Abstract
This study examines the impact of three amino acids such as proline, methionine and tryptophan on methane (95%)–propane (5%) hydrate formation with the use of different impellers. The concentration of amino acids was 1 wt% at 24.5 bar and 2 °C. Based on experimental outcomes proline behaves as inhibitor and methionine and tryptophan perform as promoters. RT experiments both formed more quickly gas hydrates and indicated higher values in rate of hydrate formation compared to PBTU and PBTD experiments showing that in radial flow bubbles are subjected to higher shear stresses, their size are reduced, so that the contact surface is increased resulting in an improved mass transfer coefficient.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Gas hydrates are crystalline compounds formed from water and suitable sized gas molecules. Depending on which gas molecules are present, hydrates form different crystal structures. Cubic structure I (sI) and structure II (sII) and hexagonal structure H (sH) are the three structures of gas hydrates [1]. Structure I hydrate has two types of cavity: a small pentagonal dodecahedral cavity consisting of 12 pentagonal rings of water and a large tetrahedral cavity consisting of 12 pentagonal and two hexagonal rings of water. Structure II hydrate also has two cavity sizes, the pentagonal dodecahedral cavity and larger hexakaidecahedral cavity consisting of 12 pentagonal and four hexagonal rings of water [2]. Methane hydrates can contain 150–180 v/v at standard temperature and pressure and they provide very good storage characteristics [3]. Gudmundsson and Parlaktuna initially and several scientists later have reported outcomes in this field [3,4,5,6,7].
Two well-known of non-pipeline methods of methane storage are liquefied natural gas (LNG) or compressed natural gas (CNG). LNG method is less energy demanding but more costly compared to storage of gas hydrates, while CNG method is only developed for small-scale systems [8]. On the other hand, gas hydrate formation guides to pipeline plugging matters throughout the period of oil and gas transportation [9,10,11,12,13,14,15,16]. To prevent hydrate formation, thermodynamic inhibitors (THIs) such as methanol [17] monoethylene Glycol (MEG) [18], ethylene glycol [19, 20] and triethylene glycol [20, 21] are commonly injected in the pipelines during the production and transportation of oil and gas. The amount of thermodynamic inhibitors is high hence the expenditures in these operations cannot be justified [22]. In such way, financial hydrate management policies have become more than an important need nowadays [23]. For that reason, another process for impeding hydrate formation is the use low dosage inhibitors (LDHIs). LDHIs can be divided into two classes of compounds: (i) kinetic hydrate inhibitors (KHIs) and (ii) anti-agglomerates (AAs) [24]. Kinetic inhibitors are mostly polymeric inhibitors while AAs are mostly surfactants. Both AAs and KHIs are used for continuous injection applications [25]. There are three main categories of kinetic inhibitors such as: (1) poly (N-vinyl lactam) polymers including PVP [26], PVCap [27], (2) polyesteramides [28] and (3) N-isopropylmethacrylamide. Other Polymeric KHIs groups are polyaspartamides [29], poly alkyl amides [30] and pyroglutamate polymers [28]. An omnibus rule for developing KHIs is that a specific grade of hydrophobicity should be reached for the KHI to desirably disintegrate the water structures. Surfactants are another category of LDIs for hydrates [30]. Anti-agglomerates (AAs) are surfactants that make the water phase to be suspended in small droplets. When hydrates are formed from water droplets, the flow characteristics are retained without occlusion. In that way there are hydrate crystals but they are very small and they are scattered in hydrocarbon liquid. Hence instead of inhibit hydrate formation; AAs inhibit hydrate plugging [8]. Sodium dodecyl sulfite (SDS) in methane hydrates when is used in submillimolar concentration (around 0.3 mM) and tetra-n-butyl ammonium-bromide (TBAB) in CO2 hydrates can display a strong inhibiting effect [31].
