Abstract
Action spectra of photoinactivation of Photosystem II (PS II) in wild-type and chlorophyll b-less barley leaf segments were obtained. Photoinactivation of PS II was monitored by the delivery of electrons from PS II to PS I following single-turnover flashes superimposed on continuous far-red light, the time course of photoinactivation yielding a rate coefficient k i. Susceptibility of PS II to photoinactivation was quantified as the ratio of k i to the moderate irradiance (I) of light at each selected wavelength. k i/I was very much higher in blue light than in red light. The experimental conditions permitted little excess light energy absorbed by chlorophyll (not utilized in photochemical conversion or dissipated in controlled photoprotection) that could lead to photoinactivation of PS II. Therefore, direct absorption of light by Mn in PS II, rather than by chlorophyll, was more likely to have initiated the much more severe photoinactivation in blue light than in red light. Mutant leaves were ca. 1.5-fold more susceptible to photoinactivation than the wild type. Neither the excess-energy mechanism nor the Mn mechanism can explain this difference. Instead, the much lower chlorophyll content of mutant leaves could have exerted an exacerbating effect, possibly partly due to less mutual shading of chloroplasts in the mutant leaves. In general, which mechanism dominates depends on the experimental conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oxygenic photosynthesis is intrinsically suicidal (van Gorkom and Schelvis 1993), meaning that Photosystem II (PS II) is photoinactivated by light during photosynthesis. This phenomenon has been studied for over a century (Ewart 1896; Powles 1984), and the mechanisms have been actively investigated in more recent years (for reviews, see Aro et al. 1993; Tyystjärvi 2008; Nishiyama et al. 2011; Oguchi et al. 2011a; Ohad et al. 2011; Vass 2011).
A large number of experimental results support a mechanism whereby PS II is inactivated by singlet oxygen, 1O2 (Hideg et al. 1994; Cser and Vass 2007; Vass and Cser 2009), particularly under excess light conditions, though this mechanism can occur even in intermittent flashes or continuous low light that delivers electrons infrequently to Q B, the secondary quinine acceptor in PS II. (Keren et al. 1995, 1997). This mechanism is often referred to as “charge recombination-induced photoinactivation” or “acceptor side photoinactivation” (Ohad et al. 2011) or excess-light photoinactivation of PS II (Kato et al. 2003). This mechanism frequently occurs when absorption of light by chlorophyll exceeds photochemical conversion (Demmig and Björkman 1987; Osmond 1994; Kornyeyev et al. 2010). It is initiated by the absorption of light by photosynthetic pigments, and is supported by the action spectrum of photoinactivation of PS II in isolated thylakoids, showing distinct peaks at red and blue wavelengths (Jones and Kok 1966), with the red peak sometimes slightly blue-shifted (Santabarbara et al. 2002).
A second mechanism is that the primary step that initiates photoinactivation of PS II is the direct absorption of light by the Mn4–O5–Ca–Cl2 cluster in PS II, followed by the removal of a Mn atom (Hakala et al. 2005; Ohnishi et al. 2005). This results in inactivation of the oxygen-evolving complex, leading to secondary events which damage the PS II complex, e.g., the existence of a longer-lived highly oxidizing P680+ or the enhancement of charge recombination which can result in highly reactive singlet oxygen. This mechanism is termed “Mn-induced donor side” inactivation or the “two-step mechanism”. Consistent with this mechanism, the action spectrum of photoinactivation of PS II, at visible wavelengths, shows that blue light is more effective than green light, which is more effective than red light in isolated chloroplasts/thylakoids (Jung and Kim 1990; Hakala et al. 2005). Further, model Mn compounds show absorption spectra similar to the action spectrum of photoinactivation of PS II (Hakala et al. 2005; Tyystjärvi 2008).
There is a third mechanism, in which the low pH in the thylakoid lumen during illumination can impair the donor-side function of PS II, most probably by removal of Ca and Cl from the Mn4–O5–Ca–Cl2 cluster. This mechanism, however, may not be important in leaves if an efflux of protons from the thylakoid lumen during prolonged illumination is coupled to the influx of Ca2+ via an antiporter (Ettinger et al. 1999).
Oguchi et al. (2009, 2011a, b) suggested that the excess-energy mechanism and the two-step mechanism need not be mutually exclusive. In this study, we set out to further investigate the action spectrum of the photoinactivation of PS II in barley leaves. We reasoned that the excess-light mechanism should show a distinct red peak corresponding to light absorption by chlorophyll in leaves, as has been reported for Chlorella (Schreiber and Klughammer 2013). The chlorina mutant of barley (used by Highkin 1950), with poorer light absorption in the green compared with the wild type, was expected to show a more distinct peak in the red. To our surprise, the red peak that we obtained was rather small. Further, light of blue wavelength was very much more effective than light of red wavelengths in causing photoinactivation of PS II. The results are better explained in terms of the two-step mechanism operating under the conditions of measurement of the action spectra of photoinactivation of PS II. In addition, we found that PS II complexes in wild-type barley leaves were about 50 % less susceptible to photoinactivation than the chlorina mutant.
