Abstract
In this paper, Fenton-like oxidation of Crystal Violet (CV) was simulated in Continuous Stirred Tank Reactor (CSTR) and Plug Flow Reactor (PFR). It was observed that the optimum decolorization rate in CSTR and PFR was occurred in 333 K and 303 K, respectively. It was shown the CV decolorization in residence time of 15 min for CSTR is 64.11% while for PFR is 98.88%. In addition, CSTR can decolorize the dye about 0.03 g/L of CV solution by 90–100%, while, that efficiency can obtain up to 0.4 g/L of CV solution by PFR. It was also shown that PFR removes CV by 14% more than that of CSTR in residence time of 120 min for 0.15 g of catalyst. So, for same decolorization efficiency, PFR requires lower temperature, less contact time and consequently less reactor volume, and less catalyst amount compared to CSTR.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution as a result of synthetic substances from textile and other industries has been faced the world with a serious environmental challenge. Presence of dyes in the water has significantly affected health of human, animals, and ecosystem, so, scientists and engineers have aimed to remove dyes from water streams [1]. Based on the nature of synthetic dye, three different methods have been suggested for water treatment including: physical, biological and chemical technologies.
Physical water treatment methods such as adsorption, ultra-filtration techniques, Dissolved Air Flotation, coagulation, membrane processes, and so on have an acceptable efficiency in elimination of contaminant from the water. However, the main disadvantage of physical separation technologies is removing color from water and transporting it into a solid material such as activated carbon, so, the pollutant is not decomposed during the process [2, 3].
The biological wastewater treatment technologies include bioremediation, phytoremediation, and mycoremediation of wastewater. During the biological wastewater treatment processes, the pollutants are decomposed and treated biologically; however, they are usually insufficient for de-colorization of textile wastewater because of non-biodegradable nature of the chromophoric groups in the textile dyes.
Chemical substances have been extensively used to accelerate destruction of non-biodegradable pollutants during a series of chemical reaction. The most brilliant materials for chemical treatment are hydrogen peroxide, chlorine, sodium chlorite, and sodium hypochlorite. The progress of oxidative processes depends on the chemical composition of dye, nature of oxidant, and structure of catalyst [2, 3]. One of non-biodegradable dyes is crystal violet.
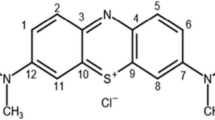
Crystal violet is a cationic triphenylmethane dye that extensively used for identifying and tracing medicine fingerprints in animals. The other application is the colorization of textiles and paper. It has been observed that the poison of crystal violet can be absorbed by ingestion, inhalation, and skin contact and faced the human with cancer or eye irritation [4, 5]. So, it is necessary to degrade existed crystal violet in wastewater to prevent its harmful effects on human beings.
According to the nature of crystal violet, Advanced Oxidation Processes (AOPs) degrade dyes to water, carbon dioxide, and small less harmful molecules [6]. Several AOPs has been proposed for CV decomposition such as photocatalytic decomposition in the presence of silver ion-doped TiO2 [4, 7] or over aqueous nano-ZnO suspension [8], a combination of a photocatalytic and biological system [9], electrochemical oxidation [10], electrocoagulation using aluminum or iron electrodes [11], Fenton-like oxidation process over several catalysts including complexes of Cu2+-amine [12], TiO2 powder or Ag-doped TiO2 catalysts, CdO/zeoLe and P25-TiO2 catalyst, CdS, nano-CdS, nano-CdS/zeoLe [1, 4, 13,14,15,16,17,18], and FeGAC/H2O2 [19]. Fenton oxidation process over the Fe2+ catalyst is the most applied and efficient of AOPs.
