Abstract
Prebiotics are regarded as the non-digestible food constituents that are selectively consumed by health-promoting bacteria (probiotics). In fact, a number of active metabolites is released due to intensive interaction between prebiotics and probiotics in the gut which exert local and systemic beneficial effects including regulation of intestinal disorders and modulation of host immunity. Turmeric is one of the most important medicinal herbaceous that is derived from Curcuma longa rhizome. Curcumin is a well-recognized component of turmeric which contributes to the prevention of multiple inflammatory diseases. Despite curcumin as a well-known compound, few researches have focused on the turmeric extract (TE) and its potential as prebiotic and anti-inflammatory compound. The aim of this study was to evaluate the prebiotic potential and some functional-structural properties of TE. The Fourier-transform-infrared spectroscopy (FTIR) spectrum of TE showed identical peaks that belonged to β configuration in pyranose and glycosidic bonds. High performance liquid chromatography (HPLC) analysis revealed the presence of potent phenolic and flavonoid anti-oxidants and curcuminoids, and some functional monosaccharides. TE demonstrated excellent resistance to artificial human gastric and intestine juice compared to the standard prebiotic (inulin) (p ≤ 0.05). Interestingly, our time course experiment showed that TE not only is digested by probiotics including Lactobacillus rhamnosus GG (LGG) and Bifidobacterium animalis BB12, but also supports the growth of these bacteria even after 72 h (p ≤ 0.05). To our knowledge, this is the first report evaluating prebiotic potential of TE and exploring its suppressive effects on LPS induced IL-8 production in HT29-19A cell line.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The huge community of microorganisms residing in our gastrointestinal tract (GI) modulate its functions. In fact, they are able to tight intestinal junctions, provide a defensive barrier against pathogens and boost the immune system. In addition, commensal bacteria play an essential role in suppression of inflammatory signals by producing anti-inflammatory metabolites such as short chain fatty acids (SCFAs) [1,2,3]. A growing body of literature has revealed the central role of dysbiosis (any dysregulation in the composition or metabolitic function of microbiota) in the innitiation of chronic diseases, so the maintenance of a healthy microbiota has received more attention as a new therapeutic target [4].
One of the promising strategies in modulating gut microbiota composition and metabolic function is the use of prebiotics. Prebiotics refer to the non-digestible food constituents or components that are selectively consumed by health-promoting bacteria that in turn facilitate bacterial colonization in GI and enhance their growth and activity. The combination of pro- and prebiotic is called “synbiotic” and it means that the health-promoting effects of probiotic and prebiotic may be enhanced when combined together. Although most of the available prebiotics are carbohydrates, recent investigations revealed that there are compounds with prebiotic properties that are not pure carbohydrate and can be metabolized and digested by probiotic bacteria [5]. In this regard, phenolic compounds are digested by a range of bacteria residing in the human GI leading to the increase of health-promoting bacteria. Expansion of these beneficial bacteria can in turn inhibits the survival and growth of opportunistic and pathogenic bacteria. Numerous studies have demonstrated the prebiotic potential of some polyphenol-rich foods extracted from natural sources [6].
Turmeric is a crude compound derived from the rhizome of Curcuma longa. It is well known as a plant with strong antioxidant and anti-inflammatory activity [7]. Turmeric consists of different components with following percentage; curcuminoid compounds 2–5%, carbohydrates nearly 40–70%, proteins 6–8%, oils 5–8%, and minerals and other elements 3–5% [8].
Although recent researches have investigated the biological effects of the turmeric on health [9], there are limited studies investigating the prebiotic potential of crude turmeric extract in the gut and its effects on probiotic microbiota. Since, turmeric is most often administrated orally, therefore, there is of great interest to explore its interaction with gut microbiota and intestinal epithelial cells during digestion. Many in vitro studies have reported the anti-inflammatory effects of turmeric on the various types of cell lines [10,11,12]. In this regard, the human colon adenocarcinoma cell line (HT29-19A) has a widespread interest due to expression of different intracellular pathways and its capability in secretion of various inflammatory and regulatory cytokines. There are also several studies indicating the ability of lactobacilli and bifidobacteria in suppression of proinflammatory-related mediator in human HT-29 cells. More recent evidences illuminate that aforementioned genus can suppress IL-8 secretion from intestinal epithelial cells. But, our knowledge concerning the prebiotic potential of turmeric extract and its combination with probiotics is largely based on very limited data [10,11,12].
