Abstract
This study compares the tracking of bisphenol A (BPA) degradation by three analytical techniques: liquid chromatography mass spectrometry (HPLC–MS), ultraviolet/visible (UV–Vis) spectroscopy and dissolved oxygen (DO) content. In each evaluation method, the photochemical behavior of TiO2 and N–TiO2 under visible light confirmed the relationship between the adsorption and photodegradation processes, with the reaction analysis indicating the possibility of two photocatalytic mechanisms: conventional photocatalyzed radical oxidation and lattice oxygen driven oxidation. The first one concerns HPLC, where the average half-lives (t1/2) were 38 min (5% N–TiO2), 60 min (1% N–TiO2) and 64 min (TiO2), these values vary in 12% if compared with the values obtained by UV–Vis spectroscopy. The second mechanism showed a decrease in the initial concentration by more than 50% (8 mg L−1) after 3 h. The solution of doped photocatalysts is tracked best by DO measurement. The results presented here confirm that the efficiency of photocatalytic oxidation (EPO) of a reaction is highly related to the DO content, given that dissolved O2 proactively causes the release of radicals on the surface of excited materials under the action of visible light, thus increasing the BPA degradation rate. The quantum yield of BPA disappearance was below 0.1 for all the materials.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water quality has deteriorated globally, and the preservation of clean water is one of the most important topics worldwide [1]. Nowadays, various toxic organic and inorganic compounds have been detected at critical levels in waste, ground and surface water [1]. At the same time, epidemiological studies have reported the increasing presence of endocrine disruptors in the environment and their potential role in the incidence of metabolic diseases [2–4]. Both of these reasons justify the complete removal of this type of substance from water using tertiary treatments, which has led to the development of various biochemical, electrochemical, sonochemical and membrane processes, as well as photochemical reactions [5].
Photocatalytic processes based on the use of TiO2 or its combination with other materials having improved properties under solar irradiation can be considered a sustainable and energy-efficient solution [6–11]. However, it is necessary to involve other parameters in the process, such as DO concentration, water temperature and the wide variety of compounds existing in the water, and accurate results are required for the large-scale application of this technology. Tracking a reaction with DO is a feasible and reliable alternative, as oxygen plays a key role in photocatalytic reactions by inducing simultaneous oxidation and reduction processes [6, 8].
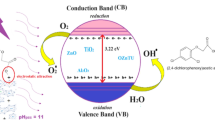
It is well known in heterogeneous photocatalysis that reactions should be preferentially carried out with a steady, external supply of air, but currently, the cost of aeration in treatment plants represents more than 60% of the operation expense for the entire process, without considering the environmental impact from energy consumption [12]. However, it is necessary to ensure that the O2 concentration does not become the rate-limiting factor. The presence of oxygen has three major roles in photocatalysis [8, 9]. The first is associated with semiconductor excitation (e.g. N–TiO2) and the resulting oxygen reduction, which facilitates the separation of electron–hole charges (e−, h+). In the second role, O2 accepts electrons generated on the TiO2 surface and is reduced to \({\text{O}}_{2}^{ - }\), H2O2, OH radicals and Ti–O. These active elements on the material surface promote the photocatalytic oxidation of organic compounds. Finally, during oxidation, O2 combines with organic radicals, which are generated by a reaction between the holes and the reactant, producing oxygen adduct intermediates, such as organoperoxy radicals [6, 8].
The aim of this paper is to show that the monitoring of DO is an alternative for tracking BPA degradation in water treatment plants that use TiO2 and N–TiO2 (N = 1 and 5 wt%) photocatalysts under visible light. For this purpose, a kinetic study was undertaken on photocatalyst performance during the photocatalytic BPA reaction, with measurements taken using different spectrophotometric methods (HPLC and UV–Vis) and a dissolved O2 probe.
