Abstract
Pigeon pea protein isolates (PPI) are an option to obtain a high yield of good quality proteins and represent a great potential for the food industry. In this work, physicochemical and structural properties of albumin (ALB), globulin (GLB), and PPI obtained at different pHs (8, 9, 10, and 11) were studied to deepen the knowledge of these proteins for future application. GLB presented protein aggregates and polypeptides characteristics of 7S vicilin subunits while ALB presented polypeptides with low molecular masses. GLB showed a more compact and less flexible structure than ALB fraction due to the distinct conformational characteristics found in DSC, fluorescence spectroscopy, Ho. These structural characteristics conferred GLB greater conformational stability (∆GH2O) than ALB fraction. The latter presented a higher proportion of β-strand in aggregated structures. PPI11 showed the highest protein recovery, but the least So with more presence of protein aggregates with the least proportion of β-strands in aggregated structures. A higher percentage of protein unfolding and exposure of hydrophobic residues to solvent was observed as the extraction pH of the isolates increased. Enthalpy change of transition decreased, and the maximum emission wavelength shifted to red in fluorescence spectroscopy. However, PPI11 showed only a slight increase in Ho (10%) with respect to PPI8. The variation in pH for protein extraction constitutes a simple, rapid, and low-cost method to obtain PPI with physicochemical and structural properties that will determine its functional properties and their use as food ingredients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pigeon pea (PP) [Cajanus cajan (L.) Millspaugh] is the sixth most popular legume crop cultivated in semi-arid tropical and subtropical regions of the world [1]. It also contributes to food security due to its potential as sustainable agriculture in regions that face the early effects of global climate changes [2]. PP crop increases soil nutrition and tolerates drought conditions and its seeds provide one of the major source of proteins for human nutrition (19–23%) with good digestibility (68%) and carbohydrates (54–58%) with a 49–51% of starch [3, 4]. PP proteins have a high content of essential amino acids such as lysine, valine, threonine, and phenylalanine. However, these seeds are generally deficient in sulfur amino acids such as cysteine and methionine [5]. Recent studies had focused on the proteome and genome of PP for crop improvement [6; additional data in ESM, Reference 1].
The main protein fractions of PP are globulins (GLB) (50–70%) and albumins (ALB) (10–15%), where the last has the largest amount of sulphur amino acids, lysine, aspartic acid, glycine, and alanine [7, 8]. Based on their sedimentation coefficients, GLB fraction is divided into two groups named 11S legumin-like globulins and 7S vicilin-like globulins, which is the most abundant [6]. There are limited studies on major protein fractions of PP and mainly they have been focused on GLB fractionation, vicilins (7S) purification and the N-terminal amino acid sequenciation of vicilin subunits [9; additional data in ESM, References 2, 3]. Also, Prema et al. [10] reported that GLB fraction from PP has an hypolipidaemic action in rats. No further information about physicochemical (thermal stability and hydrophobicity) and structural properties of major protein fractions of PP was found, mainly about ALB fraction.
Protein isolates (PI) and concentrates from PP are a valid option to obtain a high yield of good quality proteins that could be used as ingredient in food products increasing its nutritional quality and providing desirable sensory characteristics [11]. Alkaline extraction-isoelectric precipitation is currently the most practiced method in the food industry to manufacture PI and since the isoelectric point of GLB and ALB is different, mainly GLB is extracted [12]. Adenekan et al. [13] evaluated different solvent precipitation techniques on nutritional and functional properties of PP proteins, meanwhile Akintayo et al. [14] studied functional properties of PP protein concentrates. Mizubuti et al. [15] optimized the conditions of alkaline extraction of proteins from PP (pH 8.5, without NaCl), without protein characterization. Mwasaru et al. [16] analyzed the influence of alkaline extraction-isoelectric precipitation, followed by a drying process (50 °C, 48 h), and micellization on the physicochemical and functional properties of PI from PP and cowpea [17]. Since freeze-drying step preserves proteins more native than air-dried process [18], PI with different physicochemical, structural, and functional properties could be obtained. Also, a correlation between structural properties (i.e β-sheet ratio) with protein digestibility was reported [19, 20].
The aim of the present work was to characterize ALB and GLB fractions from PP in terms of physicochemical and structural properties (electrophoresis, size exclusion chromatography, thermal behavior, surface hydrophobicity, infrared and fluorescence spectroscopy, and protein solubility). Also, we evaluated the influence of pH extraction on physicochemical and structural properties of protein isolates from PP (PPI), obtained by alkaline extraction–isoelectric precipitation followed by a freeze-drying process.
