Abstract
This article is devoted to rape oil transesterification by methanol and ethanol on powder and extruded Mg/Al hydrotalcite catalyst in a stirred reactor under atmospheric pressure close to boiling point of alcohol with inserted perforated basket with extrudates. With 15 wt% of catalyst in relation to rape oil and molar ratio methanol/ester group of oil 13:1, the yield of monomethylester reached 99.5% within 48 h in the case of fresh catalyst. After recalcination, the same catalyst enabled the same yield of monomethylester within 72 h and third cycle of this catalyst after two recalcinations led to 98.5% of monomethylester yield also within 72 h. Extrudates enabled the same monoester yield as hydrotalcite powder within 24 (60–61%) up to 48 h (99%). Moreover, several experiments were done in the autoclave to assume temperature effect to reaction time. Enhanced temperature 140 °C provided nearly 99% monoester yield within 12 h. A new arrangement with extrudates in a perforated basket makes transesterification with heterogeneous hydrotalcite catalyst easily feasible. Differently from rather promising results in rape oil transesterification with methanol, ethanol worked in this reaction much more slowly. Not surprisingly water inhibits transesterification, as the reaction with 96% ethanol shows. Using 99.8% ethanol nearly without water makes transesterification significantly faster, but 51% monoester yield after 120 h reaction time is still insufficient. Nevertheless, ethanol remains a promising raw material for oil transesterification, as it can be prepared by a bioprocess, which makes the whole technology based on renewable sources.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
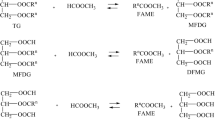
Fatty acid methylester (FAME) or fatty acid ethylester (FAEE) has been utilized as an additive in biodiesel fuel for more than ten years due to its starting material—vegetable oil, in Europe mostly rape oil. Before use, triacylglycerides have to be transformed to methyl- or ethylesters with methanol (mostly) or with ethanol, which can be prepared from biologic and renewable starting materials. In industrial scale, vegetable oils are commonly transferred to FAME by homogenous catalysis. Rarely acidic acids take place by using sulfuric acid, but mostly sodium or potassium alcoholates are used. Transesterification technologies based on homogenous catalysts face up to various technical problems—necessity of washing alkali cations, saponification and emulsion arising [1]. Moreover, these technologies, based on homogenous catalysis, result in waste water production. To avoid such troubles, heterogeneous catalysts for vegetable oil transesterification have been developed for many years.
Calcium oxide, tin (IV) oxide and their mixture were tested in babassu oil transesterification with methanol in molar ratio of oil to methanol 10:1 with 10 wt% of catalyst to oil weight for 2 h. After the first test series a mixture of CaO/SnO2 with a weight ratio 7:3 was chosen as the most efficient. At the reaction temperature 54 °C total yield of FAME reached 89.5% [2].
Potassium catalyst on mesoporous alumina as a heterogeneous catalyst was utilized for canola oil transesterification with methanol/oil molar ratio in the range 6:1 up to 15:1 at temperatures 50–70 °C. FAME yields were influenced by stirring velocity, particle size of the catalyst and reaction temperature. Stirring velocity 500 rpm enabled maximum conversions and faster stirring had no further influence to FAME yields, the smallest particle size of the catalyst (< 160 µm) led to the highest FAME yields. Under here mentioned optimum condition, reaction temperature effect to transesterification was investigated in the range 50–70 °C. FAME yields grew with increasing temperature and achieved up to more than 90% [3].
A bifunctional catalyst based on Cu and alkali earth cations was investigated in cannabis oil transesterification under pressure in an autoclave with 100 g cannabis oil batch, a molar ratio of methanol to oil 3:1 to 15:1 and 3 g of catalyst. FAME yields grew within the range of methanol/oil ratio 3:1 to 12:1, higher excess of methanol did not enhance yields in a significant way. At the molar methanol/oil ratio 12:1, FAME yields were in the range 73–96% and increased in the ascending order Cu/MgO < Cu/CaO < Cu/ BaO < Cu/SrO. Increasing CuO concentration in a catalyst increased FAME yields too, but only in the range 1 to 10 wt%, above this value yields declined. In some experiments the autoclave was under hydrogen flow, but it resulted in a moderate decrease of FAME yields. Also temperature above 60 °C caused lower FAME yields. Transesterification of triolein by ethanol runs also with an anion exchanger in –OH form as a catalyst in a batch process with 2–4 g of anion exchanger, 10 g of reaction mixture and a triolein/ethanol molar ratio 1:3 to 1:20 at 50 °C. Less crosslinked anion exchangers with smaller particle size provided highest conversions. If 4 g of ion exchanger were used instead of 2 g, reaction started faster, but maximum conversions in equilibrium remained nearly the same. Transformation yields grew in the range of triolein/ethanol molar ratio 1:3 to 1:10, further enhancement up to 1:20 had no effect. The best anion exchanger provided after 1 h conversion above 80% with a product purity > 98% [4].
