Abstract
The physical and chemical properties of tobacco (Nicotiana tabacum L.) plants are sensitive to changes in genetics and the environment. However, few studies have investigated the effect of both cultivar and regional factors on tobacco quality at the proteomic level. Here, a TMT-based quantitative proteomics method was used to investigate proteome profiling of different tobacco leaves under various geographical locations. In total, 8587 proteins were detected, among which 300 differentially abundant proteins (DAPs) were identified. Proteins associated with carbohydrate metabolism and amino acid metabolism were more abundant in tobacco plants from Yunnan. In contrast, proteins involved in the response to heat were more abundant in tobacco plants from Henan. We found that proteins related to carbon metabolism and defense signaling played an important role in the characteristics of different cultivars within the same region. In this work, we identified key proteins and pathways involved in the response of Nicotiana tabacum to environmental change and explored the proteomic differences among cultivars. Our results provide a better understanding of the effect of environment and cultivar on the tobacco leaf proteome, which will be helpful for elucidating the molecular mechanisms of the formation of tobacco characteristic quality.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Tobacco (Nicotiana tabacum L.) is an important model plant with great significance in the fields of plant genetics, breeding, physiology, and biochemistry (Liu et al. 2022; Zhao et al. 2013; Sierro et al. 2014). Tobacco is widely cultivated in many geographical regions around the world, and its growth and quality are sensitive to environmental conditions such as temperature, sun exposure time, and rainfall (Wu et al. 2013; Zhou et al. 2015; Li et al. 2021). Therefore, tobacco can be expected to develop a characteristic quality within its cultivation range. Aroma is one of the important indicators of tobacco quality. According to the geographic region, the aroma type of tobacco leaves can be generally classified into light aroma, medium aroma, and heavy aroma. In addition to the geographical region, it has been reported that cultivar also has a marked influence on the quality of tobacco leaves. Different tobacco cultivars have different adaptations to the environment (Sun et al. 2015; Tsaballa et al. 2020). Previous work has shown that the NC567 and Taiyan8 tobacco cultivars exhibit different tolerances to cold stress (Hu et al. 2018). Studies on chemical components also showed that alkaloids and some aroma substances were significantly different among different tobacco cultivars (Xu et al. 2017; Sun et al. 2013).
Numerous investigations of the effects of cultivar or geographical factors on tobacco quality have been performed. Tang et al. investigated the effects of environmental factors (soil and climatic factors) on the yield and quality of three flue-cured tobacco and determine the main regulating factors (Tang et al. 2020). Gang et al. reported that polyphenols and organic acids in tobacco leaves varied across different growing regions in China (Xiang et al. 2010). Karangwa et al. analyzed the correlation of volatile compounds in mainstream smoke and aroma types (Yin et al. 2016). Metabolomics studies have also been employed to analyze the impact of the planting environment on the metabolite profile of tobacco in recent years (Xu et al. 2020; Zhao et al. 2015; Zhang et al. 2013). However, most of these studies have been focused on the analysis of chemical components, which is insufficient for understanding the mechanism by which various geographical and cultivar factors influence tobacco quality.
Proteomic analysis provides a robust and reliable approach to simultaneously study multiple signaling pathways (Mergner and Kuster 2022; Liu et al. 2019; Gupta et al. 2015). Given the essential role of proteins in cellular functions, proteomics technology has become an important approach for investigating biological processes due to its ability to provide comprehensive qualitative and quantitative information on hundreds to thousands of proteins and to reveal functional information on the biochemical processes underlying phenotypes that are not accessible or predictable by other means. Li et al. utilized isobaric tags for relative and absolute quantification (iTRAQ) proteomics to understand the genetic regulation mechanism of tobacco roots in different soil types (Li et al. 2022). Mo et al. performed data-independent acquisition proteomics to analyze four tobacco varieties with different nicotine content (Mo et al. 2022). These studies demonstrate the potential of proteomics analysis in understanding the biological processes in tobacco.
To better understand the effect of region and cultivar on tobacco quality and to identify the main regulating factors, herein, a TMT-labeled quantitative method was used for proteomics analysis of different tobacco cultivars from three growth regions (Henan, Fujian, and Yunnan). The protein level of the Y87 cultivar grown in three different regions was compared to explore the environmental effect on tobacco aroma quality. A total of 300 differentially abundant proteins (DAPs) were detected, most of which were involved in carbohydrate metabolism, amino acid synthesis, and secondary metabolism synthesis. To further investigate the impact of cultivar factors on tobacco, the proteomic responses of different cultivars within the same region were compared. There were 324 DAPs detected in the comparison of the Z100 and Y87 cultivars from the Henan region, while 153 DAPs were found in the comparison of Y100 and Y87 from the Yunnan region. This work investigates for the first time the effect of both cultivar and geographic factors on tobacco quality development. Several key proteins and pathways associated with geographic origin and cultivar differences were identified, providing insight into the underlying mechanism of tobacco quality development under the regulation of environmental and cultivar factors.
Materials and Methods
Plant Material and Sampling
The tobacco cultivars Yunyan87 and Yunyan100 from Qujing, Yunnan Province; Yunyan87 from Nanyang, Henan Province; Zhongyan100 from Linying, Henan Province; and Yunyan 87 from Longyan, Fujian Province were collected and used for this study. The plants were cultivated according to the production standards for high-quality tobacco leaves in Yunnan, Fujian, and Henan. The plant materials were sampled from the middle leaf positions (Nos. 9–11) at the mature leaf stage. Leaves from five tobacco plants were collected as a replicate, and three replicates were collected for analysis. After harvest, the leaf samples were immediately frozen in liquid nitrogen, lyophilized to dryness, and ground to a fine powder for subsequent analyses.
