Abstract
Kabuli chickpea is traditionally consumed in Mexico. It is currently exported to countries including United States where its demand has recently increased. In order to demonstrate the effect of thermal processes on the quality of fresh chickpea, the objective of the present work was to evaluate some nutrimental and functional characteristics of raw, steamed and toasted chickpea. The partial chemical composition, total phenols, oligosaccharides, and antioxidant capacities were measured in five genotypes of chickpea. Steamed and toasted chickpea showed up to 8.4 and 25.8% less protein, respectively, than that of raw samples. Oligosaccharides, in general decreased in steamed and toasted fresh grain; however, verbascose increased on average 30.6 and 37.9% in steamed and toasted samples, respectively. Minor changes in total phenolic content were observed a result of the process. Trolox equivalent antioxidant capacity increased up to 3.5 times compared to that of antioxidant capacity of raw samples. Fresh chickpea grain, raw or processed, shows attractive nutritional and antioxidant properties that can contribute to the diet and health of the person who consumes it.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The grain-legume chickpea is the third-most important pulse crop in the world with a global production of 11.3 Mt [1]. The producing countries are India, Australia, Myanmar, Pakistan, Turkey, Iran, Mexico, United States and Canada; India contributes with approximately 74% [2]. Chickpea seeds vary in size, shape and color [3]. Based on these characteristics, chickpea genotypes are classified into two distinct types: Kabuli (white-cream seeded) and Desi (yellow-brown seeded). In Mexico Kabuli is mainly used for human consumption, both fresh and dry, while Desi is used as fodder. Kabuli shows 18.3% protein, 4.9% lipids, 50–61 mg/g total phenols and up to 11 μg/g total flavonoids [4, 5]. Chickpea is the second pulse crop consumed below common bean (Phaseolus vulgaris L.) in Mexico. The growth circle for fresh chickpea is days shorter than that of dry chickpea grain, therefore. it needs less water for irrigation and its economic value is generally grater [6]. The production of high-quality Kabuli chickpea grain in near 100,000 ha, places Mexico in the third place worldwide among exporting countries, below India and Turkey [7].
Dry chickpea contains higher protein, dietary fiber, mineral micronutrients, and oligosaccharides compared to other dry legumes [8,9,10,11]. It is also a source of phenolic compounds such as flavonoids with high antioxidant capacity [4, 12]. Therefore, chickpea could be considered as a functional food due to its beneficial effects on health [13]. Traditionally, fresh Kabuli chickpea (FKCH) is consumed steamed or toasted, processes that require short cooking times; occasionally it is consumed raw. The minimal times for FKCH cooking, allows the preservation of its nutritional and functional qualities [14]. On the other hand, FKCH consumption started few years ago in Mexico, Israel, Canada and United States. Mexico is one of the major exporters of FKCH to the United States. Recently, FKCH has appeared in the markets and it is increasing in popularity [15]. To our knowledge, there are no reports on nutritional and functional characteristics of FKCH. In order to demonstrate if thermal processes improve the quality of chickpea, the objective of the present work was to evaluate some nutrimental and functional characteristics of raw, steamed and toasted FKCH.
Materials and Methods
Chickpea Samples
Five chickpea Kabuli genotypes were used, namely Blanoro, Costa 04, Blancoson, Cuga 093, and Hoga 067. The genotypes were produced during the fall-winter 2017/18 season at the Bajío Experimental Station (INIFAP) in Celaya, Gto. Mexico (20° 34′ 48.75” N, 100° 49′ 16.490” W) as follows: The soil is a typic pellusterts organic matter and clay in texture. The experimental plot was irrigated 18 days before sowing on January 17, 2018. Chemical fertilizer was applied at a rate of 40–40-00 units of N-P-K before planting. Experimental plots consisted of four rows 6 m in length, rows 76 cm apart. Before flowering two mechanical cultivations were given to control weeds and supplemental irrigation applied at 65 days after sowing. The FKCH was harvested when grain was fully developed inside the pod but before starting the drying process. Three independent lots of each genotypes were collected: Grain of one lot was hand peeled, freeze-dried and stored until analysis, pods of the second and third lots were steamed (10 min, 90 °C) or toasted on a hot clay dish as follows: The clay dish was placed on a gas stove and preheated to 125 °C. Immediately afterwards, 30-g portions of chickpea were placed and left to cook for 10 min, stirring from time to time. Then grains were hand peeled, lyophilized, and stored until analysis.
