Abstract
In this research, M/Cs1.5H1.5PW/Al2O3 (M = Ni or/and Mo) nanocatalysts were prepared via 2 steps with the impregnation method and the effect of molybdenum on the characteristic and catalytic properties of the prepared samples was studied. The synthesized samples were characterized by X-ray diffraction (XRD), temperature programmed reduction, temperature programmed desorption (TPD), and energy dispersive X-ray spectroscopy techniques. Morphology of the samples was studied by field emission scanning electron microscope (FESEM) and surface area, pore volume and pore size of the compounds were measured by BET method. In the XRD patterns of the prepared catalysts, the H3PW12O40 phase was observed. The FESEM images showed that the synthesized particles were in nanoscale. The results of TPD studies indicated that the total acid site of Ni–Mo/Cs1.5H1.5PW/Al2O3 catalyst was more than the others. The catalytic activity of the nanocatalysts in hydrocracking of n-decane indicated that Ni–Mo/Cs1.5H1.5PW/Al2O3 nanocatalyst had the highest catalytic activity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The most popular heteropoly acids are those having the Keggin structure, H3PW12O40 (HPW). The main disadvantages of HPWs are high solubility in water and their very low surface area (less than 10 m2 g−1), which hinders accessibility to the acidic sites. In addition, the HPW has excessive acidity and an overhigh cracking activity, which increases the probability to undergo secondary reactions [1,2,3,4]. By substituting a fraction of the proton with monovalent ions such as Cs+, K+, Rb+ and Ag+, the heteropoly acids are turned into insoluble salts, which can be used as a reasonably good heterogeneous solid acid catalyst. The Cs-exchanged Keggin-type heteropoly acid is also well maintained at high temperatures, usually over 500 °C [5,6,7,8,9]. There are many studies about the effect of Cs content in HPW on the catalytic performance of these catalysts in various catalytic processes [8, 10]. H3PW12O40 and CsxH3−xPW12O40 compounds have been used as catalysts in hydrocracking of extra-heavy oils and reported the optimum Cs content (x = 2.2) which induces the best activity in this process [5]. For overcoming the low surface area of HPW, a feasible way is to prepare supported HPW catalysts. One approach to obtain supported HPW catalysts is the impregnation of a support with a heteropoly acid solution followed by evaporation of the solvent. Several supports, such as silica–alumina [11], silica gel [12], silica [13] and BEA zeolite [14] have been used to enhance the dispersion of the HPW. In this case, the accessibility to HPW acid sites has been increased, while its solubility in polar media decreased [15]. Extensive studies have been carried out over Cs salt of HPA supported on metal oxides for the hydrocracking reaction [4, 12, 16]. The effect of Cs content on the catalytic performance of the reduced Ni–CsxH3−xPW12O40/Al2O3 catalysts for hydrocracking of n-decane in the presence of thiophene and pyridine has been reported. The highest catalytic activity for these catalysts belongs to Ni–CsH2P12W40/Al2O3 catalyst [16]. In another work, the behavior of non-sulfided bimetallic Ni–Co–H3PW12O40/SiO2 catalysts has been reported. The results indicated the promotional effects of Co to hydrocracking activity of these catalysts [17]. Generally, hydrocracking supported catalysts are bifunctional, i.e. the acid sites which provide the cracking function and metal sites with a hydrogenation–dehydrogenation function [18]. Hence, active hydrocracking catalysts are composed of acidic supports such as SiO2, Al2O3, ZrO2, active carbon, modified zeolite etc., and active metals (e.g. Mo, W) with promoters (e.g. Ni, Co) [19,20,21,22,23].
Since molybdenum is an active phase in conventional catalysts, in this work, we have used both molybdenum and HPW for preparing new catalysts. Thus, the series of M/Cs1.5H1.5PW/Al2O3 (M = Ni or/and Mo) nanocatalysts by impregnation method have been prepared and the synthesized samples characterized with conventional techniques. The effect of molybdenum on the catalytic activity of the nanocatalysts in hydrocracking of n-decane has also been studied.