Protein Extraction and Digestion
Protein was extracted using the phenol extraction method. The ground leaf powder was mixed with 1 mL phenol extraction buffer, followed by the addition of an equal volume of Tris-buffered phenol buffer. The top phenolic layer was collected by centrifugation and transferred to a new tube. Then, five volumes of 100 mM ammonium acetate in methanol were added, and the mixture was incubated overnight. The pellet obtained after centrifugation was washed twice with methanol and then washed once with precooled acetone. The air-dried pellet was resuspended in 500 μL SDS lysis buffer (2% (w/v) SDS, 10 mM DTT, 100 mM Tris-HCl, pH 7.6) and incubated for 60 min at room temperature. The supernatant was collected after centrifugation at 12,000 × g for 20 min at 4 °C and stored at − 80 °C for further digestion. For in-solution digestion, 150 μg of protein from each sample was alkylated with 30 mM iodoacetamide (IAA) in the dark for 30 min at room temperature. Then, the protein was precipitated with 10% TCA/acetone overnight at − 20 °C. The protein pellet was washed with cold acetone three times the next day and digested at 37 °C for 18 h with trypsin (Promega) at a concentration of 1:50 (w:w, trypsin to protein) in 100 mM ammonium bicarbonate buffer (ABC). The peptides were dried by vacuum centrifugation for TMT labeling.
TMT Labeling and HPLC Fractionation
The peptides were resuspended in 100 mM TEAB, and the concentration was determined by the BCA assay. Samples of 30 μg peptides were labeled with TMT 10-plex reagents (Thermo Fisher Scientific, USA) according to the manufacturer’s instructions. The peptides were incubated with 0.4 mg label reagent for 1 h at room temperature, and then, the reaction was quenched with 4 μL of 5% hydroxylamine. Differentially labeled peptides were combined and desalted on a C18 3 cc vac cartridge (Waters, Milford, MA, USA). The TMT-labeled peptide mixture was fractionated using a Waters XBridge BEH130 C18 3.5 μm 2.1 × 150 mm column on an Agilent 1260 HPLC operating at 0.2 mL/min. Buffer A of the mobile phase consisted of 10 mM ammonium formate with water, and buffer B consisted of 10 mM ammonium formate with 90% (v/v) acetonitrile. Both buffers were adjusted to pH 10 with ammonium hydroxide as described previously [18]. The samples were separated and eluted using a 90-min gradient and then combined by a concatenation strategy into 14 fractions. The fractions were lyophilized and resuspended in 0.1% FA for nano LC‒MS/MS analysis.
Nanoflow Liquid Chromatography-Tandem Mass Spectrometry
The LC‒MS/MS analysis was performed using a Thermo Q Exactive HF with an online nanoflow EASY-nLC1000 HPLC system (Thermo Fisher Scientific, USA). The peptides were loaded onto a self-packed column (75 μm × 150 mm; 3 μm ReproSil-Pur C18 beads, 120 Å, Dr. Maisch GmbH, Ammerbuch) and separated with a 90-min gradient at a flow rate of 300 nL/min. Solvent A contained 100% H2O and 0.1% formic acid, and solvent B contained 100% acetonitrile and 0.1% formic acid. The mass spectrometer was operated in data-dependent acquisition (DDA) mode. The full MS scan range was set from 375 to 1500 m/z, and the resolution was set as 120,000. The AGC target value was 40,000 with a 50 ms maximum injection time. The most intense precursors were then subjected to HCD fragmentation with a 3 s duty cycle and 38% collision energy. The MS2 spectra were acquired at a resolution of 50,000 and an isolation window of 1. The dynamic exclusion was set as 45 s with a ± 10 ppm window. The AGC target value was set as 10,000 with a maximum ion injection time of 105 ms. The spray voltage was set as 2.5 kV, and the heated capillary temperature was 275 °C.
Data Analysis
The MS data were analyzed via MaxQuant software (http://maxquant.org/, version 1.6.5.0). Carbamidomethyl (C) was set as a fixed modification, while oxidation (M) and protein N-term acetylation were set as variable modifications. The MS/MS spectra were searched against the Common Tobacco UniProt FASTA database (UP000084051, containing 73605 entries), and trypsin/P was selected as the digestive enzyme with two potential missed cleavages. The false discovery rate (FDR) for peptides and proteins was controlled to < 1% by the Andromeda search engine. KEGG pathway annotations and enrichment were performed by KOBAS (Bu et al. 2021), and the results were visualized via R studio. To further annotate and classify the proteins into cellular components, molecular functions, and biological processes, the DAPs were analyzed using Gene Ontology (GO) annotation (http://www.geneontology.org/).
Results
Protein Identification
In our study, five tobacco samples from two tobacco cultivars (Yunyan 87 and Yunyan100) from Yunnan, two tobacco cultivars (Yunyan87 and Zhongyan100) from Henan, and the tobacco cultivar Yunyan87 from Fujian were analyzed (Table S1). Proteome profiling of the tobacco leaves from different regions was performed by reversed-phase HPLC and LC‒MS/MS. A total of 8587 proteins were identified in this study. As shown in the box plot (Fig. S1), the intensity data for each sample were distributed similarly, indicating good repeatability of the quantitative data. The PCA score plots of the five samples showed three distinctive strains, revealing that the leaves from different regions could be easily discriminated based on the identified protein profiling. The location of samples from Yunnan and Henan in the score plot was entirely opposite, while samples from Fujian were located between them (Fig. S2).
