Abstract
Processing is an important and essential component to enhance the digestibility of essential nutrients of grains. Dietary fibres play an important role in bringing health advantages in chickpea and help in lowering plasma cholesterol. Changes during soaking and soaking followed by cooking on cellulose, hemicellulose, lignin and pectin contents of four genotypes of desi type (KWR 108, JG 74, DCP 92-3 and BG 256), four genotypes of kabuli types (KAK 2, JKG 1, BG 1053, and L 550) and two genotypes of green seed type (BGD 112 and Sadabahar) of chickpeas (Cicer arietinum, L.) was studied. Cellulose, lignin and pectin increased during soaking and cooking, whereas hemicellulose increased during soaking but decreased drastically during cooking. Cellulose recorded an overall increase of 40% during cooking, followed by 15.7% and 15.2% increase in pectin and lignin, respectively during cooking of chickpea grain. Hemicellulose, on the contrary showed a decrease of 26.8% during cooking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The role of seed legumes in the diets of human beings is well known. Food legumes form an important part of the human diet, providing a high proportion of proteins, fats, carbohydrates, dietary fibres, B-group vitamins (thiamin, riboflavin, niacin), and minerals. Chickpea (Cicer arietinum L.) is one of the oldest and most widely consumed food legume in the world, particularly in tropical and subtropical areas. Based on seed colour and geographic distribution, the chickpea is grouped into two types: desi (Indian origin) and kabuli (Mediterranean and Middle Eastern origin). Kabuli cultivars are white to cream coloured and are used almost exclusively by cooking whole seeds as vegetable, whereas the seeds of desi cultivars are wrinkled at the beak with brown, fawn, yellow, orange, or green colour (Chavan et al. 1989).
The presence of anti-nutritional factors such as trypsin and chymotrypsin inhibitors, oligosaccharides, lectins and tannins (Lyimo et al. 1992; Muzquiz et al. 1999) limits the availability of nutrients present in the leguminous crops. Removal of these factors is achieved by soaking and cooking of dry grains of legumes. Soaking of legume grains reduces the levels of total sugars, α-galactosides, minerals, phytic acid and proteolytic enzyme inhibitors (Frias et al. 2000; Vidal-Valverde et al. 1994), which can be partly or totally solubilised and eliminated with the discarded soaking solution.
The benefits of dietary fibre in human diet is gaining importance in developed countries. A fibre rich diet helps to promote body fat loss and lower triglycerides in individuals with coronary heart disease who are overweight and have high triglycerides. Fatal and nonfatal myocardial infarctions have been inversely related with a total fibre intake (Rimm et al. 1996). Individuals who regularly take fibre rich food have lower risk of cardiovascular disease compared to individuals who do not consume adequate quantity of fibre (Jacob and Gallaghar 2004). Food legumes including chickpea is a rich source of dietary fibre and therefore helps in regulating blood cholesterol triglycerides in human beings.
Legumes are usually cooked before being used in the human diet. This improves the protein quality by destruction or inactivation of the heat-sensitive anti-nutritional factors such as trypsin inhibitors, decrease of phytic acid and α-galactoside contents (Frias et al. 2000; Iyer et al. 1989; Wang et al. 1997; Chau et al. 1997). However, cooking causes considerable losses in soluble solids, especially vitamins and minerals (Barampama and Simard 1995).
The role of these domestic processing namely soaking and cooking on food legumes and their effect on dietary fibre contents of different types of chickpeas need to be explored. Though, some information is available on dietary fibre content of some of the legumes, but no systematic study was carried out on soaking and soaking followed by cooking effect on dietary fibre components of chickpeas. Some researchers have reported the effect of soaking and cooking on dietary fibre fractions of lentil (Martin-Cabrejas et al. 2006) and effect of autoclaving on dietary fibre fractions of beans (Phaseolus vulgaris) has been reported by Martin-Cabrejas et al. (2004). The effect of soaking and cooking on insoluble dietary fibre of black grams, chickpeas, lentils, red and white kidney beans has been reported by Rehinan et al. (2004), but the information reported is very meagre. Vidal-Valverde and Frias (1991) have reported processing effect on insoluble fibres of some of the legumes. The effect of cooking on cellulose and lignin of beans and pigeonpea has been reported by Apata (2008). Marconi et al. (2000) have reported the effect of microwave cooking on the non starch polysaccharides (NSP) of chickpeas and Phaseolus vulgaris. No information is available on pectin content of chickpeas during processing. This investigation was therefore carried out to work out the changes in different dietary fibres of different type of chickpeas during soaking and cooking.
