Abstract
The core light-harvesting complexes (LH1) in bacteriochlorophyll (BChl) b-containing purple phototrophic bacteria are characterized by a near-infrared absorption maximum around 1010 nm. The determinative cause for this ultra-redshift remains unclear. Here, we present results of circular dichroism (CD) and resonance Raman measurements on the purified LH1 complexes in a reaction center-associated form from a mesophilic and a thermophilic Blastochloris species. Both the LH1 complexes displayed purely positive CD signals for their Qy transitions, in contrast to those of BChl a-containing LH1 complexes. This may reflect differences in the conjugation system of the bacteriochlorin between BChl b and BChl a and/or the differences in the pigment organization between the BChl b- and BChl a-containing LH1 complexes. Resonance Raman spectroscopy revealed remarkably large redshifts of the Raman bands for the BChl b C3-acetyl group, indicating unusually strong hydrogen bonds formed with LH1 polypeptides, results that were verified by a published structure. A linear correlation was found between the redshift of the Raman band for the BChl C3-acetyl group and the change in LH1-Qy transition for all native BChl a- and BChl b-containing LH1 complexes examined. The strong hydrogen bonding and π–π interactions between BChl b and nearby aromatic residues in the LH1 polypeptides, along with the CD results, provide crucial insights into the spectral and structural origins for the ultra-redshift of the long-wavelength absorption maximum of BChl b-containing phototrophs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Anoxygenic phototrophic purple bacteria synthesize bacteriochlorophylls (BChl) a or b as the major photosynthetic pigment. Of the 150 or more species of purple bacteria known (Imhoff et al. 2018), the majority produce BChl a and display a phylogenetically broad species diversity (Imhoff 1995). By contrast, only 8 species of purple bacteria synthesize BChl b in their light-harvesting 1 (LH1) and reaction center (RC) complexes; 4 of these species are purple nonsulfur bacteria and 4 are purple sulfur bacteria. BChl b-containing purple nonsulfur bacteria are characterized by limited morphological and phylogenetic diversities and form a tight phylogenetic cluster in the genus Blastochloris, distinct from all other purple bacteria (Hoogewerf et al. 2003; Imhoff et al. 2018). BChl b-containing phototrophs are well known for their unique spectral feature of absorbing far-infrared light in regions near to and beyond 1000 nm for the RC and LH1 complexes, respectively. These are the lowest energetic wavelengths absorbed by any known photosynthetic organism (Scheer et al. 1974). Blastochloris is also renowned for the high-resolution 3D structure of the RC from Blastochloris (Blc.) viridis, which was the first membrane protein complex revealed by X-ray crystallography (Deisenhofer et al. 1985).
In our recent study on a BChl b-containing LH1-RC complex from the mildly thermophilic phototroph Blc. tepida (Madigan et al. 2019), we have demonstrated that the LH1-RC from this bacterium is thermally more stable than its mesophilic counterpart from Blc. viridis. The mechanism of thermostability in the Blc. tepida LH1-RC complex was found to differ from that of thermostable BChl a-containing LH1-RC complexes by incorporating carotenoids that have longer C = C conjugated chains into the LH1 complex (Seto et al. 2020). During this study, we also observed that the BChl b-containing LH1 complexes from both Blc. tepida and Blc. viridis displayed circular dichroism (CD) spectra distinct from those of the BChl a-containing LH1 complexes and exhibited signatures in resonance Raman spectra that indicated a strong hydrogen bonding for the C3-acetyl group of BChl b. Since CD and resonance Raman spectra are highly sensitive to subtle changes in the conformation and the binding environment of the BChl molecules in the LH1 complex, our findings could provide new insights into the pigment arrangement and spectral origin of the ultra-redshift of the BChl b-containing LH1 complexes. Here we investigate this possibility with CD and resonance Raman results on the LH1-RC complexes from both a mesophilic and a thermophilic Blastochloris species and discuss them in the context of structural data available on these complexes and those from BChl a-containing purple bacteria.
Materials and methods
Growth conditions and preparation of LH1-RC complexes
Blc. tepida strain GIT was grown at 43 °C in a mineral salts medium containing malate as sole carbon source supplemented with thiosulfate and yeast extract (Resnick and Madigan 1989). Blc. viridis strain DSM133T was cultured at 26 °C in the same medium. Both bacteria were grown anaerobically under continuously incandescent illumination with 60 W tungsten bulbs for 7 days (Seto et al. 2020). Chromatophores from both bacteria were prepared by sonication (UD-200, 20 kHz, TOMY) of whole cells suspended in 50 mM Tris–HCl buffer (pH 8.5) followed by differential centrifugation. Preparations of the LH1-RCs from Blc. tepida and Blc. viridis employed the same method as described elsewhere (Seto et al. 2020) and the absorption spectra of purified LH1-RC complexes are given in Fig. 1a. The LH1-RC complexes from Allochromatium (Alc.) vinosum, Thermochromatium (Tch.) tepidum and Thiorhodovibrio (Trv.) strain 970 were purified as previously described (Kimura et al. 2012; Imanishi et al. 2019). In addition, BChl a and BChl b were extracted in organic solvents according to the procedure described previously (Imanishi et al. 2019).