Partial Chemical Composition
Protein (2001.11) (conversion factor, 6.25), ether extract (920.39), and ash (942.05) were determined according to the procedure described in Association of Official Analytical Chemists [16]. Dietary fiber was determined using an enzymatic-gravimetric method [17].
HPLC Oligosaccharide Determination
Samples (500 mg) were extracted with aqueous ethanol (80%) and agitation at room temperature for 10 min. After agitation procedure, samples were centrifuged (5 min 7000 rpm) and supernatant was recovered. The extraction procedure was repeated three times in each sample and supernatants were mixed, freeze-dried and resuspended in 5 mL of HPLC-grade water.
The identification and quantification of the raffinose oligosaccharide family (R-OCHF) was carried out by an Agilent 1100 series HPLC system (Agilent, USA) with a refraction index detector and a Zorbax eclipse XDB-C18 column (4.6 × 250 mm) and a pre-column Xorbax NH2 (4.6 mm ×125 mm), using acetonitrile and-water (75:25, v/v) as a mobile phase with a flow rate of 1 ml/min [18]. Oligosaccharides were identified by comparing the retention times with those of commercial standards (SIGMA).
Total Phenols and Antioxidant Capacity
Total phenols were assessed following the methodology of Wolfe et al. [19]. Meanwhile antioxidant capacities were determined following reported methods for Trolox equivalent antioxidant capacity (TEAC) [20], oxygen radical absorbance capacity (ORAC) [21], and ferric reducing ability of plasma (FRAP) [22].
Statistical Analysis
Data were reported as mean ± SD of four replicates (n = 4). The data were analyzed using JMP.5.0.1 software (A Business Unit of SAS, 1989–2003, SAS Institute, Cary, NC,
USA). Treatment results were subjected to an ANOVA (2-factor design, genotype and type of process) and means separated by Tukey test at a 0.05 level of significance.
Results and Discussion
Partial Chemical Composition
Moisture content of samples ranged from 72 to 76% (data not shown). On the other hand, steamed and toasted FKCH showed on average, 6.4 to 8.4% and 6.9 to 26.4% less (p < 0.05) protein and lipids, respectively, than the raw grain. Reduction on protein content could be attributed to several factors such as leaching, the formation of insoluble complexes with tannins [23], and thermal denaturation that leads to degradation or reaction with other components [24]. However, the analytical method employed in this study relied on determination of total nitrogen, thus it is more probably that reduction is the result of leaching. The same effect could explain the lipid reduction. The protein content of samples was almost three times lower than that reported for dry chickpea (18.7–30.95%) [8, 9, 25].
On average, lipids of steamed and toasted chickpea were reduced 1% when compared to FKCH. Lipid content in raw, steamed or toasted FKCH were two to three times lower than those reported by Garzón-Tiznado et al. [8] (6.01–9.27%) and Gupta et al. (4.25–6.98%) [26] for raw chickpea. On average, dietary fiber was minimally reduced (3%) both in steamed and toasted FCCH (Table 1), which is contrary to increments reported by Vasishtha and Srivastava [27] in chickpea. Apparently, temperature and time of steam and toast processes used here do not soft the cotyledonous tissue or lead to conversion of native protopectin to pectin to increment dietary fiber [27]. Dietary fiber content of raw, steamed, and toasted FKCH were approximately 70% lower to those reported by Tos et al. [5] for dry chickpea. The ash content in FKCH was, 4.25% higher than that reported by Garzón Tiznado et al. [8] (3.02–3.41%) for dry chickpea.
HPLC Oligosaccharide Determination
Raffinose, stachyose and verbascose were detected in all samples. Differences in R-OCHF content were observed between genotypes analyzed (Table 2); for example, Cuga 093 showed 1.9, 2.4, and 3.6 times more stachyose than Blancoson, Hoga 067 and Blanoro, respectively. The content of raffinose and verbascose in raw grain showed similar levels among genotypes with the exception of Blancoson, which showed lower content of verbascose (14.9 mg/100 g) compared to the rest of the genotypes.
Of the three oligosaccharides identified, verbascose showed the highest content on the analyzed genotypes, both raw, steamed or toasted (Table 2). The high content of verbascose showed by steamed and toasted samples cannot be explained based on the results of this work. There are no references that reports increments of R-OCHF in processed grains.
Steamed or toasted Blancoson, Cuga 093 and Hoga 067 samples showed a reduction of stachyose and steamed or toasted Blanoor, Costa 04, Blancoson, Hoga 067 samples showed a reduction of raffinose. Increments showed by several chickpea samples analyzed here were not in line with those reported for boiling, autoclaving, and microwave chickpea, lentils, soybean and pea, processes which effectively reduced R-OCHF [11].