Materials and methods
The seeds of ten genotypes varying in their type and size were collected from the crop grown at Indian Institute of Pulses Research, Kanpur, UP, India during 2006–07. The four cultivars of desi type consisting of small seeded (DCP 92–3), medium seeded (KWR 108 and JG 74), bold seeded (BG 256); four cultivars of kabuli types viz., small seeded (L550), medium seeded (BG 1053) and bold seeded (JKG 1 and KAK 2); and two cultivars of green seed type viz., small seeded (sadabahar) and bold seeded (BGD 112) were collected and divided into 3 lots. One lot of seed samples was dried at 70 °C and powdered to a uniform particle size in a seed grinder Perten model 3303 and analysed in triplicate. The second lot was subjected to soaking and third lot was used for soaking followed by cooking.
The seeds of second lot of all the genotypes of desi, kabuli and green seed varieties were soaked in distilled water in a proportion of 1:3 (w/v) seeds: soaking medium. The chickpea seeds were allowed to imbibe water at room temperature for 12h. The chickpea seeds were strained to drain off the soaking water, and the soaked seeds were freeze-dried, ground and analysed in triplicate.
The third lot of seeds after soaking overnight in distilled water were cooked for 30 min in distilled water [seed: water ratio 1:6.7 (w/v)]. The cooking water was drained off and the seeds were freeze-dried, ground and analysed in triplicate.
Determination of dietary fibre components
The cellulose was determined by the method of Updegroff (1969) and results are expressed as percent on dry weight basis. The hemicellulose was determined according to method of Goering and Van Soest (1975) and the results calculated as difference of Neutral Detergent Fibre (NDF) and Acid Detergent Fibre (ADF), and reported as g/100g of seed on dry weight basis. The lignin was determined by the method of AOAC (1980) and the Acid Detergent Lignin (ADL) is reported as lignin g/100g seed on dry weight basis. The pectin was determined by the method of Ranganna (1979) and reported as percent on dry weight basis.
Statistical analysis
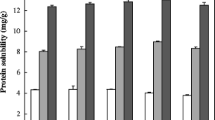
Data were analysed using SPSS 13 by one-way analysis of variance (ANOVA). A multiple comparison of the treatment means was performed by Duncan’s new multiple range test. Significance of the differences was defined as P < 0.05. The results are summarized in Table 1. The data of different genotypes for cellulose, hemicellulose, lignin and pectin are grouped into desi, kabuli and green seeded types and presented in Table 2 and 3.
Results and discussion
The study on the effect of soaking and cooking in chickpea is useful from nutritional and health point of view due to health advantages of dietary fibres. The effect of soaking and cooking may provide useful information for optimization of use of chickpea seeds as the food products, since soaking and cooking have proved beneficial for the nutritional quality of common seeds.
Cellulose
Effect of soaking and cooking on cellulose content of chickpea is reported in Table 1 and 2. Soaking had no significant effect on cellulose content of seed. However, an overall increase of 35.7% in cellulose content of seed was observed on soaking of seed (Table 1). The cellulose content in desi types increased during soaking, JG – 74 and DCP 92–3 had the highest cellulose (5.89 & 5.91%) in their seed, which was increased to 6.75 and 6.96% respectively during soaking, whereas the BG 256 and KWR 108 increased their cellulose from 4.02 to 4.47% and 4.22 to 5.78% respectively (Table 2). The overall increase in desi type genotypes during soaking was 19.5%. The green type seeds also had higher cellulose (5.22–5.35%), which increased during soaking by 13.4%. The kabuli types had the least cellulose in their seed: L 550 and BG 1053 had the lowest cellulose in their seed followed by JKG 1 and KAK 2. The cellulose content of seed during soaking increased from 1.61% to 3.59% in kabuli types, which is a remarkable increase in cellulose content. In kabuli type, highest increase of 123% in cellulose content was observed during soaking, although the content of cellulose was much lower than desi or green seed types. The cellulose in different genotypes of kabuli types was varying between 1.20% and 2.00%, which has increased enormously during soaking. Kabuli types of chickpeas have thinner seed coat as compared to desi types, which lead to greater imbibitions of water. Rehinan et al. (2004) also reported significant increase in cellulose content during water soaking of chickpea and other food legumes (black gram, lentil and kidney beans).