Experimental
In this work, we have prepared the M/Cs1.5H1.5PW/Al2O3 (M = Ni or/and Mo) nanocatalysts by impregnation and characterized the synthesized samples with conventional methods. The catalytic activity of the nanocatalysts in hydrocracking of n-decane has also been studied.
The samples were prepared via two steps with the impregnation method. First, the alumina support (Sasol Chemical Co., specific surface area 273 m2 g−1, 40–70 mesh) was impregnated with a solution containing the desired quantities of Ni(NO3)2 (BDH, 98%), (NH4)6Mo7O24·4H2O (Merck, 99%), and Cs2CO3 (Merck, 99.99%). Impregnated samples were dried overnight at 120 °C and then calcined at 400 °C for 3 h using a heating ramp of 2 °C min−1. In the second step, the obtained samples were impregnated with a solution containing the desired quantities of H3PW12O40 (Merck, 99%). The obtained products were finally dried overnight at 120 °C without calcination.
Powder X-ray diffraction (XRD) patterns were obtained on X’ Pert Pro diffractometer equipment with a copper anode (Cu Kα monochromatized radiation source, λ = 1.54056 Å). The surface area (BET), pore volume and pore size of the catalysts were measured using N2 at − 196 °C on an adsorption instrument (Quantasorb, Quantachrome). The morphologies and quantative analysis of the samples were determined by field emission scanning electron microscopy (FE-SEM) on TESCAN Mira3-XMU microscope equipped with energy dispersive X-ray spectroscopy (EDX). Temperature programmed desorption (TPD) and temperature programmed reduction (TPR) studies were performed using a semiautomatic micrometrics TPD/TPR 2900 apparatus to investigate the acid properties and reduction behavior of the catalysts, respectively.
The catalytic performance of the prepared catalyst was evaluated in a 300 ml batch type stainless steel autoclave reactor (452 HC reactor, Parr instrument). Schematic diagram of the experimental setup for hydrocracking of n-decane is shown in Fig. 1. The reaction test was carried out as follows: 20 g of n-decane (Merck, 99%) was charged into a reactor and 2 g of catalyst was added to the reactor. After the leak test of the reactor and purging with hydrogen (99.999%) for three times to remove air from the reactor, all the catalysts were reduced by a flow of H2 at 200 °C for 1 h during stirring at 300 rpm. Then the reactor was pressurized to 3 MPa with hydrogen and the reactor was heated to achieve the reaction temperature (300 °C), which took 4 h. The pressure and temperature were recorded continuously during the reaction using a data logging instrument (4842 reactor controller, Parr instrument). When reaction time elapsed, the reactor was rapidly quenched to the ambient temperature. The reactants and products were analyzed by a gas chromatograph (VARIAN model CP-3800) equipped with capillary column (100 m) and FID detector. For comparison, an industrial hydrocracking catalyst (NiMo/ASA) was applied for hydrocracking of n-decane under the same condition. Conversion of the hydrocracking process was calculated by Eq. 1 and selectivity of the catalyst to the desired product was obtained by Eq. 2.
here Pi is a desired product and Xi is mole number of Pi and nFeed is mole number of the feed.
Results and discussion
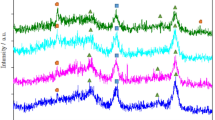
The XRD patterns of the M/Cs1.5H1.5PW/Al2O3 (M = Ni or/and Mo) samples are presented in Fig. 2. The comparison of the XRD patterns of the prepared catalysts indicate that the keggin structure of CsxH3-xPW phase (10.5°, 18°, 23.6°, 25.9°, 30°, 35.1°, 54.3° and 62.2°) (JCPDS No:50-1857) is observed in the patterns [24, 25]. It is clear that no diffraction peaks of NiO (JCPDS No:73-1519), MoO3 (JCPDS No:21-0569) and Al2O3 (JCPDS No:10-0425) appeared in the patterns. It is elucidated that Ni and Mo were dispersed well on the support and no segregation of those took place in impregnation process. The crystallite sizes of the prepared samples, calculated by the Scherrer equation using the intense peak (2θ = 26), are presented in Table 1. This table shows that the size of the prepared particles are in nanoscale.