a Absorption spectra of purified LH1-RC complexes from Blc. tepida (solid curve) and Blc. viridis (dotted curve) in 20 mM Tris–HCl containing 0.05% DDM at room temperature. The inset shows enlarged portion of Qy transitions. b Near-infrared CD spectra of purified LH1-RC complexes from Blc. tepida (solid curve) and Blc. viridis (dotted curve) in the same buffer at room temperature. The values in parentheses show the peak positions for the Blc. viridis LH1-RC. c Deconvoluted CD spectra in the region of 900–1100 nm for Blc. tepida LH1-RC. d Deconvoluted CD spectra in the region of 900–1100 nm for Blc. viridis LH1-RC
Spectroscopic measurements
Absorption spectra were recorded on a Jasco V-730Bio spectrophotometer. CD spectra were recorded in 2 mm cuvettes on a Jasco J-1500 spectropolarimeter with an InGaAs detector for the range of 800–1200 nm. Conditions for the CD measurements were 100 nm/min scan speed, 16 nm bandwidth, 4 s response time and 4 repeated scans between 800 and 1200 nm. The CD spectra were normalized with respect to the absorption bands of LH1-Qy transition (Fig. 1a). The CD spectra in the region of 900–1100 nm were deconvoluted using the procedure described previously (Kimura et al. 2015). UV and visible Raman spectra were recorded on a Jasco NRS-7100 Raman microscope. The LH1-RC dissolved in 20 mM Tris–HCl (pH 8) containing 0.1% n-dodecyl β-D-maltopyranoside (DDM) at OD = 200 (1006 nm for Blc. tepida and 1010 nm for Blc. viridis) or isolated BChls dissolved in diethyl ether were excited at 355 nm by a laser (~ 0.4 mW) and the back-scatterings from the sample solutions were collected at 25 °C (Seto et al. 2020). Each spectrum was accumulated for 10–60 s to alleviate degradation of the sample and 5–30 spectra were averaged to improve the S/N ratio.
Results
CD spectroscopy
Figure 1 shows absorption spectra in the range of 250–1100 nm and CD spectra in the near-infrared region of purified LH1-RC complexes from Blc. tepida and Blc. viridis. The most remarkable feature is that the LH1 complexes from both species displayed purely positive CD signals centered at 1000 nm for Blc. tepida and 1002 nm for Blc. viridis (Fig. 1b). To our knowledge, this is the first observation of complete CD signals with a positive sign in the near-infrared region (Qy transition) for bacterial LH complexes. The positive CD signature is in sharp contrast to those observed for BChl a-containing LH1 complexes; all such complexes reveal negative or nonconservative (larger negative amplitudes) CD signals (Sauer and Austin 1978; Hayashi et al. 1981, 1982; Bolt et al. 1981; Cogdell and Scheer 1985; Georgakopoulou et al. 2006a; Suzuki et al. 2007; Imanishi et al. 2019). These differences indicate that the BChl b-containing LH1 complexes likely have a different BChl arrangement than that in complexes containing BChl a. The LH1 CD signals (Fig. 1b) partially overlapped with the peaks at 958 nm for Blc. tepida and 962 nm for Blc. viridis and presumably arise from the Qy transition of the RC special pairs. Deconvolution of the CD spectra in the region of 900–1100 nm yielded two major components centered at 959 nm and 1001 nm for the Blc. tepida LH1-RC (Fig. 1c), and 954 nm and 1003 nm for the Blc. viridis LH1-RC (Fig. 1d). The CD spectrum of a RC-only complex from Blc. viridis was reported to show a broad positive peak at 955 nm for the special pair at room temperature in the reduced state (Philipson and Sauer 1973), and the peak position and shape are consistent with our work (Fig. 1d). The deconvoluted peak intensities of the LH1 complexes from both Blc. tepida and Blc. viridis were approximately double those of the RC special pairs. Because the positive CD signal from the RC special pair disappeared completely upon oxidation (Philipson and Sauer 1973), our result indicates that both Blc. tepida and Blc. viridis LH1-RCs were purified in a reduced form. Notably, the positive CD component at 1001 nm for the Blc. tepida LH1 exhibited a much narrower band width (full width at half maximum, FWHM = 260 cm−1) than that of Blc. viridis LH1 (FWHM = 440 cm−1), implying a greater homogeneity of the site energy for the Blc. tepida LH1.
The CD signals of both Blc. tepida and Blc. viridis LH1-RCs between 800 and 900 nm were nearly identical (Fig. 1b) and revealed a rather conservative shape with two components at 826 ( +) and 853 (−) nm; these can be assigned to the exciton splitting of the Qy transition of accessory BChl b molecules in the RC (Friesner and Won 1989). The CD spectra below 800 nm for both Blc. tepida and Blc. viridis LH1-RCs were previously published (Seto et al. 2020).
