Abstract
To keep up with the growth of human population and to circumvent deleterious effects of global climate change, it is essential to enhance crop yield to achieve higher production. Here we review mathematical models of oxygenic photosynthesis that are extensively used, and discuss in depth a subset that accounts for diverse approaches providing solutions to our objective. These include models (1) to study different ways to enhance photosynthesis, such as fine-tuning antenna size, photoprotection and electron transport; (2) to bioengineer carbon metabolism; and (3) to evaluate the interactions between the process of photosynthesis and the seasonal crop dynamics, or those that have included statistical whole-genome prediction methods to quantify the impact of photosynthesis traits on the improvement of crop yield. We conclude by emphasizing that the results obtained in these studies clearly demonstrate that mathematical modelling is a key tool to examine different approaches to improve photosynthesis for better productivity, while effective multiscale crop models, especially those that also include remote sensing data, are indispensable to verify different strategies to obtain maximized crop yields.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction: improving the efficiency of photosynthesis for a better crop yield
In oxygenic photosynthesis, plants capture light energy from the sun and produce sugars and organic biomass from CO2 and water, releasing molecular oxygen into the atmosphere as a byproduct (Blankenship 2021; Björn et al. 2023). This process has had a fundamental role in shaping the history of Earth, by contributing to the rise of atmospheric O2 and the evolution of animals (Sánchez-Baracaldo and Cardona 2020). It was and is the source of our food, fiber, biofuel, and many other useful substances. As the human population is expected to reach 10.87 billion by 2100, much more primary foodstuff would be needed to feed us all. It has been estimated that the production of the current major field crops such as wheat, rice, maize, and soybean, which provide about two thirds of calories consumed globally, must double by 2050 (see e.g., Long and Ort 2010; Long et al. 2015). Since a corresponding expansion of the available cultivable land is not sustainable (Muhie 2022), it is necessary to find ways to increase the crop yields. Moreover, the ongoing climate change has diminished the crop productivity in the last 25 years, which also highlights the necessity of having higher crop yields in the future (Long 2012; Ray et al. 2012, 2013; Arora 2019). Yet, the progress in disease protection and fertilization, as well as the classical breeding measures taken to improve production during the green revolution in the late twentieth century (such as dwarfing; see Peng et al. 1999; Parry et al. 2011), cannot continue at present to lead alone to sufficient advancement in the crop yield (Evans and Fischer 1999; Sinclair et al. 2004; Sharma-Natu and Ghildiya 2005; Long et al. 2015; Furbank et al. 2020).
Furthermore, theoretical considerations show that the efficiency (ε) of light conversion to biomass is ~ 0.03 at the best in plants, which is only one-third of the theoretical maximum of ~ 0.09, while under the field conditions it is even lower (Zhu et al. 2004a, 2008, 2010). Since improvement in photosynthesis (i.e., with a higher ε) was not addressed during the green revolution (Gifford and Evans 1981; Koester et al. 2016), the enhancement of photosynthesis is now considered an important target to increase crop productivity, as any increases in the net photosynthesis rate of individual plants may lead to increases in biomass across the entire growing season and crop canopy (Long et al. 2006; Zhu et al. 2010; Evans 2013; Long 2014; Ort et al. 2015). Experimental evidence supporting this point of view has been obtained in field experiments using the free-air CO2 enrichment (FACE) method, in which higher biomass and yield was achieved in plants grown under elevated CO2 (Ainsworth et al. 2004; Long et al. 2006; Kang et al. 2021; Ainsworth and Long 2021), as well as in studies in which the rate of photosynthesis was enhanced through genetic engineering (e.g., Kromdijk et al. 2016; Köhler et al. 2017; Lopez-Calcagno et al. 2020; De Souza et al. 2022). A variety of possible ways to improve photosynthesis have been suggested and reviewed, in which both advanced non-transgenic phenomics approaches and transgenic technologies are expected to play a major role (e.g., Long et al. 2006, 2015; Ort et al. 2015; Yin and Struik 2015; Foyer et al. 2017; Dann and Leister 2017; Wu et al. 2019; Muhie 2022; Zhu et al. 2010, 2022; Walter and Kromdijk 2022; Yin et al. 2022). These include: (i) enhancing light capture; (ii) optimizing the photosynthetic electron transport to increase NADPH and ATP production; (iii) optimizing the photoprotection mechanisms; (iv) increasing photosynthesis induction under fluctuating light; (v) reducing photorespiration losses; (vi) boosting the enzymatic activity in the Calvin-Benson-Bassham (CBB) cycle; (vii) improving the flow of CO2 through the leaf, or introducing CO2 concentrating mechanisms around Rubisco from algae and cyanobacteria in C3 plants; (viii) optimizing the resource investment among the components of the photosynthetic apparatus to maximize carbon gain; and (ix) engineering C4 or intermediary pathways in C3 grain crops, such as rice or wheat. Together, the above modifications are expected to double the yield potential of the major crops (Zhu et al. 2010). Although there are encouraging results from some of the above methods, in a number of model species under field conditions (see e.g., Kromdijk et al. 2016; South et al. 2019; Cavanagh et al. 2022), other methods (e.g., viii and ix) are long term projects. We emphasize that many of the above options to enhance plant productivity require modelling to guide the necessary changes via in-silico-assisted bioengineering (Long et al. 2015), or to provide information on the genes that would enhance crop photosynthesis (Yin et al. 2022). In view of the above, we highlight, in this review, several significant modelling studies aiming to find solutions for crop yield improvement and include the importance of solar-induced fluorescence (SIF) measurements towards this goal. We begin by providing an overview on the basics of photosynthesis and on modeling oxygenic photosynthesis with the goal of improving plant productivity.
Basic information on oxygenic photosynthesis and its modelling
Oxygenic photosynthesis: a background
It is well known that oxygenic photosynthesis is one of the most studied biological processes (Blankenship and Govindjee 2007; Braun 2020; Stirbet et al. 2020; Shevela et al. 2019, 2023), providing a great advantage for its modelling. During the initial light phase, taking place in the thylakoids (the specialized membrane system in the chloroplast), light energy is harvested by both photosystem (PS) I and PSII antenna, and transferred to their respective reaction centers, where photochemical reactions take place (Sauer 1975, 1979; Mirkovic et al. 2017). This process initiates electron transfer from water to NADP+ in a linear chain of electron carriers (Pushkar et al. 2008; Govindjee et al. 2017), leading as well to cyclic and pseudo cyclic electron transport, in addition to the formation of NADPH and ATP, the major event. In the second phase of oxygenic photosynthesis in C3 species, taking place in the chloroplast stroma, the Calvin-Benson-Bassham (CBB) cycle, the C3 cycle, is the central part of the overall carbon metabolism (Fig. 1), which uses the reducing power of NADPH and the energy from ATP, both produced by the light reactions, to do the job. The CBB cycle involves 11 enzymes that catalyze 13 reactions, where ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) is the key enzyme (for its discovery, see Sharkey 2023). It is involved in the fixation of CO2 (carboxylation) on ribulose 1,5-bisphosphate (RuBP), a five-carbon compound producing two molecules of 3-phosphoglycerate (3PG), which are used for biosynthetic reactions and the recycling of RuBP (Fig. 1). The necessary light activation of this process is achieved by covalent redox-modification of some key enzymes that are inactive in the dark. For a historical background of the CBB cycle, see Calvin (1989), Benson (2002), Bassham (2003), Nonomura et al. (2017), Sharkey (2019), and for mathematical models of the C3 photosynthesis see e.g., Farquhar et al. (1980), Poolman et al. (2000), Laisk et al. (2006), Zhu et al. (2007, 2013), Jablonsky et al. (2011), Morales et al. (2018a, b) and Bellasio (2019).

Modified from Shevela and Govindjee (2016)
A diagram of the Calvin–Benson–Bassham (CBB) cycle for carbon assimilation (CO2 fixation) in oxygenic photosynthesis. The three stages of the CBB cycle are: (i) Carboxylation the enzyme Rubisco catalyzes the incorporation of CO2 into RuBP (ribulose 1,5-bisphosphate), a molecule with five carbon atoms, resulting in the formation of two molecules of 3-phosphoglicerate (3PG), each with three atoms of carbon; (ii) Reduction NADPH (together with ATP) are used to reduce the 3PG molecules to glyceraldehyde 3-phosphate (GAP), which is used for the biosynthesis of starch and sugars; and (3) Regeneration ribulose bis phosphate (RuBP), the molecule that starts the cycle, is regenerated, in several steps, by using ATP, so that the cycle can continue.
We emphasize here that Rubisco is not very discriminating with respect to CO2 and O2 (see e.g., Farquhar et al. 1980; Zhu et al. 2008), since it also catalyzes, as a side-reaction, the fixation of molecular O2 on RuBP, which leads to the formation of one molecule of 3-phosphoglycerate (3PG), and one molecule of 2-phosphoglycolate (2PG). The 2PG is then converted to glycolate (Ogren and Bowes 1971; Bauwe 2023), which can inhibit at least three enzymes of the CBB cycle, and, thus, it must be quickly removed. The glycolate is detoxified through a complex photorespiration cycle, also known as the C2 cycle (Hodges et al. 2016; Busch 2020), involving reactions taking place in chloroplasts, mitochondria, peroxisomes, and the cytosol. The rate of photorespiration depends on the reaction kinetics of Rubisco and the atmospheric conditions: the oxygenation decreases as the [CO2] increases, and the specificity of Rubisco for CO2 relative to O2 decreases as the temperatures increase, thus increasing the rates of oxygenation. Due to photorespiration, 75% of the carbon in 2PG is recycled, and can reenter the C3 cycle, but the efficiency of C3 photosynthesis decreases significantly, since the photorespiration requires additional ATP and NADPH (Walker et al. 2016).
Although the C3 pathway for carbon assimilation in plants is predominant on Earth, there are plants which use other metabolic pathways (Fig. 2): (i) C4, also known as the Hatch-Slack pathway (Hatch and Slack 1966); and (ii) the crassulacean acid metabolism (CAM). Note that C4 and CAM pathways are adaptations that have evolved independently multiple times over the past 30 million years (see e.g., Sage et al. 2012; Schlüter and Weber 2020).

Modified from https//ib.bioninja.com.au/higher-level/topic-8-metabolism-cell/untitled-2/c3-c4-and-cam-plants.html
Schematic illustration of C3, C4, and the crassulacean acid metabolism (CAM) pathways of photosynthesis in plants. C4, malate, a four-carbon compound that releases CO2 after decarboxylation; CBB cycle, Calvin-Benson-Bassham cycle of carbon assimilation (see Fig. 1); Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase; 3PG, 3-phosphoglycerate; BPG, 1,3-bisphosphoglycerate; GAP, glyceraldehyde 3-phosphate; RuMP, ribulose 5-phosphate; RuBP, ribulose 1,5-bisphosphate.
The C4 pathway leads to increased [CO2] at Rubisco, counteracting the effect of oxygen, and enhancing the carbon assimilation efficiency, which, together with improved nitrogen and water use efficiencies, leads to higher biomass production in several crops, such as sugarcane, sorghum, and maize. To achieve the above, C4 plants have developed a special anatomy (the so-called Kranz anatomy; e.g., Hatch and Osmond 1976; Fouracre 2014), where mesophyll and bundle sheath cells have different structures and functions (see Fig. 2). However, after 2000, C4 photosynthesis was shown to occur also in single mesophyll cells of a few plant species, due to their unique intracellular compartmentalization, equivalent to that between mesophyll and bundle sheath cells in the Kranz anatomy (see reviews by Edwards et al. 2004; Sharpe and Offermann 2014). The C4 photosynthesis starts in the mesophyll cells, where the CO2 is converted into bicarbonate by carbonic anhydrase, and then the latter is ‘fixed’ on phosphoenolpyruvate (PEP, a three-carbon compound) by PEP carboxylase (PEPC), producing oxaloacetate (OAA, a four-carbon compound), which is then converted into malate (also a four-carbon compound) by malate dehydrogenase. Then, the malate is transported to the bundle sheath cells, where it is decarboxylated to release CO2, which is used by the CBB cycle in the chloroplasts of the bundle sheath cells. The remaining pyruvate is translocated back to the mesophyll cells and reconverted to PEP, using ATP. For mathematical models of C4 photosynthesis see e.g., Laisk and Edwards (2000), von Caemmerer and Furbank (2003), Zhu et al. (2008), Bellasio (2016), Wang et al. (2014a,b; 2021), von Caemmerer (2021). Based on the enzymes used to decarboxylate the C4 acids in the bundle sheath cells, the C4 plants are of the following subtypes: (i) NADP-malic enzyme (ME) (e.g., in: maize, sorghum, sugarcane, and millet); (ii) NAD-ME (e.g., in: Atriplex rosea, Panicum milioides, and Portulaca oleracea): and (iii) phosphoenolpyruvate carboxykinase (PEP-CK) (e.g., in the guinea grass Megathyrsus maximus). However, by using a modelling analysis, Wang et al. (2014a) have concluded that only the NADP-ME and NAD-ME should be considered separate C4 subtypes, as they both intrinsically involve a supplementary PEP-CK cycle. On the other hand, the optimal energetic requirements of 80% energy allocation in the bundle sheath cells and 20% in the mesophyll cells, which was determined in their theoretical analysis, cannot be fulfilled in a pure PEP-CK type plant, because, in the Kranz anatomy, the bundle sheath cells are shaded by the mesophyll cells.
Further, the CAM pathway is a special metabolism used by plants, such as the succulent ones that grow in extremely arid environments. These plants separate the photosynthetic function in time, in order to conserve water and fix CO2 at high concentrations, without photorespiration (Winter and Smith 2022). This is done in the following manner (see Fig. 2): (i) when the stomata are open at night (i.e., when it is cooler), CO2 is fixed on PEP, using PEP carboxylase, and the resulting oxaloacetate is converted to malate, which is stored in the vacuoles of the mesophyll cells; and (ii) when the stomata are closed during the day (i.e., when the temperature is high), the malate from the vacuoles is transported into the mesophyll chloroplasts and, there, it is decarboxylated releasing CO2 which then feeds into the CBB cycle, while the remaining pyruvate is converted back to PEP. For models of CAM photosynthesis, see e.g., Cheung et al. (2014) and a review by Burgos et al. (2022).
Background on mathematical modelling of oxygenic photosynthesis
Models of oxygenic photosynthesis can be classified into empirical, mechanistic, quasi-mechanistic, as well as steady-state or dynamic, depending on the assumptions made, their formulation, and applications. There is a great deal of diversity among the models as they describe the entire photosynthetic process in different ways, but they can be also incomplete, depicting either the light or the carbon reactions (or parts of these), depending on the research question raised, as well as the available data. The very early models related to carbon assimilation were empirical (e.g., Maskell 1928), being based on the relationship between the net photosynthesis rate (PN) and the environmental variables such as light intensity (I), temperature (T), and CO2 concentration (Ci or Cc, i.e., the intercellular CO2 concentration, or that in chloroplasts). This method has been used by many researchers, with numerous variations, and consists of an algebraic function containing several parameters, which is relatively simple and easy to use to fit the experimental data, but without the description of the physiological processes of photosynthesis (see e.g., Smith 1936; Jassby and Platt 1976; Eilers and Peeters 1988; Ye 2007). However, when such an empirical model is combined with a steady-state mechanistic model of photosynthesis, which links biochemical properties of the leaves to gas exchange measurements (see e.g., Farquhar et al. 1980), the newer unified model can indeed provide information on key photosynthetic parameters ‘capturing’ the underlying physiological processes of photosynthesis. For different protocols, proposed for fitting the dependence of photosynthesis or the electron transport rate (ETR, noted also as flux J) on environmental variables by using such a combined model, see e.g. (1) Ögren and Evans (1993), von Caemmerer (2000), Johnson and Murchie (2011), Ye et al. (2013a,b; 2020; 2024), and Herrmann et al. (2020) for net photosynthesis-light response curves, (PN/I) and (J/I); (2) Long and Bernacchi (2003), Sharkey et al. (2007), Dubois et al. 2007, Miao et al. (2009), and Gu et al. (2010) for net photosynthesis-intercellular CO2 response curves (PN/Ci); and (3) Medlyn et al. (2002), Hikosaka et al. (2006) and Adams et al. (2017) for net photosynthesis-temperature response curves (PN/T). We note that the PN/I curves are characteristic for the photosynthetic phenotype of plants, and a combined model (as those mentioned above) gives information on the quantum yield, the maximum photosynthetic capacity, the leaf radiation use efficiency, and the light compensation point (Johnson and Murchie 2011). The PN/Ci curves, as determined from gas exchange measurements, have been successfully used to evaluate the parameters defined by the Farquhar et al. (1980) model, or its variants (Yin et al. 2009). Since these models are commonly used to estimate the CO2 assimilation from the leaf to the global scale (Yin and Struik 2009; Bernacchi et al. 2013; Rogers et al. 2017; Wu et al. 2016, 2019), quantitative evaluation of their parameters is necessary for the estimation of crop and ecosystem productivity, and together with the results obtained from the PN/T curves, they are essential for the evaluation of climate change impact on the entire system (von Caemmerer 2013, 2021).
However, the mechanistic photosynthetic models (steady-state or dynamic) are based on biochemical and biophysical principles that describe the reactions and pathways of photosynthesis. Although they are more realistic and explanatory, they require many more parameters as well as data to be calibrated and validated (see e.g., Antal et al. 2013). Mechanistic steady-state models, as those just described above, assume that the photosynthetic reactions are in equilibrium, i.e., the concentrations of intermediates do not change over time, and thus they consist of a set of algebraic equations containing parameters characterizing the photosynthetic system. The steady-state model of C3 photosynthesis, proposed by Graham Farquhar, Susanne von Caemmerer and Joseph Berry (Farquhar et al. 1980), often referred to as the FvCB model, is one of the most widely used models in plant science. This model consists of a small number of algebraic equations describing the steady-state CO2 exchange in C3 leaves—that relates the biochemistry of CO2 assimilation to gas exchange—and accounts for the limitations imposed by Rubisco activity, electron transport capacity, and CO2 diffusion, within the leaf. The FvCB model has now many improved variants with additions such as: (1) temperature dependence of CO2 assimilation from the chloroplast to the ecosystem level (Bernacchi et al. 2001, 2002, 2013), and the mesophyll conductance process (Moore et al. 2021); (2) adjustments for C4 photosynthesis (von Caemmerer and Furbank 2003; Wang et al. 2014a,b; von Caemmerer 2021), or for C3-C4 photosynthesis (von Caemmerer 2000; Bellasio 2016); (3) inclusion of alternative electron transport pathways (Yin et al. 2004); (4) generalization of a mesophyll resistance model for different intracellular arrangements of chloroplasts and mitochondria (Yin and Struik 2017); and (5) inclusion of possible effects of cyanobacterial bicarbonate transporters at the chloroplast envelope (Price et al. 2011). The above-cited steady-state models of photosynthesis are simple, easy to use for the analysis of experimental data, and for the exploration of different ways to improve photosynthesis, such as optimizing the distribution of resources between different enzymes of the carbon metabolism, redirecting photorespiratory CO2 to implement bicarbonate pumps in C3 chloroplasts, or to insert C4, CAM or intermediate C3–C4 forms of photosynthesis into C3 plants, and for exploring potential benefits of engineering different photosynthetic traits into crops (for additional applications, see reviews by von Caemmerer 2000, 2013; Yin et al. 2021).
When investigating the response of photosynthesis to rapid variations of different environmental variables, mechanistic dynamic models are much more appropriate than the steady-state photosynthesis model, since the photosynthetic processes in this case are continuously adjusted to match the various changes of the environment (Kaiser et al. 2015). Dynamic models are usually based on the use of ordinary differential equation systems describing the kinetics of the reactions involved in photosynthesis, such as electron transport and carbon fixation, but they can be also based on stochastic or probabilistic methods, or contain related elements.
Many available models of photosynthesis have focused on the kinetics of the underlying processes related to the induction of photosynthesis during transition from the dark to the light, such as Chl fluorescence induction; for the relation between the Chl fluorescence and photosynthesis, see Papageorgiou and Govindjee (2004), Kalaji et al. (2016, 2017, 2018), Brestic and Allakhverdiev (2022), and Stirbet et al. (2019). There are models of Chl fluorescence induction that do not consider the carbon assimilation reactions (e.g., Stirbet and Strasser 1996; Stirbet et al. 1998; Lazár 2003, 2009, 2013; Tomek et al. 2003; Sušila et al. 2004; Lazár et al. 2005a; Zhu et al. 2005; Guo and Tan 2011, 2014; Ebenhöh et al. 2014; Belyaeva et al. 2019; Riznichenko and Rubin 2021), or those which include both the light and carbon assimilation reactions and other related metabolic reactions (e.g., Laisk et al. 2006, 2009a; Zhu et al. 2013); see reviews by Stirbet et al. (2014, 2020), and a book on photosynthesis in silico (Laisk et al. 2009b). On the other hand, minimized models of photosynthesis have been used by Morales et al. (2018a) and Bellasio (2019) to analyze the photosynthetic electron transport regulation and to simulate experimental data measured with different methods, or by Fu et al. (2020) to verify a feedback control framework for regulating photosynthetic activities. The effects of natural fluctuating light on the rate of photosynthesis have been also analyzed (see e.g., Way and Pearcy 2012; Morales et al. 2018b; Slattery et al. 2018); for reviews, see Morales and Kaiser 2020; Long et al. 2022), as well as artificial fluctuating light (e.g., Lazár et al. 2022a). In the latter case, multiple factors influence the rate of photosynthesis, such as changes in stomatal and mesophyll conductance, NPQ relaxation, Rubisco activation/de-activation, activation of RuBP regeneration enzymes, and metabolite pool sizes; further, the relative importance of these factors varies with the species of the plant or of the algae (e.g., Taylor and Long 2017; De Souza et al. 2022). On the other hand, simultaneous changes of several other environmental factors (e.g., variations in leaf temperature, leaf-to-air vapor pressure deficit VPDleaf-air, and atmospheric [CO2]) also induce perturbations in plants (Kaiser et al. 2017), which cause e.g., changes in Ci (Peak and Mott 2011), and thus in the rate of carbon assimilation and the crop yield (Taylor and Long 2017; Wang et al. 2021).
