Abstract
Antioxidants act as major protective factors against different infections and diseases. The search for natural antioxidants has gained significant momentum due to its associated health benefits. It prompted the investigation of the antioxidant properties of widely recognized medicinal plants, considering their prominent role in conventional medicine. The incorporation of natural antioxidants derived from medicinal plants into food products has the potential to enhance their health benefits. The present investigation is the first study on the optimization of drying and extraction techniques in Costus pictus leaves. C. pictus leaves were dried under varying conditions (40, 50 and 60 °C) and dried powders were subjected to various solvents, namely water, ethanol, methanol and ethyl acetate. The leaves dried at 60 °C and treated with ethanol showed improved activities and were subsequently selected for further extraction. Among the various extraction methods, ultrasound-assisted extraction demonstrated superior antioxidant properties and increased phytochemical contents, making it the optimal technique for our study. Fourier transform infrared spectroscopy (FTIR) reports also substantiated these quantitative results. The extraction process played a significant role in enhancing the desirable attributes and properties of the leaf extracts, surpassing the results obtained from both dried and fresh leaves. The application of liquid chromatography-mass spectrometry (LC-MS) analysis to the leaf extracts facilitated the identification of phenolic compounds and flavonoids, presenting a comprehensive insight into the composition of the extract. Exploration of antioxidant properties, phenolic compounds and flavonoids would validate the benefits and expand the applications of C. pictus in functional foods and nutraceuticals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oxidative stress, which could be attributed to lifestyle changes and environmental factors, is imperative for the cause of diabetes, cancer and neurological disorders. Lack of an efficient antioxidant defense was a vital factor in increased diabetes rates [1]. However, no appropriate curative therapies were available against these diseases. Moreover, synthetic antioxidants also have detrimental health effects owing to their toxicity [2]. So, plants have been perceived as a natural source, due to their profuse bioactive compounds, to have a positive militating impact in preventing these disorders.
Medicinal plants have been inextricably intertwined in the therapy for many medical ailments since their primeval days. The atavistic nature of utilization for healthcare applications has paved for more tapping on medicinal plants. Diverse bioactive compounds, namely alkaloids, phenolic compounds and flavonoids, contribute to a diverse array of biological properties like antioxidant and anti-inflammatory activities [3]. Furthermore, the diverse nature of phytochemicals has also intrigued the derivatization of dietary supplements from these plants [4]. It was indispensable to investigate its biological properties, to have an efficacious functional food based on plant fortification [5]. The antioxidant efficacy of the plant was influenced by the parameters involved in diverse processing techniques, which affected the availability of antioxidants and other phytochemicals. Processing techniques positively impacted carotenoids and flavonoids; while also attenuating the oxidation of polyphenols through thermal processing, which led to its elevated levels in processed forms like dried powders [6]. The stability of the flavonoids also depends on the methods employed for extraction [7].
The utilization of plants as food additives can be done by adding either dry plant powders or plant extracts obtained through a contemporary extraction approach. For the isolation of bio-actives from botanical sources, extraction acts as a vital step, and solvents play an indispensable part in the extraction. Solvent extraction (SE) is commonly utilized for plant-based extractions. Soxhlet extraction (SOX) involves repeated interaction between the sample and the solvent, and the bioactive molecules get leached away into the solvent, thus circumventing the requirement for filtration [8]. Advanced technologies like microwave-assisted extraction (MAE) and ultrasound-assisted extraction (US) are gaining more attention due to their reduced extraction time and requirement of minimal solvents [9,10,11]. The application of plant extracts in diversified areas, such as the food and pharmaceutical sectors, has been observed. Yesil-Celiktas et al. [12] have documented the potential utilization of rosemary extracts as powerful anti-cancer supplements, attributing their cytotoxic properties to their effectiveness against cancer cells.
Costus pictus originated from the sub-Himalayan parts and South and Central America and is a renowned medicinal plant. It can thrive in a tropical climate which aids its cultivation. It is highly acclaimed as the ‘Insulin plant’ and is attributable to its anti-diabetic traits. Earlier investigations on C. pictus leaves demonstrated their anti-diabetic properties by evaluating their impact on rats with induced diabetes [13]. Quantitative analysis conducted by Jayasri et al. [14] revealed the antioxidant attributes of the leaves [14]. No previous studies have explored the optimization of various processing stages for C. pictus leaves.