Proteome Profiles of Yunyan-87 (Y87) from Three Regions
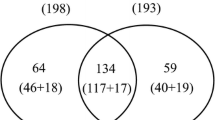
To investigate the protein response to environmental change, the protein level of the Yunyan87 (Y87) cultivar from Yunnan (YN), Fujian (FJ), and Henan (HN) was compared. Y87 tobacco from Yunnan Province (YN-Y87) is considered to have a light-aroma type with a characteristic fresh-sweet scent, while Y87 from Henan Province (HN-Y87) is considered to have a heavy aroma. Y87 from Fujian (FJ-Y87), is also regarded as having a light aroma but featuring a stronger honey-sweet scent compared to YN-Y87 (Li et al. 2016; Yang et al. 2014). Differentially abundant proteins (DAPs) with significant changes (p-value < 0.5) were selected. The cut-off point was fixed at > 2.0-fold change for up-regulated proteins and < 0.5-fold change for down-regulated proteins. Under these conditions, 300 DAPs were detected in Y87 from different regions. A total of 250, 84, and 92 DAPs were detected in the comparison of YN-Y87 vs. HN-Y87, YN-Y87 vs. FJ-Y87, and HN-Y87 vs. FJ-Y87 (Table S2). The overlaps of DAPs between any two groups are illustrated in the Venn diagram (Fig. 1A), indicating that Y87 from Henan and Yunnan has the greatest difference in abundance. This large polarity of HN-Y87 and YN-Y87 in the Venn diagram was also in accordance with the PCA result (Fig. S2). The DAPs in tobacco samples from different regions were easily discriminated by hierarchical clustering analysis (HCA) and can be classified into 5 clusters. We found that the abundances of most of the proteins showed the greatest differences in the HN-Y87 and YN-Y87 samples, with the abundances in the FJ-Y87 sample located between them. Therefore, proteins in cluster II (95 proteins) and cluster IV (119 proteins) that followed this pattern were selected for further analysis (Fig. 1B). Cluster II, with proteins from the YN region showing the highest abundance, was defined as up-regulated, while cluster IV, with a reverse order of abundance, was considered as down-regulated.
Functional Annotation and Pathway Analysis of DAPs
To refine the effect of region on tobacco leaf quality, we performed GO annotation and enrichment on selected DAPs. On the basis of the GO analysis, a total of 198 proteins were annotated in terms of biological processes, cellular components, and molecular functions. The GO annotations were assigned to 54 biological process categories, 20 cellular component categories, and 31 molecular function categories. As shown in Fig. S3, a detailed analysis of the GO enrichment results revealed that most of the proteins enriched in biological processes were associated with responses to various stresses. Response to heat was the most enriched GO category under the biological process term, while cytoplasm was the main enriched cellular component. In the molecular function term, unfolded protein binding and protein self-association were the most affected subgroups.
KEGG enrichment was performed to further investigate the biochemical pathways associated with environmental effects. The top 10 most enriched pathways of both up-regulated protein and down-regulated proteins are shown in Fig. 2. For up-regulated protein, biosynthesis of secondary metabolites, carbohydrate metabolism (glycolysis/gluconeogenesis, amino sugar, and nucleotide sugar metabolism), and amino acid metabolism (tyrosine metabolism, cysteine and methionine metabolism, alanine, aspartate and glutamate metabolism) were defined as the main affected pathway. As for down-regulated protein, the KEGG pathway was mainly DNA folding and unfolding (protein processing in the endoplasmic reticulum, endocytosis, spliceosome) and biosynthesis of secondary metabolism (porphyrin and chlorophyll metabolism, terpenoid backbone biosynthesis). Based on the KEGG enrichment results, these pathways are expected to be closely related to the characteristic formation of tobacco leaves under environmental change.
The top 10 enriched KEGG pathways from the significantly up-regulated (A) and down-regulated (B) protein. The p-value is less than 0.05, and a higher − Log(p-value) indicates greater intensiveness. The rich factor is the ratio of the number of DAPs annotated with this pathway term to the total number of proteins annotated with this pathway term
Proteomic Profiling of Different Tobacco Cultivars Within the Same Region
In addition to geographical factors, cultivar differences can influence the proteome profile and quality development in tobacco. To verify whether different cultivars would exhibit disparate responses to geographical variation, the proteomic profiles of different cultivars within the same region were investigated. Zhongyan-100 is a cultivar widely planted in Henan Province that is highly resistant to brown spot and black shank (Sun et al. 2018). The Yunyan100 cultivar from Yunnan was bred from the cross between Y87 and KX14 and therefore bears much similarity with Y87 (Zhang et al. 2015). Y100 from Yunnan (YN-Y100) and Z100 from Henan (HN-Z100) were selected for comparison with the Y87 cultivar in the same region. A total of 324 and 152 DAPs were observed in the comparison of HN-Z100 vs. HN-Y87 and YN-Y100 vs. YN-Y87, respectively. The number of DAPs induced by different cultivars was similar to the 250 DAPs induced by environmental change in Yunnan and Henan. This result suggested that cultivar factors contribute greatly to the protein level differences.
Furthermore, we analyzed the abundance of 214 selected DAPs related to environmental change in the YN-Y100 and HN-Z100 samples. The heatmaps for selected DAP expression in two cultivars from the Yunnan and Henan regions were generated and are shown in Fig. S4. In general, the two cultivars in Yunnan (Y87 and Y100) exhibited similar proteome profiles, while Zhong100 in Henan did not follow this pattern. A total of 109 proteins induced by geographic factors also showed significant differences in abundance in the two cultivars from the Henan region. This result indicates that the two cultivars in the Henan region have great differences and may have different adaptations to the environment.