Furthermore, dynamic models of photosynthesis have been used to verify hypotheses regarding spontaneous oscillations in photosynthesis (e.g., Laisk and Walker 1986; Laisk et al. 1991; Lazár et al. 2005b; see a review by Walker 1992), and to calculate the flux control coefficient (CC) of enzymes for CO2 uptake rate (Poolman et al. 2000), as well as to identify targets to enhance photosynthesis (Poolman 1999; Zhu et al. 2007; Wang et al. 2021; see also Zhu et al. 2010). In addition, we note that for the quantitative understanding of complex metabolic pathways, related to carbon assimilation reactions, with the goal of altering the distribution of metabolic flux or of designing metabolic pathways for new products, mathematical modelling of metabolism is definitely an important tool (Giersch 2000).
Finally, different multiscale crop models, having at their base available models of photosynthesis, are currently being developed, and these will be mentioned in the last section of this review: Crop models are imperative to improve crop yield.
Fine-tuning the light phase of photosynthesis to obtain a greater crop yield
Several reviews have recently been published on how to optimize/improve the light-dependent photosynthetic reactions to increase plant productivity (see e.g., Cardona et al. 2018; Murchie and Ruban 2020; Sukhova et al. 2021; Walter and Kromdijk 2022; Leister 2023; Wu et al. 2023). For this goal, the following aspects of the light-phase of photosynthesis need to be considered (see the blue stars in Fig. 3). The quality (i.e., the wavelengths) of the light absorbed by the photosystems has been shown to strongly influence the efficiency of photosynthesis (see e.g., Hamdani et al. 2019a; and a review by Lazár et al. 2022b). Moreover, since PSII and PSI work in series, any imbalance in their relative excitations will affect negatively on the photosynthetic process; however, there is regulation and re-equilibration through a complex process known as the ‘state-transition’, in which a part of the PSII antenna (a mobile LHCII complex) is relocated from PSII to PSI, or the other way around, as a function of the redox state of the plastoquinone (PQ) pool (see e.g., Papageorgiou and Govindjee 2011; see blue star 1 in Fig. 3). Moreover, if the light already absorbed by PSII is in excess, the excited state can be quenched non-photochemically as heat (Demmig et al. 1987; Jahns and Holzwarth 2012; Papageorgiou and Govindjee 2014; see blue star 2 in Fig. 3). If the absorbed light is still in excess, it leads to the formation of reactive oxygen species (ROS), which can be, however, scavenged, in a complicated manner, by the water-water cycle (WWC; see Foyer 2018 and blue star 5 in Fig. 3). An important feature of photosynthetic electron transport in the thylakoid membrane (TM) during the “light phase “of photosynthesis is that it is coupled to proton transport across the membrane, leading to acidification of the lumen (see e.g., Buchanan 1980). The low lumen pH, thus formed, not only initiates the NPQ, but it is also a part of the proton motive force (pmf) driving ATP synthesis (Mitchell 1975); in addition, it causes photosynthesis control at the level of Cyt b6/f (Wilson et al. 2021; Trinh and Masuda 2022; see blue star 3 in Fig. 3). Cyclic electron transport (CET) around PSI (see blue star 4 in Fig. 3) also contribues to acidification of lumen (e.g., Kawashima et al. 2017). As the other part of pmf is the electric potential difference, caused by redistribution of ions between the two sides of the TM, i.e., the membrane potential, ΔΨ (see blue star 6 in Fig. 3), it is important to consider the partitioning of pmf into ΔΨ and ΔpH (see blue star 7 in Fig. 3). All the processes, namely the state transitions, the NPQ, the water-water cycle, the photosynthesis control, and the membrane potential have been mathematically modelled as described below.
Schematic representation of the ‘light phase” of oxygenic photosynthesis in plants. Several protein complexes in the thylakoid membranes (TM) of the chloroplast participate in the production of ATP and NADPH needed for the CBB cycle to fix CO2: PSII and PSI operating in series, Cyt b6/f complex, PGR5/PGRL1 complex, NDH, PTOX, and CF0-CF1 ATP-synthase. The light energy harvested by LHCII and LHCI complexes is, respectively, channeled to P680 in PSII and P700 in PSI, where photochemical reactions take place, and the photogenerated electrons are then transported by several electron carriers through a linear electron transfer chain from water to NADPH (black arrows). The PGR5/PGRL1 and the chloroplast NDH complexes, the last one forming a super-complex with PSI, mediate two CET pathways around PSI shown by red arrows. Also, the water-water cycle (WWC), and the PTOX activity are indicated by dark-red arrows. In parallel with the electron transport, protons are also transported across TM by PSII, Cyt b6/f, PGR5/PGRL1 complex, NDH, and PTOX, leading to a pH difference (ΔpH) across the TM, Furthermore, the charge separation(s) in the PSI and PSII reaction centers, as well as different ion transport steps through TM, generate an electric potential difference (ΔΨ) across TM, which together with ΔpH, forms the proton motive force (pmf) that is used for ATP production at the ATP synthase. Finally, we show the CBB cycle, which works with NADPH, ATP and CO2, and few metabolic reactions in stroma, as indicated by purple arrows. Additionally, several bioengineering targets for the improvement of crop yield are marked with blue stars: (1) the antenna size; (2) the NPQ that develops in PSII antenna; (3) the photosynthesis control exerted by Cyt b6/f; (4) the influence of CET on ΔpH, and thus on NPQ and ATP/NADPH ratio; (5) the influence of WWC on ROS concentration; (6) the influence of ion transporters on ΔΨ; (7) the partitioning of pmf into ΔpH and ΔΨ components; and (8) the influence of different manipulations of the CBB cycle on CO2 fixation efficiency, such as engineering Rubisco, Rac, photorespiration, and addition of CO2 concentration mechanisms near Rubisco. Figure modified from Stirbet et al. (2020). 2.3RT/F, a physical constant; CBB cycle, Calvin–Benson–Bassham cycle of carbon assimilation; CET, cyclic electron transport around PSI; CF1 and CF0, a catalytic part, and a membrane-embedded part (containing a proton channel) of the ATP synthase; Cyt, cytochrome; ΔΨ, electric potential difference across the thylakoid membrane; ΔpH, pH difference across the thylakoid membrane; Fe-S, Rieske iron sulfur protein, an electron carrier; Fd, ferredoxin iron sulfur protein, another electron carrier; FNR, ferredoxin-NADP+ oxidoreductase; LHCI and LHCII, light‐harvesting complexes of PSI and PSII, respectively; NADP+, nicotinamide adenine dinucleotide phosphate, oxidized form, which is a terminal electron carrier; NDH, NADH dehydrogenase-like complex that is involved in antimycin A insensitive cyclic electron transport; NPQ, nonphotochemical quenching of the excited state of chlorophyll; OEC, oxygen evolving complex of PSII; P680, P700, the primary electron donors of PSII and PSI; PGR5/PGRL1, a complex containing Proton Gradient Regulation 5 and Proton Gradient Regulation Like 1 proteins that is involved in the antimycin A sensitive cyclic electron transport; pmf, proton motive force; PQ and PQH2, plastoquinone and plastoquinol; PSI and PSII, Photosystem I and II; PTOX, plastid terminal oxidase; QA, QB, first and second plastoquinone electron acceptors of PSII; Rac, Rubisco activase; ROS, reactive oxygen species; Rubisco, ribulose-1,5-bisphosphate carboxylase/oxygenase; TM, thylakoid membrane; WWC, water-water cycle
The state transitions
As mentioned earlier, the state transitions take place to provide a balanced excitation between the two photosystems (Allen et al. 1981). PQ pool reduction by the PSII under excess light leads to the activation of a kinase (STN7 in plants; Stt7 in algae) that phosphorylates a fraction of the LHCII in the PSII antenna, which, in accord with the most popular view of this process (see a review by Rochaix 2014), moves in the TM towards PSI, and attaches to its antenna. In this way, a transition from the high fluorescence State 1 to the low fluorescence State 2 takes place, since PSI has lower fluorescence yield than PSII. If this is followed by exposure to low light, the PQ pool is oxidized by the higher light absorption of PSI, which then inactivates the kinases, and the constitutively active PHP1/TAP38 phosphatase dephosphorylates the mobile LHCII complexes from PSI antenna, which then move back to PSII, leading to a State 2 to State 1 transition. Fine tuning of the state transitions and of the rates of their adjustment/accommodation in fluctuating light might lead to a better light management and thus, potentially to higher plant productivity. Indeed, Negi et al. (2020) have bioengineered a mutant of C. reinhardtii which dynamically adjusts the size of its light harvesting antenna as a function of the growth light intensity; this mutant shows higher photosynthetic rates, and two to three-fold greater biomass productivity, while preserving the ability to induce state transitions and nonphotochemical quenching (NPQ) of the excited state of Chl a (Demmig et al. 1987; see Fig. 3).
The state transitions have been mathematically modelled by Ebenhöh et al. (2014), and an essentially similar model was used by Stirbet and Govindjee (2016) for the same phenomenon. In both cases, the modelling involved the state transitions in the green alga Chlamydomonas reinhardtii, grown under low light, when the NPQ is only slightly involved. Ebenhöh et al. (2014) assessed the state-transition by using Pulse Amplitude Modulation (PAM) fluorescence measurements and simulations of changes in the maximal ChlF (FM´) under very low as well as high light. On the other hand, Stirbet and Govindjee (2016) used Chl a fluorescence induction measurements under continuous illumination and confirmed in silico that under anaerobic conditions in the darkness, the PQ pool is reduced by chlororespiration using PTOX (the plastid terminal oxidase; see Fig. 3), which triggers a State 1 to State 2 transition. In the subsequent in silico illumination under aerobic conditions, a State 2 to State 1 transition occurs, causing a slow Chl a fluorescence rise from the S-step to a maximum M in the simulated slow Chl fluorescence induction curve [Note that the “step S” follows the usual OJIP phase of the Chl a fluorescence transient, where P to S is a fluorescence decline due mostly to the ferredoxin-NADP+ oxidoreductase (FNR) activation and NPQ induction by lumen acidification, see e.g., Briantais et al. 1979]. Additionally, Stirbet and Govindjee (2016) analyzed, also in silico, how light intensity and different photosynthetic processes influence the degree of state transitions, and thus the relative amplitude of the simulated S-M increase.
The non-photochemical quenching of the excited state of chlorophyll a
Similar to the state transition, a faster induction of the NPQ under excess light and a faster relaxation of NPQ under low light, might be a way for the plants and algae to better manage the light and thus to increase plant productivity. The so-called fast NPQ (denoted as qE) is triggered by acidification of the lumen, which causes protonation of the PsbS protein and the activation of violaxanthin de-epoxidase (VDE); VDE converts violaxanthin to zeaxanthin via antheraxanthin. Further, protonated PsbS, and zeaxanthin are necessary for the induction of the NPQ in excess light (Jahns and Holzwarth 2012). Under low light (or in darkness), zeaxanthin epoxidase (ZE) converts zeaxanthin back to antheraxanthin, and then to violaxanthin, causing a decrease in NPQ. Different mathematical models have been formulated by choosing different molecular aspects of NPQ (for reviews, see Zaks et al. 2013; Bennett et al. 2018; Morris and Fleming 2018). D’Haese et al. (2004), however, focused specifically on the role of VDE and the conversion of violaxanthin to antheraxanthin and zeaxanthin. On the other hand, Ebenhöh et al. (2011) presented a more detailed model of NPQ, based on zeaxanthin being converted by VDE, which is activated by acidification of the lumen. And this was then improved by Zaks et al. (2012) and Matuszyńska et al. (2016) by the inclusion of protonated PsbS for having a more complete view of NPQ. We note that in addition to zeaxanthin and protonated PsbS, a role of lutein was included by Leuenberger et al. (2017) in their model. On the other hand, a general model of NPQ, considering only PSII in the reduced and the oxidized state being either quenched or unquenched, without going into molecular details of the quenching, has been formulated by Snellenburg et al. (2017). A similar approach was also used in the model by Sukhova et al. (2020). However, Bennett et al. (2018) related the extent of NPQ to the diffusion length of excitation, modelled as a random walk in the PSII pigment bed: the higher the quenching, the shorter the diffusion length.
Using only a phenomenological model of NPQ in response to the light history, X-G. Zhu et al. (2004a) theoretically predicted that the delay in the recovery of NPQ can lead up to a 30% decrease of canopy carbon uptake. These theoretical predictions have been qualitatively confirmed, in tobacco, in field measurements (Kromdijk et al. 2016), and in soybean transformants overexpressing VDE, PsbS, and ZE (De Souza et al. 2022). It is important to note that the increased plant productivity of the transformants over the wild-types occurred only under fluctuating light conditions, which is, however, common in the field.
On photoinhibition, and the water-water cycle
PSII is well-known to be easily photoinhited, but it is also rapidly repaired (for a review, see Vass 2012). Photoinhibition of PSII, initiated by the oxidation of amino acids of PSII D1/D2 proteins by hydroxyl radical HO• and superoxide anion radical O2•−, has been experimentally confirmed (Kale et al. 2017). On the other hand, photoinhibition of PSI is rare, and occurs only under certain specific conditions (reviewed in Sonoike 2011; Furutani et al. 2020), e.g., at low temperature (Tjus et al. 1998). Photoinhibition of PSI is usually inferred from the decrease of Pm (Terashima et al. 1994), the maximal transmittance at 820 nm, reflecting the oxidation state of P700, the primary electron donor in PSI reaction center.
However, Suorsa et al. (2012) showed clear PSI photoinhibition using Arabidopsis pgr5 mutant under fluctuating light; they observed a large decrease in its Pm; this pgr5 mutant lacks the PGR5 protein, which facilitates a type of CET around PSI (see Fig. 3). Lack of the CET in the mutant disables the photosynthesis control (see below), and decreases the outflow of electrons from PSI (Kono and Terashima 2016), both leading to the electron flow from PSI to oxygen and the formation of O2·−, which damages PSI. The O2·− undergoes enzymatic as well as non-enzymatic reactions known as the water-water cycle (WWC; see Fig. 3), leading to the formation of other reactive oxygen species (ROS), but also to a safe ROS scavenging (reviewed in Asada 1999, 2006). Since ROS also participate in signaling pathways (reviewed by Foyer and Hanke 2022), we note that we have here a fine tuning of WWC. The WWC has been mathematically modelled by Polle (2001), Valero et al. (2009, 2016), and Saadat et al. (2021); while Valero et al. (2009) have calculated steady-state fluxes through the WWC, Polle (2001) and Valero et al. (2016) have explored the dynamics of WWC function, respectively, under illumination by constant light, and under illumination by fluctuating light. On the other hand, Saadat et al. (2021) have presented the connection between the model of WWC with that of the CBB cycle; in addition, they have explored the WWC kinetics under fluctuating light.
The photosynthesis control
Apart from the central role of the acidified lumen in triggering the fast NPQ, protons already present in the lumen cause a back pressure against the movement of other protons going to the lumen during (re)oxidation of the plastoquinol (PQH2) on the luminal side of Cyt b6/f. The back pressure of protons leads to a decrease of the rate constant of PQH2 reoxidation (Rumberg and Siggel 1969), the effect being the so-called photosynthesis control (Tikhonov et al. 1981).
Increased photosynthesis control, on the one hand, results in the accumulation of P700+ (i.e., the oxidized PSI primary donor), which prevents the photoinhibition of PSI (see e.g., Sejima et al. 2014) but, on the other hand, it decreases the amount of NADPH produced, which decreases CO2 assimilation, and thus plant productivity. Since the photosynthesis control requires a more acidified lumen than that needed for the triggering of the NPQ (Takizawa et al. 2007; Schansker 2022), fine tuning of photosynthesis control and NPQ, i.e., in fact of the luminal pH, is thus important for attaining high plant productivity. In relation to that, transgenic rice lines with variable content of Rieske protein in the Cyt b6/f complex (see Fe-S in Fig. 3) indeed show enhanced leaf photosynthesis, making Cyt b6/f complex a target to increase grain yield (Yamori et al. 2016).
We note that the light-induced acidification of the lumen is caused not only by the linear electron transport from water to NADP+, but also by the CET around PSI (see Fig. 3), either via PGR5/PGRL1 protein complex, or via NDH-like complex (Kawashima et al. 2017; reviewed, e.g., in Shikanai 2016). Several models of different complexity have already been constructed; several describe the role of CET on the luminal pH and the role of photosynthesis control on the electron transport (Hope et al. 1994; Sato et al. 2014; Morales et al. 2018a; Matuszyńska et al. 2019; Vershubskii and Tikhonov 2020; Johnson and Berry 2021).
The electric potential difference across the thylakoid membrane
To dissipate the charge of protons, accumulated in the lumen due to light-driven proton-coupled electron transport, counter ions (K+, Cl−, Mg2+; Hind et al. 1974) have been shown to move thorough the TM via related ion channels, which affect the electrical potential difference across the thylakoid membrane (ΔΨ), leading to pmf (= ΔpH + (2.3RT/F)ΔΨ) partitioning (see Fig. 3), and, ultimately, this shows its impact on plant productivity (reviewed by e.g., Szabò and Spetea 2017). The role of counter ion fluxes and ΔΨ on photoprotection and photosynthetic performance have been clearly recognized, especially under fluctuating light conditions, by, e.g., Li et al. (2021); Lazár et al. (2022a); and von Bismarck et al. (2023). Mathematical models of ΔΨ of different complexity are available (see e.g., van Kooten et al. 1986; Cruz et al. 2001; Lyu and Lazár 2017a, 2017b, 2022, 2023; Li et al. 2021), and they need to be further examined and related to future experimental data.
Photosynthesis under fluctuating light
All of the processes mentioned above are, of course, important, especially under fluctuating light conditions, when their fine tuning/regulation is repeatedly required. Since the fluctuating light is more natural in the field than illumination with constant light intensity, as is mostly used in laboratory experiments, a large number of researchers are now exploring photosynthesis under fluctuating light conditions. Several models have now been formulated to describe the photosynthesis and its regulation under fluctuating light, and what is important, they have been related to plant and algal productivity (see e.g., Graham et al. 2017; Morales et al. 2018b; Steen et al. 2020; Krichen et al. 2021; Salvatori et al. 2022). For a better understanding of photosynthesis regulation at the molecular level, there is also a special interest in experiments that use illumination by light with its intensity changing as a sinus function (see: Nedbal and Březina 2002; Nedbal et al. 2003, 2005); this has now been supported not only by mathematical models, but by new experiments (Nedbal and Lazár 2021; Lazár et al. 2022a; Niu et al. 2024). By changing the period (frequency) of the sinusoidal wave, a resonance with a particular regulatory process can be established and thus the response of the regulatory processes to a particular frequency (period) of light oscillations is being revealed (Niu et al. 2023). Such an approach seems to be promising for finding frequency limits of particular regulatory processes, which can be important for obtaining higher plant productivity in the future.
Synthetic biology approach for improving the plant carbon metabolism
Synthetic biology is a highly promising interdisciplinary field that integrates molecular biology with biochemical engineering, combined with computational methods. Its objective is to modify existing organisms, or to create artificial novel life forms for newer purposes, including the improvement of photosynthesis (South et al. 2018; Stewart et al. 2018).
As discussed above (see Background on oxygenic photosynthesis), the photorespiratory cycle is a complex and energy consuming set of reactions required to recycle the 2PG resulting from the oxygenation of RuBP by Rubisco (see reviews by Foyer et al. 2009; Walker et al. 2016), which takes place mainly in chloroplasts, peroxisomes, and mitochondria. Further, photorespiration is known to be significant in C3 plants; it can increase considerably under warm and dry environments (Long et al. 2015). By using mathematical modelling, X.G. Zhu et al. (2008) showed that the maximal theoretical efficiency of gross photosynthesis in C3 plants decreases due to photorespiration by ≈ 49% at 30℃ and 380 ppm [CO2]. According to Walker et al. (2016), even a 5% reduction in photorespiration would be worth almost 540 million dollars a year in yield gain in the US Corn Belt alone! Therefore, decreasing photorespiration has been an early goal for crop yield improvement (see e.g., Long et al. 2006, 2015; Peterhansel and Maurino 2011; Evans 2013; Ort et al. 2015; Betti et al. 2016; Walker et al. 2016; Orr et al. 2017; South et al. 2018; Batista-Silva et al. 2020). However, this process also plays multiple metabolic and regulatory roles (Foyer et al. 2009; Timm et al. 2016; Bauwe 2023; Chen et al. 2023c). Thus, decreasing the efficiency of photorespiration must also lead to stability and beneficial regulation of plant metabolism (Walker et al. 2016). There are several approaches to bioengineer photorespiration that can reduce the carbon and energetic loss in C3 crops (Raines 2006; South et al. 2018), such as by (i) increasing the efficiency of Rubisco to assimilate CO2 through genetic manipulation; (ii) bioengineering new alternative metabolic pathways, i.e., photorespiration bypasses, by introducing non-native genes that are used to process the glycolate more efficiently; (iii) preventing Rubisco oxygenation reaction by enriching the [CO2] at the Rubisco site, through the installation of pyrenoid or carboxysome structures obtained from single-celled algae or cyanobacteria, and, this together with the expression of bicarbonate transporters; and (iv) by enriching [CO2] at the Rubisco site, by introducing C4 photosynthesis into C3 plants. Below, we briefly present some of these approaches.