The main aim of this study was to characterize turmeric extract (TE). Also, we assessed the prebiotic activity of TE in the presence of Lactobacillus rhamnosus GG (LGG), the most abundant flora of small intestine with potent probiotic function, as well as Bifidobacterium animalis BB12 which comprises 10% of the commensal colon microbiota [13]. In this study, we evaluated the effect of the probiotic Lactobacillus rhamnosus GG, TE, and their combination on IL-8 secretion in LPS-treated HT29-19A. Commercially, applying pure compounds with nutritional and pharmaceutical purposes is highly cost effective. Furthermore, no special anti-nutritional effects or unpleasant tastes have been reported for turmeric; hence, in this investigation we studied the structural properties of crude turmeric extract instead of each component individually.
Materials and Methods
The material and methods section is presented as online resources 1–7.
Results and Discussion
Chemical Analysis of TE
The TE consisted of 65.50% (w/w) total sugar, 8.7% (w/w) protein, 11.7% uronic acid content, 5.2% ash, fat 2.51% and 6.4% total phenolic content. Deffating and precipitation with ethanol were not able to remove all fat residues which was consistent with the finding of Kozarski et al. [14].
The high amount of uronic acid in the TE can justify many of its functional and physiological properties, such as its high water absorption capacity (Online Resource 7).
The relationship between certain carbohydrate and radicals scavenging ability has been widely investigated. It has been revealed that the compounds with structures containing certain functional groups like -OH, -SH, C=O, -NR2, -S- play an important role in radical scavenging ability of crude compounds [15]. The sugars like uronic acids, rhamnose, xylose and arabinose have these functional groups abundantly highlighting stronger radicals scavenging ability of crude compound [15].
Structural Characterization of TE
FTIR Spectroscopy
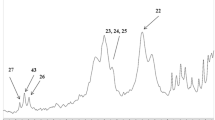
Although many studies have been carried out on the phenolic compounds of curcumin or turmeric [6], no characterization of sugar profile has ever been reported (Online Resource 2).
HPLC analysis of phenolic and sugar composition
The average amount of phenolic-based and carbohydrate compounds in TE were mentioned in Online Resource 2.
Determination of Prebiotic Properties
TE Resistance to Acidic and Enzymatic Digestion
The essential items by which the suitability of a prebiotic is assessed are the slow digestibility of their monomers while passing through the stomach and small intestine to reach the colon, as well as the ability of being digested by resident gut microbiota. The structure of low-digestible polysaccharides consists of β2 fructosyl-fructose bonds which are started with α-D-glucose portion. These carbohydrates are not absorbed in the small intestine and thus become susceptible to bacterial fermentation in the colon leading to SCFA production [16].
The results of TE digestibility were shown in Fig. 1. TE was exposed to a pH 1.2 ± 0.5 AHG buffer, and the hydrolysis degree was only 3.01 ± 0.28%. When compared to the standard prebiotic inulin (20.5 ± 0.71%), the acidic resistance of TE was significantly higher (p ≤ 0.05). Then TE was examined by AHI buffer and the degrees of hydrolysis were 3.35 ± 0.21 and 8.2 ± 4.24% for TE and inulin, respectively. Finally, the combination of AHG and AHI buffers was added to the mixture provided in latter steps and the pH of the combination buffer (AHG and AHI) raised up to 4.5 with a 1.2 unit/ml of α-amylase. There was less susceptibility of TE (1.85 ± 0.21%) to the combination buffer compared to inulin (3.85 ± 0.49%) (p ≤ 0.05). Our results suggested that TE would be a suitable prebiotic because nearly more than 91% of it, could probably pass through the stomach and small intestine. Thus, it seems to be able to reach the colon intact or without considerable digestion. In comparison with other studies, the digestability of TE was almost the same as other polysaccharides [17,18,19,20]. However, these results are just based on aformentioned tests, so further experiments such as measuring the whole carbohydrate profile by HPLC should be considered before and after digestion to precisely confirm the findings [21].
