Abstract
Oxygen effects have long been ambiguous: exacerbating, being indifferent to, or ameliorating the net photoinactivation of Photosystem II (PS II). We scrutinized the time course of PS II photoinactivation (characterized by rate coefficient k i) in the absence of repair, or when recovery (characterized by k r) occurred simultaneously in CO2 ± O2. Oxygen exacerbated photoinactivation per se, but alleviated it by mediating the utilization of electrons. With repair permitted, the gradual net loss of functional PS II during illumination of leaves was better described phenomenologically by introducing τ, the time for an initial k r to decrease by half. At 1500 μmol photons m−2 s−1, oxygen decreased the initial k r but increased τ. Similarly, at even higher irradiance in air, there was a further decrease in the initial k r and increase in τ. These observations are consistent with an empirical model that (1) oxygen increased k i via oxidative stress but decreased it by mediating the utilization of electrons; and (2) reactive oxygen species stimulated the degradation of photodamaged D1 protein in PS II (characterized by k d), but inhibited the de novo synthesis of D1 (characterized by k s), and that the balance between these effects determines the net effect of O2 on PS II functionality.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Light energy, especially in excess, damages the photosynthetic apparatus (primarily Photosystem II or PS II), decreases photochemical efficiency and consequently retards growth and productivity (Powles and Björkman 1982; Vass and Cser 2009; Nishiyama et al. 2011; Oguchi et al. 2011; Vass 2011; Tyystjärvi 2013). In nature, plants have evolved various strategies to manage photoinactivation of PS II. Among the strategies, the PS II repair cycle is crucial; when repair keeps pace with photoinactivation per se, no net loss of functional PS II occurs (Aro et al. 1993; Mulo et al. 2012). A highly efficient PS II repair cycle is demonstrated by the observation that the D1 protein in the entire PS II population may turn over during a sunny day. Therefore, understanding the mechanism(s) of the PS II photoinactivation and the repair cycle is critical for engineering the capacity of plants to resist photoinactivation.
Environmental stresses, such as salt stress (Allakhverdiev et al. 2002), oxidative stress (Allakhverdiev and Murata 2004), low temperature (Öquist and Huner 1990) and high light (Aro et al. 1993), affect the PS II repair cycle significantly. However, how oxygen affects repair remains ambiguous. Anaerobic conditions have been shown to exacerbate (Nedbal et al. 1990; Šetlik et al. 1990), be indifferent to (Satoh 1970; Powles 1984) or ameliorate (Cornic 1978; Park et al. 1996) net photoinactivation of PS II. It was hypothesized that such inconsistency was attributed to the dual roles of oxygen in the net photoinactivation of PS II; that is, oxygen can either act as an electron acceptor to mitigate against the excess excitation pressure on PS II, or stimulate photoinactivation via toxic effects of reactive oxygen species (Krause et al. 1985).
The knowledge gap of oxygen effects on PS II repair occurs partly because most reports did not separate the photoinactivation of PS II per se from the repair. Some reports applied lincomycin or chloramphenicol (both of which inhibit chloroplast-encoded protein synthesis) to separate the two processes (Aro et al. 1993; Allakhverdiev and Murata 2004; Nishiyama et al. 2001). However, this is not straightforward. For example, to quantify the rate of PS II repair, Allakhverdiev and Murata (2004) used the initial net rate of recovery which, however, included a photoinactivation component.
In order to quantify PS II repair in terms of a rate coefficient (k r) in vivo, several studies have been conducted (Kok 1956; He and Chow 2003; Campbell and Tyystjärvi 2012; Hu et al. 2013). Nevertheless, most models did not consider that k r might change with time under given environmental conditions. The PS II repair cycle includes many sub-processes such as protein phosphorylation, migration and degradation of the damaged D1 protein, synthesis of the D1 protein and the insertion of newly synthesized D1 protein, changes in any of which will affect k r. If k r were treated as a constant, simulation with these models should lead to a time-independent functional fraction of PS II when the rate of photoinactivation per se equals the rate of repair; unfortunately, this is seldom observed.
In the present study, we devised a model with a dynamic k r. We introduced a parameter τ, the time for the initial k r to decrease by half, to evaluate the dynamics of k r phenomenologically. The time course of net photoinactivation of PS II was carefully measured in leaves of spinach plants grown in full glasshouse light (sun leaves) or under shade cloth (shade leaves), the measurements conducted in ambient air or nitrogen + 0.5 % CO2. At an irradiance of 1500 μmol m−2 s−1, oxygen decreased the initial k r but increased τ. As the irradiance increased above 1500 μmol m−2 s−1, the initial k r decreased further, while τ increased further. The effect of oxygen on net photoinactivation depended on growth irradiance. Anaerobicity alleviated net PS II net photoinactivation slightly in shade leaves. By contrast, for sun leaf discs, anaerobicity first alleviated, and then exacerbated net PS II photoinactivation. The results can be simulated by considering multiple roles of oxygen in photoinactivation per se and the repair of photoinactivated PS II, thereby resolving past ambiguities of oxygen effects.
Materials and methods
Plant growth
Spinacea oleracea L. (cv. Yates hybrid 102) plants were grown in a glasshouse (approximately 30/15 °C, day/night). A nutrient mix (Aquasol, Hortico, Clayton, Australia) was supplemented by a slow release fertilizer (‘Osmocote’, Scotts Australia Pty Ltd, Castle Hill). The maximum growth irradiances of sun and shade plants were 800 and 150 μmol m−2 s−1 at noon, respectively.
Photoinhibitory treatment of leaf discs
Spinach leaf discs (1.5 cm2) were first floated on a 3-mM lincomycin solution overnight in darkness to allow sufficient uptake of the inhibitor of chloroplast-encoded protein synthesis. Leaf discs were floated with the abaxial side facing air and the adaxial side in contact with the lincomycin solution in a clear petri dish; photoinhibitory light was applied vertically up onto the adaxial side. Illumination at 1500 μmol m−2 s−1 (unless specified otherwise) using white light-emitting diodes was applied for up to 3 h (sometimes 6 h) to obtain the time course of photoinactivation per se of PS II, which yielded the rate coefficient of photoinhibition k i. The same procedure was applied to observe the net PS II photoinactivation in the absence of lincomycin while PS II repair occurred simultaneously with PS II photoinactivation per se, from which the rate coefficient of repair (k r) and τ could be deduced.
Before illumination, a constant flow of humidified gas mixtures containing either ambient air or 0.5 % CO2 in N2 was passed through the chamber for ≥30 min at 25 °C. The gas flow persisted during the whole treatment time, and after every half hour of illumination, 5 min darkness was applied before resuming photoinhibitory treatment, in order to minimize the contamination by oxygen evolved by PS II during illumination in 0.5 % CO2 in N2.