Bioengineering Rubiscos with higher rates of CO2 assimilation
More than 90% of the inorganic carbon converted into biomass, in Nature, is fixed by Rubisco (ribulose-1,5-bisphosphate carboxylase/oxygenase), which represents 50% of all soluble proteins in a leaf, and it is the most abundant protein on Earth (Bar-On and Milo 2019); for reviews, see Spreitzer and Salvucci (2002), Erb and Zarzycki (2018), and Prywes et al. (2023); for historical aspects, see Portis and Parry (2007) and Sharkey (2023). This enzyme is known to limit carbon assimilation of C3 plants, due to its activity as oxygenase, and its low turnover frequency (i.e., between 1 and 10 s−1; Erb and Zarzycki 2018). In order to increase crop production, different ways to improve the activity of Rubisco have been suggested (see e.g., Zhu et al. 2010; Raines 2003, 2011; Cummins et al. 2018; Paul 2021; Oh et al. 2023; Prywes et al. 2023). These include: (i) increasing the Rubisco content within chloroplasts (Salesse-Smith et al 2018, 2020; Yoon et al. 2020); (ii) replacing the inefficient endogenous Rubisco with a better performing one (Zhu et al. 2004b; Lin et al. 2014); (iii) decreasing photorespiration by increasing Rubisco specificity for CO2 relative to O2 through genetic modification (Zhu and Long 2009; Lin et al. 2014; Carmo-Silva et al. 2015; Sharwood 2017); and (iv) manipulating the activation of Rubisco by Rubisco activase (Rca) (Mott and Woodrow 2000; Carmo-Silva et al. 2015; Perdomo et al. 2019; Chen et al. 2023a).
On a positive note, Salesse-Smith et al. (2018) have already obtained a > 30% increase in Rubisco content in maize by overexpressing its large (LS) and/or small (SS) subunits, as well as the Rubisco assembly chaperone RUBISCO ASSEMBLY FACTOR 1 (RAF1). The transgenic plants had 15% increased CO2 assimilation (i.e., the light-saturated photosynthetic capacity, ASAT), which was correlated with higher fresh weight. The difference between the increase in Rubisco content (i.e., 30%) and that of ASAT (i.e., 15%) was accounted by a decrease in Rubisco activation state up to 23%. Furthermore, important results have been obtained also by Yoon et al. (2020), who have overexpressed Rubisco in paddy rice with its own promoter. Their study showed increases in filled spikelets, with 28% higher grain yields in paddy fields, and 11–23% higher total biomass. In addition, there was greater yield gain for nitrogen added in the transgenic rice than in the wild-type, indicating an increased nitrogen use efficiency in the former. Long (2020) suggests that this work and that of Salesse-Smith et al. (2018) “contradict the space limitation hypothesis for C3 and C4 crops”, which assumes that there is no physical space for a higher Rubisco content in their chloroplasts (Pyke and Leech 1987). Moreover, Long (2020) has also highlighted the results obtained by Yoon et al. (2020), stating that “an engineered increase in photosynthetic capacity does, in fact, result in increased grain yield under production field conditions in the world’s most important cereal — rice”.
Concerning the work on Rubisco improvement, Zhu et al. (2004b) have analyzed the potential effects on the productivity of a C3 crop plant by engineering a ‘foreign’ Rubisco with higher catalytic rates per active site (see e.g., Erb and Zarzycki 2018), and/or higher specificities for CO2 relative to O2; for this, they used a steady-state biochemical model for leaf photosynthesis coupled with a canopy biophysical microclimate model. The results obtained by Zhu et al. (2004b) suggest that introducing a Rubisco with a high carboxylation rate could, indeed, lead to increased crop yields, without an increase in the amount of Rubisco per unit leaf area. Their results show that the use of Rubisco from Amaranthus edulis (a rare C4 plant) could increase the carbon gain by 17%, while that from Griffithsia monilis (a red alga) could lead to greater than 25% carbon gain. Nonetheless, the challenges of engineering foreign Rubiscos in crop plants, or bioengineering the native ones, are still to be fully understood and exploited (Sharwood 2017; Chen et al. 2023a). Moreover, it is known that rice yields can be increased by 23%, when CO2 atmospheric concentration is raised to 627 ppm (Ainsworth 2008), since the Rubisco specificity to oxygen decreases by increasing CO2. However, Hu et al. (2022) showed that to maximize this CO2 fertilizer effect in the future, heat-resistant, high-yield hybrid rice cultivars with large sink capacity, and appropriate nitrogen supplement should be selected. For further information, see Cao et al. (2021).
Further, the role of Rubisco activase (Rca) on the Rubisco performance in non-steady-state photosynthesis was studied by Mott and Woodrow (2000) by using a photosynthesis model to predict the optimum protein allocation between Rubisco and Rca for plants under different light conditions, including light flecks of various duration; we note that Rca is an indispensable catalytic chaperone of Rubisco, which remodels its active site, helps the release of inhibitors, and restores its catalytic functions (Waheeda et al. 2023). Simulations by Mott and Woodrow (2000) showed that the protein distribution leading to the maximum steady-state rate of photosynthesis does not produce the maximum activation rate for Rubisco, and that in fluctuating light the plant must allocate more protein to the Rca than when exposed to constant light. Thus, further research is needed to find and improve Rubisco and Rca engineering based on the observations, mentioned above.
Finally, we note that, while Rubisco plays a key role in photosynthesis, the increased activity of other enzymes participating in the CBB cycle can also affect positively the carbon assimilation and plant growth (see e.g., Simkin et al. 2019). Indeed, modelling results have shown that the natural distribution of the enzymes within the CBB cycle is not optimal and could limit photosynthesis (Zhu et al. 2007), and that higher levels of ‘for example, sedoheptulose-1,7-bisphosphatase and fructose-1,6-bisphosphate aldolase, as well as of the enzymes related to sink capacity, could support an increased productivity (Kubis and Bar-Even 2019). For example, Morales et al. (2018a) have analyzed potential improvements in CO2 assimilation resulting from optimizing different regulatory processes; their simulations showed 17% improvement when the limiting steps, related to the photoactivation of the CBB cycle enzymes and stomatal opening, were removed. Further, Matuszyńska et al. (2019) have used the metabolic control analysis (MCA) to investigate the regulatory dependence between the photosynthetic electron transport and the CBB cycle, to quantify the control distribution of ‘demand and supply’ under different light conditions; Matuszyńska and coauthors found that the ‘demand’ reactions control the flux under light-saturating conditions (with seduheptulose-1,7-bisphosphatase maintaining the highest overall flux control, as shown by Poolman et al. 2000), while the ‘supply’ reactions (sustained by PSII and PSI activities) show a higher overall flux control under light-limited conditions. Furthermore, Kandoi et al. (2022), who overexpressed carbonic anhydrase βCA3 from Flaveria bidentis into Arabidopsis thaliana, observed that this led to increased amino acid content, improved photosynthesis (by 16–22%), better water-use efficiency (by 22–26%), higher starch content (by 10–19%), and higher biomass (by 14–20%). Lastly, Hamdani et al. (2019b) discovered the importance of the β-glucosidase 5 gene to obtain a higher quantum yield of photosynthesis in rice—the mechanism of which remains to be further investigated.
Reducing photorespiration by engineering synthetic bypasses routes
We now provide a glimpse of the bioengineering approach involving the introduction of alternative pathways to reduce photorespiration, i.e., the ‘photorespiratory bypasses’, such as those proposed by Carvalho (2005), Kebeish et al. (2007), Carvalho et al. (2011), and Maier et al. (2012) (also see Raines 2011; Peterhansel et al. 2013; Xin et al. 2015; Betti et al. 2016).
-
(1)
Kebeish et al. (2007) have introduced the glycolate catabolic pathway from Escherichia coli into Arabidopsis thaliana, in which the glycolate is converted to glycerate only in chloroplasts (Fig. 4, in blue), with no NH3 release, but with CO2 release into chloroplasts instead of mitochondria, which is energetically more economical; it increases the net photosynthesis, as well as the biomass.
-
(2)
Carvalho et al. (2011; also see Carvalho 2005) engineered a photorespiratory bypass in which the glyoxylate is converted to hydroxy-pyruvate in the peroxisome (Fig. 4, in green); here, the NH3 release is abolished, one-quarter of the carbon from glycolate is released as CO2 in the peroxisomes, and three-quarters of the carbon from glycolate is re-converted to PG; however, the implementation of this bypass in Nicotiana tabacum was only partially successful.
-
(3)
Maier et al. (2012) introduced a photorespiration bypass in Arabidopsis thaliana, in which the glycolate had a complete oxidation in chloroplasts (Fig. 4, in red), with the data indicating that both the photosynthesis and biomass of the transgenic plants were enhanced.
Photorespiration in plants, and bioengineering approaches for its optimization. Photorespiration in C3 plants (black), with four engineered photorespiratory bypasses to increase CO2 assimilation and/or reduce energy expenses; in blue is the pathway of Kebeish et al. (2007); in green is the pathway of Carvalho et al. (2011); in red is the pathway of Maier et al. (2012); and in purple is the pathway of South et al. (2019). Enzymatic reactions or metabolite transport steps are indicated by arrows. Figure modified from South et al. (2018)
Xin et al. (2015) have analyzed theoretically the above three bypasses by using the C3 photosynthesis model of Zhu et al. (2007), and by adding the sets of the new biochemical reactions of these bypasses. The results obtained by Xin et al. (2015) led them to the conclusion that not all photorespiration bypasses are functional or beneficial since they predict that: (i) the bypass of Kebeish et al. (2007) would increase the photosynthetic rate under a range of CO2 and light conditions; (ii) the bypass of Carvalho et al. (2011) would enhance photosynthesis, but only under low light intensity; and (iii) that the bypass of Maier et al. (2012) would reduce the photosynthetic rate, which means that the loss of CO2 in this pathway would not be compensated by the benefit of an increased CO2 concentration in the chloroplast. However, we note that the above in silico predictions were not in complete agreement with the experimental results, as the photosynthetic rate measured by Kebeish et al. (2007) increased only under low light, and that measured by Maier et al. (2012) showed a modest increase, not a decrease. Xin et al. (2015) explained that it is difficult to predict with precision if a bypass is a viable approach to optimize the photosynthetic rate since the photorespiratory pathway can interact with many other pathways, such as the nitrogen metabolism and respiration (see also Hodges et al. 2016). However, the bypasses that increase the CO2 concentration close to Rubisco in chloroplasts, and reduce the energy costs of photorespiration (by avoiding the ammonium release), are promising, and must be pursued further.
A number of other photorespiratory bypasses have been studied, and show promising results in enhancing photosynthesis in different crop plants. These include those by Nölke et al. (2014) on potato (Solanum tuberosum), by Dalal et al. (2015) on the biofuel crop plant Camelina sativa, by South et al. 2019 and Cavanagh et al. (2022) on tobacco (Nicotiana tabacum) (see this pathway in Fig. 4), and by Shen et al. (2019) and Wang et al. (2020) on rice (Oryza Sativa). Due to the relationship of photorespiration with other metabolic pathways (see the above discussion) adequate multiscale crop models (see e.g., Wu et al. 2019; Wu 2023), as well as complex laboratory and field trials (see e.g., Wang et al. 2020; Cavanagh et al. 2022), are necessary for the verification of these bypasses. In view of the advances made in genome engineering and in synthetic biology, we are extremely optimistic that other possible inducible metabolic pathways will be found in the future, including those for a fully optimized photorespiration in crop plants under field conditions.
Introducing CO2 concentrating mechanisms into C3 plants
The possible effects of the addition of a cyanobacterial CO2 concentrating mechanism (i,e., a carboxysome; see e.g., Price et al. 2011, 2013) into a C3 crop leaf were studied theoretically by McGrath and Long (2014). We note that a carboxysome has a polyhedral structure covered with a protein shell (see Fig. 5), which contains carbonic anhydrase (CA) and Rubisco arranged in a semi-ordered array (Long et al. 2007). McGrath and Long (2014) used a kinetic model containing 9 biological compartments, as well as the photosynthesis model of Farquhar et al. (1980), to evaluate the potential of the added carboxysome into the stroma (of the chloroplast) to improve C3 photosynthesis, and to determine the necessity of the introduction of other components, such as bicarbonate transporters. These simulations have shown that ≈ 60% improvement in the net CO2 uptake can be achieved without any modification of the leaf anatomy, and it could lead to 36%-60% increase in the crop yield; also, the addition of bicarbonate transporters is expected to further increase photosynthesis by 16%. Success in engineering α-carboxisomes into tobacco chloroplasts has been obtained by Long et al. (2018), who, by using a reduced gene set, have succeeded in increasing CO2 fixation and crop yield up to 60% in a transgenic tobacco, as previously predicted by McGrath and Long (2014). Furthermore, Chen et al. (2023b) have been able to generate morphologically correct α-carboxysomes, by transforming 9 carboxysome genetic components derived from a proteobacterium.
Different strategies to bioengineer carbon concentrating components in chloroplasts from C3 plants. They consist of the expression, in the chloroplast stroma, either of a functional cyanobacterial carboxysome (orange icosahedron), or an algal pyrenoid (red circle), as well as HCO3− transporters (red and orange ovals) on the inner chloroplast membrane. Figure modified from Batista-Silva et al. (2020). CBB cycle, Calvin–Benson–Bassham cycle of carbon assimilation; 2PG, 2-phosphoglycolate; 3PG, 3-phosphoglycerate; RuBP, ribulose 1,5-bisphosphate
Further, we note that the modelling work of Fei et al. (2022) has provided insights into the operating principles of the pyrenoid-based CO2-concentrating mechanism of the green alga Chlamydomonas reinhardtii (also see: Burlacot and Peltier 2023). Based on the results obtained with their model, as well as the data from the literature (Wang et al. 2016; Meyer et al. 2017; Mukherjee et al. 2019), Fei et al. (2022) suggest that a physical barrier is necessary for the pyrenoid to diminish the CO2 leakage, and to provide a proper localization of the enzymes to reduce the futile cycling between CO2 and bicarbonate; in addition, they have proposed a four-step path for engineering pyrenoids into crop plants. For a schematic presentation of the introduction of CO2 concentrating mechanisms into C3 plants, see Fig. 5.
Bioengineering C4 rice
The C4 plants have a higher potential energy-conversion efficiency and improved nitrogen and water use efficiencies than the C3 plants, particularly in hot climates, due to their CO2-concentrating mechanism at the Rubisco site in bundle sheath (BS) cells, and a reduced photorespiration (Schlüter and Weber 2020). Therefore, introducing C4 pathway into C3 plants is considered to be a highly desirable way to increase their yield (Schlüter and Weber 2016; Kubis and Bar-Even 2019). However, this is a difficult task, as key features of C4 photosynthesis must be introduced into C3 plants (Cui 2021), such as the Kranz anatomy (Hatch and Osmond 1976), which implies a spatial separation between the CO2 fixation and carbohydrate synthesis into the mesophyll (M) and BS cells. Moreover, in NADP-ME-type C4 plants, the M cells perform whole-chain electron transport, while the BS cells have low PSII activity, but high rates of CET around PSI to drive ATP synthesis, because most of NADPH is supplied by the M cells, via the malate shuttle (Munekage and Taniguchi 2017; Sales et al. 2021), which is not the case in C3 plants. An international C4 rice consortium with the aim to bioengineer C4 photosynthesis in rice began in 2008 (see https://c4rice.irri.org/), and significant progress has already been made to identify genes that are involved in the NADP-ME-type C4 pathway, and to successfully introduce some of these into rice, as published by Ermakova et al. (2020, 2021). On the other hand, important modelling work is needed in order to verify different hypotheses related to the various ways to proceed in this endeavor, and to regulate the functionality and viability of such radical changes that control morphological and biochemical conversion in rice photosynthesis. In this direction, Ermakova et al. (2020) have used a combination of the C3 model of photosynthesis by Farquhar et al. (1980) and the enzyme- limited C4 model of von Caemmerer (2000), showing that even small amounts of C4 photosynthesis introduced around the existing veins in rice could enhance photosynthesis. This is consistent with the results obtained by Wang et al. (2017a, b), in which a 3D reaction diffusion model of connected M and BS cells in a C3 rice leaf was used to answer the question: Would the photosynthetic efficiency be improved by expressing C4 metabolism into a C3 leaf structure, without removing the C3 background metabolism? Data of Wang et al. (2017a, b) show that this is indeed possible, but in the engineered C3–C4 leaf, the partitioning of the energy between C3 and C4 photosynthesis, and that of Rubisco between the M and BS cells, are the main factors that would control the photosynthetic efficiency. Furthermore, Bellasio and Farquhar (2019) have used a leaf-level biochemical model for C3, C2, C2 + C4 and C4 photosynthesis (cf. Bellasio 2016) to simulate the introduction of C2 and C4 photosynthesis in C3 rice. Besides the ATP-limited and NADPH-limited sub-model of CO2 assimilation, the above-mentioned model contains a hydromechanical and biochemical sub-model of stomatal conductance, and a mechanistic description of light reactions necessary to simulate the NADPH and ATP generation, including cyclic electron flow around PSI that can be engaged, variably, to regulate the NADPH/ATP ratios. The simulations with this model have shown that C4 photosynthesis becomes detrimental under low light, at low temperature, and at high [CO2], which agrees with the available ecophysiological observations. Additionally, Wang et al. (2021) have studied the limiting factors of the C4 photosynthesis under non-steady-state conditions, by combining gas exchange data on maize, sorghum, and sugarcane (obtained under fluctuating light regimes) with a dynamic C4 photosynthesis model including dynamic stomatal conductance, post-translational regulation of key photosynthetic enzymes (with their temperature responses), and leaf energy balance. By comparing their model outputs with the rates of CO2 uptake and leaf stomatal conductance, Wang et al. (2021) showed that Rubisco activase, pyruvate phosphate dikinase regulatory protein, and stomatal conductance are the major limitations for the efficiency of the NADP-ME-type C4 photosynthesis during dark-to-high light transitions, which indicates that these components are good possible bioengineering targets for increasing photosynthesis in some C4 crops.
Models are imperative for improving crop yield
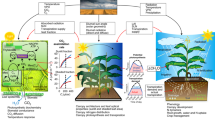
For the improvement of crop yields, canopy models are crucial, as they are key to not only basic long-term research on crop productivity, but also for applied research that provides short to medium term predictions at both field and subfield scales. Crop models can be of different types, depending on their design, such as empirical (e.g., correlative or statistical), and process-based models, which require physiological data from field experiments for parameterization (see e.g., Kasampalis et al. 2018). The process-based crop growth models describe the photosynthesis of the entire crop field, as well as the daily and seasonal integrals of CO2 assimilation rate of the canopy (Ac), which are correlated positively with daily and seasonal biomass production (X-G. Zhu et al. 2012, 2013; Song et al. 2017). Several types of canopy models have been developed to realistically simulate canopy photosynthesis under heterogeneous microenvironments, such as the big leaf model (Sellers et al. 1996), the sunlit/shaded model (De Purry and Farquhar 1997; Wu et al. 2018), and the 3D canopy photosynthetic model (Song et al. 2013; Liu et al. 2021). Additionally, the architecture of the crops—such as plant height, plant density, spike length, spike height, position in the canopy, and leaf angle—is a major factor that influences canopy photosynthesis and crop yields (see e.g., Song et al. 2017; Chang et al. 2022). For example, Chang et al. (2022) found that, in general, shorter plant height, erect leaves, lower spike position, and appropriate plant densities can improve the daily Acnet.
As mentioned earlier in the paper, increasing photosynthesis is an important target to increase crop productivity (Long et al. 2006; Evans 2013; Long 2014; Ort et al. 2015; Furbank et al. 2020; Zhu et al. 2010, 2022; Walter and Kromdijk 2022). For this reason, model-guided genetic modifications (von Caemmerer et al. 2012; South et al. 2019; Ermakova et al. 2020), and model-guided natural variations of the photosynthetic parameters (Qu et al. 2017; Ye et al. 2019; Yin et al. 2022) have already been employed to promote crop yield growth.
