Abstract
Zn–La–Zr–Si oxide composition has been investigated in the ethanol-to-butadiene process using ethanol–water mixtures with different water content. An increase of H2O content in the initial reaction mixture decreases ethanol conversion, 1,3-butadiene selectivity, yield and productivity. The results of in situ FTIR spectroscopy (with ethanol and acetone as probe molecules) have shown the main reason for a decrease in activity of the catalyst to be H2O adsorption on active sites of aldol condensation of acetaldehyde and, to a lesser extent, ethanol dehydrogenation. Zn–La–Zr–Si oxide composition is a highly active and selective catalyst for the ethanol-to-butadiene process when ethanol–water mixture of 80 vol% ethanol and 20 vol% H2O is used, 60% 1,3-butadiene yield is achieved.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
1,3-Butadiene (BD) is one of the most important conjugated dienes and is used as an intermediate for the synthesis of rubber, elastomers and polymer resins. Traditionally, BD can be produced as a side product in the steam cracking process and a process of oxidation of butenes [1]. The alternative non-fossil method of BD production is bioethanol conversion [2,3,4,5].
An overall scheme of ethanol (EtOH) conversion into BD involves multiple consecutive reaction steps such as: acetaldehyde (AA) formation from EtOH, cross-condensation of AA, Meerwein–Ponndorf–Verley (MPV) reduction of crotonaldehyde (CA) with EtOH to crotyl alcohol and AA, and dehydration of crotyl alcohol to BD [2, 3, 6, 7]. According to one of the alternative mechanisms proposed in the literature, AA reacts with EtOH carbanion or enolic species (or nucleophilic attack of a reactive enolate upon an acetaldehyde molecule) to form crotonaldehyde [8,9,10,11]. Most authors note that both catalytic activity and BD selectivity of the process correlate with the strength and amount of acid–base sites and the sites of EtOH dehydrogenation [12,13,14,15].
A rather important problem in the development of catalysts for the ethanol-to-butadiene (ETB) process is their high activity and selectivity in the conversion of EtOH–water mixtures. The feedstock with high water content is more advantageous and environmentally sustainable; 50–80 vol% EtOH solutions are easily obtained from a fermentation broth by flash separation [16]. Recently, Michelin company has been issued a patent for BD production from 80 wt% EtOH solution [17]. The stability of the ETB process catalysts to deactivation in the presence of water vapor allows using not only pure or rectified EtOH as raw material, but also EtOH of partial (not complete) rectification. At the same time, two molecules of water are formed with a single molecule of BD in ETB process in accordance with the following equation: 2C2H5OH → C4H6 + H2 + 2H2O. The calculations indicate that the content of water vapor among the products increases with the increase in the conversion (Fig. 1). The water content in the reaction mixture can reach 40–50 vol% with an EtOH conversion of 96–80%.
Rahman et al. [18] have investigated EtOH conversion on ZnO and showed that H2O blocks strong Lewis acidic sites for EtOH dehydration reaction to ethylene and diethyl ether (DEE) while leaving weak Lewis acid–base pairs for EtOH dehydrogenation; aldol-condensation of AA to CA is inhibited by water vapor too. Ochoa et al. [8] have studied the co-fed water on one-step ETB conversion on MgO/SiO2 catalysts. The decrease in BD selectivity and rising in ethylene selectivity are observed. According to the results by Fourier transform infrared (FTIR) spectroscopy with adsorbed pyridine, the observed tendencies are due to formation of Brønsted acidic sites by adsorption of molecular water on Lewis acidic sites. Zhu et al. [19] have shown the effect of water vapor of the EtOH–AA feed over MgO/SiO2 catalysts. The water amount of 10 wt% is found to inhibit the formation of 1-butanol and C6+ compounds without increasing the selectivity to EtOH dehydration products (ethylene and DEE). The effect of water vapor on higher alcohol and non-condensable gas formation in the condensed-phase ethanol Guerbet process has been investigated on Ni/La2O3/γ-Al2O3 catalysts by Jordison et al. [20]. The addition of 10 wt% water to anhydrous EtOH is reported to have a moderate effect on conversion rate but significantly reduces both n-butanol and C6+ alcohol yields and increases non-condensable gas yield.