Experimental
TiO2 preparation
TiO2 was synthesized using a modified sol–gel method, in accordance with the procedure reported elsewhere [10]. To obtain nanostructured TiO2 spheres, a hydrolysis process was used, where 1 mL of tetrabutylorthotitanate (TNBT, Fluka, 97%) was mixed with 100 mL of ethylene glycol (EG, Fisher) in an inert environment (N2) to avoid effects of humidity during hydrolysis. This solution was magnetically stirred for 20 h at room temperature. Then, 98 mL of acetone was mixed with 2 mL of distilled water and added to the first solution to precipitate the TiO2 nanoparticles. The TiO2 nanoparticles were washed and re-dispersed in an ultrasonic bath of ethanol/water (1:1 v/v) solution. This process was repeated twice, followed by centrifugal separation to remove excess ethylene glycol from the surface of the particles. Finally, the material was dried at 80 °C for 16 h and annealed in air at 400 °C for 4 h.
TiO2 doped with nitrogen
The titanium oxide samples were doped with 1 and 5 wt% nitrogen using the methodology previously reported [10]. This study used melamine as the nitrogen precursor, diluting it with ethylene glycol in an inert environment under vigorous stirring, after which TNBT was added, and the solution was magnetically stirred for 20 h at room temperature. To precipitate the TiO2 nanoparticles, 98 mL of acetone was mixed with 2 mL of distilled water and added to the first solution. The steps for the recovery, washing and annealing of doped TiO2 were similar to those previously performed on TiO2.
Characterization
The structural properties of TiO2 and TiO2 doped with nitrogen were determined by X-ray diffraction (XRD) using a Rigaku Miniflex diffractometer at a scanning rate of 0.05°/s over a 2θ range of 10–70°. X-ray photoelectron spectroscopy analysis (XPS) was conducted with an Intercovamex XPS 1100 instrument to reveal the presence of dopant in the TiO2 structure. The physical adsorption of N2 at −196 °C was performed using a Quantachrome Autosob-1C analyzer to measure the specific surface areas and adsorption–desorption isotherms [13]. Reflectance spectra of the solids were obtained in air and at room temperature with a Perkin Elmer Lambda 35 UV–Vis spectrophotometer. The bandgap was calculated by measuring the diffuse reflectance of TiO2 using the Kubelka–Munk reflectance transfer model [11]. The total organic carbon was measured using a TOC-VCSN analyzer (Shimadzu).
Photocatalytic degradation of emerging compound
The photocatalytic activity of the sample was determined by monitoring BPA decomposition at room temperature under visible light. In a dark box, a stirred solution was irradiated with a GaI3 250 W lamp, which emits visible light (quantum flux at 2886 mmol m−2 s−1) [14, 15]. The quartz lamp was placed in a refrigerated vessel containing the reactant solution (30 ppm BPA in 200 mL) and 100 mg of photocatalyst. The average pH on the reaction solutions was 5.5 ± 0.2. To achieve saturation of DO and ensure the complete adsorption of BPA onto the semiconductor, a 1 mL s−1 flux of dry air was bubbled for 60 min before the light source was turned on. The photocatalytic activity of BPA was calculated from the adsorption band at 275 nm as a function of irradiation time using a Perkin Elmer Lambda 35 UV–vis spectrophotometer. A complementary test for the photochemical reaction of BPA decomposition was conducted by liquid chromatography mass spectrometry (HPLC–MS) using an Agilent Model 6420 system with acetonitrile as the carrier.
EPO
During the photocatalytic degradation of BPA, the quantum flux was measured with an MQ-200apogee model, and the DO was measured for each material using an Orion 087003 DO probe coupled to a Thermo Scientific Orion Star A325 cell. The evolution of DO was related to the EPO using Eq. 1, as detailed below [16]:
Here E is the efficiency of photocatalytic oxidation (mg W−1 h−1), C is the demand for dissolved oxygen (C° = 8.20 mg L−1), V is the solution volume (0.2 L−1), I corresponds to the irradiation (0.0684 Mw cm−2), s is defined as the irradiated surface (65.9 cm2), and t is the exposition time (h).