FKCH contains up to 141 time less R-OCHF when compare to those reported by Xiaoli et al. [18] (2260–3870 mg/100 g), and Han and Baik [11] (7720 mg/100 g) for dry chickpea. It is well known that humans are not able to hydrolyze non-digestible fiber including R-OSCHF, which arrives intact to the large intestine where after fermentation produce several gases including carbon dioxide, hydrogen and methane contributing to flatulence that discourages human consumption [28]. The discomfort caused by R-OCHF fermentation in humans could be much lower when consuming immature chickpea grain in comparison with dry chickpea and other legumes such as lentils, yellow peas, green peas, and soybean, which show R-OCHF contents between 6570 and 7720 mg/100 g [10]. However, it should be remembered that gas production contributes to an increased volume and decreased fecal transit time [29]. In addition, the beneficial effect of chickpea on human health through fermentation of nondigestible compounds, including R-OSCHF, has been widely reported [29, 30].
Total Phenols
Similar contents of total phenols were detected in raw FKCH with exception of Blanoro cultivar who shows from 9 to 12% less concentration than those detected in the other genotypes (Table 3). Meanwhile, after steaming, cultivars Blanoro and Cuga 093 showed a reduction of total phenols from 7.4 to 9.4% compared to raw grain. The rest of genotypes showed minor losses after steaming. The toasting process did not decrease total phenol contents when compared with steamed grain, with exception of genotype Cuga 093 that showed reductions from 16.2 to 7.5%. Total phenol contents in toasted fresh grains reported here are different to those reported by Segev et al. [12] who reported a content up to 10-fold higher in toasted dry chickpea compared to those not subjected to such process.
The range in total phenols content in raw FKCH grain found in this research was similar to that reported by Thavarajah and Thavarajah [10] (260 to 370 mg/100 g) in ten different varieties. However, total phenols content was higher in steamed and toasted chickpea than those reported by Segev et al. [12] in roasted (225 mg/100 g) and frying (70 mg/100) in Kabuli cultivars; such differences might be due to process time and frying temperature used. It has been reported that oxidation of phenolics is responsible for 60% loss of the predominant phenol in olive oil after frying [31]. Based in our results we conclude that temperature of processes used here was not high enough to significatively oxidize the phenolics in FKCH. Also, the toast process does not activate the process of releasing bound polyphenols as suggested by Segev et al. [12].
Antioxidant Capacity
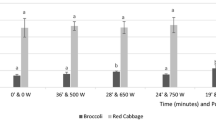
Of the three antioxidant capacities (AC) evaluated (TEAC, ORAC, and FRAP), TEAC was affected when FKCH was subjected to steaming or toasting (Fig. 1). Such effect was particularly visible on Blanoro, Costa 04, Hoga 067 and Blancoson, with increment in TEAC levels of 3.5, 1.5, 2.5 and 2.4 times respectively, compared to those of raw grain.
a) Trolox equivalent (TEAC, b) oxygen radical (ORAC), and c) ferric reducing (FRAP) antioxidant capacities (μmol TE/100 g, DB) of □ raw,  steamed, and
steamed, and  toasted fresh chickpea cultivars. Bars with the same letter are not statistically different (Tukey, 0.05). The comparison of means refers to that made between the type of process and the raw grain
toasted fresh chickpea cultivars. Bars with the same letter are not statistically different (Tukey, 0.05). The comparison of means refers to that made between the type of process and the raw grain
The FRAP measures the capacity by reducing the ferric (Fe3+) to ferrous (Fe2+) ion [32]. The ORAC method measures the ability of antioxidant compounds to specifically scavenge reactive oxygen species (ROS) through the donation of a hydrogen cation. Lastly, the TEAC method measures the ability of antioxidant compounds to scavenge the radical cation ABTS•+ by donating an electron. Raw, steamed or toasted FKCH, showed higher values of FRAP, reported as Trolox Equivalent (TE), compared to those reported by Segev et al. [12] on baking (0.6 μmol TE/100 g), frying (up to 1.2 μmol TE/100 g) and toasting (2.4 μmol TE/100 g) and by Quintero et al. [33] (0.04 to 1.18 μmol TE/100 g) for different kabuli genotypes. Consequently, antioxidant compounds in immature chickpea showed a higher capacity to reduce ferric Fe3+ to a ferrous Fe2+ ion than non-fresh grain processed and processed dry chickpea.