Resonance Raman spectroscopy
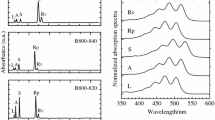
Figures 2a and b show 355-nm-excited resonance Raman spectra of the LH1-RC complexes dissolved in detergent-containing solutions from Alc. vinosum, Tch. tepidum, Trv. strain 970, Blc. viridis and Blc. tepida along with those of extracted BChl a and BChl b dissolved in diethyl ether. For the BChl monomers, the most intensive bands at 1605–1603 cm−1 are assigned to CC stretching modes of methine bridges of bacteriochlorin rings in which the central Mg2+ is penta-coordinated and believed to be a marker band for the bacteriochlorin ring core size (Fujiwara and Tasumi 1986). These bands appeared at similar positions when BChl monomers are incorporated into the LH1-RC complexes (Fig. 2). In addition, no significant differences were observed in band positions among all of the species examined, indicating that the ring core sizes of BChls were virtually identical between the BChl a- and BChl b-containing LH1-RC complexes.
Resonance Raman spectra obtained with 355-nm excitation. a Isolated BChl a (black and gray), BChl a-containing LH1-RCs from Alc. vinosum (cyan), Tch. tepidum (orange) and Trv. strain 970 (blue). b Isolated BChl b (black and gray), BChl b-containing LH1-RCs from Blc. viridis (green) and Blc. tepida (magenta). The C3-acetyl C = O stretching frequencies are shown in bold fonts. c Plots of the ∆C3-acetyl νC = O frequencies against ∆LH1-Qy peaks between each LH1-RC complex and isolated BChl molecules. Inset: plot of the frequency peaks for the C3-acetyl C = O stretching modes of the purified LH1-RC complexes against their LH1-Qy peaks
The BChl a spectrum exhibited characteristic bands at 1684 cm−1 and 1655 cm−1, which are ascribed to the C = O stretching modes of C13-keto and C3-acetyl groups, respectively (Imanishi et al. 2019). These bands were red-shifted to 1657 cm−1 and 1637 cm−1 in the Alc. vinosum LH1-RC spectrum, to 1669 cm−1 and 1629 cm−1 in the Tch. tepidum LH1-RC spectrum, and to 1671 cm−1 and 1623 cm−1 in the Trv. strain 970 LH1-RC spectrum when BChls a were incorporated into each complex. When inserted, BChl a forms specific hydrogen bonds with conserved Trp residues in the LH1 polypeptides (Sturgis et al. 1997; Kimura et al. 2012, 2017; Imanishi et al. 2019) as identified in the 1.9 Å resolution X-ray crystallographic structure of the Tch. tepidum LH1-RC complex (Yu et al. 2018). The BChl b spectrum also exhibited characteristic bands at 1693 cm−1 and 1658 cm−1, assigned to the νC=O of C13-keto and C3-acetyl groups, respectively. These νC=O bands were red-shifted to 1665–1666 cm−1 and 1620 cm−1 in both the Blastochloris spectra. Similar behavior was also noticed in a preliminary study of Blc. viridis (Robert et al. 1988). The redshifts (38 cm−1) for the C3-acetyl νC=O bands are extraordinarily large compared with those of Alc. vinosum (18 cm−1), Tch. tepidum (26 cm−1) and Trv. strain 970 (32 cm−1), strongly indicating that the C3-acetyl C = O groups of the Blastochloris LH1-RCs form unusually strong hydrogen bonds with nearby residues of the LH1 polypeptides. Indeed, the cryo-EM structure of Blc. viridis LH1-RC complex showed that the C3-acetyl C = O groups of BChl b molecules interact with α-Trp47 and β-Trp46 of LH1 polypeptides (Qian et al. 2018). Moreover, close inspection of this structure (PDB ID: 6ET5) revealed that the C3-acetyl C = O groups of α-BChl b is not only hydrogen-bonded to α-Trp47 but also to the adjacent α-Trp48 and that both the indolyl planes face each other and display π–π interactions (Fig. 3). Intriguingly, a similar interacting pattern was observed for the Trv. strain 970 LH1-RC complex that exhibits the most red-shifted LH1-Qy absorption of any BChl a-containing purple bacterium (Imanishi et al. 2019; Tani et al. 2020).
Hydrogen-bonding network formed between BChl b C3-acetyl group and the Trp residues in Blc. viridis LH1 α-polypeptide (green) based on the published structure (PDB ID: 6ET5) (Qian et al. 2018). Distances are in ångströms. Sequence scheme shows alignment of the LH1 α-polypeptides between Blc. tepida and Blc. viridis where the hydrogen-bonding Trp residues are shown in red bold fonts and the BChl b-coordinating histidine residues are shown in magenta fonts. Symbol scheme: (*) identical, (:) conservative
To further investigate the determinative origin of the far-red LH1-Qy absorptions of Blastochloris species, the νC=O values of C3-acetyl groups of all BChl molecules were plotted against their Qy peaks (inset in Fig. 2c). As the red-shifting of the LH1-Qy peaks increased, the C3-acetyl νC=O frequencies decreased by forming stronger hydrogen bonds with adjacent amino acid residues; these were identified as α-Trp46 and β-Trp45 for Tch. tepidum (Yu et al. 2018), α-Trp47/α-Trp48 and β-Trp46 for Blc. viridis (Qian et al. 2018)(PDB ID: 6ET5) and α-Trp47/α-His48 and β-Trp45 for Trv. strain 970 (Tani et al. 2020). However, it is necessary to take into account the original positions of the LH1-Qy bands and C3-acetyl C = O stretching bands in these conclusions because BChl a and BChl b monomers showed different values in the organic solvents. Therefore, we reassessed the relationship between the LH1-Qy peak and the C3-acetyl νC=O position by plotting the ∆C3-acetyl νC = O frequencies against ∆LH1-Qy peaks between each LH1-RC complex and isolated BChl molecules (Fig. 2c). The ∆C3-acetyl νC = O frequencies were linearly correlated with the ∆LH1-Qy peaks, strongly indicating that the hydrogen-bonding between BChl C3-acetyl groups and LH1 α- and β-polypeptides directly impact the LH1-Qy absorption for both BChl a- and BChl b-containing purple bacteria.