A comparison of Li/Al, Mg/Fe and Ag/Al oxides, calcined at 450 °C, in soya bean oil transesterification in a batch reactor with molar methanol/oil ratio 15:1 showed Li/Al oxides the most effective providing FAME yield 80% with 1 wt% of the catalyst after 2 h. Om the other hand, Mg/Al oxides led only to 13.6% FAME yield with 3 wt% of the catalyst after 6 h. To tell the truth, the key parameter for successful implementation of these results will be Li leakage into FAME [5].
Within the last 10–15 years, lots of research has been devoted to hydrotalcites as promising heterogeneous catalysts for transesterification of vegetable oil and similar substances. Comparison of pure MgO and pure Al2O3 with hydrotalcites of various Mg/Al ratios in transesterification of glycerol tributyrate (0.01 mol) with methanol (0.3 mol) and 0.05 g of a catalyst in 0.5 ml of hexyl ether showed zero catalytic activity for Al2O3 and 11% of glycerol tributyrate conversion for pure MgO, 42% conversion for hydrotalcite with 13 wt% of Mg and 75% conversion with 24 wt% of Mg [6].
Rape oil transesterification in a batch arrangement at 55–75 °C with a methanol/oil molar ratio 3:1 to 9:1 and 0.5–2.5 wt% of a catalyst was studied on hydrotalcites with various Mg/Al molar ratios. Conversions after 4 h reaction enhanced from 75 to 90% when Mg/Al ratio increased from 1 to 3, which corresponds to enhancement of basicity. Also higher calcination temperature of hydrotalcites (500 °C) provided a higher conversion (91%) than 86% after calcination at 400 °C and 60% at non-calcined hydrotalcites. The best of investigated catalyst was a hydrotalcite with Mg/Al molar ratio 3 calcined 12 h at 500 °C with the highest crystallinity. Increasing molar ratio of methanol/oil led to higher conversions too with 61% for methanol/oil ratio 3:1, 79% for 5:1 and 90% for 6:1. Also increasing amount of a catalyst enhanced conversions, but only to 1.5 wt% of oil. Further addition of a catalyst led to declination of yields, which was probably caused by side reactions. Vigorous stirring was necessary for optimum reaction course, the best reaction temperature was 65 °C [7].
On the other hand, investigation of soya oil transesterification by methanol in batch arrangement with methanol/oil molar ratio 12:1 and 5 wt% of a catalyst (related to oil) at 65 °C with 6 h reaction time showed the best FAME yield (92%) with hydrotalcite of a molar ratio Mg/Al 2, but only 34% FAME yield with Mg/Al 4 and 9% FAME with Mg/Al 3. All hydrotalcites were calcined at 500 °C as a standard method. Among this standard calcination method, various calcination temperatures were tested too. Calcination at 400 °C resulted in FAME yield decrease from 92 to only 4%. Enhancement of calcination temperature to 700 °C enabled prolonged activity of a catalyst even at its second and third cycle (92% and 35% of FAME). With a higher methanol/oil ratio, a smaller amount of a catalyst was necessary for nearly the same FAME yields [8].
Another article on soyabean oil transformation to methylester with calcined (to 500 °C) Mg/Al hydrotalcites (7.5 wt%) at molar ratio methanol to oil 15:1 reports 67% oil conversion after 9 h [9]. As several hydrotalcite catalysts were tested, the best results were reached with Mg/Al = 3.0, which corresponds to results in [7]. On the other hand, a work on biodiesel synthesis with Mg/Al hydrotalcites with molar ratio of Mg/Al 1.5 to 5 shows that the hydrotalcite catalysts with the highest Mg/Al ratio provided the best results of sunflower oil transformation to methylester due to their strongest basicity. Especially rehydrated hydrotalcites worked most effectively. 92% methylester yield was reached with 2% of the best catalyst and molar ratio of methanol/oil 48:1 [10].
