Abstract
The antioxidant balance, photochemical activity of photosystem II (PSII), and photosynthetic pigment content, as well as the expression of genes involved in the light signalling of callus lines of Eutrema salsugineum plants (earlier Thellungiella salsuginea) under different spectral light compositions were studied. Growth of callus in red light (RL, maximum 660 nm), in contrast to blue light (BL, maximum 450 nm), resulted in a lower H2O2 content and thiobarbituric acid reactive substances (TBARS). The BL increased the activities of key antioxidant enzymes in comparison with the white light (WL) and RL and demonstrated the minimum level of PSII photochemical activity. The activities of catalase (CAT) and peroxidase (POD) had the highest values in BL, which, along with the increased H2O2 and TBARS content, indicate a higher level of oxidative stress in the cells. The expression levels of the main chloroplast protein genes of PSII (PSBA and PSBD), the NADPH-dependent oxidase gene of the plasma membrane (RbohD), the protochlorophyllide oxidoreductase genes (POR B, C) involved in the biosynthesis of chlorophyll, and the key photoreceptor signalling genes (CIB1, CRY2, PhyB, PhyA, and PIF3) were determined. Possible mechanisms of light quality effects on the physiological parameters of callus cells are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light is an important environmental factor necessary for the growth and development of plants. The change in the spectral composition of light affects not only photosynthesis but also other processes associated with it, such as the biosynthesis of proteins and nucleic acids, formation of secondary metabolites, and antioxidant status of cells (Kendrick and Kronenberg 2012). Recent studies have shown the effect of light quality on the content of pigments, antioxidants, phenolic compounds, and other secondary metabolites. Thus, red light (RL, 640–660 nm) caused an increase in the content of anthocyanins and chlorophylls in Brassica oleracea plants (Lefsrud et al. 2008), as well as increased the content of phenolic compounds (Li and Kubota 2009), tocopherol (Samuoliene et al. 2012), and antioxidants in leaves of Lactuca sativa (Samuoliene et al. 2011). The RL had a similar stimulating effect on the antioxidant content in Sinapis alba, Spinacia oleracea, Eruca sativa, and Petroselinum sativum plants (Bliznikas et al. 2012). Like RL, the blue light (BL, 440–476 nm) caused an increase in the content of polyphenols, anthocyanins, and carotenoids (Stutte 2009; Li and Kubota 2009; Johkan et al. 2010). The combined effect of BL and RL increased the vitamin C content in Brassica rapa leaves (Massa et al. 2008). A possible consequence of the activity of the antioxidant system upon the action of BL and RL by appropriate photoreceptors may be an increase in the resistance of plants to stress (Kreslavski et al. 2013a, b; Yurina et al. 2013). In some cases, light (UV, high-intensity light, or intermittent light) can act as a stressful factor (Powles 1984; Foyer and Noctor 2005; Kosobryukhov et al. 2015) requiring a change in the pro-/antioxidative balance in plants.
There are five types of phytochromes (PhyA–PhyE) that sense the light in the RL region (620–750 nm). A special role is played by PhyA, which is activated by far-red light and PhyB, which is predominantly activated by RL from 650 to 670 nm (Bae and Choi 2008; Kreslavski et al. 2009). The effect of RL on plants is realized during seed germination, photomorphogenesis, avoidance of shade, flowering, and many other physiological processes (Bae and Choi 2008).
BL is perceived by the cryptochromes and phototropins. Cryptochromes are associated with the processes of de-etiolation, flowering and development, and stomatal development. Cryptochrome effects are based on their interaction with transcription factors and proteasome components (Kleine et al. 2007; Liu et al. 2011). Along with phytochromes, cryptochromes control the biosynthesis of anthocyanins and carotenoids and are involved in the regulation of the expression of anthocyanin biosynthesis genes.
Recent data showed that cryptochromes can participate in the generation of reactive oxygen species (ROS), including hydrogen peroxide (Consentino et al. 2015). The role of hydrogen peroxide in the transduction of BL signals with the participation of cryptochromes was considered in several articles (Wen et al. 2008; Consentino et al. 2015; Jourdan et al. 2015). In particular, it has been shown that the ROS generated by BL can act as intermediates in the transduction of the BL signal. It was also found that ROS accumulation in the nucleus can occur as a result of the reception of BL by CRY2 (Jourdan et al. 2015).
Despite the existence of a large number of studies on isolated systems (protoplasts, chloroplasts, and thylakoid membrane preparations) (Wen et al. 2008; Consentino et al. 2015; Jourdan et al. 2015) and on intact plants (Meinhard and Grill 2001; Desikan et al. 2001) that are related to ROS in light signalling (Neill et al. 2002), the mechanisms by which H2O2 transmits its signal and oxidizes its targets are not fully understood.
The question arises whether callus culture is an adequate model for studying these effects. Of particular interest is the culture of Eutrema salsugineum callus cells. This plant species is very similar to the Arabidopsis thaliana model plant but it is distinguished by its high resistance to salinity, drought, low temperatures, and UV-B irradiation. E. salsugineum plants are capable of significant activation of the antioxidant system under stress conditions (Zhao et al. 2009; Radyukina et al. 2011; Wu et al. 2012). The leaves of higher plants have a complex organization and possesses certain optical characteristics, while callus cells are homogeneous and do not have an epidermis, cuticle, or trichomes, which facilitates light penetration into tissues and simplifies the interpretation of the obtained results. This makes the callus culture of E. salsugineum a convenient model for studying the regulatory effects of light quality, regardless of its photosynthetic component, and the possible role of ROS in light signalling. In view of the photoheterotrophic growth of the callus lines cultivated on nutrient media with amino acids, vitamins, and sucrose, the trophic role of photosynthesis is obviously reduced, but this fact does not exclude the functioning of photoreceptors and the phytohormonal system.
In this work, an attempt was made to investigate the relationship between pro-/antioxidant balance and PSII activity with the level of photoreceptor gene transcription and light signalling components in callus culture of E. salsugineum exposed to different light quality.
Materials and methods
Plant materials, callus formation, and light treatments
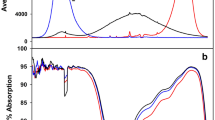
The callus culture was obtained from the leaves of 6-week-old E. salsugineum plants (earlier Thellungiella salsuginea) (Pall.) O. E. Schulz (ecotype of Shandong). Plant leaf explants were cultured on a Schenk & Hildebrandt nutrient medium (Schenk and Hildebrandt 1972) supplemented with 6-benzylaminopurine (0.2 mgl− 1), 1-naphthaleneacetic acid (0.8 mgl− 1), and sucrose (3%). Callusogenesis proceeded in the dark. The resulting calluses were grown under fluorescent lamps of 100 ± 20 μmol photons/m− 2s− 1 (ESL-S12-32/2700/E27, Uniel lighting Co. Ltd, China) at a 12-h light period and after the third passage were used for the experiment. The calluses were divided into four experimental groups: WL—illuminated with white LEDs (two-band emission spectrum with 2 maxima of 450 nm and 660 nm), RL—illuminated with red LEDs (maximum 660 nm), BL—illuminated with blue LEDs (maximum 450 nm), and WCFL—illuminated white compact fluorescent lamps (control with a full spectrum). The spectral characteristics of the light sources were determined using the USB2000 spectrometer (OceanOptics, USA) (Fig. 1). In the experiments, we used a 50-W LED matrix (Epistar, Taiwan): AlGaN blue light, AlGaN with a dusting of yellow phosphorus emitting white light, AlGaInP emitting red light, and white compact fluorescent lamps emitting white polychromatic light. The calluses were grown in Petri dishes with a 12-h light period at a light flux intensity in the range of 100 ± 15 μmol photons/m− 2s− 1 (Li-COR Li-250A, USA) and at the air temperature of 25 ± 2 °C. The duration of the light experiment was 21 days. The calluses grown under different spectral light compositions were fixed with liquid nitrogen under the same light conditions. Maternal callus cells, from which new daughter calluses were formed, were not fixed and analysed.