On the other side, surfactants can also play the role of kinetic promoters. Kalogerakis et al. was the first that investigated the performance of surfactants in methane hydrate formation without any influence in the thermodynamics [32]. Anionic surfactants that have been used to promote methane hydrates are linear alkyl benzene sulfonate (LABS), dodecyl benzene sulfonic acid (DBSA), sodium dodecyl sulfonate (SDSN), lithium dodecyl sulfate (LDS), (SO), sodium hexadecyl sulfate (SHS), sodium dodecyl benzene sulfonate (SDBS), sodium tetracyl sulfate (STS), sodium octadecyl sulfate and other sodium alkyl sulfates like sodium butyl sulfate [33,34,35,36,37]. Cationic surfactants that play the role of promoter in methane hydrates are dodecylamine hydrochloride (DAH), hexadecyl-trimethyl-ammoium bromide (HTABr), cetyl trimethyl ammonium bromide (CTAB), N-dodecylpropane-1,3-diamine hydrochloride (DN2Cl) while non-ionic surfactants such as ethoxylated nonylphenol (ENP), tergitol and polyoxyethylene (20) cetyl ether (Brij-58) have also been tested successfully as methane hydrate promoters [38,39,40,41,42].
Another group of chemicals that are used as hydrate inhibitors or promoters are amino acids. Hydrophobic amino acids such as glycine, l-alanine, and l-valine can be applied as thermodynamic hydrate inhibitors (THIs) [43]. l-serine, l-proline, asparagine, l-threonine, l-valine, l-histidine, glycine, alanine, serine, proline, arginine, l-leucine, l-tryptophan, Lysine, valine, methionine, phenylalanine, alanine, serine, glysine + ethylene glycol and glysine + 1-ethyl-3-methy limidazolium chloride have also been used as inhibitors for methane gas hydrates [44]. Other amino acids that have been used for CO2 hydrate inhibition are l-phenylalanine, l-cysteine, l-methionine l-threonine, proline, glycine, threonine, glutamine, histidine, alanine, arginine, l-methionine, l-norvaline, l-norleucine, 2 amino heptanoic acid, n-hexylamine, lysine, phenylalanine, methionine, cysteine, isoleucine, aspartic acid, asparagine, histidine, l-histine, PVP and l-tyrosine [45, 46]. Polymers and starches also have been tested successfully as hydrate promoters. Polymers that have been used for promoting hydrates are soluble hydroxyl ethyl cellulose [47], poly (2-acrylamido-2-methylopropane sulfonic acid and poly (acrylic acid) [48] and poly vinyl alcohol (PVA) [49]. Starches that successfully functioned as hydrate promoters are potato starch [50], xanthan gum and starch [51], and Maize starch [52]. There are few previous works examine mixture of methane-propane gas hydrate formation or inhibition by the use of different amino acids [53,54,55,56,57,58]. In this work, three different amino acids will be examined if they function as promoters or inhibitors with the use of three different impellers. The impellers that will be examined are pitched blade turbine upward trending (PBTU), pitched blade turbine downward trending (PBTD) and rushton turbine (RT). The first two impellers create mixed flow while rushton turbine creates radial flow.
Reactor design and experimental process
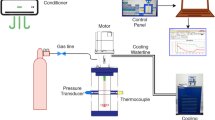
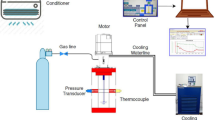
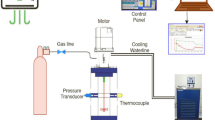
A transparent reactor with internal volume 1.56 l was used to conduct our experiments for mixture gas hydrate formation. Methane–propane mixture (95% methane and 5% propane—Hat Group Company, Kocaeli-Turkey) were used to form hydrates at medium-pressure PMMA reactor. Distilled water is the liquid phase to form hydrate (see supplementary material for dimensions and instruments of experimental process, S1, S2, S4,). The stages of experimental process are presented in supplementary material, too (S3, S5). Hydrate equilibrium line is obtained from CSMHYD (Research Center for Hydrates, Chemical Engineering Department, Colorado School of Mines).
The main objective of this study is to investigate the effect of three different amino acids and their function (inhibitors or promoters) with the use of three different impellers. Therefore, a tool must be devised to extract the kinetic data from raw experimental data. Application of real gas law (pV = znRT) for each data point with known pressure, temperature and free gas volume gives the change in number of moles of free gas with time. The gas compressibility factor of the real gas law Z is calculated by using Lee and Kesler’s (1975) compressibility factor expression [59]. A sample plot of change in free gas number of moles is given in Fig. 1 for CH4–C3H8-SI-PBTU-FB-methionine.
Fig. 2 is plotted with the same data of Fig. 1 but covering only hydrate formation period result with Eq. 1.
here n = number of moles of free gas, mol and t = time, s.
