Abstract
Nitrogen plays a crucial role in plant metabolism, growth, and development of plants, and its deficiency leads to severe growth retardation and reduced grain yield. The efficient utilization of nitrogenous fertilizers is needed to enhance crop yield and also to fetch the food demand of the world population. The accumulated nitrogen in the ecosystem leads to severe environmental pollution and health hazards to inhabited animals. However, nitrogen inside plants is regulated by a set of nitrogen metabolism genes, promoters, and transcription factors. Further, the identification and characterization of nitrogen metabolism genes in crop plants is a prerequisite for developing tailored crop plants for increased nitrogen use efficiency (NUE), grain yield, biomass, and other economic traits. Moreover, NUE is a complex trait, and breeding crops for improving NUE is still in the infancy stage. Therefore, a targeted and holistic approach is required for enhanced nitrogen uptake and its utilization. The precise modulation of key genes of nitrogen metabolism, amino acid biosynthesis, and carbon metabolism could result in enhancement of NUE, and the engineered crop plants for NUE traits were reported to be superior in terms of NUE and also incurred higher grain yield, biomass, and improved agronomical parameters as that of cultivated crop cultivars. In this review, we described the basics of nitrogen metabolism, genomics, and recently targeted genetic engineering strategies employed in crop plants for improving NUE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The last four decades witnessed a double enhancement in cereal production driven mainly by the use of high-yielding varieties and fertilizers in agriculture (Zhu et al. 2022). Nitrogen (N) shares 78% of the atmospheric gaseous composition and crops are unable to use it in this molecular form (Anas et al. 2020). Although leguminous crops fix the gaseous N into a utilizable form such as ammonia (NH3) and nitrate (NO3−), this fixed N is insufficient to meet the crop production demand of larger acreages (Ladha et al. 2022). Moreover, N is deficient in almost all agricultural soils of the world, and about 111.59 million tonnes of nitrogenous fertilizers are applied in world agricultural land to meet the crop’s N requirement (FAO 2022). To tackle N paucity, the farmers generally solicit N fertilizers externally in their fields to maintain higher crop yields (Dimkpa et al. 2020). However, the excessive N application incurs higher environmental and farmer inputs costs, with sometimes human health implications (Ren et al. 2022). Moreover, crop plants absorb only 30% of applied nitrogenous fertilizer (Pahalvi et al. 2021), and unutilized N is lost to water bodies and the atmosphere (Rahman et al. 2021). Presently, elevated demand and high cost of nitrogenous fertilizer in agriculture call for a “second green revolution,” which could be accomplished by designing nitrogen-use efficient crops. These nitrogen-use efficient crops could efficiently uptake, translocate, and assimilate N and allow a lesser quantity of cascading reactive N to be released into the ecosystem (Liu et al. 2022b). Hence, this review has been formulated to focus on both present and forthcoming nitrogen use efficiency (NUE) for sustainable agriculture.
N is essential for plant growth and development (Liu et al. 2022a; Muhammad et al. 2022), and it is a constituent of essential biomolecules such as nucleic acid, enzymes, protein, ATP, chlorophyll, and alkaloids. Also, N improves root system architecture, resulting in better water and nutrient absorption from the soil. (Jia et al. 2022; Sinha et al. 2020a). In chronic N deficiency, older leaves undergo senescence and detach from plants (Zakari et al. 2020). Moreover, a deficiency of N leads to altered metabolism in the plant (Saloner and Bernstein 2021) and ultimately results in reduced yield and biomass (Gong et al. 2019; Meise et al. 2019).
The movement of N in the environment is governed by the biogeochemical cycle, and it involves the dynamic interaction of soil and plant systems. The interaction is regulated by various processes, including N fixation, nitrification, denitrification, and leaching. (Liu et al. 2022a). The flow of chemical N in the environment leads to environmental deterioration (Huang et al. 2019). The primary reason for N leaching in the ecosystem is the low NUE of crop plants (Darjee et al. 2023). The rise of chemical N in the soil causes groundwater pollution (Shukla and Saxena 2020. The enrichment of inland and marine water bodies with nutrients, especially N and phosphorus (P), in water bodies causes eutrophication, and a rise in greenhouse gases like N2O contributes to global warming (Lenhart et al. 2019).
Genetic Approaches for Enhancing NUE
Various approaches are being utilized to enhance NUE to counteract the effects of low NUE on crop plants, economic yield, and the environment. The most prominent and efficient available method among the contemporary approaches includes conventional breeding and genetic manipulation of the transcription factors, transporters, assimilatory, and remobilization genes for nitrogen metabolism in crop plants to enhance the NUE (Lee et al. 2021; Kasemsap and Bloom 2022; Yadesa 2022).
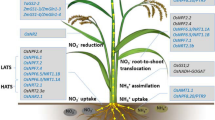
The NUE is an inherently complex trait involving gene interaction networks that regulate N uptake, assimilation, translocation, and storage (Fig. 1). The NUE is governed by complex genetic makeup that mediates the N uptake and utilization (Mahboob et al. 2023). Several candidate genes in crop plants have been identified for improving NUE (McAllister et al. 2012; Li et al. 2022b), and through genetic manipulation, these identified genes have been modulated in crop plants to enhance NUE and crop productivity (Tegeder and Masclaux-Daubresse 2018; Luo et al. 2023; Table 1). In this regard, to the present date, many plant N–metabolism-associated transporters and assimilation enzymes have been overexpressed in crop plants resulting in enhanced plant growth even under N insufficient conditions (Fiaz et al. 2021; Yadav et al. 2021; Lal et al. 2023). Furthermore, it is critical to figure out the other probable limiting processes in N metabolism while engineering the crops for enhanced NUE in plants rather than N uptake under high N input agriculture. Advancements in genome editing tools like CRISPR/Cas9 (clustered regularly interspaced short palindromic repeat/CRISPR-associated nuclease 9) technology could be utilized for enhancing NUE in plants (Tiwari et al. 2020).
The key assimilatory genes include glutamine synthetase (GS), Glutamine oxoglutarate aminotransferase (GOGAT), Alanine aminotransferase (AlaAT), Glutamate dehydrogenase (GDH), Aspartate aminotransferase (AspAT), and Asparagine synthase (ASN) play a vital role in plant N metabolism (Lebedev et al. 2021; Singh et al. 2022; Fig. 1). Further, transcription factors (TFs) include OsMAD25, OsDOF18, OsMYB305, and N transporter like NRT1, NRT2, AMT, AAP, and STP are intensively characterized for improving NUE in crop plants.
Breeding Approaches for Enhancing NUE
Plant breeders aim to develop crop cultivars that can efficiently uptake N from the soil and its utilization inside the plant system. The NUE is the economic yield per unit N supplied to the plant. In contrast, physiological N use efficiency (PNUE) is the yield in relation to the amount of N accumulated within the plant as shown in Fig. 2 (Moll et al. 1982). PNUE is being applied in rice to assess NUE (Huang et al. 2022a, b). There is the existence of genetic variabilities in cereal crops for NUE traits as revealed by field studies (Pujarula et al. 2021). At the time of grain filling in plants, carbon (C) and N substrates are required, and N molecules get remobilized from shoots to the grain (Hajibarat and Saidi 2022). About 64% of remobilized N comes from the leaf blade and leaf sheath, while the stem contributes 36% of remobilized N from vegetative tissue to panicle at the time of grain filling (Mae and Ohira 1981). Significant variation exists among cultivars for N remobilization during the reproductive stage, and prevailing variation in rice germplasm could be employed for efficient N management (He et al. 2021). NUE is well correlated with the translocation of metabolites from leaves to panicles during grain development in rice (Melino et al. 2022).
During the Green revolution era (1960s), semi-dwarf wheat and rice varieties were bred to exploit the higher doses of nitrogenous fertilizers resulting in higher yield and productivity. However, in the last few decades, excessive use of nitrogenous fertilizer has witnessed yield stagnation along with environmental hazards, which brought much attention to the plant breeders for developing N-use efficient crop varieties (Liu et al. 2022b).
NUE is defined broadly as a dual function of N uptake efficiency (NUpE) and utilization efficiency (NUtE). The availability of adequate genetic variability for component traits is a prerequisite for response to genetic gain under selection. Classical plant breeding methods have been wonderfully performed in the last three decades by selecting the most desirable features in terms of yield improvement. However, very little attention has been paid to understanding the physiological and genetic basis, particularly in relation to NUE. Most of the plant breeding efforts have been made under idealized conditions to enhance productivity, and it is quite obvious that valuable allelic diversity might have been significantly reduced in available breeding materials for tolerance to limiting N conditions.
In the past, most peer-reviewed research papers for important crops suggested that contemporary cultivars have sufficient genetic variation for selecting and enhancing NUE component traits (Hawkesford 2017; Hawkesford and Griffiths 2019). Another source of genetic diversity can be found in the secondary or tertiary gene pool or wild relatives of almost all other major food crops, and NUE has not been thoroughly characterized in these potential wild cultivars (Hawkesford 2017). therefore, there is a greater scope for the identification and introgression of the NUE-related genes in elite cultivars through backcross breeding programs.