Microscopic study
Callus cell viability
In our study, callus cell viability was microscopically determined. Callus samples of approximately 500 mg of fresh weight were suspended with 5 ml of 0.6 M mannitol and 0.03 M CaCl2 solution and then centrifuged for 15 min at 1000 rpm and 25 °C. Then, in the dark, 900 μL of these suspensions were taken and 50 μL of a solution of fluorescein diacetate and 50 μL propidium iodide were added (Sigma, USA) in microtubes. After 5 min, we detected the viable cells showing green fluorescence and nonviable cells with red fluorescence detected by a D1 fluorescence microscope (Carl Zeiss, Germany), using filters at excitation/emission wavelengths of 460/510 nm for fluorescein diacetate and 530/615 nm for propidium iodide at a total magnification of 120×(Canon PowerShot A650 IS, Japan). Cell viability was estimated using approximately 1000 cells.
A micrograph of the callus was obtained on a stereoscopic luminescent microscope SteREO Lumar V12 (Carl Zeiss, Germany).
Determination of biomass and photosynthetic pigment content
Determination of the callus biomass was performed using the gravimetric method. The chlorophyll a, b and carotenoid contents were determined using the Lichtenthaler (1987) method. The samples were triturated with 80% acetone in shadow. The absorbance of the samples was measured with a Genesys 10 UV spectrophotometer (Thermo Fisher Scientific, USA) at the wavelengths of 470, 646, and 663 nm. The content of the photosynthetic pigments was determined using the Lichtenthaler formula (Lichtenthaler 1987).
Determining of the TBARS content
TBARS were determined by the Heath and Packer method (1968), based on the formation of a coloured complex of TBARS with thiobarbituric acid through heating and with some modifications (Radyukina et al. 2009). The concentration of TBARS was determined spectrophotometrically by measuring the optical density on a Genesys 10 UV spectrophotometer (Thermo Fisher scientific, USA) at 532 and 600 nm wavelengths.
Determination of SOD, APX, CAT, and POD activities
Determination of SOD activity was carried out by the Beauchamp and Fridovich method (1971) with some modifications (Radyukina et al. 2007) after extraction with phosphate buffer. The optical density of the solution was measured at 560 nm on a Genesys 10 UV spectrophotometer (Thermo Fisher scientific, USA).
The APX activity was determined spectrophotometrically according to the rate of destruction of ascorbic acid by the Nakano and Asada method (1981) with some modifications (Soshinkova et al. 2013). The reaction was started by the addition of hydrogen peroxide and the change in optical density was measured on a Genesys 10 UV spectrophotometer at 290 nm for 3 min.
The CAT activity was determined by the Chance and Maehly method (1955) with some modifications (Radyukina et al. 2009). The dynamics of the optical density change were recorded on a Genesys 10 UV spectrophotometer for 3 min at a wavelength of 240 nm.
The POD activity was determined by the method proposed by Ridge and Osborne (1971) with some modifications (Radyukina et al. 2007). The dynamics of the changes in optical density were recorded on a spectrophotometer for 3 min at a wavelength of 470 nm. Measurements were taken every 2 s.
Determination of protein content
The protein content in the preparations was determined using a Bicinchoninic Acid Protein Assay Kit (Sigma, USA) according to the manufacturer’s instructions. The method is based on the formation of a Cu2+–protein complex under alkaline conditions, followed by the reduction of Cu2+ to Cu1+. Bicinchoninic acid forms a purple blue complex with Cu1+ in alkaline environments, thus providing a basis to monitor the reduction of alkaline Cu2+ by proteins. The protein–BCA complex absorbance was measured at 562 nm. A known concentration of BSA protein standard was used as a standard for calibration.
Evaluation of PSII photochemical activity
To evaluate the photochemical activity of PSII, PAM fluorometry based on the JUNIOR-PAM fluorometer (Walz, Germany) was used. With the help of PAM fluorometry, the values of the maximum (Fv/Fm) and effective quantum (Fm’ − Ft)/Fm’ (Y (II)) yields were determined. Here, Fm and Fm’ are the maximum chlorophyll fluorescence levels under dark- and light-adapted conditions, respectively, and Ft is the level of chlorophyll fluorescence before a saturating flash is applied. The photoinduced changes in the fluorescence of Fv are Fm − F0, where F0 is the level of initial fluorescence and Fm is the maximum level. The light was switched for 10 min [I = 190 μmol (quantum) m− 2 s− 1 PAR]. Light PAM pulse intensity was 6000 μmol (quantum) m− 2 s− 1. The JIP-test was used to evaluate the PSII state (Kalaji et al. 2014b). JIP-test measurements were provided by an installation as described in Lankin et al. (2014). Digitized data about the fast chlorophyll a fluorescence curves were recorded and then analysed (Table 1). OJIP curves were measured under illumination with blue light with an intensity of 5000 μmol (quantum) m− 2 s− 1 for 1 s. The dark adaptation period prior to the Fo and Fm measurements, as well as prior to the OJIP records, was 15 min. The fluorescent parameters, such as ABS/RC and DI0/RC, were calculated on the basis of the induction curves. The equation was DIo/RC = ABS/RC*(1 − Fv/Fm) (total energy dissipated by RC of PSII), where ABS/RC is the average absorbed by the PSII antenna photon flux per photochemically active RC and Fv/Fm is the maximum quantum yield of the primary PSII photochemistry.
Determination of H2O2
To determine the pool of H2O2, 50–100 mg of callus was powdered in liquid nitrogen. The complete extraction of H2O2 was carried out with 3 ml of 0.05 M K-phosphate buffer (pH 8.0). For the adsorption of pheophytin and carotenoids, activated carbon (100 mg) was added to 3.4 ml of homogenate, which was centrifuged for 20 min at 10,000 g. The supernatant was decanted and titrated with 2 M KOH at pH 8.0. The H2O2 content was determined in 100 µl of extract by 1 ml of a bioluminescence assay containing peroxidase:luminol in a ratio of 5 × 10− 6 M:5 × 10− 4 M (Cormier and Prichard 1968; Kreslavski et al. 2013a).
RNA extraction and RT-PCR
RNA extraction was performed using Trizol reagent (Sigma, USA). The quantity and quality of the total RNA were determined using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, USA). The cDNA synthesis was performed using the M-MLV Reverse Transcriptase Kit (Fermentas, Canada) and the oligo (dT) 21 primer (Evrogen, Russia). Gene-specific primers were selected using nucleotide sequences from the NCBI database (National Center of Bioinformatics, USA) with the program Vector NTI Suite 9 (Invitrogen, USA). The quantification of amplicons was performed on a Typhoon™ Scanner 9410 (GE Healthcare, US) after electrophoresis of PCR products on a 1% agarose gel. The levels of apoprotein transcripts of the phytochrome (PHYA, PHYB) and cryptochrome (CRY1 and CRY2) genes, as well as the genes involved in the transmission of the light signal, including constitutive photomorphogenic (COP1), cryptochrome-interacting basic-helix-loop-helix (CIB1), and phytochrome-interacting factor (PIF3), were studied. Expression of other genes encoding the main PSII proteins (PSBA and PSBD), the Rboh family gene (RbohD), encoding NADPH-dependent oxidase (the source of H2O2 in cells), and protochlorophyllide oxidoreductase genes (PORB and PORC) participating in the biosynthesis of chlorophyll have also been studied. The Actin2 and CBP20 (Cap-Binding Protein 20) genes were used as the reference genes (Table 2).