As a contribution to environmental effort, biodiesel synthesis by waste cooking oil transesterification with hydrotalcite having Mg/Al molar ratio 4:1 was described including hydrotalcite preparation by two various methods. Co-precipitation method lies in common precipitation of Mg and Al nitrates in ratio 3:1 by sodium carbonate solution, heating, aging at room temperature for 18 h, product separation and washing. Urea hydrolysis method is based on precipitation of Mg and Al nitrate solutions with molar ratio 4:1 by urea solution with urea to nitrate ratio 0.6, the following steps remained the same as in the above described co-precipitation method. Transesterification experiments were optimized with respect to reaction temperature, methanol/oil molar ratio and amount of catalyst. The best yields were reached at 60 °C with methanol/oil ratio 9:1 and 1.5 wt% of catalyst [11].
The reusability of Mg/Al hydrotalcite catalyst in sunflower oil transesterification was also investigated. First reaction conditions were optimized and the best achieved yields of FAME were 96.5% after 3 h at 70 °C with molar ratio methanol/oil 12:1. Then repeated use of catalyst was tried, the catalyst was successfully used three times with only a moderate decrease of FAME yield from 96.5 to 90% [12].
Soybean oil was also transformed to FAME by hydrotalcite at 64 °C with methanol/oil ratio 20:1 and 5% of catalyst with 94.8% yield on Mg/Al hydrotalcite after 10 h. Hydrotalcite powder was prepared by precipitation and after its separation, washing and drying it was calcined at 450 °C for 4 h under air flow [13].
Besides processing vegetable oils, transesterification between ethylene glycol monomethyl ether (EGME) and methyl laurate (ML) was studied to produce a novel biodiesel consisting of ethylene glycol monomethyl ether monolaurate (EGMEML). KF modified Ca/Al hydrotalcites were used. Biodiesel yields grew with increasing KF concentrations in catalysts up to 97.7% yield with 24.7% of KF in the best catalyst. Higher KF concentrations led to lower yields [14].
Ca/Al hydrotalcites were also modified by potassium carbonate and utilized in soybean transesterification. At optimum reaction conditions, which were methanol/oil molar ratio 13:1, temperature 65 °C, reaction time 2 h and 2 wt% of a catalyst, the catalyst was usable several times and after 4 cycles yields reached more than 87.4% [15].
A modified Ca-Mg/Al hydrotalcite prepared by impregnation with calcium nitrate and calcined at 600 °C for 30 min was also tested in transesterification between methyl acetate and ethanol with a molar ratio 6:1 and 4 wt% of catalyst. The catalyst was reusable at least 5 times, only Ca leaching has to be solved for use in a technological process [16].
Transesterification of rape oil was also investigated on a series of prepared hydrotalcite catalysts with various Mg/Al molar ratio in the range 1.8 to 7.2, which were calcined at 450 °C before use. Total concentration of basic sites decreased with increasing Mg/Al ratio, but yields of esters grew as well as surface area of catalysts. Only negligible Mg leaching was observed [17].
Surprising results of soybean oil transesterification were reached with hydrotalcites of three various Mg/Al ratios. The hydrotalcites were calcined at 400 °C, transesterification ran in an autoclave at 180 °C and 230 °C with methanol/oil ratio 8–13:1 and 3–5 wt% of a catalyst. The highest conversion (90.7%) was achieved at 230 °C, molar methanol/oil ratio 13:1 and with the catalyst having the least Mg content [18].
As a new alternative, Mg/Fe hydrotalcites with a molar ratio Mg/Fe 2.5:1 were prepared in two manners—from nitrates and chlorides of both metals. Products were calcined at 500 °C and 600 °C to compare their efficiency in rape oil transesterification. Calcined products from nitrates contained more basic sites and provided higher conversions than that synthesized from chlorides. For successful rehydration hydrotalcites from nitrates needed calcination at 500 °C, but hydrotalcites from chlorides had to be calcined at 600 °C [19, 20].
Besides most utilized Mg/Al hydrotalcites, Mg-Fe catalysts of the same structure were studied too and catalytic behaviour of both types in rape oil transesterification was compared. All catalysts had Mg/Al or Mg/Fe ratio 3:1. Before use, they were calcined to 500 °C. Reaction ran at 115 °C, 150 °C and 200 °C, long time tests verified stability and lifetime of these catalysts. At the highest temperature (200 °C) both types of catalysts had very close catalytic activity, but at lower temperatures the Mg/Al hydrotalcites provided better results [21].