Material and Methods
Pigeon Pea Seeds and Flour
Pigeon pea (PP; Cajanus cajan L. Millspaugh) seeds were obtained from Estación Experimental El Sombrero-Corrientes (Instituto Nacional de Tecnología Agropecuaria-INTA) (crop 2017). Non damaged PP seeds (with seed coat) were grounded in an electric mill (Smart-tek model, coffee grinder, China) and sieved (80 ASTM, 177 μm). Flour was defatted (hexane 10 g/100 mL) under continuous stirring (24 h, 4 °C), filtered, and flour was air-dried (24 h, 25 °C).
Protein Extraction
Albumin (ALB) and Globulin (GLB) Fractions
The sequential extraction of ALB and GLB was carried out according to their solubility in different solvents, as described by Rosa et al. [21] with some modifications. Samples were freeze-dried and kept at −20 °C until use.
Pigeon Pea Protein Isolates (PPI)
PPI was prepared according to Horax et al. (ESM, Reference 4). PPI obtained were named PPI8, PPI9, PPI10, and PPI11, according to the pH of extraction and were stored at 4 °C (additional data in ESM). Moisture and ash content of PPI were determined according to AOAC (ESM, Reference 5).
Protein Content and Yield
The protein content of flour, ALB, GLB, and PPI was determined by Kjeldahl method (N × 6.25) (ESM, Reference 5). Yield in weight and protein recovery of total seed protein extracted was calculated (ESM, eq. S1 and S2).
Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE was performed according to Acevedo et al. [4]. ALB, GLB, and PPI were dispersed (1 mg/mL protein) in sample buffer without (non-reduced conditions) or with 2-mercaptoethanol (reduced conditions, 2-ME) (5 mL/100 mL) and centrifuged (10,000×g, 20 min, 25 °C). Protein molecular weights (MW) were estimated using low molecular weight markers (St) (Pharmacia Hepar Inc., Franklin, OH, USA) (additional data in ESM).
Differential Scanning Calorimetry (DSC)
Differential scanning calorimetry (DSC) measurements were performed according to Peyrano et al. [22] (additional data in ESM).
Size Exclusion Chromatography (SEC)
Samples (20 mg of protein) were dispersed (1 h, 25 °C) in 1 mL of elution buffer A (0.05 mol/L Na2HPO4, pH 8, NaCl 0.15 mol/L). Dispersions were centrifuged (14,000 x g, 15 min, 20 °C). Supernatants (0.5 mL) were analyzed in a Superose 6 10/300 GL column linked to an ÄKTA purifier UPC10 GE Healthcare (Uppsala, Sweden) and eluted with the same buffer (0.2 mL/min). Fractions were collected and the elution profile was obtained at 214 nm. Protein peaks were pooled and precipitated with 12.5 g/100 mL trichloroacetic acid and recovered by centrifugation (14,000 x g, 20 min, 25 °C), washed with acetone and analyzed by SDS-PAGE. Column calibration was performed with High MW and Low MW gel filtration calibration kits (GE-Healthcare, Buckinghamshire, UK).
Fluorescence Spectroscopy
ALB, GLB, and PPI were dispersed (5 mg protein/mL) in buffer A, stirred (1 h, 25 °C) and centrifuged (10,000×g, 30 min, 25 °C). Intrinsic fluorescence spectra of supernatants were determined (FluoroMate FS-2 fluorescence spectrophotometer, Scinco, South Korea) at an excitation and emission wavelengths of 290 nm and 300–400 nm, respectively (slit width, 5 nm; scanning speed of 300 nm/min; 25 °C). The protein concentration was determined according to Lowry method.
Surface Hydrophobicity (Ho)
Ho of ALB, GLB, and PPI was determined according to Cardamone and Puri (ESM, Reference 6) with 1,8-aniline-naphthalene-sulfonate (ANS) as a fluorescent probe (Aldrich Chemical Co., Milwaukee, Wisconsin, USA) (additional data in ESM).
Protein Denaturation with Urea
ALB and GLB were dispersed (3 mg protein/mL) and stirred (1 h, 25 °C) in buffer A, and the experiments were done according to Quiroga et al. [23]. The intrinsic fluorescence spectra of solutions were determined as described previously (Fluorescence spectroscopy) and maximum emission wavelength (λmax) was plotted against urea concentration (additional data in ESM).
Attenuated Total Reflection Infrared Spectra (ATR-FTIR)
ALB, GLB, and PPI (10 mg of protein suspended in 100 μL of D2O) were analyzed by ATR-FTIR Thermo Nicolet iS10 spectrometer (Thermo Scientific, MA, USA). IR spectrums (4,000–400 cm−1) were registered by co-adding 16 scans (4 cm−1 spectral resolution). IR spectrum of amide I band of protein (1700–1600 cm−1) were deconvolved and fitted to Gaussian/Lorentzian profiles. Curve fitting was performed (OMNIC 8.3 software, Thermo Fisher Scientific Inc.).
