Abstract
Purpose
Fatigue is a common symptom of multiple sclerosis (MS) and can adversely affect all aspect of quality of life. The etiology of fatigue remains unclear, and its treatments are suboptimal. Characterizing the phenotypes of fatigued persons with MS may help advance research on fatigue’s etiology and identify ways to personalize fatigue interventions to improve quality of life. The purpose of this study was to identify fatigue phenotypes; examine phenotype stability overtime; and characterize phenotypes by health and function, social and environmental determinants, psychosocial factors, and engagement in healthy behaviors.
Methods
We conducted a longitudinal study over a 3-month period with 289 fatigued participants with MS. To identify fatigue phenotypes and determine transition probabilities, we used latent profile and transition analyses with valid self-report measures of mental and physical fatigue severity, the mental and physical impact of fatigue, depression, anxiety, and sleep quality. We used ANOVAs and effect sizes to characterize differences among phenotypes.
Results
The best fitting model included six subgroups of participants: Mild Phenotype, Mild-to-Moderate Phenotype, Moderate-to-Severe Phenotype, Severe Phenotype, Fatigue-dominant Phenotype, and Mental Health-dominant Phenotype. The transition analysis indicated that phenotypic membership was highly stable. Variables with a large eta squared effect size included environmental barriers, self-efficacy, and fatigue catastrophizing.
Conclusion
These results indicate that the magnitude of fatigue experienced may be more important to consider than the type of fatigue when characterizing fatigue phenotypes. Future research should explore whether tailoring interventions to environmental barriers, self-efficacy, and fatigue catastrophizing reduce the likelihood of transitioning to a more severe phenotype.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fatigue is a common and disabling symptom of multiple sclerosis (MS) [1]. Fatigue can negatively affect quality of life, including ability to perform self-care activities, maintain employment, and participate in social roles [2,3,4,5,6]. Unfortunately, the multifactorial etiology of fatigue remains unclear, and its treatments are suboptimal, with medications and behavioral interventions having modest effects on reducing fatigue [7,8,9,10,11,12]. Studies examining the multifactorial causes of MS fatigue have yielded inconsistent results [13], and there is significant heterogeneity in how participants with MS respond to fatigue interventions [14,15,16]. Identifying subgroups of participants who experience fatigue in the same way may help provide novel insights into the factors causing fatigue and how to develop personalized fatigue interventions that reduce response heterogeneity.
Studies have yielded inconsistent results on the relative importance of factors that influence MS fatigue [13, 17]. One possible explanation is that the factors that influence fatigue may vary overtime depending on the characteristics of the individual, which other symptoms are present, and social and environmental determinants, such as accessibility barriers, social support, and weather. Thus, identifying subgroups of fatigued participants who have similar characteristics and experience similar symptoms may help identify the conditional circumstances in which a factor may or may not significantly influence fatigue.
Differences in how fatigue is operationalized and measured also make it difficult to compare the inconsistent results and examine response heterogeneity in fatigue interventions [18]. Fatigue is most commonly measured using self-report questionnaires, which ask participants to recall the severity or impact of their fatigue over a specified period of time ranging from 1 week to a few months [18]. These questionnaires operationalize and measure fatigue by severity and impact, as well as its mental and physical components. The severity of mental and physical fatigue can be characterized by intensity, frequency, and duration [19], while their impact can be characterized by the extent to which they negatively affect daily activities [20]. Whether differences in the severity and impact of mental and physical fatigue need to be accounted for in studies remains unknown.
Accounting for the severity and impact of mental and physical fatigue is further complicated by their relationship with other symptoms [21, 22]. Research shows that fatigue can coexist or cluster with symptoms of depression, pain, anxiety, and insomnia [23,24,25,26]. This clustering may indicate common underlying mechanisms that an intervention could address. It may indicate a need to target multiple symptoms within a fatigue intervention. However, it is unclear how symptom clusters that include fatigue vary across individuals and under which circumstances individuals would benefit from a fatigue intervention that addresses other symptoms as well.
A promising approach to explain the heterogeneous results among MS fatigue studies and develop personalized fatigue interventions is to identify and characterize fatigue phenotypes [27, 28]. Here, we define a phenotype as a measurable, quantifiable, and distinct pattern of how fatigue is manifested and experienced overtime in relation to other symptoms and that can explain variations in clinically relevant outcomes [29, 30]. We can operationally define a phenotype as a cluster or subgroup of individuals who experience fatigue and other symptoms in the same way. Identified phenotypes can be characterized by comparing environmental and psychological characteristics within and between subgroups. To the best of our knowledge, no studies to date have identified and characterized fatigue phenotypes in people with MS.
The purpose of this study was threefold: (1) to identify phenotypes or subgroups of individuals with MS who experience fatigue in the same way in relation to symptoms of depression, anxiety, pain, and insomnia; (2) to examine membership stability within a phenotype over a 3-month period; and (3) to characterize phenotypes by health and function, social and environmental determinants, psychosocial factors, and engagement in healthy behaviors. To identify phenotypes and determine the probability of transitioning into a different phenotype overtime, we used latent profile and transition analyses with measures of mental and physical severity of fatigue, mental and physical impact of fatigue, depression, anxiety, and sleep quality.
Methods
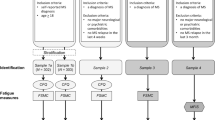
We conducted a longitudinal observational study in which we administered online questionnaires on demographics, health and disability status, environmental factors, and the utilization of healthcare and wellness services at baseline, after 6 weeks from baseline, and after 3 months from baseline. Valid and reliable questionnaires were selected to help minimize biases. A recruitment goal of 300 participants was based on a power analysis that assumed moderately correlated data and moderate effect sizes in a structural equation model. Data were collected from July, 2017 to May, 2019. We note that the baseline data from this study were previously published to identify factors that predict fatigue self-management behaviors, as well as to explore the associations between fatigue, depression, and cognitive impairment [31, 32]. The participants were recruited by asking neurologists and rehabilitation clinicians to distribute flyers, as well as by asking advocacy organizations, such as the National Multiple Sclerosis Society, to distribute flyers via email listservs and at events and support groups. Interested individuals were screened over the phone. The inclusion criteria were 18–65 years of age, a self-reported diagnosis of MS by a physician, and at least mild MS fatigue (> 2 on the Quality of Life in Neurological Disorders short form for fatigue) [33]. Individuals who mostly used a wheelchair (> 6.5 on self-reported Expanded Disability Status Scale [34]) and had severe cognitive deficits (< 12 on the short Orientation-Memory-Concentration Test [35]) were excluded.