Quantum yield
The disappearance quantum yield was calculated according with the methodology of [17] with the assumption that the optical density in the medium is low, the decrease in light intensity thereby negligible and the average wavelength is 410 nm [17]. The quantum yield (Φ) is defined as the ratio between the number of reacted molecules per unit time and unit volume (in this case see Eq. 4) and the total number of photons absorbed per unit time and unit volume:
The parameters calculated for total amount of absorbed photons I a, at overage wavelength 410 nm and for time t (s) were then: I 0 , the intensity of the incoming light (mol L−1 s−1), I the outgoing light intensity (mol L−1 s−1), ε λ the molar absorption coefficient, (L mol−1 cm−1), l is the cell path length (cm) and C the concentration of BPA (mol L−1).
Results and discussion
Physicochemical characterization
XRD
Fig. 1 shows the XRD analysis results of both TiO2 and TiO2 doped with nitrogen, prepared by the modified sol–gel method and annealed at 400 °C for 4 h. The diffractogram confirmed the presence of anatase crystals with an orthorhombic structure, whose characteristic reflections were recorded at angles of 2θ = 25.73, 37.54, 38.44, 39.10, 48.45, 54.78, 56.01, 63.13, 63.81, and 70.01°, according to card COD-96-101- 0943. There is no evidence of major structural changes in the doped materials given that the TiO2 reflections are the same; however, the level of crystallinity was affected, as evidenced by an increase in the peak width of the 5% N–TiO2 material a trend that has been reported by other authors [18–20]. A detailed description of those diffractograms is given in Fig. S1 of the supplemental material.
XPS spectroscopy
Fig. S2 (supplementary material) shows the XPS results for TiO2 and TiO2 doped with nitrogen (1% N–TiO2 and 5% N–TiO2), which was prepared by the modified sol–gel method and annealed at 400 °C. Three regions in the XPS spectra were examined: the N 1 s region at approximately 400 eV (Fig. S2-I), the Ti 2p region at approximately 458 eV (Fig. S2-II), and the O 1 s region at approximately 532.5 eV (Fig. S2-III). There are important differences in these three regions among the synthetized materials. The binding energy peak for N 1 s is greater than the typical binding energy for TiN (397.2 eV), and therefore, could be associated with interstitial nitrogen in the form of NOx [21, 22], which causes defects between the valence band and conduction band [23].
Other authors have reported that nitrogen impurities can induce the excitation of electrons in the bandgap, thus forming a new adsorption band [24, 25]. The Ti 2p signal for non-doped TiO2 was found at 1.2 eV below the assigned binding energy for TiO2. This shift is probably attributed to trace carbon from the synthesis method. Meanwhile, a clear shift toward lower binding energy was observed for the doped materials, indicating the successful incorporation of nitrogen into the TiO2 lattice [7, 22]. Finally, the three samples show O 1 s peaks at 529 eV, related to structural oxygen, and 532 eV, corresponding to oxygen adsorbed on the surface [26].
UV–Vis spectroscopy
The bandgap of both TiO2 and TiO2 doped with nitrogen was measured using the diffuse reflectance technique, after which an adjustment was performed by means of the Kubelka–Munk equation, as reported in a previous paper [10].
According to the results shown in Table 1, a 3.2 eV bandgap was observed for TiO2, which corresponds to reports in the literature [7, 10, 27]. The doped solid samples of 1% N–TiO2 and 5% N–TiO2 presented a significant bandgap shift in proximity to the visible region (~3.05 eV), showing similar behavior to the non-doped sample.
Nitrogen physisorption
Table 1 summarizes the surface area results for the materials with an anatase crystalline structure prepared using the modified sol–gel method. It is observed that while TiO2 showed a specific surface area of 60 m2 g−1, it was slightly modified to 56 m2 g−1 when nitrogen was added to the structure, constituting a variation of less than 10%. On the other hand, for the 5% N–TiO2 sample, the specific surface area was twice that of the other samples. The modification of the morphology and porosity of the TiO2 nanoparticles [6, 18, 26] caused these variations.
The adsorption and desorption isotherms for the materials used in this study are Type IV, as shown in Fig. S3. This type of isotherm is characteristic of mesoporous solids, in which the adsorption is produced in multilayers and reflected in a central zone of the ascending isotherm to the extent that more layers are adsorbed onto the solid surface, clearly describing the surface changes (desorption) that exist in the doped materials, as shown in the hysteresis of the material.