Materials and methods
Growth of plants
Barley wild type (Hordeum vulgare L.) and its chlorophyll b-less mutant (chlorine-f2; Highkin 1950) were grown in a glasshouse at approximately 28/18 °C (day/night) under natural light (maximum irradiance ~1000 µmol m−2 s−1). The plants were provided with a nutrient solution of “Aquasol” (Yates Australia, Padstow, NSW) weekly.
Measurement of the absorptance spectra of leaves
The transmittance spectrum of leaf tissue was measured in a darkened room by placing a leaf segment directly against the sensor (without cosine correction) of a spectroradiometer (PS300, Apogee Instruments, USA), and the raw spectrum of transmitted light from an incandescent source was normalized to the full transmission spectrum obtained in the absence of leaf tissue. The raw reflection spectrum R leaf (λ) was measured by placing the sensor (without cosine correction) about 1 cm vertically above three leaf segments placed side by side and held down by tape at the far edges. The incandescent light was incident on the leaf segments at an angle of about 45° from the normal to the surface. A matt white 100 % reflectance standard (Apogee Instruments, USA) was used in place of the leaf segments to generate a raw full reflectance spectrum R 100 (λ). A matt black card, of close to zero reflectance, was used to provide a baseline R 0 (λ) to be subtracted from (1) R 100 (λ), yielding the signal for 100 % reflection across the visible wavelength range, or (2) R leaf (λ), yielding the signal reflected by leaf segments across the visible wavelength range. The leaf reflectance spectrum was calculated as [R leaf (λ)−R 0 (λ)]/[R 100 (λ)−R 0 (λ)]. The absorption spectrum was obtained by subtracting the sum of the transmittance spectrum and the reflectance spectrum from 1.0.
Photoinhibitory treatment of leaf segments
For photoinactivation of PS II in the presence of lincomycin, barley leaf segments (~15 mm long) were first floated on 1 mM lincomycin solution overnight in darkness, with the abaxial side facing air, to allow uptake of the inhibitor of chloroplast-encoded protein synthesis. The next morning, leaf segments were first illuminated with white LED light at about 200 µmol m−2 s−1 for about 10 min to allow photosynthetic induction to occur before control leaf discs were assayed for the complete population of functional PS II. Leaf segments that were to be exposed to photoinhibitory light continued to be floated on a 1-mM lincomycin solution, with the abaxial side facing air, and illuminated with LED light of a selected wavelength and irradiance, by directing the light vertically upwards onto the adaxial side through a clear Petri dish. Illumination was applied for up to 6 h to obtain the first-order time course of photoinactivation of PS II, which yielded the rate coefficient of photoinactivation k i. The irradiance of light (supplied by LED arrays, LEDxxx-66-60, Roithner LaserTechnik, Vienna, Austria) at each wavelength was measured with a spectroradiometer (Ocean Optics, Winter Park, FL, USA) fitted with a cosine-corrected sensor that had been newly re-calibrated.
Assaying the functional fraction of PS II by measurement of the redox kinetics of P700
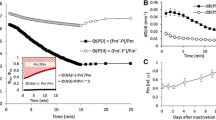
Leaf segments were used for measurement of redox changes of P700 with a dual wavelength (810/870 nm) unit (ED-P700DW) attached to a phase amplitude modulation fluorometer (PAM 101/102/103, Walz, Effeltrich, Germany) and used in the reflectance mode (Chow and Hope 2004). To obtain redox changes due to a flash superimposed on continuous far-red light, a steady state was reached by illumination with far-red light (~20 µmol photons m−2 s−1, peak wavelength 729 nm, 102-FR, Walz, Effeltrich, Germany) for ≥1 min. Then a single-turnover, saturating xenon flash (EG&G xenon flash, full width at half height = 6 μs) was applied to the adaxial side of the leaf disc. Timing of the start of data acquisition (time constant = 95 μs), the triggering of the flash, and the repetition rate were controlled by a pulse/delay generator (Model 565, Berkeley Nucleonics Corporation, USA). The analogue output from the fluorometer was digitized and stored in a computer using a program written by the late A.B. Hope. Flashes were given at 0.2 Hz, and 4 consecutive signals were averaged automatically. The maximum signal immediately after the flash was taken as the total amount of photo-oxidizable P700 and used to normalize the trace (Fig. 1). The area bounded by a trace and the horizontal dashed line corresponding to steady state in continuous weak far-red light is here termed the P700 kinetics area; when the leaf segment was photoinhibited, the P700 kinetics area was decreased. It has been shown empirically that the P700 kinetics area is directly proportional to the oxygen yield per repetitive, single-turnover flash for a number of plant species (Kou et al. 2012; Hu et al. 2013).