Measures
Identifying phenotypes
The questionnaires selected to classify the participants’ fatigue phenotypes were the Chalder Fatigue Scale (CFS) [36], Fatigue Scale for Motor and Cognitive Functions (FS) [37], Quality of Life in Neurological Disorders (Neuro-QOL) short forms for depression (DEP) and anxiety (ANX) [33; 38], Pain Effects Scale (PAIN) [39, 40], and Pittsburgh Sleep Quality Index (SLP) [41]. The questionnaires’ Cronbach’s alpha (α) and test–retest reliability (intraclass correlation coefficient, ICC) statistics that are reported below were calculated from the baseline and 6-week data. Baseline and 6-weeks were selected to include as many participants as possible in the reliability calculations while minimizing risks of capturing changes in health status.
The 11-items CFS yields two composite scores on the physical (CFS-P; α = 0.99; ICC = 0.68) and mental (CFS-M; α = 0.99; ICC = 0.73) severity of fatigue. The 20-item FS generates two composite scores on the physical (FS-P; α = 0.95; ICC = 0.90) and mental (FS-M; α = 0.99; ICC = 0.91) impact of fatigue. The eight-item Neuro-QOL DEP assesses feelings of hopelessness and negative affect over a 1-week period (α = 0.94; ICC = 0.90). The 8-item Neuro-QOL ANX measures unpleasant thoughts related to fear, worries, and hyperarousal (α = 0.93; ICC = 0.84). The six-item PAIN assesses ways in which pain interferes with daily activities and enjoyment (α = 0.97; ICC = 0.88). The SLP measures sleep quality and disturbances by generating a composite score of seven subscale scores on subjective sleep quality and duration and daytime dysfunction (ICC = 0.85).
Characterizing phenotypes
The health and function indicators used to examine the differences in phenotypes were the Community Participation Indicators Activity Ratio (CPI-AR) [42,43,44,45], Patient Determined Disease Steps (PDDS) [46, 47], and Self-Report Comorbidity Questionnaire for Multiple Sclerosis (CQMS) [48]. The 20-item CPI-AR assesses meaningful participation in specific social roles and daily activities (ICC = 0.85). A ratio is calculated using the number of important activities engaged in often enough or too much (numerator) over the number of important activities (denominator). The PDDS is a valid and reliable measure of disability in people with MS [46, 47]. The scale ranges from minimal MS impact with normal ambulatory function and no activity limitations to bedridden (ICC = 0.96). The CQMS consists of 36 co-morbidities for which participants indicate their presence or absence based on a physician diagnosis (ICC = 0.93).
Indicators of social and environmental determinants were the Craig Hospital Inventory of Environmental Factors Short-Form (CHIEF-SF) [49], utilization of health and wellness services [50,51,52], and the Modified Social Support Survey (MSSS) [39, 40]. The CHIEF-SF measures perceived barriers in the physical and social environment over the past 12 months. A composite score is calculated based on how frequently participants experience the environmental barrier and whether it posed a big or small problem (ICC = 0.85). The health and wellness services (H&W) indicator was a composite score of three separate questions on the utilization of wellness services (e.g., exercise class or nutritional counseling) and rehabilitation services (e.g., physical or occupational therapy), as well as the use of medications to manage fatigue. These three questions (yes/no) were tallied to generate a composite score ranging from 0 to 3. Finally, the five-item MSSS measures tangible and emotional social support (α = 0.88; ICC = 0.87).
Psychosocial factors were the Self-Efficacy to Manage Chronic Disease Scale (SMCD) [53] and Fatigue Catastrophizing Scale (FCS) [54]. The six-item SMCD assesses confidence in managing symptoms, emotional distress, daily activities, and medications (α = 0.88; ICC = 0.81). The 10-item FCS measures how frequently individuals have pessimistic evaluations or negative outcome expectations about the fatigue they experience (α = 0.98; ICC = 0.88).
Healthy behaviors were the Godin Leisure-Time Exercise Questionnaire (GLTEQ) [55, 56], the Meats, Eggs, Dairy, Fried foods, Baked goods, Convenience food, Fats added at the Table, and Snacks (MEDFICTS) questionnaire [57], and the Energy Conservation Strategies Survey (ECSS) [58]. The three-item GLTEQ yields a composite score based on the frequency of engagement in light leisure time activities, moderate activities, and strenuous exercises ≥ 15 min. The MEDFICTS measures adherence to the American Heart Association’s recommendations for the prevention of cardiovascular disease based on eight food categories that are weighted by weekly consumption and serving size (ICC = 0.84). The 14-item ECSS measures the frequency of engaging in fatigue self-management behaviors (α = 0.92; ICC = 0.86).
Analysis
This study’s primary objective was to identify subgroups of people with MS based on self-reported measures of mental fatigue severity, physical fatigue severity, mental fatigue impact, physical fatigue impact, depression, anxiety, and sleep using Latent Profile Analysis (LPA). LPA is an application of mixture modeling with the goal of identifying subgroups of individuals based on the joint distribution of a designated set of continuous observed variables as a function of a finite and mutually exclusive number of components [59]. LPA generates non-overlapping latent subgroups as one unobserved categorical variable, with its value representing which profile an individual belongs to with a certain degree of probability [60]. Several statistical indices and tests, including the Akaike Information Criterion (AIC), Bayesian Information Criterion (BIC), sample-size adjusted BIC, posterior probability assessment, entropy, and bootstrap likelihood ratio test (LRT) were used to determine how well the models performed and the optimal number of subgroups [61, 62]. Posterior probabilities are computed using Bayes’ theorem as the probability of belonging to a subgroup conditional to the observed data and parameters estimated in the model [59]. A full information maximum likelihood procedure (as well as the MLR option in Mplus to request robust standard errors [63]) were used to include all available data in the analysis [59]. All available data were included in the analysis and data were assumed to be missing at random.