Grain yield and biomass are primarily used to select promising phenotypes under low N and optimal N conditions. Despite the availability of adequate variability for the component traits under low N, lower overlapping values in normalized conditions make the selection more attractive (Gallais et al. 2006). However, direct selection under low input/low N status yielded desired results, mainly in wheat and maize (Presterl et al. 2003; Laperche et al. 2006). Simultaneous selection of genotypes based on grain yield in both N stress and optimal conditions has resulted in the overall improvement in both conditions. Designing an efficient breeding program is based on the kind of genetic materials, genetic parameters of variability, and stability of parameters across the environment. The ability of the genotype to efficiently take up N is a key component trait for plant NUE. The key traits for NUE include N uptake at stress conditions, harvest index (HI), N harvest index (NHI), and biomass potential are important for a breeder while developing nitrogen-use efficient lines.
Genetic variability for root size, distribution, and proliferation has been reported in several crop plants; however, it also varies with soil types and nutrient status (Matsunami et al. 2009, 2010). Genetic diversity for root traits was observed while screening spring wheat genotypes under pot and hydroponics conditions and they have found NUPE largely responsible for contributing towards NUE mainly because of high root proliferation and biomass production (Ranjan et al. 2019). Sufficient variability for NUE in modern wheat cultivars was observed under low N conditions (Hawkesford 2017). However, very little variation for grain yield suggests incorporating a more diverse range of genotypes for creating a wider range of variability for yield coupled with NUE. Some previous research with N metabolism and signalling pathway-governed genes show adequate variability in crop species for breeding the NUE efficient cultivars. The most striking variability of NUE-related genes in rice is for a GOGAT enzyme. This gene was originally reported in japonica rice and transferred in indica background and resulting in enhancement of yield (Yamaya et al. 2002).
Since NUE is an integrative complex trait, decided by the number of key traits and other morpho-physio traits that are linked or function independently. The presence of significant genotypes by environment (G × E) interactions reduces the overall effectiveness of selection using grain yield under low N conditions. In addition, the polygenic nature, linkage, and non-additive gene action coupled with low heritability further complicate the selection criterion. Indirect selection using the component traits is more realistic because of high heritability and more stability with respect to genetic and external environmental factors. The selection-based index is the most attractive approach for complex traits like NUE; it overcomes the limitation of single trait selection and allows simultaneous improvement of component traits. The appropriate selection of selection index may vary depending upon the conditions and usefulness of the breeders while working on a particular problem. N efficient wheat lines identified based on utilizing four selection indices: tolerance index (TOL), stress susceptibility index (SSI), geometric mean productivity (GMP) and yield susceptibility index (YSI) (Tyagi et al. 2020). Presently, conventional breeding methods have been greatly improved by integrating the knowledge of genetics, physiology, and biochemistry.
Exploiting genetic variability through innovative molecular breeding technology is in great demand because of the easy dissection of complex traits and assisting in phenotyping in less time. Precise phenotyping of NUE traits is cumbersome and requires vigorous exercise to monitor the component's traits. Advancement in developing next-generation markers/sequencing-based markers and bioinformatics improves the chances of locating and mapping QTLs governing the NUE traits. While breeding nitrogen-use efficient cultivars, key agronomic traits such as grain protein content, grain yield, test weight, and other NUE traits such as NHI, N remobilization capacity, and N content in grain were used in the breeding program (Karunarathne et al. 2020).
Molecular linkage genetic maps and quantitative trait locus (QTL) mapping technologies are commonly used to estimate loci’s number and precise location governing genetic variation in various genetic materials (segregating or fixed populations). The selection of appropriate parents, evaluation of effective population sizes, multi-location testing, and the availability of high-density genetic maps are all required for successful QTL mapping programs for complex traits like NUE (Han et al. 2015). N deficiency traits (NDT) have also been targeted as an indirect selection criterion for selecting nitrogen-use efficient phenes in rice (Shen et al. 2021), and several QTLs for NDT and NUE traits in rice have been identified. (Ogawa et al. 2016; Shen et al. 2021). A few of a total of six QTL contributing towards NUE or low N-responsive traits in winter wheat showed the association with photoperiod and disease resistance and suggested for exploitation of potential marker-assisted selection (MAS) for breeding soft red winter wheat (Brasier et al. 2020).
Li et al. (2015) identified 184 and 147 QTLs for NUE and root system architecture (RSA) related traits and established a genetic relationship with RSA. They also explored the genomics segment for MAS in maize to improve complex traits like NUE and others. A total of 15 QTLs showing significant effects under low N conditions were identified in a barley population (Prisma × Apex) (Kindu et al. 2014). Besides that, several other QTLs have also been identified through genome-wide association studies (GWAS) for key indicator traits of NUE in barley (Pasam et al. 2012). Fifty-two putative QTLs were reported for different NUE traits in a double haploid (DH) potato population and field trial at several locations with different N-levels, specific QTLs showing significant QTL × E interaction (Getahun et al. 2020).
NUE and its component traits are mostly polygenic and background-specific; thus, the ultimate target would be to converge all the small effect loci in one genetic background to advance the realized genetic gain (R). Genomic selection is one of the most appropriate strategies to target all the small-effect minor QTLs through a predictive equation based on genome-estimated breeding value (EBV) and facilitate overall genetic improvement of complex traits like NUE. In future research, there is a need to holistically target the key information on the agronomical aspect of NUE, physiological NUE, and absorption NUE in respect of breeding varieties for low input or low N conditions.
Engineering Root System Architecture for NUE
The gene reported to control root growth and to give a particular response towards nutrients in soil has shown to be a promising target for engineering plants for better nutrient use efficiency. OsTOND1 belongs to QTL, which confers tolerance under N deficiency in rice. It codes for a thaumatin protein and its role in the induction of primary root elongation in low N soil. OsTOND1 has been found in only some indica rice varieties, which is absent in the japonica rice varieties studied so far. Furthermore, overexpression of OsTOND1 in rice confers tolerance to nitrogen deprivation, and overexpressed rice plants growing in nitrogen-deficient conditions have increased shoot N content, grain production, panicle number, dry weight, chlorophyll content, plant height, and root length (Zhang et al. 2015; Sujata et al. 2019).
A longer root system meant for the acquisition of nutrients from deeper soil layer, a characterized QTL, DEEPER ROOTING1 (DRO1) responsible for longer root in upland land rice, affects root system architecture in Arabidopsis and Prunus species and also enhances yield under drought in rice (Uga et al. 2011, 2013; Arai-Sanoh et al. 2014; Guseman et al. 2017). The rice cultivar with the DRO1 gene exhibiting enhanced N uptake and grain yield increased by 10% compared to the rice variety with a dormant DRO1 allele (Arai-Sanoh et al. 2014; Kitomi et al. 2020). Rice transcription factor OsMADS25 is involved in N signalling and it is predominantly expressed in the root during the growth period. OsMADS25 overexpression in rice results in better root system architecture and higher shoot mass (Yu et al. 2015; Nazish et al. 2021).
Mild N deficiency enhances lateral root (LR) branching (Forde 2014). A study in Arabidopsis reveals that N deficiency causes an increase in lateral roots (Ma et al. 2014). Moreover, expression of tryptophan aminotransferase related 2 (TATAR2) was enhanced under low nitrogen in wheat, and knockdown mutant of TATAR2.1 exhibits impaired lateral root growth and phenotype defects under limiting and optimum N supply in wheat (Shao et al. 2017). Also, TaTAR2.1-3A overexpression in wheat enhanced plant growth, grain yield, primary root length, lateral root branching, and N accumulation at different N conditions while reducing expression of TaTAR2.1 have opposite effects (Shao et al. 2017; Teng et al. 2022).
Genetic Manipulation of N Transporters
The N transporters play a crucial role in N plant metabolism. Moreover, N transporters are extensively studied in rice, and its genome encodes 94 nitrate transporter 1 (NRT1)/peptide transporter (PTR), five nitrate transporter 2 (NRT2), five chloride channel (CLC), and nine slow anion channel-associated homologs (SLAC/SLAH) gene (Lee 2021). Transport of NO3− inside crops is mainly regulated through NRT1 and NRT2 transporters (Aluko et al. 2023). However, utilizing existing natural variations and overexpressing NO3– transporters resulted in enhanced NUE and grain yield in rice (Hu et al. 2015; Fan et al. 2016b; Zhang et al. 2022). The OsNPF7.6 is a member of the nitrate transporter 1/peptide transporter family (NPF) expressed in all plant tissues. Moreover, overexpression of OsNPF7.6 enhances the rice yield and NUE (Zhang et al. 2022). Furthermore, OsNPF7.3 of the NO3– NPF is expressed in lateral roots and stem and localized in the vacuolar membrane. The elevated expression of OsNPF7.3 in rice results in enhanced grain yield, tiller number, and grain N content (Fang et al. 2017). The NH4+ induces the expression of OsNRT1.1A, which belongs to the NO3– transporter1 family. In rice, OsNRT1.1A overexpression results in up to 50% enhancement in NUE, grain yield, and early maturity by 18 days. On the contrary, the OsNRT1.1A mutant shows growth retardation, yield loss, late flowering, and reduced utilization of NH4+ and NO3– (Wang et al. 2018).