Protein Solubility (So)
Protein solubility of ALB, GLB, and PPI in distilled water and in buffer A was determined following Bera and Mukherjee (ESM, Reference 7). So it was expressed as the percentage ratio between the soluble protein in the supernatants determined by Lowry method and the total protein content measured by Kjeldahl method (ESM, Reference 5). Bovine serum albumin was used as a standard.
Statistical Analysis
Two batches of each protein extraction (ALB, GLB, PPI8, PPI9, PPI10, and PPI11) were performed. The batches of each ALB, GLB, PPI8, PPI9, PPI10, and PPI11 were mixed. Each experimental measure (DSC, SEC, Fluorescence spectroscopy, Ho, protein denaturation with urea, FTIR, and So) was performed in triplicate. Analysis of variance (ANOVA) of data was performed using Infostat software (ESM, Reference 8). The least significant difference (LSD) test with interval confidence of 95% was used to compare the means of results.
Results and Discussion
Protein Extractability
ALB and GLB fractions were obtained from PP flour (protein content 22.30 ± 0.81%). Yield in weight of ALB and GLB fractions was 30.55 ± 1.18% and 6.57 ± 1.21%, respectively, while protein yield recovery was similar for ALB and GLB fractions (26.58 ± 1.19% and 22.26 ± 1.25%). Even though the protein recovery of GLB fraction was lower than those reported by other authors (50–60% of the total protein) [7, 8], the protein content was elevated (90.84 ± 2.24%). Besides, ALB and GLB fractions showed distinct sets of polypeptides in SDS-PAGE under non-reduced conditions (Fig. 1a), which is in coincidence with that informed in previous works for PP flour [4, 24]. The low protein recovery yield of GLB fraction obtained in this work could be due to the protein extraction method used [7, 8; additional data in ESM, References 1, 9] or to the type of legume analyzed [25; additional data in ESM, References 1, 10, 11].
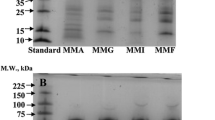
SDS-PAGE patters of Pigeon pea protein fractions and PPI. a ALB: albumin fraction; GLB: globulin fraction, under non-reduced condition; b ALB; GLB under reduced condition. c PPI: Pigeon pea protein isolates at different pHs (8, 9, 10, 11), under non-reduced condition; d PPI under reduced condition. Lane 8: PPI8; lane 9: PPI9; lane 10: PPI10; lane 11: PPI11. St: Low molecular weight standard
There were no significant differences (p ≥ 0.05) among protein recovery of PPI8, PPI9, and PPI10 (49.99 ± 1.51%, 50.10 ± 2.69%, 51.17 ± 2.46%, respectively); while PPI11 showed higher protein recovery (67.50 ± 5.64%) (ESM, Table S1). Protein recovery of PPI were higher than those reported by Mwasaru et al. [16] (35–50%), but similar to those informed by Peyrano et al. [22] for cowpea PI (56–61%). Even though, Mizubuti et al. [15] reported 74.8% of yield at the optimal protein extraction conditions, the authors did not mention the protein recovery after an isoelectric precipitation. The moisture content of PPI8 was the highest while the ash content increased with the pH of extraction, probably due to the increment in salt content (ESM, Table S1).
Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
ALB fraction presented polypeptides of 73, 63, 55, 43, 38, 28–20, and 17 kDa (Fig. 1a). GLB fraction presented protein aggregates at the top of the stacking gel, protein aggregates with molecular weight (MW) >94 kDa in the resolving gel and polypeptides of 65, 52, and 47 kDa (Fig. 1a), which belong to 7S vicilin [6]. Polypeptides with MW <30 kDa were also observed but in minor proportion than ALB fraction. Protein aggregates (MW >94 kDa) could be formed by the subunits of 7S vicilin [26]. Under reduced condition (Fig. 1b), ALB fraction showed a major intensity of some polypeptides (43, 38, and 20 kDa) and a new band (MW <14 kDa) was noted. GLB protein aggregates (stabilized by disulfide bonds) were dissociated, which was evidenced by an increase in intensity of 7S vicilin bands and the presence of polypeptides <30 kDa. On the other hand, polypeptides of legumin-like globulins (11S) are in much less extent as was previously informed by Krishna et al. [6] and they only can be noticed under reduced condition.