Photocatalytic activity of emerging compound
UV–Vis spectroscopy
This paper measured the performance of TiO2 and TiO2 doped with nitrogen using a modified sol–gel method during the photocatalytic degradation of BPA under visible light. Fig. S4 shows the evolution of the UV–Vis spectrum during the photocatalytic degradation of BPA in a 30 ppm solution over 180 min. The presence of a maximum peak in the adsorption band of BPA at 275 nm is clearly visible, which has been reported by other authors as well [27, 28]. The figure clearly shows that the spectral intensity decreases along with the contact time of the catalyst in visible light, which is evidence of BPA degradation.
Fig. 1 shows the photocatalytic behavior an increase in the activity of 60% of TiO2 after 180 min of exposure to visible light is observed with similar trend when 1% N–TiO2 (78%) is used. Nevertheless, the most active catalyst is 5% N–TiO2 (90%) reached after 120 min of reaction. On the other hand, Fig. 1 shows the calculation of kinetic parameters calculated by exponential model showed in Eq. 4 [29–31].
In this equation, X is the concentration of [BPA] at different times (mg L−1), k is the first order rate constant (min−1), whereas E is called the endpoint of the process (mg L−1). According with non-linear model for the BPA photodegradation, the rate constants reaction were calculated for the different catalyst obtaining the next results k5% N-TiO2 = 0.0162 min−1, k1% N-TiO2 = 0.0130 min−1 and kTiO2 = 0.0090 min−1. The calculated half-lives values are t1/2 = 43, 53 and 76 min for 5% N–TiO2, 1% N–TiO2 and TiO2. It is worth mentioning that although the regression coefficients were around 0.9881, the three experimental data showed that the standard deviations were not significant being located in the range 0.0011 < σk < 0.0023 (see Table 2) [31].
DO measurement
During the BPA degradation reaction described above, the DO consumption was analyzed, as described in the experimental section. Fig. 2a shows the photolysis of the BPA solution, which did not present any variation over the 3 h interval. With the addition of catalysts to the three materials, a greater consumption of DO was noted, with the highest consumption for the 5% N–TiO2 sample of 4 mg L−1 in the first hour. For 1% N–TiO2, the same level of oxygen consumption took 120 min, while finally, the O2 consumption of the non-doped TiO2 photocatalyst was very slow, recording a total consumption of less than 2.5 mg L−1 over the entire reaction time. The photocatalytic stability was also measured during the BPA degradation reaction using 5% N–TiO2, as shown in Fig. 2b. This figure shows that the catalytic activity is the same after two cycles, it means the rate of O2 evolution in the second run with intermittent evacuation was unchanged compared to the first run, and the reaction continued to proceed steadily with consume of oxygen around 85%.
Evolution of DO consumption during photodegradation reaction of BPA using different photocatalysts of TiO2 and TiO2 doped with nitrogen under action of visible light. [CO2,0] = 8 mg L−1, V = 0.2 L−1, mcatalyst = 100 mg. a DO O2 vs. time, b 5%N-TiO2, c 1/t vs. 1/[XO2 Dissolved], d Kinetic model of Langmuir Hishelwood for DO
Equation 3 represents the conventional Langmuir–Hinshelwood mechanism.
Here rmax is the maximum DO consumption and KD is the equilibrium constant for the adsorption reaction. When the adsorption sites are fully occupied by reactant molecules, rmax is equivalent to rDO [8].
By plotting the inverse of the DO decay concentration against the inverse of the time (see Fig. 2c). A linear relationship can be detected among the different materials; nevertheless, rmaxOD and the apparent constant of the material tends to approach zero as the nitrogen content decreases (K5% N–TiO2 = 0.1023 min−1 > K1% N–TiO2 = 0.0044 min−1 > KTiO2 = 0.0038 min−1), as shown in Table 2. The regression coefficients were around 0.999 for the doped materials nevertheless the three experimental data showed that the standard deviations were not significant being located in the range 0.276 < σk < 0.001 (see Table 2). The adjustment of the apparent constants using the Langmuir–Hinshelwood kinetics model is shown in Fig. 2d. This image agrees with previous reports [8, 32]. The kinetic parameters for the less active materials tend to approach zero (1/rOD < 0.0610 (mg L−1)−1) reflecting the worse adsorption equilibrium of photocatalysis, while the doped material 5% N–TiO2 [1/rOD = 0.1 (mg L−1)−1] presented the best availability to adsorb oxygen produced by oxygen vacancies generated by nitrogen doping [8].