Elemental analysis, surface area, pore volume and pore diameter of the samples are given in Table 1. The elemental composition of the prepared catalysts was examined via EDX. EDX analysis at different points on the samples also confirmed the uniform composition of the compound (Fig. 3). Table 1 also indicates that the surface area, pore volume and pore diameter of Al2O3 after impregnation decrease remarkably. It is reported that during impregnation the pore walls of Al2O3 can be eroded with acidic HPW aqueous solution and also the pores partially can be blocked with Ni/Mo species which lead to reducing the surface area of Al2O3 [12, 26, 27].
The FE-SEM images are indicated in Fig. 4. These images show that the synthesized particles are actually agglomerated and homogeneous nano-size crystallites are nearly in spherical shape.
To examine the acidic properties of the M/Cs1.5H1.5PW/Al2O3 (M = Ni or/and Mo) catalysts, NH3–TPD was carried out. According to the experimental work of Jiang and et al. the strength of acidic sites can be classified by NH3-desorption temperature as weak (150–350 °C), moderate (350–500 °C) and strong (≥ 500 °C) acidic sites [28]. The NH3–TPD profiles of the prepared samples are shown in Fig. 5. All profiles presented an intense peak which can be assigned to weak acidic sites. In addition, these profiles indicate that moderate and strong acidic sites exist in the samples. These results show that in Ni–Mo/Cs1.5H1.5PW/Al2O3 catalyst weak and moderate acidic sites are more than the other catalysts, while strong acidic site in Ni/Cs1.5H1.5PW/Al2O3 catalyst is more than the other ones. These results also confirm that total acid sites of Ni–Mo/Cs1.5H1.5PW/Al2O3 catalyst is more than the others (Table 2). Increasing total acid sites can be correlated to the formation of MoNiOx phase. This result is in agreement with the literature [29]. Meng et al. reported that in NiMo/Beta-KIT-6 catalysts, all NiMo based catalysts contained both weak and moderate acid sites in NiMo/BK catalysts.
The H2–TPR profiles of catalysts are shown in Fig. 6. It is interesting to notice that the reduction peak of NiO Bulk is not observed in these profiles. According to literature the NiO reduction peak appears at 300 °C [28]. Also, in the presence of H3PW species, NiO reduction occurs at higher temperature around 530 °C [16]. When Cs replaces with H in H3PW species, the interaction of NiO with these species is weaker and reduction occurs at lower temperature, so that the peaks observed at about 420–500 °C can be attributed to the reduction of Ni(II). The peak observed at about 490 °C for TPR profile of a sample could be assigned to reduction of Mo(VI) to Mo(IV) [30, 31]. This peak is also seen in Fig. 6b, which is covered by NiO reduction peak. The peaks at 680 °C and 710 °C can be attributed to different intermediate composition such as Al2(MoO4)3 (Fig. 6a) [32]. These peaks can also be seen in Fig. 6b containing Ni and Mo, although they are overlap with a strong peak in the 700 °C. This strong peak corresponds to the reduction of Ni(II) that interacts with the support [33]. The reduction peak near 740 °C corresponds to the reduction of Cs1.5H1.5PW species [14, 16]. It can be noticed from Table 2 that the H2 consumption per gram of catalyst in Ni–Mo/Cs1.5H1.5PW/Al2O3 catalyst is higher than that of for the other samples that indicate higher reducibility of catalyst. It seems that the higher amount of H2 consumption for Ni–Mo/Cs1.5H1.5PW/Al2O3 catalyst was related to MoNiOx phase which could be formed due to the reaction between Mo and Ni.
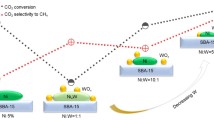
The catalytic evaluation results for the reduced M/Cs1.5H1.5PW/Al2O3 (M = Ni or/and Mo) catalysts in hydrocracking of n-decane are shown in Figs. 7 and 8 and Table 3. The catalytic performance of the prepared catalysts was compared with that of a typical NiMo/ASA industrial catalyst. By addition of Ni to the Mo/Cs1.5H1.5PW/Al2O3 catalyst, the conversion of n-decane increases. Classically, the middle acidity amount of a catalyst would favor the hydrocracking activity [14]. In the Ni–Mo/Cs1.5H1.5PW/Al2O3 catalyst, weak and moderate acidic sites are more than the other catalysts and amount of total conversion of this catalyst is more than the others.