Studies revealed diversification in rice genotypes for uptake efficiency of NO3–; the indica subspecies have higher NO3– uptake efficiency than the japonica subspecies that is due to variation of the gene sequence of OsNRT1.1B (Hu et al. 2015). Moreover, OsNRT1.1b overexpression in rice accumulated higher N and improved plant growth under low and sufficient N supply (Fan et al. 2016a). Moreover, rice contains a high-affinity transporter, OsNRT2.3 consisting of OsNRT2.3a and OsNRT2.3b, splice forms that code for plasma membrane protein. OsNRT2.3a function in association with nitrate transport accessory protein, OsNAR2.1 (Yan et al. 2011; Tang et al. 2012). The concurrently expressing OsNAR2.1 and OsNRT2.3a in rice results in enhanced uptake and transport of NO3– and NH4+, and the grain yield and NUE were enhanced by 24.6% and 28.6% in co-overexpressed lines (Chen et al. 2020). Also, overexpression of OsNRT2.3b in barley results in up to 43% enhancement in NUE. The grain weight and yield of overexpressed lines are higher than control plants (Luo et al. 2020). Furthermore, overexpression of OsNRT2.3b in rice results in up to 40% higher NUE and grain yield (Fan et al. 2016b). The study reveals that NRT2 plays a key function in increasing yield and NUE under a different N supply. However, the gene should be expressed with tissue-specific promoters to get the desired result.
The OsPTR6 and OsPTR69 belong to the PTR/NRT1 family. The OsPTR6 overexpression in rice enhances the activity of glutamine synthetase (GS), NUE, and growth of the plant as compared to control plants supplied with NH4+ at the optimum amount (Fan et al. 2014), and overexpression of OsPTR9 in rice enhances NH4+ absorption, which drives LR branching and boosts grain production. On the contrary, OsPTR9 T-DNA mutant and OsPTR9 RNAi lines exhibit growth retardation (Fang et al. 2013).
Ammonium is transported inside plants through ammonium transporter (AMT). Rice (Oryza sativa) genome encodes four AMT family members: OsAMT1, OsAMT2, OsAMT3;1, OsAMT3, and OsAMT4. The OsAMT1 members belong to high-affinity transporters, while the other three family members are low-affinity transporters (Wu et al. 2017). Crop plants primarily absorb N in NH4+ and NO3– forms. In rice, overexpression of OsAMT1-1 boosts NH4+ uptake and its translocation inside the plant at an optimum level, but under a high amount of NH4+ supply, it impairs plant growth (Ranathunge et al. 2014; Li et al. 2016). Also, overexpressing OsAMT1.3 in rice enhances plant growth, and plants able to adapt at a low concentration of NH4+ (Bao et al. 2015; Ferreira et al. 2015). In rice, activation tagging mutants co-expressing OsAMT1;2 and OsGOGAT had better grain yield and NUE under deficient N as that of wild type and double mutants of OsAMT1;2 and OsGOGAT1 exhibit higher protein and N concentration as compared to wild type (Lee et al. 2020a). Also, ZmAMT1;1a overexpression in maize shows a 17% increase in NH4+ uptake under low NH4+ supplied to plants as that of control plants (Zhao et al. 2018).
N uptake in crop plants is regulated by transcription factors (TFs) and signalling molecules (Zhang et al. 2020; Zuluaga and Sonnante 2019). Among the TFS, DNA binding with one finger 18 (OsDOF18) rice mutant has impaired NH4+ assimilation and reduced expression of NH4+ transporter genes. Rice mutant plants grow well on supplying NH4+ as NO3– (Wu et al. 2017) and N-mediated heading date-1 (Nhd1) activate the transcription of OsAMT1;3 and OsNRT2.4 and knockdown of the Nhd1 inhibits the root growth in rice (Li et al. 2022a).
The OsMYB305 encodes for transcription activator in rice. Under low nitrogen, this gene shows higher expression in the root. The overexpression of OsMYB305 in rice exhibits enhancement in tiller number, shoot dry weight, and increased N accumulation in the plant grown under low N conditions. The expression of a high-affinity NO3– transporter and nitrite reductase gene upregulated in OsMYB305 overexpressed lines (Wang et al. 2020).
The NIN-like protein (NLP) is involved in nitrate signalling by regulating the expression of several N uptakes and assimilatory genes (Mu and Luo 2019). The OsNLP4 overexpression in rice results in an enhancement in yield by 30% and increased NUE by 47% as that of the control plant. The loss of function rice mutant generated through CRISPR/Cas9 shows a significant reduction in yield under low nitrogen (LN), normal nitrogen (NN), and high nitrogen (HN) as that of wild type (Wu et al. 2021). Also, overexpressing OsNLP1 in rice improved the biomass, grain yield, and NUE on supplying with variant N supply. The knocking out of OsNLP1 reduces the grain yield and NUE (Alfatih et al. 2020). Moreover, Overexpression of AtNLP7 upregulates the expression of N uptake and assimilatory genes and improves the NUE and yield in cotton (Jan et al. 2022).
Genetic Manipulation of Key Genes of C and N Metabolism
The well-known RuBisCO plays an important role in C fixation by incorporating CO2 into Ribulose 1,5-bisphosphate (RuBP) and converting it into 3-phosphoglycerate (3-PGA). At a low concentration of CO2, RuBP combines with O2 to produce phosphoglycolate and later releases ammonium ions during photorespiration, hence affecting carbon fixation (Stitt et al. 2010). In C3 plants, released ammonium ions are returned to amino acid pools through assimilatory enzymes (McAllister et al. 2012). At a higher level of CO2, plants exhibit enhanced assimilation of N and C (Zong and Shangguan 2016). Knocking down the RuBisCO expression in crop plants affects the C and N metabolism, reducing grain yield, biomass, amino acid pools, and plant metabolites (Maheshwari et al. 2021).
Furthermore, overexpression of RuBisCO in rice causes an increase in photosynthesis and NUE under sufficient N fertilization (Yoon et al. 2020). PEPC (phosphoenolpyruvate carboxylase) is another enzyme involved in photosynthesis and N storage in plants; this enzyme is involved in vital metabolic processes in bacteria, algae, and other lower species. The product of this enzyme is oxaloacetic acid (OAA), having a vital role in other metabolic processes like tricarboxylic acid cycle (TCA) cycle, amino acid biosynthesis, gluconeogenesis, urea cycle, glyoxylate cycle, fatty acid synthesis, etc. (Doubnerová and Ryšlavá 2011). PEPC plays a crucial role in N assimilation as validated by the knockdown of chloroplastic PEPC isoforms (Masumoto et al. 2010). In rice, PEPC overexpression enhances photosynthesis, biomass, and grain yield (Behera et al. 2023). Moreover, ZmPEPC overexpression in wheat results in upregulating TCA cycle genes that signify more C generation under low and high N conditions. The total amino acid concentration was enhanced by 48.18% in overexpressed lines as that of wild-type plants (Peng et al. 2018).
Plants mostly uptake inorganic N in the form of NO3– and NH4+ from the soil, while plants take up organic N in the form of amino acids through amino acid transporter (AAT). The transporters are classified into three major families: ATF (amino acid transporter family), APC (amino acid-polyamine-choline transporter family), and UMAMIT (usually multiple acids move in and out transporter family). Among all these families, most research has focused on AAP (amino acid permeases) because of their multiple physiological functions, AAP transporters belong to ATF (Yang et al. 2020). The AAP1 gene overexpression is extensively studied in legumes and VfAAP1, specifically overexpressed in the seed of Vicia narbonensis and Pisum sativum results in up to 15% increase in total N content and enhancement in seed size up to 30% and also, co-overexpression of AAP1 and sucrose transporter, SUT1 in pea results in enhanced grain number, protein and sugar content (Grant et al. 2021; Rolletschek et al. 2005). Sugar transporters are associated with phloem loading and unloading in plants, and these transporters are involved in carbon distribution inside plants (Wen et al. 2022). Sugar transporter proteins (STPs) play crucial roles in plant sugar transport. Overexpressing STP13 (hexose transporter) in Arabidopsis results in an enhancement in glucose uptake, internal glucose amount, and biomass when plants are grown in a limiting N environment (Schofield et al. 2009). These studies suggested a link exists in plant systems for coordinating C and N metabolism. Improvement in NUE requires both C substrate and N availability, the lack of C substrate can compromise the NUE, and N availability affects C fixation (Sandhu et al. 2021). The GS/GOGAT cycle is also critical for maintaining the C/N ratio. The keto group contains amino acids involved in C metabolism utilizing glutamate as signalling molecules (Hildebrandt et al. 2015). The tuning of the C/N ratio is crucial for plant growth and development. Moreover, the NUE is regulated by the photosynthesis assimilation rate, and under the limitation of C substrate, NUE gets reduced (Liu et al. 2022a).