The study’s secondary objective was to examine stability in subgroup membership over a 3-month period using latent transition analysis (LTA). LTA is an extension of LPA that analyzes longitudinal data [59]. The LTA model is a type of latent Markov model in which the predictive portion is comprised of a multinomial logistic model used to estimate latent status membership probabilities and transition probabilities. Transition probabilities are the probabilities associated with subgroup changes (i.e., the probability of staying in a particular subgroup at the next time point).
The tertiary objective was to explore the differences in the latent subgroups of people with MS using descriptive statistics and ANOVA models. We examined whether there were differences in their health and function, social and environmental determinants, psychosocial factors, and healthy behaviors. A one-way ANOVA with a Bonferroni post-hoc test was conducted for this purpose. Given our expectation that there would be several significant differences between the latent subgroups (especially if we identified several subgroups), rather than rely only on the p-value, we used effect sizes to help gauge whether differences between subgroups were meaningful. Specifically, we used eta squared in SPSS version 28, which measures the proportion of variance associated with each effect in an ANOVA model. Eta squared ranges from 0 to 1, with a value closer to 0 indicating a low proportion of variance as explained in the model. We considered a small effect < 0.01, a medium effect 0.06, and a large effect ≥ to 0.14 [64].
All statistical tests were two-tailed. LPA and LTA were conducted using Mplus (version 8.6). R program in the R studio environment was used for graphical displays, and the Mplus Automation package was used to automate LPA estimation and interpretation [65,66,67]. Descriptive and post-hoc analyses of the latent subgroups were done in SPSS version 28.
Results
A total of 394 individuals were screened for eligibility; 17 individuals were deemed ineligible and 77 were not enrolled due to delays in returning the informed consent or were no longer interested; 289 individuals who returned the baseline questionnaire packet were included in the analyses. Table 1 shows the demographic characteristics of participants included in the analysis. The research sample was mostly representative of the population with MS, given our research sample ratio of about 1:4 male to female (85.5%), the majority of participants having relapsing–remitting MS (73.3%), and the research sample’s mean age of 52 years [68, 69]. The median PDDS score was 3, which indicates a gait disability that does not require regular use of an assistive device.
Latent profile and transition analysis
The best fitting model included 6 subgroups of participants. The entropy for this model was at near maximum (0.83), and the average posterior probability of membership for each latent subgroup ranged from 0.83 to 0.92, denoting that the model classified participants relatively well [59]. The BIC was minimized for the 6-subgroup model. The model also achieved a near minimum across the AIC and sample-size adjusted BIC, as well as statistical significance in the bootstrap LRT. There was no practical advantage for seven subgroups compared to six subgroups with these model selection measures (seven subgroups had a group with only two participants). Participants were assigned to the six classes based on the highest posterior probability of membership, which aforementioned was high on average.
Figure 1 illustrates the pattern and manifestation of symptoms for the 6 subgroups using standardized scores. Subgroup #6 had the highest fatigue, depression, anxiety, pain, and insomnia symptoms; thus, we named this subgroup the Severe Phenotype. In turn, we named Subgroup #1 the Mild Phenotype for having the lowest fatigue, depression, anxiety, pain, and insomnia symptoms. Subgroup #4 was named the Mild-to-Moderate Phenotype for having slightly elevated fatigue, depression, anxiety, pain, and insomnia symptoms compared to the Mild Phenotype. Subgroup #3 became the Moderate-to-Severe Phenotype given its higher symptoms compared to the Mild-to-Moderate Phenotype and lower symptoms compared to the Severe Phenotype. Subgroup #5 was named the Fatigue-dominant Phenotype for having moderate to severe fatigue, but lower symptoms of depression, anxiety, pain, and insomnia. Lastly, Subgroup #2 was named the Mental Health-dominant Phenotype for having relatively low fatigue, pain, and insomnia, but higher depression and anxiety symptoms. The LTA indicated that subgroup membership was highly stable over the 3-month period, that is, the latent transition probabilities for remaining in the same subgroup at 6-weeks compared to the baseline ranged from 0.84 to 0.93 and at 3-months compared to 6-weeks from 0.90 to 1.00.
Latent profile analysis of the seven self-reported measures of fatigue, depression, anxiety, pain, and sleep. Key: Class 1 = Mild Phenotype; Class 2 = Mental Health- dominant Phenotype; Class 3 = Moderate-to-Severe Phenotype; Class 4 = Mild-to-Moderate Phenotype; Class 5 = Fatigue- dominant Phenotype; Class 6 = Severe Phenotype; CFS-P = Chalder Fatigue Scale-physical; CFS-M = Chalder Fatigue Scale-mental; FS-P = Fatigue Scale for Motor and Cognitive Functions-physical; FS-M = Fatigue Scale for Motor and Cognitive Functions-mental; ANX = Neuro-QOL Anxiety; DEP = Neuro-QOL Depression; SLP = Pittsburgh Sleep Quality Index
Table 2 presents the participants’ characteristics in each phenotype along with the results of the ANOVA and effect size calculations, and Supplementary Table 1 shows the results of the post-hoc analysis. The ANOVA revealed that most participants exhibited significant differences across the six subgroups, with the only variable not significantly different across groups was physical activity (i.e., GLTEQ) and utilization of health and wellness services (i.e., H&W). The variables that had a large eta squared effect (> 0.14) on phenotypic membership were community engagement (i.e., CPI-AR), number of comorbidities (i.e., CQMS), environmental barriers (i.e., CHIEF-SF), self-efficacy (i.e., SMCD), and fatigue catastrophizing (i.e., FCS). Using the Mild Phenotype as a reference, the Bonferroni post-hoc analysis showed that community engagement was significantly worse in the Moderate-to-Severe and Severe Phenotypes; the number of comorbidities was significantly higher in the Moderate-to-Severe, Severe, and Fatigue-dominant Phenotypes; environmental barriers were significantly worse for the Moderate-to-Severe, Severe, and Fatigue-dominant Phenotypes; and self-efficacy and fatigue catastrophizing were significantly worse for all phenotypes compared to the Mild Phenotype.