Statistical data processing
The experiments were performed in three biological replicates and three analytical replicates. The expression level of each gene was measured in three independent experiments. For each of these experiments, at least three parallel independent measurements were performed. The arithmetic mean values of the expression level of each gene and the standard deviations were calculated in Excel 2010 (Microsoft, USA). The significance of the differences between the variants is calculated by a t test at a 5% significance level unless otherwise specified. Letters indicate significant differences (t test: p < 0.05) between the experiment variants. The mRNA levels of the genes were expressed as a percentage of the corresponding value for the WCFL plants. The data represent mean ± SD (n = 3 independent experiments).
Results
Pigment content
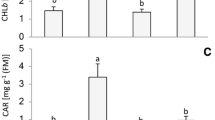
The callus cells grown under WCFL were characterized by the highest photosynthetic pigment content (Fig. 2A). In the cells grown under RL, the photosynthetic pigment content, especially the carotenoids, was significantly lower than in other variants. The calluses grown under BL conditions differed from the WL variant by reduced Chla and Chlb contents but not reduced Car content.
Photosynthetic pigment content (A), Chla/Chlb ratio (B), Car/Chl ratio (C), callus fresh weight (D), and callus cell viability (E) of E. salsugineum callus cells grown under WCFL, WL, BL, and RL. The means ± standard errors (n = 3) are presented. Letters indicate significant differences (t test: p < 0.05) between experimental variants
The Chla/Chlb ratio increased in the WCFL variant, while the RL caused a decrease in the Chla/Chlb ratio (Fig. 2B). The Chla/Chlb ratio in the WL and BL varieties had little difference but was less than that in the WCFL cells. The Car/Chl ratio was the lowest in the WL and was slightly higher in WCFL and BL, but the highest Car/Chl ratio was observed in the RL variant (Fig. 2C).
Biomass
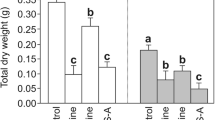
Calluses grown under WCFL, unlike the other variants (Fig. 2D), had the largest fresh weight. With all other types of light sources, the fresh weight did not differ significantly, but it was lower than that under WCFL.
Cell viability
During the experiments, the amounts of live and dead callus cells were counted under the influence of different light quality (Fig. 2E). Under the BL, the number of living cells was the least among all the studied variants. The RL caused the death of a small amount of cells compared to the BL. The highest amount of living cells was detected under WL and WCFL. Thus, BL caused the highest decrease in the number of living cells.
Photochemical activity of PSII
The maximum quantum yield of PSII was higher under WL (0.42 ± 0.03), slightly lower at RL (0.35 ± 0.04), lower under WCFL, and the lowest under BL (0.17 ± 0.05) (Table 1). The effective quantum yield (Y (II)) was sufficiently low, so it was possible to find a significant difference in this parameter only between the WL and BL variants. It follows that the photochemical activity of PSII was the highest under WL and the lowest under BL. The callus grown on RL was intermediate. In addition, the amount of dissipated energy by one RC predominantly in the form of heat (DI0/RC) was the highest in the BL samples. This means that the energy losses in the RC of PSII were the highest in cells grown in BL. The energy flux absorbed by one active RC of PSII (ABS/RC) was maximal in the BL samples (4.8 compared with 2.0 in RL cells and 2.6 in WL cells).
Hydrogen peroxide and TBARS contents
The levels of H2O2 and TBARS largely depend on the light source used (Fig. 3A, B). Thus, the H2O2 content was the lowest in RL, while on the contrary the content of H2O2 in the BL and WCFL variants was maximal. The content of H2O2 in WL was between those in the BL and RL variants. The content of TBARS in the BL cells exceeded their content in all other experimental variants. The content of TBARS in WCFL cells was the lowest. The levels of TBARS in the WL and RL variants were close to each other, but they were lower than those in the BL variant.
Levels of H2O2 (A), TBARS (B), SOD (C), CAT (D), APX (E), and POD (F) activities. Three different isoforms of POD, including soluble (dark colour), ion-bound (grey colour), and covalently bound (white colour), are shown. The means ± standard errors (n = 3) are presented. Letters indicate significant differences (t test: p < 0,05) between the experimental variants
SOD, APX, CAT, and POD activities
The SOD activity was slightly changed. Considering the error of the mean, it can be said that light does not have a significant effect on the enzyme activity (Fig. 3C).
The CAT activity was the highest in callus cells on the BL and the lowest on the RL (Fig. 3D). The CAT activity on WL and WCFL did not differ significantly.
Unlike WL and WCFL, the cultivation of cells on narrow-band RL or BL was accompanied by an increase in the APX activity. The activity of this enzyme under WCFL was the lowest (Fig. 3E).
The activities of three POD fractions (soluble, ionically bound, and covalently bound) were determined. Cell samples under BL and RL differed by the high POD activity of the soluble fraction. The activity of soluble POD in WL and WCFL was two times lower than that in RL and BL. Differences in the activity of soluble POD in cells grown under WL and WCFL were insignificant (Fig. 3F).
The effect of different light quality on the activity of the ion-bound POD had different characteristics. Its highest activity was found in cells grown under BL, which exceeded the enzyme activity under other light conditions. The activity of ionically bound POD upon exposure of calluses to RL and WL was not so high, but the lowest activity of the enzyme was detected in cells grown under WCFL.
The activity of the covalently bound POD was the highest when exposed to BL. In the RL variant, the POD activity was 30% lower than that under BL. Upon cultivation of cells under BL and WCFL, the activity of covalently bound POD was significantly lower than that under RL and the biggest difference was observed between BL and RL.
Changes in transcript level of photoreceptors and light signalling genes
The results obtained (Fig. 4A) show that the cultivation of cells under different light conditions caused a decrease in the expression of the COP1 gene, although the highest decrease in the transcript level was observed in BL (up to 40% of WCFL control). Cryptochrome-interacting basic-helix-loop-helix (CIB1) interacts with the blue light photoreceptor CRY2 and participates in flowering activation. In our experiments, the levels of transcripts of these two genes changed in a similar way. The highest decrease in the intensity of their expression occurred under BL, while an increase in the expression was observed under RL (Fig. 4A, B). The expression of the PHYA apoprotein gene was not changed under different light quality. At the same time, the level of PHYB apoprotein gene transcripts increased more in BL than in WL or RL (Figs. 4B).
Effect of light quality on the transcript levels of different groups of genes in E. salsugineum callus cells. (A) Cryptochrome-interacting basic-helix-loop-helix (CIB1), constitutive photomorphogenic1 (COP1), and phytochrome-interacting factor3 (PIF3) genes; (B) main photoreceptor genes; (C) main chloroplast proteins PSII (PSBA and PSBD), the Rboh family gene (RbohD), and encoding NADPH-dependent oxidase; and (D) protochlorophyllide oxidoreductase genes (PORB, PORC) involved in the biosynthesis of chlorophyll. The transcript levels are determined by PCR after reverse transcription and are expressed as a percentage of the reference values of the mRNA for plants grown under a WCFL. All values were normalized to the mRNA of the Actin2 and CBP20 genes. The bars indicate the standard errors (n = 3). In all the experiments, the relative signal intensity under WCFL was considered as 100%. Letters indicate significant difference (t test: p < 0.05) between the experimental variants
In our experiments in callus cells, the highest expression level of the PIF3 gene was observed in RL and the lowest was observed in WL (Fig. 4A). This is consistent with a significant decrease in the biosynthesis of chlorophyll under RL, which is confirmed by the pigment content, which demonstrated the lowest value at RL (Fig. 2A). The level of the protochlorophyllide oxidoreductase (POR) family gene transcripts was multidirectional. Thus, RL caused a significant increase in the expression of the PORB gene relative to WCFL, but the level of PORC transcripts was reduced (Fig. 4D). Similarly, under BL, the expression of both genes was slightly lower than that in the RL variant. WL increases the expression of only PORC relative to WCFL, while the level of PORB gene transcripts was within the static error of the WCFL variant.
