Abstract
In this paper a series of zirconium oxide supported on silica composite oxides were studied as catalysts for the production of 1,3-butadiene from bioethanol and acetaldehyde. The highest selectivity observed was 91.43%. Different silica materials with varied pore diameters in the range of 3.6–11.6 nm and the service life of the catalyst have been initially investigated. The catalysts were characterized by a nitrogen adsorption analysis, X-ray diffraction, scanning electron micrography and 29Si solid-state NMR spectroscopy. The catalytic results show that the pore sizes are important factors determining the activity when catalyst contains a subtle balance of the acid and base.
Graphical Abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
A main challenge facing the bulk chemical industry in the twenty-first century is the sustainability of its supply chains. It has been advocated that future high-value bio-based chemicals need to be produced from bio-refineries in addition to the lower-value biofuels [1]. One of the most abundant sustainable and practicable raw materials is bioethanol, produced from biomass [2, 3]. There are many applications for the conversion of bioethanol into commodity chemicals [4]. In this paper we report a systematic study of ZrO2 supported on series silica catalysts for the bioethanol conversion into 1,3-butadiene (BD).
BD is an important building block in the chemical industry and generally used as a material for synthetic polymers and rubbers [5]. While BD is currently obtained as by-product of ethylene production in the process of hydrocarbon steam cracking [6]. So there is a desire to produce BD from a sustainable source, bioethanol. This is by no means a new technology, during the first decades of the twentieth century, two processes were developed for the synthesis of BD from ethanol, that is, the Ostromisslenski [7] and Lebedev [8] processes.
Different classes of catalysts have been adopted in this process [9,10,11]. As early as 1947, transition metal oxides supported on silica were used in the two-step process of BD formation. Highest BD selectivities 67 and 59% were obtained on Ta2O5/SiO2 and ZrO2/SiO2, respectively [12]. Later on, Corson et al. [13] have studied more than 350 oxides as catalysts on BD formation from ethanol and concluded that Ta2O5–SiO2 and ZrO2–SiO2 exhibited preferable catalytic activity in the two-step process. Jones et al. [14] have concluded that Zr–Zn/SiO2 was supposed as the most promising catalyst with the highest BD selectivity 67.4%. Chae et al. [15] have tested a series of ordered mesoporous silica supported tantalum oxide samples as catalysts for conversion ethanol and acetaldehyde to BD and their research have found that pore structure has an influence on the reaction activity. Along with research thorough, we can draw a conclusion that ingenious combination of ZrO2 and SiO2 can meet the demand of ethanol and acetaldehyde to BD reaction and the activity of catalysts would improve further through tailoring other factors, which is a meaningful task.
In this study, a variety of ZrO2 supported on SiO2 catalysts were synthesized. Moreover, the effect of the physical structure and properties of the catalysts on the performance of BD formation from bioethanol and acetaldehyde reaction was examined. In this case, we used NanoSiO2, SBA-15, MCM-41 as silicon source. This is the first study to apply series silica as a support in Zr-containing catalysts.
2 Experimental
2.1 Preparation of Catalysts
All of the silica-supported 2 wt% ZrO2 catalysts were obtained by incipient wetness impregnation of silica series with zirconium oxynitrate solutions. After impregnation all samples were treated by ultrasounding for 2 h. Then, all samples were dried at 393 K and calcined at 923 K for 6 h (ramping rate, 5° C/min) in a flow of dry air.
2.2 Catalyst Characterization
Nitrogen adsorption isotherms were measured at 77 K on a Micromeritics Tristar 3000 volumetric adsorption analyzer. Powder X-ray diffraction patterns were recorded on a Rigaku Multiplex instrument using Cu-K radiation operated at 40 kV and 150 mA. Scanning electron micrograph images were obtained with a Philips XL-30S FEG scanning electron microscope. 29Si MAS NMR spectra were recorded on a Bruker AMX300 spectrometer.