Effects of TE on Probiotic Growth
One of the important characteristics of a potential prebiotic is the ability to promote the probiotic bacteria rather than other bacteria in the gut. In this study, the probiotics (Lactobacillus rhamnosus GG-ATCC 53103 and Bifidobacterium animalis BB12) were cultured in sugar free MRS and sugar free TOS-MUP, either supplemented with 2% of glucose, inulin or TE. Bacterial populations and pH were measured within 24 h intervals. The bacteria showed a positive growth in the TE supplemented medium (Fig. 2a and b). Since glucose is preferred by all bacteria and consumed earlier, during the first 24 h of fermentation, the bacterial proliferation in glucose supplemented MRS or TOS MUP base was increased, but after 48 h this population was decreased, whereas the bacterial population in the TE containing media gradually was elevated. The difference between bacterial number in the first hour of fermentation and 72 h fermentation was calculated as the proliferative index (PI).
Effect of TE on microbial population of Lactobacillus rhamnosus GG ATCC 53103 (a) and Bifidobacterium animalis BB12 (b) in sugar free MRS (sfMRS), sfMRS medium supplemented with 2% (w/v) TE, inulin and glucose. The growth was monitored by measuring bacteria counts at the 24, 48 and 72 h. Negative control: sfMRS; positive control: sfMRS + glucose (p ≤ 0.05)
The PI of Lactobacillus rhamnosus GG-ATCC 53103 was increased in the order of sfMRS-base (−4.96 ± 0.13), sfMRS+ glucose (−0.19 ± 0.06), sfMRS+ inulin (0.97 ± 0.06) and sfMRS + TE (1.02 ± 0.14), and the groups were significantly different (p ≤ 0.05).
The PI of Bifidobacterium animalis BB12 was increased in the order of sfTOS MUP-base (−6.01 ± 0.15), sfTOS MUP + glucose (−1.74 ± 0.16), sfTOS MUP + inulin (0.98 ± 0.08) and sfTOS MUP + TE (2.5 ± 0.16), and the groups were significantly different (p < 0.05).
Although there was no difference between the PI of LGG in the TE and inulin supplemented media, there was a significant difference between the PI of B. animalis in the mentioned media (p < 0.05) which means that B. animalis might be a better candidate for using with TE in nutraceuticals. In addition, the PI of LGG and B. animalis in TE supplemented medium were significantly different (p < 0.05) which indicated that the B. animalis could specifically digest the TE [21].
Glucose was consumed initially by both strains and enhanced the bacterial population more than TE and inulin, as it is a simple sugar and easier to digest. After 72 h, the number of bacteria was decreased in the sfMRS+ glucose while the bacterial population was increased in the inulin and TE supplemented medium. Both TE and inulin showed the ability to be digested by LGG and B. animalis gradually, keeping the probiotics alive even after 72 h. The results showed that this spice have a complex carbohydrate that could be used by probiotics as a carbon source which in turn increased their survival. TE (see Fig. 2a and b) had a surviving effect on cell growth after 24 h as well as showed a stimulatory effect on cell growth after 48 h (from 5.23 ± 0.26 l to 6.50 ± 0.12 log cfu/ml for LGG and from 6.66 ± 0.29 to 8.83 ± 0.32 log cfu/ml for B.animalis). A reasonable explanation for a decrease in the number of bacteria after 24 h may be the complexity of the TE and inulin which caused a delay for bacterial adaptation. Previously, the prebiotic potential of various polysaccharides extracted from acorn and pistachio hull was suggested based on their stimulatory effects on the growth of Lactobacillus plantarum A7 PTCC 1896 and Lactobacillus rhamnosus GG [18, 19]. The stimulatory effects of these polysaccharides were the same as inulin on mentioned bacteria which are in line with Lactobacillus rhamnosus GG in our findings. However, the stimulatory effect of TE on the growth of Bifidobacterium animalis BB12 was significantly higher that inulin and was in line with other carbohydrate extracted from different plants regarding the stimulatory effects of probiotic bacteria; oligosaccharides extracted from white-flesh dragon fruit had a stimulatory effect on Lactobacillus delbrueckii BCC13296 and increased the number of cells by almost two logarithmic cycles [22]. Bamboo shoots induced a 5-time increase in cell density of bifidobacterial and lactobacilli strains [17].