The primary enzyme in sucrose production is sucrose phosphate synthase (SPS). The overexpression of SPS in alfalfa plants results in an enhancement in growth and nodule development, and overexpression of SPS in sugarcane enhances growth and increases the height, sucrose and biomass content (Kaur et al. 2019; Anur et al. 2020). N assimilatory gene, AlaAT converts pyruvate to alanine, and this conversion is activated during hypoxia; thus, AlaAT links Glycolysis and TCA Cycle (Rocha et al. 2010). Further, the genetic manipulation of HvAlaAT using root and stress inducible promoter enhances NUE and grain yield in rice, barley, wheat, oilseed rape (Brassica napus) and sugarcane under low N conditions (Tiong et al. 2021; Good et al. 2007; Shrawat et al. 2008; Snyman et al. 2015).
The Dof gene of maize has been utilized for improving N assimilation by regulating the TCA cycle. The overexpression of Dof1 in rice enhances the C flow, which results in improved plant growth under low nitrogen (LN) environments (Kurai et al. 2011). Also, overexpression TaDof1 in wheat shows enhanced expression of citrate synthase, phosphoenolpyruvate carboxylase, isocitrate dehydrogenase, pyruvate kinase, and overexpressed wheat transgenic lines show improved agronomical traits under limiting N supply as compared to wild type (Hasnain et al. 2020).
Amino Acid Metabolism Regulates NUE
Glutamine synthetase (GS) catalyzes the ATP-dependent condensation of ammonia and Glutamate (Glu) to Glutamine (Gln), and GOGAT transfers the amide group of Gln to 2-oxoglutarate to form two molecules of Glu. Plant encodes two forms of GS, the cytosolic (GS1) and chloroplastic (GS2). In plants, GOGAT exists in two ferredoxin-GOGAT (Fd-GOGAT) and NADH-GOGAT that are expressed in chloroplast and cytoplasm, respectively. GS enzyme is the prime target of researchers for enhancing the NUE of crop plants (Hakvoort et al. 2017). GS modulated in plants through genetic engineering and tobacco plants overexpressing Arabidopsis GS1, GS2 grown under low nitrogen exhibits higher sugar, protein, and chlorophyll content (Wang et al. 2013). The cisgenic HvGS1;1 overexpressed line of barley exhibited enhanced grain yield and NUE compared to control plants at different N-supplied (Gao et al. 2019). Studies in durum wheat reveal GS2 alleles co-localized with grain protein content QTL, which makes the possibility of GS2 imparting a role in enhancing grain protein content (Gadaleta et al. 2011). Also, GS-overexpressed rice contains enhanced protein, amino acid, and total nitrogen content compared to wild type (Cai et al. 2009). Moreover, transgenic wheat expressing the plastidic GS2 isoform under low and high N regime conditions shows higher yield, NUE, and grain N concentration like wild type (Hu et al. 2018). Furthermore, Concurrent co-overexpression of OsGS1;1 and OsGS2 increased NUE, yield, and biomass in indica rice (Lal et al. 2023).
Plant roots exhibit plasticity rather than preference in the acquisition of NH4+ and NO3– (Chalk and Smith 2021). On supply of NH4+ to Arabidopsis, NADH-GOGAT shows higher expression and accumulation in the roots. T-DNA mutant line having non-functional NADH-GOGAT shows a reduction in biomass when the major N source is supplied as NH4+ (Konishi et al. 2014). In addition, glutamate dehydrogenase (GDH) is also a key enzyme in ammonia assimilation that converts 2-oxoglutarate to glutamate. The rice genome encodes four putative genes for Glutamate dehydrogenase (OsGDH1-4). There is differential regulation of OsGDH family members that takes place under P and N deprivation (Qiu et al. 2009). Overexpression of Novel TrGDH (Trichurus GDH) in rice results in improved plant growth and grain weight per plant (Du et al. 2019).
Aspartate aminotransferase (AAT; aspartate:2-oxoglutarate aminotransferase) catalyzes the reversible transamination reaction of glutamate and oxaloacetate to form aspartate and 2-oxoglutarate. AAT plays a key role in the C and N metabolism in plants. In rice, overexpression of aspartate aminotransferase (AAT) using CaMV 35S promoter enhanced AAT activity in the leaf and higher protein and seed amino acid content (Zhou et al. 2009). Another enzyme, asparagine synthetase (ASN) forms asparagine by condensing ammonia and aspartate or through the transamination of glutamine and aspartate. In Arabidopsis, ASN is encoded by three genes: ASN1, ASN2, and ASN3 (Gaufichon et al. 2016). In Arabidopsis, Asparagine synthetase 2 (ASN2) overexpression leads to amino acid accumulation under NH4+ as a sole N source, which depicts the role of ASN2 in NH4+ detoxification and N assimilation. Asparagine content was reduced in plants that under-express ASN2 (Igarashi et al. 2009). On overexpressing OsASN1 in rice enhanced the expressions of OsAMT1;1, OsAMT1;2, and OsAMT1;3. In overexpressed lines, Asparagine content in grain was enhanced to 3.4-fold, and N content in grain increased to 140% as that of wild type. (Lee et al. 2020a, b). Also, asparagine synthetase 4 (ZmTHP9) overexpressed maize lines exhibit enhanced asparagine content and seed protein content (Huang et al. 2022a, b). Furthermore, alanine:2-oxoglutarate aminotransferase (AlaAT) catalyzes the reversible transamination of glutamate and pyruvate to produce 2-oxoglutarate and alanine in the plant cytoplasm and mitochondria. Map-based cloning reveals floury endosperm12 (flo12) encodes alanine aminotransferase1 (OsAlaAT1) in rice. However, the starch synthesizing genes downregulated in flo12 mutants. Further, the amylose content was reduced, and protein content was enhanced in flo12 grain. This study depicts how OsAlaAT1 regulates the C and N metabolism (Zhong et al. 2019).
Future Prospects
For developing nitrogen use-efficient crops, the genes involved in N uptake, mobilization, and translocation need to be stacked and expressed inside the crop plants (Fig. 3). For enhancing NUE in crop plants, tissue-specific, inducible promoters could be utilized. Further, studies have revealed that enhancement in NUE is linked with C metabolism (Behera et al. 2023; Jin et al. 2022; Rosolem et al. 2022). Therefore, the C and N metabolism genes could be stacked together to get desired and consistent results.
The microbial communities are involved in biological N fixation and its uptake. For example, mycorrhiza mobilizes N and P and is also a reservoir of C (Scartazza et al. 2023). Additionally, the microbial genes involved in N fixation and uptake could be extensively screened, and desired NUE genes could be isolated from microbes. Furthermore, these genes can be transferred to crop plants for enhancing NUE. In addition to that, the mutant alleles imparting high NUE in crop plants could be made using CRISPR/Cas9 gene editing technology and mutant alleles imparting high NUE could be transferred in elite crop cultivars through conventional breeding. However, field trials of reported candidate genes associated with NUE need to be extensively conducted to reach at a final conclusion. Moreover, this study aids in understanding recent advancements and developments in improving crop NUE and yield. Therefore, the genetic approaches mentioned in this study can be adopted to obtain “tailored crop plants” having efficient NUE capabilities.
Concluding Remarks
In the present scenario of high N input agriculture, one of the major bottlenecks that dampen the farmer’s confidence and increase the agriculture–associated costs are the low NUE of crop plants (Govindasamy et al. 2023). Thus, in this scenario, one of the suitable solutions for maintaining crop productivity without increasing fertilizer usage is the development of crop plants with improved NUE via transgenic and gene editing technology (Karunarathne et al. 2022; Zhang et al. 2021; Sinha et al. 2020b; Fiaz et al. 2021). Moreover, intensive cultivation, adoption of high-yielding crop varieties, and hybrids in agriculture require enormous application of nitrogenous fertilizers in soils (Shukla et al. 2022). The N-efficient and responsive crop varieties need to be developed to reduce the nitrogen requirement. Developing such varieties is a bit challenging and complex task for the breeders. The identification and incorporation of potent genes, alleles, and QTLs for efficient nitrogen use efficiency into cultivated varieties through genetic engineering methods could be a promising alternative to minimize the use of fertilizers in agriculture (Karunarathne et al. 2022; Dong et al. 2022).
The cross-talk pathway related to nitrogen use efficiency needs to be modified to enhance NUE in crop plants. The carbon–nitrogen and nitrogen-phosphorus could be the prime targets for improving nitrogen use efficiency in crop plants. The most potent target for enhancing NUE could be the nitrogen transport pathways; the key transporters and activators of such pathways must be modified to channel nitrogen molecules into plant systems. Apart from this, the prime target could be the signalling pathway that directly/indirectly regulates nitrogen uptake, transport, assimilation, and translocation The geneticist and plant biotechnologist’s role is crucial for modifying the pathway in a specific and tailored way. The key genes involved in the pathway could be stacked to get a better phenotype for nitrogen use efficiency in crop plants. The developed designer N uses efficient crop plants, so-called super crops could provide a path and road map for an evergreen revolution.
Data Availability
Not applicable.