A detailed study on the influence of calcination temperature of Mg/Al hydrotalcite with a molar Mg/Al ratio 3.2:1 to oil transesterification results was done too. The reaction ran at 117 °C for 8 h with molar ratio methanol/oil 24:1 and 4 wt% of the catalyst to oil. Increase in calcination temperature from 450 °C to 550 °C led to larger MgO crystallites, larger number of basic centres and BET surface area increase. On the contrary, higher calcination temperatures (650–150 °C) had an opposite effect. The highest ester yield (77%) was achieved with a hydrotalcite calcined at 550 °C [22].
Mixed Ca-Mg/Al hydrotalcites were also used for sunflower oil transesterification with methanol in non-calcined and calcined (at 600 °C) state. The reaction ran at 60 °C with molar methanol/oil ratio 15:1 and 2.5 wt% of a catalyst for 6 h. The best results were achieved after impregnation of non-calcined Mg4Al2hydrotalcite with 40% Ca and calcination at 600 °C, where FAME yield was 95% [23].
A question of regeneration and repeated use of Mg/Al hydrotalcite in soya bean oil transesterification was also studied. The reaction ran at 220 °C and 55 bar. Washing the catalyst in situ 4 h with methanol at 100 °C did not lead to its original activity after its exhaustion, but washing with acetone at 70 °C for the same time provided the same catalytic activity and lifetime as at the beginning [24].
Within environmental effort, transesterification of waste cooking oil was investigated with Mg/Al hydrotalcite catalyst having Mg/Al ratio 3:1, calcined at 500 °C [25]. Before transesterification, the waste cooking oil involved preliminary treatment. First, mechanical impurities (bones, paper, vegetables etc.) were removed, then clay was added to sorb further impurities and after its removal, free acids were washed out by extraction and water removed up to moisture content under 0.5%. Finally, a small addition of NaOH was needed to neutralize the oil to pH 7. For transesterification, a mixture of methanol and ethanol was used. The highest monoester yield (95%) was achieved with alcohol/oil molar ratio 6:1 at 80 °C.
Besides powders as heterogeneous catalysts, Mg–Al hydrotalcite with sepiolite as a binder wash-coated to metal monoliths fixed to blades of a propeller was used for sunflower oil transesterification. Before use, the metal monolites fixed to blades were calcined at 500 °C for 6 h, then rehydrated in boiling deionized water and dried in vacuum at 60 °C. With molar methanol/oil ratio 48:1 and 2% of catalyst, after 10 h 62% conversion of oil was done with 10% of sepiolite and 77% oil conversion on the same reaction conditions, when the catalyst contained only 5% of sepiolite [26]. Also γ-Al2O3 and pseudo-boehmite were used as binders to prepare particles of a catalyst containing hydrotalcite, γ-Al2O3 and pseudo-boehmite in ratios 7.5:10:2 [27]. Also magnetite containing extrudates from γ-Al2O3 and sepiolite as binders and potassium carbonate as the active ingredient were prepared and tested in transesterification of sunflower oil by ethanol. Magnetic properties of extrudates enable their separation, which can be advantageous for larger scale implementations [28].
Nearly all above given articles deal with powder catalysts. Only two last published papers inform about formed hydrotalcite catalysts with binders. From published articles it follows, that among heterogeneous catalysts, hydrotalcite powders provide most promising results. Anyway, to implement scientific results into industrial scale, powders as catalysts are encumbered by a drawback of necessary separation of the powder from a reaction mixture. In this article, transesterification of rape oil by methanol and ethanol with binderless extruded hydrotalcite catalyst in a reactor with inserted perforated basket is studied. Differently from powder, this arrangement enables simple manipulation with a catalyst without any separation processes. The stainless steel basket is only pulled out from a reactor, inserted into a kiln to calcine it and then the extrudates can be used once more. As heterogeneous catalysis still does not enable such fast reaction as direct transesterification of oil with sodium methanolate, several transesterification tests with hydrotalcite under higher pressure at 140 °C were done.
Experimental
Hydrotalcite characterization
The crystalline structure of as synthesized powder hydrotalcite before calcination was determined by X-ray diffraction method (XRD) on the diffractometer BRUKER AXS D8 Advance.