The time course of redox changes in the P700+ signal in Chl b-less barley leaves when a single-turnover, saturating flash was superimposed on continuous background far-red light. Weak continuous far-red light resulted in the photo-oxidation of about 85 % of the total photo-oxidizable P700. The single-turnover saturating flash photo-oxidized the remainder of the P700, giving the spike (set to 1.0). Subsequent to the flash, electrons arrived from PS II to P700+, but the background far-red light brought the [P700+] back to the steady-state level. Each signal trace is an average of four scans. The “P700 kinetics area”, bounded by a signal trace and the horizontal line corresponding to the steady-state oxidation level of P700, decreased as the photoinhibition pretreatment time increased from 0, to 40 and 80 min using light at 453 nm and irradiance 580 µmol m−2 s−1
Measurement of the excess fraction of absorbed energy
The excess fraction of absorbed energy E that is neither utilized in photochemical conversion nor dissipated by non-photochemical means of photoprotection was calculated as
(Demmig-Adams et al. 1996; Kato et al. 2003; Hikosaka et al. 2004; Kornyeyev et al. 2010; Eq. 1 of Oguchi et al. 2011a). In this definition of E,
where \(F_{\text{m}}^{'}\) and \(F_{{}}^{'}\) are the actinic light-adapted maximum and steady-state yield of Chl fluorescence yield (detected at >650 nm), respectively. \(F_{\text{m}}^{\prime}\) was measured with a saturating pulse of irradiance 5000 µmol m−2 s−1; \(F_{\text{o}}^{\prime}\) is the light-adapted Chl fluorescence yield when PS II reaction centre traps are open, and was calculated from F m (measured on a dark-adapted leaf segment with a saturating pulse of irradiance 5000 µmol m−2 s−1), F o (measured on a dark-adapted leaf segment with a modulated blue excitation light of irradiance 0.05 µmol m−2 s−1), and \(F_{\text{m}}^{\prime}\) (Oxborough and Baker 1997); and \(F_{\text{v}}^{\prime}\) = \(F_{\text{m}}^{\prime}\) − \(F_{\text{o}}^{\prime}\). The actinic light, supplied to the adaxial side by LEDs of selected wavelengths, was 300 µmol m−2 s−1 in each case.
Results
Absorptance spectra of wild-type and Chl b-less barley leaves
The absorptance spectrum of wild-type barley leaves showed a shallow trough around 550 nm. By contrast, Chl b-less mutant leaves showed a noticeably deeper trough in the same wavelength range (Fig. 2). However, even at green wavelengths, about 60 % of the light was absorbed by the paler green leaves.
The rate coefficients of photoinactivation of PS II (k i) determined using a simple P700 kinetics area that indicates the relative abundance of functional PS II in the whole tissue
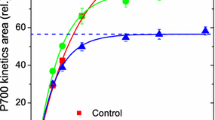
The maximum P700 kinetic area of a control sample at flash saturation (see Methods and Fig. 1) is taken to represent the fraction f = 1.0 of functional PS II. The exponential decrease (Kou et al. 2012) of f from the value 1.0 during the onset of photoinactivation of PS II in the absence of repair (i.e., in the presence of lincomycin) gives the rate coefficient of photoinactivation k i at a given irradiance. At any time t, the rate of photoinactivation PS II is directly proportional to the fraction of functional PS II complexes that are available to be photoinactivated:
In other words, f declines exponentially from an initial value of 1.0: f = exp(- k i t), as given by the example in Fig. 3a. Curve fitting of the data points using the software Origin 7 (Microcal Software Inc, Northhampton, MA, USA) allowed the determination of k i. When k i was plotted against the irradiance of LED light of a particular wavelength used to photoinactivate PS II, a straight line was fitted to the data points and was constrained to pass through the origin [the point (0,0) in Fig. 3b; see also Tyystjärvi and Aro 1996; Lee et al. 2001; Kato et al. 2003]. The slope of the straight line (k i/I) is independent of irradiance, and has the meaning of probability of photoinactivation of PS II per unit photon exposure (Lee et al. 1999). Therefore, we have used this parameter as a measure of the susceptibility of PS II to photoinactivation at any selected wavelength.