PSII includes D1 and D2 proteins, which bind the main electron transfers. Proteins D1 and D2 are encoded by the PSBA and PSBD genes, respectively. As our data showed, the expression of the main PSII proteins decreased in all the light variants (Fig. 4C). However, it should be noted that the expression of the PSII genes was higher in BL and RL cells than in WL.
Genes of the RBOH family encode the NADPH-dependent oxidase. In our experiments, the level of RBOHD transcripts was the highest in the WL and BL variants, whereas RL had about half as much expression as RBOHD relative to WL and BL (Fig. 4C).
Interestingly, we did not detect transcripts of a number of genes in the tissue of E. salsugineum, despite the fact that all these genes were actively expressed in the leaves of adult plants. These genes include genes of the large subunit of RuBisCO, cryptochrome 1 (CRY1), and protochlorophyllide oxidoreductase A (PORA) (data not shown).
Discussion
Callus tissues are actively used to study the biosynthesis of secondary metabolites. They are a convenient model system to study the mechanisms of basic physiological processes. Nevertheless, many patterns of callus tissue growth, metabolic regulation, and responses to external factors are not sufficiently studied. In this work, we tried to determine how far the callus E. salsugineum cultivated under heterotrophic conditions retains the ability to respond to different spectral compositions of light by changing the photochemical activity of PSII and the activity of the antioxidant system, as well as the expression of the photoreceptor genes and main components of the light signalling chain.
Different spectral light compositions significantly influenced the content of the main photosynthetic pigments and their ratio (Fig. 2A–C). The WCFL callus samples differed from all other variants with the largest content of the photosynthetic pigments, and the Chla and Car levels were two times higher than the other variants (Fig. 2A). On the one hand, the lack of photosynthetic pigments leads to a decrease in the photosynthetic activity. On the other hand, an excessive increase in pigment content may indicate a lack of light of the required spectrum, which increases the pigments needed for light-harvesting complexes (Jin et al. 2016). Since all variants used light equalized by the number of falling quanta, the high pigment content in the WCFL variant and “nonoptimal” spectral range of these light sources can be considered as chromatic adaptation of cells (Fig. 1). The minimum content of pigments was observed in the RL variant and the largest contribution to this decrease was made by Chla.
An important characteristic of the reaction of plant cells to the quality of light is the pigment ratio. The minimum Chla/Chlb ratio observed in the RL variant (Fig. 2B) is more likely due to the decrease in Chla, but not the increase in Chlb (Fig. 2B). For terrestrial plants, unlike algae, adaptation to the intensity of light is more important than adaptation to the spectral composition. On the other hand, in the leaves of shade-tolerant plants, a relatively higher content of Chlb and an increase in total pigment content can be observed, which is regarded as a demonstration of chromatic adaptation (far-red light) (Dai et al. 2009). It can be assumed that a decrease in the Chla/Chlb ratio appears to be due to the increase in the size of the light-harvesting antenna of PSII, which correlates with the Chlb content inherent only in the PSII antenna. Chla is a component of both the core complexes and the peripheral antenna of PSI and PSII, whereas Chlb is predominantly a component of the peripheral antenna of both photosystems (Kalaji et al. 2014a). In this connection, a change in the Chla/Chlb ratio indicates a change in the ratio between the reaction centre complexes of the photosystem and the light-harvesting complex.
Another important indicator for the adaptation of plants to the quality of light is the carotenoid/chlorophyll ratio. Since the Car level in the WL, BL, and RL variants did not differ significantly, the chlorophyll component contributes the most to the Car/Chl ratio. This explains why the maximum Car/Chl ratio was observed in the RL variant (Fig. 2C), which probably also indicates a decrease in chlorophylls due to the absence of BL and the high potential of the photodynamic capacity of the cells. It should be noted that the Car/Chl ratio in the BL variant was lower than that in the RL, but much higher than that in the WL variant, in which both the red and blue components of the spectrum are shown (Fig. 1).
All experimental conditions led to a general decrease in the pigment content, as well as both chlorophylls and carotenoids (Fig. 2A–C). At the same time, the highest ratio of Car/Chl is noted in the RL variant, which is highly related to the drop in the amount of Chl (in particular Chlb) than Car. A high increase in the ratio of Car/Chl in RL and a significant increase in BL probably indicate the accelerated senescing of callus cells, since it is known that increases in the relative concentration of Car are often observed when plants are subjected to stress. Some articles show that Cars persist longer than Chla in senescing leaves. Additionally, it is shown that the Car/Chl ratio generally increases in senescing and unhealthy plants and decreases in healthy grown plants (Penuelas et al. 1995). An additional confirmation is the fact that the effect of narrow-band light reduced the viability and biomass of calluses (Fig. 2D, E).
Because of long-term exposure to light of different spectral compositions on the photomorphogenesis of E. salsugineum calluses (Fig. 5), we observed their response at the level of antioxidant enzyme activity (Fig. 4C, D), hydrogen peroxide and TBARS contents (Fig. 4A, B), and functioning of PSII (Table 1), as well as the expression of light-dependent genes involved in primary photochemical processes and photoreceptor signalling (Fig. 4).
The highest TBARS content, as well as the maximum activity of POD and CAT, was observed in the BL variant (Fig. 3B, D, F). The APX activity was the highest in callus cells under both blue and red light (Fig. 3E). At RL, the H2O2 content was lower compared to BL and WL (Fig. 3A). This can be explained by the more efficient generation of ROS by BL compared to RL, which corresponds to the data of Sarvikas et al. (2006) on the inhibition of photosynthesis by blue and red light. The activity of SOD did not change in the studied samples (Fig. 3C). Even though under RL conditions the lowest hydrogen peroxide content was found, the SOD activity remained unchanged. On the other hand, the highest level of TBARS, unlike other variants, and the largest number of dead cells in the BL variant (Fig. 2E) indicated that the cells were under strong oxidative stress, which can lead to their premature senescence and/or apoptosis. It should be noted that the observed oxidative stress and cell death in BL cannot be explained only by the low SOD activity (Fig. 3C). Of particular interest is the fact that the gene of the large subunit of the key enzyme of photosynthesis RuBisCO was not expressed in callus cells at all (data not shown). This may indicate that secondary photosynthetic processes are largely suppressed due to the heterotrophic type of nutrition of callus tissues. Nevertheless, primary processes are activated by different spectral light compositions, which is consistent with the presence of photochemical activity of PSII (Table 1). One of the possible reasons for the activation of primary photosynthetic processes and the absence of active RuBisCO is simply the need of oxygen by cells, which is used by the cells in the respiration process.
To understand the ways in which primary photochemical processes are activated in callus cells, it is necessary to study the expression of the main photoreceptor genes. Increases in transcript levels of phytochromes—photoreceptors of red light, such as PHYA, PHYB—and decreases in the level of transcripts of blue light photoreceptors (Fig. 4B) when cells grow on BL and RL can be explained either by the nonspecific reaction of the phytochromes to the action of BL in callus cells (Ahmad et al. 1998) or by a high level of phytohormones. These are also known to regulate the expression of photoreceptor genes (Imaizumi et al. 2002). Although transcripts of the CRY1 gene in callus cells were not detected, the apoprotein genes PHYA, PHYB, and CRY2 were expressed at a significant level (Fig. 4B).