The objective of the study is to (i) investigate the influence of various solvents on antioxidant properties of C. pictus leaves processed at different drying temperatures, (ii) analyze various extraction methods, and validate by Fourier-transform infrared spectroscopy. The study also analyzed the efficacy of processing conditions on the antioxidant characteristics and flavonoid content. The objective is to enhance the extract to achieve its maximum potential in terms of antioxidant properties and flavonoid composition through optimization, thus enabling its utilization in various industrial applications.
Materials and Methods
The Materials and Methods has been provided as supplemental information.
Results and Discussion
All figures and tables were presented in the supplementary material section.
Optimization of the Solvent and Drying Temperature
Fresh C. pictus leaves and leaf powders dried at different conditions were dispersed in various solvents. It could give a brief understanding of the role of these solvents on the antioxidant properties and phytochemicals. The effect of temperature and solvents on various quantitative assays of the dried powders was illustrated in Fig. (S1).
For the 2,2–diphenyl–1–picrylhydrazyl (DPPH) free radical scavenging assay, IC50 values were far lesser at 60 °C, followed by 50 and 40 °C, respectively. There was a 4-fold reduction in the IC50 values at 60 °C compared to the fresh samples for ethanol and ethyl acetate dilutes, whereas a 3-fold reduction was noticed for water and methanol samples. Among the solvents, methanolic dilute had fewer IC50 values and ethanol samples (267.958 ± 3.08 µg/mL) only had very minimal differences from the methanol samples (272.570 ± 2.42 µg/mL). IC50 values increased in water and a vast increase was seen in the ethyl acetate samples (Fig. S1 1a). Methanolic samples at 60 °C (1.641 ± 0.019 µg/g Trolox equivalents (TE)) revealed better results for 2,2’-azino‐bis(3‐ethylbenzothiazoline‐ 6‐sulfonic acid) (ABTS) assay; nevertheless, only minimal variations were seen among the different drying temperatures (40 °C – 1.593 ± 0.013 µg/g TE and 50 °C – 1.618 ± 0.006 µg/g TE) and the polar solvents. Ethyl acetate dilutes had very minimal values for the dried and fresh samples (Fig. S1 1b). Ferric reducing antioxidant potential (FRAP) analysis results signified that higher temperature (60 °C) had more appreciable antioxidant properties than the other temperatures and was significantly higher than the fresh leaves (approximately 4-fold increase). Methanol (11.034 ± 0.02 mM/g TE at 60 °C) gave the best results and ethyl acetate samples (4.590 ± 0.09 mM/g TE at 60 °C) revealed the lowest antioxidant properties (Fig. S1 1c). Phosphomolybdenum (PM) assay also revealed methanol and ethanol showed better antioxidant properties; however, the latter had minor variations than the former solvent. Minimal changes were noticed among the different temperatures and antioxidant activity was considerably better than in the fresh leaves (Fig. S1 1d). Total phenol content (TPC) was higher in the ethanol samples and was consecutively lesser in methanol, water and ethyl acetate samples. Ethanol revealed greater TPC due to its high affinity for phenolic constituents. Leaves dried at 60 °C (water − 12.979 ± 0.03 mg/g gallic acid equivalents (GAE), ethanol − 22.338 ± 0.06 mg/g GAE, methanol − 22.068 ± 0.04 mg/g GAE and ethyl acetate – 6.619 ± 0.04 mg/g GAE) displayed similar TPC to the fresh leaves (water − 12.108 ± 0.02 mg/g GAE, ethanol − 20.463 ± 0.02 mg/g GAE, methanol – 16.321 ± 0.04 mg/g GAE and ethyl acetate – 5.053 ± 0.01 mg/g GAE), thus confirming the slightest variation caused to the phenolic compounds owing to the less drying time of the samples (Fig. S1 1e). Flavonoid content (FC) was more evident in methanol and ethanol samples than in water and ethyl acetate and was more at 60 °C compared to other drying temperatures and the fresh leaves (Fig. S1 1f).