Discussion
In this study, we proposed a classification of self-reported symptom manifestations based on the identification of distinct subgroups of fatigued individuals with MS. To the best of our knowledge, this is the first study to examine the clustering of mental and physical fatigue severity and impact with symptoms of depression, anxiety, pain, and insomnia using LPA and LTA. Notable findings include homogeneity in how the six different phenotypes experienced mental and physical fatigue severity and impact; the identification of the Fatigue- and Mental Health-dominant Phenotypes; and the large effect size differences between phenotypes in community engagement (i.e., CPI-AR, environmental barriers (i.e., CHIEF-SF), self-efficacy (i.e., SMCD), and fatigue catastrophizing (i.e., FCS). Our findings provide insight on the patterns and clustering of different types of fatigue and their relationship with other symptoms, which may have implications for the development and testing of personalized fatigue interventions.
There were negligible differences between the phenotypes concerning the mental and physical severity of fatigue and the mental and physical impact of fatigue. None of the phenotypes were characterized as having a distinct type of fatigue, such as mental or physical fatigue, and were rather characterized by having low, moderate, or high levels of all types of fatigue. These results indicate that the magnitude or extent of fatigue experienced may be a more important factor to consider than the type of fatigue experienced. Research is varied on the similarities and distinctions between the severity and impact of mental and physical fatigue. Pust et al. [70] concluded from a factor analysis that only total fatigue composite scores should be used rather than the subscales of mental and physical fatigue. Other studies have identified two-factor solutions or a bi-factor model with one general fatigue factor and two subfactors of severity and impact of fatigue or mental and physical fatigue [36, 37, 71]. Further research is thus needed on the dimensions of self-reported measures of fatigue.
Nonetheless, the identification of the Fatigue-dominant and Mental Health-dominant Phenotypes provides support for distinguishing between fatigue, anxiety, and depression. The distinctions between these phenotypes may have implications for selecting the most appropriate fatigue intervention based on an individual’s characteristics. For example, future research can explore whether those in the Mental Health-dominant Phenotype benefit more from an intervention focused on managing emotions, while those in the Fatigue-dominant Phenotype could benefit more from an intervention focused on energy management. Although it may be difficult to clinically distinguish between fatigue and depression, evidence-based approaches have been identified using adjusted depression screening scales based on differential item functioning [72].
There were no significant differences between the Fatigue-dominant and Mental Health- dominant Phenotypes on indicators of insomnia, health and function, social and environmental determinants, psychosocial factors, and engagement in healthy behaviors. This is consistent with prior research showing a moderate correlation between the impact of fatigue and the impact of depression on daily activities [73,74,75]. However, we did find that participants in the Fatigue-dominant Phenotype had significantly higher levels of pain compared to those in the Mental Health-dominant Phenotype. Together, these findings have several implications for further research. First, they provide a rationale for conducting clinical trials that combine evaluations of fatigue and depression interventions using a common distal outcome, such as participation in social roles. Second, they may indicate a need for further research to test whether those in the Fatigue-dominant Phenotype may benefit from additional intervention strategies to manage pain. Lastly, these findings offer support to further explore the supposition that fatigue arising from interoceptive networks may be modulated by pain pathways [18].
In contrast to the Fatigue- and Mental Health-dominant Phenotypes, latent Subgroups #1, #3, #4, and #6 were categorized into phenotypes based on low, moderate, and high levels of fatigue, depression, anxiety, pain, and insomnia symptoms. Individuals in the Moderate-to-Severe and Severe Phenotypes had the worse indicators of health and function, social and environmental determinants, psychosocial factors, and engagement in healthy behaviors, whereas individuals in the Mild and Mild-to-Moderate Phenotype had the most positive indicators of these concepts. Despite the clear need for individuals in the Severe Phenotype to utilize health and wellness services, they reported the lowest service utilization. Thus, individuals in the more severe phenotypes likely experience more bodily impairments due the pathophysiology of MS, which is further aggravated by environmental barriers, inaccessible health and wellness services, low self-efficacy, high fatigue catastrophizing, and difficulties engaging in self-care behaviors.
Interestingly, environmental barriers, self-efficacy to manage symptoms, and fatigue catastrophizing provided the greatest overall effect on explaining group membership. These 3 variables consistently showed the sharpest declines or decrements between the Mild-to-Moderate and Moderate-to-Severe Phenotypes. Both behavior change theories and interventional studies have indicated that it is possible to reduce the impact of environmental barriers by improving problem solving, decision making, and systems thinking skills; increase self-efficacy by fostering modeling and mastery experiences using verbal persuasion and teaching appropriate emotional attributions; and decrease fatigue catastrophizing by helping individuals reframe how they think about fatigue [76,77,78,79]. A possible strategy for future research is to tailor interventions specifically to environmental barriers, self-efficacy, and fatigue catastrophizing to reduce the likelihood of transitioning to a more severe phenotype.