References
Alfatih A, Wu J, Zhang ZS, Xia JQ, Jan SU, Yu LH, Xiang CB (2020) Rice NIN-LIKE PROTEIN 1 rapidly responds to nitrogen deficiency and improves yield and nitrogen use efficiency. J Exp Bot 71(19):6032–6042
Aluko OO, Kant S, Adedire OM, Li C, Yuan G, Liu H, Wang Q (2023) Unlocking the potentials of nitrate transporters at improving plant nitrogen use efficiency. Front Plant Sci 14:1074839
Anas M, Liao F, Verma KK, Sarwar MA, Mahmood A, Chen ZL, Li Q, Zeng XP, Liu Y, Li YR (2020) Fate of nitrogen in agriculture and environment: agronomic, eco-physiological and molecular approaches to improve nitrogen use efficiency. Biol Res 53(1):1–20
Anur RM, Mufithah N, Sawitri WD, Sakakibara H, Sugiharto B (2020) Overexpression of sucrose phosphate synthase enhanced sucrose content and biomass production in transgenic sugarcane. Plants 9(2):200
Arai-Sanoh Y, Takai T, Yoshinaga S, Nakano H, Kojima M, Sakakibara H, Kondo M, Uga Y (2014) Deep rooting conferred by DEEPER ROOTING 1 enhances rice yield in paddy fields. Sci Rep 4(1):5563
Bao A, Liang Z, Zhao Z, Cai H (2015) Overexpressing of OsAMT1-3, a high affinity ammonium transporter gene, modifies rice growth and carbon-nitrogen metabolic status. Int J Mol Sci 16(5):9037–9063
Behera D, Swain A, Karmakar S, Dash M, Swain P, Baig MJ, Molla KA (2023) Overexpression of Setaria italica phosphoenolpyruvate carboxylase gene in rice positively impacts photosynthesis and agronomic traits. Plant Physiol Biochem 194:169–181
Brasier K, Ward B, Smith J, Seago J, Oakes J, Balota M, Davis P, Fountain M, Brown-Guedira G, Sneller C, Thomason W (2020) Identification of quantitative trait loci associated with nitrogen use efficiency in winter wheat. PLoS ONE 15(2):e0228775
Cai H, Zhou Y, Xiao J, Li X, Zhang Q, Lian X (2009) Overexpressed glutamine synthetase gene modifies nitrogen metabolism and abiotic stress responses in rice. Plant Cell Rep 28:527–537
Chalk P, Smith C (2021) On inorganic N uptake by vascular plants: can 15N tracer techniques resolve the NH4+ versus NO3− “preference” conundrum? Eur J Soil Sci 72(4):1762–1779
Chen J, Liu X, Liu S, Fan X, Zhao L, Song M, Fan X, Xu G (2020) Co-overexpression of OsNAR2. 1 and OsNRT2. 3a increased agronomic nitrogen use efficiency in transgenic rice plants. Front Plant Sci 11:1245
Darjee S, Shrivastava M, Langyan S, Singh G, Pandey R, Sharma A, Khandelwal A, Singh R (2023) Integrated nutrient management reduced the nutrient losses and increased crop yield in irrigated wheat. Arch Agron Soil Sci 69(8):1298–1309
Dimkpa CO, Fugice J, Singh U, Lewis TD (2020) Development of fertilizers for enhanced nitrogen use efficiency–trends and perspectives. Sci Total Environ 731:139113
Dong G, Zhou Y, Zhang J, Wang J, Zhou J, Chen C, Zhang X, Hua H, Shu X, Gao Y, Ju J (2022) Introgression of qPE9-1/DEP1, a major QTL for rice panicle erectness, drastically improves nitrogen use efficiency under limited nitrogen supply. Eur J Agron 133:126444
Doubnerová V, Ryšlavá H (2011) What can enzymes of C4 photosynthesis do for C3 plants under stress? Plant Sci 180(4):575–583
Du CQ, Lin JZ, Dong LA, Liu C, Tang DY, Yan L, Chen MD, Liu S, Liu XM (2019) Overexpression of an NADP (H)-dependent glutamate dehydrogenase gene, TrGDH, from Trichurus improves nitrogen assimilation, growth status and grain weight per plant in rice. Breed Sci 69(3):429–438
Fan X, Xie D, Chen J, Lu H, Xu Y, Ma C, Xu G (2014) Over-expression of OsPTR6 in rice increased plant growth at different nitrogen supplies but decreased nitrogen use efficiency at high ammonium supply. Plant Sci 227:1–11
Fan X, Tang Z, Tan Y, Zhang Y, Luo B, Yang M, Lian X, Shen Q, Miller AJ, Xu G (2016b) Overexpression of a pH-sensitive nitrate transporter in rice increases crop yields. Proc Natl Acad Sci 113(26):7118–7123
Fan X, Feng H, Tan Y, Xu Y, Miao Q, Xu G (2016a) A putative 6‐transmembrane nitrate transporter OsNRT1. 1b plays a key role in rice under low nitrogen. J Integr Plant Biol 58(6):590–599
Fang Z, Xia K, Yang X, Grotemeyer MS, Meier S, Rentsch D, Xu X, Zhang M (2013) Altered expression of the PTR/NRT 1 homologue OsPTR 9 affects nitrogen utilization efficiency, growth and grain yield in rice. Plant Biotechnol J 11(4):446–458
Fang Z, Bai G, Huang W, Wang Z, Wang X, Zhang M (2017) The rice peptide transporter OsNPF7. 3 is induced by organic nitrogen, and contributes to nitrogen allocation and grain yield. Front Plant Sci 8:1338
Ferreira LM, de Souza VM, Tavares OC, Zonta E, Santa-Catarina C, de Souza SR, Fernandes MS, Santos LA (2015) OsAMT1. 3 expression alters rice ammonium uptake kinetics and root morphology. Plant Biotechnol Rep 9:221–229
Fiaz S, Wang X, Khan SA, Ahmar S, Noor MA, Riaz A, Ali K, Abbas F, Mora-Poblete F, Figueroa CR, Alharthi B (2021) Novel plant breeding techniques to advance nitrogen use efficiency in rice: a review. GM Crops & Food 12(2):627–646
Food and Agriculture Organization, 2022. https://www.fao.org
Forde BG (2014) Nitrogen signalling pathways shaping root system architecture: an update. Curr Opin Plant Biol 21:30–36
Gadaleta A, Nigro D, Giancaspro A, Blanco A (2011) The glutamine synthetase (GS2) genes in relation to grain protein content of durum wheat. Funct Integr Genomics 11:665–670
Gallais A, Coque M, Quilléré I, Prioul JL, Hirel B (2006) Modelling post silking nitrogen fluxes in maize (Zea mays) using 15N-labelling field experiments. New Phytol 172(4):696–707
Gao Y, de Bang TC, Schjoerring JK (2019) Cisgenic overexpression of cytosolic glutamine synthetase improves nitrogen utilization efficiency in barley and prevents grain protein decline under elevated CO2. Plant Biotechnol J 17(7):1209–1221
Gaufichon L, Rothstein SJ, Suzuki A (2016) Asparagine metabolic pathways in Arabidopsis. Plant Cell Physiol 57(4):675–689
Getahun BB, Visser RG, van der Linden CG (2020) Identification of QTLs associated with nitrogen use efficiency and related traits in a diploid potato population. Am J Potato Res 97:185–201
Gong X, Li J, Ma H, Chen G, Dang K, Yang P, Wang M, Feng B (2019) Nitrogen deficiency induced a decrease in grain yield related to photosynthetic characteristics, carbon–nitrogen balance and nitrogen use efficiency in proso millet (Panicum miliaceum L.). Arch Agron Soil Sci 398–413
Good AG, Johnson SJ, De Pauw M, Carroll RT, Savidov N, Vidmar J, Lu Z, Taylor G, Stroeher V (2007) Engineering nitrogen use efficiency with alanine aminotransferase. Botany 85(3):252–262
Govindasamy P, Muthusamy SK, Bagavathiannan M, Mowrer J, Jagannadham PT, Maity A, Halli HM, GK S, Vadivel R, TK D, Raj R, (2023) Nitrogen use efficiency—a key to enhance crop productivity under a changing climate. Front Plant Sci 14:1121073
Grant JE, Ninan A, Cripps-Guazzone N, Shaw M, Song J, Petřík I, Novák O, Tegeder M, Jameson PE (2021) Concurrent overexpression of amino acid permease AAP1 (3a) and SUT1 sucrose transporter in pea resulted in increased seed number and changed cytokinin and protein levels. Funct Plant Biol 48(9):889–904
Guseman JM, Webb K, Srinivasan C, Dardick C (2017) DRO 1 influences root system architecture in Arabidopsis and Prunus species. Plant J 89(6):1093–1105
Hajibarat Z, Saidi A (2022) Senescence-associated proteins and nitrogen remobilization in grain filling under drought stress condition. J Genet Eng Biotechnol 20(1):101
Hakvoort TB, He Y, Kulik W, Vermeulen JL, Duijst S, Ruijter JM, Runge JH, Deutz NE, Koehler SE, Lamers WH (2017) Pivotal role of glutamine synthetase in ammonia detoxification. Hepatology 65(1):281–293