The results were analogous to the other studies in C. pictus, wherein methanol was reported as the best solvent [13, 15]. As methanol was a class 2 GRAS (Generally Recognized As Safe) solvent, its utilization was prone to the concentration guidelines due to its toxic effects. Therefore, using it would hinder the application of dried powders as food additives, as it might exceed the utilization limit of 3000 ppm. Moreover, the results of ethanolic samples were on par with methanol; therefore, ethanol was chosen for further extraction. Similar results were observed for another medicinal plant, Limnophila aromatica, wherein ethanol gave superior results compared to other solvents [16].
The antioxidant activity of dried powders was more than the fresh leaves and leaves dried at 60 °C had the best antioxidant properties. The increment in the activity of the dried leaves could be due to the unbinding of bound antioxidants caused by cell structural disruption on drying. Another contributing factor could be the potential thermal inactivation of enzymes, including polyphenol oxidase, which may play a role in suppressing oxidation and thereby maintaining the integrity of the antioxidant property [17]. The observed outcomes matched the results of Pinela et al. [18], wherein dried samples had better antioxidant properties than wild plants.
Drying expedited the release of phenolic compounds, leading to higher total phenolic content in dried samples compared to fresh leaves. Moreover, some plants might remain metabolically active on drying and they recognize moisture loss as a stress inducer and produce more phenolics during drying. It might also contribute to the increased TPC levels in dried leaves. Enzymes, polyphenol oxidase and peroxidase, found in fresh leaves can facilitate the oxidation of phenolic compounds, resulting in lower TPC levels. However, during the drying process, these enzymes become thermally inactive, leading to an increase in TPC in the dried leaf powders [19]. Therefore, the dried leaf powders displayed higher total phenolic content, with a notable increase observed at a drying temperature of 60 °C. These results followed a similar pattern to the dried leaves of Camellia sinensis [19] and Urtica dioica [17]. FC was higher in the leaves dried at higher drying temperatures owing to the shorter drying time, thereby minimizing the prolonged exposure of flavonoids to heat. Thus, 60 °C had more pronounced FC. The findings were consistent with the hot air drying of lemon myrtle leaves, which also demonstrated increased phytochemical content at higher drying temperatures. Sustained exposure to lower temperatures could potentially induce the degradation of phytochemicals, while higher temperatures reduced drying time and minimized phytochemical loss [20].
Hence, leaves dried at 60 °C revealed unrivaled results and were taken for extraction. These observations suggested that dried powder could be ideal for incorporation in various nutraceutical and food formulations owing to its better antioxidant properties and improved phytochemical contents.
Optimisation of the Extraction Method
Ethanol was used as the solvent and the dried powders were subjected to various extraction techniques. Different extraction criteria were set for diverse methods and the optimal conditions were found depending on its phytochemical contents and antioxidant properties. Fig. (S2) illustrates the optimum extraction conditions for all the extraction methods.
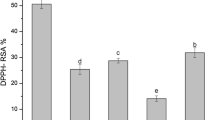
The higher yield was noticed in ultrasonicated extracts and was nearly 2-fold higher than solvent extraction (SE) and Soxhlet extracts. Microwave-assisted extracts showed a minimal extraction yield. Ultrasonicated extracts demonstrated enhanced antioxidant properties than other extracts and displayed the highest values in all four quantitative assays (Fig. S3 a). The pulsed and continuous modes in the ultrasound-assisted extraction revealed minor variations. The results of the antioxidant assays varied among themselves due to the variations in the radical neutralizing mechanism. Similar antioxidant properties were reported for the leaf extracts of Psidium cattleianum [21]. TPC was highest in US extracts (61.000 ± 0.011 mg/g GAE), followed by SE (57.540 ± 0.002 mg/g GAE), MAE (49.278 ± 0.007 mg/g GAE) and SOX (48.595 ± 0.004 mg/g GAE). A similar trend was also seen in the flavonoids, where the US had the maximum FC (159.19 ± 0.002 mg/g quercetin equivalents (QE)) and SOX had the minimum FC (34.83 ± 0.003 mg/g QE) (Fig. S3 b).