The derivative of Eq. 1 results with the gas consumption rate (Eq. 2) which can be considered as the hydrate formation rate.
here \(\frac{dn}{dt}\) = gas consumption rate, mol/s and t = time, s.
Comparison of gas consumption rates of different experiments will be done by utilizing gas consumption rate equations (Eq. 2 is an example) with four different time values, namely 1, 600, 1200 and 1800s. Table 1 presents the gas consumption rates of experiment CH4-C3H8-SI-PBTU-FB-methionine, as an example.
here Vwater is the volume of water (l) in the reactor, R30 is the rate of hydrate growth (mol × s−1) calculated by fitting the gas uptake due to hydrate growth versus time for the first 30 min after the induction time.
Results and discussion
Table 2 shows the outcomes of single impeller such as PBTU, PBTD and RT, methane (95%)–propane (5%) mixture experiments with pure water and the amino acids of aspartic acid, methionine and proline. Hydrate formation almost began immediately in all RT experiments in contrast with PBTU and PBTD ones. Amino acids behaved as promoters and not as inhibitors (from perspective of induction time) with the objection that hydrate formation is a stochastic procedure. The different kind of impellers was a way to duplicate the experiments due to their stochastic process and take some conclusions in case the different flow may influence together with the chemistry of amino acids the rate of hydrate formation.
Column 5 shows the hydrate productivity. In all three different impeller experiments showed that experiments with leucine have the highest value of hydrate productivity while the lowest value of hydrate productivity belongs to experiments with water. From Table 2 and last column there is observation that third-order polynomial fits of experimental data very well since all R2 are above 0.97. The change in the number of moles of free gas after the initiation of hydrate formation was used to calculate the rate of hydrate formation at four different times (1 s, 10, 20 and 30 min). Figs. 3, 4 and 5 present the hydrate formation rates of PBTU, PBTD and RT impeller experiments.
Figs. 3, 4 and 5 show that experiments with methionine have the highest hydrate formation rate with 15.0 × 10–8 mol/s, 14.60 × 10−8 mol/s and 16.60 × 10−8 mol/s for PBTU,PBTD and RT impellers respectively. The explanation is that promotion conduct of methionine is due to hydrophobic chain length and the synergistic effect of hydrophilic amino and carboxyl groups. Hence methionine practical helps in hydrate formation energy storage application since it forms stronger hydrogen bond with water molecules than hydrogen bonds between water molecules [60]. Tryptophan shows also promotion conduct since it belongs to non-polar hydrophobic amino acids. Moreover, the existence of the aromatic side chain in the amino acids also assists positively in mixture hydrate formation compared to aliphatic side chain [61]. On the other hand the lowest hydrate formation rate from amino acids with 12.80 × 10–8 mol/s, 10.60 × 10−8 mol/s and 13.40 × 10−8 mol/s for PBTU, PBTD and RT impellers belongs to proline experiments. Proline is an aliphatic non polar amine which shows inhibition conduct. The experimental results demonstrated that the inhibition effects of uncharged side chain amino acids augment with increasing hydrophobicity values [62, 63].
The below outcomes are concluded from perspective of hydrodynamic analysis. Hydrate formation rates of RT experiments are always higher than hydrate formation rates of PBTD and PBTU experiments. This shows that radial flow experiments present a behavior which nominates a better level of gas–liquid contact by giving the permission to mass transfer impedances to be appreciably diminished which eventually guided at beneficial mixing intensity [64,65,66]. Bubbles are subjected to higher shear stresses, their size are decreased, so that the contact surface is augmented leading in an ameliorated mass transfer coefficient [67, 68]. This indicates better mutual practice between the gas and the liquid phases when radial flow is near the surface. Better pumping competence, unchanging in form shear field and good touch efficiency can be misdoubted to be the cause of this effect [69,70,71]. Last column of Table 2 shows the values of standard error for 1 and 600 s. The values of standard errors range from 1.21 to 1.81 for the first 1 s and from 1.07 to 1.69 for the first 600 s confirming the quality of our experiments.