Cooking had no significant effect on cellulose content of seed, however an overall increase of 40.0% in cellulose content of seed was observed in chickpea during cooking of seed (Table 1). Cellulose of desi and green seed types increased cellulose by 16.4 and 20.4% respectively during cooking, whereas kabuli type recorded 99% increase in cellulose content during cooking (Table 2). The increase in cellulose was due to losses of soluble sugars, reduction in phytates and minerals, and amylase activity during soaking, before the process of cooking. Vidal-Valverde and Frias (1991) reported an increase in cellulose content of chickpeas and beans during cooking. Ramulu and Udayasekhararao (1997) also reported an increase in total and insoluble dietary fibre in chickpea, pigeonpea and lentil during cooking. An increase in insoluble fibre during cooking can be attributed to Maillard’s reaction. It is possible that thermal processing may have caused production of Maillard reaction products and thus increase its IDF value. Similar results were reported by Chang and Morris (1990); Vidal-Valverde and Frias (1991).
Hemicellulose
The effect of soaking and cooking on hemicelluloses content of chickpeas is also reported in Table 1 and 2. Soaking had no significant effect on hemicellulose content of seed. However, an overall increase of 14.1% in hemicellulose content of seed was observed during soaking of seed. Desi varieties showed an increase of 9.8% in hemicellulose content during soaking as compared to 4.3% increase in green seed types, and the kabuli types recorded highest increase of 17.4%. Desi and green seed types had relatively high hemicellulose in the range of 3.02–3.52% as compared to kabuli types. JKG 1 of kabuli type had the least hemicellulose, whereas other genotypes namely KAK 2, L 550 and BG 1053 were at par in their hemicellulose content.
Cooking led to a significant reduction of 26.8% in hemicellulose content of chickpea (Table 1). Seeds of desi and green types showed a reduction of 24.0% and 34.4% respectively in hemicellulose content, whereas kabuli types decreased their hemicellulose by 26.9% during cooking (Table 2). Rehinan et al. (2004) also reported a reduction of 37.6–42.4% in hemicellulose content during pressure cooking of legumes. Hemicellulose is a heteropolymer (matrix polysaccharides) present in almost all plant cell walls along with cellulose, while cellulose is crystalline and strong. Hemicellulose has a random, amorphous structure with little strength. It is easily hydrolyzed by dilute acid or base as well as enzymes. Unlike cellulose, hemicellulose consists of shorter chains 500–3000 sugar units as opposed to 7,000–15,000 glucose molecules per polymer seen in cellulose. In addition, hemicellulose is a branched polymer, while cellulose is unbranched. On cooking, hemicellulose may reduce due to loss of its branched structure. A reduction in hemicellulose content of beans and lentils has also been reported by Vidal-Valverde and Frias (1991).