Bacterial enumeration and pH evaluation were monitored during the first 72 h of fermentation at 24 h intervals. In Fig. 3a and b, the pH was reduced sharply after 24 h of growth of the probiotic bacteria in the glucose containing medium. When the amount of glucose in the medium is very high, bacteria consume this carbon source very fast which leads to dropping pH immediately. This acid production may cause catabolic inhibition in the bacteria, preventing further growth. On the other hand, in the media supplemented with TE or inulin, the abundance of complex carbohydrates, which was consumed very gently by LGG and B. animalis BB12, kept the pH at 5.7 after 24 h. The more complex a carbohydrate, the slower its consumption by the probiotic, leading to more gradual reduction of the pH which allowing alternative metabolic pathways to be activated. The different metabolites of the other pathways may even increase the pH of medium which facilities bacterial growth and survival. The dominant pH of the medium mostly depends on the type of metabolites secreted by bacteria. In this regard, stronger acidic metabolites decrease pH and weaker acids with some minerals help buffers to maintain the pH. Some metabolites like SCFA increase the pH [23].
Effect of TE on pH of media caused by Lactobacillus rhamnosus GG ATCC 53103 (a) and Bifidobacterium animalis BB12 (b) in sugar free MRS (sfMRS), sfMRS medium supplemented with 2% (w/v) TE, inulin and glucose. The growth was monitored by measuring bacteria counts at the 24, 48 and 72 h. Negative control: sfMRS; positive control: sfMRS + glucose (p ≤ 0.05)
Determination of Anti-Inflammatory Properties
Cell Viability Assay
Curcumin and turmeric have been shown to exert cytotoxicity effects on cancer cell lines [24]. Thus, we conducted the cell viability assay to find out the best concentration of curcumin, TE, and DMSO (as solvent) with the lowest toxicity on the HT29-19A cell line. The cells were treated with different concentrations of curcumin and TE (1, 3, 6, 12, 25, 50 and 100 μM) for 6 h. The viability was less than 80% at 12 μM of curcumin whereas the viability was not statistically significant for the first three concentrations (1, 3, 6 μM) (unpublished data). Therefore, we used these concentrations (1, 3, 6 μM) in the subsequent experiments. As shown in Fig. 4a and b, the combination of curcumin and TE with LGG did not have any negative effect on the viability of HT29-19A cells. It is also indicated that 1000 ng/ml LPS could not have any cytotoxicity effect on the cells which is consistent with the other’s studies [25,26,27,28].
HT29-19A cells were seeded in 96-well plates at the density of 10,000 cells/well. The cells were incubated with curcumin and TE with the concentrations of 1, 3 and 6 μm + 1% of the 109 cfu/ml Lactobacillus rhamnosus GG, for 6 h (6 wells for each condition). Cell viability was determined by MTT assay. The data are presented as mean ± SEM of three independent experiments. LGC1,3,6 = LGG + curcumin 1,3,6 μM, LGT1,3,6 = LGG + TE 1,3,6 μM, HT29-19A = human colorectal adenocarcinoma cell line
IL-8 Suppression by Curcumin, TE and TE-LGG Mixture
HT-29 cell line driven from human intestinal epithelial cells are able to release pro-inflammatory cytokines (TNF-α, IL-1β and IL-6), immune-modulatory cytokines (granulocyte colony-stimulating factor and IL-3) and a remarkable amount of IL-8 as a potent pro-inflammatory chemokine. IL-8 can excite acute inflammatory responses in all over the body specially intestine [12].
We aimed to assess whether LGG alone or combination of LGG and curcumin/TE impacts on the IL-8 secretion from LPS-treated HT29-19A cells. Thus, cells were pre-incubated with 1% of 109 cfu/ml LGG or 1% of 109 cfu/ml LGG + different concentrations of curcumin (1, 3, 6 μM) or 1% of 109 cfu/ml LGG + different concentrations of TE (1, 3, 6 μM) then stimulated with LPS (1000 ng/ml). As shown in Fig. 5a and b, the IL-8 in LPS stimulated group was significantly increased compared to untreated cells as the control group (p < 0.0001). The LGG alone was able to reduce the amount of IL-8 (p < 0.05) but when the LGG was applied with curcumin or TE (1 μM or 3 μM), we observed more reduction in the IL-8 level. This might be due to the synergistic anti-inflammatory activity of TE/curcumin and LGG on the cells.