Han M, Okamoto M, Beatty PH, Rothstein SJ, Good AG (2015) The genetics of nitrogen use efficiency in crop plants. Annu Rev Genet 49:269–289
Hasnain A, Irfan M, Bashir A, Maqbool A, Malik KA (2020) Transcription factor TaDof1 improves nitrogen and carbon assimilation under low-nitrogen conditions in wheat. Plant Mol Biol Rep 38:441–451
Hawkesford MJ (2017) Genetic variation in traits for nitrogen use efficiency in wheat. J Exp Bot 68(10):2627–2632
Hawkesford MJ, Griffiths S (2019) Exploiting genetic variation in nitrogen use efficiency for cereal crop improvement. Curr Opin Plant Biol 49:35–42
He H, Xie Y, Zhao A, Hu W, Guo X, Miller AJ, Wu X, Chen B, Zhang R, Tian H, Gao Y (2021) Genotypic variation in nitrogen utilization efficiency in oilseed rape is related to the coordination of leaf senescence and root N uptake during reproductive stage. Plant Soil 463:291–306
Hildebrandt TM, Nesi AN, Araújo WL, Braun HP (2015) Amino acid catabolism in plants. Mol Plant 8(11):1563–1579
Hu B, Wang W, Ou S, Tang J, Li H, Che R, Zhang Z, Chai X, Wang H, Wang Y, Liang C, Liu L, Piao Z, Deng Q, DengK XuC, Liang Y, Zhang L, Li L, Chu C (2015) Variation in NRT1.1B contributes to nitrate-use divergence between rice subspecies. Nat Genet 47:834–838
Hu M, Zhao X, Liu Q, Hong X, Zhang W, Zhang Y, Sun L, Li H, Tong Y (2018) Transgenic expression of plastidic glutamine synthetase increases nitrogen uptake and yield in wheat. Plant Biotechnol J 16(11):1858–1867
Huang L, Yang D, Li X, Peng S, Wang F (2019) Coordination of high grain yield and high nitrogen use efficiency through large sink size and high post-heading source capacity in rice. Field Crops Res 233:49–58
Huang M, Chen J, Cao F (2022a) The fraction of intercepted radiation to nitrogen absorption as an indicator for assessing physiological nitrogen use efficiency in rice. Agron 12(7):1603
Huang Y, Wang H, Zhu Y, Huang X, Li S, Wu X, Zhao Y, Bao Z, Qin L, Jin Y, Cui Y (2022b) THP9 enhances seed protein content and nitrogen-use efficiency in maize. Nature 612(7939):292–300
Igarashi D, Ishizaki T, Totsuka K, Ohsumi C (2009) ASN2 is a key enzyme in asparagine biosynthesis under ammonium sufficient conditions. Plant Biotechnol 26(1):153–159
Jan Su, Liaqat A, Zhu Y, Li J, Zhang H, Abdalla M, Wu J, Xiang C, Wu S, alfatih A, (2022) Arabidopsis NLP7 improves nitrogen use efficiency and yield in cotton. J Cotton Res 5(1):2
Jia Z, Giehl RF, von Wirén N (2022) Nutrient–hormone relations: driving root plasticity in plants. Mol Plant 15(1):86–103
Jin Y, He J, Zhu Y, Siddique KH (2022) Nodule formation and nitrogen use efficiency are important for soybean to adapt to water and P deficit conditions. Agriculture 12(9):1326
Karunarathne SD, Han Y, Zhang XQ, Li C (2020) Advances in understanding the molecular mechanisms and potential genetic improvement for nitrogen use efficiency in barley. Agron 10(5):662
Karunarathne SD, Han Y, Zhang XQ, Li C (2022) CRISPR/Cas9 gene editing and natural variation analysis demonstrate the potential for HvARE1 in improvement of nitrogen use efficiency in barley. J Integr Plant Biol 64(3):756–770
Kasemsap P, Bloom AJ (2022) Breeding for higher yields of wheat and rice through modifying nitrogen metabolism. Plants 12(1):85
Kaur H, Peel A, Acosta K, Gebril S, Ortega JL, Sengupta-Gopalan C (2019) Comparison of alfalfa plants overexpressing glutamine synthetase with those overexpressing sucrose phosphate synthase demonstrates a signaling mechanism integrating carbon and nitrogen metabolism between the leaves and nodules. Plant Direct 3(1):e00115
Kindu GA, Tang J, Yin X, Struik PC (2014) Quantitative trait locus analysis of nitrogen use efficiency in barley (Hordeum vulgare L.). Euphytica 199:207–221
Kitomi Y, Hanzawa E, Kuya N, Inoue H, Hara N, Kawai S, Kanno N, Endo M, Sugimoto K, Yamazaki T, Sakamoto S (2020) Root angle modifications by the DRO1 homolog improve rice yields in saline paddy fields. Proc Natl Acad Sci 117(35):21242–21250
Konishi N, Ishiyama K, Matsuoka K, Maru I, Hayakawa T, Yamaya T, Kojima S (2014) NADH-dependent glutamate synthase plays a crucial role in assimilating ammonium in the Arabidopsis root. Physiol Plant 152(1):138–151
Kurai T, Wakayama M, Abiko T, Yanagisawa S, Aoki N, Ohsugi R (2011) Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low-nitrogen conditions. Plant Biotechnol J 9(8):826–837
Ladha JK, Peoples MB, Reddy PM, Biswas JC, Bennett A, Jat ML, Krupnik TJ (2022) Biological nitrogen fixation and prospects for ecological intensification in cereal-based cropping systems. Field Crops Res 283:108541
Lal SK, Mehta S, Raju D, Achary VM, Venkatapuram AK, Yadav SK, Parmar H, Pandey R, Panditi V, Sheri V, Singh AK, Chinnusamy V, Reddy MK (2023) Concurrent overexpression of rice GS1; 1 and GS2 genes to enhance the nitrogen use efficiency (NUE) in transgenic rice. J Plant Growth Regul 1–22
Laperche A, Devienne-Barret F, Maury O, Le Gouis J, Ney B (2006) A simplified conceptual model of carbon/nitrogen functioning for QTL analysis of winter wheat adaptation to nitrogen deficiency. Theor Appl Genet 113(6):1131–1146
Lebedev VG, Popova AA, Shestibratov KA (2021) Genetic engineering and genome editing for improving nitrogen use efficiency in plants. Cells 10(12):3303
Lee S, Marmagne A, Park J, Fabien C, Yim Y, Kim SJ, Kim TH, Lim PO, Masclaux-Daubresse C, Nam HG (2020a) Concurrent activation of OsAMT1; 2 and OsGOGAT1 in rice leads to enhanced nitrogen use efficiency under nitrogen limitation. Plant J 103(1):7–20
Lee S, Park J, Lee J, Shin D, Marmagne A, Lim PO, Masclaux-Daubresse C, An G, Nam HG (2020b) OsASN1 overexpression in rice increases grain protein content and yield under nitrogen-limiting conditions. Plant Cell Physiol 61(7):1309–1320
Lee S, Park J, Yim Y (2021) Genetic modification of rice for efficient nitrogen utilization. Plant Biotechnol Rep 15(5):573–583
Lee S (2021) Recent advances on nitrogen use efficiency in rice. Agron 11(4)-753
Lenhart K, Behrendt T, Greiner S, Steinkamp J, Well R, Giesemann A, Keppler F (2019) Nitrous oxide effluxes from plants as a potentially important source to the atmosphere. New Phytol 221(3):1398–1408
Li P, Chen F, Cai H, Liu J, Pan Q, Liu Z, Gu R, Mi G, Zhang F, Yuan L (2015) A genetic relationship between nitrogen use efficiency and seedling root traits in maize as revealed by QTL analysis. J Exp Bot 66(11):3175–3188
Li K, Zhang S, Tang S, Zhang J, Dong H, Yang S, Qu H, Xuan W, Gu M, Xu G (2022a) The rice transcription factor Nhd1 regulates root growth and nitrogen uptake by activating nitrogen transporters. Plant Physiol 189(3):1608–1624
Li Q, Lu X, Wang C, Shen L, Dai L, He J, Yang L, Li P, Hong Y, Zhang Q, Dong G (2022b) Genome-wide association study and transcriptome analysis reveal new QTL and candidate genes for nitrogen-deficiency tolerance in rice. Crop J 10(4):942–951
Li C, Tang Z, Wei J, Qu H, Xie Y, Xu G (2016) The OsAMT1. 1 gene functions in ammonium uptake and ammonium–potassium homeostasis over low and high ammonium concentration ranges. J Genet Genomics 43(11):639–649
Liu Q, Wu K, Song W, Zhong N, Wu Y, Fu X (2022a) Improving crop nitrogen use efficiency toward sustainable green revolution. Annu Rev Plant Biol 73:523–551
Liu X, Hu B, Chu C (2022b) Nitrogen assimilation in plants: current status and future prospects. J Genet Genomics 49(5):394–404
Luo J, Hang J, Wu B, Wei X, Zhao Q, Fang Z (2023) Co-overexpression of genes for nitrogen transport, assimilation, and utilization boosts rice grain yield and nitrogen use efficiency. Crop J 11(3):785–799