Lignin
The lignin content of seed was not influenced significantly during soaking and cooking. However, an overall increase by 19.3% in lignin content of seed was observed during soaking of chickpea (Table 1). The lignin content of desi and green seed types was higher than kabuli types (Table 3). The desi and green seed types had lignin in their seed in the range of 2.15–3.01% and 2.52–2.61% respectively, which was much higher than kabuli types (1.10–1.86%). The desi and green seed varieties showed an increase of 19.8 and 14.8% respectively in lignin content of seed as compared to 27.5% increase in kabuli types. Cooking led to an overall increase of 15.2% in lignin content. The desi varieties showed an increase of 17.6% in lignin content of seed as compared to 15.6% in green seed varieties and 18.8% in kabuli types. Porres et al. (2002) reported an increase in ADL content of lentils from 0.62% to 1.95% during autoclaving of lentils. Vidal-Valverde and Frias (1991) reported an increase in lignin content of chickpeas and beans during soaking and cooking. Vidal-Valverde et al. (1992) also reported an increase in lignin content of lentil during cooking of soaked seeds. Ramulu and Udayasekhararao (1997) also reported an increase in insoluble dietary fiber (IDF) during cooking. Similarly Rehinan et al. (2004) reported an increase of 15.2–27.8% in lignin content of different food legumes.
Pectin
Soaking and cooking had no significant effect on pectin content of seed. However, an overall increase of 11.4% in pectin content of seed was observed during soaking (Table 1). The pectin content in desi types was much lower than kabuli and green seed types (Table 3). The kabuli types had pectin in the range of 4.41–6.34% in raw seed, whereas desi types had pectin in the range of 2.27–2.56%. The green seed types were also having high pectin (above 5%). KAK 2 genotype of kabuli type had highest pectin (6.34%) in raw seed. Desi varieties showed an increase of 19.2%, whereas kabuli and green seed types increased their pectin by 11.9 and 4.7% respectively during soaking. Cooking had no significant effect on pectin content of seed. However, an overall increase of 15.7% in pectin content of chickpea was observed during cooking. The desi varieties showed an increase in pectin by 28.0%, whereas kabuli types increased their pectin by 14.8% only and the green seed types showed the least increase of 8.4%. The softening of legumes during cooking is due to disintegration of the cotyledonous tissue in individual cells. This is caused by the conversion of native protopectin to pectin, which quickly depolymerises on heating. The middle lamella of the cell walls, which consists of pectins and strengthens the tissue disintegrates in the process (Belitz et al. 2009). Remarkable increase in soluble dietary fibre has been reported in mungbean on soaking and cooking by Azizah and Zainon (1997). Vidal-Valverde et al. (1992) also reported an increase in pectin content of lentil during cooking.
Conclusion
Cellulose, hemicellulose, lignin and pectin of seed are changing drastically in all the genotypes of different type of chickpeas during soaking and cooking. These components constitute dietary fibre of chickpea, hence are important from health point of view. Soaking and cooking help in improving their concentration and ultimately enhances health benefits of the food legumes. Chickpea is a rich source of dietary fibre, therefore can be recommended as health food for longevity. Dietary fibres play an important role in lowering blood cholesterol, control of colon cancer and help in body weight management, therefore chickpea of different types can be used for health benefits depending on their availability and processing techniques.
References
AOAC (1980) Official methods of analysis of the Association of Official Analytical Chemists, 13th edn, Washington DC, p 134–135
Apata DF (2008) Effect of cooking methods on available and unavailable carbohydrates of some tropical grain legumes. African J Biotechnol 7:2940–2945
Azizah AH, Zainon H (1997) Effect of processing on dietary fibre contents of selected legumes and cereals. Malaysian J Nut 3:131–136
Barampama Z, Simard RE (1995) Effect of soaking, cooking and fermentation on composition, in-vitro starch digestibility and nutritive value of common beans. Plant Foods Hum Nutr 48:349–365