a An example of a decrease in the P700 kinetics area with photoinhibition pretreatment time, fitted with a single-exponential decay curve to yield the rate coefficient of photoinactivation of PS II. Chl b-less barley leaf segments were pretreated with light of wavelength 653 nm and irradiance 834 µmol m−2 s−1. b The rate coefficient k i plotted as a function of pretreatment irradiance (at 653 nm). The straight line was fitted by constraining the line to pass through the origin, (0,0)
The action spectrum of photoinactivation of PS II
The action spectrum of photoinactivation of PS II in the visible wavelength range consisted of a plot of the slope k i/I against wavelength. In the semi-log plot of Fig. 4, in either the Chl b-less mutant or the wild type, blue light was more than an order of magnitude as effective as red light in causing photoinactivation of PS II. Further, at a particular blue or red wavelength, PS II in the mutant was approximately 1.5-fold more susceptible to photoinactivation by blue light compared with that by red light. A small peak was discernible in the red region. For comparison, the extinction coefficients of two Mn model compounds are included in Fig. 4, scaled to approximate the data points at 520 nm.
Action spectra of photoinactivation of PS II in wild-type (solid circles) and Chl b-less barley leaves (open circles). The parameter on the y-axis, plotted on a logarithmic scale, is k i/I [in units of m2 (µmol photons)−1], which is interpreted as the probability of photoinactivation per unit photon exposure (see text). The relative extinction coefficient (ε) spectra of Mn(IV) gluconate (adapted from Bodini et al. 1976) and of a Mn(III/IV)-oxo mixed-valence compound (adapted from Wei et al. 2011) are also shown, scaled to approximate the data points at 520 nm
The excess fraction of absorbed energy (E) as a function of wavelength
At the same irradiance of 300 µmol m−2 s−1, the excess fraction of absorbed energy (E) that was neither utilized photochemically nor dissipated in controlled photoprotection (see Methods) exhibited no large variation across the visible wavelength range (Fig. 5). In both the mutant and the wild type, E was very small, between ~0.05 and ~0.20. Further, E of the Chl b-less mutant was even smaller than that of the wild type.
The excess fraction of absorbed energy E, neither utilized in photochemical conversion nor in controlled photoprotective dissipation, for wild-type (solid circles) and Chl b-less barley leaves (open circles), each point being the average of nine leaf segments of the wild type and eight of the mutants
Discussion
The action spectrum of photoinactivation of PS II
The most surprising feature of the action spectrum of photoinactivation of PS II was the very steep rise in k i /I as the wavelength decreased. In both the mutant and the wild type, blue light was greater than an order of magnitude more effective than red light in causing photoinactivation of PS II. In this study, only a moderate irradiance of blue light was used (typically ~300 µmol m−2 s−1). At this irradiance, E was small at all wavelengths, so there was only a little excess light energy that was neither utilized in photosynthesis nor dissipated safely in controlled non-photochemical quenching; consequently, there would be little chance of charge recombination that may lead to the formation of reactive singlet oxygen. Therefore, the excess-energy hypothesis is not likely to explain the high effectiveness of blue light. Instead, it is more likely that direct absorption, by Mn, of blue light, and to some extent green light, was responsible for initiating the photoinactivation process, as advocated by Hakala et al. (2005) and Ohnishi et al. (2005).
At red wavelengths, we needed much higher irradiances (up to about 1000 µmol m−2 s−1) to photoinactivate PS II in leaf tissue to a sufficient extent in a few hours. At a higher irradiance of red light, there was more chance for an increase in excess-energy-induced photoinactivation of PS II. Even so, the action spectra of photoinactivation of PS II showed only a small peak that corresponded to absorption of red light by chlorophyll. Thus, there was only a relatively weak effect of red light in inducing photoinactivation of PS II in leaf discs under the chosen experimental conditions.
Other studies have reported a less extreme difference in susceptibility of PS II to photoinactivation between blue and red light. For example, Sarvikas et al. (2006) observed that blue light at 400 nm (1600 µmol m−2 s−1) was about 7-fold more effective at photoinactivating PS II in wild-type Arabidopsis than red light at 650 nm of the same irradiance. Similarly, Schreiber and Klughammer (2013) showed that, at equal electron transport rate in Chlorella, blue light at 440 nm (1598 µmol m−2 s−1) was approximately 6-fold more effective than red light at 625 nm (4435 µmol m−2 s−1). The several-fold difference reported in these studies is relatively small, compared with the much greater difference observed in the present study, and could be partly attributed to the use of high irradiance (≥1600 µmol m−2 s−1) which would favour the operation of the excess-energy mechanism. Indeed, Schreiber and Klughammer (2013) deconvoluted the wavelength dependence of the two mechanisms in Chlorella. The mechanism initiated by Mn absorption of light is negligible at wavelengths longer than 550 nm. On the other hand, the mechanism initiated by PS II turnover (light absorption by photosynthetic pigments) shows two peaks of comparable heights: one peak in the blue and the other (extrapolated) peak in the red regions. Therefore, the relative contributions of the two mechanisms will determine the overall difference in effectiveness of blue and red light.