Discussion
Tobacco is one of the most widely cultivated industrial crops and an important model plant for studies of fundamental biological processes. The quality of tobacco leaves can be affected by various factors, such as cultivar, temperature, sun exposure time, and rainfall. Several investigations of the effects of cultivar or geographical factors on tobacco quality have been performed using metabolomic and proteomic methods. However, previous proteomics studies on exploring the geographical factors on tobacco quality have mostly been limited to 2D gel electrophoresis, a method that leads to inadequate quantification and an incomplete proteomics comparison. In our study, TMT-based quantitative proteomic analysis was performed to obtain a global view of the proteomic profiles of tobacco leaves from different regions. First, Y87 cultivars grown in Yunnan, Fujian, and Henan provinces were analyzed to investigate proteomics differences under various environmental conditions. A total of 300 DAPs were detected in the comparison among three samples to help elucidate the mechanism by which environmental factors affect the development of tobacco quality. Based on the KEGG enrichment results, several candidate DAPs taking part in the most enriched KEGG pathway were found. In addition, we analyzed the proteome profile of different tobacco cultivars grown in the same region. The major differentially expressed proteins and related metabolic pathways of these DAPs are discussed in the following sections.
Proteins Associated with Carbohydrate Metabolism
Plant carbohydrates are fundamental to plant growth, development, and stress responses and play an essential role in metabolic regulation within a changing environment (Herrmann et al. 2019). The analysis of the chemical composition of tobacco leaves with different aroma types indicated that the leaf quality was mainly influenced by carbohydrates and nitrogen-containing compounds and their ratios (Song et al. 2007). In this study, several DAPs involved in carbohydrate metabolism, including UGP1, SuSy, ATP-PFK, ADH, and PDC, were identified, and their pathways were illustrated (Fig. 3). The role of UGP1 has been studied extensively in plants to increase agricultural production. In plant leaves, UGP1 is a key part of the sucrose biosynthesis pathway, is responsible for supplying uridine diphosphate glucose to sucrose phosphate synthase, and is also involved in UDP-glucose metabolic processes and cellular carbohydrate biosynthetic processes (Sghaier-Hammami et al. 2009). Two sucrose synthases (SuSy) were found to increase in abundance in the YN-Y87 sample by ~ 2.3-fold over HN-Y87. Previous studies have reported that the upregulation activity of SuSy and UGP1 is associated with an increase in total sugar content (Coleman et al. 2006). Research on chemical content has demonstrated that the total content of water-soluble sugars in leaves with a light aroma is significantly higher than that in medium-aroma and heavy-aroma leaves, which is basically in accordance with our results. Though tobacco in Fujian is also regarded as a light aroma type, the level of proteins related to sugar regulation in FJ-Y87 is located between the level of that in YN-Y87 and HN-Y87. According to previous studies, sufficient sun exposure time and higher altitude can enhance sugar content (Zhao et al. 2013). There were obvious differences in ecological climate among the three different regions. Yunnan shows the ecological characteristics of lengthy sun exposure time in the early stage of tobacco growth and relatively weak sunlight, lower temperature, and a long rainy season in the late growth period, which is beneficial to the accumulation of sugar. For tobacco cultivars growing in Fujian Province, the weak light intensity and long rainy period in the prosperous stage diminished the accumulation of sugar, which may be one of the main reasons for the low sugar content. The climate in Henan presents high temperatures, and long sunlight exposure in the later stage of tobacco growth may also lead to a decrease in sugar content. Our results indicate that the sugar content difference of tobacco leaves from three regions induced by their characteristic climate could be reflected by protein level.
Proteins associated with glycolysis/gluconeogenesis, including ATP-PFK, PDC, and ADH, also showed significantly high abundance in Y87 samples from Yunnan. All these enzymes are involved in the key processes that directly regulate glycolysis/gluconeogenesis and the production of material for various secondary metabolites. By catalyzing the reduction of acetaldehyde to ethanol and the decarboxylation of pyruvate to acetaldehyde, alcohol dehydrogenase (ADH) and pyruvate decarboxylase (PDC) are especially important enzymes associated with abiotic stress, such as low temperature, low oxygen, and salt stress (Strommer 2011; Kimmerer 1987). In addition, alcohol derived from pyruvate provides a major source for the synthesis of esters, which constitute an important fraction of aroma in various plant species (Osorio et al. 2010). In the volatile aroma production process, ADH also acts as a key enzyme by participating in fatty acid metabolism and amino acid metabolism pathways, playing a key role in aldehyde/alcohol conversion to form precursors for aroma synthesis. These volatiles, including aldehydes, alcohols, and esters, are known as “green scents” in fresh leaves and have been shown to mediate both indirect and direct defenses against insect predators (Strommer 2011; Pichersky and Gershenzon 2002). The dihydrolipoamide acetyltransferase component of the pyruvate dehydrogenase (PDH) complex and the acetyl-CoA carboxytransferase (ACC) in pyruvate metabolism were observed in lowest abundance in YN-Y87. We surmise that the difference in the abundance of these proteins may be the origin of the light aroma of tobacco from Yunnan.