Conclusion
The main problem that gas hydrates create to oil and gas industry is the blockage of pipelines. Amino acids can play such role because are environmental friendly, biodegradable and can be used in small quantities. In this study there was examination of conduct in three amino acids. The outcomes indicated that proline works as inhibitor while methionine and tryptophan work as promoters. The highest rate of hydrate formation of methane (95%)–propane (5%) gas hydrate took place in radial flow experiments compared to mixed flow ones. The induction time of radial flow experiments is smaller compared to mixed flow ones showing that radial flow has better liquid gas contact compared to mixed flow.
Abbreviations
- LNG:
-
Liquefied natural gas
- KHI:
-
Kinetic hydrate inhibitors
- CNG:
-
Compressed natural gas
- LDHI:
-
Low dosage hydrate inhibitors
- THI:
-
Thermodynamic hydrate inhibitor
- AA:
-
Anti agglomerates
- MEG:
-
Monoethylene glycol
- SDS:
-
Sodium dodecyl sulfite
- TBAB:
-
Tetra-n-butyl ammonium-bromide
- PVP:
-
Polyvinylpyrrolidone
- CO2 :
-
Carbon dioxide
- LABS:
-
Linear alkyl benzene sulfonate
- DBSA:
-
Dodecyl benzene sulfonic acid
- SDSN:
-
Sodium dodecyl sulfonate
- LDS:
-
Lithium dodecyl sulfate
- SO:
-
Sodium oleate
- SHS:
-
Sodium hexadecyl sulfate
- SDBS:
-
Sodium dodecyl benzene sulfonate
- STS:
-
Sodium tetracyl sulfate
- DAH:
-
Dodecylamine hydrochloride
- HTABr:
-
Hexadecyl-trimethyl-ammoium bromide
- CTAB:
-
Cetyl trimethyl ammonium bromide
- ENP:
-
Ethoxylated nonylphenol
- DN2CL:
-
N-Dodecylpropane-1,3-diamine hydrochloride
- PBTU:
-
Pitched blade turbine upward
- PBTD:
-
Pitched blade turbine downward
- RT:
-
Rushton turbine
- dn/dt:
-
Gas consumption rate mol/s
- t:
-
Time, s
- NR30 :
-
Hydrate productivity mol/s × l
- R30 :
-
Hydrate formation for first 30 min, mol/s
- Vwater :
-
Volume of experiment
References
Longinos SN, Longinou DD, Achinas S (2020) Natural gas hydrates: possible environmental issues. Contemporary environmental issues and challenges in era of climate change. Springer, Singapore
Longinos SN, Bulbul S, Parlaktuna M (2019) Potential effects of methane hydrates to the environment, PESXM-12th Pan-Hellenic scientific conference in chemical engineering. Greece, Athens
Longinos S (2019) Potential environmental challenges for gas hydrates. LAP Lambert Academic Publishing, Beau Basin
Gudmundsson J, Borrehaug A (1996) Frozen hydrate for transport of natural gas. In: NGH 96: 2nd international conference on natural Gaz hydrates (Toulouse, June 2–6, 1996), pp 415–422
Shirota H, Aya I, Namie J (2002) Measurements of methane hydrate dissociation for application to natural gas storage and transportation. In: Proceedings of the fourth international conference on natural gas hydrates, Yokohama, pp 972–977
Akhmetzhan A, Abeu N, Longinos SN, Tashenov A, Myrzakhmetova N, Amangeldi N, Kuanyshova Z, Ospanova Z, Toktarbay Z (2021) Synthesis and heavy metal sorption studies of N,N-dimethylacrylamide based hydrogels. Polymers 13(18):3084. https://doi.org/10.3390/polym13183084
Dauletov Y, Nuraje N, Abdiyev K, Toktarbay Z, Zhursumbaeva M (2019) Copolymers of diallyldimethylammonium chloride and vinyl ether of monoethanolamine: synthesis, flocculating, and antimicrobial properties. J Surfactants Deterg 22(5):1129–1137
Bai Y, Bai Q (2005) Hydrates, Subsea Pipelines and Rivers, Ocean Engineering Series. Elsevier Ltd, The Boulevard, Langford Lane, Kidlington, Oxford, UK, 357–38
Merey S, Longinos SN (2019) The gas hydrate potential of the eastern Mediterranean sea. Bull Min Res Exp 160:117–134. https://doi.org/10.19111/bulletinofmre.502275