HT29-19A cells primed with TE and curcumin, are characterized by release of IL-8. HT-29 cells were incubated with curcumin and TE with the concentrations of 1, 3 and 6 μm + 1% of the 109 cfu/ml Lactobacillus rhamnosus GG, for 6 h (6 wells for each condition). After 6 h, half of the wells stimulated with 1000 ng/ml LPS and maintained for additional 12 h. Medium-treated (McCoy) HT29-19A served as negative control and HT29-19A by LPS (1000 ng/ml) as positive control for functional HT29-19A stimulation. Supernatants were analyzed for secretion of IL-8 in curcumin (a) and IL-8 in TE (b) by ELISA. Results are presented as mean ± SEM, three independent experiments are shown. **** p < 0.0001, ** p < 0.01, ** p < 0.01one-way ANOVA and post hocBonferroni’s multiple comparisons test. LGC1,3,6 = LGG + curcumin 1,3,6 μM, LGT1,3,6 = LGG + TE 1,3,6 μM, HT29-19A = human colorectal adenocarcinoma cell line
Various mechanisms have been suggested concerning the suppressive effects of probiotics on IL-8 release. Bai et al. [11] proposed that the suppression of IL-8 in HT29 cells after pre-incubation with lactobacilli and bifidobacteria was mediated by the reduction of NF-қB expression. The results were in line with the study conducted by Chen et al. [10] revealing that NF-κBs had a remarkable impact on the up-regulation of iNOS, PTGS2, and IL-8 expression and were suppressed by live Lactobacillus acidophillus. Many studies have been published on the ability of probiotics to adhere to intestinal epithelial cells to both prevent establishment of enteric pathogens [29] and suppress inflammation [30]. Another study conducted by Aggarwal et al. [8] showed that curcumin-free turmeric, specially water-soluble peptides, such as tumerin and turmeric antioxidant protein (TAP) are still able to represent many anti-inflammatory and anticancer activities.
Conclusion
High resistance of TE to GI enzymes along with its high phenolic and carbohydrate content such as pentosans support its potential as a prebiotic in real food. In addition, characterization of polysaccharides in TE, evaluation of their prebiotic potential, and use of whole TE or TE derivatives in the real food can be considered in future studies.
We have detected the considerable amount of mannose, rhamnose, arabinose and xylose in TE which might be due to the existence of pentosans. Experimental studies indicate that these polymers such as pentosans, especially arabinose, can reinforce the immune system [31]. Measurement the quality and the quantity of the metabolites such as SCFA secreted by LGG or B. animalis together with TE is highly recommended in further investigations.
Our study provided a blueprint for using the TE as a nutritional compound for modulating immune system via: 1- maintaining health-promoting bacteria in the gut; 2- suppressing the inflammations. In addition it might be used in a pharma-nutrition approach to increase the effectiveness of pharmaceuticals.