Luo B, Xu M, Zhao L, Xie P, Chen Y, Harwood W, Xu G, Fan X, Miller AJ (2020) Overexpression of the high-affinity nitrate transporter OsNRT2. 3b driven by different promoters in barley improves yield and nutrient uptake balance. Int J Mol Sci 21(4):1320
Ma W, Li J, Qu B, He X, Zhao X, Li B, Fu X, Tong Y (2014) Auxin biosynthetic gene TAR 2 is involved in low nitrogen-mediated reprogramming of root architecture in Arabidopsis. Plant J 78(1):70–79
Mae T, Ohira K (1981) The remobilization of nitrogen related to leaf growth and senescence in rice plants (Oryza sativa L.). Plant Cell Physiol 22(6):1067–1074
Mahboob W, Yang G, Irfan M (2023) Crop nitrogen (N) utilization mechanism and strategies to improve N use efficiency. Acta Physiol Plant 45(4):52
Maheshwari C, Coe RA, Karki S, Covshoff S, Tapia R, Tyagi A, Hibberd JM, Furbank RT, Quick WP, Lin HC (2021) Targeted knockdown of ribulose-1, 5-bisphosphate carboxylase-oxygenase in rice mesophyll cells. J Plant Physiol 260:153395
Masumoto C, Miyazawa SI, Ohkawa H, Fukuda T, Taniguchi Y, Murayama S, Kusano M, Saito K, Fukayama H, Miyao M (2010) Phosphoenol pyruvate carboxylase intrinsically located in the chloroplast of rice plays a crucial role in ammonium assimilation. Proc Natl Acad Sci 107(11):5226–5231
Matsunami M, Matsunami T, Kokubun M (2009) Growth and yield of new rice for Africa (NERICAs) under different ecosystems and nitrogen levels. Plant Prod Sci 12(3):381–389
Matsunami M, Matsunami T, Kokubun M (2010) Comparison of nitrogen uptake, transpiration rate and exudation rate between upland NERICAs and Japanese cultivars. Plant Prod Sci 13(4):347–350
McAllister CH, Beatty PH, Good AG (2012) Engineering nitrogen use efficient crop plants: the current status. Plant Biotechnol J 10(9):1011–1025
Meise P, Seddig S, Uptmoor R, Ordon F, Schum A (2019) Assessment of yield and yield components of starch potato cultivars (Solanum tuberosum L.) under nitrogen deficiency and drought stress conditions. Potato Res 62:193–220
Melino VJ, Tester MA, Okamoto M (2022) Strategies for engineering improved nitrogen use efficiency in crop plants via redistribution and recycling of organic nitrogen. Curr Opin Biotechnol 73:263–269
Moll RH, Kamprath EJ, Jackson WA (1982) Analysis and interpretation of factors which contribute to efficiency of nitrogen utilization 1. Agron J 74(3):562–564
Mu X, Luo J (2019) Evolutionary analyses of NIN-like proteins in plants and their roles in nitrate signaling. Cell Mol Life Sci 76(19):3753–3764
Muhammad I, Yang L, Ahmad S, Farooq S, Al-Ghamdi AA, Khan A, Zeeshan M, Elshikh MS, Abbasi AM, Zhou XB (2022) Nitrogen fertilizer modulates plant growth, chlorophyll pigments and enzymatic activities under different irrigation regimes. Agron 12(4):845
Nazish T, Arshad M, Jan SU, Javaid A, Khan MH, Naeem MA, Baber M, Ali M (2001) Transporters and transcription factors gene families involved in improving nitrogen use efficiency (NUE) and assimilation in rice (Oryza sativa L.). Transgenic Res 15:1–20
Ogawa S, Valencia MO, Lorieux M, Arbelaez JD, McCouch S, Ishitani M, Selvaraj MG (2016) Identification of QTLs associated with agronomic performance under nitrogen-deficient conditions using chromosome segment substitution lines of a wild rice relative, Oryza rufipogon. Acta Physiol Plant 38:1–10
Pahalvi HN, Rafiya L, Rashid S, Nisar B, Kamili AN (2021) Chemical fertilizers and their impact on soil health. Microbiota and Biofertilizers. Ecofriendly Tools for Reclamation of Degraded Soil Environs 2:1–20
Pasam RK, Sharma R, Malosetti M, van Eeuwijk FA, Haseneyer G, Kilian B, Graner A (2012) Genome-wide association studies for agronomical traits in a world wide spring barley collection. BMC Plant Biol 12(1):1–22
Peng C, Xu W, Hu L, Li Y, Qi X, Wang H, Hua X, Zhao M (2018) Effects of the maize C4 phosphoenolpyruvate carboxylase (ZmPEPC) gene on nitrogen assimilation in transgenic wheat. Plant Growth Regul 84:191–205
Presterl T, Seitz G, Landbeck M, Thiemt EM, Schmidt W, Geiger HH (2003) Improving nitrogen-use efficiency in European maize: estimation of quantitative genetic parameters. Crop Sci 43(4):1259–1265
Pujarula V, Pusuluri M, Bollam S, Das RR, Ratnala R, Adapala G, Thuraga V, Rathore A, Srivastava RK, Gupta R (2021) Genetic variation for nitrogen use efficiency traits in global diversity panel and parents of mapping populations in pearl millet. Front Plant Sci 12:625915
Qiu X, Xie W, Lian X, Zhang Q (2009) Molecular analyses of the rice glutamate dehydrogenase gene family and their response to nitrogen and phosphorous deprivation. Plant Cell Rep 28:1115–1126
Rahman MM, Biswas JC, Sutton MA, Drewer J, Adhya TK (2021) Assessment of reactive nitrogen flows in Bangladesh’s agriculture sector. Sustainability 14(1):272
Ranathunge K, El-Kereamy A, Gidda S, Bi YM, Rothstein SJ (2014) AMT1; 1 transgenic rice plants with enhanced NH4+ permeability show superior growth and higher yield under optimal and suboptimal NH4+ conditions. J Exp Bot 65(4):965–979
Ranjan R, Yadav R, Kumar A, Mandal SN (2019) Contributing traits for nitrogen use efficiency in selected wheat genotypes and corollary between screening methodologies. Acta Agriculturae Scandinavica, Section B-Soil & Plant Science 69(7):588–595
Ren C, Zhang X, Reis S, Gu B (2022) Socioeconomic barriers of nitrogen management for agricultural and environmental sustainability. Agric Ecosyst Environ 333:107950
Rocha M, Sodek L, Licausi F, Hameed MW, Dornelas MC, Van Dongen JT (2010) Analysis of alanine aminotransferase in various organs of soybean (Glycine max) and in dependence of different nitrogen fertilizers during hypoxic stress. Amino Acids 39:1043–1053
Rolletschek H, Hosein F, Miranda M, Heim U, Götz KP, Schlereth A, Borisjuk L, Saalbach I, Wobus U, Weber H (2005) Ectopic expression of an amino acid transporter (VfAAP1) in seeds of Vicia narbonensis and pea increases storage proteins. Plant Physiol 137(4):1236–1249
Rosolem CA, Batista TB, Dias PP, Motta Neto LV, Calonego JC (2022) The joint application of phosphorus and ammonium enhances soybean root growth and P uptake. Agric 12(6):88
Saloner A, Bernstein N (2021) Nitrogen supply affects cannabinoid and terpenoid profile in medical cannabis (Cannabis sativa L.). Ind Crops Prod 167:113516
Sandhu N, Sethi M, Kumar A, Dang D, Singh J, Chhuneja P (2021) Biochemical and genetic approaches improving nitrogen use efficiency in cereal crops: a review. Front Plant Sci 12:657629
Scartazza A, Sbrana C, D’Andrea E, Matteucci G, Rezaie N, Lauteri M (2023) Above-and belowground interplay: canopy CO2 uptake, carbon and nitrogen allocation and isotope fractionation along the plant-ectomycorrhiza continuum. Plant Cell Environ 46(3):889–900
Schofield RA, Bi YM, Kant S, Rothstein SJ (2009) Over-expression of STP13, a hexose transporter, improves plant growth and nitrogen use in Arabidopsis thaliana seedlings. Plant Cell Environ 32(3):271–285
Shao A, Ma W, Zhao X, Hu M, He X, Teng W, Li H, Tong Y (2017) The auxin biosynthetic tryptophan aminotransferase related TaTAR2.1–3A increases grain yield of wheat. Plant Physiol 174(4):2274–2288
Shen C, Chen K, Cui Y, Chen J, Mi X, Zhu S, Zhu Y, Ali J, Ye G, Li Z, Xu J (2021) QTL mapping and favorable allele mining of nitrogen deficiency tolerance using an interconnected breeding population in rice. Front Genet 12:616428
Shrawat AK, Carroll RT, DePauw M, Taylor GJ, Good AG (2008) Genetic engineering of improved nitrogen use efficiency in rice by the tissue-specific expression of alanine aminotransferase. Plant Biotechnol J 6(7):722–732
Shukla AK, Behera SK, Chaudhari SK, Singh G (2022) Fertilizer use in Indian agriculture and its impact on human health and environment. Indian J Fertil 18:218–237
