982. https://doi.org/10.1021/acsami.8b09225
Wang JY, Ma CL, Bao YM, Xu PS (2012) Lipase entrapment in protamine-induced bio-zirconia particles: characterization and application to the resolution of (R, S)-1-phenylethanol. Enzyme Microb Tech 51(1):40–46. https://doi.org/10.1016/j.enzmictec.2012
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 21978077), Postgraduate Scientific Research Innovation Project of Hunan Province (CX20211194).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, S., Wu, Y., Sun, B. et al. Experimental and optimization for kinetic resolution of 1-(4-(trifluoromethyl)phenyl)ethanol enantiomers by lipase-catalyzed transesterification in organic phase. Reac Kinet Mech Cat 136, 183–204 (2023). https://doi.org/10.1007/s11144-022-02339-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-022-02339-y