2.3 Catalytic Test
The production of BD from ethanol and acetaldehyde was performed in a fixed bed reactor system. A mixture of ethanol and acetaldehyde with ethanol to acetaldehyde volume ratio of 3.5 was fed into the catalytic reactor by a flow pump [16]. The catalyst was loaded in the middle of the quartz tube. Before the reaction, the catalyst was pretreated to the reaction temperature with an N2 flow as the carrier gas. The reaction was then performed with a weight hourly space velocity (WHSV) of 1.8 h−1 at 593 K. Products were detected online by Agilent 7890A. In this study, total conversions and BD selectivities were calculated by the following equations.
3 Results and Discussion
3.1 Properties of Silica Materials Supported ZrO2 Catalysts
Figure 1 shows low-angle XRD patterns of silica materials supported 2 wt% zirconium oxide catalysts. Both the ZrO2/NanoSiO2 and ZrO2/SiO2 (2 µm) catalysts exhibit patterns typical for amorphous materials without ordered mesoporous structure. There are three distinct peaks shown for ZrO2/SBA-15. These can be indexed as (1 0 0), (1 1 0) and (2 0 0). The low angle XRD patterns of ZrO2/MCM-41 show one major (100) reflection and two minor reflections corresponding to the (110) and (220) planes, pointing to a highly ordered hexagonal pore structure.
The structural properties of the catalysts are summarized in Table 1. Series of the silica support with zirconium results in decreasing of the surface area of the samples. This decrease may occur due to the formation of highly dispersed zirconium species. These species occupy a part of the internal and external surface of series silica particles. However, the average pore sizes of samples increase except ZrO2/SBA-15 and ZrO2/MCM-41 catalysts. These data increase in turn ZrO2/MCM-41, ZrO2/SiO2 (2 µm), ZrO2/SBA-15 and ZrO2/NanoSiO2. Most interesting, the BD selectivity also increases in this turn. The reasons maybe large pore makes both the reagent and product move freely in and out of the catalysts. These results indicate that the pore sizes are important factors determining the activity when catalyst contains a subtle balance of the acid and base, which is in good agreement with other recent studies [14, 15].
SEM images of series ZrO2/SiO2 samples are displayed in Fig. 2. ZrO2/NanoSiO2 shows relatively uniform particles shown in Fig. 2a. Figure 2b shows ZrO2/SBA-15, showing a hexagonal rod-like SBA-15 morphology. However, the ZrO2/MCM-41 and ZrO2/SiO2 (2 µm) samples show randomly amorphous particles and small sphere-like primary particles with particle diameters of around 2 µm, respectively (Fig. 2c and d).
Figure 3 displays the 29Si MAS NMR spectra for ZrO2 supported on silica materials. All of the ZrO2 supported silica catalysts show resonances in the range of − 108.5 ppm which are attributed to Q4 (Si (4Si)) species. Besides, for ZrO2/MCM-41 a shoulder at − 100 ppm points to the presence of Q3 (Si (OH, 3Si)) defects [17]. This means that the interactions between Zr and Si are weak in the ZrO2/SiO2 materials.
3.2 Catalytic Test for BD Production from Ethanol and Acetaldehyde
Table 2 shows catalytic performance of 2 wt% ZrO2 supported SiO2 catalysts in BD production at 593 K. Obviously, ZrO2/NanoSiO2 exhibited the best performance with the 91.43% BD selectivity. As discussed in the part of structural properties of the catalysts, BD yield increases in the turn of ZrO2/MCM-41, ZrO2/SiO2 (2 µm), ZrO2/SBA-15 and ZrO2/NanoSiO2, which is the same as pore sizes order of increasing. Thus, the higher total conversion and BD selectivity were associated with its larger pore sizes. It is supposed that pore sizes are important factors determining the activity when catalyst contains a subtle balance of the acid and base.