Scanning electron microscopy (SEM) of hydrotalcite powder was performed on JEOL JSMIT500HR. The samples sprinkled throughout the carbon tape were sputtered with gold before analysis. Adsorption properties, surface area and porosity of catalysts were characterized by measurements of nitrogen sorption and desorption isotherms at 77 K on Quantachrome Autosorb iQ. Prior to analysis, the samples were heated to 395 °C for 19 h at pressure 0.13–0.15 Pa. Total surface area, total pore volume, micropore and mesopore volumes were calculated by NLDFT method with a model for silica with cylindrical pores. For external surface area determination, t-plot analysis was used.
Chemical analysis of hydrotalcite catalyst was measured on extrudates after calcination (described below) by X-ray fluorescence (XRF) using the spectrometer BRUKER AXS S8 Tiger with a 5–10% error.
Forming powder catalyst by extrusion
Mg/Al hydrotalcite powder (100 g, Sigma-Aldrich) was inserted into a kneader HKV-1 (IKA), glycerol (40 g, Penta) and isopropanol (15 g, Penta) were added and the whole mixture was kneaded while stirring with 30 Hz frequency for 10 min. Resulting thick paste was pushed in the device CALEVA VDE with a single screw auger through a die with circle slots of 2.2 mm diameter, extrudates were saved in a desiccator and before use they were calcined in air flow in a kiln (LAC) with a temperature increase to 550 °C within 8 h and further 8 h dwell. Immediately after calcination the extrudates were saved in a desiccator with KOH under a grate to prevent catalyst from humidity and CO2. These extrudates after calcination did not contain any binder neither any other ingredient besides pure calcined hydrotalcite.
Transesterification tests under atmospheric pressure
Doses of reactants for tests with methanol
Rape oil (Brölio) 40 g (45.7 mmol of triacylglyceride).
Hydrotalcite 2.8 g (7 wt% of oil) or 4 g (10 wt% of oil) or 6 g (15 wt% of oil).
Methanol (LachNer) 43.92 g (1.371 mol) for molar ratio CH3OH/ester group of oil = 10 or 57.09 g (1.781 mol) for molar ratio CH3OH/ester group of oil = 13.
Doses of reactants for tests with ethanol
Rape oil 30 g (34.3 mmol of triacylglyceride).
Hydrotalcite 4.5 g (15 wt% of oil).
Ethanol (LachNer, 99.8% and 96%) 61.69 g (1.336 mol for 99.8% C2H5OH) and 64.14 g (1.336 mol for 96% C2H5OH) for molar ratio C2H5OH/ester group of oil = 13.
Procedure with hydrotalcite extrudates
In a two-neck round bottom glass flask (NZ 45/40 neck for a cooler with a desiccant end cap (KOH) and NZ 14/23 neck for a thermometer and sampling) a stainless steel perforated basket with hydrotalcite extrudates was immersed in a mixture of rape oil and alcohol. The flask was immersed in oil bath of 69 °C temperature on a heated magnetic stirrer, the whole reaction mixture was vigorously stirred (700 rpm) and held on 63 °C for methanol or 78 °C for ethanol. The time when the flask was inserted into the oil bath, was assumed the beginning of the reaction. During the whole catalytic test, sampling was done usually after every 24 h. When the samples settled, their top phase was taken away. The bottom glycerol phase was not analysed. After methanol evaporation the samples were analysed by gas chromatography.
Procedure with hydrotalcite powder
A catalytic test under the same conditions and with the same doses of reactants as the optimum test with methanol was done to compare results reached with powder and extrudates. A reaction mixture in this comparative test contained 40 g of oil, 57.09 g of methanol (CH3OH/ester group of oil = 13) and 6 g of hydrotalcite powder (15 wt% of oil). The only difference was, that no perforated basket was used and the powder catalyst was added directly into the reaction mixture.
Transesterification tests in a laboratory autoclave with powder hydrotalcite
Instead of one test with several samplings, several individual tests with powder hydrotalcite (15 wt% of oil), rape oil and methanol (with molar ratio to ester group of oil = 13) in a laboratory autoclave with various reaction times were done to see the whole reaction course. Powder hydrotalcite was calcined before test in the same way as extrudates (see above).
For catalytic tests, a batch of rape oil (20 g), methanol (28.54 g) and calcined hydrotalcite powder (3 g) was used always of the same composition. The catalytic tests were carried out in an autoclave stirred by a propeller with a velocity 700 rpm at 140 °C. During heating period, the temperature in the autoclave increased to 130 °C within 11 min and this time was assumed the beginning of the reaction time. Further heating led to reaction temperature 140 °C within 4 min. Individual experiments ran with reaction times 3 h, 6 h and 9 h. After that the heating coat of the autoclave was removed and the internal vessel was cooled by immersing in cold water. Internal pressure reached 9.9 bar and during experiment it decreased to 9.6 bar due to lower methanol concentration after its partial conversion to methyl ester.