There are limitations to this study that should be noted. The study solely relied on self-report measures, used convenience sampling methodology, did not obtain verification of MS and depression diagnoses, and did not confirm the identified fatigue phenotypes in other research samples of people with MS. Although we confirmed that membership in the phenotypes was stable over a 3-month period, future research conducted over a longer period of time in different research samples is necessary. The low number of participants in the Severe Phenotype also prevented the examination of categorical variables that could provide additional insights. Consistent with other MS studies, people with more severe disabilities and from historically marginalized backgrounds were not adequately represented in our research study either. Future research is therefore required to develop strategies to recruit a more diverse sample to fully understand the determinants of individuals who are categorized into the Mild, Moderate, and Severe Phenotypes. Future research is also needed on examining the influence of disease modifying therapies and other medications on phenotypes and using performance-based measures of fatiguability and disability as well as state measures of perceived fatiguability to classify participants into phenotypes [18].
References
Rooney, S., Wood, L., Moffat, F., & Paul, L. (2019). Prevalence of fatigue and its association with clinical features in progressive and non-progressive forms of multiple sclerosis. Multiple Sclerosis and Related Disorders, 28, 276–282. https://doi.org/10.1016/j.msard.2019.01.011
Olsson, M., Lexell, J., & Soderberg, S. (2005). The meaning of fatigue for women with multiple sclerosis. Journal of Advanced Nursing, 49(1), 7–15. https://doi.org/10.1111/j.1365-2648.2004.03258.x
Fisk, J., Pontefract, A., Ritvo, P., Archibald, C., & Murray, T. (1994). The impact of fatigue on patients with multiple sclerosis. Canadian Journal of Neurological Sciences, 21(1), 9–14.
Smith, M. M., & Arnett, P. A. (2005). Factors related to employment status changes in individuals with multiple sclerosis. Multiple Sclerosis Journal, 11(5), 602–609. https://doi.org/10.1191/1352458505ms1204oa
Skerrett, T. N., & Moss-Morris, R. (2006). Fatigue and social impairment in multiple sclerosis: The role of patients’ cognitive and behavioral responses to their symptoms. Journal of Psychosomatic Research, 61(5), 587–593. https://doi.org/10.1016/j.jpsychores.2006.04.018
Hersche, R., Roser, K., Weise, A., Michel, G., & Barbero, M. (2021). Fatigue self-management education in persons with disease-related fatigue: A comprehensive review of the effectiveness on fatigue and quality of life. Patient Education and Counseling. https://doi.org/10.1016/j.pec.2021.09.016
Nourbakhsh, B., Revirajan, N., Morris, B., Cordano, C., Creasman, J., Manguinao, M., Krysko, K., Rutatangwa, A., Auvray, C., & Aljarallah, S. (2021). Safety and efficacy of amantadine, modafinil, and methylphenidate for fatigue in multiple sclerosis: A randomised, placebo-controlled, crossover, double-blind trial. Lancet Neurology, 20(1), 38–48. https://doi.org/10.1016/S1474-4422(20)30354-9
Moss-Morris, R., Harrison, A. M., Safari, R., Norton, S., van der Linden, M. L., Picariello, F., Thomas, S., White, C., & Mercer, T. (2021). Which behavioural and exercise interventions targeting fatigue show the most promise in multiple sclerosis? A systematic review with narrative synthesis and meta-analysis. Behaviour Research and Therapy, 137, 103464. https://doi.org/10.1016/j.brat.2019.103464
Salomè, A., SassoD’Elia, T., Franchini, G., Santilli, V., & Paolucci, T. (2019). Occupational therapy in fatigue management in multiple sclerosis: an umbrella review. Multiple Sclerosis International, 2019, 2027947. https://doi.org/10.1155/2019/2027947
Chalah, M. A., & Ayache, S. S. (2018). Cognitive behavioral therapies and multiple sclerosis fatigue: A review of literature. Journal of Clinical Neuroscience, 52, 1–4. https://doi.org/10.1016/j.jocn.2018.03.024
Miller, P., & Soundy, A. (2017). The pharmacological and non-pharmacological interventions for the management of fatigue related multiple sclerosis. Journal of the Neurological Sciences, 381, 41–54. https://doi.org/10.1016/j.jns.2017.08.012
Phyo, A. Z. Z., Demaneuf, T., De Livera, A. M., Jelinek, G. A., Brown, C. R., Marck, C. H., Neate, S. L., Taylor, K. L., Mills, T., O’Kearney, E., Karahalios, A., & Weiland, T. J. (2018). The efficacy of psychological interventions for managing fatigue in people with multiple sclerosis: A systematic review and meta-analysis. Frontiers in Neurology. https://doi.org/10.3389/fneur.2018.00149
Manjaly, Z. M., Harrison, N. A., Critchley, H. D., Do, C. T., Stefanics, G., Wenderoth, N., Lutterotti, A., Muller, A., & Stephan, K. E. (2019). Pathophysiological and cognitive mechanisms of fatigue in multiple sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry, 90(6), 642–651. https://doi.org/10.1136/jnnp-2018-320050
Plow, M., Motl, R. W., Finlayson, M., & Bethoux, F. (2020). Response heterogeneity in a randomized controlled trial of telerehabilitation interventions among adults with multiple sclerosis. Journal of Telemedicine and Telecare. https://doi.org/10.1177/1357633x20964693
Finlayson, M., Preissner, K., & Cho, C. (2012). Outcome moderators of a fatigue management program for people with multiple sclerosis. American Journal of Occupational Therapy, 66(2), 187–197. https://doi.org/10.5014/ajot.2012.003160
Ehde, D. M., Arewasikporn, A., Alschuler, K. N., Hughes, A. J., & Turner, A. P. (2018). Moderators of treatment outcomes after telehealth self-management and education in adults with multiple sclerosis: A secondary analysis of a randomized controlled trial. Archives of Physical Medicine and Rehabilitation, 99(7), 1265–1272. https://doi.org/10.1016/j.apmr.2017.12.012
Plow, M., & Nicka, A. (2018). Fatigue in multiple sclerosis. In F. Robert, A. Rae-Grant, & F. Bethoux (Eds.), Multiple sclerosis and related disorders: Clinical guide to diagnosis, medical management, and rehabilitation. Springer Publishing Company.