Sloan ED (2001) Hydrate engineering, vol 21. SPE, Richardson
Merey S, Longinos SN (2018) The role of natural gas hydrates during natural gas transportation. OHU J Eng Sci 7(2):937–953. https://doi.org/10.28948/ngumuh.445410
Sloan ED, Koh CA, Sum A (2011) Natural gas hydrate in flow assurance. Gulf Professional Publishing, New York
Wang W, Li Y, Liu H, Zhao P (2015) Study of agglomeration characteristics of hydrate particles in oil/gas pipelines. Adv Mech Eng 7(1):457050
Merey S, Longinos SN (2018) Investigation of gas seepages in Thessaloniki mud volcano in the Mediterranean Sea. J Petrol Sci Eng 168:81–97. https://doi.org/10.1016/j.petrol.2018.05.014
Merey S, Longinos SN (2018) Does the Mediterranean sea have potential for producing gas hydrates? J Nat Gas Sci Eng 55:113–134. https://doi.org/10.1016/j.jngse.2018.04.029
Abdiyev KZ, Toktarbay Z, Zhenissova AZ, Zhursumbaeva MB, Kainazarova RN (2015) Copolymerization of N, N-dimethyl-N, N-diallylammonium chloride with N,N-dimethylacrylamide. Polym Sci Ser B 57(3):217–223
Anderson FE, Prausnitz JM (1986) Inhibition of gas hydrates by methanol. AIChE J 32(8):1321–1333
Akhfash M, Arjmandi M, Aman ZM, Boxall JA, May EF (2017) Gas hydrate thermodynamic inhibition with MDEA for reduced MEG circulation. J Chem Eng Data 62(9):2578–2583
Ayatzhan A, Tashenov A, Nurgeldi A, Zhanar O, Zhexenbek T, Kaldibek A, Nuraje N (2021) P (DADMAAC-co-DMAA): synthesis, thermal stability, and kinetics. Polym Adv Technol 32(7):2669–2675
Mohammadi AH, Afzal W, Richon D (2008) Experimental data and predictions of dissociation conditions for ethane and propane simple hydrates in the presence of methanol, ethylene glycol, and triethylene glycol aqueous solutions. J Chem Eng Data 53(3):683–686
Dauletov Y, Abdiyev K, Toktarbay Z, Nuraje N, Zhursumbaeva M, Kenzhaliyev B (2018) Radical polymerization and kinetics of N, N-diallyl-N, N-dimethylammonium chloride and vinyl ether of monoethanolamine. Fibers Polym 19(10):2023–2029
Jensen L, Thomsen K, von Solms N (2011) Inhibition of structure I and II gas hydrates using synthetic and biological kinetic inhibitors. Energy Fuels 25(1):17–23
Kinnari K, Hundseid J, Li X, Askvik KM (2015) Hydrate management in practice. J Chem Eng Data 60(2):437–446
Kelland MA (2006) History of the development of low dosage hydrate inhibitors. Energy Fuels 20(3):825–847
Ke W, Kelland MA (2016) Kinetic hydrate inhibitor studies for gas hydrate systems: a review of experimental equipment and test methods. Energy Fuels 30(12):10015–10028
Kvamme BB, Huseby G, Forrisdahl OK (1997) Molecular dynamics simulations of PVP kinetic inhibitor in liquid water and hydrate/liquid water systems. Mol Phys 90(6):979–992
Kvamme B, Kuznetsova T, Aasoldsen K (2005) Molecular dynamics simulations for selection of kinetic hydrate inhibitors. J Mol Graph Model 23(6):524–536
Ajiro H, Takemoto Y, Akashi M, Chua PC, Kelland MA (2010) Study of the kinetic hydrate inhibitor performance of a series of poly (N-alkyl-N-vinylacetamide)s. Energy Fuels 24(12):6400–6410
Chua PC, Sæbø M, Lunde A, Kelland MA (2011) Dual kinetic hydrate inhibition and scale inhibition by polyaspartamides. Energy Fuels 25(11):5165–5172
Villano LD, Kommedal R, Fijten MW, Schubert US, Hoogenboom R, Kelland MA (2009) A study of the kinetic hydrate inhibitor performance and seawater biodegradability of a series of poly (2-alkyl-2-oxazoline) s. Energy Fuels 23(7):3665–3673
Veluswamy HP, Kumar A, Seo Y, Lee JD, Linga P (2018) A review of solidified natural gas (SNG) technology for gas storage via clathrate hydrates. Appl Energy 216:262–285