According to its acceptable efficiency, the de-colorization over Fe2+ catalyst during Fenton-like oxidation process has been investigated for degrading of phenol [20, 21], ethanol [22], and some of carboxylic acids [23], Orange II [24,25,26], Rhodamine 6G [27], Reactive Brilliant Blue KN-R [28], and Reactive Red 141 [29]. Although Fenton-like oxidation process over the Fe2+ catalyst has been implemented for different types of wastewater except for alkaline sludge and solutions with high buffering capacity; the challenge of this process is production of iron sludge as a secondary contaminant [30,31,32].
ZeoLes are aluminosilicate materials with high surface area whose recovery from water and regeneration are not too difficult. Metal-containing zeoLes do potentially have a substantial advantage over zeoLes in the greater ease in which metal ions can be introduced into the frameworks. The brilliant advantage of Fe/ZSM-5 zeoLe in Fenton-like oxidation process is that fixing iron ions into the frameworks of zeoLe structure, prevents them from leaching and release into the water. In other words, recovery of Fe2+ on the zeoLe surface, suspended in treated water, is significantly simpler than those ions dissolved directly in the water [33, 34].
In this study, decomposition of crystal violet by Fenton-like oxidation process over the iron-loaded ZSM-5 catalyst has been investigated. Since the treatment processes are studied in a batch stirred reactor; it was considered that based on the kinetics of oxidation reaction, plug flow regime influences the conversion of reaction, residence time, reactor geometry, and other reaction parameters. For this purpose, degrading of crystal violet by Fenton-like oxidation process in CSTR (continuous stirred tank reactor) and in PFR (plug flow reactor) has been simulated by HYSYS 3.2 and the results for effects of temperature, initial dye concentration, Fe2+ amount, and H2O2 concentration compared with each other.
Governing equations
Fenton-like oxidation reactions over ferrous ions are as follows [35]:
The produced hydroxyl radicals react with CV which the main reaction is presented as follows [14]:
During Eq. 5, CV decomposes by produced \(\dot{OH}\) in Fenton-like oxidation process. Kinetic relation for the decomposition of CV is as follows [36]:
Here CCV and COH represent the concentration of crystal violet and \(\dot{OH}\) in mol/L.
Simulation procedure
In this paper, simulation of crystal violet decolorization by Fenton-like oxidation process has been performed by HYSYS 3.2. The prediction of the thermodynamic behavior of substances was performed by NRTL equation of state.
Simulation of CV decolorization in continuous stirred tank reactor (CSTR)
The simulations of present study have been carried out based on kinetic model and experimental set up of Unnu et al., [36]. They performed the oxidative degradation of CV under isothermal conditions in a shaded temperature- controlled glass batch reactor equipped with a mechanical stirrer at about 280 rpm. They used hydrogen peroxide solution (35% in mass), and FeZSM-5 (Si/Al = 42 and 1.825 g of Fe powder) catalyst prepared by the ion exchange method.
Fig. 1 shows the simulation of CV de-colorization process in a Continuous Stirred Tank Reactor (CSTR). During the process, H2O2 produces radical hydroxyl and other products over iron ions according to Eqs. 1–4 in an equilibrium reactor. The effluent is sent to a CSTR to react with CV according to Eq. 5. In the experiment for this process, all of the reactions take place in one reactor; however, in Aspen HYSYS, it is impossible to simulate equilibrium and kinetic reactions in a unique reactor; so, a couple of equilibrium and stirred reactors have been employed for simulation of CV decomposition. Table 1 presents the condition of feed based on Unnu et al., [36].
Verifying the validity of simulation results
In order to verify the validity of results, a comparison has been performed between simulation results for CSTR and experimental data reported by Unnu et al., [36]. The comparison has been developed for the effects of three parameters of temperature, hydrogen peroxide, and CV initial concentration on dye decolorization.