Enoka, R. M., Almuklass, A. M., Alenazy, M., Alvarez, E., & Duchateau, J. (2021). Distinguishing between fatigue and fatigability in multiple sclerosis. Neurorehabilitation & Neural Repair, 35(11), 960–973. https://doi.org/10.1177/15459683211046257
Lyon, D. E., McCain, N. L., Pickler, R. H., Munro, C., & Elswick, R. K., Jr. (2011). Advancing the biobehavioral research of fatigue with genetics and genomics. Journal of Nursing Scholarship, 43(3), 274–281. https://doi.org/10.1111/j.1547-5069.2011.01406.x
Fisk, J. D., Ritvo, P. G., Ross, L., Haase, D. A., Marrie, T. J., & Schlech, W. F. (1994). Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clinical Infectious Diseases, 18(Suppl 1), S79–S83.
Miaskowski, C., Barsevick, A., Berger, A., Casagrande, R., Grady, P. A., Jacobsen, P., Kutner, J., Patrick, D., Zimmerman, L., Xiao, C., Matocha, M., & Marden, S. (2017). Advancing symptom science through symptom cluster research: Expert panel proceedings and recommendations. Journal of the National Cancer Institute. https://doi.org/10.1093/jnci/djw253
Shahrbanian, S., Duquette, P., Kuspinar, A., & Mayo, N. E. (2015). Contribution of symptom clusters to multiple sclerosis consequences. Quality of Life Research, 24(3), 617–629. https://doi.org/10.1007/s11136-014-0804-7
Kwekkeboom, K. L., Tostrud, L., Costanzo, E., Coe, C. L., Serlin, R. C., Ward, S. E., & Zhang, Y. (2018). The role of inflammation in the pain, fatigue, and sleep disturbance symptom cluster in advanced cancer. Journal of Pain and Symptom Management, 55(5), 1286–1295. https://doi.org/10.1016/j.jpainsymman.2018.01.008
DeVon, H. A., Vuckovic, K., Ryan, C. J., Barnason, S., Zerwic, J. J., Pozehl, B., Schulz, P., Seo, Y., & Zimmerman, L. (2017). Systematic review of symptom clusters in cardiovascular disease. European Journal of Cardiovascular Nursing, 16(1), 6–17. https://doi.org/10.1177/1474515116642594
Jenkins, B. A., Athilingam, P., & Jenkins, R. A. (2019). Symptom clusters in chronic obstructive pulmonary disease: A systematic review. Applied Nursing Research, 45, 23–29. https://doi.org/10.1016/j.apnr.2018.11.003
Motl, R. W., Suh, Y., & Weikert, M. (2010). Symptom cluster and quality of life in multiple sclerosis. Journal of Pain and Symptom Management, 39(6), 1025–1032. https://doi.org/10.1016/j.jpainsymman.2009.11.312
De Meo, E., Portaccio, E., Giorgio, A., Ruano, L., Goretti, B., Niccolai, C., Patti, F., Chisari, C. G., Gallo, P., Grossi, P., Ghezzi, A., Roscio, M., Mattioli, F., Stampatori, C., Simone, M., Viterbo, R. G., Bonacchi, R., Rocca, M. A., De Stefano, N., … Amato, M. P. (2021). Identifying the distinct cognitive phenotypes in multiple sclerosis. JAMA Neurology, 78(4), 414–425. https://doi.org/10.1001/jamaneurol.2020.4920
Leavitt, V. M., Tosto, G., & Riley, C. S. (2018). Cognitive phenotypes in multiple sclerosis. Journal of Neurology, 265(3), 562–566. https://doi.org/10.1007/s00415-018-8747-5
Kim, M. T., Radhakrishnan, K., Heitkemper, E. M., Choi, E., & Burgermaster, M. (2021). Psychosocial phenotyping as a personalization strategy for chronic disease self-management interventions. American Journal of Translational Research, 13(3), 1617.
Wojczynski, M. K., & Tiwari, H. K. (2008). Definition of phenotype. Advances in genetics, 60, 75–105.
Wang, E., Chang, J. H. C., & Plow, M. (2022). Predictors of fatigue self-management behaviors in adults with multiple sclerosis. NeuroRehabilitation, 50(1), 75–87. https://doi.org/10.3233/nre-210179
Plow, M., & Gunzler, D. D. (2022). Disentangling self-reported fatigue, depression, and cognitive impairment in people with multiple sclerosis. Multiple Sclerosis and Related Disorders, 61, 103736. https://doi.org/10.1016/j.msard.2022.103736
Cella, D., Lai, J. S., Nowinski, C. J., Victorson, D., Peterman, A., Miller, D., Bethoux, F., Heinemann, A., Rubin, S., Cavazos, J. E., Reder, A. T., Sufit, R., Simuni, T., Holmes, G. L., Siderowf, A., Wojna, V., Bode, R., McKinney, N., Podrabsky, T., … Moy, C. (2012). Neuro-QOL: Brief measures of health-related quality of life for clinical research in neurology. Neurology, 78(23), 1860–1867. https://doi.org/10.1212/WNL.0b013e318258f744
Bowen, J., Gibbons, L., Gianas, A., & Kraft, G. H. (2001). Self-administered expanded disability status scale with functional system scores correlates well with a physician-administered test. Multiple Sclerosis Journal, 7, 201–206. https://doi.org/10.1177/135245850100700311
Katzman, R., Brown, T., Fuld, P., Peck, A., Schechter, R., & Schimmel, H. (1983). Validation of a short orientation-memory-concentration test of cognitive impairment. American Journal of Psychiatry, 140(6), 734–739.