To investigate the effect of methyl viologen (MV) on photoinactivation when chloroplast-encoded protein synthesis was blocked by lincomycin, leaf discs were first floated on a 3-mM lincomycin solution overnight in darkness, then were vacuum infiltrated with 20 μM MV + 3 mM lincomycin, blotted with absorbent paper, and allowed to evaporate off excess intercellular water, followed by photoinhibitory treatment. Floating leaf discs on 3 mM lincomycin plus 20 μM MV overnight in darkness would inactivate about 20 % of total functional PS II, and so was not adopted.
PS II functionality determined by redox kinetics of P700
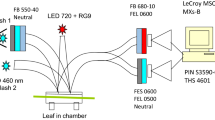
The functional fraction of PS II was determined from the flash-induced redox kinetics of P700, the special chlorophyll pair in PS I, according to the method of Kou et al. (2012) and Hu et al. (2013). This method relies on the delivery of electrons from PS II to P700+ after a saturating single-turnover flash. Leaf segments were used for measurement of redox changes of P700 with a dual wavelength (810/870 nm) unit (ED-P700DW) attached to a phase amplitude modulation fluorometer (PAM 101, Walz, Effeltrich, Germany). To obtain redox changes due to a flash superimposed on continuous far-red light, a steady state was sought by illumination with far-red light (12 µmol photons m−2 s−1, peak wavelength 729 nm, 102-FR, Walz, Effeltrich, Germany). Then a single-turnover, saturating xenon flash (XST 103, full width at half height = 6 μs) was applied to the adaxial side of the leaf disc. The start of data acquisition (time constant = 95 μs), the triggering of the flash and the repetition rate were controlled by a pulse/delay generator (Model 555, Berkeley Nucleonics Corporation, USA). The analogue output was digitized and stored using a program written by Alexander Hope. Flashes were given at 0.2 Hz; 4–6 consecutive signals were averaged automatically. The maximum signal immediately after the flash, representing the total amount of photo-oxidizable P700, was used to normalize the trace. Subsequent to the flash, electrons were delivered from PS II to PSI, tending to reduce P700+, while the background far-red light brought the P700+ concentration back to the steady state in far-red light. The area between the dipping curve and the horizontal line representing the steady state [P700+] in continuous far-red light is a simple empirical measure of the electrons delivered by PS II. The P700 kinetics area was measured about 1 min after the end of high-light pre-treatment, in order to diminish the effect of energy-dependent quenching of excitation energy, while minimizing any substantial recovery in prolonged darkness (Hu et al. 2013).
The so-called P700 kinetics area is directly proportional to the relative content of functional PS II (determined from the oxygen yield per single-turnover repetitive flash), as demonstrated with different species, including spinach (Kou et al. 2012; Chow et al. 2012; Hu et al. 2013; Jia et al. 2014). The rationale for using a PS I signal to assay PS II functionality is based on the fact that the two photosystems work in series in electron transfer; a single-turnover flash can deliver one electron from each functional PS II to PS I, thereby reducing P700+. Therefore, the size of P700 kinetics area directly depends on the relative content of functional PS II. Since the continuous far-red light is very weak, no electrons would return from the acceptor side of around PS I in a cyclic electron flux. Indeed, infiltration with MV, which is known to intercept electrons on the acceptor side of PS I, did not reduce the P700 kinetics area (Jia et al. 2014). One advantage of using the P700 kinetics area is that it is a whole-tissue measurement, unlike Chl fluorescence which is detected from an unspecified depth in the leaf tissue. Another advantage is that the measurement is fast and non-intrusive, and can be made within one minute after the cessation of illumination if the flash is sufficiently strong; therefore any recovery of photoinactivated PS II in prolonged darkness can be avoided.
To test if the single-turnover flash at maximum intensity could yield the maximum P700 kinetics area, we decreased the flash intensity (I) gradually by neutral density films (Lee filters, UK). An extrapolated maximum area (M fitted) could be obtained by fitting a curve of the form y = M fitted (1 − e−kI), where k is a constant coefficient. The experimental maximum (M exp) was found to be almost identical to the extrapolated maximum (M fitted) for control leaf discs (M exp and M fitted were 100 and 100.4, respectively); leaf discs photoinhibited without lincomycin (M exp and M fitted were 82.0 and 79.1, respectively), and leaf discs photoinhibited in the presence of lincomycin (M exp and M fitted were 58.2 and 56.6, respectively) (Fig. 1). This maximum corresponded to the capacity for flash-induced delivery of electrons from the functional PS II complexes to P700+.
The P700 kinetics area as a function of relative flash intensity (I). High-light-acclimated spinach leaf discs were either used as control or photoinactivated (PI) for 1 h at 1500 μmol m−2 s−1 in the presence or absence of lincomycin. Each dataset was fitted by an equation of the form y = M fitted (1 − e−kI), yielding both M fitted (horizontal dashed lines) and the coefficient k. The experimental maximum, M exp, at 100 % of flash intensity was close to the M fitted value in each case. Each point is a mean of 4 replicates ± SE
A simple kinetic model containing the three parameters k i, τ, and initial k r
Assuming that both photoinactivation of the functional fraction of PS II (F) and recovery of the non-functional fraction of PS II (1 − F) are first-order reactions, the reaction scheme:
is described by the differential equation:
where t is the time, k r is the repair rate coefficient, and k i is the photoinactivation rate coefficient. This model sets k r as a constant and is regarded as a static k r model in the present study.
In general, the PS II repair rate coefficient may change with illumination time. To describe this variation of k r phenomenologically, we introduced an empirical parameter τ, the time for the initial k r to decrease by half. Equation (1) can be modified to be
which is solved with the aid of a Matlab program (Matlab, R2010b, the MathWorks, Natick, Massachusetts, USA) to yield the function:
where \(F\left( T \right)\) represents the current fraction of active PS II after cumulative photoinhibitory treatment time T, with the boundary condition that F = 1.0 at T = 0.
Equation (3) is obtained by the following Matlab code:syms F k r τ
where F represents the functional PS II fraction.
This model is termed a dynamic k r model in the present study. The maximum P700 kinetics area of a control sample is taken to represent the fraction F = 1.0. The k r in Eqs. 2 and 3 is regarded as the initial k r at the beginning of photoinhibitory treatment. To obtain k i, lincomycin was applied to block the de novo synthesis of chloroplast D1 protein, leading to k r = 0 in Eq. (2), which yields a first-order reaction for loss of active PS II centres:
Equation (4) was used to fit the data points for photoinactivation in the presence of lincomycin to obtain k i, using the software Origin 7 (Microcal Software Inc, Northhampton, MA, USA), allowing k i to vary from an initial estimate until a stable value is obtained after a number of iterations.