PPI8, PPI9, PPI10, and PPI11 resulted in similar electrophoretic patterns (Fig. 1c), since polypeptides of the same MW were found (>94, 65, 52, 47, 40, <30 kDa); polypeptides corresponding to 7S vicilin were in greater proportion than those from ALB fraction, similar to those reported by Acevedo et al. [4] for PP flour. At non-reduced condition, as the pH of protein extraction increased the soluble protein aggregates (MW >94 kDa) decreased in the resolving gel, while a higher proportion of large aggregates that did not enter to the stacking gel were found (Fig. 1c, arrow). The increment of protein aggregates could be attributed to protein structure rearrangements associated with protein denaturation provoked by pH. Under reduced condition (Fig. 1d), the intensity of protein aggregates decreased, mainly in PPI8 and PPI9, but did not disappear whereas an increase in the intensity of bands related to 7S vicilin and to polypeptides of 40 and 23–18 kDa were found. Polypeptides of 30 kDa were also detected in PPI9, PPI10, and PPI11. The presence of protein aggregates that remain even under reduced condition suggests that other interactions than disulfide bonds could be operating as a result of denaturation induced by pH. Other authors informed similar behavior when denaturation process by other treatments was studied [22, 27; additional data in ESM, Reference 12].
Differential Scanning Calorimetry (DSC)
ALB fraction did not present a detectable peak (ESM, Fig. S1), which could be due to the high flexibility in the tertiary conformation of its polypeptides, as was reported by Tang and Wang [28] for buckwheat ALB fraction. Avanza et al. [25] informed low enthalpy change of transition (ΔH) (0.46–1.77 J/g) for ALB of cowpea. GLB fraction showed one endothermic peak (ESM, Fig. S1) with a denaturation temperature (Td) value of 90.37 ± 0.05 °C and ∆H of 10.28 ± 0.05 J/g (Table 1), which suggest that thermal denaturation is more associated to the rupture of hydrogen bond associations (endothermic) than to the weakening of hydrophobic interactions and aggregation of proteins (exothermic). Similar Td but lower ∆H (0.79–1.58 J/g) for cowpea GLB fraction were informed [25], indicating that PP GLB fraction might be more stable to heat.
PPI showed only one endothermic peak (ESM, Fig. S1) and the Td increased with the increment of pH of protein extraction up to 10 (Table 1), resulting in PPI more stable to heat; the increment in thermal stability could be associated to the increment of salts (NaCl) formed during the protein extraction [27]. The increment of salts also was evidenced by the increment in ash content (Table S1). ΔH of PPI decreases slightly with increasing pH, where the lowest value was obtained at pH 11 (Table 1) denoting protein denaturation as a consequence of the increment in pH. Protein denaturation by pH could promote the aggregation of PP proteins as it was observed in SDS-PAGE (Fig. 1c, d). PPI obtained in this work are more stable to heat (higher Td and ΔH) than those obtained by Mwasaru et al. [16], which means that proteins were less denaturated during the extraction steps. The fact that food-based proteins have high Td (as PPI) facilitates the application of thermal processes on food matrices without causing major changes in protein structure. The complete denaturation of proteins would be expected to limit their functional properties [29] and to affect their digestibility resulting in less benefit from the nutritional point of view.
Size Exclusion Chromatography (SEC)
ALB fraction (Fig. 2a, grey line) showed three peaks (1′, 2′, and 3′); where species of MW ≈ 135 kDa eluted at peak 1′, species of MW ≈ 60–19.5 kDa at peak 2′ and species of MW <13.7 kDa at peak 3′. The SDS-PAGE profile of ALB-peaks showed that peak 1` had not well-defined bands of polypeptides, while in peak 2′ polypeptides of 45 and 20 kDa were found and in peak 3′ no band was observed (not shown). GLB fraction (Fig. 2a, black line) was resolved in four main peaks (1, 2, 3, 4), where species eluted had higher molecular weight than those obtained for ALB fraction. Based on chromatographic profile, soluble protein aggregates eluted at void volume (V0) (peak 1), species of MW ≈ 250 kDa eluted at peak 2, species of MW ≈ 63 kDa at peak 3 and species of MW ≈ 19.4 kDa at peak 4. The SDS-PAGE profile of GLB-peaks showed the presence of soluble aggregates (MW >94 kDa) (peak 1) and mainly polypeptides of 7S vicilin (peak 1 and peak 2), a major wide band of 45 kDa and a faint band of 20 kDa (peak 3), whereas this last band was the principal component of peak 4.