Protein Associated with Amino Acid Metabolism and Alkaloid Biosynthesis
Amino acids are a dispensable component in organisms. Amino acid metabolism plays a pivotal role in the regulation of C and N metabolism (Fritz et al. 2006; McAllister et al. 2016). Three aspartate aminotransferases (AATs) were identified in the clusters of proteins with increased levels. AAT catalyzes the reversible transamination between glutamate and oxaloacetate to generate aspartate and 2-oxoglutarate and was identified and found to engage in the synthesis of several amino acids (de la Torre et al. 2014). Previous studies on metabolism profiles have reported a higher amino acid content in light-aroma tobacco (Zhao et al. 2015), which also aligns with our results. Additionally, AAT serves as a key enzyme in the production of aroma amino acids (AAAs) through the shikimate pathway (Fritz et al. 2006). The existence of AAAs had a more significant effect on the sugar profile. As reported in previous studies, tobacco lines that exhibit higher AAA levels show an increase in the levels of several sugars, such as fructose and glucose (Oliva et al. 2021). Therefore, we predicted that the upregulation of AAT, together with SuSy and UGP1, all resulted in higher levels of sugar in Y87 from the Yunnan region. Aspartate aminotransferase was also associated with the pathway of isoquinoline alkaloid biosynthesis and tropane, piperidine, and pyridine alkaloid biosynthesis. Alkaloids are plant secondary metabolites of the tobacco stress response and also serve as main aroma precursors that contribute greatly to the quality of tobacco leaves (Liu et al. 2022). Our results indicate that the environmental condition in Yunnan may be beneficial to the accumulation of sugar and alkaloid biosynthesis in tobacco, thus contributing to the formation of the light aroma of tobacco from the Yunnan region. Previous studies also reported that tobacco with a light aroma type had significantly higher values of sugar and total nitrogen content, which is in accordance with the regulation of related proteins (Yang et al. 2014).
Various secondary metabolites that function in the plant defense response are generated from aroma amino acids through the shikimate-phenylpropanoid pathway (Zhang and Liu 2015). Two phenylalanine ammonia lyases (PALs), which are the first key step enzymes in the phenylpropanoid pathway, were detected in the highest abundance in the HN-Y87 sample, followed by the FJ-Y87 sample. The PAL abundance in YN-Y87 was found to be the lowest. PAL activity has been reported to have a direct effect on the accumulation of phenylpropanoid products, including chlorogenic acid (CGA), lignin, and rutins (Bate et al. 1994; Zhang and Liu 2015; Tsaballa et al. 2020). In addition, a higher abundance of caffeoyl-CoA O-methyltransferase (CCoAOMT), the key enzyme in the biosynthesis of lignin, was observed in the HN-Y87 sample. Those phenylpropanoid products are closely related to climate change and contribute greatly to the aroma type of tobacco leaves. This result revealed that PAL and CCoAOMT in the phenylpropanoid pathway may play a crucial role in the scent of the heavy aroma of tobacco leaves.
Proteins Associated with Protein Folding and Unfolding
Cells are repeatedly exposed to environmental or endogenous stresses. Heat shock proteins (HSPs) are a large family of molecular chaperones that are well known for their roles in protein maturation, refolding, and degradation (Timperio et al. 2008). They would get induced in cells during various stress conditions produced by cellular insult, environmental changes, temperature, and infections to ensure tissue integrity and function. In our study, the abundances of 11 HSPs were increased in HN-Y87. HSPs play an essential role in chloroplast function under heat stress and have been found to stabilize chloroplast development and the functioning of PSII (Seydel et al. 2022). Considering the relatively high temperature in the Henan region during the later stage of tobacco growth, we assumed that the increased abundance of HSP in the Henan region was caused by the response to temperature stress.
Proteins Involved in Pigment Synthesis
Two metabolic pathways involved in pigment synthesis were enriched. Magnesium chelatase and magnesium-protoporphyrin IX monomethyl ester cyclase (MgPME) are essential enzymes in chlorophyll biosynthesis (Kong et al. 2016; Tomiyama et al. 2014). Two geranylgeranyl pyrophosphate synthases (GGPSs) in the terpenoid biosynthesis pathway showed higher abundances in HN-Y87. GGPS catalyzes the conversion of farnesyl pyrophosphate (FPP) and isopentenyl pyrophosphate (IPP) to produce geranylgeranyl pyrophosphate (GGPP), which plays a vital role in terpenoid biosynthesis and is essential for plant growth and development (Wu et al. 2020b). It has also been reported that the expression of chloroplast-localized GGPS in tobacco plants has conferred fast plant growth (Tata et al. 2016). Considering the higher temperature and strong UV exposure in Henan, the upregulation of GGPPs may result from increased photosynthesis, which could foster plant growth.
The Effect of Cultivar on Tobacco Proteome Profiles
To investigate the proteomic response of different cultivars planted under the same geographic conditions, we compared the proteomic profiles of HN-Z100 vs. HN-Y87 and YN-Y100 vs. YN-Y87. Of the 324 DAPs identified from HN-Z100 vs. HN-Y87, 27 DAPs were heat shock proteins, and all had decreased levels in HN-Z100 (Fig. 4). The heat tolerance of different species varies significantly. Considering the important role of HSPs in plant heat response and tolerance mechanisms, the differentially expressed HSPs may suggest that the Z100 cultivar is less sensitive to high-temperature stress. Carbon metabolism has profound effects on the production of accessible energy, resistance metabolites, and carbon skeletons for biological activities during the growth and development of plants (Nunes-Nesi et al. 2010; Zhao et al. 2015). All the proteins associated with carbon fixation and over 80% of proteins related to carbon metabolism decreased in abundance in the Z100 cultivar compared with Y87 from Henan. 6-Phosphogluconate dehydrogenase (6PGD), which participates in carbon metabolism, was reduced by 0.08-fold in the Z100 cultivar compared with HN-Y87. Several key proteins involved in carbon metabolism, such as glutamate-glyoxylate aminotransferase 2-like (GGAT), fructose bisphosphate aldolase (FBA), D-fructose-1,6-bisphosphate 1-phosphohydrolase (Fru-1,6P2ase), pyruvate kinase (PK), phosphoglycerate kinase (PGK), and malic enzyme, had significantly decreased levels in HN-Z100. Proteins involved in the production of defense-related metabolites had mostly increased levels in HN-Z100 compared with HN-Y87. In the present study, seven endochitinases and two chitinases were found to increase in abundance in the comparison of HN-Z100 vs. HN-Y87. These proteins are associated with both mitogen-activated protein kinase (MAPK) signaling and amino sugar and nucleotide sugar metabolism and are essential for the transduction of defense-related signals and plant immunity actions against various environmental stresses (Bi et al. 2018). Previous studies have reported overexpression of chitinase in several biotic and abiotic stresses (Wu et al. 2020a). Thus, we assume that the differential expression of these proteins resulted from the difference in the stress tolerance of the two cultivars.