ZrO2–SiO2 composition is intensively studied as a potential basis for the development of industrial catalysts for ETB process [21,22,23,24,25]. A modification of the composition with Cu, Ag, Au and ZnO is shown to substantially improve its activity [24,25,26,27,28]. Zhang and coworkers [29] have demonstrated that ZnO is an especially good promoter of ZrO2–SiO2 catalyst for the EtOH/acetaldehyde conversion into BD. Zinc oxide is usually considered to be responsible for EtOH dehydrogenation step of ETB process [3, 30]. However, it has been recently shown that ZnO in ZnO/MgO–SiO2 catalysts also participates in the formation of Lewis acidic sites for further conversion of acetaldehyde to BD [27, 31]. Similar effect can be also expected for ZnO-doped ZrO2–SiO2 catalyst [29, 32]. To further increase in selectivity of ETB process over Zn–Zr–Si oxide compositions, lanthanum compounds are used as the dopants. The relatively high process indices (BD selectivity of 74% at T = 598 K and WHSV = 0.3 gEtOH g −1cat h−1) are observed even in the case of using 92 vol% EtOH solution, but the maximum BD productivity does not exceed 0.09 g g −1cat h−1 [33].
We have previously found that in the presence of Zn–La–Zr–Si oxide composition high BD selectivity, yield and productivity of 0.71 g g −1cat h−1 are achieved [34, 35]. The aim of the present study is to find out the effect of water content in the initial reaction mixture and H2O formed during the reaction on the ETB process indices, the reasons for a decrease in the catalyst activity in the presence of water vapor, and to determine the ways to reduce the effect of this negative factor. The influence of water vapor on the key steps of the process (ethanol dehydrogenation and aldol condensation) is studied using in situ FTIR spectroscopy with ethanol and acetone as probe molecules.
Experimental details
Catalyst preparation
KSKG industrial silica gel (silica gel granular with large pore, specific surface area is 283 m2 g−1) was treated with dilute nitric acid, washed with doubly distilled water, and calcined at 773 K. Then, the silica gel was sequentially impregnated to incipient wetness with aqueous solutions of chemically-pure-grade La(NO3)3·6H2O and Zn(O2CCH3)2 with intermediate drying at 393 K for 2 h and calcining at 773 K for 3 h. Introduction of zirconium into the sample was carried out by wet kneading (fraction < 0.1 mm) with analytical grade ZrO(NO3)2·2H2O, followed by drying (393 K, 2 h) and calcining (773 K, 3 h). The 2 wt% ZnO–7 wt% La2O3–1 wt% ZrO2–SiO2 catalyst was prepared and marked as Zn–La–Zr–Si oxide composition [34].
Catalyst characterization
In situ FTIR spectra were recorded on a Spectrum One FTIR spectrometer (Perkin Elmer, USA) accumulating 24 scans at a spectral resolution of 1 cm−1 using handmade quartz cell with NaCl glasses. The catalyst sample was pressed at ~ 2 ton cm−2 into thin wafers of ca. 12 mg cm−2 and placed inside the IR cell. Before injection of EtOH, AA, crotonaldehyde and acetone, the catalyst-containing IR cell was heated in a constant Ar flow (~ 60 mL min−1). Once 773 K was reached, treatment lasted 1 h.
In the case of ethanol adsorption, the sample was cooled to 648 K, a baseline was recorded. To inject EtOH into the IR cell containing the catalyst, Ar was passed through EtOH-containing gas bubbler for 30 min, and the spectra of adsorbed molecules were recorded. To study water effect on EtOH adsorption/transformation, after the treatment at 773 K and cooling to 648 K, Ar with H2O vapor were passed through the IR cell containing the catalyst (for this Ar was passed through H2O-containing gas bubbler for 20 min). Then, to inject EtOH into the IR cell, Ar was passed through a gas bubbler containing an EtOH–water mixture (50 vol% EtOH and 50 vol% H2O) for 30 min, and the spectra of adsorbed molecules were recorded. In the case of AA and CA, the sample was cooled to 648 K and 0.5 mL of AA or crotonaldehyde were injected into an Ar flow. The spectra of adsorbed molecules were recorded 10 min after the injection. In the case of acetone, the sample was cooled to 373 K, and a baseline was recorded. To inject the probe molecule into the IR cell containing the catalyst, Ar was passed through acetone-containing gas bubbler for 10 min. After that, Ar was passed through the IR cell, and the spectra of adsorbed molecules were recorded at different temperatures once the intensity of the bands of adsorbed acetone became unchanged.