The 1574 cm−1 band in the BChl b monomer (Fig. 2b) is assigned to the C = C stretching mode of ethylidene group at C8 position, which was slightly upshifted to 1580 cm−1 or 1578 cm−1 in the Blc. viridis or Blc. tepida LH1-RC spectra. This suggests that the C8 = C81 bond length was shortened due to interactions with a neighboring BChl and/or surrounding amino acid residues upon incorporation into the LH1 complex.
Discussion
The unique Qy absorption of BChl b-containing complexes was difficult to study for many years because most conventional spectroscopic instruments could not reach wavelengths beyond 1000 nm. For example, there was no full-range CD spectrum reported for a BChl b-containing LH1 complex although partial CD spectra up to 1010 nm were measured for the BChl b-containing LH1 complexes from Halorhodospira (Hlr. formerly Ectothiorhodospira) halochloris and Hlr. abdelmalekii (Steiner and Scheer 1985). Thus, one of the most striking features observed in our spectrally wide-range study of purified BChl b-containing LH1-RCs from Blc. tepida and Blc. viridis is that the LH1 complexes displayed purely positive CD signals for the Qy transition in contrast to those reported for BChl a-containing LH1 complexes. Typically, opposite CD signals indicate different forms of pigment aggregation with opposite chiralities (Georgakopoulou et al. 2002; Kuroha et al. 2019).
Two possible explanations can be forwarded for the CD differences observed in our work. One is that the BChl b molecule has a transition dipole slightly different from that of BChl a because BChl b has a conjugated double bond between C8 and C81. The difference in the transition dipole moments between BChl b and BChl a may be too small to yield different CD spectra for the RC special pairs because both BChl b- and BChl a-containing RCs reveal essentially the same positive CD signatures (Philipson and Sauer 1973). From structural studies (Deisenhofer et al. 1985; Allen et al. 1987; Nogi et al. 2000), the bacteriochlorin rings of the RC special pairs from both BChl a- and b-containing species all have a face-to-face configuration, a similar orientation of the transition dipole moment and His-coordination to the central Mg atom from the back of the bacteriochlorin ring (Fig. 4). This difference could be large enough to yield different CD spectra for the LH1 complexes that contain a large number of excitonically interacting BChl molecules. Since the structures for both BChl b- and BChl a-containing LH1 complexes are available, this possibility could be tested by computational chemistry but may encounter difficulties. It is known that the transition dipoles in LH1 complexes that have a larger ring size are oriented more in the plane of the ring than that in the LH2 with smaller ring size; this results in a cancellation of all contributions to the CD signals that consequently become sensitive to small variations in the orientation and position of the transition dipoles (Robert et al. 2003; Georgakopoulou et al. 2006b).
a BChl a configuration of the special pair in Tch. tepidum RC (PDB ID: 5Y5S). b BChl b configuration of the special pair in Blc. viridis RC (PDB ID: 6ET5). c A pair of BChl a molecules in the Tch. tepidum LH1 with a back-to-back configuration (PDB ID: 5Y5S). d A pair of BChl a molecules in the Tch. tepidum LH1 with a face-to-face configuration (PDB ID: 5Y5S). Black arrows represent transition dipoles of the BChl molecules
Another possibility to explain our CD results is that a different BChl arrangement exists in BChl b-containing LH1 complexes compared to BChl a-containing LH1 complexes. This would require a review on the organization of the BChl b molecules reported in the Blc. viridis LH1, which was almost the same as that in a BChl a-containing LH1 (see Extended Data Figs. 5 and 6a in (Qian et al. 2018) and (Gardiner et al. 2020)). In BChl a-LH1 complexes, all BChl a molecules are coordinated from the front side of the bacteriochlorin ring by a histidine residue and form two groups with different orientations of their transition dipole moments (Fig. 4) (Yu et al. 2018). In the back-to-back pair of BChl a (Fig. 4c), the dipole moments point toward each other, whereas in the face-to-face pair, the dipole moments point outward from each other (Fig. 4d). An isolated LH1 subunit (B820) composed of one pair of αβ polypeptides and two BChl a molecules revealed a nonconservative CD shape with a small positive peak at 776 nm and a much larger negative peak at 816 nm at room temperature (Visscher et al. 1992; Georgakopoulou et al. 2006a). A similar shape was observed at 77 K (Visschers et al. 1991). This B820 subunit shows an absorption maximum at 820 nm at room temperature and a reconstituted B820 complex was analyzed to have a face-to-face configuration (Fig. 4d) by solution NMR (Wang et al. 2002). Overall, the unique arrangement of the BChl a molecules along the large LH1 ring is thought to be responsible for its characteristic CD signals with negative or nonconservative (larger negative amplitudes) shapes for the LH1-Qy transition (Sauer and Austin 1978; Hayashi et al. 1981, 1982; Bolt et al. 1981; Cogdell and Scheer 1985; Suzuki et al. 2007; Imanishi et al. 2019). It has also been reported that carotenoids in LH1 could affect near-infrared CD spectra of the LH1 complexes (Georgakopoulou et al. 2006b, 2006a). However, the possibility that the different CD shapes observed from BChl a- and b-containing LH1 complexes are due to different carotenoid compositions can be excluded because another BChl b-containing LH1 complex largely deficient in carotenoids isolated from the halophilic purple sulfur bacterium Hlr. halochloris also exhibits a positive CD signal for the LH1-Qy transition (data not shown).