Volcano plots displaying the statistical p-value with the magnitude of abundance changes between two cultivars from Yunnan (A) and Henan (B). Blue circles indicate up-regulated proteins, and orange circles indicate down-regulated proteins. KEGG pathways of DAPs identified in the comparison of YN-Y100 vs. YN-Y87 (C) and HN-Z100 vs. HN-Y87 (D). nta01100, metabolic pathways; nta01110, biosynthesis of secondary metabolites; nta04141, protein processing in endoplasmic reticulum; nta01200, carbon metabolism; nta00710, carbon fixation in photosynthetic organisms; nta00030, pentose phosphate pathway; nta00520, amino sugar and nucleotide sugar metabolism; nta00010, glycolysis/gluconeogenesis; nta01230, biosynthesis of amino acids; nta00940, phenylpropanoid biosynthesis; nta00051, fructose and mannose metabolism; nta04016, MAPK signaling pathway—plant. nta00480, glutathione metabolism; nta00220, arginine biosynthesis; nta03010, ribosome; nta00250, alanine, aspartate, and glutamate metabolism; nta00710, carbon fixation in photosynthetic organisms
For the comparison of the two cultivars in Yunnan, 152 DAPs were identified in YN-Y100 vs. YN-Y87. This was half the number of DAPs identified in HN-Z100 vs. HN-Y87. The KEGG analysis revealed that the amino sugar and nucleotide sugar metabolism, the MAPK signaling pathway, and glutathione metabolism were most enriched in the DAPs. The levels of endochitinase, chitinase 134, and pathogenesis-related proteins involved in MAPK signaling were both increased in the Y100 cultivar, while proteins associated with glutathione metabolism were all found at low abundances. The levels of GGAT, FBA, and 6PGD, which are involved in carbon metabolism, were significantly decreased in the Y100 cultivar. Notably, the expression abundance of 6PGD was greatly decreased (by 0.06-fold) in YN-Y100. We found that defense signal-related proteins, such as endochitinase and chitinase, and carbon metabolism-related proteins, including GGAT, FBA, and 6PGD, were differentially expressed in both HN-Z100 vs. HN-Y87 and YN-Y100 vs. YN-Y87. This result suggests that carbon metabolism and MAPK signaling might contribute to metabolism regulation in different tobacco cultivars.
Conclusion
In this study, we investigated the effect of environmental and cultivar factors on the proteome profiles of tobacco leaves. The proteomic results of Y87 show a distinct pattern in different growing areas. The levels of proteins related to carbohydrate metabolism, carbon metabolism, amino acid synthesis, alkaloid biosynthesis, protein folding and unfolding, and pigment synthesis show obvious differences in the tobacco leaf sample from the three regions. This finding indicates that the above metabolic process might play a critical role in the regulation of the metabolic network to adapt to environmental changes. The upregulation of protein associated with sugar, amino acid, and alkaloid synthesis pathway may be the main contributor to the formation of light aroma for tobacco from the Yunnan region, while the PAL and CCoAOMT in the phenylpropanoid pathway may contribute greatly to the scent of heavy-aroma tobacco leaves. The identification of those aroma substance–related proteins is important to support tobacco planning for industries and help highlight and optimize the aroma type of tobacco in specific regions. In addition, the proteomic comparison of different tobacco cultivars in the Henan or Yunnan area demonstrated that the cultivar factor also has a great effect on proteomic profiles. The abundance of proteins involved in carbon metabolism and defense signaling was found to be significantly regulated in both comparisons from Henan and Yunnan (HN-Z100 vs. HN-Y87 and YN-Y100 vs. YN-Y87), suggesting that those proteins might be essential for metabolism regulation related to tobacco quality. By investigating the proteome profiling of different tobacco cultivars with changing geographic origins, this work defines the key proteins and pathways related to cultivar and environmental variation and highlights the effect of environment and cultivar on tobacco leaf quality.
Availability of data and materials
Not applicable.