ORAC values in FKCH were up to 100-fold higher than those reported by Garzón-Tiznado et al. [8] (0.439–0.539 μmol TE/100 g) for dry chickpea cultivars, demonstrating that FKCH showed higher capacity to turn off ROS ions. Although the ORAC and TEAC methods cannot be compared because they measure different mechanisms to turn off free radicals, there are no reports on the TEAC antioxidant capacity in FKCH. We found that TEAC values were similar to those of ORAC especially in processed FKCH. Aguilera et al. [34] identified 25 phenolic components in chickpea by HPLC, being isoflavones the main group. The authors emphasize the concordance between the high antioxidant capacity and the presence of isoflavones.
Conclusions
The results of this work show that steam and toast processes reduce the nutritional quality, the phenolic content and the antioxidant activity levels of FKCH, with the exception of verbascose which is significantly increased. However, we demonstrated that steamed and toasted FKCH retain attractive protein, fiber, fat and total phenolic contents as high levels of antioxidant capacity compared to FKCH. Given the popularity of this food, raw, steamed of toasted chickpea will provide more compounds with biological activity that many snacks, especially in underdeveloped countries. Thus, this staple food can contribute positively to the nutrition and health in regular consumers.
Abbreviations
- FKCH:
-
Fresh Kabuli chickpea
- FRAP:
-
Ferric reducing ability
- HPLC:
-
High performed liquid chromatography
- R-OCHF:
-
Raffinose oligosaccharide family
- TEAC:
-
Trolox equivalent antioxidant capacity
- ORAC:
-
Oxigen radical antioxidant capacity
References
FAOSTAT (2014) Statistics data. Food and Agriculture Organization. Rome, Italy. http://faostat.fao.org/. Accessed on January 2020
http://atlasbig.com/en-us/countries-chickpea-production. Accessed on January 2020
Singh U, Subrahmanyam N, Kumar J (1991) Cooking quality and nutritional attributes of some newly developed cultivars of chickpea (Cicer arientum). J Sci Food Agric 55:37–46
Segev A, Badani H, Galili L, Hovav R, Kapulnik Y, Galili S, Shomer I (2011) Total phenolic content and antioxidant activity of chickpea (Cicer arietinum L.) as affected by soaking and cooking conditions. Food Nut Sci 2:724–730
Tosh SM, Farnworth ER, Brummer Y, Duncan AM, Wright AJ, Boye JI, Marcotte M, Benali AM (2013) Nutritional profile and carbohydrate characterization of spray-dried lentil, pea and chickpea ingredients. Foods 2:338–349
Badani H, Katsir I, Shemesh D, Gera G (2010) Influence of sowing date on yield of fresh-harvested chickpea. J Agric Sci 2(4):83–88
SIAP (2013) Agricultural and fisheries information service. Agricultural statistics of agricultural production by crop. SAGARPA http://www.siap.gob.mx/cierre-de-la-produccion-agricola-por-cultivo/ accessed on January 2020
Garzón-Tiznado JA, Ochoa-Lugo MI, Heiras-Palazuelos MJ, Domínguez-Arispuro DM, Cuevas-Rodríguez EO, Gutiérrez-Dorado R, Milán-Carrillo J, Reyes-Moreno C (2012) Acceptability properties and antioxidant potential of desi chickpea (Cicer arietinum L.) cultivars. Food Nutr Sci 3:1281–1289
Vasishtha H, Srivastava RP, Verma P (2014) Effect of dehusking and cooking on protein and dietary fiber of different genotypes of desi, kabuli and green type chickpeas (Cicer arientum). J Food Sci Technol 51(12):4090–4095
Thavarajah D, Thavarajah P (2012) Evaluation of chickpea (Cicer arientum) micronutrients composition: biofortification opportunities to combat global micronutrient malnutrition. Food Res Int 49:99–104
Han IH, Baik B-K (2006) Oligosaccharide content and composition of legumes and their reduction by soaking, cooking, ultrasound, and high hydrostatic pressure. Cereal Chem 83(4):428–433
Segev A, Badani H, Galili L, Hovav R, Kapulnik Y, Galili S, Shomer I, Galili S (2012) Effect of baking, toasting and frying on total polyphenols and antioxidant activity in colored chickpea seeds. Food Nutr Sci 3:369–376
Jukanti AK, Gaur PM, Gowda CLL, Chibbar RN (2012) Nutritional quality and health benefits of chickpea (Cicer arietinum L.). British J Nutr 108:S11–S26