Simple scale crop models can provide explanations for specific biological phenomena and are capable of guiding the development of measurement techniques, as well as providing suggestions for the modification of plant traits in a direction that is beneficial for the humankind. However, these models cannot track key interactions and gaps resulting from the complex system characteristics, especially the nonlinear behavior that may appear through the feedback of the interaction of crop growth, interspecific competition, and dynamic micro-environments (Qu et al. 2011; Wu et al. 2019). Several studies have, however, already shown that the effect of manipulating photosynthesis can become weaker at the canopy scale, because of the complexities in the light interception process at the canopy level (Zhu et al. 2012; Song et al. 2013; Yin and Struik 2017; Wu 2023; Wu et al. 2016, 2018, 2019, 2023); in fact, light absorption, even by a single leaf. is a nonlinear function, see e.g., Baránková et al. (2016) and Nauš et al. (2018). In addition, nitrogen deficiency has been shown to reduce the yield-enhancing effects of increased CO2 on photosynthesis as well as growth (Wu et al. 2019). Therefore, cross-scale crop growth models should prevail in crop modelling, as they can reveal new, the so-called emergent features that cannot be “seen” by a simple scale model, obtained by the interaction of models at different spatial and temporal scales (Fig. 6). Indeed, the crop mechanistic models should integrate seamlessly the multiscale physical and molecular processes in the crops, by including sub-models of different processes of crop growth and development in cells, tissues, organs, individual plants, and even the entire crop (see Xiao et al. 2017; Chang et al. 2019a; Hammer et al. 2019). Further, the crop models may also contain different types of data, depending on the goal of the study (see e.g., Xiao et al. 2017; Benes et al. 2020). For example, there can be (i) crop models which include remote sensing data (Kasampalis et al. 2018); (ii) spatialized crop models, from small fields to global level (Pasquel et al. 2022); (iii) metabolic engineering models (Cavanagh et al. 2022); and (iv) systems models including phenomics, genomics, and/or high-throughput photosynthetic measurements data (Chang et al. 2019b; Baslam et al. 2020; Yin et al. 2022).
Figure 6 shows the basic framework of a cross-scale model, from the genes to the stand; further, it incorporates information on the climate and the nutritional factors. Such models not only help us visualize the physiological state of the crops but allow us to predict how the growth and the development of plants may respond, comprehensively, to different environmental signals (Lynch 2007); in addition, they can guide the experimental design and the technology development to increase crop yields and to ensure that the crops grow in suitable environments (see e.g., Srinivasan et al. 2017).
At present, cross-scale crop growth models are widely used to study the response mechanisms of crop productivity under different environmental and management conditions, and to explore potential ways to improve crop yields (Benes et al. 2020; Chang et al. 2022; Wu et al. 2016, 2018, 2019, 2023). For example, the CERES (Crop Environment Resource Synthesis) model ranges from gene-based modelling, nutrient and water stress, farm and precision management to regional assessment of climate change impacts. This cross-scale system model has been widely used in the analysis of agricultural experiments, in yield forecast, and in production risk assessment (Jones et al. 2003). Also, APSIM (Agricultural Production System Simulator) is a modular crop system model that is used to simulate the biophysical processes in agricultural systems. We note that APSIM allows independent modules describing the key components of agricultural systems to be “plugged” into the platform, so that the ‘master- model’ becomes suitable for relatively accurate prediction of the crop yield under different conditions, under different climate, soil, and management factors, while analyzing long-term water resource management issues (Brown et al. 2014; Holzworth et al. 2014).
Table 1 is a summary of what is known, as well as the differences between the four commonly used cross-scale crop growth models, namely WOFOST (World Food Studies), Aqua Crop, CERES (Crop Environment Resource Synthesis) and APSIM (Agricultural Production Systems Simulator). In addition, the cross-scale crop growth models have been widely used in optimal management studies under different stress conditions, such as water stress (Yang et al. 2017; Wang et al. 2017a, b; Zhuo et al. 2022), nutrient stress (Shibu et al. 2010; Saengwilai et al. 2014; Galindo-Castaneda et al. 2018), and weather stress (Rosenzweig et al. 2013; Gabaldón-Leal et al. 2016; Rincent et al. 2019). The key to establish a cross-scale crop model is to have a series of basic data, including soil properties and crop parameters. However, there are difficulties in obtaining and calibrating some of the model parameters. At present, most parameters of crop system models are default or empirical parameters, which often lead to deviations in the accuracy of the model simulation. In addition, as a complex system model, the cross-scale crop models require verification and evaluation of experimental data to describe the rationality of its single processes and the interaction relationship between the various processes. The current acquisition of crop characteristics is mostly based on their manual measurement, which is time-consuming, and laborious. Further application of spectral technology, agricultural big data, agricultural internet of things, computer vision technology, and citizen science approach will, in the future, make it easier for the acquisition of crop characteristics.
For overcoming the challenges ahead of us, further research in modelling is essential, especially by using advances in promising and fast developing domains, such as artificial intelligence (see e.g., Xia et al. 2023) and remote sensing (RS) techniques (Kasampalis et al. 2018; Zhou et al. 2022). Since the climate change is affecting the agriculture through increased temperature and atmospheric CO2 concentration, as well as by modification of the weather patterns, it is important to monitor the status of growth during the entire lifecycle of the crops, as it can provide valuable information on the ‘health’ and the yield potential of the crops. Remote sensing (RS) has already proven to be an effective method for monitoring crop growth and for the estimation of the yield, due to its ability to provide large-scale, non-destructive, low-cost, and highly efficient approach to monitor crops (see e.g., Abdulridha et al. 2019; Mahlein et al. 2019). However, solar-induced (chlorophyll) fluorescence (SIF) is a much more accurate way to measure the photosynthetic activity of plants than the traditional indices of RS, such as the Normalized Difference Vegetation Index (NDVI), the Enhanced Vegetation Index (EVI), the Photochemical Reflectance Index (PRI) and the Leaf Area Index (LAI), which are only slightly sensitive to the actual photosynthesis (Meroni et al. 2009; Zhang et al. 2016; Song et al. 2020). For example, Pandiyan et al. (2021) have successfully used the SIF signal to monitor the effect of drought on photosynthesis. Further, Guanter et al. (2014) showed that SIF is highly correlated with the gross primary production (GPP) and is much more sensitive to environmental changes than the other indices mentioned above (see also Frankenberg and Berry 2018). Thus, SIF has a distinct advantage in obtaining information on photosynthesis and in predicting crop yield, especially under the climate change (Peng et al. 2020). Further, Magney et al. (2019) have concluded, based on the interpretation of SIF signals, that the wavelengths used by satellites are stable enough to even track the downregulation of photosynthesis resulting from stress, while spectral shape changes respond more strongly to dynamic changes in canopy structure and chlorophyll concentration. An example in this direction has been the use of hyperspectral imagery and SIF retrievals from aerial and satellite remote sensing for the detection of variations in the rates of photosynthesis in many crops (Camino et al. 2019).
Finally, although the current satellite SIF data have relatively low spatial resolution, which limits their accuracy in estimating crop yield at finer scales (Zhang et al. 2019), a convolutional neural network (CNN) framework has been proposed by Kang et al. (2022) to downscale the SIF data from a resolution of 0.05° to 0.0005°. This newer method has been quite effective, the results showing a high level of agreement with the referenced SIF (i.e., the spatial continuous SIF; see Kang et al. 2022). This research emphasizes the significance of using higher resolution SIF data (i.e., CNN-SIF) to obtain precise crop yield estimates, particularly for farms that are not contiguous. Further, by using other remote sensing indices together with SIF to predict crop yields, it is possible to obtain better results than by using only one alone (Liu et al. 2022). Therefore, we suggest that it is essential to conduct further research to evaluate the effectiveness of high-resolution SIF data in predicting yields of various crops. A new satellite called FLEX (Fluorescence Explorer) by European Space Agency is planned to be launched in 2025; it has been designed especially for the high-resolution detection of SIF (see https://earth.esa.int/eogateway/missions/flex) should be helpful in this.
Data availability
Data will be made available, if required.
Abbreviations
- C i, C m, C c :
-
Intercellular, mesophyll, or chloroplast CO2 concentration
- CAM:
-
Crassulacean acid metabolism, a pathway of CO2 assimilation
- CBB cycle:
-
Calvin-Benson-Bassham cycle of CO2 assimilation
- CCM:
-
CO2 concentrating mechanism
- CET:
-
Cyclic electron transport
- Chl:
-
Chlorophyll
- Cyt:
-
Cytochrome
- ΔΨ:
-
Electric potential difference across the thylakoid membrane
- ΔpH:
-
pH difference across the thylakoid membrane
- ETR:
-
Electron transport rate
- Fe-S:
-
Rieske iron sulfur protein, an electron carrier
- Fd:
-
Ferredoxin, an electron carrier
- FNR:
-
Ferredoxin-NADP+ oxidoreductase
- LHCI, LHCII:
-
Light-harvesting complexes in PSI and PSII antenna
- NADP+ :
-
Oxidized form of nicotinamide adenine dinucleotide phosphate, an electron carrier
- NDH:
-
NADH dehydrogenase-like complex involved in antimycin A insensitive cyclic electron transport
- NPQ:
-
Nonphotochemical quenching of the excited state of chlorophyll
- OEC:
-
Oxygen evolving complex of PSII
- P680, P700:
-
The primary electron donor of PSII and of PSI, respectively
- PGR5/PGRL1:
-
A complex containing Proton Gradient Regulation 5 and Proton Gradient Regulation Like 1 proteins involved in antimycin A sensitive cyclic electron transport
- pmf :
-
Proton motive force
- P N :
-
The net photosynthetic rate
- PQ and PQH2 :
-
Plastoquinone and its reduced form, plastoquinol
- PSI, PSII:
-
Photosystem I and II
- PTOX:
-
Plastid terminal oxidase, involved in chlororespiration
- QA, QB :
-
First and second plastoquinone electron acceptors of PSII
- Rac:
-
Rubisco activase
- ROS:
-
Reactive oxygen species
- Rubisco:
-
Ribulose-1,5-bisphosphate carboxylase/oxygenase
- SIF:
-
Solar induced fluorescence
- TM:
-
Thylakoid membrane
- WWC:
-
Water-water cycle
References
Abdulridha J, Ampatzidis Y, Kakarla SC, Roberts P (2019) Detection of target spot and bacterial spot diseases in tomato using UAV-based and benchtop-based hyperspectral imaging techniques. Precis Agric 21:955–978. https://doi.org/10.1007/s11119-019-09703-4
Adams MP, Collier CJ, Uthicke S, Ow YX, Langlois L, O’Brien KR (2017) Model fit versus biological photosynthesis-temperature models for three tropical seagrass species. Sci Rep 7:39930. https://doi.org/10.1038/srep39930
Adnan AA, Diels J, Jibrin JM et al (2019) Options for calibrating CERES-maize genotype specific parameters under data-scarce environments. PLoS ONE 14:e0200118. https://doi.org/10.1371/journal.pone.0200118
Ainsworth EA (2008) Rice production in a changing climate: a meta-analysis of responses to elevated carbon dioxide and elevated ozone concentration. Glob Change Biol 14:1642–1650. https://doi.org/10.1111/j.1365-2486.2008.01594.x
Ainsworth EA, Long SP (2021) 30 years of free-air carbon dioxide enrichment (FACE): What have we learned about future crop productivity and its potential for adaptation? Glob Change Biol 27:27–49. https://doi.org/10.1111/gcb.15375
Ainsworth EA, Rogers A, Nelson R, Long SP (2004) Testing the ‘source-sink’ hypothesis of down-regulation of photosynthesis in elevated [CO2] in the field with single gene substitutions in Glycine max. Agric for Meteorol 122:85–94. https://doi.org/10.1016/j.agrformet.2003.09.002
Allen J, Bennett J, Steinback K, Arntzen CJ (1981) Chloroplast protein phosphorylation couples plastoquinone redox state to distribution of excitation energy between photosystems. Nature 291:25–29. https://doi.org/10.1038/291025a0
Amarasingha R, Suriyagoda L, Marambe B, Gaydon D, Galagedara LW, Punyawardena R, Silva GLLP, Nidumolu UB, Howden M (2015) Simulation of crop and water productivity for rice (Oryza sativa L.) using APSIM under diverse agro-climatic conditions and water management techniques in Sri Lanka. Agric Water Manag 160:132–143. https://doi.org/10.1016/j.agwat.2015.07.001
Antal TK, Kovalenko IB, Rubin AB, Tyystlärvi E (2013) Photosynthesis-related quantities for education and modeling. Photosynth Res 117:1–30. https://doi.org/10.1007/s11120-013-9945-8
Arora NK (2019) Impact of climate change on agriculture production and its sustainable solutions. Environ Sustain 2:95–96. https://doi.org/10.1007/s42398-019-00078-w
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639. https://doi.org/10.1146/annurev.arplant.50.1.601
Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141:391–396. https://doi.org/10.1104/pp.106.082040
Bahri H, Annabi M, Cheikh M’Hamed H, Frija A (2019) Assessing the long-term impact of conservation agriculture on wheat-based systems in Tunisia using APSIM simulations under a climate change context. Sci Total Environ 692:1223–1233. https://doi.org/10.1016/j.scitotenv.2019.07.307
Baránková B, Lazár D, Nauš J (2016) Analysis of the effect of chloroplast arrangement on optical properties of green tobacco leaves. Remote Sens Environ 174:181–196. https://doi.org/10.1016/j.rse.2015.12.011
Bar-On YM, Milo R (2019) The global mass and average rate of rubisco. Proc Natl Acad Sci USA 116:4738–4743. https://doi.org/10.1073/pnas.1816654116
Baslam M, Mitsui T, Hodges M, Priesack E, Herritt MT, Aranjuelo I, Sanz-Sáez Á (2020) Photosynthesis in a changing global climate: scaling up and scaling down in crops. Front Plant Sci 11:882. https://doi.org/10.3389/fpls.2020.00882
Bassham JA (2003) Mapping the carbon reduction cycle: a personal retrospective. Photosynth Res 76:35–52. https://doi.org/10.1023/A:1024929725022
Batista-Silva W, da Fonseca-Pereira P, Martins AO, Zsögön A, Nunes-Nesi A, Araújo WL (2020) Engineering improved photosynthesis in the era of synthetic biology. Plant Commun 1:100032. https://doi.org/10.1016/j.xplc.2020.100032
Bauwe H (2023) Photorespiration—Rubisco’s repair crew. J Plant Physiol 280:153899. https://doi.org/10.1016/j.jplph.2022.153899
Bellasio C (2016) A generalized stoichiometric model of C3, C2, C2+C4, and C4 photosynthetic metabolism. J Exp Bot 68:269–282. https://doi.org/10.1093/jxb/erw303
Bellasio C (2019) A generalized dynamic model of leaf-level C3 photosynthesis combining light and dark reactions with stomatal behaviour. Photosynth Res 141:99–118. https://doi.org/10.1007/s11120-018-0601-1
Bellasio C, Farquhar GD (2019) A leaf-level biochemical model simulating the introduction of C2 and C4 photosynthesis in C3 rice: gains, losses and metabolite fluxes. New Phytol 223:150–166. https://doi.org/10.1111/nph.15787
Belyaeva NE, Bulychev AA, Riznichenko GYu, Rubin AB (2019) Analyzing both the fast and the slow phases of chlorophyll a fluorescence and P700 absorbance changes in dark-adapted and preilluminated pea leaves using a thylakoid membrane model. Photosynth Res 140:1–19. https://doi.org/10.1007/s11120-019-00627-8
Benes B, Guan K, Lang M, Long SP, Lynch JP, Marshall-Colon A, Peng B, Schnable J, Sweetlove LJ, Turk MJ (2020) Multiscale computational models can guide experimentation and targeted measurements for crop improvement. Plant J 103:21–31. https://doi.org/10.1111/tpj.14722
Bennett DIG, Fleming GR, Amarnath K (2018) Energy-dependent quenching adjusts the excitation diffusion length to regulate photosynthetic light harvesting. Proc Natl Acad Sci USA 115:E9523–E9531. https://doi.org/10.1073/pnas.1806597115
Benson AA (2002) Following the path of carbon in photosynthesis: a personal story. Photosynth Res 73:29–49. https://doi.org/10.1023/A:1020427619771
Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR, Long SP (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ 24:253–259. https://doi.org/10.1111/j.1365-3040.2001.00668.x
Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP (2002) Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol 130(4):1992–1998. https://doi.org/10.1104/pp.008250
Bernacchi CJ, Bagley JE, Serbin SP, Ruiz-Vera UM, Rosenthal DM, Vanloocke A (2013) Modelling C3 photosynthesis from the chloroplast to the ecosystem. Plant Cell Environ 36:1641–1657. https://doi.org/10.1111/pce.12118
Betti M, Bauwe H, Busch FA, Fernie AR, Keech O, Levey M, Ort DR, Parry MAJ, Sage R, Timm S, Walker B, Weber APM (2016) Manipulating photorespiration to increase plant productivity: recent advances and perspectives for crop improvement. J Exp Bot 67:2977–2988. https://doi.org/10.1093/jxb/erw076
Biswas R, Banerjee S, Bhattacharyya B (2018) Impact of temperature increase on performance of kharif rice at Kalyani, West Bengal using WOFOST model. J Agrometeorol 20:28–30. https://doi.org/10.54386/jam.v20i1.498
Björn LO, Shevela D, Govindjee G (2023) What is photosynthesis?—A broader and inclusive vew. In: Dalal VK, Misra AN (eds) A closer look at photosynthesis (Chapter 1). Biochemistry and molecular biology in post genomic era. Plant science research and practices. Nova Science Publishers, Hauppauge
Blankenship RE (2021) Molecular mechanisms of photosynthesis, 3rd edn. Wiley-Blackwell, Oxford, p 320
Blankenship R, Govindjee G (2007) Photosynthesis. In: The Encyclopedia of science and technology, vol 13, 10th ed. McGraw Hill Publishers, New York, pp 468‒475. https://doi.org/10.1036/1097-8542.511700
Boogaard H, Wolf J, Supit I, Niemeyer S, van Ittersum M (2013) A regional implementation of WOFOST for calculating yield gaps of autumn-sown wheat across the European Union. Field Crops Res 143:130–142. https://doi.org/10.1016/j.fcr.2012.11.005
Braun A (2020) Quantum electrodynamics of photosynthesis. Mathematical description of light, life and matter. De Gruyter, Boston
Brestic M, Allakhverdiev SI (2022) Photosynthesis under biotic and abiotic environmental stress. Cells 11:3953. https://doi.org/10.3390/cells11243953
Briantais J-M, Vernotte C, Picaud M, Krause GH (1979) A quantitative study of the slow decline of chlorophyll a fluorescence in isolated chloroplasts. Biochim Biophys Acta 548:128–138. https://doi.org/10.1016/0005-2728(79)90193-2