A program was developed to fit the net photoinactivation data points in the absence of lincomycin by using Eq. (3) to obtain initial k r, τ and the coefficient of correlation. This program was designed based on an enumeration method; a best fit must guarantee the least sum of squares error between observed and calculated data among all potential fits.
Meanwhile, there has been a more elaborate model developed by Tyystjärvi et al. (1994), in which there are inactive PS II centres still containing the D1 protein and those with the D1 protein degraded. In this coupled first-order reactions model, besides k
i, and instead of k
r, rate coefficients for degradation (k
d) and synthesis (k
s) were introduced: where F1 is the fraction of active PS II complexes, F2 is the fraction of inactive PS II complexes containing the D1 protein, and F3 is the fraction of inactive PS II complexes in which the D1 protein has been degraded. This model was only analysed by Tyystjärvi et al. (1994) for the special case where k
s = 0. We used this model in full to simulate the net photoinactivation of PS II in leaves when repair is permitted.
where F1 is the fraction of active PS II complexes, F2 is the fraction of inactive PS II complexes containing the D1 protein, and F3 is the fraction of inactive PS II complexes in which the D1 protein has been degraded. This model was only analysed by Tyystjärvi et al. (1994) for the special case where k
s = 0. We used this model in full to simulate the net photoinactivation of PS II in leaves when repair is permitted.
The reaction scheme is described by a set of three differential equations:
An analytical solution for the coupled differential equations can be obtained by the Matlab program, via the code below:
Syms F1 F2 F3 k i k d k s
The analytical solution for the active PS II fraction as a function of time is
In this equation,
However, k d and k s in Eq. (5) are not separable in curve fitting; they can only be derived independently with the aid of additional data such as the D1 protein degradation.
Measurement of electron transport rates
The total electron transport rate through PS I (ETR1) was obtained as the product Y(I) × irradiance × 0.85 × 0.5, where Y(I) is the photochemical yield of PS I, 0.85 is the leaf absorptance, and 0.5 (close to an experimental estimate for spinach by Kou et al. 2013) is assumed to be the fraction of excitation energy partitioned to PS I. The rate of linear electron flow through PS II (ETR2) was estimated as the product ϕ PS II × irradiance × 0.85 × 0.5, where ϕ PS II is the effective photochemical yield of PS II, averaged over open and closed PS II traps. Both Y(I) and ϕ PS II were determined using a Dual-PAM (Walz, Effeltrich, Germany), as described by Miyake et al. (2005).
Statistical analysis
One-way ANOVA was applied to determine the effects of gas composition and growth irradiance on the photoinactivation and photorepair processes. When necessary, data were transformed to meet the assumptions of ANOVA. All analyses were performed with SPSS 11.0 (SPSS, Chicago, USA)
Results
Photoinactivation of PS II in the presence of lincomycin with or without oxygen
The P700 kinetics area is a rapid, empirical, whole-tissue and non-intrusive measurement of the fraction of functional PS II (Kou et al. 2012; Hu et al. 2013). We investigated the time course of net photoinactivation of PS II in the absence of repair by floating leaf discs with the adaxial side in contact with a lincomycin solution and the abaxial side facing air or nitrogen + 0.5 % CO2, while illuminating the adaxial side at an irradiance of 1500 μmol m−2 s−1. Figure 2 shows that the time courses in the presence of lincomycin exhibited first-order kinetics (Tyystjärvi and Aro 1996). Nitrogen + 0.5 % CO2 slowed the decrease of functional PS II in sun leaf discs (p < 0.001) (Fig. 2a). However, there was no significant difference between ambient air and nitrogen + 0.5 % CO2 in shade leaves (p = 0.582, Fig. 2b). Under air conditions, the k i value of a sun leaf was marginally (and non-significantly) smaller than that of a shade leaf (p = 0.227), while under nitrogen + 0.5 % CO2, sun leaves had a considerably smaller k i than shade leaves (p < 0.001) (Table 1).
The time course of photoinactivation of PS II in sun leaf discs (a) or shade leaves (b) in the presence of lincomycin in nitrogen + 0.5 % CO2 (filled square), in air (open circle), or in the presence of MV + lincomycin in air (solid triangles). Leaf discs were pre-floated on a 3-mM lincomycin solution in darkness overnight. Illumination (1500 μmol m−2 s−1) was directed at the adaxial side which was in contact with lincomycin solution. Data points were fitted by the equation: \(F\left( t \right) = e^{{ - k_{\text{i}} t}}\), where F is the fraction of functional PS II assayed by the P700 kinetics area (rel.), k i is the rate coefficient of photoinactivation, and t is the photoinhibition time. Each point is a mean of 4 replicates ± SE
As the effect of oxygen on PS II photoinactivation might be attributed to direct damage by reactive oxygen species (ROS) to which O2 is a precursor, we maximized ROS production by infiltrating leaf discs with MV. MV indeed accelerated photoinactivation of PS II in the presence of lincomycin for both sun and shade leaves in air (Fig. 2). The k i value of sun leaf discs was 1.49 times higher than the control (p < 0.001), and that of shade leaf discs was 1.24 times higher than control (p < 0.001) (Table 1).
PS II recovery in the light in the absence of lincomycin with or without oxygen
Figure 3 depicts the time course of net photoinactivation of PS II in sun and shade leaves at an irradiance of 1500 μmol m−2 s−1, when PS II repair was permitted, in air or nitrogen + 0.5 % CO2. The adaxial side of each floating leaf discs was in contact with water at 25 °C and was illuminated by directing the light vertically upwards. Due to the simultaneous repair, PS II functionality in the absence of lincomycin decreased more slowly than in the presence of lincomycin.
The time course of net photoinactivation of PS II in sun leaf discs (a) or shade leaves (b) in the absence of lincomycin in air (open circle) or in nitrogen + 0.5 % CO2 (filled square). Data points were fitted by a dynamic k r model described in “Materials and methods” section. The correlation coefficients are displayed in the panels. Each point is a mean of 4 replicates ± SE
Oxygen had different impacts on net PS II photoinactivation in sun and shade leaves when PS II repair was permitted. Flushing with nitrogen + 0.5 % CO2 first alleviated and then exacerbated net PS II photoinactivation in sun leaves (Fig. 3a), but alleviated net PS II photoinactivation slightly in shade leaves at all times (Fig. 3b). To quantitatively evaluate the oxygen impact on PS II repair, a dynamic model with three parameters (k i obtained by experiment, initial k r and τ) was developed for curve fitting using Eq. (3). Figure 4 shows the curves fitted by the static k r model and the dynamic k r model to experimental data for net photoinactivation of PS II in sun leaf discs in air. The dynamic k r model gave a much higher coefficient of correlation than the static k r model (0.98 vs. 0.90). Indeed, the coefficients of correlation of curving fitting were improved greatly by the dynamic k r model in all cases in this study.