Galili S, Kitain S, Badani H (2008) Developing new uses for chickpea emphasis on healthy food. Nir Vatelem 3:8–10
Merga B, Haji J (2019) Economic importance of chickpea: production, value, and world trade. Cogent Food Agric 5:1615718. (https://doi.org/10.1080/23311932.2019.1615718)
AOAC (2005) Official method of analysis. Association of Official Analytical Chemists (AOAC), Washington DC, USA, 18th ed, 2005
Prosky L, Asp NG, Shcweizer TF, DeVaries J, Wand Furda I (1988) Determinacion of insoluble, soluble and total dietary fiber in foods and food products: interlaboratory study. J Assoc Off Anal Chem 71:10-17-1023
Xiaoli X, Liyi Y, Shuang H, Wei L, Yi S, Hao M, Jusong Z, Xiaoxiong Z (2008) Determination of oligosacharides contents in 19 cultivars of chickpea (Cicer arietinum L) seeds by high-performanse liquid chromatography. Food Chem 111:215–219
Wolfe K, Wu X, Liu RH (2003) Antioxidant activity of apple peels. J Agric Food Chem 51:609–614
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med 26:1231–1237
Ou B, Hampsh-Woodill M, Prior R (2001) Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as a fluorescent probe. J Agric Food Chem 49:4619–4626
Pulido R, Bravo L, Saura-Calixto F (2000) Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/ antioxidant power assay. J Agric Food Chem 48:3396–3402
Adamczyk B, Simon J, Kitunen V, Admaczyk S, Smolander A (2017) Tannins and their complex interactions with different organic nitrogen compounds and enzymes: old paradigms versus recent advances. ChemistryOpen 6:610–614. https://doi.org/10.1002/open.201700113
Duston TR, Orcutt MW (1984) Chemical changes in proteins produced by thermal processing. J Chem Educ 61(4):303–308
El-adawy TA (2002) Nutritional composition and antinutritional factors of chickpea (Cicer arietium L.) undergoing different cooking methods and germination. Plant Foods Hum Nutr 57(1):83–97. https://doi.org/10.1023/a:1013189620528
Gupta S. Liu C, Sathe SK (2019) Quality of a chickpea-based high protein snack. J Food Sci 84(6):1621-1630
Vasishtha H, Srivastava RP (2013) Effect of soaking and cooking on dietary fibre components of different type of chickpea genotypes. J Food Sci Technol 50(3):579–584
Cummings JH (1984) Constipation, dietary fiber and the control of large bowel function. Postgrad Med J 60:811–819
Tungland BC, Meyer D (2002) Nondigestible oligo- and polysaccharides: their physiology and role in human health and food. Comp Rev Food Sci Food Saf 3:90–109
Rachwa-Rosiak D, Nebesny E, Budryn G (2015) Chickpeas –composition, nutritional value, health benefits, application to bread and snacks: a review. Crit Rev Food Sci Nutr 55(8):1137–1145
Kaskalaki D, Kefi G, Kotsiov K, Tasioula-Margari M (2009) Evaluation of phenolic compound degradation in virgin olive oil during storage and heating. J Food Nutr Res 48(1):31–41
Benzie IFF, Strain JJ (1996) The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power: the FRAP assay. Anal Biochem 239:70–76
Quintero MF, Saracho-Peña A, Chave-Ontiveros J, Garzon-Tiznado JA, Pineda-Hidalgo KV, Delgado-Vargas F, Lopez-Valenzuela JA (2018) Phenolic profiles and their contribution to the antioxidant activity of selected chickpea genotypes from Mexico and ICRISAT collections. Plant Foods Hum Nutr 73:122–129. https://doi.org/10.1007/s11130-018-0661-61
Aguilera Y, Duñas M, Estrella I, Hernandez T, Benitez V, Esteban RM, Matin-Cabrejas MA (2011) Phenolic profile and antioxidant capacity of chickpeas (Cicer aritinum L.) as affected by a dehydration process. Plant Foods Hum Nutr 66(2):187–195. https://doi.org/10.1007/s11130-011-0230-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Human and Animal Rights
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Arevalo, I., Guzmán-Maldonado, S.H., Sanchez, S.M.M. et al. Steaming and Toasting Reduce the Nutrimental Quality, Total Phenols and Antioxidant Capacity of Fresh Kabuli Chickpea (Cicer arietinum L.). Plant Foods Hum Nutr 75, 628–634 (2020). https://doi.org/10.1007/s11130-020-00857-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-020-00857-5