Size exclusion chromatography and SDS-PAGE of eluted fractions. a ALB: albumin fraction, GLB: globulin fraction. b Pigeon pea protein isolates (PPI) obtained at pH 8 (PPI8) and pH 11 (PPI11). Arrows indicate the elution volumes of standard proteins (a-e). V0: Void volume (7.1 mL), a: 669 kDa, b: 150 kDa, c: 67 kDa, d: 43 kDa, e: 13.7 kDa. St: Low molecular weight standard
The chromatographic profiles of all PPI analysed were similar (five peaks), only slight differences in the proportion of species that eluted in peak 1 (V0) among PPI11 and the rest of PPI were observed; therefore, PPI8 and PPI11 are only shown in Fig. 2b. The chromatographic profiles of PPI were similar to GLB (Fig. 2a, black line). The SDS-PAGE of peaks (Fig. 2b) showed protein aggregates (MW >94 kDa) that eluted at peak 1 (V0) of PPI8, but they were absent in PPI11 (peak 1′). Peaks 2 and 3′ (3’a and 3’b) showed typical polypeptides characteristic of GLB fraction (Fig. 1a), while peaks 4 and 4′ showed polypeptides of 45 and 20 kDa, similar to those observed in ALB fraction (peak 2′) and in GLB fraction (peaks 3 and 4) (Fig. 2a). The higher denaturation of PPI11 favoured the exposure of the interior of proteins that was not exposed in native conditions and allowed the formation of protein aggregates (Fig. 1c, d). At pH 11, the protein aggregates formed were less soluble under conditions used in SEC (Buffer A, pH = 8, μ = 0.3), therefore a reduction in the proportion of larger species eluted in peak 1 was observed. Similar results were found by Abugoch et al. [30] for quinoa proteins.
Fluorescence Spectroscopy
The fluorescence spectrum of proteins is related to the polarity of the environment surrounding the residues of tryptophan (Trp). The maximum emission wavelength (λmax) of ALB fraction was 346 nm with higher fluorescence intensity (FI) than GLB fraction, whose λmax was 333 nm (Table 1). These results suggest that Trp residues of ALB fraction were exposed to a more hydrophilic environment -i.e., closer to the surface- [23] and that GLB fraction presented a more compact tertiary structure. Similar results were reported for cowpea [25].
The increase in pH of extraction induced a red shift of λmax in PPI due to protein unfolding (Table 1), with greater exposure of aromatic residues to a more polar environment (ESM, Fig. S2). FI was higher in PPI8 than in the other PPI (Table 1). The FI decrease could be due to an increase in the distance between Trp and Tyr residues because of the unfolding process or to the presence of quenching agents like disulfide bonds [22] that were in PPI aggregates (Fig. 1c, d). Yin et al. [31] informed that protein isolates from Phaseolus vulgaris (pH 8) showed a λmax close to that obtained in this work, which is characteristic of Trp residues surrounded by a relatively hydrophobic environment.
Surface Hydrophobicity (Ho)
Surface hydrophobicity (Ho) is an important structural property related to functional properties of proteins, like solubility, emulsifying, and foaming properties. ALB fraction presented lower Ho than GLB fraction (Table 1), agreeing with Ho reported by Avanza et al. [25] for ALB and GLB fractions of cowpea. Ho increased from PPI8 to PPI11 only 10% despite the greater exposure of Trp residues to the solvent (red shift of λmax of PPI) (Table 1). Ho measure depends on the probe used to sense a hydrophobic zone (it must be an hydrophobic patch of several residues arranged close to each other, not only a Trp residue) and on the presence of hydrophobic residues inside the protein aggregates that could hinder the interaction ANS-hydrophobic regions. The Ho of PPI11 could favoured the formation of protein aggregates (Fig. 1c) and the presence of NaCl could reinforced the hydrophobic interactions [27]. Ho not only affect physicochemical properties, but also bioavailability and nutritional quality. The concentration in hydrophobic amino acids is unusually high in legume oligomeric proteins, and their presence on protein surface play a major role in protein stabilization and could decrease its susceptibility to proteolysis [32]. Ho of PPI were lower than those reported by Mwasaru et al. [16] for PPI and Peyrano et al. [22] for cowpea PI.
Protein Denaturation with Urea
Conformational stability of ALB and GLB fractions was studied by denaturation with urea, where the proposed mechanisms suggest that urea interface between water and natively buried parts of the protein and interrupts intramolecular hydrogen bond associations (ESM, Reference 13). ALB and GLB fractions presented a red shift of λmax as urea concentration increases between 2 and 8 mol/L (ESM, Fig. S3), evidencing a progressive unfolding and exposure of Trp residues on the protein surface [23], though the FI may either increase or decrease (ESM, Reference 14). Yin et al. [31] observed similar behavior of PI from Phaseolus vulgaris treated with urea (≥ 1.5 mol/L).
U80/U20 value of GLB fraction (2.85 ± 0.09%) was lower than ALB fraction (5.27 ± 0.02%) (ESM, Table S2), suggesting that denaturation processes in GLB fraction occurred more cooperatively [23] than in ALB fraction (protein segments unstablized by urea cooperate with one another to unfold the protein). The higher free energy denaturation (∆GH2O) of GLB fraction (2.37 ± 0.10 kcal/mol) suggests a higher conformational stability than ALB fraction (1.32 ± 0.10 kcal/mol), which agree with that found for amaranth globulins [23]. These results and those obtained by DSC and fluorescence spectroscopy, indicate that GLB fraction has a less flexible and more compact structure than ALB fraction.