Our resonance Raman measurements on the Blc. tepida and Blc. viridis LH1 complexes revealed by far the most red-shifted Raman bands for the C3-acetyl C = O group of BChl molecules including BChls a in the BChl a-containing LH1 complexes. These data indicate that unusually strong hydrogen bonds form between the C = O group and surrounding polypeptides in the BChl b-containing LH1 complexes. This is verified by the cryo-EM structure of the Blc. viridis LH1-RC complex (Qian et al. 2018) in which the C3-acetyl C = O of LH1 α-BChl b is coordinated by two consecutive Trp residues in the LH1 α-polypeptide (Fig. 3) forming a triangular network through hydrogen bonding and aromatic π–π stacking. Collectively, these interactions significantly stabilize the conformation of BChl b-containing LH1-rings and contribute to their Qy redshift in a similar fashion to how Ca2+ stabilizes BChl a-containing LH1 complexes in purple bacteria that display LH1-Qy redshifts above 900 nm (Yu et al. 2018; Imanishi et al. 2019; Tani et al. 2020). In the BChl a-containing LH1 and LH2 complexes, linear relationships exist between the redshift of the Raman band of the BChl a C3-acetyl stretching mode and the LH absorption maximum (Sturgis and Robert 1997; Uyeda et al. 2010; Kimura et al. 2012, 2017). The Raman band redshift observed in the Trv. strain 970 LH1 that has an absorption at 960 nm (the most red-shifted Qy of all BChl a-containing purple bacteria) is the largest among all BChl a-containing LH complexes reported (Imanishi et al. 2019). Here we demonstrate that this correlation can be extended to BChl b-containing LH1 complexes as well (Fig. 2c), explaining the spectral origin of its ultra-redshift of the Qy transition. In this regard, the Trv. strain 970 LH1 can be viewed as a “transitional” structure that bridges classical BChl a-containing LH1 complexes with those containing BChl b.
All BChl b-containing phototrophs reported have a long-wavelength absorption maximum around 1010–1015 nm arising from LH1 absorbance (Hoogewerf et al. 2003), about 130 nm longer than those of BChl a-containing species. The decisive cause for this ultra-redshifted LH1 absorption has remained a long-standing unsolved issue in bacterial photosynthesis. It cannot be solely explained by intrinsic differences in the molecular structure of BChl a and BChl b because their Qy transitions differ by only ~ 20 nm in organic solvents (Canniffe and Hunter 2014). Protein–pigment interaction was listed as one of the factors affecting the LH1-Qy transitions in the structure paper of B. viridis LH1-RC (Qian et al. 2018) in which the hydrogen bonds between the C3-acetyl groups of BChls b with α-Trp47 and β-Trp46 were mentioned. However, this is thought insufficient because such a hydrogen-bonding pattern is observed in all LH1 complexes with published structures irrespective of their Qy positions. Our work has pointed out for the first time that α-Trp48 likely plays a crucial role in the ultra-redshift of the BChl b-containing LH1 complexes, at least as one of the decisive factors, by forming an additional hydrogen bond with the BChl b C3-acetyl group (Fig. 3). This further strengthens BChl b–LH1 interactions through the triangular hydrogen-bonding network and π–π stackings among the two consecutive Trp residues that have large aromatic ring and the bacteriochlorin. The enhanced strength of hydrogen bonding is evidenced by the resonance Raman measurements in our study that shows good correlation with LH1-Qy peak position (Fig. 2), and the importance of hydrogen bonding has been demonstrated in the Trv. strain 970 LH1 where a Ca2+-mediated triangular hydrogen-bonding network was also observed in which one of the Trp residues is replaced by His, which has a smaller aromatic ring (Tani et al. 2020).