Catalytic activity measurement
Catalytic activity tests were carried out in a fixed-bed flow quartz reactor with inner diameter of 4 mm at 648–673 K and atmospheric pressure. Samples with grains of 0.25–0.5 mm were loaded into the reactor. Ethanol–water mixture feed (96–20 vol% EtOH–4–80 vol% H2O) was introduced to an evaporator via a syringe infusion pump, and argon with flow of 7 mL min−1 was the feed into the reactor. The reaction was carried out at WHSV by EtOH of 0.25–1.2 gEtOH g −1cat h−1 [marked as WHSV(e)] or by the EtOH–water mixture—1.04–5 gEtOH+ H2O g −1cat h−1 [marked as WHSV(m)]. Before the reaction, the catalysts were annealed at 773 K under flowing argon for 1 h. The reagents and reaction products were analyzed on a gas chromatograph (KristalLyuks 4000M, MetaChrome) equipped with a TCD detector and a packed column (10% NiSO4 on coal, 3 m × 3 mm) for CO and CO2, and a FID detector and a capillary column (HP-FFAP, 50 m × 0.32 mm) for organic compounds.
Catalytic activity of the catalysts was characterized by EtOH conversion (X), selectivity to products (Si), BD yield (YBD) and BD productivity (PBD):
here \(\text{n}_{{\text{EtOH}}}^{\text{0}}\) is the initial amount of C1 moles of EtOH; nEtOH and ni are the amount of C1 moles of unreacted EtOH and product i in the stream of the reaction products, respectively; 0.587 is the maximum possible amount of BD (g) that can be produced from 1 g of EtOH from a stoichiometric equation:
To examine the water effect on EtOH conversion into BD, we have simulated two different experimental conditions:
-
(1)
the amount of EtOH feed, regardless of the water content, is the same (WHSV(e) = const). This condition allows us to evaluate the effect of water in the initial EtOH–water mixture on ETB process and demonstrates a feasibility of using bioethanol to obtain BD.
-
(2)
the amount of the reaction mixture feed, regardless of EtOH content, is the same (WHSV(m) = constant). This condition makes possible to estimate how the water formed during EtOH conversion (according to Fig. 1) can affect ETB process indices and to check the process sustainability when using excess water in the feed.
Results and discussion
The Zn–La–Zr–Si oxide composition is an X-ray amorphous material containing Lewis acidic sites, formed by Zn2+ and Zr4+ (medium and strong ones) and La3+ (weak ones). Brønsted acidic sites are not identified. Weak basic sites formed by OH groups associated with lanthanum are also detected on the sample surface. For more information about physical–chemical properties of the Zn–La–Zr–Si oxide composition, see Ref. [34].
Water vapor effect on activity of Zn–La–Zr–Si oxide catalyst in ETB process
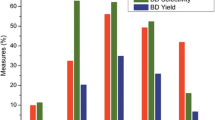
Fig. 2 shows the dependences of conversion of EtOH–water mixtures in the presence of Zn–La–Zr–Si oxide composition, selectivity to products, BD yield and productivity on EtOH content in the reaction feed at the same WHSV (by EtOH). The process is carried out at the temperature range in which the highest BD yield has been observed (648–673 K) [34].
The use of anhydrous ethanol for carrying out the process makes it possible to achieve EtOH conversion of 80% and BD selectivity of 65% at temperature of 648 K. Adding small amounts of water (4 vol%) into the reaction mixture leads to insignificant increase in these indicators. A similar effect of 5% H2O has been observed by the authors of the work [32]. For the reaction mixture containing 80 vol% EtOH and 20 vol% H2O, the EtOH conversion is around 71% at 648 K, BD selectivity—61%. This is comparable with those (80 and 66%) for the mixture containing 96 vol% EtOH and 4 vol% H2O.