As can be observed in Fig. 7, the hydrocracking activity of Mo/Cs1.5H1.5PW/Al2O3 catalyst is low. By partially and completed substitution of Ni for Mo in the Mo/Cs1.5H1.5PW/Al2O3 catalyst, the order of catalysts activity is: Ni–Mo/Cs1.5H1.5PW/Al2O3 > Ni/Cs1.5H1.5PW/Al2O3 > Mo/Cs1.5H1.5PW/Al2O3. Meanwhile, the activities of the prepared catalysts are more than activity of industrial catalyst. Furthermore, the order of the acidity of the catalysts is coincident with the order of catalytic activity. It can be concluded that higher acidity leads to higher catalytic activity. It is evident that the activity of the Mo/Cs1.5H1.5PW/Al2O3 catalyst with addition of Ni increased from 28.54% to 49.24% while, the activity of Ni/Cs1.5H1.5PW/Al2O3 catalyst is lower than that of Ni–Mo/Cs1.5H1.5PW/Al2O3 catalyst. This result could be due to interaction between Ni and Mo, which leads to increasing the reducibility of Ni in this catalyst. This result is also proved by TPR experiment. Similar results were reported by B. Qiu and et al. for enhancing the reducibility of Ni–H3PW/SiO2 catalyst by Co, which increases the hydrocracking activity of such bimetallic catalyst [17].
The distribution of the obtained products in the presence of the prepared catalysts for hydrocracking of n-decane is shown in Fig. 8. For all the prepared catalysts the main products is C6 hydrocarbons while the commercial catalyst leads to production of heavier hydrocarbon such as C10 hydrocarbons including 3-methyl C9, 2-3-dimethyl C8 and 3-3-4 trimethyl C7. For all samples the amounts of < C4 hydrocarbons are negligible and selectivity of the catalysts for these compounds are not considered in Fig. 8.
The production of C10 hydrocarbons in the presence of all the catalysts indicates that isomerization process is also occurred. However, this process is more notable for industrial catalyst. In addition, 94% of the C6 components are branched hydrocarbons which confirm the isomerization process. Also, according to the results, it can be explained that the Ni–Mo/Cs1.5H1.5PW/Al2O3 catalyst has higher selectivity to C6 products than the other catalysts. Thus, it is more likely that this catalyst produce middle distillate hydrocarbons in hydrocracking process. The use of the commercial catalyst resulted in a comparatively lower C6 product selectivity (2.8%) compared to that of Ni–Mo/Cs1.5H1.5PW/Al2O3 catalyst (58.6%). In fact, all of the prepared catalysts were more proper than the commercial catalyst for producing the middle distillate products (see Table 3). Generally, the hydrocracking process usually carried out using a metal/acid bifunctional catalyst [14], over which the alkanes are dehydrogenated-hydrogenated on the metallic sites and hydrocracked on the acidic sites. The hydrocracking of n-decane on the M/Cs1.5H1.5PW/Al2O3 (M = Ni or/and Mo) catalysts occurs through bifunctional mechanism as well, hydrocracking mechanism on the acidic sites and hydrogenation reaction mechanism on the metallic sites. According to the aforementioned discussion, the high activity of Ni–Mo/Cs1.5H1.5PW/Al2O3 catalyst for hydrocracking was apparently attribute to the unique structure of the HPW, which did not mere act as an acid site, but also cooperated with the hydrogenation metal site to act as hydrogen supply.
Conclusions
M/Cs1.5H1.5PW/Al2O3 (M = Ni or/and Mo) catalysts were successfully prepared by impregnation method and evaluated as catalyst for hydrocracking of n-decane. The obtained results indicated that using both molybdenum and nickel in these catalysts has an important role in chemical and physical properties and catalytic behavior of them in n-decane hydrocracking. The NH3-TPD results indicate that in Ni–Mo/Cs1.5H1.5PW/Al2O3 catalyst weak and moderate acidic sites are more than the other catalysts, the catalyst shows the highest activity. The best result was obtained on the Ni–Mo/Cs1.5H1.5PW/Al2O3 catalyst with the C6 selectivity of 58.5% and n-decane conversion of 49.24%. The results show that C6 and C10 component are branched hydrocarbons which confirm the isomerization process is also occurred during hydrocracking process. Moreover, the interaction between the metal (Ni or/and Mo) and the Cs1.5H1.5PW of the catalyst offers the sufficient reactive hydrogen on the surface of the catalysts.