Considerable efforts have been devoted to engineering BChl biosynthesis pathways in order to synthesize BChl b molecules in a BChl a-producing species (Canniffe and Hunter 2014; Tsukatani et al. 2015). Despite having observed accumulation of BChl b in the engineered cells, the membrane suspension only showed an absorption maximum at ~ 900 nm (Tsukatani et al. 2015), indicating an important role of the protein environment in modulating the absorption behavior of the assembled complexes. Our results here provide evidence that the unique feature in the sequences of LH1 polypeptides of BChl b-containing species may be responsible, at least in part, for their ultra-redshifted LH1 complexes. Experiments have shown that in vitro reconstitution of the Blc. viridis LH1 αβ-polypeptides with BChl a in detergent solutions does not yield a complex with longer Qy absorbance than those using the LH1 αβ-polypeptides from BChl a-containing species, implying that the Blc. viridis LH1 αβ-polypeptides do not have special characteristics (Parkes-Loach et al. 1994). On the other hand, reconstitution of BChl b with the LH1 αβ-polypeptides from BChl a-containing species yielded a subunit form with absorption maximum at ~ 852 nm and a complex form at 896 nm (Parkes-Loach et al. 1990), indicating that polypeptides may play a role in the ultra-redshifted Qy. A reconstituted LH1 subunit using the Blc. viridis αβγ-polypeptides and BChl b exhibited an absorption maximum at 888–895 nm and the CD spectrum with a positive peak at 851 nm and a negative peak at 898 nm (Parkes-Loach et al. 1994), a similar CD shape to that reconstituted using the Blc. viridis αβ-polypeptides and BChl a. Moreover, reconstitution of a detergent-dissociated Blc. viridis LH1 complex containing all components (polypeptides, BChl b and carotenoid) yielded ~ 25% of reassociated LH1 with an absorption maximum at 1016 nm, whereas reconstitution using isolated, purified Blc. viridis LH1 α- and β-polypeptides with BChl b did not form an LH1-like complex (Parkes-Loach et al. 1994). These indicate that intimate interactions between the pigments and polypeptides in the BChl b-containing LH1 complexes are necessary to form a fully functional structure. The factors and their relationships that govern the long-wavelength absorption maximum in BChl b-containing phototrophs could be further explored using an in vivo expression system such as that established for producing chimeric LH1-RC in BChl a-containing species (Nagashima et al. 2017).
In summary, we have observed separate CD signals with positive signs for both Qy transitions of LH1 and the RC special pair from the BChl b-containing LH1-RC complexes of two Blastochloris species. This contrasts with the BChl a-containing LH1-RC for which the CD signals from the RC special pair were typically buried in the LH1 signals because the latter are closely positioned and have opposite signs. The origin of the positive CD signals for the BChl b-containing LH1-Qy remains to be further investigated. However, based on our resonance Raman analysis thus far, we propose that the Raman band redshift of the C3-acetyl C = O group of BChl correlates with changes in the LH1-Qy transition in the LH1 complexes of all purple bacteria, regardless if the complex contains BChl a or BChl b. In addition, the unusually strong hydrogen bonding and π–π interactions between BChl b and nearby aromatic residues in the LH1 polypeptide of BChl b-containing purple bacteria may also contribute to the ultra-redshifted Qy absorption maximum of these species.
Abbreviations
- Alc :
-
Allochromatium
- BChl:
-
Bacteriochlorophyll
- Blc :
-
Blastochloris
- CD:
-
Circular dichroism
- DDM:
-
n-dodecyl β-D-maltopyranoside
- FWHM:
-
Full width at half maximum
- Hlr :
-
Halorhodospira
- LH:
-
Light-harvesting
- RC:
-
Reaction center
- Tch :
-
Thermochromatium
- Trv :
-
Thiorhodovibrio
References
Allen JP, Feher G, Yeates TO, Komiya H, Rees DC (1987) Structure of the reaction center from Rhodobacter sphaeroides R-26: The cofactors. Proc Natl Acad Sci USA 84:5730–5734
Bolt JD, Sauer K, Shiozawa JA, Drews G (1981) Linear and circular dichroism of membranes from Rhodopseudomonas capsulata. Biochim Biophys Acta 635:535–541
Canniffe DP, Hunter CN (2014) Engineered biosynthesis of bacteriochlorophyll b in Rhodobacter sphaeroides. Biochim Biophys Acta 1837:1611–1616
Cogdell RJ, Scheer H (1985) Circular dichroism of light-harvesting complexes from purple photosynthetic bacteria. Photochem Photobiol 42:669–678
Deisenhofer J, Epp O, Miki K, Huber R, Michel H (1985) Structure of the protein subunits in the photosynthetic reaction centre of Rhodopseudomonas viridis at 3Å resolution. Nature 318:618–624
Friesner RA, Won Y (1989) Spectroscopy and electron transfer dynamics of the bacterial photosynthetic reaction center. Biochim Biophys Acta 977:99–122