Thus, ultrasonicated extracts exhibited improved antioxidant activities with the greatest TPC and significantly higher FC. As the ultrasound-assisted extraction was a non-thermal method, it aided in sustainment of the functionality of plant bioactive, thus imposing a great advantage to it. Moreover, shorter extraction time and minimal solvent utilization also favored it [22]. Acoustic cavitation was the chief principal behind this extraction and these cavitations accelerated the solubilization of the bioactive compounds, thereby yielding superior results. Also, the ultrasonication performed with an ultrasonic probe imparted an additional benefit over an ultrasonic bath due to the uniform energy distribution and high-intensity ultrasonic waves, thereby causing better cavitation [23]. Higher extraction time and increased utilization of solvents were the major setbacks for solvent extracts. Microwave extracts also showed lower results, as microwaves could cause degradation of the heat sensitive phytochemicals. Soxhlet extracts had minimal antioxidant property and phytochemicals due to thermal decomposition of the bioactive compounds. However, the requirement of an additional concentration step also makes Soxhlet extraction less favourable [24]. Hence, the C. pictus leaf extracts had better antioxidant properties than their dried powders.
FTIR Analysis
Optimal extracts from all the extraction methods were subjected to FTIR analysis to analyze the impact of different extraction methods on the dried C. pictus leaf samples (Fig. S4). All the leaf extracts revealed characteristic ethanol bands in the FTIR spectra at ~ 3700 cm− 1, as ethanol was the solvent in all the extraction processes. Peaks at 3366 cm-1 and 3273 cm− 1 indicated O-H stretching and C-H stretching was seen at 2924 cm− 1 and 2883 cm− 1 [25]. A broad peak attributed to O-H stretching, along with a peak for C = O at 1270 cm− 1, signified the occurrence of aliphatic carboxylic acid; it was uniformly present in all the extracts. C-H stretching vibrations in the aromatic aldehyde resulted in a peak around 2300 cm− 1 and were reported only in the US samples.
Peaks associated with C-O stretching in the saturated primary alcohol and asymmetric stretching of S = O in sulfonamides were noticed at 1057.7 and 1327 cm− 1, respectively, for all the methods [26]. All C. pictus leaf extracts exhibited peaks around 3320 and 1045 cm− 1 and implied phenolic O-H stretching and phenolic C-OC bending, respectively. The presence of phenolic compounds was indicated by peaks at 1237 cm-1 and 1245 cm-1, corresponding to the stretching of phenolic C-OH and C-O bonds in the aryl ether ring, respectively [27]; it was seen in the US and SE samples, and it was comparatively lesser in MAE extracts. These peaks were absent in SOX extracts. These substantiated the variations in the polyphenol content among these extraction methods.
A sharp peak at 1045 cm− 1 signified the symmetric stretching observed in the C-O groups of unmodified polyflavonoids and was seen in all the extracts. Peak around 1445 cm− 1 denoted the bending vibrations in the C-H groups of the flavonoids [28]. In-plane deformations in the C-H group of the flavonoids were observed in the wavenumbers from 600 to 980 cm− 1 [29]. This range was observed as a broad peak in ultrasound-assisted extracts and solvent-extracted extracts; nevertheless, the former had a more intense peak than the latter, which could have contributed to the differences in the flavonoid content. The intensities of the peaks in this range were lesser in microwave-assisted extracts followed by the Soxhlet extracts and might be related to their lower flavonoid concentrations. Thus, these FTIR results confirmed that ultrasound-assisted extracts had better phytochemical contents and were concordant with the quantitative assays.
Identification of Compounds
Following quantitative analysis, the optimized ultrasound-assisted extract underwent compound identification through liquid chromatography-mass spectrometry. The resulting chromatogram (Fig. S5) and relevant studies on plant extracts [30,31,32,33,34] aided in the tentative identification of compounds, as presented in Table S1. Phenolic acids, including caffeic acid, 4-O-feruloylquinic acid, and dihydroxybenzoic acid pentoside, were noticed in the leaf extract. Additionally, there were observed ionizations with mass-to-charge ratios of 173 and 453, suggesting the occurrence of phenol derivatives. In the optimized leaf extracts, flavonoids such as quercetin and its derivatives, as well as kaempferol 3-O-glucoside, were detected. Ashwini et al. [35] reported major flavonoids such as kaempferol 3-O-glucoside, isoquercetin, and quercetin in C. pictus leaf extract and our results align with those findings.