It cannot be ruled out that BL-activated cryptochromes stimulate hydrogen peroxide formation (Consentino et al. 2015), which is likely involved in intracellular signalling of the BL, leading to the activation of antioxidant enzymes and the long-term action of premature senescence and apoptosis of callus cells.
To understand why the reduction of the hydrogen peroxide content and increase in APX activity in callus cells on RL occur, it is necessary to consider possible sources of ROS generation in plant cells.
One of the primary ROS formed by a cell is the superoxide anion radical, which rapidly turns into H2O2 in the presence of SOD. In turn, H2O2 enters into a series of reactions as a substrate and is destroyed by the cell antioxidant system. The ROS forming systems include the mitochondrial electron transport chains, chloroplasts, a family of plasma membrane NADPH oxidase complexes, and a number of other oxidases. One of the main functions of NADPH oxidases is in the superoxide anion formation. The gene respiratory burst oxidase homolog (Rboh) encodes plant NADPH oxidase (Sagi and Fluhr 2006). In our experiments, the level of plasma membrane-localized RbohD transcripts was higher in the BL and WL variants compared to the RL variant (Fig. 4C). In addition, one should note that singlet oxygen can form in callus cells due to the interaction between chlorophyll triplets (3Chl) and the ground triplet state of molecular oxygen (Schmitt et al. 2014). Here, triplet is populated either via intersystem crossing of the Chl antenna or via radical pair recombination in the reaction centres of PSII. There are also enzymatic sources of O2 −•/H2O2 generation, such as cell wall-bound peroxidases, amino oxidases, flavin-containing oxidases, and oxalate and plasma membrane NADPH oxidases.
In addition to the pro-oxidant function, H2O2 can act as a signalling molecule. It is very likely that a significant amount of H2O2 is generated when CRY2 is activated. Previously, it was shown that, in vitro, the cryptochrome CRY1 of A. thaliana in BL can cause the transformation of molecular oxygen to its active forms, including H2O2 (Consentino et al. 2015; Sellaro et al. 2010). In the work of Consentino et al. (2015), the physiological role of H2O2 generated with the participation of CRY1 was demonstrated in an A. thaliana protoplast culture, as well as the possible role of hydrogen peroxide in the transmission of a light signal under the action of BL. In E. salsugineum cells, we were not able to detect the expression of the CRY1 gene. It cannot be ruled out that its lack of activity plays a stress-protective role, reducing the sensitivity of cells to the high-intensity light. It is very likely that in callus culture active generation of H2O2 can also be carried out by CRY2 in the nucleus of the cell (Jourdan et al. 2015). In our experimental conditions, a long high-intensity light exposure was used, which could cause the formation of a large amount of ROS. In this case, H2O2 could act not only as a signalling molecule but also as a damaging factor. Utilization of electrons at the PSI acceptor side likely occurs via the water–water cycle. RuBisCO activity is absent. Therefore, electrons from the photosynthetic electron transport chain are not used for carbon assimilation, but they can be used by the water–water cycle, which functions as the main electron sink under the suppressed photosynthesis condition (Allakhverdiev et al. 1997; Asada 1999; Miyake 2010). Here, electrons produced from H2O are transported to PSI and used to reduce O2 to superoxide anion radical (.O2 −) in chloroplasts. As a result of these processes, superoxide, H2O2, and some other ROS are produced. The produced ROS are detoxified by the scavenging enzymes, including superoxide dismutase, ascorbate peroxidase, and several other enzymes. The function of this cycle can explain the activity of antioxidative enzymes, such as SOD and APX. Here, one of the sources of increased ROS generation under BL can also be the oxygen-evolving water-oxidizing complex of PSII, which is more efficiently inhibited by BL compared to RL.
The known two-step mechanism for photodamage to PSII (Allakhverdiev and Murata 2004; Ohnishi et al. 2005; Nishiyama et al. 2006) can likely explain the strong photoinhibition of PSII under blue light. According to this mechanism, the first step occurs at the oxygen-evolving complex of PSII by UV and strong blue light, and the second occurs at the photochemical reaction centre of PSII by light absorbed by the photosynthetic pigments (Mohanty et al. 2007). The action spectrum of the first step corresponded to the absorption spectrum of the Mn-containing water-oxidizing complex. Photodamage to the oxygen-evolving complex was accompanied by the release of Mn ions from the PSII reaction centre, suggesting the photoinduced disruption of the Mn-containing cluster. The action spectrum of the second step is similar to the absorption spectrum of Chl (Allakhverdiev 2011). The data obtained showed that ROS act primarily by inhibition of the translation of psbA transcripts without direct damage of PSII. One can suggest that the addition of Mn to callus samples could decrease H2O2 and TBARS formation. However, there are a number of other possible pathways for ROS generation. For example, the study of Pathak et al. (2017) using PSII membranes showed that 1O2 can be formed by decomposition of hydroperoxide protein in PSII membranes deprived of Mn4O5Ca complex.
Gene expression
To understand whether light signalling genes are activated in callus cells, the expression of a number of key genes necessary for intact plants has been studied. These include the constitutive photomorphogenic1 (COP1) gene, which encodes the COP1 protein. This protein is part of the E3 Ubiquitin ligase complex and affects the expression of more than 20% of the genes in A. thaliana plants when grown in the dark, and it is associated with the repression of photomorphogenesis genes (Lau and Deng 2012). COP1 is involved in the proteolysis of transcription factors associated with light regulation (Cluis et al. 2004; Yamamoto et al. 1998). In our work, we observed a decrease in the expression level of the gene in all variants; however, the highest decrease in the COP1 transcripts level was observed in BL (up to 40% of WCFL control) (Fig. 4A). On this basis, it can be suggested that the signalling system associated with COP1 is repressed. CIB1 interacts with the blue light photoreceptor CRY2 and participates in the activation of flowering. Moreover, the interaction of CRY2–CIB1 is activated by high-intensity BL (Liu et al. 2008). The expression of the two genes CRY2 and CIB1 changed in a similar way. The greatest decrease in the expression of both genes occurred on BL, and an increase in the expression was found in the WL and RL variants (Fig. 4A, B). The observed decrease in the expression of these genes under BL can be explained by their ontogenetic control. Another reason for inhibiting the activity of the CRY2 and CIB1 genes may be a change in the hormonal balance of the callus cells due to the presence of hormones in the nutrient medium.
The level of PHYA gene transcripts did not change under different light quality. At the same time, the expression of the PHYB gene was more activated in BL than in WL or RL (Fig. 4B). This observation is similar to the intact higher plants in which PHYA plays a large role in etiolated plants, and PHYB in green plants (Casal 2000). This may also indicate the possible crosstalk between the different photoreceptors (Fraser et al. 2016; Franklin and Whitelam 2004).
Phytochrome-interacting factors (PIFs) are transcription factors that interact in the nucleus with active forms of phytochromes (Jeong et al. 2016). This interaction is due to the inhibition of PIF activity, followed by dissociation from the target promoters or by phosphorylation and subsequent degradation by 26S proteasomes. The highest level of the PIF3 gene was observed in RL (Fig. 4A). With an elevated content of the active form of phytochrome in RL, the level of PIF is likely decreased, which may result in phytochrome gene up-regulation. In contrast, with a minimum expression of phytochrome in BL, the expression level of the PIF3 gene is also minimal. In addition, the chlorophyll content was lowered in RL, but under RL the maximum level of the PORB gene was observed (Fig. 4D). It can be assumed that the synthesis of pigments is regulated more at a posttranscriptional level.