Oxygen had dramatic and contrasting effects on the initial k r and on τ in sun and shade leaves (Fig. 5). Irrespective of growth irradiance, nitrogen + 0.5 % CO2 greatly increased initial k r (Fig. 5a), but significantly decreased τ (Fig. 5b) compared with air. Thus, k r decreased faster in N2 + 0.5 % CO2, i.e., repair was retarded faster in anaerobic conditions. Compared with a shade leaf, a sun leaf in air had a 2.2-fold greater initial k r and a 2.0-fold greater τ (p < 0.001), suggesting a higher initial capacity to repair damaged PS II complexes in a sun leaf in air, and that such capacity could be maintained for a longer period than in a shade leaf in air. In nitrogen + 0.5 % CO2, a sun leaf still had a superior initial repair capacity (the initial k r being 1.8 fold higher than in a shade leaf, p < 0.01); however, such capacity diminished very rapidly during the treatment at 1500 μmol m−2 s−1 (τ being only 0.4 of that in a shade leaf, p < 0.01).
Initial rate coefficients (k r) of PS II repair and the time (τ) in which the repair rate coefficient decreases to half of the initial k r in air or in nitrogen + 0.5 % CO2, for sun and shade leaves exposed to an irradiance of 1500 μmol m−2 s−1. k r and τ were derived from curve fittings in Figs. 2 and 3, based on a dynamic k r model. Each bar is a mean of 4 replicates ± SE
Response of initial k r and τ to irradiance above 1500 μmol m−2 s−1
The response of initial k r and τ of high-light-acclimated spinach to irradiance ≥1500 μmol m−2 s−1 is shown in Fig. 6. Curve fitting to the time-course data based on the dynamic k r model worked very well at all three irradiances (Fig. 6a). When the incident light increased from 1500 to 2100 μmol m−2 s−1, the initial k r decreased from 2.4 to 0.2 h−1, and τ increased from 3 to about 12 h (Fig. 6b). At an irradiance ≤1200 μmol m−2 s−1, there was little net loss of functional PS II in sun leaves (data not shown), so curve fitting was not attempted.
a The time course of net photoinactivation of PS II in sun leaf discs in the absence of lincomycin at irradiances of 1500, 1800, and 2100 μmol m−2 s−1 in air. b The relationship between the initial rate coefficients (k r) of PS II repair and τ for sun leaves under the three irradiances. The rate coefficients of PS II photoinactivation per se (k i) at 1800 and 2100 μmol m−2 s−1 were deduced from the k i value of 1500 μmol m−2 s−1, based on the strict proportionality between irradiance and k i (Tyystjärvi and Aro 1996). A dynamic k r model as described in “Materials and methods” section was applied to obtain initial k r and τ
Response of electron transport to irradiance of sun- and shade-leaves
The total rate of electron transport through PS I (ETR1) assayed by the P700 signal, and the rate of linear electron transport through PS II (ETR2) assayed by chlorophyll fluorescence are depicted in Fig. 7. As expected, sun leaf discs had significantly higher rates than shade ones when the irradiance exceeded 200 μmol m−2 s−1.
Total electron transport rate through PS I (ETR1) and linear electron transport rate through PS II (ETR2) of sun and shade leaves as a function of irradiance. ETR1 and ETR2 were determined using the P700 signal and Chl fluorescence, respectively. The abaxial side faced air while the adaxial side was in contact with water. Illumination with red LED light was provided on the adaxial side, using an RG9 filter to block the actinic light that normally is supplied along with the 810/870 nm measuring light by the Dual-PAM, leaving only the actinic light that is supplied along with the Chl fluorescence excitation light. Each point is a mean of 4 replicates ± SE
Discussion
O2 has dual roles in the PS II photoinactivation process per se
In the presence of lincomycin, nitrogen + 0.5 % CO2 alleviated PS II photoinactivation in sun leaves exposed to 1500 μmol m−2 s−1, though not in shade leaves (Table 1; Fig. 2), suggesting that the formation of ROS associated with atmospheric oxygen can photodamage PS II directly. This finding is contrary to some studies (Nishiyama et al. 2001, 2011; Takahashi and Murata 2008), but consistent with others (Hideg et al. 2007; Kornyeyev et al. 2010; Blot et al. 2011; Hideg et al. 2011). The reason for such a discrepancy among results is unknown. One possibility is that only qualitative description of photoinactivation curves without statistical significance test of k i values cannot unveil the difference between treatments. Second, the difference in methods and procedures adopted in different studies might also lead to such a discrepancy. For example, application of Chl a fluorescence to represent PS II functionality suffers from the fact that the signal is detected from an unspecified depth in the leaf tissue, and that the depth of signal detection may well vary during the course of illumination (Oguchi et al. 2011). Third, the direct damage to PS II by ROS depends on light conditions and ROS concentration (Blot et al. 2011). In the present study, except for the treatment with MV, the ROS that induced photoinactivation of PS II is probably mainly singlet oxygen (Vass and Cser 2009; Vass 2011), formed in leaves on exposure to strong visible light, with hardly any superoxide free radical (Hideg et al. 2002). Singlet oxygen is formed within PS II: in the reaction centre by charge recombination and in the PS II antenna by intersystem crossing (Krieger-Liszkay 2005). The formation of singlet oxygen is negligible at 200 μmol m−2 s−1, but substantial at 1000 μmol m−2 s−1, and even more so at 2000 μmol m−2 s−1 (Hideg et al. 1998). Singlet oxygen can cause oxidative modification of the reaction centre polypeptides, selective destruction of pigments including P680 chlorophylls and de-stabilization of Mn clusters on the donor side of PS II (Hideg et al. 2011).
On the other hand, O2 can also play a photoprotective role in various electron sinks, notably photorespiration (Osmond 1981) and, to a lesser extent, the Mehler reaction and the plastid terminal oxidase reaction. By mitigating against the excess excitation pressure on PS II, these electron sinks diminish the probability of production of singlet O2 in the vicinity of PS II reaction centres (Cornic 1978; Park et al. 1996). Singlet O2 is regarded as the dominant player in PS II photodamage, according to an acceptor-side photoinactivation hypothesis (Rehman et al. 2013). Indeed, such a protective role is not inconsistent with our observation that there was no significant net difference between shade leaves flushed with nitrogen + 0.5 % CO2 and those flushed with ambient air in the presence of lincomycin (Table 1; Fig. 2): while anaerobicity tended to alleviate photoinactivation by minimizing ROS formation (k i tending to be smaller), it did not permit the photoprotective O2-mediated electron sinks to operate (k i tending to be larger). That is, the atmospheric O2 effect on the PS II photoinactivation process is a balance between the dual roles of oxygen on k i: the damaging effect of excess ROS versus the protective effect of O2-mediated electron sinks. In sun leaves, the observation of overall photoprotection by anaerobic conditions could be due to the relief of substantial oxidative stress in sun leaves.