Conclusion
The present investigation explored the efficacy of different solvents on fresh and dried samples of C. pictus leaves. Antioxidant properties and phytoconstituents acted as crucial elements for the optimization. Leaf samples dried at 60 °C and ethanol were chosen for further extraction. Dried powders were extracted using different methods and the US emerged as an unequivocal technique based on the critical optimization parameters. FTIR analysis also substantiated these experimental outcomes. Thus, the leaf extracts were more desirable than dried powders and fresh leaves. As C. pictus was widely acknowledged for its anti-diabetic properties, these leaf extracts would be a propitious choice for functional foods and pharmaceutical industries, as they would nutritionally enrich it. This study also suggested further phytochemical characterization and clinical trials with these leaf extracts as it would give a comprehensive overview of this medicinal plant and aid its implementation in varied industrial applications.
Data Availability
The published article incorporates all the generated and analyzed data.
Abbreviations
- ABTS:
-
2,2'-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)
- DPPH:
-
2,2-diphenyl-1-picrylhydrazyl
- FC:
-
Flavonoid content
- FRAP:
-
Ferric reducing antioxidant potential
- FTIR:
-
Fourier transform infrared spectroscopy
- GAE:
-
Gallic acid equivalents
- LC-MS:
-
Liquid chromatography-mass spectrometry
- MAE:
-
Microwave-assisted extraction
- PM:
-
Phosphomolybdenum
- QE:
-
Quercetin equivalents
- SE:
-
Solvent extraction
- SOX:
-
Soxhlet extraction
- TE:
-
Trolox equivalents
- TPC:
-
Total phenolic content
- TPTZ:
-
2,4,6-Tripyridyl-S-triazine
- US:
-
Ultrasound-assisted extraction
References
Kaviarasan S, Vijayalakshmi K, Anuradha CV (2004) Polyphenol-rich extract of fenugreek seeds protect erythrocytes from oxidative damage. Plant Foods Hum Nutr 59:143–147. https://doi.org/10.1007/s11130-004-0025-2
Sreelatha S, Padma PR (2009) Antioxidant activity and total phenolic content of Moringa oleifera leaves in two stages of maturity. Plant Foods Hum Nutr 64:303–311. https://doi.org/10.1007/s11130-009-0141-0
Ulewicz-Magulska B, Wesolowski M (2019) Total phenolic contents and antioxidant potential of herbs used for medical and culinary purposes. Plant Foods Hum Nutr 74:61–67. https://doi.org/10.1007/s11130-018-0699-5
Joana Gil-Chávez G, Villa JA, Fernando Ayala‐Zavala J, Basilio Heredia J, Sepulveda D, Yahia EM, González‐Aguilar GA (2013) Technologies for extraction and production of bioactive compounds to be used as nutraceuticals and food ingredients: an overview. Compr Rev Food Sci Food Saf 12(1):5–23. https://doi.org/10.1111/1541-4337.12005
Foss K, Przybyłowicz KE, Sawicki T (2022) Antioxidant activity and profile of phenolic compounds in selected herbal plants. Plant Foods Hum Nutr 77(3):383–389. https://doi.org/10.1007/s11130-022-00989-w
Kaur C, Kapoor HC (2001) Antioxidants in fruits and vegetables–the millennium’s health. Int J Food Sci Technol 36(7):703–725. https://doi.org/10.1111/j.1365-2621.2001.00513.x
Biesaga M (2011) Influence of extraction methods on stability of flavonoids. J Chromatogr A 1218(18):2505–2512. https://doi.org/10.1016/j.chroma.2011.02.059
Kalantari K, Moniri M, Boroumand Moghaddam A, Abdul Rahim R, Bin Ariff A, Izadiyan Z, Mohamad R (2017) A review of the biomedical applications of zerumbone and the techniques for its extraction from ginger rhizomes. Molecules 22(10):1645. https://doi.org/10.3390/molecules22101645