Analyses of reaction mixtures after transesterifications
Samples from transesterifications were derivatized first. To 5 µl of a sample, N-trimethylsilyl-N-methyl trifluoroacetamide (MSTFA, 60.8 µl), 100 µl of pyridine and 4 ml of cyclohexane were added. The sample was let react 2 h at room temperature, then it was transferred into a vial and analysed on GC Agilent 7890 with a column Agilent cp 90,787 (10 m × 30 µm × 0,1 µm) with FID. Evaluation was done from a calibration curve. As a standard, pure rape oil monoester, purified by molecular distillation, was used.
Leaching inorganic elements from catalysts into reaction mixtures was evaluated at the end of the reaction by inductively coupled plasma emission spectroscopy using ICP-OES Agilent VDV 5110. The following elements were determined in oil phases after transesterification tests: Ag, Al, As, B, Ba, Be, Bi, Ca, Cd, Co, Cr, Cu, Fe, K, Li, Mg, Mn, Mo, Na, Ni, P, Pb, S, Sb, Se, Sr, Ti, Tl, V, Zn.
Evaluation of transesterification courses
Monoester yields and their dependence on time were evaluated by using first order reaction mechanism, as a very high excess of alcohol enables this approximation. So the following equation was used:
Here Y stands for yield, t stands for time and A, B are regression constants, calculated by the least squares method. The sum of squares of deviations from experimental points was calculated by using a data analysis in EXCEL. Equation 1 with calculated constants was used for calculating the curves fitting experimental data in the Figs. 4 and 5.
Results and discussion
The SEM photo of calcined powder hydrotalcite and calcined extrudates are shown in the Fig. 1a, b and a photo of calcined extrudates is shown in the Fig. 2. Hydrotalcite composition, determined as concentrations of all elements detected by XRF method in wt%, is given in the Table 1. With regards to XRF limitations, where only elements with atomic numbers equal to fluorine and higher are detectable, oxygen was not determined. Hydrogen from hydroxylic groups was probably not present after 8 h calcination at 550 °C. Molar Mg/Al ratio in binderless calcined hydrotalcite extrudates was 2.066. This from Sigma Aldrich purchased hydrotalcite contained also some impurities probably from manufacture process, as it is given in the Table 1. XRD plot of crystalline hydrotalcite structure of original Sigma Aldrich hydrotalcite powder is depicted in the Fig. 3. Surface area and porosity data of calcined hydrotalcite powder and extrudates from NLDFT analysis of nitrogen sorption and desorption isotherms are given in the Table 2. No micropores were found in hydrotalcite powder and nearly none in extrudates. All calculated parameters (total surface area, total pore volume and micropore volume) are significantly higher in extrudates, which shows internal porosity of extrudates, necessary for good transport of a substrate to catalytic sites.
Optimizations of methanol/triglyceride molar ratio and weight ratio of hydrotalcite extrudates to oil are depicted in the Fig. 4 and show obvious sensitivity of the reaction to catalyst concentration in the range 7–15% of catalyst to oil, where reaction rate is rather proportional to increasing amount of the catalyst, especially at the beginning. Also increase in molar ratio of methanol to ester group of oil from 10 to 13 leads to faster reaction course and higher monoester yields. Repeated use of hydrotalcite extrudates after re-calcination is depicted in the Fig. 5 and illustrates the same transesterification course after second re-calcination in the third use of hydrotalcite extrudates as in their second utilization after first re-calcination. Comparison of these reused extrudates shows a bit slower reaction, but nearly 100% monoester yields were reached within 72 h with recycled extrudates. When fresh extrudates provided nearly 100% yields within 48 h, recycled catalyst in its second and third use led to about 90% monoester yields at the same time.
The calculated curves of monoester yields in dependence on time fit experimental data rather well with one exception. It is clear that these curves do not go through zero. First order as a reaction mechanism was chosen due to high surplus of alcohol resulting in its nearly constant concentration for the whole reaction time. Nevertheless, the whole transesterification of triglycerides to monoester is rather complicated and passes through diglycerides and further intermediates. Therefore initial periods of the reaction cannot be controlled by first order mechanism.