Kalogerakis N, Jamaluddin AKM, Dholabhai PD, Bishnoi PR (1993, March) Effect of surfactants on hydrate formation kinetics. In SPE international symposium on oilfield chemistry. OnePetro
Daimaru T, Yamasaki A, Yanagisawa Y (2007) Effect of surfactant carbon chain length on hydrate formation kinetics. J Pet Sci Eng 56(1–3):89–96
Okutani K, Kuwabara Y, Mori YH (2008) Surfactant effects on hydrate formation in an unstirred gas/liquid system: an experimental study using methane and sodium alkyl sulfates. Chem Eng Sci 63:183–194
Ando N, Kodama T, Kondo W, Mori YH (2012) Clathrate hydrate formation from a methane+ ethane+ propane mixture in an unstirred surfactant-containing system. Energy Fuels 26(3):1798–1804
Wang F, Jia ZZ, Luo SJ, Fu SF, Wang L, Shi XS, Wang CS, Guo RB (2015) Effects of different anionic surfactants on methane hydrate formation. Chem Eng Sci 137:896–903
Dicharry C, Diaz J, Torré JP, Ricaurte M (2016) Influence of the carbon chain length of a sulfate-based surfactant on the formation of CO2, CH4 and CO2–CH4 gas hydrates. Chem Eng Sci 152:736–745
Link DD, Ladner EP, Elsen HA, Taylor CE (2003) Formation and dissociation studies for optimizing the uptake of methane by methane hydrates. Fluid Phase Equilib 211(1):1–10
Di Profio P, Arca S, Germani R, Savelli G (2007) Novel nanostructured media for gas storage and transport: clathrate hydrates of methane and hydrogen. J Fuel Cell Sci Technol. https://doi.org/10.1115/1.2393304
Fazlali A, Kazemi SA, Keshavarz-Moraveji M, Mohammadi AH (2013) Impact of different surfactants and their mixtures on methane-hydrate formation. Energy Technol 1(8):471–477
Du J, Li H, Wang L (2014) Effects of ionic surfactants on methane hydrate formation kinetics in a static system. Adv Powder Technol 25(4):1227–1233
Saw VK, Gudala M, Udayabhanu G, Mandal A, Laik S (2014) Kinetics of methane hydrate formation and its dissociation in presence of non-ionic surfactant Tergitol. J Unconv Oil Gas Resour 6:54–59
Sa JH, Lee BR, Park DH, Han K, Chun HD, Lee KH (2011) Amino acids as natural inhibitors for hydrate formation in CO2 sequestration. Environ Sci Technol 45(13):5885–5891
Sa JH, Kwak GH, Han K, Ahn D, Cho SJ, Lee JD, Lee KH (2016) Inhibition of methane and natural gas hydrate formation by altering the structure of water with amino acids. Sci Rep 6(1):1–9
Prasad PS, Kiran BS (2018) Are the amino acids thermodynamic inhibitors or kinetic promoters for carbon dioxide hydrates? J Nat Gas Sci Eng 52:461–466
Bavoh CB, Lal B, Osei H, Sabil KM, Mukhtar H (2019) A review on the role of amino acids in gas hydrate inhibition, CO2 capture and sequestration, and natural gas storage. J Nat Gas Sci Eng 64:52–71
Mohammad-Taheri M, Moghaddam AZ, Nazari K, Zanjani NG (2012) Methane hydrate stability in the presence of water-soluble hydroxyalkyl cellulose. J Nat Gas Chem 21(2):119–125
Al-Adel S, Dick JA, El-Ghafari R, Servio P (2008) The effect of biological and polymeric inhibitors on methane gas hydrate growth kinetics. Fluid Phase Equilib 267(1):92–98
Karaaslan U, Parlaktuna M (2002) Promotion effect of polymers and surfactants on hydrate formation rate. Energy Fuels 16(6):1413–1416
Fakharian H, Ganji H, Far AN, Kameli M (2012) Potato starch as methane hydrate promoter. Fuel 94:356–360
Ganji H, Manteghian M, Mofrad HR (2007) Effect of mixed compounds on methane hydrate formation and dissociation rates and storage capacity. Fuel Process Technol 88(9):891–895
Babakhani SM, Alamdari A (2015) Effect of maize starch on methane hydrate formation/dissociation rates and stability. J Nat Gas Sci Eng 26:1–5
Longinos S (2020) The effect of experimental process on natural gas hydrate formation, PhD Thesis, petroleum & natural gas engineering department, Middle East Technical University, Ankara, Turkey