The deconvoluted action spectrum of Schreiber and Klughammer (2013) showed that the Mn-initiated mechanism ceased at wavelengths longer than 550 nm. In a comparable way, above this cut-off wavelength, the (re-scaled, relative) extinction coefficient of Mn(IV) gluconate (Bodini et al. 1976) began to fall below the observed k i/I, while that of the Mn(III/IV)-oxo mixed-valence compound (Wei et al. 2011) shows a sharp decline above 555 nm (Fig. 4). The residual action at long visible wavelengths was almost certainly due to light absorption by photosynthetic pigments rather than Mn.
Interestingly, the deconvoluted action spectrum for the mechanism initiated by Mn absorption of light in Chlorella has a steep increase by a factor of 10 when the wavelength decreases from 520 to 440 nm (Schreiber and Klughammer 2013). In a comparable way, though not to quite the same extent, the action spectrum observed in this study changed by a factor of about 3 over the same wavelength range, in both the wild type and the Chl b-less mutant (Fig. 4). In the case of a model compound, Mn(IV) gluconate, the extinction coefficient increases by a factor of 2.5 when the wavelength is decreased from 520 to 440 nm (thick line, Fig. 4). Another model compound, a Mn(III/IV)-oxo mixed-valence compound, has an extinction coefficient that increases by a factor of 8 when the wavelength decreases from 520 to 440 nm (open circles, Fig. 4). When the extinction coefficient of Mn(IV) gluconate was scaled to approximate k i/I of both the wild type and mutant at 520 nm, it roughly tracked the data points down to about 440 nm. By contrast, the increase in extinction coefficient of the Mn(III/IV)-oxo mixed-valence compound was even greater than that of k i/I in either the WT or the b-less mutant. This shows that there is considerable variation in the extinction coefficients of model Mn compounds. Unfortunately, the extinction coefficient of the Mn cluster in vivo is not known.
At 400 nm, the observed k i/I was greater than the (re-scaled, relative) extinction coefficients of the two Mn model compounds. It is not clear what caused this difference, which was quite large when comparing k i/I with the extinction coefficient of Mn(IV) gluconate. Our method of measurement of PS II functionality could have had an impact. As a hypothetical example, suppose at 400 nm (and perhaps also shorter wavelengths) there was an additional mechanism (other than the Mn-initiated mechanism) that resulted in partial photoinactivation of PS II in which the complex, although capable of charge separation and charge stabilization, had enhanced recombination of the electron on \(Q_{\text{A}}^{ - }\) or \(Q_{\text{B}}^{ - }\) with the S states during the water oxidation cycle. In such PS II complexes, an enhanced charge recombination to the ground state would mean that the electron transfer would be wasted. Since our method of measuring PS II activity used in the present study requires electrons from PS II to be delivered to P700+ after a single-turnover flash, the charge recombination would have been equivalent to the absence of charge separation in a completely inactive PS II. In other words, our method would register fewer electrons arriving at P700+ after each flash (i.e., showing greater susceptibility to photoinactivation or a larger k i/I). By contrast, assaying PS II functionality by the rate of oxygen evolution under saturating continuous light (Sarvikas et al. 2006) may register a lesser loss of PS II activity in the sample. This is because, in saturating continuous light, an unsuccessful forward transfer of an electron in a partially inactivated PS II could be compensated for by the actions of other photons, some of which resulting in successful whole-chain electron transport. Thus, the method of using saturating continuous light would not detect partially inactivated PS II complexes that are capable of charge separation, though prone to charge recombination. Further work is needed to elucidate the apparent large observed k i/I at 400 nm and shorter wavelengths.
Comparison of Chl b-less barley with the wild type
PS II in the barley mutant was approximately 1.5-fold more susceptible to photoinactivation than in the wild type (Fig. 4). This confirms the findings of Leverenz et al. (1992) that the Chl b-less mutant of barley is more susceptible to photoinactivation of PS II than the wild type. E, if anything, was lower in the mutant than in the wild type, almost certainly because a smaller light-harvesting antenna of PS II resulted in a smaller fraction of excess energy. Therefore, the excess-energy hypothesis per se cannot explain the greater susceptibility of the mutant to photoinactivation of PS II. Equally, the Mn-initiated mechanism cannot explain the 1.5-fold difference between the mutant and the wild type, which occurred even at red wavelengths. Therefore, other explanations are needed.