References
Dai Y, Li BD, Quan HD, Lu CX (2010) [Hmim]3PW12O40: a high-efficient and green catalyst for the acetalization of carbonyl compounds. Chin Chem Lett 21:678–681
Sheng JY, Qiu B, Jin H, Yi XD, Fang WP (2010) Alumina supported H3PW12O40 and Ni catalysts for hydrocracking of n-decane. Asian J Chem 22:4439–4449
Bhure MH, Kumar I, Natu AD, Chikate RC, Rode CV (2008) Phosphotungstic acid on silica with modified acid sites as a solid catalyst for selective cleavage of tert-butyldimethylsilyl ethers. Catal Commun 9:1863–1868
Qiu B, Yi XD, Lin L, Fang WP, Wan HL (2008) The hydrocracking of n-decane over bifunctional Ni-H3PW12O40/SiO2 catalysts. Catal Today 131:464–471
Eom HJ, Lee D-W, Kim S, Chung S-H, Hur YG, Lee KY (2014) Hydrocracking of extra-heavy oil using Cs-exchanged phosphotungstic acid (CsxH3-xPW12O40, x = 1–3) catalysts. Fuel 126:263–270
Misono M (2013) Catalysis of heteropoly compounds (polyoxometalates). In: Makoto M (ed) Studies in surface science and catalysis. Elsevier, New York, pp 97–155
Zeng ZX, Cui L, Xue WL, Ma KN (2013) Study on adsorption behavior of 12-phosphotungstic acid on silica gel. Ind Eng Chem Res 52:8070–8078
Shin H-Y, An S-H, Sheikh R, Park YH, Bae S-Y (2012) Transesterification of used vegetable oils with a Cs-doped heteropolyacid catalyst in supercritical methanol. Fuel 96:572–578
Geboers J, Van de Vyver S, Carpentier K, Jacobs P, Sels B (2011) Hydrolytic hydrogenation of cellulose with hydrotreated caesium salts of heteropoly acids and Ru/C. Green Chem 13:2167–2174
Parida KM, Rana S, Mallick S, Rath D (2010) Cesium salts of heteropoly acid immobilized mesoporous silica: an efficient catalyst for acylation of anisole. J Colloid Interface Sci 350:132–139
De Mattos FCG, De Carvalho ENCB, Freitas EFD, Paiva MF, Ghesti GF, Macedo JLD, Dias SC, Dias JA (2017) Acidity and characterization of 12-tungstophosphoric acid supported on silica-alumina. J Braz Chem Soc 28:336–347
Jin H, Yi X, Sun X, Qiu B, Fang W, Weng W, Wan H (2010) Influence of H4SiW12O40 loading on hydrocracking activity of non-sulfide Ni–H4SiW12O40/SiO2 catalysts. Fuel 89:1953–1960
Jovic A, Bajuk-Bogdanovi D, Vasiljevi BN, Milojevi –Raki M, Krajisnik D, Dondur V, Popa A, Uskokovi-Markovi S, Holclajtner-Antunovi I (2017) Synthesis and characterization of 12-phosphotungstic acid supported on BEA zeolite. Mater Chem Phys 186:430–437
Jin H, Guo D, Sun X, Sun S, Liu J, Zhu H, Yang G, Yi X, Fang W (2013) Direct synthesis, characterization and catalytic performance of non-sulfided Ni–CsxH3-xPW12O40/SiO2 catalysts for hydrocracking of n-decane. Fuel 112:134–139
Haber J, Pamin K, Matachowski L, Mucha D (2003) Catalytic performance of the dodecatungstophosphoric acid on different supports. Appl Catal A 256:141–152
Jin H, Yi X, Sun S, Liu J, Yang G, Zhu H, Fan W (2012) Hydrocracking of n-decane over non-sulfided Ni-CsxH3-xPW12O40/Al2O3 catalysts. Fuel Process Technol 97:52–59
Qiu B, Yi X, Li Lin, Fang W, Wan H (2009) Influence of the incorporation of cobalt on non-sulfided Ni–H3PW12O40/SiO2 hydrocracking catalysts. Catal Commun 10:1296–1299