Abbreviations
- LC‒MS/MS:
-
Liquid chromatography-tandem mass spectrometry
- RP-HPLC:
-
Reverse-phase high-performance liquid chromatography
- TCA:
-
Tricarboxylic acid
- UGP1:
-
UTP—glucose-1-phosphate uridylyltransferase
- ATP-PFK:
-
ATP-dependent 6-phosphofructokinase
- PS II:
-
Photosystem
- TEAB:
-
Triethylammonium bicarbonate
References
Bate NJ, Orr J, Ni W et al (1994) Quantitative relationship between phenylalanine ammonia-lyase levels and phenylpropanoid accumulation in transgenic tobacco identifies a rate-determining step in natural product synthesis. Proc Natl Acad Sci 91:7608–7612
Bi G, Zhou Z, Wang W et al (2018) Receptor-like cytoplasmic kinases directly link diverse pattern recognition receptors to the activation of mitogen-activated protein kinase cascades in arabidopsis. Plant Cell 30:1543–1561. https://doi.org/10.1105/tpc.17.00981
Bu D, Luo H, Huo P et al (2021) KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res 49:W317–W325. https://doi.org/10.1093/nar/gkab447
Coleman HD, Ellis DD, Gilbert M, Mansfield SD (2006) Up-regulation of sucrose synthase and UDP-glucose pyrophosphorylase impacts plant growth and metabolism. Plant Biotechnol J 4:87–101. https://doi.org/10.1111/j.1467-7652.2005.00160.x
de la Torre F, Cañas RA, Pascual MB et al (2014) Plastidic aspartate aminotransferases and the biosynthesis of essential amino acids in plants. J Exp Bot 65:5527–5534
Fritz C, Mueller C, Matt P et al (2006) Impact of the C-N status on the amino acid profile in tobacco source leaves. Plant Cell Environ 29:2055–2076
Gupta, DB, Shekhar, S, Agrawal, L (2015) Plant Proteomics: Technologies and Applications. In: Barh D, Khan M, Davies E (eds) PlantOmics: The Omics of Plant Science. Springer, New Delhi. https://doi.org/10.1007/978-81-322-2172-2_8
Herrmann HA, Schwartz JM, Johnson GN (2019) Metabolic acclimation - a key to enhancing photosynthesis in changing environments? J Exp Bot 70:3043–3056
Hu R, Zhu X, Xiang S et al (2018) Comparative proteomic analysis reveals differential protein and energy metabolisms from two tobacco cultivars in response to cold stress. Acta Physiol Plant 40:1–9. https://doi.org/10.1007/s11738-017-2582-7
Kimmerer TW (1987) Alcohol dehydrogenase and pyruvate decarboxylase activity in leaves and roots of eastern cottonwood ( Populus deltoides Bartr.) and soybean ( Glycine max L.). Plant Physiol 84:1210–1213. https://doi.org/10.1104/pp.84.4.1210
Kong W, Yu X, Chen H et al (2016) The catalytic subunit of magnesium-protoporphyrin IX monomethyl ester cyclase forms a chloroplast complex to regulate chlorophyll biosynthesis in rice. Plant Mol Biol 92:177–191
Li Y, Ren K, Hu M et al (2021) Cold stress in the harvest period: effects on tobacco leaf quality and curing characteristics. BMC Plant Biol 21:1–15
Li Z, Xia Y, Wang Y et al (2016) The similarities and differences of flue-cured tobacco aroma-note between Yunnan and Fujian. In: In Food Hygiene, Agriculture and Animal Science: Proceedings of the 2015 International Conference on Food Hygiene, Agriculture and Animal Science. pp 284–290. https://doi.org/10.1142/9789813100374_0036
Li J, Yang R, Jiang Y et al (2022) Comparative proteomic analysis by isobaric tags for the relative and absolute quantification reveals the responses of tobacco (Nicotiana tabacum L.) Roots to Different Soil Types. Front Plant Sci. https://doi.org/10.3389/fpls.2022.847388
Liu Y, Lu S, Liu K et al (2019) Proteomics: a powerful tool to study plant responses to biotic stress. Plant Methods 15:1–20
Liu A, Yuan K, Xu H et al (2022) Proteomic and metabolomic revealed differences in the distribution and synthesis mechanism of aroma precursors in Yunyan 87 tobacco leaf, stem, and root at the seedling stage. ACS Omega. https://doi.org/10.1021/acsomega.2c03877
McAllister CH, Wolansky M, Good AG (2016) The impact on nitrogen-efficient phenotypes when aspartate aminotransferase is expressed tissue-specifically in Brassica napus. New Negat Plant Sci 3:1–9
Mergner J, Kuster B (2022) Plant proteome dynamics. Annu Rev Plant Biol 73:67–92
Mo Z, Duan L, Pu Y et al (2022) Proteomics and co-expression network analysis reveal the importance of hub proteins and metabolic pathways in nicotine synthesis and accumulation in tobacco (Nicotiana tabacum L.). Front Plant Sci. https://doi.org/10.3389/fpls.2022.860455
Nunes-Nesi A, Fernie AR, Stitt M (2010) Metabolic and signaling aspects underpinning the regulation of plant carbon nitrogen interactions. Mol Plant 3:973–996
Oliva M, Guy A, Galili G et al (2021) Enhanced production of aromatic amino acids in tobacco plants leads to increased phenylpropanoid metabolites and tolerance to stresses. Front Plant Sci 11:604349
Osorio S, Muñoz C, Valpuesta V (2010) Physiology and biochemistry of fruit flavors. Handbook of Fruit and Vegetable Flavors 25:43
Pichersky E, Gershenzon J (2002) The formation and function of plant volatiles: perfumes for pollinator attraction and defense. Curr Opin Plant Biol 5:237–243
Seydel C, Kitashova A, Fürtauer L, Nägele T (2022) Temperature-induced dynamics of plant carbohydrate metabolism. Physiol Plant 174:e13602
Sghaier-Hammami B, Valledor L, Drira N, Jorrin-Novo JV (2009) Proteomic analysis of the development and germination of date palm [Phoenix dactylifera L.) zygotic embryos. Proteomics 9:2543–2554. https://doi.org/10.1002/pmic.200800523
Sierro N, Battey JND, Ouadi S et al (2014) The tobacco genome sequence and its comparison with those of tomato and potato. Nat Commun 5:1–9. https://doi.org/10.1038/ncomms4833