ATR FTIR
ALB fraction, GLB fraction, and PPI present the characteristic amide I band of proteins (ESM, Fig. S4). The sensitivity of amide I to conformational changes makes possible the study of protein folding-unfolding and aggregation processes [19]. From the deconvolution of amide I band, the percentages of each secondary structure were obtained (ESM, Table S3). ALB and GLB fractions presented a higher proportion of β-sheet (41.57 ± 0.09% and 37.34 ± 6.50%, respectively) than α-helix (10.84 ± 1.19% and 13.71 ± 1.06%, respectively), which is in agreement with results reported for vicilins from PP [9]. ALB fraction presented a greater proportion (p < 0.05) of β-strand (15.56 ± 1.92%) in aggregated structures than GLB fraction (7.87 ± 2.69%).
PPI9, PPI10, and PPI11 presented a similar proportion of α-helix (14–16%), β-turns (18–20%), and random coil (25–32%). PPI8 showed a higher proportion of α-helix (22.36 ± 2.21%), a lower proportion of β-turns (9.35 ± 1.68%), and the absence of β-strands in aggregated structures. Also, PPI10 had a slight minor proportion of β-sheet structure than other PPI. Our results agree with those reported for proteins from legume seeds (common bean, chickpea, and lentil) [19; additional data in ESM, Reference 15]. The β-sheet values of PPI were lower and random coil values were higher to those obtained for ALB and GLB fractions; this was probably due to partial unfolding during protein extraction.
The proportion of β-sheet and β-strand had been related with protein digestibility [19, 20], and a decrease of β-sheet and/or an increment of random coil contributes to increase protein digestibility [20]. Considering that ALB and GLB fractions presented equal proportion of β-sheet, GLB fraction could be more digestible than ALB fraction because of the minor proportion of β-strands [19]. Because of the absence of β-strands in PPI8 and the minor proportion of β-sheet in PPI10, both could be more digestible than the other PPI.
Protein Solubility (So)
Solubility of ALB, GLB, and PPI was analyzed as an important criterion for the applicability of seed proteins in food matrices. ALB fraction presented similar So in all solutions while GLB fraction exhibited a higher So in buffer A compared to distilled water (21%). The fact that GLB fraction was highly soluble under conditions of buffer A is favorable thinking on using these proteins as food ingredients. So of PPI in distilled water was 60–75%, but decreased in buffer A (Table 1) due to a “salting out” effect because of the presence of salts in buffer composition (μ = 0.3). Also, the formation of aggregates in PPI changes the solubility properties of proteins. While PPI10 presented the highest So in all solutions, PPI11 had the lowest So, which could be related to the highest hydrophobicity of PPI11 (Table 1) and hence the formation of insoluble aggregates was favoured; this fact is in concordance with data from SEC, where a decrease of soluble aggregates was observed (Fig. 2b). So of PPI in distilled water was similar to those reported by Fernández Sosa et al. [24] for PP flour and higher than those reported by Mwasaru et al. [16] for PPI (<10%), which may be due to lower denaturation degree of proteins.
Conclusion
The results obtained in this work showed that GLB fraction in PP presented a more compact and less flexible structure than ALB fraction due to the distinct conformational characteristics found in DSC, fluorescence spectroscopy, Ho. Furthermore, GLB fraction had a greater conformational stability with a more cooperative denaturation process than ALB fraction. The latter presented a higher proportion of β-strand in aggregated structures. PPI showed physicochemical properties and electrophoretic patterns closer to GLB fraction than ALB fraction. Also, the pH of extraction influenced on conformational changes reflected on PPI physicochemical properties. PPI11 showed the highest protein recovery, but the least So in distilled water and buffer A with more presence of protein aggregates with the least proportion of β-strands in aggregated structures. The protein structure was more open and thermally stable, but more denatured than the other PPI. Even though, protein fractions and PPI are different and complex structures, the study of similarities and differences on their physicochemical and structural properties enriches and deepens the knowledge of physicochemical behavior of protein isolates. Although, no extraction methods can be claimed to be the best in all criterion, the variation of pH for protein extraction constitutes a simple, rapid, and low-cost method to obtain PPI with physicochemical and structural properties that will determine its functional properties and their use as food ingredients.