In our previous work [16], it was reported the ZrO2–SiO2 catalyst sample synthesized by sol–gel method contained the same ZrO2 content of 2 wt%, reaching the lower selectivity of 1,3-butaidene (69.7%), and the main difference between them is the average pore size of the catalyst. Jones et al. [14] and Chae et al. [15] also reported that the larger pore size of catalysts was a favorable factor to enhance the 1,3-butadiene selectivity. It could be supposed that the pore sizes parameter would become the important determining factors to improve the catalytic activity, when the catalyst surface contained appropriate active sites.
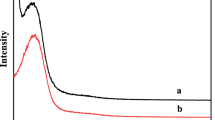
Finally, the catalytic durability was also preliminarily explored as shown in Fig. 4, which shows the longevity of ZrO2/NanoSiO2 catalyst. ZrO2/NanoSiO2 catalyst developed in this work has relatively longer catalyst life. In other words, besides initial BD selectivity and conversion, the long-term stability of catalytic performance of ZrO2/NanoSiO2 was also obtained. This may due to its larger pore size which enhances the resistance to coke formation.
4 Conclusions
A series of catalysts based on ZrO2–SiO2 have been prepared, characterized and tested for the formation of BD from ethanol and acetaldehyde. The most promising catalyst investigated is ZrO2/NanoSiO2 with the highest selectivity achieved being 91.43% and the total conversion obtained being 52.39%. The investigation also indicates that the pore sizes are more important factors determining the activity when catalyst contains a subtle balance of the acid and base. These effects of the pore size could be explained by the better accessibility of the reactants and products and the better coke tolerance of the catalysts.
References
Bozell JJ, Petersen GR (2010) Green Chem 12:539–554
Alvira P, Tomás-Pejó E, Ballesteros M, Negro MJ (2010) Bioresour Technol 101(13):4851–4861
John RP, Anisha GS, Nampoothiri KM, Pandey A (2011) Bioresour Technol 102(1):186–193
Llorca J, de la Piscina PR, Sales J, Homs N (2001) Chem Commun 7:641–642
Makshina EV, Dusselier M, Janssens W, Degrève J, Jacobs PA, Sels BF (2014) Chem Soc Rev 43:7917–7953
White WC (2007) Chem Boil Interact 166:10–14
Dunn JT, Toussaint WJ (1947) US Patent 2,421,361
Lebedev SV, Yakubchik AO (1929) J Chem Soc 1929:220–225
Ordomsky VV, Sushkevich VL, Ivanova II (2010) J Mol Catal A 333:85–93
León M, Díaz E, Ordóñez S (2011) Catal Today 164:436–442
Baerdemaeker TD, Feyen M, Müller U, Yilmaz B, Xiao FS, Zhang WP, Yokoi T, Bao XH, Gies H, De Vos DE (2015) ACS Catal 5:3393–3397
Quattlebaum WM, Toussaint WJ, Dunn JTJ (1947) Am Chem Soc 69:593–599
Corson B, Jones H, Welling C, Hinckley J, Stahly E (1950) Ind Eng Chem Res 42:359–373
Jones MD, Keir CG, Iulio CD, Robertson RAM, Williams CV, Apperley DC (2011) Catal Sci Technol 1:267–272
Chae H-J, Kim T-W, Moon Y-K, Kim H-K, Jeong K-E, Kim C-U, Jeong SY (2014) Appl Catal B 150:596–604
Han Z, Li X, Zhang MH, Liu ZZ, Gao MX (2015) RSC Adv 5:103982–103988
Wang XX, Lefebvre F, Patarin J, Basset JM (2001) Microporous Mesoporous Mater 42:269–276
Acknowledgements
The authors thank the key laboratory for green chemical technology of ministry of education and collaborative innovation center of chemical science and engineering, for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, M., Zhang, M. & Jiang, H. 1,3-Butadiene Production from Bioethanol and Acetaldehyde over Zirconium Oxide Supported on Series Silica Catalysts. Catal Surv Asia 22, 118–122 (2018). https://doi.org/10.1007/s10563-018-9243-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10563-018-9243-8