A subsequent increase in water content in the feed flow decreases significantly both EtOH conversion and BD selectivity, while selectivity to AA, ethylene and diethyl ether (DEE) rises. Conversion of the EtOH-water mixture containing 20% EtOH and 80% H2O is 12%, BD selectivity does not exceed 9%. The main products under these conditions are ethylene and AA. The same tendency is observed at the temperature of 673 K, BD selectivity of the ETB process with 20 vol% EtOH mixture is 13%, opposed to 45% for ethylene and 34% for AA.
It should be mentioned that regardless of the water content in the reaction mixture, an increase in the content of crotonaldehyde in the reaction products is not observed. Crotonaldehyde selectivity does not exceed 0.1–0.2% at all studied temperatures. Thus, MPV reduction of AA formed during aldol condensation does not limit ETB process.
BD productivity is markedly decreased with changing the feed to those with higher water content. The use of the mixture containing 20 vol% EtOH and 80 vol% H2O allows obtaining only 0.017 gBD g −1cat h−1 at 648 K and 0.006 gBD g −1cat h−1 at 673 K.
The rising content of DEE in the reaction products with the increase in water content in the feed can be explained by the fact that H2O acts as an esterification co-catalyst by its reaction with enolates [36]. At the same time, the rising selectivity of ethylene and DEE may be due to the increase in concentration of active sites of EtOH dehydration. In the presence of water vapor Brønsted acidic sites are formed from Lewis acidic sites (LAS), as shown by Ochoa et al. [8]. The competing adsorption of H2O and AA/enolic species is suggested to be on active sites of aldol condensation (LAS).
Comparing ETB process indices at different temperatures, it can be concluded that in the case of EtOH–water mixtures an increase in the process temperature practically does not lead to a decrease in BD selectivity, while EtOH conversion, as well as BD yield, is increased. As a rule, the temperature rising promotes an increase in EtOH conversion and, at the same time, leads to the BD selectivity declining [30, 34, 37]. This is primarily due to the fact that the rate of ethanol dehydration to ethylene is increased with temperature [38]; an inverse temperature dependence is observed for aldol condensation [4]. Higher temperature is likely to contribute to intensification of H2O desorption from surface active sites of the catalyst. Besides, the temperature rising from 648 to 673 K contributes to a significant increase in the productivity of the catalyst to BD. A similar result has been also noted for the process of propylene production from an ethanol–water mixture [39].
Fig. 3 shows the dependences of conversion of EtOH–water mixtures in the presence of Zn–La–Zr–Si oxide catalyst, selectivity to products, BD yield and productivity on EtOH content in the reaction feed at the same WHSV(m). This approach allows us to analyze how much the process indices are changed with replacement of pure (rectified) EtOH by its water mixture at a constant flow rate of the feed. In addition, these dependences help estimating how the process indices are changed with the changing of the reaction mixture composition (adding of water as a reaction product) along the length of a catalyst bed.
The use of 80/20 vol% EtOH–water mixture results in slightly decreasing in EtOH conversion and BD selectivity, BD productivity remains high enough (0.256 gBD g −1cat h−1 at 648 K and 0.358 gBD g −1cat h−1 at 673 K). An increase in water content in the reaction medium at the same WHSV(m) results in the rising selectivity of the dehydration products (in particular, ethylene). The typical dependence of selectivity on conversion has been shown earlier in the paper [25, 30]; it can be due to the effect of water on ETB process. Thus, the use of proposed catalyst makes it possible to convert 80% EtOH into BD with high selectivity and yield.
In situ FTIR studies of Zn–La–Zr–Si oxide catalyst
To estimate the effect of water vapor on EtOH dehydrogenation, in situ FTIR spectroscopy studies of the Zn–Zr–La–Si catalyst have been carried out under EtOH and EtOH–water mixture flows (Fig. 4). For comparison, the spectra of adsorbed AA and CA are shown, too.