DO measurements can also be used to define the EPO in contact with the solution per watt of energy (see Eq. 1) as shown in Fig. 3. The 5% N–TiO2 photocatalyst attained the best performance (150 W mg−1 L−1) in the first 60 min of reaction, while 1% N–TiO2 and TiO2 were less sensitive to optical phenomena and reached their maximum value in the second hour, recording 90 and 59 W mg−1 L−1, respectively. These behaviors correspond to the BPA degradation reaction observed using UV–Vis spectroscopy, the results of which are described above. These results show that a 50% reduction in BPA concentration occurs after 60 min of reaction using the 5% N–TiO2 photocatalyst.
Table 3 shows the quantum yields of BPA disappearance under visible light using TiO2 and N–TiO2 prepared by modified sol–gel method. No major differences between the disappearance quantum yield of TiO2 and 1% N–TiO2 at pH 5.5 were observed, while the disappearance quantum yield of 5% N–TiO2 was two times higher. That value are according with previous reports for BPA [15].
At this point, it is necessary to compare the relationship between the adsorption and photodegradation processes, as the reaction analysis indicates the possibility of two photocatalytic mechanisms: conventional photocatalyzed radical oxidation and lattice oxygen driven oxidation [33, 34]. The former involves the formation of both h+ and ·OH species on the surface of the catalyst, which are associated with the reduction of DO during the photocatalytic process [9], while in the solid phase, the structural and optical properties play an important role in the bandgap [30]. In addition, the amphoteric character of TiO2 can lead to a change in the concentration of H+ and OH− when nitrogen is added, thus modifying the surface charge [35]. A negative charge on the surface indicates that cationic BPA ions have been efficiently adsorbed on the surface [35, 36].
HPLC–MS
Fig. 4 shows the evolution over time of BPA conversion using doped and non-doped titanium oxides. The results obtained with this technique show similar behavior to the photocatalytic assays analyzed with UV–Vis spectroscopy. The 5% N–TiO2 photocatalyst achieved conversions greater than 90% after 3 h of light exposure. This was followed by the 1% N–TiO2 photocatalyst, which reached a conversion level of 80% over the same time period, and finally, by TiO2, with a conversion of 65%.
The kinetic non-linear model summarized in Table 2 corroborates that the different photocatalysts present a behavior with rate constants of k5% N–TiO2 = 0.0181 min−1, k1% N–TiO2 = 0.0114 min−1 and kTiO2 = 0.0108 mg L−1 min−1. The average half-lives obtained are t1/2 = 38 min, t1/2 = 60 min and t1/2 = 64 min for 5% N–TiO2, 1% N–TiO2 and TiO2, respectively. The regression coefficients were around 0.9724.
Table 2 confirms that the percentage of TOC removal for the 5% N–TiO2 sample was >85% after 3 h. All samples reached complete mineralization after 24 h. These efficiencies were comparable with the results for materials reported by Subagio [35], which had similar surface areas. While the identification of intermediates produced by the oxidation reaction is beyond the scope of this study, some mechanisms have been proposed for the photocatalytic degradation of BPA. According to the literature, the reaction could be initiated by ·OH attacking the electron-rich C3 in the phenyl group [35, 37]. This reaction is followed by the cleavage of the phenyl group into 4-isopropanolphenol, 4-isopropylphenol, 4-isopropenylphenol or 4-hydroxyacetonphenol, either sequentially or directly through the formation of transient intermediates or radicals [•H and •C(CH3)2C6H4OH)]. In addition, immediate ring cleavage caused by OH− attacking BPA to form 3-hydroxy-1,3,5-hexadiene has been previously proposed for N–TiO2 [35, 37]. However, according to CAS, in all cases, the byproducts are non-toxic [38].