Brown HE, Huth NI, Holzworth DP, Teixeira EI, Zyskowski RF, Hargreaves JNG, Moot DJ (2014) Plant modelling framework: software for building and running crop models on the APSIM platform. Environ Model Softw 62:385–398. https://doi.org/10.1016/j.envsoft.2014.09.005
Buchanan BB (1980) Role of light in the regulation of chloroplast enzymes. Annu Rev Plant Physiol 31:341–374. https://doi.org/10.1146/annurev.pp.31.060180.002013
Burgos A, Miranda E, Vilaprinyo E, Meza-Canales ID, Alves R (2022) CAM models: lessons and implications for CAM evolution. Front Plant Sci 13:893095. https://doi.org/10.3389/fpls.2022.893095
Burlacot A, Peltier G (2023) Energy crosstalk between photosynthesis and the algal CO2-concentrating mechanisms. Trends Plant Sci 28:795–807. https://doi.org/10.1016/j.tplants.2023.03.018
Busch FA (2020) Photorespiration in the context of Rubisco biochemistry, CO2 diffusion and metabolism. Plant J 101:919–939. https://doi.org/10.1111/tpj.14674
Calvin M (1989) Forty years of photosynthesis and related activities. Photosynth Res 23:3–16. https://doi.org/10.1007/BF00047170
Camino C, Gonzalez-Dugoa V, Hernandeza P, Zarco-Tejada PJ (2019) Radiative transfer Vcmax estimation from hyperspectral imagery and SIF retrievals to assess photosynthetic performance in rainfed and irrigated plant phenotyping trials. Remote Sens Environ 231:111186. https://doi.org/10.1016/j.rse.2019.05.005
Cao J, Zhang Z, Tao F, Zhang L, Luo Y, Zhang J, Han J, Xie J (2021) Integrating multi-source data for rice yield prediction across China using machine learning and deep learning approaches. Agric for Meteorol 297:108275. https://doi.org/10.1016/j.agrformet.2020.108275
Cardona T, Shao S, Nixon PJ (2018) Enhancing photosynthesis in plants: the light reactions. Essay Biochem 62:85–94. https://doi.org/10.1042/EBC20170015
Carmo-Silva E, Scales JC, Madgwick P, Parry MAJ (2015) Optimising Rubisco and its regulation for greater resource use efficiency. Plant Cell Environ 38:1817–1832. https://doi.org/10.1111/pce.12425
Carvalho JFC (2005) Manipulating carbon metabolism to enhance stress tolerance (short circuiting photorespiration in tobacco). PhD thesis. University of Lancaster, Lancaster, UK. https://ethos.bl.uk/OrderDetails.do?uin=uk.bl.ethos.435874
Carvalho JFC, Madgwick PJ, Powers SJ, Keys AJ, Lea PJ, Parry MA (2011) An engineered pathway for glyoxylate metabolism in tobacco plants aimed to avoid the release of ammonia in photorespiration. BMC Biotechnol 11:111. https://doi.org/10.1186/1472-6750-11-111
Cavanagh AP, South PF, Bernacchi CJ, Ort DR (2022) Alternative pathway to photorespiration protects growth and productivity at elevated temperatures in a model crop. Plant Biotechnol J 20:711–721. https://doi.org/10.1111/pbi.13750
Chang TG, Zhao H, Wang N, Song QF, Xiao Y, Qu M, Zhu XG (2019a) A three-dimensional canopy photosynthesis model in rice with a complete description of the canopy architecture, leaf physiology, and mechanical properties. J Exp Bot 70:2479–2490. https://doi.org/10.1093/jxb/ery430
Chang TG, Chang S, Song QF, Perveen S, Zhu XG (2019b) Systems models, phenomics and genomics: three pillars for developing high-yielding photosynthetically efficient crops. In Silico Plants 2019:diy003. https://doi.org/10.1093/insilicoplants/diy003
Chang TG, Shi Z, Zhao HL, Song QF, He ZH, Van Rie J, Boer BD, Galle A, Zhu XG (2022) 3dCAP-wheat: An open-source comprehensive computational framework precisely quantifies wheat foliar, nonfoliar, and canopy photosynthesis. Plant Phenomics 2022:9758148. https://doi.org/10.34133/2022/9758148
Chapman S, Cooper M, Podlich D, Hammer G (2003) Evaluating plant breeding strategies by simulating gene action and dryland environment effects. Agron J 95:99–113. https://doi.org/10.2134/agronj2003.9900
Chen Q, Lan Y, Li Q, Kong M, Mi H (2023a) Inactivation of photosynthetic cyclic electron transports upregulates photorespiration for compensation of efficient photosynthesis in Arabidopsis. Front Plant Sci 14:1061434. https://doi.org/10.3389/fpls.2023.1061434
Chen T, Riaz S, Davey P, Zhao Z, Sun Y, Dykes GF, Zhou F, Hartwell J, Lawson T, Nixon PJ, Lin Y, Liu LN (2023b) Producing fast and active Rubisco in tobacco to enhance photosynthesis. Plant Cell 35:795–807. https://doi.org/10.1093/plcell/koac348
Chen T, Hojka M, Davey P, Sun Y, Dykes GF, Zhou F, Lawson T, Nixon PJ, Lin Y, Liu LN (2023c) Engineering α-carboxysomes into plant chloroplasts to support autotrophic photosynthesis. Nat Commun 14:2118. https://doi.org/10.1038/s41467-023-37490-0
Cheng Z, Meng J, Qiao Y, Wang Y, Dong W, Han Y (2018) Preliminary study of soil available nutrient simulation using a modified WOFOST model and time-series remote sensing observations. Remote Sens 10:64
Cheung CY, Poolman MG, Fell DA, Ratcliffe RG, Sweetlove LJ (2014) A Diel Flux Balance model captures interactions between light and dark metabolism during day-night cycles in C3 and Crassulacean Acid Metabolism leaves. Plant Physiol 165:917–929. https://doi.org/10.1104/pp.113.234468
Cichota R, Vogeler I, Sharp J, Verburg K, Huth N, Holzworth D, Dalgliesh N, Snow V (2021) A protocol to build soil descriptions for APSIM simulations. Methods X 8:101566. https://doi.org/10.1016/j.mex.2021.101566
Connolly RD, Bell M, Huth N, Freebairn DM, Thomas G (2002) Simulating infiltration and the water balance in cropping systems with APSIM-SWIM. Aust J Soil Res 40:221–242. https://doi.org/10.1071/SR01007
Cruz JA, Sacksteder CA, Kanazawa A, Kramer DM (2001) Contribution of electric field (ΔΨ) to steady-state trans-thylakoid proton motive force (pmf) in vivo and in vitro. Control of pmf parsing into ΔΨ and ΔpH by ionic strength. Biochemistry 40:1226–1237. https://doi.org/10.1021/bi0018741
Cui H (2021) Challenges and approaches to crop improvement through C3-to-C4 engineering. Front Plant Sci 12:715391. https://doi.org/10.3389/fpls.2021.715391
Cummins PL, Kannappan B, Gready JE (2018) Directions for optimization of photosynthetic carbon fixation: RuBisCO’s efficiency may not be so constrained after all. Front Plant Sci 9:183. https://doi.org/10.3389/fpls.2018.00183
D’Haese D, Vandermeiren K, Caubergs RJ, Guisez Y, De Temmerman L, Horemans N (2004) Non-photochemical quenching kinetics during the dark to light transition in relation to the formation of antheraxanthin and zeaxanthin. J Theor Biol 227:175–186. https://doi.org/10.1016/j.jtbi.2003.10.011
Dalal J, Lopez H, Vasani NB, Hu ZH, Swift JE, Yalamanchili R, Dvora M, Lin XL, Xie DY, Qu RD, Sederoff HW (2015) A photorespiratory bypass increases plant growth and seed yield in biofuel crop Camelina sativa. Biotechnol Biofuels 8:175. https://doi.org/10.1186/s13068-015-0357-1
Dann M, Leister D (2017) Enhancing (crop) plant photosynthesis by introducing novel genetic diversity. Philos Trans R Soc B 372:20160380. https://doi.org/10.1098/rstb.2016.0380
De Pury D, Farquhar G (1997) Simple scaling of photosynthesis from leaves to canopies without the errors of big-leaf models. Plant Cell Environ 20:537–557. https://doi.org/10.1111/j.1365-3040.1997.00094.x
De Souza AP, Burgess SJ, Doran L, Manukyan L, Maryn N, Gotarkar D, Leonelli L, Niyogi KK, Long SP (2022) Soybean photosynthesis and crop yield are improved by accelerating recovery from photoprotection. Science 377:851–854. https://doi.org/10.1126/science.adc9831
Demmig B, Winter K, Krüger A, Czygan F-C (1987) Photoinhibition and zeaxanthin formation in intact leaves: a possible role of the xanthophyll cycle in the dissipation of excess light energy. Plant Physiol 84:218–224. https://doi.org/10.1104/pp.84.2.218
Dubois JB, Fiscus EL, Booker FL, Flowers MD, Reid CD (2007) Optimizing the statistical estimation of the parameters of the Farquhar-von Caemmerer-Berry model of photosynthesis. New Phytol 176:402–414. https://doi.org/10.1111/j.1469-8137.2007.02182.x
Ebenhöh O, Houwaart T, Lokstein H, Schlede S, Tirok K (2011) A minimal mathematical model of nonphotochemical quenching of chlorophyll fluorescence. BioSystems 103:196–204. https://doi.org/10.1016/j.biosystems.2010.10.011
Ebenhöh O, Fucile G, Finazzi GG, Rochaix J-D, Goldschmidt-Clermont M (2014) Short-term acclimation of the photosynthetic electron transfer chain to changing light: a mathematical model. Philos Trans R Soc Lond B 369:20130223. https://doi.org/10.1098/rstb.2013.0223
Edwards GE, Franceschi VR, Voznesenskaya EV (2004) Single-cell C(4) photosynthesis versus the dual-cell (Kranz) paradigm. Annu Rev Plant Biol 55:173–196. https://doi.org/10.1146/annurev.arplant.55.031903.141725
Eilers PHC, Peeters JCH (1988) A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. Ecol Model 42:199–215. https://doi.org/10.1016/0304-3800(88)90057-9
Erb TJ, Zarzycki J (2018) A short history of RubisCO: the rise and fall (?) of Nature’s predominant CO2 fixing enzyme. Curr Opin Biotechnol 49:100–107. https://doi.org/10.1016/j.copbio.2017.07.017
Ermakova M, Danila FR, Furbank RT, von Caemmerer S (2020) On the road to C4 rice: advances and perspectives. Plant J 101:940–950. https://doi.org/10.1111/tpj.14562
Ermakova M, Arrivault S, Giuliani R, Danila F, Alonso-Cantabrana H, Vlad D, Ishihara H, Feil R, Guenther M, Borghi GL, Covshoff S, Ludwig M, Cousins AB, Langdale JA, Kelly S, Lunn JE, Stitt M, von Caemmerer S, Furbank RT (2021) Installation of C4 photosynthetic pathway enzymes in rice using a single construct. Plant Biotech J 19:575–588. https://doi.org/10.1111/pbi.13487
Evans JR (2013) Improving photosynthesis. Plant Physiol 162:1780–1793. https://doi.org/10.1104/pp.113.219006
Evans LT, Fischer RA (1999) Yield potential: Its definition, measurement and significance. Crop Sci 39:1549–1551. https://doi.org/10.2135/cropsci1999.3961544x
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90. https://doi.org/10.1007/BF00386231
Fei C, Wilson AT, Mangan NM, Wingreen NS, Jonikas MC (2022) Modelling the pyrenoid-based CO2-concentrating mechanism provides insights into its operating principles and a roadmap for its engineering into crops. Nat Plants 8:583–595. https://doi.org/10.1038/s41477-022-01153-7
Fouracre JP, Ando S, Langdale JA (2014) Cracking the Kranz enigma with systems biology. J Exp Bot 65:3327–3339. https://doi.org/10.1093/jxb/eru015
Foyer CH (2018) Reactive oxygen species, oxidative signaling and the regulation of photosynthesis. Environ Exp Bot 154:134–142. https://doi.org/10.1016/j.envexpbot.2018.05.003
Foyer CH, Hanke G (2022) ROS production and signalling in chloroplasts: cornerstones and evolving concepts. Plant J 111:642–661. https://doi.org/10.1111/tpj.15856
Foyer CH, Bloom AJ, Queval G, Noctor G (2009) Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu Rev Plant Biol 60:455–484. https://doi.org/10.1146/annurev.arplant.043008.091948
Foyer CH, Ruban AV, Nixon PJ (2017) Photosynthesis solutions to enhance productivity. Philos Trans R Soc B 372:20160374. https://doi.org/10.1098/rstb.2016.0374
Frankenberg C, Berry J (2018) Solar induced chlorophyll fluorescence: Origins, relation to photosynthesis and retrieval. In: Liang S (ed) Comprehensive Remote Sensing, vol 3. Elsevier, Oxford, pp 143–162
Fu L, Govindjee G, Tan J, Guo Y (2020) Development of a minimized model structure and a feedback control framework for regulating photosynthetic activities. Photosynth Res 146:213–225. https://doi.org/10.1007/s11120-019-00690-1
Furbank RT, Sharwood R, Estavillo GM, Silva-Perez V, Condon AG (2020) Photons to food: genetic improvement of cereal crop photosynthesis. J Exp Bot 71:2226–2238. https://doi.org/10.1093/jxb/eraa077
Furutani R, Ifuku K, Suzuki Y, Noguchi K, Shimakawa G, Wada S, Makino A, Sohtome T, Miyake C (2020) P700 oxidation suppresses the production of reactive oxygen species in photosystem I. Adv Bot Res 20:151–176. https://doi.org/10.1016/bs.abr.2020.08.001
Gabaldón-Leal C, Webber H, Otegui ME, Slafer GA, Ordóńez RA, Gaiser T, Lorite IJ, Ruiz-Ramos M, Ewert F (2016) Modelling the impact of heat stress on maize yield formation. Field Crops Res 198:226–237. https://doi.org/10.1016/j.fcr.2016.08.013
Galindo-Castaneda T, Brown KM, Lynch JP (2018) Reduced root cortical burden improves growth and grain yield under low phosphorus availability in maize. Plant Cell Environ 41:1579–1592. https://doi.org/10.1111/pce.13197
Ghosh K, Singh A, Mohanty UC, Acharya N, Pal RK, Singh KK, Pasupalak S (2015) Development of a rice yield prediction system over Bhubaneswar, India: combination of extended range forecast and CERES-rice model. Meteorol Appl 22:525–533. https://doi.org/10.1002/met.1483
Giersch C (2000) Mathematical modelling of metabolism. Curr Opin Plant Biol 3:249–253. https://doi.org/10.1016/S1369-5266(00)80073-4
Gifford RM, Evans LT (1981) Photosynthesis, carbon partitioning, and yield. Annu Rev Plant Physiol 32:485–509. https://doi.org/10.1146/annurev.pp.32.060181.002413
Gilardelli C, Confalonieri R, Cappelli GA, Bellocchi G (2018) Sensitivity of WOFOST-based modelling solutions to crop parameters under climate change. Ecol Modell 368:1–14. https://doi.org/10.1016/j.ecolmodel.2017.11.003
Govindjee G, Shevela D, Björn L (2017) Evolution of the Z-scheme of photosynthesis: a perspective. Photosynth Res 133:5–15. https://doi.org/10.1007/s11120-016-0333-z
Graham PJ, Nguyen B, Burdyny T, Sinton D (2017) A penalty on photosynthetic growth in fluctuating light. Sci Rep 7:12513. https://doi.org/10.1038/s41598-017-12923-1
Gu LH, Pallardy SG, Tu K, Law BE, Wullschleger SD (2010) Reliable estimation of biochemical parameters from C3 leaf photosynthesis intercellular carbon dioxide response curves. Plant Cell Environ 33:1852–1874. https://doi.org/10.1111/j.1365-3040.2010.02192.x
Guanter L, Zhang Y, Jung M, Joiner VM (2014) Global and time-resolved monitoring of crop photosynthesis with chlorophyll fluorescence. Proc Natl Acad Sci USA 111:1327–1333. https://doi.org/10.1073/pnas.1320008111
Guo Y, Tan J (2011) Modeling and simulation of the initial phases of chlorophyll fluorescence from photosystem II. BioSystems 103:152–157. https://doi.org/10.1016/j.biosystems.2010.10.008
Guo Y, Tan J (2014) Kinetic Monte Carlo simulation of the initial phases of chlorophyll fluorescence from photosystem II. BioSystems 115:1–4. https://doi.org/10.1016/j.biosystems.2013.10.004
Hamdani S, Khan N, Perveen S, Qu M, Jiang J, Govindjee G, Zhu X-G (2019a) Changes in the photosynthesis properties and photoprotection capacity in rice (Oryza sativa) grown under red, blue, or white light. Photosynth Res 139:107–121. https://doi.org/10.1007/s11120-018-0589-6
Hamdani S, Wang H, Zheng G, Perveen S, Qu M, Khan N, Khan W, Jiang J, Li M, Liu X, Zhu X, Govindjee G, Chu C, Zhu XG (2019b) Genome-wide association study identifies variation of glucosidase being linked to natural variation of the maximal quantum yield of photosystem II. Physiol Plant 166:105–119. https://doi.org/10.1111/ppl.12957
Hammer G, Messina C, Wu A, Cooper M (2019) Biological reality and parsimony in crop models—why we need both in crop improvement! In Silico Plants 2019:diz010. https://doi.org/10.1093/insilicoplants/diz010
Hatch MD, Osmond CB (1976) Compartmentation and transport in C4 photosynthesis. In: Stocking CR, Heber U (eds) Transport in plants III. Encyclopedia of plant physiology, vol 3. Springer, Berlin, pp 144–184. https://doi.org/10.1007/978-3-642-66417-5_5
Hatch MD, Slack CR (1966) Photosynthesis by sugar-cane leaves: a new carboxylation reaction and the pathway of sugar formation. Biochem J 101:103–111. https://doi.org/10.1042/bj1010103
Herrmann HA, Schwartz J-M, Johnson GN (2020) From empirical to theoretical models of light response curves—linking photosynthetic and metabolic acclimation. Photosynth Res 145:5–14. https://doi.org/10.1007/s11120-019-00681-2
Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y (2006) Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J Exp Bot 57:291–302. https://doi.org/10.1093/jxb/erj049
Hind G, Nakatani HY, Izawa S (1974) Light-dependent redistribution of ions in suspensions of chloroplast thylakoid membranes. Proc Natl Acad Sci USA 71:1484–1488. https://doi.org/10.1073/pnas.71.4.1484
Hodges M, Dellero Y, Keech O, Betti M, Raghavendra AS, Sage R, Zhu XG, Allen DK, Weber AP (2016) Perspectives for a better understanding of the metabolic integration of photorespiration within a complex plant primary metabolism network. J Exp Bot 67:3015–3026. https://doi.org/10.1093/jxb/erw145
Holzworth DP, Huth NI, deVoil PG, Zurcher EJ, Herrmann NI, McLean G, Chenu K, van Oosterom EJ, Snow V, Murphy C, Moore AD, Brown H, Whish JPM, Verrall S, Fainges J, Bell LW, Peake AS, Poulton PL, Hochman Z, Thorburn PJ, Gaydon DS, Dalgliesh NP, Rodriguez D, Cox H, Chapman S, Doherty A, Teixeira E, Sharp J, Cichota R, Vogeler I, Li FY, Wang E, Hammer GL, Robertson MJ, Dimes JP, Whitbread AM, Hunt J, van Rees H, McClelland T, Carberry PS, Hargreaves JNG, MacLeod N, McDonald C, Harsdorf J, Wedgwood S, Keating BA (2014) APSIM—evolution towards a new generation of agricultural systems simulation. Environ Modell Softw 62:327–350. https://doi.org/10.1016/j.envsoft.2014.07.009
Hope AB, Valente P, Matthews DB (1994) Effects of pH on the kinetics of redox reactions in and around the cytochrome bf complex in an isolated system. Photosynth Res 42:111–120. https://doi.org/10.1007/BF02187122
Hu S, Chen W, Tong K, Wang Y, Jing L, Wang Y, Yang L (2022) Response of rice growth and leaf physiology to elevated CO2 concentrations: a meta-analysis of 20-year FACE studies. Sci Total Environ 807:151017. https://doi.org/10.1016/j.scitotenv.2021.151017
Jablonsky J, Bauwe H, Wolkenhauer O (2011) Modeling the Calvin-Benson cycle. BMC Syst Biol 5:185. https://doi.org/10.1186/1752-0509-5-185
Jahns P, Holzwarth AR (2012) The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim Biophys Acta 1817:182–193. https://doi.org/10.1016/j.bbabio.2011.04.012
Jassby AD, Platt T (1976) Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. Limnol Oceanogr 21:540–547. https://doi.org/10.4319/lo.1976.21.4.0540
Johnson J, Berry JA (2021) The role of cytochrome b6f in the control of steady-state photosynthesis: a conceptual and quantitative model. Photosynth Res 148:101–136. https://doi.org/10.1007/s11120-021-00840-4
Johnson GN, Murchie E (2011) Gas exchange measurements for determination of photosynthetic efficiency in Arabidopsis leaves. Methods Mol Biol 775:311–326. https://doi.org/10.1007/978-1-61779-237-3_17
Jones JW, Hoogenboom G, Porter CH, Boote KJ, Batchelor WD, Hunt LA, Wilkens PW, Singh U, Gijsman AJ, Ritchie JT (2003) The DSSAT cropping system model. Eur J Agron 18:235–265. https://doi.org/10.1016/S1161-0301(02)00107-7
Kaiser E, Morales A, Harbinson J, Kromdijk J, Heuvelink E, Marcelis LFM (2015) Dynamic photosynthesis in different environmental conditions. J Exp Bot 66:2415–2426. https://doi.org/10.1093/jxb/eru406
Kaiser E, Kromdijk J, Harbinson J, Heuvelink E, Marcelis LFM (2017) Photosynthetic induction and its diffusional, carboxylation and electron transport processes as affected by CO2 partial pressure, temperature, air humidity and blue irradiance. Ann Bot 119:191–205. https://doi.org/10.1093/aob/mcw226
Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska IA, Cetner MD, Łukasik I, Goltsev V, Ladle RJ (2016) Chlorophyll a fluorescence as a tool to monitor physiological status of plants under abiotic stress conditions. Acta Physiol Plant 38:102. https://doi.org/10.1007/s11738-016-2113-y
Kalaji MH, Goltsev VN, Żuk-Gołaszewska K, Zivcak M, Brestic M (2017) Chlorophyll fluorescence: understanding crop performance—basics and applications. CRC Press, Boca Raton