Chalder, T., Berelowitz, G., Pawlikowska, T., Watts, L., Wessely, S., Wright, D., & Wallace, E. P. (1993). Development of a fatigue scale. Journal of Psychosomatic Research, 37(2), 147–153. https://doi.org/10.1016/0022-3999(93)90081-p
Penner, I., Raselli, C., Stöcklin, M., Opwis, K., Kappos, L., & Calabrese, P. (2009). The fatigue scale for motor and cognitive functions (FSMC): Validation of a new instrument to assess multiple sclerosis-related fatigue. Multiple Sclerosis, 15(12), 1509–1517. https://doi.org/10.1177/1352458509348519
Miller, D. M., Bethoux, F., Victorson, D., Nowinski, C. J., Buono, S., Lai, J. S., Wortman, K., Burns, J. L., Moy, C., & Cella, D. (2016). Validating Neuro-QoL short forms and targeted scales with people who have multiple sclerosis. Multiple Sclerosis, 22(6), 830–841. https://doi.org/10.1177/1352458515599450
Ritvo, P., Fischer, J. S., Miller, D. M., Andrews, H., Paty, D., & LaRocca, N. (1997). Multiple Sclerosis Quality of Life Inventory: A user’s manual (pp. 1–65). New York: National Multiple Sclerosis Society.
Rudick, R., Antel, J., Confavreux, C., Cutter, G., Ellison, G., Fischer, J., Lublin, F., Miller, A., Petkau, J., Rao, S., Reingold, S., Syndulko, K., Thompson, A., Wallenberg, J., Weinshenker, B., & Willoughby, E. (1996). Clinical outcomes assessment in multiple sclerosis. Annals of Neurology, 40(3), 469–479. https://doi.org/10.1002/ana.410400321
Buysse, D. J., Reynolds, C. F., 3rd., Monk, T. H., Berman, S. R., & Kupfer, D. J. (1989). The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research, 28(2), 193–213. https://doi.org/10.1016/0165-1781(89)90047-4
Hammel, J., Magasi, S., Heinemann, A., Whiteneck, G., Bogner, J., & Rodriguez, E. (2008). What does participation mean? An insider perspective from people with disabilities. Disability and Rehabilitation, 30(19), 1445–1460. https://doi.org/10.1080/09638280701625534
Heinemann, A. W., Lai, J. S., Magasi, S., Hammel, J., Corrigan, J. D., Bogner, J. A., & Whiteneck, G. G. (2011). Measuring participation enfranchisement. Archives of Physical Medicine and Rehabilitation, 92(4), 564–571. https://doi.org/10.1016/j.apmr.2010.07.220
Heinemann, A. W., Magasi, S., Bode, R. K., Hammel, J., Whiteneck, G. G., Bogner, J., & Corrigan, J. D. (2013). Measuring enfranchisement: Importance of and control over participation by people with disabilities. Archives of Physical Medicine and Rehabilitation, 94(11), 2157–2165. https://doi.org/10.1016/j.apmr.2013.05.017
Plow, M. A., Finlayson, M., Gunzler, D., & Heinemann, A. W. (2015). Correlates of participation in meaningful activities among people with multiple sclerosis. Journal of Rehabilitation Medicine. https://doi.org/10.2340/16501977-1948
Learmonth, Y. C., Motl, R. W., Sandroff, B. M., Pula, J. H., & Cadavid, D. (2013). Validation of patient determined disease steps (PDDS) scale scores in persons with multiple sclerosis. BMC Neurology, 13(1), 37. https://doi.org/10.1186/1471-2377-13-37
Hohol, M. J., Orav, E. J., & Weiner, H. L. (1999). Disease steps in multiple sclerosis: A longitudinal study comparing disease steps and EDSS to evaluate disease progression. Multiple Sclerosis, 5(5), 349–354. https://doi.org/10.1177/135245859900500508
Horton, M., Rudick, R. A., Hara-Cleaver, C., & Marrie, R. A. (2010). Validation of a self-report comorbidity questionnaire for multiple sclerosis. Neuroepidemiology, 35(2), 83–90. https://doi.org/10.1159/000311013
Whiteneck, G. G., Harrison-Felix, C. L., Mellick, D. C., Brooks, C. A., Charlifue, S. B., & Gerhart, K. A. (2004). Quantifying environmental factors: A measure of physical, attitudinal, service, productivity, and policy barriers. Archives of Physical Medicine and Rehabilitation, 85(8), 1324–1335. https://doi.org/10.1016/j.apmr.2003.09.027
Finlayson, M., Plow, M., & Cho, C. (2010). Use of physical therapy services among middle-aged and older adults with multiple sclerosis. Physical Therapy, 90(11), 1607–1618. https://doi.org/10.2522/ptj.20100072
Finlayson, M., Garcia, J. D., & Cho, C. (2008). Occupational therapy service use among people aging with multiple sclerosis. American Journal of Occupational Therapy, 62(3), 320–328. https://doi.org/10.5014/ajot.62.3.320
Plow, M., Cho, C., & Finlayson, M. (2010). Utilization of health promotion and wellness services among middle-aged and older adults with multiple sclerosis in the mid-west US. Health Promotion International, 25(3), 318–330. https://doi.org/10.1093/heapro/daq023
Lorig, K. (1996). Outcome measures for health education and other health care interventions. Sage Publications.
Jacobsen, P. B., Azzarello, L. M., & Hann, D. M. (2004). Relation of catastrophizing to fatigue severity in women with breast cancer. Journal of Consulting and Clinical Psychology, 72(2), 355–361. https://doi.org/10.1037/0022-006X.72.2.355
Godin, G. (2011). The Godin-Shephard leisure-time physical activity questionnaire. The Health & Fitness Journal of Canada, 4(1), 18–22.
Godin, G., & Shephard, R. J. (1985). A simple method to assess exercise behavior in the community. Canadian Journal of Applied Sport Sciences, 10(3), 141–146.
Mochari, H., Gao, Q., & Mosca, L. (2008). Validation of the MEDFICTS dietary assessment questionnaire in a diverse population. Journal of the American Dietetic Association, 108(5), 817–822. https://doi.org/10.1016/j.jada.2008.02.021
Mallik, P. S., Finlayson, M., Mathiowetz, V., & Fogg, L. (2005). Psychometric evaluation of the energy conservation strategies survey. Clinical Rehabilitation, 19(5), 538–543. https://doi.org/10.1191/0269215505cr789oa
Gunzler, D. D., Perzynski, A. T., & Carle, A. C. (2021). Structural equation modeling for health and medicine. Chapman and Hall/CRC.