MV increased the rate coefficient of photoinactivation of PS II (k i) by 49 % in sun leaves and 24 % in shade leaves (Table 1; Fig. 2). Hakala et al. (2005) reported also a slight increase in k i induced by the presence of MV in isolated pumpkin thylakoids. However, there is a difference between these two systems in terms of other responses. MV stimulated electron transport in isolated thylakoids, thereby decreasing the excess photons not utilized in photochemistry and decreasing the excitation pressure in PS II (greater qP) (Hakala et al. 2005), as would be expected; thus, the increase in k i must have come about despite the lowering of the excess of photons, the lowering of excitation pressure and the increase in the photoprotective trans-thylakoid pH gradient. By contrast, spinach leaves infiltrated with MV showed a slight decrease in qP (i.e., a slight increase in excitation pressure), resulting in a lowering of ϕ PS II which translates into a lower linear electron transport rate (Fan et al. 2009); in this situation, there was an increase in k i, as might be expected. In both thylakoids and leaves, notwithstanding these different responses, there might have been another effect of MV which dominated and which led to the overall exacerbation of photoinactivation. As a speculative example, if MV could lodge itself on the acceptor side of PS II as has been hypothesized (Fan et al. 2009), it might accelerate the formation of superoxide locally in PS II via MV to such an extent as to overwhelm any action by superoxide dismutase; this effect would be equivalent to knocking out iron superoxide dismutase with consequent instability in PS II (Zhang et al. 2011), thereby increasing k i. Although overexpression of iron superoxide dismutase does little to alleviate photoinactivation of PS II (Tyystjärvi et al. 1999), the observation could be explained if the abundant superoxide produced by MV locally in PS II overwhelms any action by the iron superoxide dismutase which may not have ready access to PS II in the granal stacks.
PS II repair cycle as revealed by a dynamic k r model
Our study clearly demonstrated that the oxygen effect on net photoinactivation in the absence of lincomycin depended on growth irradiance. Flushing with nitrogen + 0.5 % CO2 alleviated net PS II photoinactivation slightly in shade leaves (Fig. 3b). Curiously, for sun leaf discs, anaerobicity firstly alleviated, and then exacerbated net PS II photoinactivation (Fig. 3a). However, it is still unclear how oxygen affected the PS II repair cycle, until a proper model is found to separate photoinactivation per se from repair.
In comparison with a previous model (Kok 1956; Kato et al. 2002; He and Chow 2003; Hu et al. 2013) which sets the rate coefficient of PS II repair (k r) as a constant under a given set of conditions (static k r model), the present model (dynamic k r model) hypothesizes that k r changes during the time course of net photoinactivation, and the parameter τ was introduced. The dynamic k r model greatly improves the coefficients of correlation between observed and fitted data (e.g. Fig. 4); it describes the phenomenon better than the static k r model.
How did the initial k r increase in the absence of oxygen?
When flushed with nitrogen + 0.5 % CO2, both sun and shade leaf discs had a significantly higher initial k r at 1500 μmol photons m−2 s−1 than in air (Fig. 5). The increase in k r in the absence of oxygen is attributable to the avoidance of toxic ROS effects on PS II repair. Oxygen being a precursor to ROS, in the presence of oxygen, various ROS such as O •2 , H2O2 and OH•, are generated by photosynthetic electron transport (Gill and Tuteja 2010), but the dominant presence of singlet oxygen in strong light (Hideg et al. 2002) should not be overlooked. It has been demonstrated that environmental stresses such as salt stress (Allakhverdiev et al. 2002), low temperature (Öquist and Huner 1990), drought (Allakhverdiev and Murata 2004) and high light itself increase the intracellular level of ROS in air; ROS in turn inhibit the repair of photoinactivated PS II (Allakhverdiev et al. 2002; Nishiyama et al. 2011). Further, other circumstances such as disruption of CO2 fixation (Takahashi and Murata 2005), impairment of photorespiration pathway (Takahashi et al. 2007), loss of cyclic electron transfer around PS I (Takahashi et al. 2009), restriction of CO2 entry into leaf tissue during photoinhibition (Hu et al. 2013), all significantly affect the PS II repair via enhanced ROS formation. Nishiyama et al. (2001, 2011) have demonstrated that ROS disrupt PS II repair through inhibiting the de novo synthesis of the D1 protein at the translational level, possibly through the specific inactivation of elongation factor G. Further, such photoinactivation takes place via the formation of an intramolecular disulphide bond (Nishiyama et al. 2011).
How did τ decrease in the absence of oxygen?
Despite a significant increase of initial k r for both sun and shade leaf discs, the absence of oxygen led to a sharp decrease in τ. That is, k r decreased faster, suggesting that PS II repair quickly slowed down in the absence of oxygen (Figs. 3, 5). A plausible explanation for this is that the accumulation of non-degraded photodamaged D1 protein in the PS II reaction centre under anaerobic conditions impaired the repair cycle. Anaerobicity inhibits D1 protein degradation significantly because the degradation of D1 protein is associated with ROS produced during photoinhibition in the presence of oxygen; the D1 protein remains intact during anaerobic photoinhibitory treatment, until oxygen is introduced, whereupon D1 protein is lost rapidly (Vass et al. 1992), although the specific ROS responsible for D1 protein degradation remains unclear (Edelman and Mattoo 2008). The loss of D1 protein could come about if, for example, a conformational change in PS II caused by ROS triggers proteolytic cleavage of the D1 protein (Miyao et al. 1995). Only after degradation of photodamaged D1 proteins can the synthesis of the D1 protein and its insertion into the thylakoid membrane be completed (Mulo et al. 2012). The loss of D1 protein tends to lag behind the photoinhibitory loss of PS II activity (Aro et al. 1993); this was most obvious for photoinhibitory treatment at a low temperature which resulted in a much faster loss of active PS II compared with D1 protein, though photoinhibition at room temperature resulted in the loss of D1 and active PS II with the same time course (Osmond and Chow 1988). Therefore, in the absence of oxygen, an inactive PS II could possess D1, the degradation of which could be a limiting step in the repair process; in that case, the initial k r could decline much faster in anaerobic conditions (τ decreased).