Temperature effect on CV decolorization
Fig. 2 demonstrates the effect of temperature variation on CV decolorization from water in different residence times in a CSTR. It is shown that initially, CV decolorization quickly ascends by increasing the residence time, while the rate of increase slow down as contact time increases, due to decreasing in mass transfer driving force. In addition, it can observe that CV decolorization reaction has higher conversion in higher temperatures due to the endothermic nature of reactio, i.e., the simulation results demonstrate that CV decolorization in residence time of 120 min is 83.08 at 313 K which the average tolerance with experimental data of Unnu et al., [36] is 2.08%. So, it is seen that the simulation results are in line with experimental data.
H2O2 concentration effect on CV decolorization
Fig. 3 shows a comparison of simulation results with experimental data for crystal violet decolorization on the concentration of hydrogen peroxide of 7.5 mmol/0.15 L. It is shown that at a residence time of 120 min, the decolorization percent is 86.98%. A comparison of simulation results with experimental data shows an average error of 2.98% for the mentioned points.
Dye concentration effect on CV decolorization
Fig. 4 compares the simulation and experimental results of crystal violet decolorization at different residence times based on concentrations of initial contaminant. According to the Fig. 4, the simulation results are in the line with experimental data.
According to Figs. 2, 3, and 4, it is clear that the simulation results are in good agreement with experimental data [36]. So, it can extend the simulation for the suggested process for CV decolorization.
The proposed process for CV decolorization by Fenton-like reaction
In chemical reactor design, those reactions strongly affected by the concentration of reactants, are usually performed in a Plug Flow Reactor (PFR). Since the concentration of H2O2 along with CV have significant effects on the crystal violet decomposition process; it seems that implementing a PFR would improve the decomposition reaction. So, in this paper, simulation of CV decolorization by Fenton-like oxidation reaction over ZSM-5 catalyst has been performed in a PFR. Fig. 5 illustrates the simulation of the proposed process. For the mentioned process, the effects of temperature of reaction, initial concentration of CV, the concentration of H2O2, and concentration of Fe2+ have been studied.
Temperature effects on CV decolorization in PFR
Table 2 demonstrates the effects of temperature on CV decolorization in PFR and CSTR. For this purpose, four temperatures of 303 K, 313 K, 323 K, and 333 K have been examined. It is clear that increasing temperature increases CV decolorization in both PFR and CSTR; however, PFR has significantly better efficiency compared to CSTR in low temperatures. For instance, by increasing temperature from 303 to 323 K at a residence time of 30 min, the decolorization of CV for CSTR increases from 50.78 to 68.86% while the ascending value for PFR is from 89.02 to 98.04%. Higher decolorization rate of PFR in low temperatures is due to that the uniform motion of fluid causes temperature reduction take place differentially in the length of the reactor. So, the conversion of the endothermic reactions increases. According to Table 2, it is evident that the best temperature for reaction in CSTR is 333 K; the reaction temperature for PFR can be selected of 313 K for reaching the maximum decolorization rate of CV.
H2O2 concentration effects on CV decolorization in PFR
Table 3 and Fig. 6 show the effect of concentration of hydrogen peroxide variation on CV decolorization over iron-loaded ZSM-5 catalyst in CSTR and PFR. As it is seen, hydrogen peroxide has an optimum concentration for both CSTR and PFR. In other word, the optimum concentration of H2O2 for CV decolorization is determined of 7.5 mM and higher and lower concentrations are not as efficient as the optimum concentration. Because by increasing the concentration of H2O2, it reacts with radical hydroxyl according to Eq. 4, so, its concentration for CV decomposition decreases, and consequently dye decolorization descends. However, at the optimum concentration of hydrogen peroxide, the efficiency of the CV decolorization process in PFR is significantly higher than CSTR. For instance, CV decolorization in the optimum concentration of hydrogen peroxide and residence time of 30 min is 75.73% for CSTR, while the value for PFR is 100%.