PS II complexes at shallow depths are more susceptible to photoinactivation of PS II than in deeper tissue (Oguchi et al. 2011b). The mutant leaves, with no light-harvesting complex II (LHCII), contained less than half as much Chl per unit leaf area as the wild type (e.g., Leverenz et al. 1992). Therefore, the shielding of PS II complexes against photoinactivation would have been less effective in the Chl b-less mutant. By contrast, the large antenna of any photoinactivated PS II complexes in the shallow tissue of wild-type leaves, which had much higher Chl content per unit leaf area, would still absorb light, and dissipate it in non-photochemical quenching, thereby more effectively protecting functional PS II complexes in deeper tissue. This may be a reason for the difference in susceptibility of PS II to photoinactivation between the Chl b-less mutant and the wild type.
Another explanation was put forward by Leverenz et al. (1992). They hypothesized that there is limited energy migration among PS II complexes in the Chl b-less mutant, presumably due to poor connectivity between them. By contrast, a high probability of migration of excitons from closed PS II centres to the remaining open centres or to quenching centres should provide better photoprotection in the wild type (Leverenz et al. (1992).
Comparison of the action spectrum of photoinactivation of PS II in mutant barley leaves with that of isolated spinach thylakoids
Jones and Kok (1966) reported an action spectrum of photoinactivation of PS II in isolated spinach thylakoids, in which there was a distinct peak at about 670 nm, with a peak height comparable to that in the blue wavelength range. The thylakoids were suspended in an unstacking buffer, such that the two photosystems were randomly mixed and spillover of excitation energy from PS II to PS I was maximal. The structural organization of thylakoid membranes in leaves of the barley mutant would have been not too different from that of unstacked spinach thylakoids, since the Chl b-less mutant has poor granal stacking and, therefore, spillover from PS II to PS I would also have been enhanced. Indeed, Searle et al. (1979) concluded that a short-lived Chl fluorescence component in the Chl b-less mutant (lifetime τ1 ≈ 50 ps, amplitude ≈ 80 %) must be a mixture of the emissions from PS I and from those light-harvesting/PS II pigment beds that are strongly coupled with PS I, i.e., spillover from PS II to PS I was facilitated in the mutant. There is one difference between the two experimental systems; however, the isolated thylakoids (Jones and Kok 1966) were illuminated in the absence of an electron acceptor, whereas mutant barley leaf segments (in the present study) were capable of carbon assimilation during photoinhibitory treatment. Conceivably, reactive oxygen species, particularly singlet oxygen, would have been much more abundant in the isolated thylakoids under illumination in the absence of an electron acceptor. This might have rendered the excess-energy hypothesis much more relevant; therefore, light absorption by chlorophyll became much more important, resulting in comparable peak heights of the action spectra in light of red and blue wavelengths. By contrast, the two-step mechanism was much more relevant in barley leaf segments in the present study since there was little excess energy when carbon assimilation was allowed to occur under the moderate lights of selected wavelengths; therefore, direct absorption of light by Mn was more important, and red light had little effect because of poor absorption of red light by Mn.
Conclusions
Using the P700 kinetics area as a convenient and whole-tissue measure of functional PS II, we obtained the rate coefficients of photoinactivation k i for wild-type and Chl b-less leaf segments. The ratio of k i to irradiance (I) increased very steeply with the decrease in the wavelength of the light used to photoinactivate PS II. This observation is consistent with the two-step mechanism in which direct absorption of light by Mn initiates the photoinactivation process, but contrasts with other findings where the experimental conditions favour the excess-energy mechanism. PS II in the mutant leaves was approximately 1.5-fold more susceptible to photoinactivation than that in wild-type leaves, possibly partially due to less mutual shading of chloroplasts in the mutant leaves. In light of red wavelengths, there was a small peak in the action spectrum, indicating that light absorbed by chlorophyll did lead to photoinactivation of PS II; however, the peak was small, presumably because the experimental conditions did not result in a large excess of energy. Therefore, which mechanism operates to initiate photoinactivation of PS II depends on the experimental conditions.