O2 has dual roles in the PS II repair cycle
The different effects of oxygen on initial k r and τ strongly suggest that O2 has dual roles in the PS II repair cycle. The formation of ROS could stimulate the degradation of photodamaged D1 proteins on the one hand, but inhibit de novo synthesis of D1 proteins on the other, and the balance between them determines the net effect of O2 on the PS II repair cycle. Therefore, the rate of D1 protein synthesis per se is not the only factor limiting the PS II repair cycle in higher plants; the rate of D1 protein degradation also plays a critical role. To further substantiate this hypothesis, we applied the coupled first-order reactions model (Tyystjärvi et al. 1994) (see “Materials and methods” section). The simulation outputs for both sun and shade spinach leaves are shown in Fig. 8. For suitable values of k d and k s in air, and assuming that the absence of oxygen would lead to a lower k d but a higher k s, the resulting simulation curves in Fig. 8 are comparable to the observations (Fig. 3). However, as k s and k d cannot be derived solely from the time course of net PS II photoinactivation, further experimentation is required for a quantitative evaluation of how oxygen affects PS II repair based on the coupled first-order reactions model.
A simulation of the time course of net PS II photoinactivation (simultaneous repair permitted in the absence of lincomycin), based on the coupled first-order reactions model (Tyystjärvi et al. 1994). In this model, besides k i, rate coefficients of D1 protein degradation (k d) and synthesis (k s) were introduced. a The time course of net PS II photoinactivation in sun leaves in air (line) or in nitrogen + 0.5 % CO2 (dashed line). b The time course of net PS II photoinactivation of shade leaves in air (line) or in nitrogen + 0.5 % CO2 (dashed line). Values of k i were deduced from Fig. 2. k d and k s were set manually, assuming: (1) the values of sun leaf were higher than that of shade leaf; and (2) anaerobicity increased k s but decreased k d. The assumed values of k d and k s of sun and shade-leaves in air or N2 + 0.5 % CO2 are displayed in the panels
Additional evidence to support the dual roles of oxygen on PS II repair is the response of initial k r and τ to increasing strong irradiance (Fig. 6). The initial k r of sun leaf discs in air decreased while τ increased with the increase of irradiance above 1500 μmol m−2 s−1. Indeed, increasing irradiance increases the rate of OH• accumulation in Chlorella vulgaris (Hirayama et al. 1995) and singlet oxygen in leaves (Hideg et al. 1998), while the rate of D1 protein degradation also increases (Aro et al. 1993), and the rate of de novo D1 synthesis decreases (Sundby et al. 1993), the last effect being due to oxidative stress (He and Chow 2003; Hu et al. 2013). Thus, the increase in ROS accumulation hastened D1 degradation (Aro et al. 1993), while impairing D1 protein synthesis at the translational level (Nishiyama et al. 2001, 2011). The increase in τ (slowing of the decline of k r) as the irradiance increased above 1500 μmol m−2 s−1 came about possibly because D1 protein degradation is one of the rate limiting steps in PS II repair, but this limiting step was alleviated by increased ROS production at a higher irradiance. The dual roles of ROS on PS II repair may be one of the reasons why the evidence of protection by quenchers and scavengers of ROS against PS II photoinactivation has been elusive (summarized by Tyystjärvi 2013). Nevertheless, other mechanisms may also need to be taken into account. For example, mutants lacking FtsH (var1 and var2) are defective in D1 degradation even though ROS accumulates (Kato et al. 2009).
The different behaviours of sun and shade leaves in PS II photoinactivation and repair
In air, the difference in k i values between the low- and high-light-acclimated spinach was not significant. By contrast, sun leaves showed significantly greater τ and initial k r than shade leaves, demonstrating the importance of repair to protect against net photoinactivation of PS II in sun leaves (Fig. 5). The similar k i values but different PS II recovery kinetics between sun and shade leaves have been reported in the literature (Aro et al. 1993); indeed, sun and high-light plants have a higher capacity for PS II repair (Aro et al. 1993; Greer et al. 1986) and D1 synthesis (Sundby et al. 1993). Growth irradiance affects the rate of D1 degradation rate, with sun leaves having a higher degradation rate (Aro et al. 1993) compared with shade leaves when illuminated at the same irradiance.
High-light-acclimated plants contain more abundant enzymes, ribosomes, etc., which may facilitate the operation of the PS II repair cycle. For example, the FtsH (protein responsible for D1 degradation) content increases with growth light, in parallel with an increase in the rate constant for removal of D1 protein (Campbell et al. 2013). The structure of thylakoid membranes may also participate in the regulation of PS II repair cycle. Despite the paucity of experimental evidence, it is supposed that lower degradation rate of shade leaf is partly associated with slow migration of photodamaged D1 protein from appressed regions to non-appressed ones for degradation (Aro et al. 1993; Kato et al. 2002; Oguchi et al. 2008; Järvi et al. 2015). Although both sun and shade leaves increased their initial k r by a similar factor (2–3) in response to anaerobicity, sun leaves lowered τ more than tenfold compared with three fold in shade leaves in response to anaerobicity; this suggests that sun leaves had a higher sensitivity of PS II repair to oxygen, consistent with another report (Blot et al. 2011). That is, in sun leaves in air, the initial k r declined much more slowly, presumably aided by a relatively efficient degradation of the D1 protein. A small extent of granal stacking in sun leaves may be one factor that facilitates migration of photodamaged D1 protein from appressed to non-appressed regions for degradation of the D1 protein (Oguchi et al. 2008).
Conclusions
Our results suggest that oxygen has dual roles in the PS II photoinactivation process per se: a damaging role as a precursor of ROS and a photoprotective role in mediating electron sinks. Oxygen also had dual roles in the repair process. The dynamic k r model developed in the present study greatly increases the correlation coefficients between observed and fitted data, compared to a static k r model (Fig. 4), suggesting that it describes the real situation better. Oxygen decreased the initial k r while increasing τ at 1500 μmol photons m−2 s−1. Similarly, as irradiance increased above 1500 μmol m−2 s−1, the initial k r decreased while τ increased. Based on these observations, our hypothesis still stands. That is, on the one hand, formation of ROS associated with oxygen reduction in electron transport could stimulate the degradation of photodamaged D1 proteins, thereby avoiding the accumulation of non-functional PS II with D1 not degraded, which would impair the repair cycle and shorten τ; on the other hand, ROS inhibited the de novo synthesis of D1 proteins. The balance between them determines the net effect of O2 on PS II repair.