Rostagno MA, Palma M, Barroso CG (2003) Ultrasound-assisted extraction of soy isoflavones. J Chromatogr A 1012(2):119–128. https://doi.org/10.1016/S0021-9673(03)01184-1
Wang L, Weller CL (2006) Recent advances in extraction of nutraceuticals from plants. Trends Food Sci Technol 17(6):300–312. https://doi.org/10.1016/j.tifs.2005.12.004
Mandal V, Mohan Y, Hemalatha S (2007) Microwave assisted extraction—an innovative and promising extraction tool for medicinal plant research. Phcog Rev 1(1):7–18
Yesil-Celiktas O, Sevimli C, Bedir E, Vardar-Sukan F (2010) Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum Nutr 65:158–163. https://doi.org/10.1007/s11130-010-0166-4
Jothivel N, Ponnusamy SP, Appachi M, Singaravel S, Rasilingam D, Deivasigamani K, Thangavel S (2007) Anti-diabetic activity of methanol leaf extract of Costus pictus D. Don in alloxan-induced diabetic rats. J Health Sci 53(6):655–663. https://doi.org/10.1248/jhs.53.655
Jayasri MA, Lazar M, Radha A (2009) A report on the antioxidant activity of leaves and rhizomes of Costus pictus D. Don. Int J Integr Biol 5(1):20–26
Shiny CT, Saxena A, Gupta SP (2013) Phytochemical investigation of the insulin plant “Costus pictus” D. Don. Int J Pharm Biomed Res 4(2):97–104
Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ismadji S, Ju YH (2014) Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal 22(3):296–302. https://doi.org/10.1016/j.jfda.2013.11.001
Garcìa LM, Ceccanti C, Negro C, De Bellis L, Incrocci L, Pardossi A, Guidi L (2021) Effect of drying methods on phenolic compounds and antioxidant activity of Urtica dioica L. leaves. Horticulturae 7(1):10. https://doi.org/10.3390/horticulturae7010010
Pinela J, Barros L, Dueñas M, Carvalho AM, Santos-Buelga C, Ferreira IC (2012) Antioxidant activity, ascorbic acid, phenolic compounds and sugars of wild and commercial Tuberaria lignosa samples: effects of drying and oral preparation methods. Food Chem 135(3):1028–1035. https://doi.org/10.1016/j.foodchem.2012.05.038
Roshanak S, Rahimmalek M, Goli SAH (2016) Evaluation of seven different drying treatments in respect to total flavonoid, phenolic, vitamin C content, chlorophyll, antioxidant activity and color of green tea (Camellia sinensis or C. assamica) leaves. J Food Sci Technol 53(1):721–729. https://doi.org/10.1007/s13197-015-2030-x
Saifullah M, McCullum R, McCluskey A, Vuong Q (2019) Effect of different drying methods on extractable phenolic compounds and antioxidant properties from lemon myrtle dried leaves. Heliyon 5(12):e03044. https://doi.org/10.1016/j.heliyon.2019.e03044
Ho R, Violette A, Cressend D, Raharivelomanana P, Carrupt PA, Hostettmann K (2012) Antioxidant potential and radical-scavenging effects of flavonoids from the leaves of Psidium cattleianum grown in french polynesia. Nat Prod Res 26(3):274–277. https://doi.org/10.1080/14786419.2011.585610
Mohammadpour H, Sadrameli SM, Eslami F, Asoodeh A (2019) Optimization of ultrasound-assisted extraction of Moringa peregrina oil with response surface methodology and comparison with Soxhlet method. Ind Crops Prod 131:106–116. https://doi.org/10.1016/j.indcrop.2019.01.030
Kumar K, Srivastav S, Sharanagat VS (2021) Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: a review. Ultrason Sonochem 70:105325. https://doi.org/10.1016/j.ultsonch.2020.105325
Mohammad Azmin SNH, Abdul Manan Z, Wan Alwi SR, Chua LS, Mustaffa AA, Yunus NA (2016) Herbal processing and extraction technologies. Sep Purif Rev 45(4):305–320. https://doi.org/10.1080/15422119.2016.1145395