The band at 3674 cm−1 in the spectra recorded in the presence of ethanol and EtOH–water mixture is identified as OH stretching mode related to the presence of alcohols and vibration mode of water [40]. Several bands between 2988 and 2700 cm−1 are observed in C–H region of the spectra and attributed to combination of ν(CH3) and ν(CH2) vibrational modes of EtOH, AA and CA with the corresponding bending modes located at 1450 cm−1 and wagging modes between 1405 and 1380 cm−1. In the C=O region of the spectra, there are the bands at 1761 and 1744 cm−1 corresponding to AA, and the band at 1724 cm−1 corresponding to CA, accordingly to the spectroscopic data of AA and CA both obtained under experimental conditions and reported earlier [7, 41, 42]. The band at 1640 cm−1 corresponding to C=C vibrations is not fixed in in situ FTIR spectra of the Zn–Zr–La–Si catalyst under EtOH and EtOH–water mixture flows.
Comparing the spectra recorded under EtOH and EtOH–water flows, AA can be concluded to be formed in both cases, regardless of the presence of water. However, the intensity of the bands related to C=O vibrations in the spectra recorded under EtOH–water flow is lower by ~ 1/5 than those under EtOH flow. So, water adsorbs on the part of EtOH dehydrogenation sites, and the first step of ETB process slows down. However, EtOH conversion is varied not so much at the same WHSV(m), while AA selectivity is increased at times. So, it is likely that the effect of water on EtOH dehydrogenation is not critical, and step (1) is not limiting for ETB process as a whole.
To estimate the concentration of LASs on a catalyst surface, FTIR spectroscopic study has been done with ketone (particularly acetone) as a probe molecule adsorbed on an active site by a carbonyl group [43]. Such technique allows evaluating not just total LAS concentration, but the concentration of LASs on which the reaction molecules can be adsorbed by a carbonyl group (in our case, acetaldehyde). We hypothesize that the use of FTIR spectroscopy of adsorbed acetone in the absence and presence of water will allow estimating the number of active sites available for aldol condensation of AA during ETB process.
Fig. 5 shows the spectra of adsorbed acetone on Zn–La–Zr–Si oxide catalyst before and after contact with water vapor. The band at 1707 cm−1 may be assigned to acetone adsorbed on hydroxylated silica gel (C=O interacting with SiOH). The bands between 1698 and 1683 cm−1 may be assigned to acetone (C=O interacting) coordinated to medium and strong LAS. The weak, broad shoulder centered around 1624 cm−1 is assigned to ν(C=C) vibrations originating from minority condensation species of adsorbed acetone and/or minority adsorbed molecules (presumably diacetone alcohol) coordinated to stronger LAS. The bands at 1423 cm−1 and 1369 cm−1 are related to δ(CH) vibrations [34, 36, 38]. By comparing the intensity of the bands between 1698 and 1683 cm−1 in the absence and presence of water, one can be unambiguously concluded that the water molecules are adsorbed on LASs, active sites of adsorption of a carbonyl group.
Consequently, surface active sites for aldol condensation are blocked, and adsorptive and catalytic interactions of EtOH/AA are largely suppressed. A similar conclusion has been reached by Ochoa et al. [8] during the investigation of ETB process over MgO/SiO2 catalysts. This result also agrees with the study of water effect on aldol condensation of AA and Guerbet coupling of ethanol, made by Flaherty and coworkers and Moteki and Flaherty [11, 44]. To increase BD yield during the conversion of EtOH–water mixtures, it is necessary to develop catalysts with a high content of aldol condensation active sites.
Conclusions
In the ethanol-to-butadiene process on Zn–La–Zr–Si oxide composition EtOH conversion and BD selectivity are reduced with an increase in a water content in an ethanol–water mixture feed. The results of in situ FTIR spectroscopy (with acetone as a probe molecule) have shown that the main reason for a decrease in activity of the catalyst in the presence of water in the reaction mixture is H2O adsorption on Lewis acidic sites: water blocks active sites of aldol condensation of acetaldehyde. Water vapor has a significant effect on aldol condensation, while ethanol dehydrogenation is less inhibited, thus, AA accumulates in the reaction mixture.