Table 2 shows a summary of the kinetic constants and the half-lives of BPA degradation during the photocatalytic assays analyzed by UV–Vis spectroscopy and HPLC–MS. The rate constants show a negligible difference between the most active samples identified by both analyses (less than 10%). However, at lower degradation values, the difference is more sensitive for HPLC–MS analysis, which identified a value 10% smaller than that obtained by UV–Vis spectroscopy. This difference is probably due to the higher perturbation of the optical phenomena.
Finally, Fig. 5 shows the concentration profile of BPA, as identified by HPLC–MS, UV–Vis spectroscopy and DO content over time, using the 5% N–TiO2 photocatalyst under visible light. This figure reveals similar behavior regardless of the measurement method, which can be considered good evidence that degradation reactions can be tracked by measuring the DO content.
Conclusion
The physicochemical characterization of the materials, prepared with a modified sol–gel method and annealed at 400 °C, shows the resulting TiO2 to be in the anatase phase with a specific surface area of 60 m2 g−1 and a bandgap of 3.2 eV. These properties are modified, enhancing their activation in the visible region, with the addition of 1 and 5% of nitrogen.
Tracking BPA degradation by three different methods (UV–Vis spectroscopy, HPLC–MS and dissolved oxygen content) showed non-significant standard deviations in the range (0.0011 < σk < 0.0023 and 0.0015 < σk < 0.0039) for UV–Vis spectroscopy and HPLC–MS. After the adjustment of the spectrophotometer results to a kinetic model, the photocatalysts showed the following average half-lives: 38 min (5% N–TiO2), <60 min (1% N–TiO2) and <64 min (TiO2).
The Langmuir–Hinshelwood model showed the ability of BPA adsorption on the surface (5% N–TiO2), which improve the rate of reaction for dissolved O2.
The results presented here confirm that the quantum efficiency of a reaction is highly related to the dissolved oxygen content, given that dissolved O2 proactively causes the release of radicals on the surface of excited materials under the action of visible light, thus increasing the BPA degradation rate.
Measurement of the DO concentration was implemented as an alternative for confirming BPA mineralization, leading to the observation that the presence of oxygen is a key factor in the development of an advanced oxidation process, such as heterogeneous photocatalysis. Although the use of semiconductor material causes activity in the visible region of the electromagnetic spectrum, it is important to measure and maintain the DO concentration to assure complete mineralization.
References
Bhatnagar A, Sillanpäa M, Witek-Krowrak A (2015) Agricultural waste peels as versatile biomass for water purification—a review. Chem Eng J 270:244–271
Lorber M, Schecter A, Paepke O, Shropshire W, Christensen K, Birnbaum L (2015) Exposure assessment of adult intake of bisphenol A (BPA) with emphasis on canned food dietary exposures. Environ Inter 77:55–62
Carlson JC, Stefan MI, Parnis JM, Ch D (2015) Direct UV photolysis of selected pharmaceuticals, personal care products and endocrine disruptors in aqueous solution. Water Res 84:350–361
Chao-Yin K, Chung-Hsin W, Jui-Tai W, Yu-Ren C (2015) Synthesis and Characterization of a phosphorus-doped TiO2 immobilized bed for the photodegradation of bisphenol A under UV and sunlight irradiation. Reac Kinet Mech Cat 114:453–766
Kralchevska R, Milanova M, Bistan M, Pintar A, Todorovsky D (2013) Photocatalytic degradation of some endocrine disruptin compounds by modified TiO2 under UV or halogen lamp illumination. Reac Kinet Mech Cat 109:355–373