Spurk, D., Hirschi, A., Wang, M., Valero, D., & Kauffeld, S. (2020). Latent profile analysis: A review and “how to” guide of its application within vocational behavior research. Journal of Vocational Behavior, 120, 103445. https://doi.org/10.1016/j.jvb.2020.103445
Schwarz, G. (1978). Estimating the dimension of a model. The Annals of Statistics, 6(2), 461–464.
Muthén, B., & Shedden, K. (1999). Finite mixture modeling with mixture outcomes using the EM algorithm. Biometrics, 55(2), 463–469.
Muthén, L. K., & Muthén, B. (2017). Mplus user’s guide: Statistical analysis with latent variables, user’s guide. Muthén & Muthén.
Adams, M. A., & Conway, T. L. (2014). Eta squared. In A. C. Michalos (Ed.), Encyclopedia of quality of life and well-being research (pp. 1965–1966). Springer, Netherlands.
Muthén, L. K., & Muthén, B. O. (2021). Mplus: The comprehensive modeling program for applied researchers: User’s guide. Muthén & Muthén.
Venables, W. N., Smith, D. M., & Team, R. D. C. (2009). An introduction to R. Citeseer.
Hallquist, M. N., & Wiley, J. F. (2018). MplusAutomation: An R package for facilitating large-scale latent variable analyses in M plus. Structural Equation Modeling: A Multidisciplinary Journal, 25(4), 621–638.
Walton, C., King, R., Rechtman, L., Kaye, W., Leray, E., Marrie, R. A., Robertson, N., La Rocca, N., Uitdehaag, B., & van der Mei, I. (2020). Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS. Multiple Sclerosis Journal, 26(14), 1816–1821. https://doi.org/10.1177/1352458520970841
Gilmour, H., Ramage-Morin, P. L., & Wong, S. L. (2018). Multiple sclerosis: Prevalence and impact. Health Reports, 29(1), 3–8.
Pust, G. E. A., Pottgen, J., Randerath, J., Lau, S., Heesen, C., Gold, S. M., & Penner, I. K. (2019). In search of distinct MS-related fatigue subtypes: Results from a multi-cohort analysis in 1.403 MS patients. Journal of Neurology, 266(7), 1663–1673. https://doi.org/10.1007/s00415-019-09311-2
Chilcot, J., Norton, S., Kelly, M. E., & Moss-Morris, R. (2016). The Chalder fatigue questionnaire is a valid and reliable measure of perceived fatigue severity in multiple sclerosis. Multiple Sclerosis Journal, 22(5), 677–684. https://doi.org/10.1177/1352458515598019
Gunzler, D. D., Perzynski, A., Morris, N., Bermel, R., Lewis, S., & Miller, D. (2015). Disentangling multiple sclerosis and depression: An adjusted depression screening score for patient-centered care. Journal of Behavioral Medicine, 38(2), 237–250. https://doi.org/10.1007/s10865-014-9574-5
Kratz, A. L., Fritz, N. E., Braley, T. J., Scott, E. L., Foxen-Craft, E., & Murphy, S. L. (2018). Daily temporal associations between physical activity and symptoms in multiple sclerosis. Annals of Behavioral Medicine, 53(1), 98–108. https://doi.org/10.1093/abm/kay018
Salter, A., Fox, R. J., Tyry, T., Cutter, G., & Marrie, R. A. (2019). The association of fatigue and social participation in multiple sclerosis as assessed using two different instruments. Multiple Sclerosis and Related Disorders, 31, 165–172. https://doi.org/10.1016/j.msard.2019.04.014
Biernacki, T., Sandi, D., Kincses, Z. T., Füvesi, J., Rózsa, C., Mátyás, K., Vécsei, L., & Bencsik, K. (2019). Contributing factors to health-related quality of life in multiple sclerosis. Brain and behavior, 9(12), e01466. https://doi.org/10.1002/brb3.1466
Bandura, A. (1986). Social foundations of thought and action: A social cognitive theory. Prentice-Hall.
Moore, S. M., Jones, L., & Alemi, F. (2016). Family self-tailoring: Applying a systems approach to improving family healthy living behaviors. Nursing Outlook, 64(4), 306–311. https://doi.org/10.1016/j.outlook.2016.05.006
Plow, M., Moore, S., Chang, J., & Bachhal, F. (2020). Randomized controlled trial of SystemCHANGE weight management intervention in neurological conditions. Patient Education and Counseling, 103(1), 112–119. https://doi.org/10.1016/j.pec.2019.08.007
Lukkahatai, N., & Saligan, L. N. (2013). Association of catastrophizing and fatigue: A systematic review. Journal of Psychosomatic Research, 74(2), 100–109. https://doi.org/10.1016/j.jpsychores.2012.11.006
Funding
This work was supported by the Office of the Assistant Secretary of Defense for Health Affairs, through the Multiple Sclerosis Research Program under Award No. W81XWH-17-l-0089. Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense.
Author information
Authors and Affiliations
Contributions
MP and DG contributed to the study conception and design. Material preparation, data collection and analysis were performed by MP, DG, and JC. The first draft of the manuscript was written by MP and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Matthew Plow and Julia Chang declare they have no financial interests. Douglas Gunzler has a book royalty agreement with Taylor & Francis Publishing.
Ethical approval
The study was approved by the Institutional Review Board at the University Hospitals Cleveland Medical Center and the Human Research Protection Office of the U.S. Department of Defense.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
Not relevant.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Plow, M., Gunzler, D.D. & Chang, J.H.C. Characterizing fatigue phenotypes with other symptoms and clinically relevant outcomes among people with multiple sclerosis. Qual Life Res 32, 151–160 (2023). https://doi.org/10.1007/s11136-022-03204-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-022-03204-0