Zn–La–Zr–Si oxide composition is a highly active and selective catalyst for the ethanol-to-butadiene process using ethanol–water mixture of 80 vol% ethanol and 20 vol% H2O, 60% BD yield is achieved. A possible way to intensify the conversion of ethanol–water mixtures into BD is an optimization of a temperature regime of a catalytic reactor and use of catalytic compositions able to catalyze aldol condensation in the presence of water vapor.
References
White WC (2007) Butadiene production process overview. Chem Biol Interact 166:10–14. https://doi.org/10.1016/j.cbi.2007.01.009
Makshina EV, Dusselier M, Janssens W et al (2014) Review of old chemistry and new catalytic advances in the on-purpose synthesis of butadiene. Chem Soc Rev 43:7917–7953. https://doi.org/10.1039/c4cs00105b
Pomalaza G, Capron M, Ordomsky V, Dumeignil F (2016) Recent breakthroughs in the conversion of ethanol to butadiene. Catalysts 6:203–238. https://doi.org/10.3390/catal6120203
Angelici C, Weckhuysen BM, Bruijnincx PCA (2013) Chemocatalytic conversion of ethanol into butadiene and other bulk chemicals. ChemSusChem 6:1595–1614. https://doi.org/10.1002/cssc.201300214
Jones MD (2014) Catalytic transformation of ethanol into 1,3-butadiene. Chem Cent J 8:53–58. https://doi.org/10.1186/s13065-014-0053-4
Mueller P, Wang S-C, Burt S, Hermans I (2017) Influence of metal-doping on the Lewis-acid catalyzed production of butadiene from ethanol studied by modulated operando DRIFTS-MS. ChemCatChem. https://doi.org/10.1002/cctc.201700698
Taifan W, Yan GX, Baltrusaitis J (2017) Surface chemistry of MgO/SiO2 catalysts during the ethanol catalytic conversion to 1,3-butadiene: in situ DRIFTS and DFT study. Catal Sci Technol 7:4648–4668. https://doi.org/10.1039/C7CY01556A
Ochoa JV, Bandinelli C, Vozniuk O et al (2016) An analysis of the chemical, physical and reactivity features of MgO–SiO2 catalysts for butadiene synthesis with the Lebedev process. Green Chem 18:1653–1663. https://doi.org/10.1039/C5GC02194D
Chieregato A, Ochoa JV, Bandinelli C et al (2015) On the chemistry of ethanol on basic oxides: revising mechanisms and intermediates in the Lebedev and Guerbet reactions. ChemSusChem 8:377–388. https://doi.org/10.1002/cssc.201402632
Sushkevich VL, Ivanova II (2017) Mechanistic study of ethanol conversion into butadiene over silver promoted zirconia catalysts. Appl Catal B 215:36–49. https://doi.org/10.1016/j.apcatb.2017.05.060
Zhang H, Ibrahim MYS, Flaherty DW (2018) Aldol condensation among acetaldehyde and ethanol reactants on TiO2: experimental evidence for the kinetically relevant nucleophilic attack of enolates. J Catal 361:290–302. https://doi.org/10.1016/j.jcat.2018.02.030
Patil PT, Liu D, Liu Y et al (2017) Improving 1,3-butadiene yield by Cs promotion in ethanol conversion. Appl Catal A 543:67–74. https://doi.org/10.1016/j.apcata.2017.05.025
Klein A, Keisers K, Palkovits R (2016) Formation of 1,3-butadiene from ethanol in a two-step process using modified zeolite-ß catalysts. Appl Catal A 514:192–202. https://doi.org/10.1016/j.apcata.2016.01.026
Angelici C, Velthoen MEZ, Weckhuysen BM, Bruijnincx PCA (2015) Influence of acid–base properties on the Lebedev ethanol-to-butadiene process catalyzed by SiO2–MgO materials. Catal Sci Technol 5:2869–2879. https://doi.org/10.1039/C5CY00200A
Larina OV, Kyriienko PI, Trachevskii VV et al (2016) Effect of mechanochemical treatment on acidic and catalytic properties of MgO-SiO2 composition in the conversion of ethanol to 1,3-butadiene. Theor Exp Chem 51:387–393. https://doi.org/10.1007/s11237-016-9440-3