Kalaji HM, Rastogi A, Zivcak M, Brestic M, Daszkowska-Golec A, Sitko K, Alshrafa KY, Lotfi R, Stypinski P, Samborska IA, Cetner MD (2018) Prompt chlorophyll fluorescence as a tool for crop phenotyping: an example of barley landraces exposed to various abiotic stress factors. Photosynthetica 56:953–961. https://doi.org/10.1007/s11099-018-0766-z
Kale R, Hebert AE, Frankel LK, Sallans L, Bricker TM, Pospíšil P (2017) Amino acid oxidation of the D1 and D2 proteins by oxygen radicals during photoinhibition of photosystem II. Proc Natl Acad Sci USA 114:2988–2993. https://doi.org/10.1073/pnas.1618922114
Kandoi D, Ruhil K, Govindjee G, Tripathy BC (2022) Overexpression of cytoplasmic C4 Flaveria bidentis carbonic anhydrase in C3 Arabidopsis thaliana increases amino acids, photosynthetic potential, and biomass. Plant Biotechnol J 20:1518–1532. https://doi.org/10.1111/pbi.13830
Kang H, Zhu T, Zhang Y, Ke X, Sun W, Hu Z, Zhu XG, Shen H, Huang Y, Tang Y (2021) Elevated CO2 enhances dynamic photosynthesis in rice and wheat. Front Plant Sci 12:727374. https://doi.org/10.3389/fpls.2021.727374
Kang XY, Huang CH, Zhang LF, Zhang Z, Lv X (2022) Downscaling solar-induced chlorophyll fluorescence for field-scale cotton yield estimation by a two-step convolutional neural network. Comput Electron Agric 201:107260. https://doi.org/10.1016/j.compag.2022.107260
Kasampalis DA, Alexandridis TK, Deva C, Challinor A, Moshou D, Zalidis G (2018) Contribution of remote sensing on crop models: a review. J Imaging 4:52. https://doi.org/10.3390/jimaging4040052
Kawashima R, Sato R, Harada K, Masuda S (2017) Relative contributions of PGR5- and NDH-dependent photosystem I cyclic electron flow in the generation of a proton gradient in Arabidopsis chloroplasts. Planta 246:1045–1050. https://doi.org/10.1007/s00425-017-2761-1
Kebeish R, Niessen M, Thiruveedhi K, Bari R, Hirsch HJ, Rosenkranz R, Stäbler N, Schönfeld B, Kreuzaler F, Peterhänsel C (2007) Chloroplastic photorespiratory bypass increases photosynthesis and biomass production in Arabidopsis thaliana. Nat Biotechnol 25:593–599. https://doi.org/10.1038/nbt1299
Koester RP, Nohl BM, Diers BW, Ainsworth EA (2016) Has photosynthetic capacity increased with 80 years of soybean breeding? An examination of historical soybean cultivars. Plant Cell Environ 39:1058–1067. https://doi.org/10.1111/pce.12675
Köhler IH, Ruiz-Vera UM, VanLoocke A, Thomey ML, Clemente T, Long SP, Ort DR, Bernacchi CJ (2017) Expression of cyanobacterial FBP/SBPase in soybean prevents yield depression under future climate conditions. J Exp Bot 68:715–726. https://doi.org/10.1093/jxb/erw435
Kono M, Terashima I (2016) Elucidation of photoprotective mechanisms of PSI against fluctuating light photoinhibition. Plant Cell Physiol 57:1405–1414. https://doi.org/10.1093/pcp/pcw103
Krichen E, Rapaport A, Le Floc’h E, Fouilland E (2021) A new kinetics model to predict the growth of micro-algae subjected to fluctuating availability of light. Algal Res 58:102362. https://doi.org/10.1016/j.algal.2021.102362
Kromdijk J, Głowacka K, Leonelli L, Gabilly ST, Iwai M, Niyogi KK, Long SP (2016) Improving photosynthesis and crop productivity by accelerating recovery from photoprotection. Science 354:857–861. https://doi.org/10.1126/science.aai8878
Kubis A, Bar-Even A (2019) Synthetic biology approaches for improving photosynthesis. J Exp Bot 70(5):1425–1433. https://doi.org/10.1093/jxb/erz029
Laisk A, Edwards GE (2000) A mathematical model of C-4 photosynthesis: the mechanism of concentrating CO2 in NADP-malic enzyme type species. Photosynth Res 66:199–224. https://doi.org/10.1023/A:1010695402963
Laisk A, Walker DA (1986) Control of phosphate turnover as a rate-limiting factor and possible cause of oscillations in photosynthesis: a mathematical model. Proc R Soc Lond B 227:281–302. https://doi.org/10.1098/rspb.1986.0024
Laisk A, Siebke K, Gerst U, Eichelmann H, Oja V, Heber U (1991) Oscillations in photosynthesis are initiated and supported by imbalances in the supply of ATP and NADPH to the Calvin cycle. Planta 185:554–562. https://doi.org/10.1007/BF00202966
Laisk A, Eichelmann H, Oja V (2006) C3 photosynthesis in silico. Photosynth Res 90:45–66. https://doi.org/10.1007/s11120-006-9109-1
Laisk A, Eichelmann H, Oja V (2009a) Leaf C3 photosynthesis in silico: integrated carbon/nitrogen metabolism. In: Laisk A, Nedbal L, Govindjee G (eds) Photosynthesis in silico: understanding complexity from molecules to ecosystems. Advances in photosynthesis and respiration, vol 29. Springer, Dordrecht, pp 295–322. https://doi.org/10.1007/978-1-4020-9237-4_13
Laisk A, Nedbal L, Govindjee G (eds) (2009b) Photosynthesis in silico: understanding complexity from molecules to ecosystems. Advances in photosynthesis and respiration, vol 29. Springer, Dordrecht. https://doi.org/10.1007/978-1-4020-9237-4
Lazár D (2003) Chlorophyll a fluorescence rise induced by high light illumination of dark-adapted plant tissue studied by means of a model of photosystem II and considering photosystem II heterogeneity. J Theor Biol 220:469–503. https://doi.org/10.1006/jtbi.2003.3140
Lazár D (2009) Modelling of light-induced chlorophyll a fluorescence rise (O-J-I-P transient) and changes in 820 nm-transmittance signal of photosynthesis. Photosynthetica 47:483–498. https://doi.org/10.1007/s11099-009-0074-8
Lazár D (2013) Simulations show that a small part of variable chlorophyll a fluorescence originates in photosystem I and contributes to overall fluorescence rise. J Theor Biol 335:249–264. https://doi.org/10.1016/j.jtbi.2013.06.028
Lazár D, Ilík P, Kruk J, Strzałka K, Nauš J (2005a) A theoretical study on effect of the initial redox state of cytochrome b559 on maximal chlorophyll fluorescence level (FM): implications for photoinhibition of photosystem II. J Theor Biol 233:287–300. https://doi.org/10.1016/j.jtbi.2004.10.015
Lazár D, Kaňa R, Klinkovský T, Nauš J (2005b) Experimental and theoretical study on high temperature induced changes in chlorophyll a fluorescence oscillations in barley leaves upon 2% CO2. Photosynthetica 43:13–27. https://doi.org/10.1007/s11099-005-3027-x
Lazár D, Niu Y, Nedbal L (2022a) Insights on the regulation of photosynthesis in pea leaves exposed to oscillating light. J Exp Bot 73:6380–6393. https://doi.org/10.1093/jxb/erac283
Lazár D, Stirbet A, Björn LO, Govindjee G (2022b) Light quality, oxygenic photosynthesis and more. Photosynthetica 60:25–58. https://doi.org/10.32615/ps.2021.055
Leister D (2023) Enhancing the light reactions of photosynthesis: strategies, controversies, and perspectives. Mol Plant 16:4–22. https://doi.org/10.1016/j.molp.2022.08.005
Leuenberger M, Morris JM, Chan AM, Leonelli L, Niyogi KK, Fleming GR (2017) Dissecting and modeling zeaxanthin- and lutein-dependent nonphotochemical quenching in Arabidopsis thaliana. Proc Natl Acad Sci USA 114:E7009–E7017. https://doi.org/10.1073/pnas.170450211
Li Z, Song M, Feng H, Zhao Y (2016) Within-season yield prediction with different nitrogen inputs under rain-fed condition using CERES-Wheat model in the northwest of China. J Sci Food Agric 96:2906–2916. https://doi.org/10.1002/jsfa.7467
Li M, Svoboda V, Davis G, Kramer D, Kunz HH, Kirchhoff H (2021) Impact of ion fluxes across thylakoid membranes on photosynthetic electron transport and photoprotection. Nat Plants 7:979–988. https://doi.org/10.1038/s41477-021-00947-5
Lin MT, Occhialini A, Andralojc PJ, Parry MAJ, Hanson MR (2014) A faster Rubisco with potential to increase photosynthesis in crops. Nature 513:547–550. https://doi.org/10.1038/nature13776
Liu F, Song Q, Zhao J, Mao L, Bu H, Hu Y, Zhu XG (2021) Canopy occupation volume as an indicator of canopy photosynthetic capacity. New Phytol 232:941–956. https://doi.org/10.1111/nph.17611
Liu YY, Wang SQ, Wang XB, Chen B, Chen JH (2022) Exploring the superiority of solar-induced chlorophyll fluorescence data in predicting wheat yield using machine learning and deep learning methods. Comput Electron Agric 192:106612. https://doi.org/10.1016/j.compag.2021.106612
Long SP (2012) Virtual special issue on food security: greater than anticipated impacts of near-term global atmospheric change on rice and wheat. Glob Change Biol 18:1489–1490. https://doi.org/10.1111/j.1365-2486.2012.02676.x
Long SP (2014) We need winners in the race to increase photosynthesis in rice, whether from conventional breeding, biotechnology or both. Plant Cell Environ 37:19–21. https://doi.org/10.1111/pce.12193
Long SP (2020) Photosynthesis engineered to increase rice yield. Nat Food 1:105. https://doi.org/10.1038/s43016-020-0038-5
Long SP, Bernacchi CJ (2003) Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J Exp Bot 54:2393–2401. https://doi.org/10.1093/jxb/erg262
Long SP, Ort DR (2010) More than taking the heat: crops and global change. Curr Opin Plant Biol 13:241–248. https://doi.org/10.1016/j.pbi.2010.04.008
Long SP, Zhu XG, Naidu SL, Ort DR (2006) Can improvement in photosynthesis increase crop yields? Plant Cell Environ 29:315–330. https://doi.org/10.1111/j.1365-3040.2005.01493.x
Long BM, Badger MR, Whitney SM, Price GD (2007) Analysis of carboxysomes from Synechococcus PCC7942 reveals multiple Rubisco complexes with carboxysomal proteins CcmM and CcaA. J Biol Chem 282:29323–29335. https://doi.org/10.1074/jbc.M703896200
Long SP, Marshall-Colon A, Zhu XG (2015) Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161:56–66. https://doi.org/10.1016/j.cell.2015.03.019
Long BM, Hee WY, Sharwood RE, Rae BD, Kaines S, Lim YL, Nguyen ND, Massey B, Bala S, von Caemmerer S, Badger MR, Price GD (2018) Carboxysome encapsulation of the CO2-fixing enzyme Rubisco in tobacco chloroplasts. Nat Commun 9(1):3570. https://doi.org/10.1038/s41467-018-06044-0
Long SP, Taylor SH, Burgess SJ, Carmo-Silva E, Lawson T, De Souza AP, Leonelli L, Wang Y (2022) Into the shadows and back into sunlight: photosynthesis in fluctuating light. Annu Rev Plant Biol 73:617–648. https://doi.org/10.1146/annurev-arplant-070221-024745
Lopez-Calcagno PE, Brown KL, Simkin AJ, Fisk SJ, Vialet-Chabrand S, Lawson T, Raines CA (2020) Stimulating photosynthetic processes increases productivity and water-use efficiency in the field. Nat Plants 6:1054–1063. https://doi.org/10.1038/s41477-020-0740-1
Lorite IJ, García-Vila M, Santos C, Ruiz-Ramos M, Fereres E (2013) AquaData and Aqua-GIS: two computer utilities for temporal and spatial simulations of water-limited yield with AquaCrop. Comput Electron Agric 96:227–237. https://doi.org/10.1016/j.compag.2013.05.010
Luo Z, Wang E, Fillery IRP, Macdonald LM, Huth N, Baldock J (2014) Modelling soil carbon and nitrogen dynamics using measurable and conceptual soil organic matter pools in APSIM. Agric Ecosyst Environ 186:94–104. https://doi.org/10.1016/j.agee.2014.01.019
Lynch JP (2007) Roots of the second green revolution. Aust J Bot 55:493–512. https://doi.org/10.1071/BT06118
Lyu H, Lazár D (2017a) Modeling the light-induced electric potential difference (ΔΨ), the pH difference (ΔpH) and the proton motive force across the thylakoid membrane in C3 leaves. J Theor Biol 413:11–23. https://doi.org/10.1016/j.jtbi.2016.10.017
Lyu H, Lazár D (2017b) Modeling the light-induced electric potential difference ΔΨ across the thylakoid membrane based on the transition state rate theory. Biochim Biophys Acta 1858:239–248. https://doi.org/10.1016/j.bbabio.2016.12.009
Lyu H, Lazár D (2022) Analyzing the effect of ion binding to the membrane-surface on regulating the light-induced transthylakoid electric potential (ΔΨ). Front Plant Sci 13:945675. https://doi.org/10.3389/fpls.2022.945675
Lyu H, Lazár D (2023) Effect of ion fluxes on regulating the light-induced transthylakoid electric potential difference. Plant Physiol Biochem 194:60–69. https://doi.org/10.1016/j.plaphy.2022.10.028
Ma G, Huang J, Wu W, Fan J, Zou J, Wu S (2013) Assimilation of MODIS-LAI into the WOFOST model for forecasting regional winter wheat yield. Math Comput Model 58:634–643. https://doi.org/10.1016/j.mcm.2011.10.038
Magney TS, Frankenberg C, Kӧhler P, North G, Davis TS, Dold C, Dutta D, Fisher JB, Grossmann K, Harrington A, Hatfield J, Stutz J, Sun Y, Porcar-Castell A (2019) Disentangling changes in the spectral shape of chlorophyll fluorescence: implications for remote sensing of photosynthesis. J Geophys Res 124:1491–1507. https://doi.org/10.1029/2019JG005029
Mahlein AK, Kuska MT, Thomas S, Wahabzada M, Behmann J, Rascher U, Kersting K (2019) Quantitative and qualitative phenotyping of disease resistance of crops by hyperspectral sensors: seamless interlocking of phytopathology, sensors, and machine learning is needed! Curr Opin Plant Biol 50:156–162. https://doi.org/10.1016/j.pbi.2019.06.007
Maier A, Fahnenstich H, von Caemmerer S, Engqvist MKM, Weber APM, Flügge UI, Maurino VG (2012) Transgenic introduction of a glycolate oxidative cycle into A. thaliana chloroplasts leads to growth improvement. Front Plant Sci 3:38. https://doi.org/10.3389/fpls.2012.00038
Maskell EJ (1928) Experimental researches on vegetable assimilation and respiration. XVIII. The relation between stomatal opening and assimilation. A critical study of assimilation rates and porometer rates in leaves of cherry laurel. Proc R Soc B 102:488–533. https://doi.org/10.1098/rspb.1928.0021
Matuszyńska A, Heidari S, Jahns P, Ebenhöh O (2016) A mathematical model of non-photochemical quenching to study short-term light memory in plants. Biochim Biophys Acta 1857:1860–1869. https://doi.org/10.1016/j.bbabio.2016.09.003
Matuszyńska A, Saadat NP, Ebenhöh O (2019) Balancing energy supply during photosynthesis—a theoretical perspective. Physiol Plant 166:392–402. https://doi.org/10.1111/ppl.12962
McGrath JM, Long SP (2014) Can the cyanobacterian carbon-concentrating mechanism increase photosynthesis in crop species? A theoretical analysis. Plant Physiol 164:2247–2261. https://doi.org/10.1104/pp.113.232611
Medlyn BE, Dreyer E, Ellsworth D, Forstreuter M, Harley PC, Kirschbaum MUF, Le Roux X, Montpied P, Strassemeyer J, Walcroft A, Wang K, Loustau D (2002) Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ 25:1167–1179. https://doi.org/10.1046/j.1365-3040.2002.00891.x
Meroni M, Rossini M, Guanter L, Alonso L, Rascher U (2009) Remote sensing of solar-induced chlorophyll fluorescence: review of methods and applications. Remote Sens Environ 113:2037–2051. https://doi.org/10.1016/j.rse.2009.05.003
Meyer MT, Whittaker C, Griffiths H (2017) The algal pyrenoid: key unanswered questions. J Exp Bot 68:3739–3749. https://doi.org/10.1093/jxb/erx178
Miao Z, Xu M, Lathrop RG Jr, Wang Y (2009) Comparison of the A-Cc curve fitting methods in determining maximum ribulose 1.5-bisphosphate carboxylase/oxygenase carboxylation rate, potential light saturated electron transport rate and leaf dark respiration. Plant Cell Environ 32:109–122. https://doi.org/10.1111/j.1365-3040.2008.01900.x
Mirkovic T, Ostrumov EE, Anna JM, Van Grondelle R, Govindjee G, Scholes GD (2017) Light absorption and energy transfer in the antenna complexes of photosynthetic organisms. Chem Rev 117:249–293. https://doi.org/10.1021/acs.chemrev.6b00002
Mitchell P (1975) The protonmotive Q cycle: a general formulation. FEBS Lett 59:137–139. https://doi.org/10.1016/0014-5793(75)80359-0
Moore CE, Meacham-Hensold K, Lemonnier P, Slattery RA, Benjamin C, Bernacchi CJ, Lawson T, Cavanagh AP (2021) The effect of increasing temperature on crop photosynthesis: from enzymes to ecosystems. J Exp Bot 72:2822–2844. https://doi.org/10.1093/jxb/erab090
Morales A, Kaiser E (2020) Photosynthetic acclimation to fluctuating irradiance in plants. Front Plant Sci 11:268. https://doi.org/10.3389/fpls.2020.00268
Morales A, Yin X, Harbinson J, Driever SM, Molenaar J, Kramer DM, Struik PC (2018a) In silico analysis of the regulation of the photosynthetic electron transport chain in C3 plants. Plant Physiol 176:1247–1261. https://doi.org/10.1104/pp.17.00779
Morales A, Kaiser E, Yin X, Harbinson J, Molenaar J, Driever SM, Struik PC (2018b) Dynamic modelling of limitations on improving leaf CO2 assimilation under fluctuating irradiance. Plant Cell Environ 41:589–604. https://doi.org/10.1111/pce.13119
Morris JM, Fleming GR (2018) Quantitative modeling of energy dissipation in Arabidopsis thaliana. Environ Exp Bot 154:99–109. https://doi.org/10.1016/j.envexpbot.2018.03.021
Mott KA, Woodrow IE (2000) Modelling the role of Rubisco activase in limiting non-steady-state photosynthesis. J Exp Bot 51:399–406. https://doi.org/10.1093/jexbot/51.suppl_1.399
Muhie SH (2022) Optimization of photosynthesis for sustainable crop production. CABI Agric Biosci 3:50. https://doi.org/10.1186/s43170-022-00117-3
Mukherjee A, Lau CS, Walker CE, Rai AK, Prejean CI, Yates G, Emrich-Mills T, Lemoine SG, Vinyard DJ, Mackinder LCM, Moroney JV (2019) Thylakoid localized bestrophin-like proteins are essential for the CO2 concentrating mechanism of Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 116:16915–16920. https://doi.org/10.1073/pnas.1909706116
Munekage YN, Taniguchi YY (2017) Promotion of cyclic electron transport around Photosystem I with the development of C4 photosynthesis. Plant Cell Physiol 57:897–903. https://doi.org/10.1093/pcp/pcw012
Murchie EH, Ruban AV (2020) Dynamic non-photochemical quenching in plants: from molecular mechanism to productivity. Plant J 101:885–896. https://doi.org/10.1111/tpj.14601
Nauš J, Lazár D, Baránková B, Arnoštová B (2018) On the source of non-linear light absorbance in photosynthetic samples. Photosynth Res 136:345–355. https://doi.org/10.1007/s11120-017-0468-6
Nedbal L, Březina V (2002) Complex metabolic oscillations in plants forced by harmonic irradiance. Biophys J 83:2180–2189. https://doi.org/10.1016/S0006-3495(02)73978-7
Nedbal L, Lazár D (2021) Photosynthesis dynamics and regulation sensed in the frequency domain. Plant Physiol 187:646–661. https://doi.org/10.1093/plphys/kiab317
Nedbal L, Březina V, Adamec F, Štys D, Oja V, Laisk A, Govindjee (2003) Negative feedback regulation is responsible for the non-linear modulation of photosynthetic activity in plants and cyanobacteria exposed to a dynamic light environment. Biochim Biophys Acta 1607:5–17. https://doi.org/10.1016/j.bbabio.2003.08.005
Nedbal L, Březina V, Červený J, Trtílek M (2005) Photosynthesis in dynamic light: systems biology of unconventional chlorophyll fluorescence transients in Synechocystis sp. PCC 6803. Photosynth Res 84:99–106. https://doi.org/10.1007/s11120-004-6428-y
Negi S, Perrine Z, Friedland N, Kumar A, Tokutsu R, Minagawa J, Berg R, Barry A, Govindjee G, Sayre R (2020) Light-regulation of light harvesting antenna size substantially enhances photosynthetic efficiency and biomass yield in green algae. Plant J 103(2):584–603. https://doi.org/10.1111/tpj.14751
Niu Y, Lazár D, Holzwarth AR, Kramer DM, Matsubara S, Fiorani F, Poorter H, Schrey SD, Nedbal L (2023) Plants cope with fluctuating light by frequency-dependent non-photochemical quenching and cyclic electron transport. New Phyol. 239:1869–1886. https://doi.org/10.1111/nph.19083
Niu Y, Matsubara S, Nedbal L, Lazár D (2024) Dynamics and interplay of photosynthetic regulatory processes depend on the amplitudes of oscillating light. Plant Cell Environ. https://doi.org/10.1111/pce.14879
Nölke G, Houdelet M, Kreuzaler F, Peterhänsel C, Schillberg S (2014) The expression of a recombinant glycolate dehydrogenase polyprotein in potato (Solanum tuberosum) plastids strongly enhances photosynthesis and tuber yield. Plant Biotechnol J 12:734–742. https://doi.org/10.1111/pbi.12178