The main proteins of PSII—D1 and D2 encoded by genes PSBA and PSBD, respectively, were reduced in all variants, which may be the reason for the low efficiency of the recovery of light-damaged PSII and, in part, the low quantum efficiency of electron transfer in PSII (Fig. 4C; Table 1). Protochlorophyllide oxidoreductase gene (PORB and PORC) was the most expressed in BL and RL; however, the expression did not correlate with the pigment content. On this basis, it can be assumed that the POR expression does not limit pigment biosynthesis. It cannot be excluded that the relatively low level of photosynthetic pigments is sufficient for normal growth of the callus culture in BL and RL when PSII activity is low.
It is interesting that we did not find CRY1 gene expression in the callus cells. It cannot be ruled out that the content of a number of photoreceptors has been reduced in cell culture, since the growth and proliferation of cells, but not flowering and fruiting, play a key role in this system (Beel et al. 2012).
PSII activity and generation of ROS
The value of ABS/RC reflects the ratio between the content of chlorophyll molecules in the fluorescence-emitting antenna complexes and in the active reaction centres (Strasser et al. 2010). The increase in this parameter in cells grown in BL and WCFL can be explained by a decrease in the number of active QB-absorbing reactive centres of PSII, which probably serve as a defence mechanism against ROS formation. Another protective mechanism is the dissipation of absorbed energy into heat. In cells under BL, dissipation of absorbed energy (mainly to heat) is very effective, since the value of DI0/RC in these cells is much higher than that in cells grown in RL and WL (Table 1). A protective role against ROS can also be played by carotenoids. In addition to light energy harvesting, Cars play essential photoprotective roles in chloroplasts by quenching the triplet Chl and scavenging singlet oxygen and other reactive oxygen species (Havaux and Niyogi 1999; Schmitt et al. 2014). Additionally, the photoprotective role of xanthophylls for dissipation of excess excitation energy under irradiance stress has been demonstrated. The inter-conversion of violaxanthin into zeaxanthin occurring in the light-harvesting complexes (LHC) regulates light harvesting and subsequent energy dissipation. B-carotene in PSII RC plays a protective role against singlet oxygen (Tefler 2002).
Clear evidence for ROS quenching by carotenoids has also been found in recent reviews (Schmitt et al. 2014). Concerning this, the increased carotenoid content in callus cells in the WCFL variant can lead to the lowest TBARS content compared to other light quality (Figs. 2A, 3B), although CRY2 expression in BL samples was slightly different from the other light variants. More important than the cryptochrome content is the efficiency of formation of their semi-reduced active form, which can determine the content of inducible ROS molecules. Another cause of high H2O2 levels in BL and WCFL in comparison with WL and RL seems to be more preferable, such as the smaller rate of H2O2 formation at the sites of its generation in RL and WL, photosynthetic ETC, mitochondria, peroxisomes, and plasmalemma. The up-regulation of RbohD gene expression conforms to this version (Fig. 4C). It is worth noting that BL induces photoinhibition of the photosynthetic process more than RL. This is consistent with the known photoinhibitory spectra of higher plants (Sarvikas et al. 2006; Allakhverdiev et al. 2007).
Conclusion
Under the different light spectral conditions, the composition of callus cells changed the expression of main photoreceptors, light signalling genes, and primary photochemical processes. We believe that light causes an increase in the hydrogen peroxide content in cells through the activation of plasma membrane NADH oxidase and/or possibly through the inactivation of the water-decomposing PSII complex. The increase in the activity of CAT and various POD species under BL is probably associated with the activation of ROS formation, metabolic acceleration, senescence processes, and/or apoptosis. The maximum contents of H2O2 and TBARS in BL and WCFL are probably associated with a higher level of oxidative stress in the cells compared to RL. This is also consistent with the higher activity of CAT and total POD in BL and a smaller number of living cells. The obtained data indicate that in callus cells, despite the practically heterotrophic nutrition, primary photochemical processes are manifested, such as PSII functions, which may be important for the generation of O2. In the callus culture, no active expression of the large subunit RuBisCO gene was detected, because under these conditions carbohydrates enter cells from the nutrient medium and the dark photosynthesis stage is not necessary. Moreover, callus cells contain a sufficiently low chlorophyll content, which is consistent with the absence of transcripts of the PORA gene. At the same time, the genes of the main photoreceptors and light signalling components are expressed and the antioxidant enzyme activity is changed, which is important for maintaining pro-/antioxidant balance, the formation of chloroplasts, and the number of metabolic processes. It can be concluded that the callus culture cells can be used as a convenient model for studying the complex primary photochemical reactions. However, for a number of studied parameters, this model occupies a middle position between microalgae and intact plants.
Abbreviations
- APX:
-
Ascorbate peroxidase
- SOD:
-
Superoxide dismutase
- POD:
-
Peroxidase
- CAT:
-
Catalase
- TBARS:
-
Thiobarbituric acid reactive substances
- BL:
-
Blue light from light-emitting diodes 450 nm
- WL:
-
White light from light-emitting diodes 450 nm + 660 nm
- RL:
-
Red light from light-emitting diodes 660 nm
- WCFL:
-
Light from white compact fluorescent lamps
- CRY:
-
Cryptochrome
- PHY:
-
Phytochrome
- PSII:
-
Photosystem II
- ROS:
-
Reactive oxygen species
- ETC:
-
Electron transport chain
- Chla :
-
Chlorophyll a
- Chlb :
-
Chlorophyll b
- Car:
-
Carotenoids
References
Ahmad JV, Smirnova O, Cashmore AR (1998) The CRY1 blue light photoreceptor of Arabidopsis interacts with phytochrome A in vitro. Mol Cell 1:939–948. doi:10.1016/S1097-2765(00)80094-5
Allakhverdiev SI (2011) Recent progress in the studies of structure and function of photosystem II. J Photoch Photobio B 104: 1–8. doi:10.1016/j.jphotobiol.2011.03.010
Allakhverdiev SI, Murata N (2004). Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage–repair cycle of photosystem II in Synechocystis sp. PCC 6803. Biochimica et Biophysica Acta (BBA)-Bioenergetics. 1657:23–32. doi:10.1016/j.bbabio.2004.03.003
Allakhverdiev SI, Klimov VV, Carpentier R (1997) Evidence for the involvement of cyclic electron transport in the protection of photosystem II against photoinhibition: influence of a new phenolic compound. Biochem 36:4149–4154. doi:10.1021/bi962170n