Rossetti I, Lasso J, Compagnoni M et al (2015) H2 production from bioethanol and its use in fuel-cells. Chem Eng Trans 43:229–234. https://doi.org/10.3303/CET1543039
Dastillung R, Fischer B, Jacquin M, Huyghe R (2017) Method for the production of butadiene from ethanol in one low-water- and low-energy-consumption reaction step. US 20170267604 A1 17
Rahman MM, Davidson SD, Sun J, Wang Y (2016) Effect of water on ethanol conversion over ZnO. Top Catal 59:37–45. https://doi.org/10.1007/s11244-015-0503-9
Zhu Q, Wang B, Tan T (2016) Conversion of ethanol and acetaldehyde to butadiene over MgO–SiO2 catalysts: effect of reaction parameters and interaction between MgO and SiO2 on catalytic performance. ACS Sustain Chem Eng 5:722–733. https://doi.org/10.1021/acssuschemeng.6b02060
Jordison TL, Peereboom L, Miller DJ (2016) Impact of water on condensed phase ethanol Guerbet reactions. Ind Eng Chem Res 55:6579–6585. https://doi.org/10.1021/acs.iecr.6b00700
Han Z, Li X, Zhang M et al (2015) Sol-gel synthesis of ZrO2-SiO2 catalysts for the transformation of bioethanol and acetaldehyde into 1,3-butadiene. RSC Adv 5:103982–103988. https://doi.org/10.1039/C5RA22623F
Jones MD, Keir CG, Di Iulio C et al (2011) Investigations into the conversion of ethanol into 1,3-butadiene. Catal Sci Technol 1:267–272. https://doi.org/10.1039/c0cy00081g
Cheong JL, Shao Y, Tan SJR et al (2016) Highly active and selective Zr/MCF catalyst for production of 1,3-butadiene from ethanol in a dual fixed bed reactor system. ACS Sustain Chem Eng 4:4887–4894. https://doi.org/10.1021/acssuschemeng.6b01193
Kurmach MM, Larina OV, Kyriienko PI et al (2018) Hierarchical Zr-MTW zeolites doped with copper as catalysts of ethanol conversion into 1,3-butadiene. ChemistrySelect 3:8539–8546. https://doi.org/10.1002/slct.201801971
Sushkevich VL, Ivanova II, Ordomsky VV, Taarning E (2014) Design of a metal-promoted oxide catalyst for the selective synthesis of butadiene from ethanol. ChemSusChem 7:2527–2536. https://doi.org/10.1002/cssc.201402346
Makshina EV, Janssens W, Sels BF, Jacobs PA (2012) Catalytic study of the conversion of ethanol into 1,3-butadiene. Catal Today 198:338–344. https://doi.org/10.1016/j.cattod.2012.05.031
Larina OV, Kyriienko PI, Soloviev SO (2015) Effect of the addition of zirconium dioxide on the catalytic properties of ZnO/MgO-SiO2 compositions in the production of 1,3-butadiene from ethanol. Theor Exp Chem 51:252–258. https://doi.org/10.1007/s11237-015-9424-8
Kyriienko PI, Larina OV, Soloviev SO et al (2017) Ethanol conversion into 1,3-butadiene by the Lebedev method over MTaSiBEA zeolites (M = Ag, Cu, Zn). ACS Sustain Chem Eng 5:2075–2083. https://doi.org/10.1021/acssuschemeng.6b01728
Xu Y, Liu Z, Han Z, Zhang M (2017) Ethanol/acetaldehyde conversion into butadiene over sol–gel ZrO2–SiO2 catalysts doped with ZnO. RSC Adv 7:7140–7149. https://doi.org/10.1039/C6RA25139K
Hayashi Y, Akiyama S, Miyaji A et al (2016) Experimental and computational studies of the roles of MgO and Zn in talc for the selective formation of 1,3-butadiene in the conversion of ethanol. Phys Chem Chem Phys 18:25191–25209. https://doi.org/10.1039/C6CP04171J
Larina OV, Kyriienko PI, Soloviev SO (2015) Ethanol conversion to 1,3-butadiene on ZnO/MgO–SiO2 catalysts: effect of ZnO content and MgO:SiO2 ratio. Catal Lett 145:1162–1168. https://doi.org/10.1007/s10562-015-1509-4