Thybaut JW, Laxmi Narasimhan CS, Denayer JF, Baron GV, Jacobs PA, Martens JA (2005) Acid-metal balance of a hydrocracking catalyst: ideal versus nonideal behavior. Ind Eng Chem Res 44:5159–5169
Cui Q, Zhou Y, Wei Q, Yu G, Zhu L (2013) Performance of Zr- and P-modified USY-based catalyst in hydrocracking of vacuum gas oil. Fuel Process Technol 106:439–446
Looi PY, Mohamed AR, Tye CT (2012) Hydrocracking of residual oil using molybdenum supported over mesoporous alumina as a catalyst. Chem Eng J 181–182:717–724
Tiwari R, Rana BS, Kumar R, Verma D, Kumar R, Joshi RK (2011) Hydrotreating and hydrocracking catalysts for processing of waste soya-oil and refinery-oil mixtures. Catal Commun 12:559–562
Leyva C, Rana MS, Trejo F, Ancheyta J (2009) NiMo supported acidic catalysts for heavy oil hydroprocessing. Catal Today 141:168–175
Leyva C, Rana MS, Trejo F (2007) On the use of acid-base-supported catalysts for hydroprocessing of heavy petroleum. Ind Eng Chem Res 46:7448–7466
Corma A, Martinez A, Martinez C (1996) Acidic Cs+, NH4 +, and K+ salts of 12-tungstophosphoric acid as solid catalysts for isobutane/2-butene alkylation. J Catal 164:422–432
Narasimharao K, Brown DR, Lee AF, Newman AD, Siril PF, Avener SJ (2007) Structure–activity relations in Cs-doped heteropolyacid catalysts for biodiesel production. J Catal 248:226–234
Ameen M, Azizan MT, Ramli A, Yusup S, Yasir M (2016) Physicochemical properties of Ni-Mo/γ-Al2O3 catalysts synthesized via sonochemical method. Procedia Eng 148:64–71
Liu HP, Lu GZ, Guo Y, Wang YQ, Guo YL (2009) Synthesis of mesoporous Pt/Al2O3 catalysts with high catalytic performance for hydrogenation of acetophenone. Catal Commun 10:1324–1329
Jiang J, Dong Z, Chen H, Sun J, Yang Ch, Cao F (2013) The Effect of additional zeolites in amorphous silica–alumina supports on hydrocracking of semirefined paraffinic wax. Energy Fuels 27:1035–1042
Meng J, Wang Z, Ma Y, Lu J (2017) Hydrocracking of low-temperature coal tar over NiMo/Beta-KIT-6 catalyst to produce gasoline oil. Fuel Process Technol 165:62–71
Solı´s D, Agudo AL, Ramı´rez J, Klimova T (2006) Hydrodesulfurization of hindered dibenzothiophenes on bifunctional NiMo catalysts supported on zeolite–alumina composites. Catal Today 116:469–477
Qu L, Zhang W, Kooyman PJ, Prins R (2003) MAS NMR, TPR, and TEM studies of the interaction of NiMo with alumina and silica–alumina supports. J Catal 215:7–13
Brito J, Laine J (1986) Characterization of supported MoO3 by temperature programmed reduction. Polyhedron 5:179–182
Marzari JA, Rajagopal S, Miranda R (1995) Bifunctional mechanism of pyridine hydrodinitrogenation. J Catal 156:255–264
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amirmoghadam, H., Sadr, M.H., Aghabozorg, H. et al. The effect of molybdenum on the characteristics and catalytic properties of M/Cs1.5H1.5PW12O40/Al2O3 (M = Ni or/and Mo) nanocatalysts in the hydrocracking of n-decane. Reac Kinet Mech Cat 125, 983–994 (2018). https://doi.org/10.1007/s11144-018-1456-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-018-1456-3