Longinos SN, Parlaktuna M (2021) Are the amino acids inhibitors or promoters on methane (95%) – propane (5%) hydrate formation? React Kinet Mech Cat 132(2):771–794. https://doi.org/10.1007/s11144-021-01959-0
Longinos SN, Parlaktuna M (2021) Examination of behavior of lysine on methane (95%) – propane (5%) hydrate formation by the use of different impellers. J Pet Explor Prod Technol 11(4):1823–1831. https://doi.org/10.1007/s13202-021-01146-w
Longinos SN, Parlaktuna M (2021) Kinetic analysis of arginine, glycine and valine on methane (95%) – propane (5%) hydrate formation. React Kinet Mech Cat 133(2):741–751. https://doi.org/10.1007/s11144-021-02018-4
Longinos SN, Parlaktuna M (2021) Kinetic study of amino acids on methane (95%) – propane (5%) hydrate formation. React Kinet Mech Cat 133(2):753–763. https://doi.org/10.1007/s11144-021-02023-7
Longinos SN, Parlaktuna M (2021) Examination of asparagine, aspartic acid and threonine in methane (95%)-propane (5%) gas hydrates as kinetic inhibitors. React Kinet Mech Cat. https://doi.org/10.1007/s11144-021-02052-2
Lee BI, Kesler MG (1975) A generalized thermodynamic correlation based on three-parameter corresponding states. AIChE J 21(3):510–527
Cai Y, Chen Y, Li Q, Li L, Huang H, Wang S, Wang W (2017) CO2 hydrate formation promoted by a natural amino acid l-methionine for possible application to CO2 capture and storage. Energy Technol 5(8):1195–1199
Veluswamy HP, Lee PY, Premasinghe K, Linga P (2017) Effect of biofriendly amino acids on the kinetics of methane hydrate formation and dissociation. Ind Eng Chem Res 56(21):6145–6154
Maddah M, Maddah M, Peyvandi K (2018) Molecular dynamics simulation of methane hydrate formation in presence and absence of amino acid inhibitors. J Mol Liq 269:721–732
Liu Y, Chen B, Chen Y, Zhang S, Guo W, Cai Y, Tan B, Wang W (2015) Methane storage in a hydrated form as promoted by leucines for possible application to natural gas transportation and storage. Energy Technol 3(8):815–819
Longinos SN, Parlaktuna M (2020) The effect of experimental conditions on methane (95%)–propane (5%) hydrate formation. Energies 13(24):6710
Longinos SN, Parlaktuna M (2021) Kinetic analysis of methane–propane hydrate formation by the use of different impellers. ACS Omega 6(2):1636–1646
Longinos SN, Parlaktuna M (2021) Kinetic analysis of dual impellers on methane hydrate formation. Int J Chem React Eng 19(2):155–165
Misyura SY, Donskoy IG (2020) Dissociation kinetics of methane hydrate and CO2 hydrate for different granular composition. Fuel 262:116614
Longinos SN, Parlaktuna M (2021) The effect of experimental conditions on methane hydrate formation by the use of single and dual impellers. React Kinet Mech Cat 132:771–794
Longinos SN, Parlaktuna M (2021) Kinetic analysis of CO2 hydrate formation by the use of different impellers. React Kinet Mech Cat 133:85–100
Longinos SN, Parlaktuna M (2021) Examination of methane hydrate formation by the use of dual impeller combinations. React Kinet Mech Cat 133(2):729–740. https://doi.org/10.1007/s11144-021-02017-5
Longinos SN, Longinou DD, Celebi E, Toktarbay Z, Parlaktuna M (2021) Kinetic study of methane hydrate formation with the use of surface baffle. React Kinet Mech Cat. https://doi.org/10.1007/s11144-021-02058-w
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Longinos, S.N., Longinou, DD., Parlaktuna, M. et al. The impact of methionine, tryptophan and proline on methane (95%)–propane (5%) hydrate formation. Reac Kinet Mech Cat 134, 653–664 (2021). https://doi.org/10.1007/s11144-021-02089-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02089-3