Abbreviations
- Chl:
-
Chlorophyll
- E :
-
Excess fraction of absorbed energy
- F :
-
Functional fraction of PS II
- F o, F m :
-
Minimum and maximum Chl fluorescence yield of a dark-adapted leaf, respectively
- \(F_{\text{o}}^{ \prime}\), \(F_{\text{m}}^{ \prime}\), \(F_{\text{s}}^{ \prime}\), \(F_{\text{v}}^{ \prime}\) :
-
Minimum, maximum, steady-state, and variable Chl fluorescence yield of a light-adapted leaf, respectively
- I :
-
Irradiance
- k i :
-
Rate coefficient of photoinactivation of PS II
- LED:
-
Light-emitting diode
- LHCII:
-
Light-harvesting complex II
- P680, P700:
-
Special Chl in the PS II, PS I reaction centre, respectively
- PS:
-
Photosystem
- Q A, Q B :
-
Primary, secondary quinone acceptor in PS II, respectively
- qP :
-
Photochemical quenching coefficient
References
Aro E-M, Virgon I, Andersson B (1993) Photoinhibition of Photosystem II. Inactivation, protein damage and turnover. Biochim Biophys Acta 1143:113–134
Bodini M, Willis LA, Riechel TL, Sawyer DT (1976) Electrochemical and spectrostudies of manganese (II), -(III), and –(IV) glyconate complexes. 1. Formulas and oxidation-reduction stoichiometry. Inorg Chem 15:1538–1543
Chow WS, Hope AB (2004) Electron fluxes through Photosystem I in cucumber leaf discs probed by far-red light. Photosynth Res 81:77–89
Cser K, Vass I (2007) Radiative and non-radiative charge recombination pathways in Photosystem II studied by thermoluminescence and chlorophyll fluorescence in the cyanobacterium Synechocystis 6803. Biochim Biophys Acta 1767:233–243
Demmig B, Björkman O (1987) Comparison of the effect of excessive light on chlorophyll fluorescence (77 K) and photon yield of O2 evolution in leaves of higher-plants. Planta 171:171–184
Demmig-Adams B, Adams WW III, Barker DH, Logan BA, Bowling DR, Verhoeven AS (1996) Using chlorophyll fluorescence to assess the fraction of absorbed light allocated to thermal dissipation of excess excitation. Physiol Plant 98:253–264
Ettinger WF, Clear AM, Fanning KJ, Peck ML (1999) Identification of a Ca2+/H+ antiport in the plant chloroplast thylakoid membrane. Plant Physiol 119:1379–1385
Ewart AJ (1896) On assimilatory inhibition in plants. J Linn Soc 31:364–461
Hakala M, Tuominen I, Keränen M, Tyystjärvi T, Tyystjärvi E (2005) Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of Photosystem II. Biochim Biophys Acta 1706:68–80
Hideg E, Spetea C, Vass I (1994) Singlet oxygen production in thylakoid membranes during photoinhibition as described by EPR spectroscopy. Photosynth Res 39:191–199
Highkin HS (1950) Chlorophyll studies on barley mutants. Plant Physiol 25:294–306
Hikosaka K, Kato MC, Hirose T (2004) Photosynthetic rates and partitioning of absorbed light energy in photoinhibited leaves. Physiol Plant 121:699–708
Hu Y-Y, Fan D-Y, Losciale P, Chow WS, Zhang W-F (2013) Whole-tissue determination of the rate coefficients of photoinactivation and repair of Photosystem II in cotton leaf discs based on flash-induced P700 redox kinetics. Photosynth Res 117:517–528
Jones LW, Kok B (1966) Photoinhibition of chloroplast reactions. Kinetics and action spectra. Plant Physiol 41:1037–1043
Jung J, Kim H-S (1990) The chromophores as endogenous sensitizers involved in the photogeneration of singlet oxygen in spinach thylakoids. Photochem Photobiol 52:1003–1009
Kato MC, Hikosaka K, Hirotsu N, Makino A, Hirose T (2003) The excess light energy that is neither utilized in photosynthesis nor dissipated by photoprotective mechanisms determines the rate of photoinactivation in Photosystem II. Plant Cell Physiol 44:318–325
Keren N, Gong H, Ohad I (1995) Oscillations of reaction center II-D1 protein degradation in vivo induced by repetitive light flashes. J Biol Chem 270:806–814
Keren N, Berg A, van Kan PJM, Levanon H, Ohad I (1997) Mechanism of photosystem II photoinactivation and D1 protein degradation at low light: the role of back electron flow. Proc Natl Acad Sci USA 94:1579–1584
Kornyeyev D, Logan BA, Holaday AS (2010) Excitation pressure as a measure of the sensitivity of photosystem II to photoinactivation. Funct Plant Biol 37:943–951
Kou J, Oguchi R, Fan D-Y, Chow WS (2012) The time course of photoinactivation of photosystem II in leaves revisited. Photosynth Res 113:157–164
Lee H-Y, Chow WS, Hong Y-N (1999) Photoinactivation of photosystem II in leaves of Capsicum annuum. Physiol Plant 105:377–384
Lee H-Y, Hong Y-N, Chow WS (2001) Photoinactivation of photosystem II complexes and photoprotection by non-functional neighbours in Capsicum annuum leaves. Planta 212:332–342
Leverenz JW, Öquist G, Wingsle G (1992) Photosynthesis and photoinhibition in leaves of chlorophyll b-less barley in relation to absorbed light. Physiol Plant 85:495–502