As normal boiling point of methanol limits reaction velocity, rape oil transesterifications were performed also in an autoclave at 140 °C. Unfortunately, our laboratory autoclave is too small to insert a basket with extrudates inside, so these experiments were done with calcined hydrotalcite powder at the same ratios of reactants and catalyst as in optimized tests before (15 wt% of hydrotalcite to oil and molar methanol/triglyceride ratio = 13). Not surprisingly increase in reaction temperature from 63 to 140 °C led to significant higher reaction velocity, when almost 99% monoester yield was achieved within 12 h in an autoclave instead of 48 h at normal atmospheric pressure. Anyway, it was not certain whether only temperature caused this difference in reaction velocity or also much smaller particle size of powder than size of extrudates could have a significant influence. Therefore a comparative transesterification test with powder at normal atmospheric pressure and 63 °C was done. Utilization of extrudates and hydrotalcite powder shows nearly the same results (see Fig. 6). Only a little bit smaller reaction velocity with extrudates in comparison to powder is observed in the early reaction stage, but within 24 h this small difference disappears and finally both powder and extrudates provide the same monoester yields (99%) within 48 h. These results entitle us to suggest a feasible industrial oil transesterification arrangement lying in an autoclave with a perforated stainless steel basket placed inside eccentrically outside a stirrer. After the reaction the basket can be pulled out, hydrotalcite extrudates can be easily re-calcined in the basket and used once more.
Differently from rather promising results in rape oil transesterification with methanol, ethanol worked in this reaction much more slowly (see Fig. 7). Not surprisingly water inhibits transesterification, as the reaction with 96% ethanol shows. Using 99.8% ethanol nearly without water makes transesterification significantly faster, but 51% monoester yield after 120 h reaction time is still insufficient.
ICP analyses of liquid phases after transesterifications with extrudates determined all examined elements under detection limits, which were different for various elements and moved in the range 2–5 ppm except S (7.5 ppm). In one transesterification test with hydrotalcite powder at normal pressure, 3 ppm of Ca in liquid phase were found. Other elements were under detection limits. Liquid phases after autoclave tests with hydrotalcite powder at 140 °C contained some impurities: Al and Mg in roughly 60 ppm level, Fe 9 ppm, Ca, Cr and S about 5 ppm. This contamination was probably caused by not well filterable reaction mixtures and also by corroded walls of the autoclave.
Conclusions
A new arrangement with effective use of hydrotalcite extrudates in perforated basket was developed. After reaction, extrudates can be calcined directly in the basket and used once more. Comparison of extrudates with powder hydrotalcite showed nearly the same reaction course only with very small differences in the early reaction stage. As normal boiling point of methanol limits reaction velocity, a basket with hydrotalcite extrudates can be inserted into an autoclave where reaction can run at 140 °C or even higher temperature. On such conditions, 99% monoester yield can be reached within 12 h. Our laboratory autoclave was too small to perform transesterification tests with basket of extrudates inside, so this suggestion was proved indirectly by comparison of reaction course with powder and extrudates of hydrotalcite at 63 °C and normal atmospheric pressure: Results reached with powder hydrotalcite and extrudates were very close.
Differently from rather promising results in rape oil transesterification with methanol, ethanol worked in this reaction much more slowly. Not surprisingly water inhibits transesterification, as the reaction with 96% ethanol shows. Using 99.8% ethanol nearly without water makes transesterification significantly faster, but 51% monoester yield after 120 h reaction time is still insufficient. Nevertheless, ethanol remains a promising raw material for oil transesterification, as it can be prepared by a bioprocess, which makes the whole technology based on renewable sources.