Allakhverdiev SI, Los DA, Mohanty P, Nishiyama Y, Murata N (2007) Glycinebetaine alleviates the inhibitory effect of moderate heat stress on the repair of photosystem II during photoinhibition. Biochimica et Biophysica Acta (BBA)-Bioenergetics 1767(12):1363–1371. doi:10.1016/j.bbabio.2007.10.005
Asada K (1999) The water–water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639. doi:10.1146/annurev.arplant.50.1.601
Bae G, Choi G (2008) Decoding of light signals by plant phytochromes and their interacting proteins. Annu Rev Plant Biol 59:281–311. doi:10.1146/annurev.arplant.59.032607.092859
Beauchamp C, Fridovich I (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Beel B, Prager K, Spexard M et al (2012) A flavin binding cryptochrome photoreceptor responds to both blue and red light in Chlamydomonas reinhardtii. Plant Cell 24:2992–3008. doi:10.1105/tpc.112.098947
Bliznikas Z, Zukauskas A, Samuoliene G, Viršile A, Brazaityte A, Jankauskiene J, Novičkovas A (2012) The effect of supplementary pre-harvest LED lighting on the antioxidant and nutritional properties of green vegetables. Acta Hortic 939:85–91
Casal JJ (2000) Phytochromes, cryptochromes, phototropin, photoreceptor interactions in plants. Photochem Photobiol 71:1–11
Chance B, Maehly AC (1955) Assay of catalases and peroxidases. Method Enzymol 2:764–775. doi:10.1016/S0076-6879(55)02300-8
Cluis CP, Mouchel CF, Hardtke CS (2004) The Arabidopsis transcription factor HY5 integrates light and hormone signaling pathways. Plant J 38:332–347. doi:10.1111/j.1365-313X.2004.02052.x
Consentino L, Lambert S, Martino C, Jourdan N, Bouchet PE, Witczak J, Ahmad M (2015) Blue-light dependent reactive oxygen species formation by Arabidopsis cryptochrome may define a novel evolutionarily conserved signaling mechanism. New Phytol 206:1450–1462. doi:10.1111/nph.13341
Cormier MJ, Prichard PM (1968) An investigation of the mechanism of the luminescent peroxidation of luminol by stopped flow techniques. J Biol Chem 243:4706–4714
Dai Y, Shen Z, Liu Y, Wang L, Hannaway D, Lu H (2009) Effects of shade treatments on the photosynthetic capacity, chlorophyll fluorescence, and chlorophyll content of Tetrastigma hemsleyanum Diels et Gilg. Environ Exp Bot 65:177–182. doi:10.1016/j.envexpbot.2008.12.008
Desikan R, Soheila AH, Hancock JT, Neill SJ (2001) Regulation of the Arabidopsis transcriptome by oxidative stress. Plant Physiol 127(1):159–172. doi:10.1104/pp.127.1.159
Foyer CH, Noctor G (2005) Oxidant and antioxidant signalling in plants: a re-evaluation of the concept of oxidative stress in a physiological context. Plant Cell Env 28:1056–1071. doi:10.1111/j.1365-3040.2005.01327.x
Franklin KA, Whitelam GC (2004) Light signals, phytochromes and crosstalk with other environmental cues. J Exp Bot 55:271–276. doi:10.1093/jxb/erh026
Fraser DP, Hayes S, Franklin KA (2016) Photoreceptor crosstalk in shade avoidance. Curr Opin Plant Biol 33:1–7. doi:10.1016/j.pbi.2016.03.008
Havaux M, Niyogi KK (1999) The violaxanthin cycle protects plants from photooxidative damage by more than one mechanism. Proc Natl Acad Sci USA 96(15):8762–8767. doi:10.1073/pnas.96.15.8762
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: I. Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys 125:189–198. doi:10.1016/0003-9861(68)90654-1
Imaizumi T, Kadota A, Hasebe M, Wada M (2002) Cryptochrome light signals control development to suppress auxin sensitivity in the moss Physcomitrella patens. Plant Cell 14:373–386. doi:10.1105/tpc.010388
Jeong J, Kim K, Kim ME, Kim HG, Heo GS, Park OK, Oh E (2016) Phytochrome and ethylene signaling integration in arabidopsis occurs via the transcriptional regulation of genes co-targeted by PIFs and EIN3. Front Plant Sci 7:1055. doi:10.3389/fpls.2016.01055
Jin H, Li M, Duan S, Fu M, Dong X, Liu B, Wang HB (2016) Optimization of light harvesting pigment improves photosynthetic efficiency. Plant Physiol. doi:10.1104/pp.16.00698
Johkan M, Shoji K, Goto F, Hashida SN, Yoshihara T (2010) Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortSci 45:1809–1814
Jourdan N, Martino CF, El-Esawi M, Witczak J, Bouchet P-E, d’Harlingue A, Ahmad M (2015) Blue-light dependent ROS formation by Arabidopsis cryptochrome-2 may contribute toward its signaling role. Plant Signal Behav 10:e1042647. doi:10.1111/nph.13341
Kalaji HM, Schansker G, Ladle RJ, Goltsev V, Bosa K, Allakhverdiev SI, Elsheery NI (2014a) Photosynth Res 122:121–158. doi:10.1007/s11120-014-0024-6
Kalaji HM, Jajoo A, Oukarroum A, Brestic M, Zivcak M, Samborska I, Ahmad P (2014b) The use of chlorophyll fluorescence kinetics analysis to study the performance of photosynthetic machinery in plants. Emerg Technol Manag Crop Stress Toler 2:347–385. doi:10.1016/B978-0-12-800875-1.00009-0
Kendrick RE, Kronenberg GH (eds) (2012). Photomorphogenesis in plants. Springer, Dordrecht. doi:10.1007/978-94-011-1884-2
Kleine T, Kindgren P, Benedict C, Hendrickson L (2007) Strand A Genome-wide gene expression analysis reveals a critical role for CRYPTOCHROME1 in the response of Arabidopsis to high irradiance. Plant Physiol 127:1391–1406. doi:10.1104/pp.107.098293
Kosobryukhov AA, Lyubimov VYu, Kreslavski VD. (2015) Adaptive mechanisms of photosynthetic apparatus to UV radiation. In: Stress response in plants, Tripathi BN, Müller M (eds). Springer, Dordrecht, Chap. 3, pp 59–78. doi:10.1007/978-3-319-13368-3_3
Kreslavski VD, Carpentier R, Klimov VV, Allakhverdiev SI (2009) Transduction mechanisms of photoreceptor signals in plant cells. J Photochem Photobiol C 10:63–80. doi:10.1016/j.jphotochemrev.2009.04.001
Kreslavski VD, Shirshikova GN, Lyubimov VY, Shmarev AN, Boutanaev AM, Kosobryukhov AA, Allakhverdiev SI (2013a) Effect of preillumination with red light on photosynthetic parameters and oxidant-/antioxidant balance in Arabidopsis thaliana in response to UV-A. J Photochem Photobiol B 127:229–236. doi:10.1016/j.jphotobiol.2013.08.008
Kreslavski VD, Lyubimov VY, Shirshikova GN, Shmarev AN, Kosobryukhov AA, Schmitt FJ, Allakhverdiev SI (2013b) Preillumination of lettuce seedlings with red light enhances the resistance of photosynthetic apparatus to UV-A. J Photochem Photobiol B 122:1–6. doi:10.1016/j.jphotobiol.2013.02.016
Lankin AV, Kreslavski VD, Khudyakova AY, Zharmukhamedov SK, Allakhverdiev SI (2014) Effect of naphthalene on photosystem 2 photochemical activity of pea plants. Biochem Moscow 79:1216–1225. doi:10.1134/S0006297914110091
Lau OS, Deng XW (2012) The photomorphogenic repressors COP1 and DET1: 20 years later. Trends Plant Sci 17:584–593. doi:10.1016/j.tplants.2012.05.004
Lefsrud MG, Kopsell DA, Sams CE (2008) Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. HortSci 43:2243–2244
Li Q, Kubota C (2009) Effects of supplemental light quality on growth and phytochemicals of baby leaf lettuce. Env Exp Bot 67:59–64. doi:10.1016/j.envexpbot.2009.06.011