Abbreviations
- Chl:
-
Chlorophyll
- D1 protein:
-
psbA gene product in the PS II reaction centre
- ETR1, ETR2:
-
The rate of electron transport through PS I, PS II, respectively
- F :
-
The functional fraction of PS II
- I :
-
Irradiance
- k d, k i, k r, k s :
-
Rate coefficients of D1 protein degradation, PS II photoinactivation, PS II repair, D1 protein synthesis, respectively
- MV:
-
Methyl viologen
- ϕ PS II :
-
The effective photochemical yield of PS II during actinic illumination
- P700:
-
Special chlorophyll pair in PS I
- PS II, PS I:
-
Photosystem II, Photosystem I, respectively
- qP :
-
Photochemical quenching parameter
- ROS:
-
Reactive oxygen species
- τ :
-
Time for the initial k r to decrease by half
- Y(I):
-
The effective photochemical yield of PS I during actinic illumination
References
Allakhverdiev SI, Murata N (2004) Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage—repair cycle of Photosystem II in Synechocystis sp. PCC 6803. Biochim Biophys Acta 1657:23–32
Allakhverdiev SI, Nishiyama Y, Miyairi S, Yamamoto H, Inagaki N, Kanesaki Y, Murata N (2002) Salt stress inhibits the repair of photodamaged Photosystem II by suppressing the transcription and translation of psbAGenes in synechocystis. Plant Physiol 130:1443–1453
Aro EM, McCaffery S, Anderson JM (1993) Photoinhibition and D1 protein degradation in peas acclimated to different growth irradiances. Plant Physiol 103:835–843
Blot N, Mella-Flores D, Six C, Le Corguillé G, Boutte C, Peyrat A, Monnier A, Ratin M, Gourvil P, Campbell DA, Garczarek L (2011) Light history influences the response of the marine cyanobacterium Synechococcus sp. WH7803 to oxidative stress. Plant Physiol 156:1934–1954
Campbell DA, Tyystjärvi E (2012) Parameterization of photosystem II photoinactivation and repair. Biochim Biophys Acta 1817:258–265
Campbell D, Hossain Z, Cockshutt A, Zhaxybayeva O, Wu H, Li G (2013) Photosystem II protein clearance and FtsH function in the diatom Thalassiosira pseudonana. Photosynth Res 115:43–54
Chow W, Fan D-Y, Oguchi R, Jia H, Losciale P, Park Y-I, He J, Öquist G, Shen Y-G, Anderson J (2012) Quantifying and monitoring functional photosystem II and the stoichiometry of the two photosystems in leaf segments: approaches and approximations. Photosynth Res 113:63–74
Cornic G (1978) La photorespiration se déroulant dans un air sans CO2 a-t-elle une fonction? Can J Bot 56:2128–2137
Edelman M, Mattoo A (2008) D1-protein dynamics in photosystem II: the lingering enigma. Photosynth Res 98:609–620
Fan D-Y, Jia H, Barber J, Chow WS (2009) Novel effects of methyl viologen on photosystem II function in spinach leaves. Eur Biophys J 39:191–199
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Greer DH, Berry JA, Björkman O (1986) Photoinhibition of photosynthesis in intact bean leaves: role of light and temperature, and requirement for chloroplast-protein synthesis during recovery. Planta 168:253–260
Hakala M, Tuominen I, Keranen M, Tyystjärvi T, Tyystjärvi E (2005) Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of photosystem II. Biochim Biophys Acta 1706:68–80
He J, Chow WS (2003) The rate coefficient of repair of photosystem II after photoinactivation. Physiol Plant 118:297–304
Hideg É, Kálai T, Hideg K, Vass I (1998) Photoinhibition of photosynthesis in vivo results in singlet oxygen production detection via nitroxide-induced fluorescence quenching in broad bean leaves. Biochem 37:11405–11411
Hideg É, Barta C, Kálai T, Vass I, Hideg K, Asada K (2002) Detection of singlet oxygen and superoxide with fluorescent sensors in leaves under stress by photoinhibition or UV radiation. Plant Cell Physiol 43:1154–1164
Hideg É, Kós PB, Vass I (2007) Photosystem II damage induced by chemically generated singlet oxygen in tobacco leaves. Physiol Plant 131:33–40
Hideg É, Deák Z, Hakala-Yatkin M, Karonen M, Rutherford AW, Tyystjärvi E, Vass I, Krieger-Liszkay A (2011) Pure forms of the singlet oxygen sensors TEMP and TEMPD do not inhibit Photosystem II. Biochim Biophys Acta 1807:1658–1661
Hirayama S, Ueda R, Sugata K (1995) Effect of hydroxyl radical on intact microalgal photosynthesis. Energy Convers Manag 36:685–688
Hu Y-Y, Fan D-Y, Losciale P, Chow W, Zhang W-F (2013) Whole-tissue determination of the rate coefficients of photoinactivation and repair of photosystem II in cotton leaf discs based on flash-induced P700 redox kinetics. Photosynth Res 117:517–528
Järvi S, Suorsa M, Aro E-M (2015) Photosystem II repair in plant chloroplasts—regulation, assisting proteins and shared components with photosystem II biogenesis. Biochim Biphys Acta. doi:10.1016/j.bbabio.2015.01.006
Jia H, Dwyer SA, Fan D-Y, Han Y, Badger MR, von Caemmerer S, Chow WS (2014) A novel P700 redox kinetics probe for rapid, non-intrusive and whole-tissue determination of photosystem II functionality, and the stoichiometry of the two photosystems in vivo. Physiol Plant 152:403–413
Kato MC, Hikosaka K, Hirose T (2002) Photoinactivation and recovery of photosystem II in Chenopodium album leaves grown at different levels of irradiance and nitrogen availability. Funct Plant Biol 29:787–795
Kato Y, Miura E, Ido K, Ifuku K, Sakamoto W (2009) The variegated mutants lacking chloroplastic FtsHs are defective in D1 degradation and accumulate reactive oxygen species. Plant Physiol 151:1790–1801
Kok B (1956) On the inhibition of photosynthesis by intense light. Biochim Biophys Acta 21:234–244
Kornyeyev D, Logan BA, Holaday AS (2010) Excitation pressure as a measure of the sensitivity of photosystem II to photoinactivation. Funct Plant Biol 37:943–951
Kou J, Oguchi R, Fan D-Y, Chow WS (2012) The time course of photoinactivation of photosystem II in leaves revisited. Photosynth Res 113:157–164
Kou J, Takahashi S, Oguchi R, Fan D-Y, Badger MR, Chow WS (2013) Estimation of the steady-state cyclic electron flux around Photosystem I in spinach leaf discs in white light, CO2-enriched air and other varied conditions. Funct Plant Biol 40:1018–1028
Krause GH, Köster S, Wong SC (1985) Photoinhibition of photosynthesis under anaerobic conditions studied with leaves and chloroplasts of Spinacia oleracea L. Planta 165:430–438