Effects of initial dye concentration
Table 4 shows the effect of CV concentration on its decolorization rate during the Fenton-like oxidation process in CSTR and PFR. As it is shown in Table 4, as the initial dye concentration increases, the CV decolorization in both reactors decreases. Because by increasing the concentration of contaminant, more hydroxyl radicals are required for decolorization process and leakage of it leads to descending in CV decomposition. However; in a CSTR, the concentration of the reactants decreases once the compounds enter the reactor, so, the available hydroxyl radicals for reaction reduce. While in PFR, the hydroxyl radical concentration gradually decreases in the reactor length, which causes the CV molecules be in contact with the higher concentration of hydroxyl radicals, and decolorization efficiency increases. According to Table 4, the lowest CV decolorization is related to the dye concentration of 0.035 g/L at a residence time of 15 min for CSTR is 56.45% while the value for PFR is 94.7%.
The highest conversion for CSTR is related to the concentration of 0.005 g/L in the residence time of 45, 60, 90, and 120 min, the concentration of 0.015 g/dm3 in the residence time of 90 and 120 min and the concentration of 0.025 g/L in a contact time of 120 min. While, in PFR, the decolorization value at the times higher than 45 min for all concentrations and in 15 and 30 min for concentrations of 0.005 g/L and 0.015 g/L conversion are 100%.
The simultaneous effects of H2O2 and initial CV concentration on the dye decolorization
Fig. 7 shows the simultaneous effects of concentration of H2O2 and initial contaminant on the decolorization of CV in the CSTR and PFR. As it is seen, for both CSTR and PFR, the diagram has a maximum point based on the optimum concentration of hydrogen peroxide (7.5 mM). As it is seen, the optimum concentration of hydrogen peroxide for both CSTR and PFR is 7.5 mM. The optimum point is due to the reaction of hydrogen peroxide with hydroxyl radicals according to Eq. 4, thus, available \(\dot{OH}\) for cv decomposition declines. Since the oxidative potential of HO2 radical is much smaller OH radicals, further increasing of H2O2 concentration has negative effects on CV decolorization. More ever, by increasing CV concentration, the decolorization efficiency for both reactors descends. However, the CSTR can remove only 60–70% contaminant in most initial concentrations of CV (0.025 to 0.5 g/L), and the area under the curve for 90% CV decolorization is much smaller compared to the curve related to 60–70% dye decolorization. While, the PFR can remove 90–100% of CV with initial concentration of 0.046–1 g/L in the optimum concentration of H2O2.
Investigation of Fenton-like oxidation reaction in CSTR and PFR shows that for same decolorization efficiency for PFR occurs in less temperature, lower resident time and consequently lower reaction volume, and less catalyst amount compared to CSTR.
Conclusion
In this paper, simulation of crystal violet decolorization from wastewater by Fenton-like oxidation reactions over Fe/ZSM-5 catalyst has been performed by HYSYS 3.2. The simulations have been taken place in CSTR and PFR. Evaluation of the effective parameters in both reactors shows that the maximum decolorization rate in CSTR has been occurred in 333 K, while the same efficiency has been observed in 303 K in PFR. In addition, it has been shown that CSTR can treat only diluted wastewaters about 0.03 g/L of CV in water by 90–100%, however, that efficiency can obtain up to 0.4 g/L of CV in water by PFR. The studying of Fe2+ concentration showed that for 0.15 g catalyst, PFR removes crystal violet 14% more than CSTR in residence time of 120 min.