Abbreviations
- ALB:
-
albumin fraction
- GLB:
-
globulin fraction
- ΔH:
-
enthalpy change of transition
- FI:
-
fluorescence intensity
- ∆GH2O :
-
free energy of denaturation
- λmax :
-
maximum emission wavelength
- 2-ME:
-
2-mercaptoethanol
- MW:
-
molecular weight
- PI:
-
protein isolates
- PP:
-
pigeon pea
- PPI:
-
pigeon pea protein isolates
- SEC:
-
size exclusion chromatography
- So:
-
protein solubility
- Td:
-
denaturation temperature
- Trp:
-
Tryptophan
- V0 :
-
void volume of the column
References
FAO (2018) Legumbres. Pequeñas semillas, grandes soluciones. Ciudad de Panamá
Varshney RK, Saxena RK, Jackson SA (2017) The Pigeonpea genome. Compendium of plant genomes. Springer, Cham
Chitra U, Singh U, Rao VR (1996) Phytic acid, in vitro protein digestibility, dietary fiber, and minerals of pulses as influenced by processing methods. Plant Foods Hum Nutr 49:307–331. https://doi.org/10.1007/BF01091980
Acevedo BA, Avanza MV, Chaves MG, Ronda F (2013) Gelation, thermal and pasting properties of pigeon pea (Cajanus cajan L.), dolichos bean (Dolichos lablab L.) and jack bean (Canavalia ensiformis) flours. J Food Eng 119(1):65–71. https://doi.org/10.1016/j.jfoodeng.2013.05.014
Akande KE, Abubakar MM, Adegbola TA, Bogoro SE, Doma UD (2010) Chemical evaluation of the nutritive quality of pigeon pea Cajanus cajan L. Millsp. Int J Poultry Sci 9:63–65
Krishnan HB, Natarajan SS, Oehrle NW, Garrett WM, Darwish O (2017) Proteomic analysis of pigeon pea (Cajanus cajan) seeds reveals the accumulation of numerous stress-related proteins. J Agric Food Chem 65:4572–4581. https://doi.org/10.1021/acs.jafc.7b00998
Singh U, Jambunathan R (1982) Distribution of seed protein fractions and amino acids in different anatomical parts of chickpea (Cicer arietinum L.) and pigeonpea (Cajanus cajan L.). Plant Foods Hum Nutr 31:347–354. https://doi.org/10.1007/BF01094046
Singh U, Jambunathan R, Saxena K, Subrahmanyam N (1990) Nutritional quality evaluation of newly developed high-protein genotypes of pigeonpea (Cajanus cajan). J Sci Food Agric 50(2):201–209
Mawal Y, Mawal M, Ranjekar P (1990) Unusual denaturation properties of vicilin from Cajanus cajan. Biochem Biophys Res Commun 166(3):1446–1452. https://doi.org/10.1016/0006-291X(90)91029-R
Prema L, Kurup PA (1973) Effect of protein fractions from Cajanus cajan (redgram) and Dolichos biflorus (horsegram) on the serum, liver and aortic lipid levels in rats fed a high-fat-high-cholesterol diet. Atherosclerosis 18(3):369–377. https://doi.org/10.1016/0021-9150(73)90067-1
Shevkani K, Singh N, Kaur A, Rana JC (2015) Structural and functional characterization of kidney bean and field pea protein isolates: a comparative study. Food Hydrocoll 43:679–689. https://doi.org/10.1016/j.foodhyd.2014.07.024
Tanger C, Engel J, Kulozik U (2020) Influence of extraction conditions on the conformational alteration of pea protein extracted from pea flour. Food Hydrocoll 107:105949. https://doi.org/10.1016/j.foodhyd.2020.105949
Adenekan MK, Fadimu GJ, Odunmbaku LA, Oke EK (2017) Effect of isolation techniques on the characteristics of pigeon pea (Cajanus cajan) protein isolates. Food Sci Nutr 6:146–152. https://doi.org/10.1002/fsn3.539
Akintayo ET, Oshodi AA, Esuoso KO (1999) Effects of NaCl, ionic strength and pH on the foaming and gelation of pigeon pea (Cajanus cajan) protein concentrates. Food Chem 66:51–56. https://doi.org/10.1016/S0308-8146(98)00155-1
Mizubuti IY, Biondo Júnior O, De Oliveira Souza LW, Dos Santos Ferreira Da Silva RS, Ida EI (2000) Response surface methodology for extraction optimization of pigeon pea protein. Food Chem 70(2):259–265. https://doi.org/10.1016/S0308-8146(00)00078-9
Mwasaru MA, Muhammad K, Bakar J, Man YBC (1999a) Effects of isolation technique and conditions on the extractability, physicochemical and functional properties of pigeonpea (Cajanus cajan) and cowpea (Vigna unguiculata) protein isolates. I. Physicochemical properties. Food Chem 67(4):435–443. https://doi.org/10.1016/S0308-8146(99)00150-8
Mwasaru MA, Muhammad K, Bakar J, Man YBC (1999b) Effects of isolation technique and conditions on the extractability, physicochemical and functional properties of pigeonpea (Cajanus cajan) and cowpea (Vigna unguiculata) protein isolates. II. Functional properties. Food Chem 67(4):445–452. https://doi.org/10.1016/S0308-8146(99)00151-X