Krieger-Liszkay A (2005) Singlet production in photosynthesis. J Exp Bot 56:337–346
Miyake C, Miyata M, Shinzaki Y, K-i Tomizawa (2005) CO2 response of cyclic electron flow around PS I (CEF-PS I) in tobacco leaves—relative electron fluxes through PS I and PS II determine the magnitude of non-photochemical quenching (NPQ) of Chl fluorescence. Plant Cell Physiol 46:629–637
Miyao M, Ikeuchi M, Yamamoto N, T-a Ono (1995) Specific degradation of the D1 protein of Photosystem II by treatment with hydrogen peroxide in darkness: implications for the mechanism of degradation of the D1 protein under illumination. Biochemistry 34:10019–10026
Mulo P, Sakurai I, Aro E-M (2012) Strategies for psbA gene expression in cyanobacteria, green algae and higher plants: from transcription to PS II repair. Biochim Biophys Acta 1817:247–257
Nedbal L, Masojídek J, Komenda J, Prášil O, Šetlik I (1990) Three types of Photosystem II photoinactivation. 2. Slow processes. Photosynth Res 24:89–97
Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N (2001) Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J 20:5587–5594
Nishiyama Y, Allakhverdiev SI, Murata N (2011) Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol Plant 142:35–46
Oguchi R, Jia H, Barber J, Chow W (2008) Recovery of photoinactivated photosystem II in leaves: retardation due to restricted mobility of photosystem II in the thylakoid membrane. Photosynth Res 98:621–629
Oguchi R, Terashima I, Kou J, Chow WS (2011) Operation of dual mechanisms that both lead to photoinactivation of Photosystem II in leaves by visible light. Physiol Plant 142:47–55
Öquist G, Huner NA (1990) Effects of cold acclimation on the susceptibility of photosynthesis to photoinhibition. In: Baltscheffsky M (ed) Current research in photosynthesis. Springer, Netherlands, pp 1431–1434
Osmond CB (1981) Photorespiration and photoinhibition. Some implications for the energetics of photosynthesis. Biochim Biophys Acta 639:77–89
Osmond CB, Chow WS (1988) Ecology of photosynthesis in the sun and shade: summary and prognostications. Aust J Plant Physiol 15:1–9
Park Y II, Chow W, Osmond CB, Anderson J (1996) Electron transport to oxygen mitigates against the photoinactivation of Photosystem II in vivo. Photosynth Res 50:23–32
Powles SB (1984) Photoinhibition of photosynthesis induced by visible-light. Annu Rev Plant Physiol Plant Mol Biol 35:15–44
Powles S, Björkman O (1982) Photoinhibition of photosynthesis: effect on chlorophyll fluorescence at 77 K in intact leaves and in chloroplast membranes of Nerium oleander. Planta 156:97–107
Rehman AU, Cser K, Sass L, Vass I (2013) Characterization of singlet oxygen production and its involvement in photodamage of Photosystem II in the cyanobacterium Synechocystis PCC 6803 by histidine-mediated chemical trapping. Biochim Biophys Acta 1827:689–698
Satoh K (1970) Mechanism of photoinactivation in photosynthetic systems III. Site and mode of photoinactivation in photosystem I. Plant Cell Physiol 11:187–197
Šetlik I, Allakhverdiev SI, Nedbal L, Šetlikova E, Klimov VV (1990) Three types of Photosystem II photoinactivation. Photosynth Res 23:39–48
Sundby C, McCaffery S, Anderson JM (1993) Turnover of the photosystem II D1 protein in higher plants under photoinhibitory and nonphotoinhibitory irradiance. J Biol Chem 268:25476–25482
Takahashi S, Murata N (2005) Interruption of the Calvin cycle inhibits the repair of Photosystem II from photodamage. Biochim Biophys Acta 1708:352–361
Takahashi S, Murata N (2008) How do environmental stresses accelerate photoinhibition? Trends Plant Sci 13:178–182
Takahashi S, Bauwe H, Badger M (2007) Impairment of the photorespiratory pathway accelerates photoinhibition of Photosystem II by suppression of repair but not acceleration of damage processes in Arabidopsis. Plant Physiol 144:487–494
Takahashi S, Milward SE, Fan D-Y, Chow WS, Badger MR (2009) How does cyclic electron flow alleviate photoinhibition in Arabidopsis? Plant Physiol 149:1560–1567
Tyystjärvi E (2013) Photoinhibition of Photosystem II. Int Rev Cell Mol Biol 300:243–303
Tyystjärvi E, Aro EM (1996) The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci USA 93:2213–2218
Tyystjärvi E, Mäenpää P, Aro E-M (1994) Mathematical modelling of photoinhibition and Photosystem II repair cycle. I. Photoinhibition and D1 protein degradation in vitro and in the absence of chloroplast protein synthesis in vivo. Photosynth Res 41:439–449
Tyystjärvi E, Marjukka R, Arisi A-CM, Kettunen R, Jouanin L, Foyer CH (1999) Photoinhibition of photosystem II in tobacco plants overexpressing glutathione reductase and poplars overexpressing superoxide dismutase. Phyiol Plant 105:409–416
Vass I (2011) Role of charge recombination processes in photodamage and photoprotection of the photosystem II complex. Physiol Plant 142:6–16
Vass I, Cser K (2009) Janus-faced charge recombinations in photosystem II photoinhibition. Trends Plant Sci 14:200–205
Vass I, Styring S, Hundal T, Koivuniemi A, Aro E, Andersson B (1992) Reversible and irreversible intermediates during photoinhibition of photosystem II: stable reduced QA species promote chlorophyll triplet formation. Proc Natl Acad Sci USA 89:1408–1412
Zhang Y, Ding S, Lu Q, Yang Z, Wen X, Zhang L, Lu C (2011) Characterization of photosystem II in transgenic tobacco plants with decreased iron superoxide dismutase. Biochim Biophys Acta 1807:391–403
Acknowledgments
The support of this work by an Australian Research Council Grant (DP1093872) awarded to W.S.C. and a Knowledge Innovation Program of the Chinese Academy of Sciences Grant (KZCX2-XB3-09-02) and a Grant of NNSF of China (No. 31370424) to D.-Y. F. is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fan, DY., Ye, ZP., Wang, SC. et al. Multiple roles of oxygen in the photoinactivation and dynamic repair of Photosystem II in spinach leaves. Photosynth Res 127, 307–319 (2016). https://doi.org/10.1007/s11120-015-0185-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-015-0185-y