Fujiwara M, Tasumi M (1986) Resonance Raman and infrared studies on axial coordination to chlorophylls a and b in vitro. J Phys Chem 90:250–255
Gardiner AT, Nguyen-Phan TC, Cogdell RJ (2020) A comparative look at structural variation among RC-LH1 'Core' complexes present in anoxygenic phototrophic bacteria. Photosynth Res 145:83–96
Georgakopoulou S, Frese RN, Johnson E, Koolhaas C, Cogdell RJ, van Grondelle R, van der Zwan G (2002) Absorption and CD spectroscopy and modeling of various LH2 complexes from purple bacteria. Biophys J 82:2184–2197
Georgakopoulou S, van der Zwan G, Olsen JD, Hunter CN, Niederman RA, van Grondelle R (2006a) Investigation of the effects of different carotenoids on the absorption and CD signals of light harvesting 1 complexes. J Phys Chem B 110:3354–3361
Georgakopoulou S, van Grondelle R, van der Zwan G (2006b) Explaining the visible and near-infrared circular dichroism spectra of light-harvesting 1 complexes from purple bacteria: A model study. J Phys Chem B 110:3344–3353
Hayashi H, Nozawa T, Hatano M, Morita S (1981) Circular dichroism of bacteriochlorophyll a in light harvesting bacteriochlorophyll protein complexes from Chromatium vinosum. J Biochem 89:1853–1861
Hayashi H, Nozawa T, Hatano M, Morita S (1982) Circular dichroism of bacteriochlorophyll a in light harvesting bacteriochlorophyll-protein complexes from Rhodopseudomonas palustris. J Biochem 91:1029–1038
Hoogewerf GJ, Jung DO, Madigan MT (2003) Evidence for limited species diversity of bacteriochlorophyll b-containing purple nonsulfur anoxygenic phototrophs in freshwater habitats. FEBS Microbiol Lett 218:359–364
Imanishi M, Takenouchi M, Takaichi S, Nakagawa S, Saga Y, Takenaka S, Madigan MT, Overmann J, Wang-Otomo Z-Y, Kimura Y (2019) A dual role for Ca2+ in expanding the spectral diversity and stability of light-harvesting 1 reaction center photocomplexes of purple phototrophic bacteria. Biochemistry 58:2844–2852
Imhoff JF (1995) Taxonomy and physiology of phototrophic purple bacteria and green sulfur bacteria. In: Blankenship RE, Madigan MT, Bauer CE (eds) Anoxygenic Photosynthetic Bacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 1–15
Imhoff JF, Rahn T, Künzel S, Neulinger SC (2018) Photosynthesis is widely distributed among Proteobacteria as demonstrated by the phylogeny of PufLM reaction center proteins. Front Microbiol 8:2679
Kimura Y, Inada Y, Numata T, Arikawa T, Li Y, Zhang J-P, Wang Z-Y, Ohno T (2012) Metal cations modulate the bacteriochlorophyll-protein interaction in the light-harvesting 1 core complex from Thermochromatium tepidum. Biochim Biophys Acta 1817:1022–1029
Kimura Y, Kawakami T, Yu L-J, Yoshimura Y, Kobayashi M, Wang-Otomo Z-Y (2015) Characterization of the quinones in purple sulfur bacterium Thermochromatium tepidum. FEBS Lett 589:1761–1765
Kimura Y, Lyu S, Okoshi A, Okazaki K, Nakamura N, Ohashi A, Ohno T, Takaichi S, Madigan MT, Wang-Otomo Z-Y (2017) Effects of calcium ions on the thermostability and spectroscopic properties of the LH1-RC complex from a new thermophilic purple bacterium Allochromatium tepidum. J Phys Chem B 121:5025–5032
Kuroha M, Nambu S, Hattori S, Kitagawa Y, Niimura K, Mizuno Y, Hamba F, Ishii K (2019) Chiral supramolecular nanoarchitectures from macroscopic mechanical rotations: effects on enantioselective aggregation behavior of phthalocyanines. Angew Chem Int Ed 58:18454–18459
Madigan MT, Resnick SM, Kempher ML, Dolnalkova AC, Takaichi S, Wang-Otomo Z-Y, Toyota A, Kurokawa K, Mori H, Tsukatani Y (2019) Blastochloris tepida, sp. nov., a thermophilic species of the bacteriochlorophyll b-containing genus Blastochloris. Arch Microbiol 201:1351–1359
Nagashima KVP, Sasaki M, Hashimoto K, Takaichi S, Nagashima S, Yu L-J, Abe Y, Gotou K, Kawakami T, Takenouchi T, Shibuya Y, Yamaguchi A, Ohno T, Shen J-R, Inoue K, Madigan MT, Kimura Y, Wang-Otomo Z-Y (2017) Probing structure–function relationships in early events in photosynthesis using a chimeric photocomplex. Proc Natl Acad Sci USA 114:10906–10911
Nogi T, Fathir I, Kobayashi M, Nozawa T, Miki K (2000) Crystal structures of photosynthetic reaction center and high-potential iron-sulfur protein from Thermochromatium tepidum: Thermostability and electron transfer. Proc Natl Acad Sci USA 97:13561–13566
Parkes-Loach PS, Michalski TJ, Bass WJ, Smith U, Loach PA (1990) Probing the bacteriochlorophyll binding site by reconsititution of the light-harvesting complex of Rhodospirillum rubrum with bacteriochlorophyll a analogues. Biochemistry 29:2951–2960
Parkes-Loach PS, Jones SM, Loach PA (1994) Probing the structure of the core light-harvesting complex (LH1) of Rhodopseudomonas viridis by dissociation and reconstitution methodology. Photosynth Res 40:247–261
Philipson KD, Sauer K (1973) Comparative study of the circular dichroism spectra of reaction centers from several photosynthetic bacteria. Biochemistry 12:535–539