Nonomura AM, Holtz B, Biel KY, Cooney R, Lorimer G, Govindjee G (2017) The paths of Andrew A. Benson: a radioautobiography. Photosynth Res 134:93–105. https://doi.org/10.1007/s11120-017-0410-y
Nyakudya IW, Stroosnijder L (2014) Effect of rooting depth, plant density and planting date on maize (Zea mays L.) yield and water use efficiency in semi-arid Zimbabwe: Modelling with AquaCrop. Agric Water Manag 146:280–296. https://doi.org/10.1016/j.agwat.2014.08.024
Ogren WL, Bowes G (1971) Ribulose diphosphate carboxylase regulates soybean photorespiration. Nat New Biol 230:159–160. https://doi.org/10.1038/newbio230159a0
Ögren E, Evans JR (1993) Photosynthetic light-response curves. The influence of CO2 partial pressure and leaf inversion. Planta 189:182–190. https://doi.org/10.1007/BF00195075
Oh ZG, Askey B, Gunn LH (2023) Red Rubiscos and opportunities for engineering green plants. J Exp Bot 74:520–542. https://doi.org/10.1093/jxb/erac349
Orr DJ, Pereira AM, da Fonseca Pereira P, Pereira-Lima IA, Zsogon A, Araujo WL (2017) Engineering photosynthesis: progress and perspectives. F1000Res 6:1891. https://doi.org/10.12688/f1000research.12181.1
Ort DR, Merchant SS, Alric J, Barkan A, Blankenship RE, Bock R, Croce R, Hanson MR, Hibberd JM, Long SP, Moore TA, Moroney J, Niyogi KK, Parry MAJ, Peralta-Yahya PP, Prince RC, Redding KE, Spalding MH, van Wijk KJ, Vermaas WFJ, von Caemmerer S, Weber APM, Yeates TO, Yuan JS, Zhu XG (2015) Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc Natl Acad Sci USA 112:8529–8536. https://doi.org/10.1073/pnas.1424031112
Pandiyan S, Govindjee G, Meenatchi S, Prasanna S, Gunasekaran G, Guo Y (2021) Evaluating the impact of summer drought on vegetation growth using space-based solar-induced chlorophyll fluorescence across extensive spatial measures. Big Data 10:230–245. https://doi.org/10.1089/big.2020.0350
Papageorgiou GC, Govindjee G (eds) (2004) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht. https://doi.org/10.1016/j.jphotobiol.2011.03.008
Papageorgiou GC, Govindjee G (2011) Photosystem II fluorescence: slow changes–scaling from the past. J Photochem Photobiol B 104:258–270. https://doi.org/10.1016/j.jphotobiol.2011.03.008
Papageorgiou GC, Govindjee G (2014) The non-photochemical quenching of the electronically excited state of chlorophyll a in plants: definitions, timelines, viewpoints, open questions. In: Demmig-Adams B, Garab G, Adams WW III, Govindjee G, Sharkey TD (eds) Nonphotochemical quenching and energy dissipation in plants, algae and cyanobacteria. Advances in photosynthesis and respiration, vol 40. Springer, Dordrecht, pp 1–44. https://doi.org/10.1007/978-94-017-9032-1_1
Parry MAJ, Reynolds M, Salvucci ME, Raines C, Andralojc PJ, Zhu XG, Price GD, Condon AG, Furbank RT (2011) Raising yield potential of wheat. II. Increasing photosynthetic capacity and efficiency. J Exp Bot 62:453–467. https://doi.org/10.1093/jxb/erq304
Pasquel D, Roux S, Richetti J, Cammarano D, Tisseyre B, Taylor JA (2022) A review of methods to evaluate crop model performance at multiple and changing spatial scales. Precision Agric 23:1489–1513. https://doi.org/10.1007/s11119-022-09885-4
Paul MJ (2021) Improving photosynthetic metabolism for crop yields: what is going to work? Front Plant Sci 12:743862. https://doi.org/10.3389/fpls.2021.743862
Peak D, Mott KA (2011) A new, vapour-phase mechanism for stomatal responses to humidity and temperature. Plant Cell Environ 34:162–178. https://doi.org/10.1111/j.1365-3040.2010.02234.x
Pembleton K, Cullen B, Rawnsley R, Harrison M, Ramilan T (2016) Modelling the resilience of forage crop production to future climate change in the dairy regions of Southeastern Australia using APSIM. J Agric Sci 154:1131–1152. https://doi.org/10.1017/S0021859615001185
Peng JR, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pelica F, Sudhakar D, Christou P, Snape JW, Gale MD, Harberd NP (1999) ‘Green revolution’ genes encode mutant gibberellin response modulators. Nature 400:256–261. https://doi.org/10.1038/22307
Peng B, Guan K, Zhou W, Jiang C, Frankenberg C (2020) Assessing the benefit of satellite-based solar-induced chlorophyll fluorescence in crop yield prediction. Int J Appl Earth Obs Geoinf 90:102126. https://doi.org/10.1016/j.jag.2020.102126
Perdomo JA, Degen GE, Worrall D, Carmo-Silva E (2019) Rubisco activation by wheat Rubisco activase isoform 2β is insensitive to inhibition by ADP. Biochem J 476:2595–2606. https://doi.org/10.1042/BCJ20190110
Peterhansel C, Maurino VG (2011) Photorespiration redesigned. Plant Physiol 155:49–55. https://doi.org/10.1104/pp.110.165019
Peterhansel C, Blume C, Offermann S (2013) Photorespiratory bypasses: how can they work? J Exp Bot 64:709–715. https://doi.org/10.1093/jxb/ers247
Polle A (2001) Dissecting the superoxide dismutase-ascorbate-glutathione-pathway in chloroplasts by metabolic modeling. computer simulations as a step towards flux analysis. Plant Physiol 126:445–462. https://doi.org/10.1104/pp.126.1.445
Poolman MG (1999) Computer modeling applied to the Calvin cycle. PhD thesis. Oxford Brookes University, Oxford
Poolman MG, Fell DA, Thomas S (2000) Modelling photosynthesis and its control. J Exp Bot 51:319–328. https://doi.org/10.1093/jexbot/51.suppl_1.319
Portis AR Jr, Parry MA (2007) Discoveries in Rubisco (Ribulose 1,5-bisphosphate carboxylase/oxygenase): a historical perspective. Photosynth Res 94:121–143. https://doi.org/10.1007/s11120-007-9225-6
Price GD, Badger MR, von Caemmerer S (2011) The prospect of using cyanobacterial bicarbonate transporters to improve leaf photosynthesis in C3 crop plants. Plant Physiol 155:20–26. https://doi.org/10.1104/pp.110.164681
Price GD, Pengelly JJL, Forster B, Du J, Whitney SM, von Caemmerer S, Badger MR, Howitt SM, Evans JR (2013) The cyanobacterial CCM as a source of genes for improving photosynthetic CO2 fixation in crop species. J Exp Bot 64:753–768. https://doi.org/10.1093/jxb/ers257
Prywes N, Philips NR, Tuck OT, Valentin Alvarado LE, Savage DF (2023) Rubisco function, evolution, and engineering. Annu Rev Biochem. https://doi.org/10.1146/annurev-biochem-040320-101244
Pushkar Y, Yano J, Sauer K, Boussac A, Yachandra VK (2008) Structural changes in the Mn4Ca cluster and the mechanism of photosynthetic water splitting. Proc Natl Acad Sci USA 105:1879–1884. https://doi.org/10.1073/pnas.0707092105
Pyke KA, Leech RM (1987) Cellular levels of ribulose 1,5 bisphosphate carboxylase and chloroplast compartment size in wheat mesophyll cells. J Exp Bot 38:1949–1956. https://doi.org/10.1093/jxb/38.12.1949
Qu Z, Garfinkel A, Weiss JN, Nivala M (2011) Multi-scale modeling in biology: how to bridge the gaps between scales? Prog Biophys Mol Biol 107:21–31. https://doi.org/10.1016/j.pbiomolbio.2011.06.004
Qu M, Zheng G, Hamdani S, Essemine J, Song Q, Wang H, Chu C, Sirault X, Zhu XG (2017) Leaf photosynthetic parameters related to biomass accumulation in a global rice diversity survey. Plant Physiol 175:248–258. https://doi.org/10.1104/pp.17.00332
Raines CA (2003) The Calvin cycle revisited. Photosynth Res 75:1–10. https://doi.org/10.1023/A:1022421515027
Raines CA (2006) Transgenic approaches to manipulate the environmental responses of the C3 carbon fixation cycle. Plant Cell Environ 29:331–339. https://doi.org/10.1111/j.1365-3040.2005.01488.x
Raines CA (2011) Increasing photosynthetic carbon assimilation in C3 plants to improve crop yield. Current and future strategies. Plant Physiol 155:36–42. https://doi.org/10.1104/pp.110.168559
Ray DK, Ramankutty N, Mueller ND, West PC, Foley JA (2012) Recent patterns of crop yield growth and stagnation. Nat Commun 3:1293. https://doi.org/10.1038/ncomms2296
Ray DK, Mueller ND, West PC, Foley JA (2013) Yield trends are insufficient to double global crop production by 2050. PLoS ONE 8:e66428. https://doi.org/10.1371/journal.pone.0066428
Rincent R, Malosetti M, Ababaei B, Touzy G, Mini A, Bogard M, Martre P, Le Gouis J, van Eeuwijk F (2019) Using crop growth model stress covariates and AMMI decomposition to better predict genotype-by-environment interactions. Theor Appl Genet 132:3399–3411. https://doi.org/10.1007/s00122-019-03432-y
Riznichenko GY, Rubin AB (2021) Mathematical modeling in biology. Part 1. Dynamic models of primary photosynthesis processes. Biol Bull Rev 11:93–109. https://doi.org/10.1134/S2079086421020079
Rochaix J-D (2014) Regulation and dynamics of the light-harvesting system. Annu Rev Plant Biol 65:287–309. https://doi.org/10.1146/annurev-arplant-050213-040226
Rogers A, Medlyn BE, Dukes JS, Bonan G, von Caemmerer S, Dietze MC, Kattge J, Leakey ADB, Mercado LM, Niinemets U, Prentice IC, Serbin SP, Sitch S, Way DA, Zaehle S (2017) A roadmap for improving the representation of photosynthesis in Earth system models. New Phytol 213:22–42. https://doi.org/10.1111/nph.14283
Rosenzweig C, Elliott J, Deryng D, Ruane AC, Müller C, Arneth A, Boote KJ, Folberth C, Glotter M, Khabarov N, Neumann K, Piontek F, Pugh TAM, Schmid E, Stehfest E, Yang H, Jones JW (2013) Assessing agricultural risks of climate change in the 21st century in a global gridded crop model intercomparison. Proc Natl Acad Sci USA 111:3268–3273. https://doi.org/10.1073/pnas.1222463110
Rumberg B, Siggel U (1969) pH changes in the inner phase of the thylakoids during photosynthesis. Naturwissenschaften 56:130–132. https://doi.org/10.1007/BF00601025
Saadat NP, Nies T, van Aalst M, Hank B, Demirtas B, Ebenhöh O, Matuszyńska A (2021) Computational analysis of alternative photosynthetic electron flows linked with oxidative stress. Front Plant Sci 12:750580. https://doi.org/10.3389/fpls.2021.750580
Saengwilai P, Nord EA, Chimungu JG, Brown KM, Lynch JP (2014) Root cortical aerenchyma enhances nitrogen acquisition from low nitrogen soils in maize. Plant Physiol 166:726–735. https://doi.org/10.1104/pp.114.241711
Sage RF, Sage TL, Kocacinar F (2012) Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol 63:19–47. https://doi.org/10.1146/annurev-arplant-042811-105511
Sales CRG, Wang Y, Evers JB, Kromdijk J (2021) Improving C4 photosynthesis to increase productivity under optimal and suboptimal conditions. J Exp Bot 72:5942–5960. https://doi.org/10.1093/jxb/erab327
Salesse-Smith CE, Sharwood RE, Busch FA, Kromdijk J, Bardal V, Stern DB (2018) Overexpression of Rubisco subunits with RAF1 increases Rubisco content in maize. Nat Plants 4:802–810. https://doi.org/10.1038/s41477-018-0252-4
Salesse-Smith CE, Sharwood RE, Busch FA, Stern DB (2020) Increased Rubisco content in maize mitigates chilling stress and speeds recovery. Plant Biotechnol J 18:1409–1420. https://doi.org/10.1111/pbi.13306
Salvatori N, Carteni F, Giannino F, Alberti G, Mazzoleni S, Peressotti A (2022) A system dynamics approach to model photosynthesis at leaf level under fluctuating light. Front Plant Sci 12:787877. https://doi.org/10.3389/fpls.2021.787877
Sánchez-Baracaldo P, Cardona T (2020) On the origin of oxygenic photosynthesis and cyanobacteria. New Phytol 225:1440–1446. https://doi.org/10.1111/nph.16249
Sato R, Ohta H, Masuda S (2014) Prediction of respective contribution of linear electron flow and PGR5-dependent cyclic electron flow to non-photochemical quenching induction. Plant Physiol Biochem 81:190–196. https://doi.org/10.1016/j.plaphy.2014.03.017
Sauer K (1975) Primary events and the trapping of energy. In: Govindjee G (ed) Bioenergetics of photosynthesis. Academic Press, New York, pp 115–181
Sauer K (1979) Photosynthesis—the light reactions. Annu Rev Phys Chem 30:155–178. https://doi.org/10.1146/annurev.pc.30.100179.001103
Schansker G (2022) Determining photosynthetic control, a probe for the balance between electron transport and Calvin-Benson cycle activity, with the DUAL-KLAS-NIR. Photosynth Res 153:191–204. https://doi.org/10.1007/s11120-022-00934-7
Schlüter U, Weber AP (2016) The road to C4 photosynthesis: evolution of a complex trait via intermediary states. Plant Cell Physiol 57:881–889. https://doi.org/10.1093/pcp/pcw009
Schlüter U, Weber APM (2020) Regulation and evolution of C4 photosynthesis. Annu Rev Plant Biol 71:183–215. https://doi.org/10.1146/annurevarplant-042916-040915
Sejima T, Fukayama H, Makino A, Miyake C (2014) Repetitive short-pulse light mainly inactivates photosystem I in sunflower leaves. Plant Cell Physiol 55:1184–1193. https://doi.org/10.1093/pcp/pcu061
Sellers PJ, Randall DA, Collatz GJ, Berry JA, Field CB, Dazlich DA, Zhang C, Collelo GD, Bounoua L (1996) A revised land surface parameterization (SiB2) for atmospheric GCMs. Part 1: model formulation. J Clim 9:676–705. https://doi.org/10.1175/1520-0442(1996)009%3c0676:ARLSPF%3e2.0.CO;2
Sharkey TD (2019) Discovery of the canonical Calvin-Benson cycle. Photosynth Res 140:235–252. https://doi.org/10.1093/jxb/erac254
Sharkey TD (2023) The discovery of rubisco. J Exp Bot 74:510–519. https://doi.org/10.1093/jxb/erac254
Sharkey TD, Bernacchi CJ, Farquhar GD, Singsaas EL (2007) In practice: fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant Cell Environ 30:1035–1040. https://doi.org/10.1111/j.1365-3040.2007.01710.x
Sharma-Natu P, Ghildiyal MC (2005) Potential targets for improving photosynthesis and crop yield. Curr Sci 88:1918–1928. https://doi.org/10.1104/pp.113.219006
Sharpe RM, Offermann S (2014) One decade after the discovery of single-cell C4 species in terrestrial plants: what did we learn about the minimal requirement of C4 photosynthesis? Photosynth Res 119:169–180. https://doi.org/10.1007/s11120-013-9810-9
Sharwood RE (2017) Engineering chloroplasts to improve Rubisco catalysis: prospects for translating improvements into food and fiber crops. New Phytol 213:494–510. https://doi.org/10.1111/nph.14351
Shen BR, Wang LM, Lin XL, Zhen Yao XuHW, Zhu CH, Teng HY, Cui LL, Liu EE, Zhang JJ, He ZH, Peng XX (2019) Engineering a new chloroplastic photorespiratory bypass to increase photosynthetic efficiency and productivity in rice. Molec Plant 12:199–214. https://doi.org/10.1016/j.molp.2018.11.013
Shevela D, Govindjee G (2016) Oxygenic photosynthesis. Agrisera Educational Poster 1. https://doi.org/10.13140/RG.2.2.26291.58409
Shevela D, Björn LO, Govindjee G (2019) Photosynthesis: solar energy for life. World Scientific, Singapore, p 188. https://doi.org/10.1142/10522
Shevela D, Kern JF, Govindjee G, Messinger J (2023) Solar energy conversion by photosystem II: principles and structures. Photosynth Res 156:279–307. https://doi.org/10.1007/s11120-022-00991-y
Shibu ME, Leffelaar PA, van Keulen H, Aggarwal PK (2010) LINTUL3, a simulation model for nitrogen-limited situations: application to rice. Eur J Agron 32:255–271. https://doi.org/10.1016/j.eja.2010.01.003
Shikanai T (2016) Regulatory network of proton motive force: contribution of cyclic electron transport around photosystem I. Photosynth Res 129:253–260. https://doi.org/10.1007/s11120-016-0227-0
Simkin AJ, López-Calcagno PE, Raines CA (2019) Feeding the world: improving photosynthetic efficiency for sustainable crop production. J Exp Bot 70:1119–1140. https://doi.org/10.1093/jxb/ery445
Sinclair TR, Purcell LC, Sneller CH (2004) Crop transformation and the challenge to increase yield potential. Trends Plant Sci 9:70–75. https://doi.org/10.1016/j.tplants.2003.12.008
Slattery RA, Walker BJ, Weber AP, Ort DR (2018) The impacts of fluctuating light on crop performance. Plant Physiol 176:990–1003. https://doi.org/10.1104/pp.17.01234
Smith EL (1936) Photosynthesis in relation to light and carbon dioxide. Proc Natl Acad Sci USA 22(8):504–511. https://doi.org/10.1073/pnas.22.8.504
Snellenburg JJ, Johnson MP, Ruban AV, van Grondelle R, van Stokkum IHM (2017) A four state parametric model for the kinetics of the non-photochemical quenching in Photosystem II. Biochim Biophys Acta 1858:854–864. https://doi.org/10.1016/j.bbabio.2017.08.004
Soddu A, Deidda R, Marrocu M, Meloni R, Paniconi C, Ludwig R, Sodde M, Mascaro G, Perra E (2013) Climate variability and durum wheat adaptation using the AquaCrop Model in Southern Sardinia. Procedia Environ Sci 19:830–835. https://doi.org/10.1016/j.proenv.2013.06.092
Song Q, Zang G, Zhu XG (2013) Optimal crop canopy architecture to maximise canopy photosynthetic CO2 uptake under elevated CO2—a theoretical study using a mechanistic model of canopy photosynthesis. Funct Plant Biol 40:109–124. https://doi.org/10.1071/FP12056
Song Q, Wang Y, Qu M, Ort DR, Zhu XG (2017) The impact of modifying photosystem antenna size on canopy photosynthetic efficiency—development of a new canopy photosynthesis model scaling from metabolism to canopy level processes. Plant Cell Environ 40:2946–2957. https://doi.org/10.1111/pce.13041
Song Y, Wang J, Wang LX (2020) Satellite solar-induced chlorophyll fluorescence reveals heat stress impacts on wheat yield in India. Remote Sens 12:3277. https://doi.org/10.3390/rs12203277
Sonoike K (2011) Photoinhibition of photosystem I. Physiol Plant 142:56–64. https://doi.org/10.1111/j.1399-3054.2010.01437.x
South PF, Cavanagh AP, Lopez-Calcagno PE, Raines CA, Ort DR (2018) Optimizing photorespiration for improved crop productivity. J Integr Plant Biol 60:1217–1230. https://doi.org/10.1111/jipb.12709
South PF, Cavanagh AP, Liu HW, Ort DR (2019) Synthetic glycolate metabolism pathways stimulate crop growth and productivity in the field. Science 363:eaat9077. https://doi.org/10.1126/science.aat9077
Spreitzer RJ, Salvucci ME (2002) RUBISCO: structure, regulatory interactions, and possibilities for a better enzyme. Annu Rev Plant Biol 53:449–475. https://doi.org/10.1146/annurev.arplant.53.100301.135233
Srinivasan V, Kumar P, Long SP (2017) Decreasing, not increasing, leaf area will raise crop yields under global atmospheric change. Global Change Biol 23:1626–1635. https://doi.org/10.1111/gcb.13526
Steen CJ, Morris JM, Short AH, Niyogi KK, Fleming GR (2020) Complex roles of PsbS and xanthophylls in the regulation of nonphotochemical quenching in Arabidopsis thaliana under fluctuating light. J Phys Chem B 124:10311–10325. https://doi.org/10.1021/acs.jpcb.0c06265
Stewart CN Jr, Patron N, Hanson AD, Jez JM (2018) Plant metabolic engineering in the synthetic biology era: plant chassis selection. Plant Cell Rep 37:1357–1358. https://doi.org/10.1007/s00299-018-2342-1
Stirbet A, Govindjee G (2016) The slow phase of chlorophyll a fluorescence induction in silico: origin of the S-M fluorescence rise. Photosynth Res 130:193–213. https://doi.org/10.1007/s11120-016-0243-0
Stirbet A, Strasser RJ (1996) Numerical simulation of the in vivo fluorescence in plants. Math Comput Simul 42(2–3):245–253. https://doi.org/10.1016/0378-4754(95)00114-X
Stirbet A, Govindjee G, Strasser BJ, Strasser RJ (1998) Chlorophyll a fluorescence induction in higher plants: modelling and numerical simulation. J Theor Biol 193:131–151. https://doi.org/10.1006/jtbi.1998.0692
Stirbet A, Riznichenko GYu, Rubin AB, Govindjee G (2014) Modeling chlorophyll a fluorescence transient: relation to photosynthesis. Biochem Mosc 79:291–323. https://doi.org/10.1134/S0006297914040014
Stirbet A, Lazar D, Papageorgiou CG, Govindjee G (2019) Chlorophyll a fluorescence in cyanobacteria: relation to photosynthesis. In: Mishra AK, Tiwari DN, Rai AN (eds) Cyanobacteria—from basic science to applications. Academic Press, London, pp 79–130. https://doi.org/10.1016/B978-0-12-814667-5.00005-2
Stirbet A, Lazár D, Guo Y, Govindjee G (2020) Photosynthesis: basics, history and modelling. Ann Bot 126:511–537. https://doi.org/10.1093/aob/mcz171