Nishiyama Y, Allakhverdiev SI, Murata N (2011) Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol Plant 142:35–46
Oguchi R, Terashima I, Chow WS (2009) The involvement of dual mechanisms of photoinactivation of photosystem II in Capsicum annuum L. plants. Plant Cell Physiol 50:1815–1825
Oguchi R, Terashima I, Kou J, Chow WS (2011a) Operation of dual mechanisms that both lead to photoinactivation of Photosystem II in leaves by visible light. Physiol Plant 142:47–55
Oguchi R, Douwstra P, Fujita T, Chow WS, Terashima I (2011b) Intra-leaf gradients of photoinhibition induced by different color lights: implications for the dual mechanisms of photoinhibition and for the application of conventional chlorophyll fluorometers. New Phytol 191:146–159
Ohad I, Berg A, Berkowicz SM, Kaplan A, Keren N (2011) Photoinactivation of photosystem II: is there more than one way to skin a cat? Physiol Plant 142:79–86
Ohnishi N, Allakhverdiev SI, Takahashi S, Higashi S, Watanabe M, Nishiyama Y, Murata N (2005) Two-step mechanism of photodamage to photosystem II: step 1 occurs at the oxygen-evolving complex and step 2 occurs at the photochemical reaction center. Biochemistry 44:8494–8499
Osmond CB (1994) What is photoinhibition? Some insights from comparisons of shade and sun plants. In: Baker NR, Bowyer JR (eds) Photoinhibition of photosynthesis: from molecular mechanisms to the field. BIOS Scientific Publishing Ltd, Oxford, pp 1–24
Oxborough K, Baker NR (1997) Resolving chlorophyll a fluorescence images of photosynthetic efficiency into photochemical and non-photochemical components—calculation of qP and \(F_{\text{v}}^{\prime}/F_{\text{m}}^{\prime}\;\text{and}\; F_{\text{o}}^{\prime}.\) Photosynth Res 54:135–142
Powles SB (1984) Photoinhibition of photosynthesis induced by visible light. Annu Rev Plant Physiol 35:15–44
Santabarbara S, Cazzalini I, Rivadossi A, Garlaschi FM, Zucchelli G, Jenning RC (2002) Photoinhibition in vivo and in vitro involves weakly coupled chlorophyll-protein complexes. Photochem Photobiol 75:613–618
Sarvikas P, Hakala M, Pätsikkä E, Tyystjärvi T, Tyystjärvi E (2006) Action spectrum of photoinhibition in leaves of wild type and npq1-2 and npq4-1 mutants of Arabidopsis thaliana. Plant Cell Physiol 47:391–400
Schreiber U, Klughammer C (2013) Wavelength-dependent photodamage to Chlorella investigated with a new type of multi-color PAM chlorophyll fluometer. Photosynth Res 114:165–177
Searle GFW, Tredwell CJ, Barber J, Porter G (1979) Picosecond time-resolved fluorescence study of chlorophyll organization and excitation-energy distribution in chloroplasts from wild-type barley and a mutant lacking chlorophyll-b. Biochim Biophys Acta 545:496–507
Tyystjärvi E (2008) Photoinhibition of Photosystem II and photodamage of the oxygen evolving manganese cluster. Coord Chem Rev 252:361–376
Tyystjärvi E, Aro E-M (1996) The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci USA 93:2213–2218
van Gorkom HJ, Schelvis JPM (1993) Kok’s oxygen clock: what makes it tick? The structure of P680 and consequences of its oxidizing power. Photosynth Res 38:297–301
Vass I (2011) Role of charge recombination processes in photodamage and photoprotection of the photosystem II complex. Physiol Plant 142:6–16
Vass I, Cser K (2009) Janus-faced charge recombinations in photosystem II photoinhibition. Trends Plant Sci 14:200–205
Wei Z, Cady CW, Brudvig GW, Hou HJM (2011) Photodamage of a Mn(III/IV)-oxo mixed-valence compound and photosystem II: evidence that a high-valence manganese species is responsible for UV-induced photodamage of the oxygen-evolving complex in photosystem II. J Photochem Photobiol B 104:118–125
Acknowledgments
The support of this work by a grant awarded to J.H. by the Singapore Millennium Fund (Project code: SMF-Farming System), to WSC by the ARC (DP120100872) and to D.-Y. Fan by the Chinese Academy of Sciences (KZCX2-XB3-09) and the National Natural Science Foundation (31070356) is gratefully acknowledged. W.S.C. values the friendship with Professor Kuang Tingyun that started at a conference in Yangzhou in China in 1984. He wishes Professor Kuang good health and great success in her long-lasting mentoring of young researchers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Jie He and Wenquan Yang contributed equally to this work.
Rights and permissions
About this article
Cite this article
He, J., Yang, W., Qin, L. et al. Photoinactivation of Photosystem II in wild-type and chlorophyll b-less barley leaves: which mechanism dominates depends on experimental circumstances. Photosynth Res 126, 399–407 (2015). https://doi.org/10.1007/s11120-015-0167-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-015-0167-0