Qian P, Siebert CA, Wang P, Canniffe DP, Hunter CN (2018) Cryo-EM structure of the Blastochloris viridis LH1-RC complex at 2.9 Å. Nature 556:203–208
Resnick SM, Madigan MT (1989) Isolation and characterization of a mildly thermophilic nonsulfur purple bacterium containing bacteriochlorophyll b. FEMS Microbiol Lett 65:165–170
Robert B, Vermeglio A, Steiner R, Scheer H, Lutz M (1988) BChl a/b in antenna complexes of purple bacteria. In: Scheer H, Schneider S (eds) Photosynthetic Light-Harvesting Systems. Walter de Gruyter & Co., Berlin, New York, pp 355–363
Robert B, Cogdell RJ, van Grondelle R (2003) The Light-harvesting system of purple bacteria. In: Green BR, Parson WW (eds) Light-Harvesting Antennas in Photosynthesis. Kluwer Academic Publishers, Dordrecht, pp 169–194
Sauer K, Austin LA (1978) Bacteriochlorophyll-protein complexes from the light-harvesting antenna of photosynthetic bacteria. Biochemistry 17:2011–2019
Scheer H, Svec WA, Cope BT, Studier MH, Scott RG, Katz JJ (1974) Structure of bacteriochlorophyll b. J Am Chem Soc 96:3714–3716
Seto R, Takaichi S, Kurihara T, Kishi R, Honda M, Takenaka S, Tsukatani Y, Madigan MT, Wang-Otomo Z-Y, Kimura Y (2020) Lycopene-family carotenoids confer thermostability on photocomplexes from a new thermophilic bacterium. Biochemistry 59:2351–2358
Steiner R, Scheer H (1985) Characterization of a B800/1020 antenna from the photosynthetic bacteria Ectothiorhodospira halochloris and Ectothiorhodospira abdelmalekii. Biochim Biophys Acta 807:278–284
Sturgis JN, Robert B (1997) Pigment binding-site and electronic properties in light-harvesting proteins of purple bacteria. J Phys Chem 101:7227–7231
Sturgis JN, Olsen JD, Robert B, Hunter CN (1997) Functions of conserved tryptophan residues of the core light-harvesting complex of Rhodobacter sphaeroides. Biochemistry 36:2772–2778
Suzuki H, Hirano Y, Kimura Y, Takaichi S, Kobayashi M, Miki K, Wang Z-Y (2007) Purification, characterization and crystallization of the core complex from thermophilic purple sulfur bacterium Thermochromatium tepidum. Biochim Biophys Acta—Bioenergetics 1767:1057–1063
Tani K, Kanno R, Makino Y, Hall M, Takenouchi M, Imanishi M, Yu L-J, Overmann J, Madigan MT, Kimura Y, Mizoguchi A, Humbel BM, Wang-Otomo Z-Y (2020) Cryo-EM structure of a Ca2+-bound photosynthetic LH1-RC complex containing multiple αβ-polypeptides. Nat Commun 11:4955
Tsukatani Y, Harada J, Nomata J, Yamamoto H, Fujita Y, Mizoguchi T, Tamiaki H (2015) Rhodobacter sphaeroides mutants overexpressing chlorophyll a oxidoreductase of Blastochloris viridis elucidate functions of enzymes in late bacteriochlorophyll biosynthetic pathways. Sci Rep 5:9741
Uyeda U, Williams JC, Roman M, Mattioli TA, Allen JP (2010) The influence of hydrogen bonds on the electronic structure of light-harvesting complexes from photosynthetic bacteria. Biochemistry 49:1146–1159
Visscher RW, Nunn R, Calkoen F, van Mourik F, Hunter CN, Rice DW, van Grondelle R (1992) Spectroscopic characterization of B820 subunits from light-harvesting complex I of Rhodospirillum rubrum and Rhodobacter sphaeroides prepared with the detergent n-octyl-rac-2,3-dipropylsulfoxide. Biochim Biophys Acta 1100:259–266
Visschers RW, Chang MC, van Mourik F, Parkes-Loach PS, Heller BA, Loach PA, van Grondelle R (1991) Fluorescence polarization and low-temperature absorption spectroscopy of a subunit form of light-harvesting complex I from purple photosynthetic bacteria. Biochemistry 30:5734–5742
Wang Z-Y, Muraoka Y, Shimonaga M, Kobayashi M, Nozawa T (2002) Selective detection and assignment of the solution NMR signals of bacteriochlorophyll a in a reconstituted subunit of a light-harvesting complex. J Am Chem Soc 124:1072–1078
Yu L-J, Suga M, Wang-Otomo Z-Y, Shen J-R (2018) Structure of photosynthetic LH1-RC supercomplex at 1.9 Å resolution. Nature 556:209–213
Acknowledgements
We thank Ms. Madoka Yamashita of Kindai University for her experimental assistance, JASCO Corporation (Japan) for measurements of CD spectra, Ms. Satoko Suzuki, Mr. Yoshiro Kondo and Ms. Miwako Kobayashi of JASCO Corporation for assistance in CD measurements, Kao Corporation for providing detergent LDAO. This work was supported in part by Grants-in-aid for Scientific Research (C) (19K06563) to Y.K., JSPS KAKENHI Grant Numbers JP16H04174, JP18H05153, JP20H05086 and JP20H02856, Takeda Science Foundation, the Kurata Memorial Hitachi Science and Technology Foundation, Japan to Z.-Y.W.-O. and the National Key R&D Program of China (No. 2019YFA0904600) to L.-J. Y.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding authors state that there is no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kimura, Y., Yamashita, T., Seto, R. et al. Circular dichroism and resonance Raman spectroscopies of bacteriochlorophyll b-containing LH1-RC complexes. Photosynth Res 148, 77–86 (2021). https://doi.org/10.1007/s11120-021-00831-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-021-00831-5