Turchi CS, Ollis DF (1990) Photocatalytic degradation of organic water contaminants: mechanisms involving hydroxyl radical attack. J Catal 122(1):178–192
Manzo-Robledo A, Cruz López A, Flores Caballero AA, Zaldivar Cadena AA, López M, Vázquez Cuchillo O (2015) Photoelectrochemical properties of sol–gel synthesized titanium dioxide nano-particles using different acids: X-ray photoelectron spectroscopy reveals the induced effect of hydrolysis precursor. Mater Sci Semi Proc 31:94–99
Hirakawa T, Koga C, Negishi N, Takeuchi K, Matsuzawa S (2009) An approach to elucidating photocatalytic reaction mechanisms by monitoring dissolved oxygen: effect of H2O2 on photocatalysis. Appl Catal B 87:46–55
García-Fernández I, Fernández-Calderero I, Polo-López MI, Fernández-Ibañez P (2015) Disinfection of urban effluents using solar TiO2 photocatalysis: a study of significance of dissolved oxygen, temperature, type of microorganism and water matrix. Catal Today 240:30–38
Del Ángel-Sánchez K, Vázquez-Cuchillo O, Salazar-Villanueva M, Sánchez Ramírez JF, Cruz-López A, Aguilar-Elguezabal A (2011) Preparation, characterization and photocatalytic properties of TiO2nanostructured spheres synthesized by the Sol-Gel method modified with ethylene glicol. J Sol–Gel Sci Technol 38:360–365
Yunjin Y, Jiacheng Q, Chen H, Fengyu W, Xueting L, Jianlong W, Shaobin W (2015) one-pot approach for synthesis of N-doped TiO2/ZnFe2O4 hydbrid as an efficient photocatalyst for degradation of aqueous organic pollutants. J Hazard Mater 291:28–37
Pablo-Romero MP, De Jesús J (2016) Economic growth and energy consumption: the Energy-Environmental Kuznets Curve for Latin America and the Caribbean. Renew Sustain Energy 60:1343–1350
Zhang YZ, Song XF, Kondoh A, Xia J, Tang ChY (2011) Behavior, mass inventories and modeling evaluation of xenobiotic endocrine-disrupting chemicals along an urban receiving wastewater river in Henan Province, China. Water Res 45:292–302
Kitsinelis S, Zissis G, Fokitis E (2009) A strategy towards the next generation of low pressure discharge lamps: lighting after mercury. J Phys D 42:1–8
Klauson D, Gromyko I, Dedova T, Pronina N, Krichevskaya M, Budarnaja O, Oja Acik I, Volobujeva O, Sildos I, Utt K (2015) Study on photocatalytic activity of ZnO nanoneedles, nanorods, pyramids and hierarchical structures obtained by spray pyrolysis method. Mater Sci Semi Proc 31:315–324
Del Ángel-Sanchez K, Vázquez-Cuchillo O, Aguilar-Elguezabal A, Cruz-López A, Herrera-Gómez A (2013) Photocatalytic degradation of 2,4-dichlorophenoxyacetic acid under visible light: effect of synthesis route. Mater Chem Phys 139:423–430
Ericksson J, Rahm S, Green N, Bergman Ǻ, Jakobsson E (2004) Photochemical transformation of tetrabromobispehnol A and related phenols in water. Chemosphere 54(2004):117–126
Xie Y, Zhao X, Li Y, Zhao Q, Zhou X, Yuan Q (2008) CTAB-assisted synthesis of mesoporous F-N-codoped TiO2 powders with high visible-light-driven catalytic activity and adsorption capacity. J Solid State Chem 181:1936–1942
Dong F, Zhao W, Wu Z, Guo S (2009) Band structure and visible ligbt photocatalytic activity of multi.type nitrogen doped TiO2 nanoparticles prepared by termal descomposition. J Hazard Mater 162:763–770
Di Valentin C, Finazzi E, Pacchioni G, Selloni A, Livraghi S, Paganini MC, Giamello E (2007) N-doped TiO2: theory and experiment. Chem Phys 339:44–56
Lee S, Cho I-S, Lee DK, Kim DW, Noh TH, Kwak CH, Park S, Hong KS, Lee J-K, Jung HS (2010) Influence of nitrogen chemical states on photocatalytic activities of nitrogen-doped TiO2 nanoparticles under visible light. J Photochem Photobiol A 213:129–135
Chen X, Burda C (2004) Photoelectrons investigation of Nitrogen-doped titania nanoparticles. J Phys Chem B 108:15446–15449
Ashi R, Morikawa T, Ohwaki T, Aoki K, Taga Y (2001) Visible-light photocatalysis in nitrogen-doped titanium oxide. Science 239:269–271