Abbreviations
- C:
-
Concentration
- CSTR:
-
Continuous Stirred Tank Reactor
- CV:
-
Crystal violet
- NRTL:
-
The non-random two-liquid model
- PFR:
-
Plug flow reactor
References
Jana S, Purkait MK, Mohanty K (2010) Decolorization of crystal violet by advanced oxidation and microfiltration. Appl Surf Sci 50:337–341
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77:247–255
Slokar YM, Le Marechal AM (1998) Methods of decolorization of textile wastewaters. Dyes Pigm 37(4):335–356
Sahoo C, Gupta AK, Pal A (2005) Photocatalytic degradation of crystal violet (C.I. basic violet 3) on silver ion doped TiO2. Dyes Pigm 66:189–196
Shouman MA, Khedr SA, Attia AA (2012) Basic dye adsorption on low cost biopolymer: kinetic and equilibrium studies. J Appl Chem 2:27–36
Chen Y, Wang K, Lou L (2004) Photodegradation of dye pollutants on silica gel supported TiO2 particles under visible light irradiation. J Photochem Photobiol A 163:281–287
Gupta AK, Pal A, Sahoo C (2006) Photocatalytic degradation of a mixture of crystal violet (basic violet 3) and methyl red dye in aqueous suspensions using AgC doped TiO2. Dyes Pigm 69:224–232
Habib A, Muslim M, Shahadat T, Islam N, Ismail IMI, Islam TSA, Mahmood AJ (2013) Photocatalytic decolorization of crystal violet in aqueous nano-ZnO suspension under visible light irradiation. J Nanostruct Chem 3:1–10
Chen C, Kuo J, Yang H, Chung Y (2013) A coupled biological and photocatalysis pretreatment system for the decolorization of crystal violet from wastewater. Chemosphere 92:695–701
Zhang H, Wu J, Wang Z, Zhang D (2010) Electrochemical oxidation of crystal violet in the presence of hydrogen peroxide. J Chem Technol Biotechnol 85:1436–1444
Durango-Usuga P, Guzman-Duque F, Mosteo R, Vazquez MV, Penuela G, Torres-Palma RA (2010) Experimental design approach applied to the elimination of crystal violet in water by electrocoagulation with Fe or Al electrodes. J Hazard Mater 179:120–126
Salem IA (2001) Activation of H2O2 by Amberlyst-15 resin supported with copper (II)-complexes towards oxidation of crystal violet. Chemosphere 44:1109–1119
Senthilkumaar S, Porkodi K (2005) Heterogeneous photocatalytic decomposition of crystal violet in UV-illuminated sol–gel derived nanocrystalline TiO2 suspensions. J Colloid Interface Sci 288:184–189
Alshamsi FA, Albadwawi AS, Alnuaimi MM, Rauf MA, Ashraf SS (2007) Comparative efficiencies of the degradation of crystal violet using UV/hydrogen peroxide and Fenton’s reagent. Dyes Pigm 74:283–287
Fan H, Huang S, Chung W, Jan J, Lin W, Chen C (2009) Degradation pathways of crystal violet by Fenton and Fenton-like systems: condition optimization and intermediate separation and identification. J Hazard Mater 171:1032–1044
Nezamzadeh-Ejhieh A, Banan Z (2011) A comparison between the efficiency of CdS nanoparticles/zeoLe A and CdO/zeoLe A as catalysts in photodecolorization of crystal violet. Desalination 279:146–151
Nezamzadeh-Ejhieh A, Banan Z (2012) Sunlight assisted photodecolorization of crystal violet catalyzed by CdS nanoparticles embedded on zeoLe A. Desalination 284:157–166
Ju Y, Fang J, Liu X, Xu Z, Ren X, Sun C, Yang S, Ren Q, Ding Y, Yu K, Wang L, Wei Z (2011) Photodegradation of crystal violet in TiO2 suspensions using UV-vis irradiation from two microwave-powered electrodeless discharge lamps (EDL-2), products, mechanism and feasibiLy. J Hazard Mater 185:1489–1498