Lichtenthaler HK (1987) Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol 148:350–382. doi:10.1016/0076-6879(87)48036-1
Liu H, Yu X, Li K, Klejnot J, Yang H, Lisiero D, Lin C (2008) Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322:1535–1539. doi:10.1126/science.1163927
Liu H, Liu B, Zhao C, Pepper M, Lin C (2011) The action mechanisms of plant cryptochromes. Trends Plant Sci 16:684–691. doi:10.1016/j.tplants.2011.09.002
Massa GD, Kim HH, Wheeler RM, Mitchell CA (2008) Plant productivity in response to LED lighting. HortSci 43:1951–1956
Meinhard M, Grill E (2001) Hydrogen peroxide is a regulator of ABI1, a protein phosphatase 2C from Arabidopsis. Febs Lett 508:443–446. doi:10.1016/S0014-5793(01)03106-4
Miyake С (2010) Alternative electron flows (water–water cycle and cyclic electron flow around psi) in photosynthesis: molecular mechanisms and physiological functions. Plant Cell Physiol 51:1951–1963. 10.1093/pcp/pcq173
Mohanty P, Allakhverdiev SI, Murata N (2007) Application of low temperatures during photoinhibition allows characterization of individual steps in photodamage and the repair of photosystem II. Photosynth Res 94:217–224. 10.1007/s11120-007-9184-y
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880. doi:10.1093/oxfordjournals.pcp.a076232
Neill S, Desikan R, Hancock J (2002) Hydrogen peroxide signalling. Curr Opin Plant Biol 5:388–395. 10.1016/S1369-5266(02)00282-0
Nishiyama Y, Allakhverdiev SI, Murata N (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta. 10.1016/j.bbabio.2006.05.013
Ohnishi N, Allakhverdiev SI, Takahashi S, Higashi S, Watanabe M, Nishiyama Y, Murata N (2005) Two-step mechanism of photodamage to photosystem II: step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. BioChemistry 44(23):8494–8499. doi:10.1021/bi047518q
Pathak V, Prasad A, Pospısil P.(2017). Formation of singlet oxygen by decomposition of protein hydroperoxide in photosystem II. PLoS One 12(7):e0181732. doi:10.1371/journal.pone.0181732
Penuelas J, Baret F, Filella I (1995) Semi-empirical indices to assess carotenoids/chlorophyll a ratio from leaf spectral reflectance. Photosynthetica 31(2):221–230
Powles SB (1984) Photoinhibition of photosynthesis induced with visible light. Annu Rev Plant Physiol 35:15–44. doi:10.1146/annurev.pp.35.060184.000311
Radyukina NL, Ivanov YV, Kartashov AV, Shevyakova NI, Rakitin VY, Khryanin VN, Kuznetsov VV (2007) Inducible and constitutive mechanisms of salt stress resistance in Geum urbanum L. Russ J Plant Physiol 54:612–618. doi:10.1134/S102144370705007X
Radyukina NL, Mapelli S, Ivanov YV, Kartashov AV, Brambilla I, Kuznetsov VV (2009) Homeostasis of polyamines and antioxidant systems in roots and leaves of Plantago major under salt stress. Russ J Plant Physiol 56(3):323–331
Radyukina NL, Ivanov YV, Kartashov AV, Pashkovskiy PP, Shevyakova NI, Kuznetsov VV (2011) Regulation of gene expression governing proline metabolism in Thellungiella salsuginea by NaCl and paraquat. Russ J Plant Physiol 58:643–652. doi:10.1134/S102144371104011X
Ridge I, Osborne DJ (1971) Role of peroxidase when hydroxyproline-rich protein in plant cell walls is increased by ethylene. Nature 229:205–208. 10.1038/newbio229205a0 doi
Sagi M, Fluhr R (2006) Production of reactive oxygen species by plant NADPH oxidases. Plant Physiol 141:336–340. doi:10.1104/pp.106.078089
Samuolienė G, Brazaitytė A, Sirtautas R, Novičkovas A, Duchovskis P (2011) Supplementary red-LED lighting affects phytochemicals and nitrate of baby leaf lettuce. JFAE 9:271–274
Samuolienė G, Sirtautas R, Brazaitytė A, Duchovskis P (2012) LED lighting and seasonality effects antioxidant properties of baby leaf lettuce. Food Chem 134:1494–1499. doi:10.1016/j.foodchem.2012.03.061
Sarvikas P, Hakala M, Patsikka E, Tyystjarvi T, Tyystjarvi E (2006) Action spectrum of photoinhibition in leaves of wild type and npq1-2 and npq4-1 mutants of Arabidopsis thaliana. Plant Cell Physiol 47:391–400. doi:10.1093/pcp/pcj006
Schenk RU, Hildebrandt AC (1972) Medium and techniques for induction and growth of monocotyledonous and dicotyledonous plant cell cultures. Can J Bot 50:199–204. doi:10.1139/b72-026
Schmitt FJ, Renger G, Friedrich T, Kreslavski VD, Zharmukhamedov SK, Los DA, Kuznetsov VV, Allakhverdiev SI. 2014. Reactive oxygen species: reevaluation of generation, monitoring and role in stress-signalling in phototrophic organisms. Biochim Biophys Acta 1837:835–848. doi:10.1016/j.bbabio.2014.02.005
Sellaro R, Crepy M, Trupkin SA, Karayekov E, Buchovsky AS, Rossi C, Casal JJ (2010) Cryptochrome as a sensor of the blue/green ratio of natural radiation in Arabidopsis. Plant Physiol 154:401–409. doi:10.1104/pp.110.160820
Soshinkova TN, Radyukina NL, Korolkova DV, Nosov AV (2013) Proline and functioning of the antioxidant system in Thellungiella salsuginea plants and cultured cells subjected to oxidative stress. Russ J Plant Physiol 60:41–54. doi:10.1134/S1021443713010093
Strasser RJ, Tsimilli-Michael M, Qiang S, Goltsev V (2010) Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim Biophys Acta 1797:1313–1326. doi:10.1016/j.bbabio.2010.03.008
Stutte GW (2009) Light-emitting diodes for manipulating the phytochrome apparatus. HortSci 44:231–234
Tefler A (2002) What is b-carotene doing in the photosystem II reaction centre? Phil Trans R Soc Lond B 357:1431–1440
Wen F, Xing D, Zhang L (2008) Hydrogen peroxide is involved in high blue light-induced chloroplast avoidance movements in Arabidopsis. J Exp Bot 59:2891–2901. doi:10.1093/jxb/ern147
Wu HJ, Zhang Z, Wang JY, Oh DH, Dassanayake M, Liu B, Huang Q, Sun HX, Xia R, Wu Y, Wang YN (2012) Insights into salt tolerance from the genome of Thellungiella salsuginea. Proc Natl Acad Sci USA 109:12219–12224. doi:10.1073/pnas.1209954109
Yamamoto YY, Matsui M, Ang LH, Deng XW (1998) Role of a COP1 interactive protein in mediating light-regulated gene expression in Arabidopsis. Plant Cell 10:1083–1094. doi:10.1105/tpc.10.7.1083
Yurina NP, Mokerova DV, Odintsova MS (2013) Light-inducible stress plastid proteins of phototrophs. Russ J Plant Physiol 60:577–588. doi:10.1134/S1021443713050154
Zhao X, Tan HJ, Liu YB, Li XR, Chen GX (2009) Effect of salt stress on growth and osmotic regulation in Thellungiella and Arabidopsis callus. PCTOC 98:97–103. doi:10.1007/s11240-009-9542-x
Acknowledgements
This work was supported by the Russian Foundation for Basic Research (RFBR) on 15-04-01199a and by the Program of the Presidium of the Russian Academy of Sciences “Molecular and Cellular Biology” (MCB RAS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pashkovskiy, P.P., Soshinkova, T.N., Korolkova, D.V. et al. The effect of light quality on the pro-/antioxidant balance, activity of photosystem II, and expression of light-dependent genes in Eutrema salsugineum callus cells. Photosynth Res 136, 199–214 (2018). https://doi.org/10.1007/s11120-017-0459-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-017-0459-7