Belitz HD, Gorsch W, Schieberle P (2009) Legumes. In Food Chemistry, 4th revised edition, Springer Publications, Berlin Heidelberg, p 746–769
Chang MC, Morris WC (1990) Effect of heat treatments on chemical analysis of dietary fibre. J Food Sci 55:1647–1650
Chau CF, Cheung PC, Wong YS (1997) Effect of cooking on content of amino acids and antinutrients in three Chinese indigenous legume seeds. J Sci Food Agric 75:447–452
Chavan JK, Kadam SS, Salunkhe DK (1989) Chickpea. In: Salunkhe DK, Kadam SS (eds) Handbook of world food legumes: nutritional chemistry, processing technology and utilization, Vol I. CRC, Boca Raton, pp 247–288
Frias J, Vidal-Valverde C, Sotomayor C, Diaz-Pollan C, Urbano G (2000) Influence of processing on available carbohydrate content and antinutritional factors of chickpeas. Eur Food Res Technol 210:340–345
Goering HD, Van Soest PJ (1975) Forage fibre analysis. U.S. Department of Agriculture, Agricultural Research Service, Washington
Iyer V, Kadam SS, Salunkhe DK (1989) Cooking. In: Salunkhe DK, Kadam SS (eds) Handbook of world food legumes: nutritional chemistry, processing technology and utilization, Vol III. CRC, Boca Raton, pp 141–163
Jacob DR, Gallaghar DD (2004) Whole grain intake and cardiovascular disease: a review. Cur Athero Rep 6:415–423
Lyimo M, Mugula J, Elias T (1992) Nutritive composition of broth from selected bean varieties cooked for various periods. J Sci Food Agric 58:535–539
Marconi E, Ruggeri S, Cappelloni M, Leonardi D, Carnovale E (2000) Physicochemical, nutritional, and microstructural characteristics of chickpeas (Cicer arietinum L.) and common beans (Phaseolus vulgaris L.) following microwave cooking. J Agric Food Chem 48:5986–5994
Martın-Cabrejas MA, Sanfiz B, Vidal A, Molla E, Esteban RM, Lopez-Andreu FJ (2004) Effect of fermentation and autoclaving on dietary fibre fractions and antinutritional factors of beans (Phaseolus vulgaris L.). J Agric Food Chem 52:261–266
Martın-Cabrejas MA, Aguilera Y, Benitez V, Molla E, Lopez-Andreu FJ, Esteban RM (2006) Effect of industrial dehydration on soluble carbohydrates and dietary fibre fractions in legumes. J Agric Food Chem 54:7652–7657
Muzquiz M, Burbano C, Ayet G, Pedrosa MM, Cuadrado C (1999) The investigation of antinutritional factors in Phaseolus vulgaris Environmental and varietal differences. Biotechnol Agron Soc Environ 3:210–216
Porres MJ, Urbano G, Fernandez-Figares I, Prieto C, Perez L, Aguilera JF (2002) Digestive utilization of protein and amino acids from raw and heated lentils by growing rats. J Sci Food Agric 82:1740–1747
Ramulu P, Udayasekhararao P (1997) Effect of processing on dietary fibre content of cereals and pulses. Pl Foods Hum Nut 50:249–257
Ranganna S (1979) Manual of analysis of fruits and vegetable products. Tata McGraw-Hill Publ Co Ltd, New Delhi, p 634
Rehinan Z, Rashid M, Shah WH (2004) Insoluble dietary fibre components of food legumes as affected by soaking and cooking processes. Food Chem 85:245–249
Rimm EB, Ascherio A, Giovannucci E (1996) Vegetables, fruits and cereal fibre intake and risk of coronary heart disease among men. JAMA 275:447–451
Updegroff DM (1969) Semi-micro determination of cellulose in biological material. Anal Biochem 32:420–424
Vidal-Valverde C, Frias J (1991) Legume processing effects on dietary fibre components. J Food Sci 56:1350–1352
Vidal-Valverde C, Frias J, Valverde S (1992) Effect of processing on the soluble carbohydrate content of lentils. J Food Prot 55:301–306
Vidal-Valverde C, Frias J, Estrella I, Gorospe MJ, Ruiz R, Bacon J (1994) Effect of processing on some antinutritional factors of lentils. J Agric Food Chem 42:2291–2295
Wang N, Lewis MJ, Brennan JG, Westby A (1997) Effect of processing methods on nutrients and anti-nutritional factors in cowpea. Food Chem 58:59–68
Acknowledgements
The first author (Hina Vasishtha) wishes to thank Indian Council of Medical Research, New Delhi, India for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vasishtha, H., Srivastava, R.P. Effect of soaking and cooking on dietary fibre components of different type of chickpea genotypes. J Food Sci Technol 50, 579–584 (2013). https://doi.org/10.1007/s13197-011-0366-4
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13197-011-0366-4