Abbreviations
- (LGG):
-
Lactobacillus rhamnosus GG
- (TE):
-
Turmeric extract
References
Mangell P, Nejdfors P, Wang M, Ahrné S, Weström B, Thorlacius H, Jeppsson B (2002) Lactobacillus plantarum 299v inhibits Escherichia coli-induced intestinal permeability. Dig Dis Sci 47:511–516. https://doi.org/10.1023/A:1017947531536
Hsieh CY, Osaka T, Moriyama E, Date Y, Kikuchi J, Tsuneda S (2015) Strengthening of the intestinal epithelial tight junction by Bifidobacterium bifidum. Phys Rep 3:1–17. https://doi.org/10.14814/phy2.12327
Blackwood BP, Yuan CY, Wood DR, Nicolas JD, Grothaus JS, Hunter CJ (2017) Probiotic Lactobacillus species strengthen intestinal barrier function and tight junction integrity in experimental necrotizing enterocolitis. J Probiotics Heal 5:457–464. https://doi.org/10.4172/2329-8901.1000159
Koh JH, Kim WU (2017) Dysregulation of gut microbiota and chronic inflammatory disease: from epithelial defense to host immunity. Exp Mol Med 49:e337. https://doi.org/10.1038/emm.2017.55
Bindels LB, Delzenne NM, Cani PD, Walter J (2015) Opinion: towards a more comprehensive concept for prebiotics. Nat Rev Gastroenterol Hepatol 12:303–310. https://doi.org/10.1038/nrgastro.2015.47
Tomé-Carneiro J, Visioli F (2016) Polyphenol-based nutraceuticals for the prevention and treatment of cardiovascular disease: review of human evidence. Phytomedicine 23:1145–1174. https://doi.org/10.1016/j.phymed.2015.10.018
Zeng Z, Shen ZL, Zhai S, Xu JL, Liang H, Shen Q, Li QY (2017) Transport of curcumin derivatives in Caco-2 cell monolayers. Eur J Pharm Biopharm 117:123–131. https://doi.org/10.1016/j.ejpb.2017.04.004
Aggarwal BB, Yuan W, Li S, Gupta SC (2013) Curcumin-free turmeric exhibits anti-inflammatory and anticancer activities: identification of novel components of turmeric. Mol Nutr Food Res 57:1529–1542. https://doi.org/10.1002/mnfr.201200838
Menon VP, Sudheer AR (2007) Antioxidant and anti-inflammatory properties of curcumin. Adv Exp Med Biol 595:105–125. https://doi.org/10.1007/978-0-387-46401-5_3
Chen K, Liang N, Luo X, Zhang TC (2013) Lactobacillus acidophilus strain suppresses the transcription of proinflammatory-related factors in human HT-29 cells. J Microbiol Biotechnol 23:64–68. https://doi.org/10.4014/jmb.1208.04067
Bai AP, Ouyang Q, Zhang W, Wang CH, Li SF (2004) Probiotics inhibit TNF-α-induced interleukin-8 secretion of HT29 cells. World J Gastroenterol 10:455–457. https://doi.org/10.3748/wjg.v10.i3.455
Verhoeckx K, Cotter P, López-Expósito I, et al (2015) The impact of food bioactives on health: in vitro and ex vivo models. COST Action FA1005, Springer. https://doi.org/10.1007/978-3-319-16104-4
Arboleya S, Watkins C, Stanton C, Ross RP (2016) Gut bifidobacteria populations in human health and aging. Front Microbiol 7:1–9. https://doi.org/10.3389/fmicb.2016.01204
Kozarski M, Klaus A, Jakovljevic D, Todorovic N, Niksic M, Vrvic MM, van Griensven LJLD (2014) Dietary polysaccharide extracts of Agaricus brasiliensis fruiting bodies: chemical characterization and bioactivities at different levels of purification. Food Res Int 64:53–64. https://doi.org/10.1016/j.foodres.2014.05.075
Xu RB, Yang X, Wang J, Zhao HT, Lu WH, Cui J, Cheng CL, Zou P, Huang WW, Wang P, Li WJ, Hu XL (2012) Chemical composition and antioxidant activities of three polysaccharide fractions from pine cones. Int J Mol Sci 13:14262–14277. https://doi.org/10.3390/ijms131114262
Wong JM, Jenkins DJ (2007) Carbohydrate digestibility and metabolic effects. J Nutr 137:2539–2546. https://doi.org/10.1093/jn/137.11.2539S
Azmi AFMN, Mustafa S, Hashim DM, Manap YA (2012) Prebiotic activity of polysaccharides extracted from Gigantochloa Levis (buluh beting) shoots. Molecules 17:1635–1651. https://doi.org/10.3390/molecules17021635