Cui L, Bandillo N, Wang Y, Ohm J-B, Chen B, Rao J (2020) Functionality and structure of yellow pea protein isolate as affected by cultivars and extraction pH. Food Hydrocoll 108:106008. https://doi.org/10.1016/j.foodhyd.2020.106008
Carbonaro M, Maselli P, Nucara A (2012) Relationship between digestibility and secondary structure of raw and thermally treated legume proteins: a Fourier transform infrared (FT-IR) spectroscopic study. Amino Acids 43(2):911–921. https://doi.org/10.1007/s00726-011-1151-4
Sun X, Ohanenye IC, Ahmed T, Udenigwe CC (2020) Microwave treatment increased protein digestibility of pigeon pea (Cajanus cajan) flour: elucidation of underlying mechanisms. Food Chem 329:127196. https://doi.org/10.1016/j.foodchem.2020.127196
Rosa MJS, Ferreira RB, Teixeira AR (2000) Storage proteins from Lathyrus sativus seeds. J Agric Food Chem 48(11):5432–5439. https://doi.org/10.1021/jf000447r
Peyrano F, Speroni F, Avanza MV (2016) Physicochemical and functional properties of cowpea protein isolate treated with temperature or high hydrostatic pressure. Innov Food Sci Emerg 33:38–46. https://doi.org/10.1016/j.ifset.2015.10.014
Quiroga A, Martínez EN, Rogniaux H, Geairon A, Añón MC (2009) Globulin-p and 11S-globulin from Amaranthus hypochondriacus: are two isoforms of the 11S-globulin. Protein J 28:457–467. https://doi.org/10.1007/s10930-009-9214-z
Fernández Sosa E, Thompson C, Chaves MG, Acevedo BA, Avanza MV (2019) Legume seeds treated by high hydrostatic pressure: effect on functional properties of flours. Food Bioprocess Technol 13(2):323–340. https://doi.org/10.1007/s11947-019-02386-9
Avanza MV, Acevedo BA, Chaves MG, Aphalo P, Añón MC (2015) Physicochemical and structural properties of major protein fractions of two varieties of NEA-cowpea (Vigna unguiculata L.): a comparative study. Int J Food Nutr Sci 4(4):240–247
Petruccelli S, Añón MC (1995) Soy protein isolate components and their interactions. J Agric Food Chem 43:1762–1767. https://doi.org/10.1021/jf00055a004
Peyrano F, de Lamballerie M, Avanza MV, Speroni F (2017) Calorimetric study of cowpea protein isolates. Effect of calcium and high hydrostatic pressure. Food Biophys 12(3):374–382. https://doi.org/10.1007/s11483-017-9493-4
Tang CH, Wang XY (2010) Physicochemical and structural characterization of globulin and albumin from common buckwheat (Fagopyrum esculentum Moench) seeds. Food Chem 121(1):119–126. https://doi.org/10.1016/j.foodchem.2009.12.016
Arntfield SD, Murray ED (1981) The influence of processing parameters on food protein functionality. I Differential scanning calorimetry as an indicator of protein denaturation. Can Inst Food Sci Technol J 14(4):289–294. https://doi.org/10.1016/S0315-5463(81)72929-8
Abugoch LE, Romero N, Tapia C, Silva J, Rivera MJ (2008) Study of some physicochemical and functional properties of quinoa (Chenopodium quinoa Willd) protein isolates. J Agric Food Chem 56:4745–4750. https://doi.org/10.1021/jf703689u
Yin SW, Tang CH, Yang XQ, Wen QB (2011) Conformational study of red kidney bean (Phaseolus vulgaris L.) protein isolate (KPI) by tryptophan fluorescence and differential scanning calorimetry. J Agric Food Chem 59(1):241–248. https://doi.org/10.1021/jf1027608
Carbonaro M, Maselli P, Nucara A (2015) Structural aspects of legume proteins and nutraceutical properties. Food Res Int 76:19–30. https://doi.org/10.1016/j.foodres.2014.11.007
Funding
The authors acknowledge the financial support from the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) and the Universidad Nacional del Nordeste (UNNE) and CONICET, Argentina. M. V. Avanza and A. V. Quiroga are research members of the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Declaration of Interest
We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 340 kb)
Rights and permissions
About this article
Cite this article
Fernández Sosa, E.I., Chaves, M.G., Quiroga, A.V. et al. Comparative Study of Structural and Physicochemical Properties of Pigeon Pea (Cajanus cajan L.) Protein Isolates and its Major Protein Fractions. Plant Foods Hum Nutr 76, 37–45 (2021). https://doi.org/10.1007/s11130-020-00871-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-020-00871-7