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Carlini M, Castellucci S, Cocchi S (2014) Energy Procedia 45:198–206
Solis JL, Alejo L, Kiros Y (2016) J Environ ChemEng 4:4870–4877
Wu W, Zhu M, Zhang D (2017) ApplCatal A 530:166–173
Shibasaki N, Honda H, Kuribayashi H (2007) Biores Technol 98:416–421
Shumaker JL, Crofcheck C, Tackett SA, Santillan-Jimenez E, Morgan T, Ji Y, Crocker M, Toops TJ (2008) Appl Catal B Environ 82:120–130
Cantrell DG, Gillie LJ, Lee AF (2005) ApplCatal A 287:183–190
Zeng H, Feng Z, Deng X, Li Y (2008) Fuel 87:3071–3076
Gomes JFP, Puna JFB, Goncalves LM (2011) Energy 36:6770–6778
Xie W, Peng H, Chen L (2006) J Mol Catal A: Chem 246:24–32
Navajas A, Campo I, Moral A, Echave J, Sanz O, Montes M, Odriozola JA (2018) Fuel 211:173–181
Faiz K, Shuhaib E, Niranjana U, Nimisha S, Nagapadma M, Gopinath N (2018) Asian J ApplSciTechnol 2:1013–1020
Srivastava P, Jain P (2015) Int J TechnolEnhancEmergEng Res 3:14–17
Martins MI, Pires RF, Alves MJ, Hori CE, Reis MHM, Cardoso VL (2013) Chem Eng Trans 32:817–822
Chen J, Jia L, Guo X, Xiang L, Lou S (2014) RSC Adv 4:60025–60033
Sun C, Qiu F, Yang D, Ye B (2014) Fuel Process Technol 126:383–391
Castro CS, Garcia Júnior LCF, Assaf JM (2014) Fuel Process Technol 125:73–78
Hájek M, Kutálek P, Smoláková L, Troppová I, Čapek L, Kubička D, Kocík J, Thanh DN (2015) Chem Eng J 263:160–167
Silva CCCM, Ribeiro NFP, Souza MMVM, Aranda DAG (2010) Fuel Process Technol 91:205–210
Hájek M, Kocík J, Frolich K, Vávra A (2017) J Clean Prod 161:1423–1431
Hájek M, Tomášová A, Kocík J, Podzemná V (2018) Appl Clay Sci 154:28–35
Frolich K, Vávra A, Kocík J, Hájek M, Jílková A (2019) Renew Energy 143:1259–1267
Čapek L, Kutálek P, Smoláková L, Hájek M, Troppová I, Kubička D (2013) Top Catal 56:586–593
Dahdah E, Estephane J, Haydar R, Youssef Y, Khoury BE, Gennequin C, Aboukaïs A, Abi-Aad E, Aouad S (2020) Renew Energy 146:1242–1248
Serio MD, Mallardo S, Carotenuto G, Tesser R, Santacesaria E (2012) Catal Today 195:54–58
Ma Y, Wang Q, Zheng L, Gao Z, Wang Q, Ma Y (2016) Energy 107:523–531
Reyero I, Velasco I, Sanz O, Montes M, Arzamendi G, Gandía LM (2013) Catal Today 216:211–219
Yan-zhen W, Jing-Tao Y, Li G, Chun-min S, Hong-ling D, Xiang-rong M (2014) AdvMateri Res 953–954:1053–1062
Silveira Junior EG, Justo OR, Perez VH, Reyero I, Serrano-Lotina A, Ramirez LC, Dias DFDS (2018). Adv Mater SciEng. https://doi.org/10.1155/2018/3980967
Acknowledgements
This work is a result of the project CACTU, Reg. No. CZ.02.1.01/0.0/0.0/17_049/0008397, which has been co-financed by European Union from the European Regional Development Fund through the Operational Programme Research, Development and Education. The project CACTU has been integrated into the National Sustainability Programme I of the Ministry of Education, Youth and Sports of the Czech Republic (MEYS) through the project Development of the UniCRE Centre (LO1606). The result was achieved using the infrastructure of the project Efficient Use of Energy Resources Using Catalytic Processes (LM2018119) which has been financially supported by MEYS within the targeted support of large infrastructures.
Funding
This work is a result of the project CACTU, Reg. No. CZ.02.1.01/0.0/0.0/17_049/0008397, which has been co-financed by European Union from the European Regional Development Fund through the Operational Programme Research, Development and Education. The project CACTU has been integrated into the National Sustainability Programme I of the Ministry of Education, Youth and Sports of the Czech Republic (MEYS) through the project Development of the UniCRE Centre (LO1606). The result was achieved using the infrastructure of the project Efficient Use of Energy Resources Using Catalytic Processes (LM2018119) which has been financially supported by MEYS within the targeted support of large infrastructures.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Hydrotalcite extrudate preparation and transesterification tests were performed by AK, analysis of rape oil mixtures after transesterifications were done by JK according to a method developed by JH, the research was conducted and manuscript was written by VT. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kašpárek, A., Kocík, J. & Tokarová, V. Rape oil transesterification by methanol and ethanol on extruded hydrotalcite catalyst. Reac Kinet Mech Cat 132, 203–218 (2021). https://doi.org/10.1007/s11144-020-01915-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01915-4