Chen C, Chen W, Chiou M, Chen S, Chen Y, Fan H (2011) Degradation of crystal violet by an FeGAC/H2O2 process. J Hazard Mater 196:420–425
Fajerwerg K, Debellefontaine H (1996) Wet oxidation of phenol by hydrogen peroxide using heterogeneous catalysis FeZSM-5: a promising catalyst. Appl Catal B 10:L229–L235
Phu NH, Hoa TTK, Tan NV, Thang HV, Ha PL (2001) Characterization and activity of Fe-ZSM-5 catalysts for the total oxidation of phenol in aqueous solutions. Appl Catal B 34:267–275
Kuznetsova EV, Savinov EN, Vostrikova LA, Parmon VN (2004) Heterogeneous catalysis in the Fenton-type system FeZSM-5/H2O2. Appl Catal B 51:165–170
Centi G, Perathoner S, Torre T, Verduna MG (2000) Catalytic wet oxidation with H2O2 of carboxylic acids on homogeneous and heterogeneous Fenton-type catalysts. Catal Today 55:61–69
Bolova E, Gunduz G, Dukkancı M, Yılmaz S, Yaman YC (2011) Fe Containing ZSM-5 zeoLe as catalyst for wet peroxide oxidation of Orange II. Int J Chem React Eng 9:1–20
Bolova E, Gunduz G, Dukkancı M (2012) Heterogeneous Fenton-like degradation of orange II in water using FeZSM-5 zeoLe catalyst. Int J Chem React Eng 10:1–21
Duarte F, Madeıra LM (2009) Azo-dye Orange II degradation by Fenton’s reaction using Fe/ZSM-5 zeoLe as catalyst. In: 2nd European Conference on Environmental Applications of Advanced Oxidation Processes, EAAOP2, Nicosia, Cyprus
Dukkancı M, Gunduz G, Yılmaz S, Yaman YC, Prikhodko RV, Stolyarova IV (2010) Characterization and catalytic activity of CuFeZSM-5 catalysts for oxidative degradation of Rhodamine 6G in aqueous solutions. Appl Catal B 95:270–278
Chen A, Ma X, Sun H (2008) Decolorization of KN-R catalyzed by Fe-containing Y and ZSM-5 zeoLes. J Hazard Mater 156:568–575
Yaman YC, Gunduz G, Dukkancı M (2013) Degradation of CI reactive red 141 by heterogeneous Fenton-like process over iron-containing ZSM-5 zeoLes. Color Technol 129:69–75
Dutta K, Mukhopadhyay S, Bhattacharjee S, Chaudhuri B (2001) Chemical oxidation of methylene blue using a Fenton-like reaction. J Hazard Mater B84:57–71
Torrades F, Garcia-Montano J, Garcia-Hortal JA, Nunez L, Domenech X, Peral J (2004) Decolorization and mineralization of homo- and hetero-bireactive dyes under Fenton and photo-Fenton conditions. Color Technol 120:188–194
Guedes AMFM, Madeira LMP, Boaventura RAR, Costa CAV (2003) Fenton oxidation of cork cooking wastewater overall kinetic analysis. Water Res 37:3061–3069
Stolyarova IV, Kovban IB, Prikhodko RV, Kushko AO, Sychev MV, Goncharuk VV (2007) Relationship between the catalytic behavior of ZSM-5 zeoLes in oxidative degradation of dyes and nature of their active centers. Russ J Appl Chem 80(5):746–753
Pirkanniemi K, Sillanpaa M (2002) Heterogeneous water phase catalysis as an environmental application: a review. Chemosphere 48:1047–1060
Medien HAA, Khalil SME (2010) Kinetics of the oxidative decolorization of some organic dyes utilizing Fentonlike reaction in water. J King Saud Univ (Science) 22:147–153
Unnu BA, Gunduz G, Dukkancı M (2015) Heterogeneous Fenton-like xidation of crystal violet using an iron loaded ZSM-5 zeoLe. Desalin Water Treat 57:1–15
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Modarresi-Motlagh, S., Bahadori, F., Ghadiri, M. et al. Enhancing Fenton-like oxidation of crystal violet over Fe/ZSM-5 in a plug flow reactor. Reac Kinet Mech Cat 133, 1061–1073 (2021). https://doi.org/10.1007/s11144-021-02001-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-021-02001-z