Tadayoni M, Sheikh-Zeinoddin M, Soleimanian-Zad S (2015) Isolation of bioactive polysaccharide from acorn and evaluation of its functional properties. Int J Biol Macromol 72:179–184. https://doi.org/10.1016/j.ijbiomac.2014.08.015
Akbari-Alavijeh S, Soleimanian-Zad S, Sheikh-Zeinoddin M, Hashmi S (2018) Pistachio hull water-soluble polysaccharides as a novel prebiotic agent. Int J Biol Macromol 107:808–816. https://doi.org/10.1016/j.ijbiomac.2017.09.049
Wichienchot S, Prasertsan P, Hongpattarakere T, Gibson GR, Rastall RA (2006) In vitro fermentation of mixed linkage gluco-oligosaccharides produced by Gluconobacter. Curr Issues Intest Microbiol 7:7–12
Hansawasdi C, Kurdi P (2017) Potential prebiotic oligosaccharide mixtures from acidic hydrolysis of rice bran and cassava pulp. Plant Foods Hum Nutr 72:396–403. https://doi.org/10.1007/s11130-017-0636-z
Wichienchot S, Jatupornpipat M, Rastall RA (2010) Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem 120:850–857. https://doi.org/10.1016/j.foodchem.2009.11.026
den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, Bakker BM (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54:2325–2340. https://doi.org/10.1194/jlr. R036012
Yadav VS, Mishra KP, Singh DP, Mehrotra S, Singh VK (2005) Immunomodulatory effects of curcumin. Immunopharmacol Immunotoxicol 27:485–497. https://doi.org/10.1080/08923970500242244
Guo Y, Shu L, Zhang C, Su ZY, Kong ANT (2015) Curcumin inhibits anchorage-independent growth of HT29 human colon cancer cells by targeting epigenetic restoration of the tumor suppressor gene DLEC1. Biochem Pharmacol 94:69–78. https://doi.org/10.1016/j.bcp.2015.01.009
Altamimi M, Abdelhay O, Rastall RA (2016) Effect of oligosaccharides on the adhesion of gut bacteria to human HT-29 cells. Anaerobe 39:136–142. https://doi.org/10.1016/j.anaerobe.2016.03.010
Duary RK, Batish VK, Grover S (2014) Immunomodulatory activity of two potential probiotic strains in LPS-stimulated HT-29 cells. Genes Nutr 9(398):398. https://doi.org/10.1007/s12263-014-0398-2
Lehmann S, Hiller J, Van Bergenhenegouwen J et al (2015) In vitro evidence for immune-modulatory properties of non-digestible oligosaccharides: direct effect on human monocyte derived dendritic cells. PLoS One 10:1–15. https://doi.org/10.1371/journal.pone.0132304
Serafini F, Strati F, Ruas-Madiedo P, Turroni F, Foroni E, Duranti S, Milano F, Perotti A, Viappiani A, Guglielmetti S, Buschini A, Margolles A, van Sinderen D, Ventura M (2013) Evaluation of adhesion properties and antibacterial activities of the infant gut commensal Bifidobacterium bifidum PRL2010. Anaerobe 21:9–17. https://doi.org/10.1016/j.anaerobe.2013.03.003
Candela M, Perna F, Carnevali P, Vitali B, Ciati R, Gionchetti P, Rizzello F, Campieri M, Brigidi P (2008) Interaction of probiotic Lactobacillus and Bifidobacterium strains with human intestinal epithelial cells: adhesion properties, competition against enteropathogens and modulation of IL-8 production. Int J Food Microbiol 125:286–292. https://doi.org/10.1016/j.ijfoodmicro.2008.04.012
Tan Y-F, Li H-L, Lai W-Y, Zhang J-Q (2013) Crude dietary polysaccharide fraction isolated from jackfruit enhances immune system activity in mice. J Med Food 16:663–668. https://doi.org/10.1089/jmf.2012.2565
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
This research was supported by Isfahan University of Technology (IUT) and Utrecht University. The authors declare that they have no conflict of interest.
Human and Animal Studies
This article does not contain any studies with human or animal subjects.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 778 kb)
Rights and permissions
About this article
Cite this article
Ghiamati Yazdi, F., Soleimanian-Zad, S., van den Worm, E. et al. Turmeric Extract: Potential Use as a Prebiotic and Anti-Inflammatory Compound?. Plant Foods Hum Nutr 74, 293–299 (2019). https://doi.org/10.1007/s11130-019-00733-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-019-00733-x