Sukhova EM, Khlopkov A, Vodeneev V, Sukhov V (2020) Simulation of a nonphotochemical quenching in plant leaf under different light intensities. Biochim Biophys Acta 1861:148138. https://doi.org/10.1016/j.bbabio.2019.148138
Sukhova EM, Vodeneev VA, Sukhov VS (2021) Mathematical modeling of photosynthesis and analysis of plant productivity. Biochem (moscow) Suppl Series A 15:52–72. https://doi.org/10.1134/S1990747821010062
Suorsa M, Järvi S, Grieco M, Nurmi M, Pietrzykowska M, Rantala M, Kangasjärvi S, Paakkarinen V, Tikkanen M, Jansson S, Aro E-M (2012) Proton gradient regulation5 is essential for proper acclimation of Arabidopsis photosystem I to naturally and artificially fluctuating light conditions. Plant Cell 24:2934–2948. https://doi.org/10.1105/tpc.112.097162
Sušila P, Lazár D, Ilík P, Tomek P, Nauš J (2004) The gradient of exciting radiation within a sample affects the relative height of steps in the fast chlorophyll a fluorescence rise. Photosynthetica 42:161–172. https://doi.org/10.1023/B:PHOT.0000040586.39903.db
Szabò I, Spetea C (2017) Impact of the ion transportome of chloroplasts on the optimization of photosynthesis. J Exp Bot 68:3115–3128. https://doi.org/10.1093/jxb/erx063
Takizawa K, Cruz JA, Kanazawa A, Kramer DM (2007) The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochimica et Biophysica Acta 1767 (2007) 1233–1244. https://doi.org/10.1016/j.bbabio.2007.07.006
Taylor SH, Long SP (2017) Slow induction of photosynthesis on shade to sun transitions in wheat may cost at least 21% of productivity. Philos Trans R Soc B 372:20160543. https://doi.org/10.1098/rstb.2016.0543
Terashima I, Funayama S, Sonoike K (1994) The site of photoinhibition in leaves of Cucumis sativus L. at low temperature is photosystem I, not photosystem II. Planta 193:300–306. https://doi.org/10.1007/BF00192544
Tikhonov AN, Khomutov GB, Ruuge EK, Blumenfeld LA (1981) Electron transport control in chloroplasts. Effects of photosynthetic control monitored by the intrathylakoid pH. Biochim Biophys Acta 637:321–333. https://doi.org/10.1016/0005-2728(81)90171-7
Timm S, Florian A, Fernie AR, Bauwe H (2016) The regulatory interplay between photorespiration and photosynthesis. J Exp Bot 67(10):2923–2929. https://doi.org/10.1093/jxb/erw083
Tjus SE, Møller BL, Scheller HV (1998) Photosystem I is an early target of photoinhibition in barley illuminated at chilling temperatures. Plant Physiol 116(2):755–764. https://doi.org/10.1104/pp.116.2.755
Tomek P, Ilík P, Lazár D, Štroch M, Nauš J (2003) On the determination of QB-non-reducing photosystem II centers from chlorophyll a fluorescence induction. Plant Sci 164:665–670. https://doi.org/10.1016/S0168-9452(03)00029-3
Trinh MDL, Masuda S (2022) Chloroplast pH homeostasis for the regulation of photosynthesis. Front Plant Sci 13:919896. https://doi.org/10.3389/fpls.2022.919896
Valero E, González-Sánchez MI, Macià H, García-Carmona F (2009) Computer simulation of the dynamic behavior of the glutathione-ascorbate redox cycle in chloroplasts. Plant Physiol 149:1958–1969. https://doi.org/10.1104/pp.108.133223
Valero E, Macià H, De la Fuente IM, Hernández JA, González-Sánchez MI, García-Carmona F (2016) Modeling the ascorbate-glutathione cycle in chloroplasts under light/dark conditions. BMC Syst Biol 10:11. https://doi.org/10.1186/s12918-015-0239-y
Van Kooten O, Snel JFH, Vredenberg WJ (1986) Photosynthetic free energy transduction related to the electric potential changes across the thylakoid membrane. Photosynth Res 9:211–227. https://doi.org/10.1007/BF00029745
Vass I (2012) Molecular mechanisms of photodamage in the photosystem II complex. Biochim Biophys Acta 1817:209–217. https://doi.org/10.1016/j.bbabio.2011.04.014
Vershubskii AV, Tikhonov AN (2020) pH-dependent regulation of electron and proton transport in chloroplasts in situ and in silico. Biochem (moscow) Suppl Series A 14:154–165. https://doi.org/10.1134/S1990747819030218
von Bismarck T, Korkmaz K, Ruß J, Skurk K, Kaiser E, Correa Galvis V, Cruz JA, Strand DD, Köhl K, Eirich J, Finkemeier I, Jahns P, Kramer DM, Armbruster U (2023) Light acclimation interacts with thylakoid ion transport to govern the dynamics of photosynthesis in Arabidopsis. New Phytol 237:160–176. https://doi.org/10.1111/nph.18534
von Caemmerer S (2000) Biochemical models of leaf photosynthesis, vol 2. CSIRO Publishing, Collingwood. https://doi.org/10.1071/9780643103405
von Caemmerer S (2013) Steady-state models of photosynthesis. Plant Cell Environ 36:1617–1630. https://doi.org/10.1111/pce.12098
von Caemmerer S (2021) Updating the steady-state model of C4 photosynthesis. J Exp Bot 72:6003–6017. https://doi.org/10.1093/jxb/erab266
von Caemmerer S, Furbank RT (2003) The C4 pathway: an efficient CO2 pump. Photosynth Res 77:191–207. https://doi.org/10.1023/A:1025830019591
von Caemmerer S, Quick WP, Furbank RT (2012) The development of C4 rice: current progress and future challenges. Science 336:1671–1672. https://doi.org/10.1126/science.1220177
Waheeda K, Kitchel H, Wang Q, Chiu P-L (2023) Molecular mechanism of Rubisco activase: dynamic assembly and Rubisco remodeling. Front Mol Biosci 10:1125922. https://doi.org/10.3389/fmolb.2023.1125922
Walker DA (1992) Concerning oscillations. Photosynth Res 34:387–395. https://doi.org/10.1007/BF00029813
Walker BJ, VanLoocke A, Bernacchi CJ, Ort DR (2016) The cost of photorespiration to food production now and in the future. Annu Rev Plant Biol 67:107–129. https://doi.org/10.1146/annurev-arplant-043015-111709
Walter J, Kromdijk J (2022) Here comes the sun: how optimization of photosynthetic light reactions can boost crop yields. J Integr Plant Biol 64(2):564–591. https://doi.org/10.1111/jipb.13206
Wang Y, Bräutigam A, Weber APM, Zhu XG (2014a) Three distinct biochemical subtypes of C4 photosynthesis? A modelling analysis. J Exp Bot 65:3567–3578. https://doi.org/10.1093/jxb/eru058
Wang Y, Long SP, Zhu XG (2014b) Elements required for an efficient NADP-malic enzyme type C4 photosynthesis. Plant Physiol 164:2231–2246. https://doi.org/10.1104/pp.113.230284
Wang L, Yamano T, Takane S, Niikawa Y, Toyokawa C, Ozawa SI, Tokutsu R, Takahashi Y, Minagawa J, Kanesaki Y, Yoshikawa H, Fukuzawa H (2016) Chloroplast-mediated regulation of CO2-concentrating mechanism by Ca2+-binding protein CAS in the green alga Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 113:12586–12591. https://doi.org/10.1073/pnas.1606519113
Wang S, Tholen D, Zhu X-G (2017a) C4 photosynthesis in C3 rice: a theoretical analysis of biochemical and anatomical factors. Plant Cell Environ 40:80–94. https://doi.org/10.1111/pce.12834
Wang XP, Liu GM, Yang JS, Huang GH, Yao RJ (2017b) Evaluating the effects of irrigation water salinity on water movement, crop yield and water use efficiency by means of a coupled hydrologic/crop growth model. Agric Water Manag 85:13–26. https://doi.org/10.1016/j.agwat.2017.01.012
Wang LM, Shen BR, Li BD, Zhang CL, Lin M, Tong PP, Cui LL, Zhang ZS, Peng XX (2020) A Synthetic photorespiratory shortcut enhances photosynthesis to boost biomass and grain yield in rice. Mol Plant 13:1802–1815. https://doi.org/10.1016/j.molp.2020.10.007
Wang Y, Chan KX, Long SP (2021) Toward a dynamic photosynthesis model to guide yield improvement in C4 crops. Plant J 107:343–359. https://doi.org/10.1111/tpj.15365
Way DA, Pearcy RW (2012) Sunflecks in trees and forests: from photosynthetic physiology to global change biology. Tree Physiol 32(9):1066–1081. https://doi.org/10.1093/treephys/tps064
Wellens J, Raes D, Traore F, Denis A, Djaby B (2013) Performance assessment of the FAO AquaCrop model for irrigated cabbage on farmer plots in a semi-arid environment. Agric Water Manag 127:40–47. https://doi.org/10.1016/j.agwat.2013.05.012
Wilson S, Johnson MP, Ruban AV (2021) Proton motive force in plant photosynthesis dominated by ΔpH in both low and high light. Plant Physiol 187:263–275. https://doi.org/10.1093/plphys/kiab270
Winter K, Smith JAC (2022) CAM photosynthesis: the acid. New Phytol 233:599–609. https://doi.org/10.1111/nph.17790
Wu A (2023) Modelling plants across scales of biological organization for guiding crop improvement. Funct Plant Biol. https://doi.org/10.1071/FP23010
Wu A, Song Y, van Oosterom EJ, Hammer GL (2016) Connecting biochemical photosynthesis models with crop models to support crop improvement. Front Plant Sci 7:1518. https://doi.org/10.3389/fpls.2016.01518
Wu A, Doherty A, Farquhar GD, Hammer GL (2018) Simulating daily field crop canopy photosynthesis: an integrated software package. Funct Plant Bio 45:362–377. https://doi.org/10.1071/FP17225
Wu A, Hammer GL, Doherty A, von Caemmerer S, Farquhar GD (2019) Quantifying impacts of enhancing photosynthesis on crop yield. Nat Plants 5:380–388. https://doi.org/10.1038/s41477-019-0398-8
Wu A, Brider J, Busch FA, Chen M, Chenu K, Clarke VC, Collins B, Ermakova M, Evans JR, Farquhar GD, Forster B, Furbank RT, Groszmann M, Hernandez-Prieto MA, Long BM, Mclean G, Potgieter A, Price GD, Sharwood RE, Stower M, van Oosterom E, von Caemmerer S, Whitney SM, Hammer GL (2023) A cross-scale analysis to understand and quantify the effects of photosynthetic enhancement on crop growth and yield across environments. Plant Cell Environ 46:23–44. https://doi.org/10.1111/pce.14453
Xia Q, Tang H, Fu L, Tan J, Govindjee G, Guo Y (2023) Determination of Fv/Fm from chlorophyll a fluorescence without dark adaptation by an LSSVM model. Plant Phenomics. https://doi.org/10.34133/plantphenomics.0034
Xiao Y, Chang T, Song Q, Wang S, Tholen D, Wang Y, Xin C, Zheng G, Zhao H, Zhu XG (2017) ePlant for quantitative and predictive plant science research in the big data era—lay the foundation for the future model guided crop breeding, engineering and agronomy. Quant Biol 5:260–271. https://doi.org/10.1007/s40484-017-0110-9
Xin CP, Tholen D, Devloo V, Zhu XG (2015) The benefits of photorespiratory bypasses: how can they work? Plant Physiol 167:574–585. https://doi.org/10.1104/pp.114.248013
Yamori W, Kondo E, Sugiura D, Terashima I, Suzuki Y, Makino A (2016) Enhanced leaf photosynthesis as a target to increase grain yield: insights from transgenic rice lines with variable Rieske FeS protein content in the cytochrome b6 /f complex. Plant Cell Environ 39:80–87. https://doi.org/10.1111/pce.12594
Yang H, Grassini P, Cassman KG, Aiken RM, Coyne PI (2017) Improvements to the hybrid-maize model for simulating maize yields in harsh rained environments. Field Crops Res 204:180–190. https://doi.org/10.1016/j.fcr.2017.01.019
Ye ZP (2007) A new model for relationship between irradiance and the rate of photosynthesis in Oryza sativa. Photosynthetica 45:637–640. https://doi.org/10.1007/s11099-007-0110-5
Ye ZP, Robakowski P, Suggett DJ (2013a) A mechanistic model for the light response of photosynthetic electron transport rate based on light harvesting properties of photosynthetic pigment molecules. Planta 237:837–847. https://doi.org/10.1007/s00425-012-1790-z
Ye ZP, Suggett DJ, Robakowski P, Kang HJ (2013b) A mechanistic model for the photosynthesis–light response based on the photosynthetic electron transport of photosystem II in C3 and C4 species. New Phytol 199:110–120. https://doi.org/10.1111/nph.12242
Ye M, Peng SB, Li Y (2019) Intraspecific variation in photosynthetic nitrogen-use efficiency is positively related to photosynthetic rate in rice (Oryza sativa L.) plants. Photosynthetica 57:311–319. https://doi.org/10.32615/ps.2019.011
Ye ZP, Kang H-J, An T, Duan H-L, Wang F-B, Yang X-L, Zhou S-X (2020) Modeling light response of electron transport rate and its allocation for ribulose biphosphate carboxylation and oxygenation. Front Plant Sci 11:581851. https://doi.org/10.3389/fpls.2020.581851
Ye Z-P, An T, Govindjee G, Robakowski P, Stirbet A, Yang X-L, Hao X-Y, Kang H-J, Wang F-B (2024) Addressing the long- standing limitations of double exponential and non-rectangular hyperbolic models in quantifying light-response of electron transport rates in different photosynthetic organisms under various conditions. Front. Plant Sci. 15:1332875. https://doi.org/10.3389/fpls.2024.1332875
Yin X, Struik PC (2009) C3 and C4 photosynthesis models: an overview from the perspective of crop modelling. NJAS-Wagen J Life Sci 57:27–38. https://doi.org/10.1016/j.njas.2009.07.001
Yin X, Struik PC (2015) Constraints to the potential efficiency of converting solar radiation into phytoenergy in annual crops: from leaf biochemistry to canopy physiology and crop ecology. J Exp Bot 66:6535–6549. https://doi.org/10.1093/jxb/erv371
Yin X, Struik PC (2017) Simple generalisation of a mesophyll resistance model for various intracellular arrangements of chloroplasts and mitochondria in C3 leaves. Photosynth Res 132:211–220. https://doi.org/10.1007/s11120-017-0340-8
Yin X, van Oijen M, Schapendonk AHCM (2004) Extension of a biochemical model for the generalized stoichiometry of electron transport limited C3 photosynthesis. Plant Cell Environ 27:1211–1222. https://doi.org/10.1111/j.1365-3040.2004.01224.x
Yin X, Struik PC, Romero P, Harbinson J, Evers JB, van der Putten PE, Vos J (2009) Using combined measurements of gas exchange and chlorophyll fluorescence to estimate parameters of a biochemical photosynthesis model: a critical appraisal and a new integrated approach applied to leaves in a wheat (Triticum aestivum) canopy. Plant Cell Environ 32:448–464. https://doi.org/10.1111/j.1365-3040.2009.01934.x
Yin X, Busch FA, Struik PC, Sharkey TD (2021) Evolution of a biochemical model of steady-state photosynthesis. Plant Cell Environ 44:2811–2837. https://doi.org/10.1111/pce.14070
Yin X, Gu JF, Dingkuhn M, Struik PC (2022) A model-guided holistic review of exploiting natural variation of photosynthesis traits in crop improvement. J Exp Bot 73:3173–3188. https://doi.org/10.1093/jxb/erac109
Yoon D-K, Ishiyama K, Suganami M, Tazoe Y, Watanabe M, Imaruoka S, Ogura M, Ishida H, Suzuki Y, Obara M, Mae T, Makino A (2020) Transgenic rice overproducing Rubisco exhibits increased yields with improved nitrogen use efficiency in an experimental paddy field. Nat Food 1:134–139. https://doi.org/10.1038/s43016-020-0033-x
Zaks J, Amarnath K, Kramer DM, Niyogi KK, Fleming GR (2012) A kinetic model of rapidly reversible nonphotochemical quenching. Proc Natl Acad Sci USA 109:15757–15762. https://doi.org/10.1073/pnas.1211017109
Zaks J, Amarnath K, Sylak-Glassman EJ, Fleming GR (2013) Models and measurements of energy dependent quenching. Photosynth Res 116:389–409. https://doi.org/10.1007/s11120-013-9857-7
Zhang Y, Xiao X, Jin C, Dong J, Zhou S, Wagle P (2016) Consistency between sun-induced chlorophyll fluorescence and gross primary production of vegetation in North America. Remote Sens Environ 83:154–169. https://doi.org/10.1016/j.rse.2016.05.015
Zhang L, Zhang Z, Luo Y, Cao J, Tao F (2019) Combining optical, fluorescence, thermal satellite, and environmental data to predict county-level maize yield in China using machine learning approaches. Remote Sens 12:21. https://doi.org/10.3390/rs12010021
Zhou WM, Liu YJ, Ata-UI-Karim ST, Ge QS, Li X, Xiao J (2022) Integrating climate and satellite remote sensing data for predicting country-level wheat yield in China using machine learning methods. Int J Appl Earth Obs Geoinf 111:102861. https://doi.org/10.1016/j.jag.2022.102861
Zhu X-G, Long SP (2009) Can increase in Rubisco specificity increase carbon gain by whole canopy? A modeling analysis. In: Laisk A, Nedbal L, Govidjee G (eds) Photosynthesis in silico: understanding complexity from molecules to ecosystems, advances in photosynthesis and respiration, vol 29. Springer, Dordrecht, pp 401–416. https://doi.org/10.1007/978-1-4020-9237-4_17
Zhu X-G, Ort DR, Whitmarsh J, Long SP (2004a) The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. J Exp Bot 55:1167–1175. https://doi.org/10.1093/jxb/erh141
Zhu X-G, Portis ARJR, Long SP (2004b) Would transformation of C3 crop plants with foreign Rubisco increase productivity? A computational analysis extrapolating from kinetic properties to canopy photosynthesis. Plant Cell Environ 27:155–165. https://doi.org/10.1046/j.1365-3040.2004.01142.x
Zhu X-G, Govindjee G, Baker NR, deSturler E, Ort DR, Long SP (2005) Chlorophyll a fluorescence induction kinetics in leaves predicted from a model describing each discrete step of excitation energy and electron transfer associated with photosystem II. Planta 223:114–133. https://doi.org/10.1007/s00425-005-0064-4
Zhu X-G, de Sturler E, Long SP (2007) Optimizing the distribution of resources between enzymes of carbon metabolism can dramatically increase photosynthetic rate: a numerical simulation using an evolutionary algorithm. Plant Physiol 145:513–526. https://doi.org/10.1104/pp.107.103713
Zhu X-G, Long SP, Ort DR (2008) What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr Opin Biotechnol 19:153–159. https://doi.org/10.1016/j.copbio.2008.02.004
Zhu X-G, Long SP, Ort DR (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61:235–261. https://doi.org/10.1146/annurev-arplant-042809-112206
Zhu X-G, Song Q, Ort DR (2012) Elements of a dynamic systems model of canopy photosynthesis. Curr Opin Plant Biol 15:237–244. https://doi.org/10.1016/j.pbi.2012.01.010
Zhu X-G, Wang Y, Ort DR, Long SP (2013) e-Photosynthesis: a comprehensive dynamic mechanistic model of C3 photosynthesis: from light capture to sucrose synthesis. Plant Cell Environ 36:1711–1727. https://doi.org/10.1111/pce.12025
Zhu J, Zeng W, Ma T, Lei G, Zha Y, Fang Y, Wu J, Huang J (2018) Testing and improving the WOFOST model for sunflower simulation on saline soils of inner Mongolia, China. Agronomy 8:172. https://doi.org/10.3390/agronomy8090172
Zhu X-G, Hasanuzzaman M, Jajoo A, Lawson T, Lin R, Liu CM, Liu LN, Liu Z, Lu C, Moustakas M, Roach T, Song Q, Yin X, Zhang W (2022) Improving photosynthesis through multidisciplinary efforts: The next frontier of photosynthesis research. Front Plant Sci 13:967203. https://doi.org/10.3389/fpls.2022.967203
Zhuo W, Fang SB, Wu D, Wang L, Li MQ, Zhang JS, Gao XR (2022) Integrating remotely sensed water stress factor with a crop growth model for winter wheat yield estimation in the North China Plain during 2008–2018. Crop J 10:1470–1482. https://doi.org/10.1016/j.cj.2022.04.004
Acknowledgements
GG thanks Jeff Haas; Karl Schlief; and Tom Uebele, of the Life Sciences Office of Information Technology at the University of Illinois at Urbana- Champaign for computer-related help. YG was supported by the Natural Science Foundation of China (No: 51961125102, No: 31771680), Jiangsu Agricultural Science and Technology Innovation Fund (SCX(22)3669). DL was supported by European Regional Development Fund project ‘Plants as a tool for sustainable global development’ (CZ.02.1.01/0.0/0.0/16_019/0000827) and by European Innovation Council, HORIZON-EIC-2021-PATHFINDER OPEN project DREAM (Grant Agreement No. 101046451).
Author information
Authors and Affiliations
Contributions
All the authors (AS, YG, DL and GG) contributed to the writing and the editing of this manuscript; figures were prepared by AS.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
This is a review dedicated to the memory of Kenneth (Ken) Sauer (1931–2022), a friend of one of us (Govindjee).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Stirbet, A., Guo, Y., Lazár, D. et al. From leaf to multiscale models of photosynthesis: applications and challenges for crop improvement. Photosynth Res 161, 21–49 (2024). https://doi.org/10.1007/s11120-024-01083-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-024-01083-9