Song Q, Zheng-yin W, Jun-xiong S (2007) Quality characteristics of tobacco leaves with different aromatic styles from Guizhou Province, China. Agric Sci China 6:220–226. https://doi.org/10.1016/S1671-2927(07)60038-8
Strommer J (2011) The plant ADH gene family. Plant J 66:128–142. https://doi.org/10.1111/j.1365-313X.2010.04458.x
Sun B, Xue SL, Zhang F et al (2015) A quantitative real-time PCR-based strategy for molecular evaluation of nicotine conversion in burley tobacco. Int J Mol Sci 16:27422–27432. https://doi.org/10.3390/ijms161126038
Sun B, Zhang F, Chu GH et al (2013) Effects of different environmental locations on alkaloid accumulation in tobacco leaves in China. J Food Agric Environ 11:1337–1342
Sun B, Zheng AH, Zhang F et al (2018) Metabolic profiles of Cuibi-1 and Zhongyan-100 flue-cured tobacco leaves in different growing regions by gas chromatography/mass spectrometry. R Soc Open Sci 5:180261. https://doi.org/10.1098/rsos.180261
Tang Z, Chen L, Chen Z et al (2020) Climatic factors determine the yield and quality of Honghe flue-cured tobacco. Sci Rep. https://doi.org/10.1038/s41598-020-76919-0
Tata SK, Jung J, Kim Y et al (2016) Heterologous expression of chloroplast-localized geranylgeranyl pyrophosphate synthase confers fast plant growth, early flowering and increased seed yield. Plant Biotechnol J 14:29–39
Timperio AM, Egidi MG, Zolla L (2008) Proteomics applied on plant abiotic stresses: role of heat shock proteins (HSP). J Proteomics 71:391–411
Tomiyama M, Inoue S, Tsuzuki T et al (2014) Mg-chelatase I subunit 1 and Mg-protoporphyrin IX methyltransferase affect the stomatal aperture in Arabidopsis thaliana. J Plant Res 127:553–563
Tsaballa A, Sarrou E, Xanthopoulou A et al (2020) Comprehensive approaches reveal key transcripts and metabolites highlighting metabolic diversity among three oriental tobacco varieties. Ind Crops Prod 143:111933. https://doi.org/10.1016/j.indcrop.2019.111933
Wu W, Tang XP, Yang C et al (2013) Investigation of ecological factors controlling quality of flue-cured tobacco (Nicotiana tabacum L.) using classification methods. Ecol Inform 16:53–61. https://doi.org/10.1016/j.ecoinf.2013.04.008
Wu S, Cao G, Adil MF et al (2020a) Changes in water loss and cell wall metabolism during postharvest withering of tobacco (Nicotiana tabacum L.) leaves using tandem mass tag-based quantitative proteomics approach. Plant Physiol Biochem 150:121–132. https://doi.org/10.1016/j.plaphy.2020.02.040
Wu S, Guo Y, Adil MF et al (2020b) Comparative proteomic analysis by iTRAQ reveals that plastid pigment metabolism contributes to leaf color changes in tobacco (Nicotiana tabacum) during curing. Int J Mol Sci 21:2394
Xiang G, Yang H, Yang L et al (2010) Multivariate statistical analysis of tobacco of different origin, grade and variety according to polyphenols and organic acids. Microchem J 95:198–206. https://doi.org/10.1016/j.microc.2009.12.001
Xu J, Zhou Y, Xu Z et al (2020) Combining physiological and metabolomic analysis to unravel the regulations of coronatine alleviating water stress in tobacco (Nicotiana tabacum L.). Biomolecules. https://doi.org/10.3390/biom10010099
Xu H, Lü D, Zhang Y et al (2017) Identification of flue-cured tobacco flavor style using GC/MS fingerprint of aroma component extracted by dichloromethane. Tobacco Science and Technology. https://doi.org/10.16135/j.issn1002-0861.2016.0342
Yang C, Wu W, Wu SC et al (2014) Aroma types of flue-cured tobacco in China: spatial distribution and association with climatic factors. Theor Appl Climatol 115:541–549. https://doi.org/10.1007/s00704-013-0914-0
Yin F, Zhang X, Song S et al (2016) Identification of aroma types and their characteristic volatile compounds of Chinese faint-scent cigarettes based on descriptive sensory analysis and GC–MS and partial least squares regression. Eur Food Res Technol 242:869–880. https://doi.org/10.1007/s00217-015-2593-9
Zhang X, Liu C-J (2015) Multifaceted regulations of gateway enzyme phenylalanine ammonia-lyase in the biosynthesis of phenylpropanoids. Mol Plant 8:17–27
Zhang L, Wang X, Guo J et al (2013) Metabolic profiling of chinese tobacco leaf of different geographical origins by GC-MS. J Agric Food Chem 61:2597–2605. https://doi.org/10.1021/jf400428t
Zhao Y, Zhao C, Lu X et al (2013) Investigation of the relationship between the metabolic profile of tobacco leaves in different planting regions and climate factors using a pseudotargeted method based on gas chromatography/mass spectrometry. J Proteome Res 12:5072–5083. https://doi.org/10.1021/pr400799a
Zhao Y, Zhao J, Zhao C et al (2015) A metabolomics study delineating geographical location-associated primary metabolic changes in the leaves of growing tobacco plants by GC-MS and CE-MS. Sci Rep 5:1–11. https://doi.org/10.1038/srep16346
Zhou G, Yin X, Li Y et al (2015) Optimal planting timing for corn relay intercropped with flue-cured tobacco. Crop Sci 55:2852–2862. https://doi.org/10.2135/cropsci2014.05.0396
Author information
Authors and Affiliations
Contributions
Y.X. Hu, D. Wu, and X.M. Zhang conceived and designed the research. Y.X. Hu performed the experiments and data analysis. Y.X. Hu, M. Chen, and G. Li wrote the paper. D. Wu and X.M. Zhang reviewed and edited the paper. All the authors drafted and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, Y., Chen, M., Li, G. et al. A Comparative Proteomics Study Revealing the Impact of Growing Region and Cultivar on Protein Expression in the Leaves of Nicotiana tabacum Plants. Plant Mol Biol Rep (2024). https://doi.org/10.1007/s11105-024-01444-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11105-024-01444-7