Oliveira RN, Mancini MC, Oliveira FCSD, Passos TM, Quilty B, Thiré RMDSM, McGuinness GB (2016) FTIR analysis and quantification of phenols and flavonoids of five commercially available plants extracts used in wound healing. Matéria (Rio J) 21:767–779. https://doi.org/10.1590/S1517-707620160003.0072
Suresh J, Pradheesh G, Alexramani V, Sundrarajan M, Hong SI (2018) Green synthesis and characterization of zinc oxide nanoparticle using insulin plant (Costus pictus D. Don) and investigation of its antimicrobial as well as anticancer activities. Adv Nat Sci: Nanosci Nanotechnol 9(1):015008. https://doi.org/10.1088/2043-6254/aaa6f1
Prasetyaningrum A, Jos B, Ratnawati R, Rokhati N, Riyanto T, Prinanda GR (2022) Sequential microwave-ultrasound assisted extraction of Flavonoid from Moringa oleifera: product characteristic, antioxidant and antibacterial activity. Indones J Chem 22(2):303–316. https://doi.org/10.22146/ijc.65252
García DE, Delgado N, Aranda FL, Toledo MA, Cabrera-Barjas G, Sintjago EM, Escobar-Avello G, Paczkowski S (2018) Synthesis of maleilated polyflavonoids and lignin as functional bio-based building-blocks. Ind Crops Prod 123:154–163. https://doi.org/10.1016/j.indcrop.2018.06.065
Pavia DL, Lampman GM, Kriz GS, Vyvyan JA (2014) Introduction to spectroscopy. Cengage learning, USA
López-Fernández O, Domínguez R, Pateiro M, Munekata PE, Rocchetti G, Lorenzo JM (2020) Determination of polyphenols using liquid chromatography–tandem mass spectrometry technique (LC–MS/MS): a review. Antioxidants 9(6):479. https://doi.org/10.3390/antiox9060479
Sokkar NM, El-Hawary SM, Slem AM, Talaat Z (2016) The phenolic composition of the hepatoprotective and antioxidant fractions of Albizia lebbeck L. Quim Nova 39:973–978. https://doi.org/10.5935/0100-4042.20160112
Araujo NMP, Arruda HS, Dos Santos FN, de Morais DR, Pereira GA, Pastore GM (2020) LC-MS/MS screening and identification of bioactive compounds in leaves, pulp and seed from Eugenia calycina Cambess. Food Res Int 137:109556. https://doi.org/10.1016/j.foodres.2020.109556
Oliveira BG, Pimentel EF, Pereira ACH, Tosato F, Pinto FE, Ventura JA, Endringer DC, Romao W (2020) Phenolic and glycidic profiling of bananas Musa sp associated with maturation stage and cancer chemoprevention activities. Microchem J 153:104391. https://doi.org/10.1016/j.microc.2019.104391
Göger G, Köse YB, Demirci F, Göger F (2021) Phytochemical characterization of phenolic compounds by LC-MS/MS and biological activities of Ajuga reptans L., Ajuga salicifolia (L.) Schreber and Ajuga genevensis L. from Turkey. Turk J Pharm Sci 18(5):616. https://doi.org/10.4274/tjps.galenos.2021.33958
Ashwini S, Bobby Z, Joseph M, Jacob SE, Padmapriya R (2015) Insulin plant (Costus pictus) extract improves insulin sensitivity and ameliorates atherogenic dyslipidaemia in fructose induced insulin resistant rats: molecular mechanism. J Funct Foods 17:749–760. https://doi.org/10.1016/j.jff.2015.06.024
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Saranya Selvakumarasamy: Conceptualization, Methodology, Analysis, Data curation, Writing, review and editing of the manuscript, Balakrishnaraja Rengaraju: Supervision, Validation, Review and manuscript revision.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors affirm that there are no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Selvakumarasamy, S., Rengaraju, B. Enhancement of Antioxidant Properties of the Medicinal Plant, Costus Pictus by Optimization of its Drying and Extraction Criteria. Plant Foods Hum Nutr 78, 546–551 (2023). https://doi.org/10.1007/s11130-023-01083-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-023-01083-5