Barolo G, Livraghi S, Chiesa M, Paganini MC, Giamello E (2012) Mechanism of the photoactivity under visible light of N-doped titanium dioxide. Charge carriers migration in irradiated N-TiO2 investigated by electron paramagnetic resonance. J Phys Chem C 116(39):20887–20894
Hoang S, Berglund SP, Hahn NT, Bard AJ, Mullins CB (2012) Enhancing visible light photo-oxidation of water with TiO2 nanowire arrays via co-treatment with H2 and NH3: synergistic effects between Ti3+ and N. J Am Chem Soc 134(8):3659–3662
Frontistis Z, Daskalaki VM, Katsaounis A, Poulios I, Mantzavinos D (2011) Electrochemical enhancement of solar photocatalysis: degradation of endocrine disruptor bisphenol-A on Ti/TiO2 films. Water Res 45:2996–3004
Hou CH, Huang S-H, Chou PH, Den W (2015) Removal of bisphenol A from aqueous solutions by electrochemical polymerization on a carbon aerogel electrode. J Taiwan Inst Chem Eng 51:103–108
Tsai W-T, Lee M-K, Su T-S, Chang YM (2009) Photodegradation of bisphenol-A in a batch TiO2 suspension reactor. J Hazard Mater 168:269–275
Levenspiel O (2015) Ingeniería de las reacciones químicas. Limusa, Mexico
Lente G (2016) Editorial. React Kinet Mech Cat 119(1):3–4
Lente G (2015) Deterministic kinetics in chemistry and systems biology. The dinamics of complex reaction networks. Spinger, Berlin
Yanxiang L, Xujie L, Feng W, Nansheng D (2011) Adsorption and photooxidation of pharmaceuticals and personal care products on clay minerals. Reac Kinet Mech Cat 104:61–73
Ali AM, Emanuelsson EAC, Petterson DA (2011) Conventional versus lattice photocatalysed reactions: implications of the lattice oxygen participation in the liquid phase photocatalytic oxydation with nanostructired ZnO thin film on reaction products and mechanism at both 254 and 340 nm. Appl Catal B 106:323–336
Miyauchi M, Ikezawa A, Tobimatsu H, Irie H, Hashimoto K (2004) Zeta potential and photocatalytic activity of nitrogen doped TiO2 thin films. Phys Chem Chem Phys 6:865–870
Subagio DP, Srinivasan M, Lim M, Lim TT (2010) Photocatalytic degradation of bisphenol-A by nitrogen-doped TiO2 hollow sphere in a vis-LED photoreactor. Appl Catal B 95:414–422
Zhang X, Ding Y, Tang H, Han X, Zhu L, Wang N (2014) Degradation of bisphenol A by hydrogen peroxide activated with CuFeO2 microparticles as a heterogeneous Fenton-like catalyst: efficiency, stability and mechanism. Chem Eng J 246:251–262
Kondrakov AO, Ignatev AN, Lunin VV, Frimmel FH, Bräse S, Horn H (2016) Roles of water and dissolved oxygen in photocatalytic generation of free OH radicals in aqueous TiO2suspensions: an isotope labeling study. Appl Catal B 182:424–430
Almeida AR, Moulijn JA, Mul G (2011) Photocatalytic oxydation of cyclohexane over TiO2: evidence for a Mars-van Krevelen mechanism. J Phys Chem C 115:1330–1338
Acknowledgements
DLS wishes to thank CONACyT for the financial support (Master and mixed scholarship). Dr. Santiago I. Suárez are gratefully acknowledged for valuable support of PROMEP Nuevo PTC DSA/103.5/16/10510. R. Zanella acknowledges the financial support granted by PAPIIT 105416, UNAM, Mexico. Authors thank to PAICYT IT 404-15 and IT 510-15. The authors thank the Materials’ Laboratory of Facultad de Ingenieria Civil-UANL for allowing them to perform the described experiments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
López-Serna, D., Suárez-Vázquez, S.I., Durán-Álvarez, J.C. et al. Kinetic study of photocatalytic degradation of the emerging contaminant bisphenol A using N–TiO2 in visible light: a study of the significance of dissolved oxygen. Reac Kinet Mech Cat 122, 655–670 (2017). https://doi.org/10.1007/s11144-017-1200-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-017-1200-4