Zhang M, Tan X, Zhang T et al (2018) The deactivation of ZnO doped ZrO2-SiO2 catalyst in the conversion of ethanol/acetaldehyde to 1,3-butadiene. RSC Adv 8:34069–34077. https://doi.org/10.1039/C8RA06757K
Lewandowski M, Ochenduszko A, Jones MD (2014) Process for the production of 1,3-butadiene. WO 2014/180778 A1 14
Larina OV, Kyriienko PI, Soloviev SO (2016) Effect of lanthanum in Zn-La(-Zr)-Si oxide compositions on their activity in the conversion of ethanol into 1,3-butadiene. Theor Exp Chem. https://doi.org/10.1007/s11237-016-9450-1
Grim RG, To AT, Farberow CA et al (2019) Growing the bioeconomy through catalysis: a review of recent advancements in the production of fuels and chemicals from syngas-derived intermediates. ACS Catal 9:4145–4172. https://doi.org/10.1021/acscatal.8b03945
Wang S, Iglesia E (2016) Substituent effects and molecular descriptors of reactivity in condensation and esterification reactions of oxygenates on acid-base pairs at TiO2 and ZrO2 surfaces. J Phys Chem C 120:21589–21616. https://doi.org/10.1021/acs.jpcc.6b07304
Akiyama S, Miyaji A, Hayashi Y et al (2018) Selective conversion of ethanol to 1,3-butadiene using germanium talc as catalyst. J Catal 359:184–197. https://doi.org/10.1016/j.jcat.2018.01.001
Rossetti I, Compagnoni M, De Guido G et al (2017) Ethylene production from diluted bioethanol solutions. Can J Chem Eng 95:1752–1759. https://doi.org/10.1002/cjce.22828
Matheus CRV, Chagas LH, Gonzalez GG et al (2018) Synthesis of propene from ethanol: a mechanistic study. ACS Catal 8:7667–7678. https://doi.org/10.1021/acscatal.8b01727
Quesada J, Faba L, Díaz E, Ordóñez S (2017) Role of the surface intermediates in the stability of basic mixed oxides as catalyst for ethanol condensation. Appl Catal A 542:271–281. https://doi.org/10.1016/j.apcata.2017.06.001
Mueller P, Burt SP, Love AM et al (2016) Mechanistic study on the Lewis-acid catalyzed synthesis of 1,3-butadiene over Ta-BEA using modulated operando DRIFTS-MS. ACS Catal 6:6823–6832. https://doi.org/10.1021/acscatal.6b01642
Yan T, Dai W, Wu G et al (2018) Mechanistic insights into one-step catalytic conversion of ethanol to butadiene over bifunctional Zn–Y/beta zeolite. ACS Catal 8:2760–2773. https://doi.org/10.1021/acscatal.8b00014
Zaki MI, Hasan MA, Pasupulety L (2001) Surface reactions of acetone on Al2O3, TiO2, ZrO2, and CeO2: IR spectroscopic assessment of impacts of the surface acid-base properties. Langmuir 17:768–774. https://doi.org/10.1021/la000976p
Moteki T, Flaherty DW (2016) Mechanistic insight to C-C bond formation and predictive models for cascade reactions among alcohols on Ca- and Sr-hydroxyapatites. ACS Catal 6:4170–4183. https://doi.org/10.1021/acscatal.6b00556
Acknowledgements
This work was financially supported by programs of National Academy of Sciences of Ukraine KPKVK 6541230 “Support for the development of priority areas of scientific research” (Grant No. 0116U000061) and KPKVK 6541030 “New functional substances and materials of chemical production” (Grant No. 0119U101562).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Larina, O.V., Remezovskyi, I.M., Kyriienko, P.I. et al. 1,3-Butadiene production from ethanol–water mixtures over Zn–La–Zr–Si oxide catalyst. Reac Kinet Mech Cat 127, 903–915 (2019). https://doi.org/10.1007/s11144-019-01618-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-019-01618-5