Shukla S, Saxena A (2020) Sources and leaching of nitrate contamination in groundwater. Curr Sci (00113891) 25:118(6)
Singh P, Kumar K, Jha AK, Yadava P, Pal M, Rakshit S, Singh I (2022). Global gene expression profiling under nitrogen stress identifies key genes involved in nitrogen stress adaptation in maize (Zea mays L.). Sci Rep 12(1):4211
Sinha SK, Kumar A, Tyagi A, Venkatesh K, Paul D, Singh NK, Mandal PK (2020a) Root architecture traits variation and nitrate-influx responses in diverse wheat genotypes under different external nitrogen concentrations. Plant Physiol Biochem 148:246–259
Sinha VB, Jangam AP, Raghuram N (2020b) Biological determinants of crop nitrogen use efficiency and biotechnological avenues for improvement. Just Enough Nitrogen: perspectives on how to get there for regions with too much and too little nitrogen. 157–171
Snyman SJ, Hajari E, Watt MP, Lu Y, Kridl JC (2015) Improved nitrogen use efficiency in transgenic sugarcane: phenotypic assessment in a pot trial under low nitrogen conditions. Plant Cell Rep 34:667–669
Stitt M, Lunn J, Usadel B (2010) Arabidopsis and primary photosynthetic metabolism–more than the icing on the cake. Plant J 61(6):1067–1091
Sujata SB, Nirakar SN, Batta BB, Nagireddy RK, Sabarinathan S, Subudhi HN, Meher J, Reddy JN, Anandan A (2019) Understanding the physiological responses to low nitrogen and molecular screening of selected rice genotypes for TOND1 gene. Oryza-an Int J Rice 56(2):185–192
Tang Z, Fan X, Li Q, Feng H, Miller AJ, Shen Q, Xu G (2012) Knockdown of a rice stelar nitrate transporter alters long-distance translocation but not root influx. Plant Physiol 160(4):2052–2063
Tegeder M, Masclaux-Daubresse C (2018) Source and sink mechanisms of nitrogen transport and use. New Phytol 217(1):35–53
Teng W, He X, Tong Y (2022) Genetic control of efficient nitrogen use for high yield and grain protein concentration in wheat: A Review. Plants 11(4):492
Tiong J, Sharma N, Sampath R, MacKenzie N, Watanabe S, Metot C, Lu Z, Skinner W, Lu Y, Kridl J, Baumann U (2021) Improving nitrogen use efficiency through overexpression of alanine aminotransferase in rice, wheat, and barley. Front Plant Sci 12:628521
Tiwari JK, Buckseth T, Singh RK, Kumar M, Kant S (2020) Prospects of improving nitrogen use efficiency in potato: lessons from transgenics to genome editing strategies in plants. Front Plant Sci 23(11):597481
Tyagi BS, Foulkes J, Singh G, Sareen S, Kumar P, Broadley MR, Gupta V, Krishnappa G, Ojha A, Khokhar JS, King IP (2020) Identification of wheat cultivars for low nitrogen tolerance using multivariable screening approaches. Agron 10(3):417
Uga Y, Okuno K, Yano M (2011) Dro1, a major QTL involved in deep rooting of rice under upland field conditions. J Exp Bot 62(8):2485–2494
Uga Y, Sugimoto K, Ogawa S, Rane J, Ishitani M, Hara N, Kitomi Y, Inukai Y, Ono K, Kanno N, Inoue H (2013) Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat Genet 45(9):1097–1102
Wang D, Xu T, Yin Z, Wu W, Geng H, Li L, Yang M, Cai H, Lian X (2020) Overexpression of OsMYB305 in rice enhances the nitrogen uptake under low-nitrogen condition. Front Plant Sci 11:369
Wang Y, Fu B, Pan L, Chen L, Fu X, Li K (2013) Overexpression of Arabidopsis Dof1, GS1 and GS2 enhanced nitrogen assimilation in transgenic tobacco grown under low-nitrogen conditions. Plant Mol Biol Rep 886–900
Wang W, Hu B, Yuan D, Liu Y, Che R, Hu Y, Ou S, Liu Y, Zhang Z, Wang H, Li H (2018) Expression of the nitrate transporter gene OsNRT1. 1A/OsNPF6. 3 confers high yield and early maturation in rice. Plant Cell 30(3):638–651
Wen S, Neuhaus HE, Cheng J, Bie Z (2022) Contributions of sugar transporters to crop yield and fruit quality. J Exp Bot 73(8):2275–2289
Wu Y, Yang W, Wei J, Yoon H, An G (2017) Transcription factor OsDOF18 controls ammonium uptake by inducing ammonium transporters in rice roots. Mol Cells 40(3):178
Wu J, Zhang ZS, Xia JQ, Alfatih A, Song Y, Huang YJ, Wan GY, Sun LQ, Tang H, Liu Y, Wang SM (2021) Rice NIN-LIKE PROTEIN 4 plays a pivotal role in nitrogen use efficiency. Plant Biotechnol J 19(3):448–461
Yadav B, Jogawat A, Lal SK, Lakra N, Mehta S, Shabek N, Narayan OP (2021) Plant mineral transport systems and the potential for crop improvement. Planta 253:1–30
Yadesa L (2022) Review on breeding cultivars for N-low stress tolerance in major food crops. Am J Life Sci 10(4):58–71
Yamaya T, Obara M, Nakajima H, Sasaki S, Hayakawa T, Sato T (2002) Genetic manipulation and quantitative-trait loci mapping for nitrogen recycling in rice. J Exp Bot 53(370):917–925
Yan M, Fan X, Feng H, Miller AJ, Shen Q, Xu G (2011) Rice OsNAR2. 1 interacts with OsNRT2. 1, OsNRT2. 2 and OsNRT2. 3a nitrate transporters to provide uptake over high and low concentration ranges. Plant Cell Environ 34(8):1360–72
Yang G, Wei Q, Huang H, Xia J (2020) Amino acid transporters in plant cells: a brief review. Plants 9(8):967
Yoon DK, Ishiyama K, Suganami M, Tazoe Y, Watanabe M, Imaruoka S, Ogura M, Ishida H, Suzuki Y, Obara M, Mae T (2020) Transgenic rice overproducing Rubisco exhibits increased yields with improved nitrogen-use efficiency in an experimental paddy field. Nat Food 1(2):134–139
Yu C, Liu Y, Zhang A, Su S, Yan A, Huang L, Ali I, Liu Y, Forde BG, Gan Y (2015) MADS-box transcription factor OsMADS25 regulates root development through affection of nitrate accumulation in rice. PLoS ONE 10(8):e0135196
Zakari SA, Asad MA, Han Z, Zhao Q, Cheng F (2020) Relationship of nitrogen deficiency-induced leaf senescence with ROS generation and ABA concentration in rice flag leaves. J Plant Growth Regul 39:1503–1517
Zhang Y, Tan L, Zhu Z, Yuan L, Xie D, Sun C (2015) TOND1 confers tolerance to nitrogen deficiency in rice. Plant J 81(3):367–376
Zhang J, Zhang H, Li S, Li J, Yan L, Xia L (2021) Increasing yield potential through manipulating of an ARE1 ortholog related to nitrogen use efficiency in wheat by CRISPR/Cas9. J Integr Plant Biol 63(9):1649–1663
Zhang Z, Hu B, Chu C (2020) Towards understanding the hierarchical nitrogen signalling network in plants. Curr Opin Plant Biol 5560–5565
Zhang M, Lai L, Liu X, Liu J, Liu R, Wang Y, Liu J, Chen J (2022) Overexpression of nitrate transporter 1/peptide gene OsNPF7.6 increases rice yield and nitrogen use efficiency. Life 12(12):1981
Zhao Y, Liu Z, Duan F, An X, Liu X, Hao D, Gu R, Wang Z, Chen F, Yuan L (2018) Overexpression of the maize ZmAMT1;1a gene enhances root ammonium uptake efficiency under low ammonium nutrition. Plant Biotechnol Rep 12:47–56
Zhong M, Liu X, Liu F, Ren Y, Wang Y, Zhu J, Teng X, Duan E, Wang F, Zhang H, Wu M (2019) FLOURY ENDOSPERM12 encoding alanine aminotransferase 1 regulates carbon and nitrogen metabolism in rice. J Plant Biol 62:61–73
Zhou Y, Cai H, Xiao J, Li X, Zhang Q, Lian X (2009) Over-expression of aspartate aminotransferase genes in rice resulted in altered nitrogen metabolism and increased amino acid content in seeds. Theor Appl Genet 118:1381–1390
Zhu P, Burney J, Chang J, Jin Z, Mueller ND, Xin Q, Xu J, Yu L, Makowski D, Ciais P (2022) Warming reduces global agricultural production by decreasing cropping frequency and yields. Nat Clim Change 12(11):1016–1023
Zong YZ, Shangguan ZP (2016) Increased sink capacity enhances C and N assimilation under drought and elevated CO2 conditions in maize. J Integr Agric 15(12):2775–2785
Zuluaga DL, Sonnante G (2019) The use of nitrogen and its regulation in cereals: structural genes, transcription factors, and the role of miRNAs. Plants 8(8):294
Funding
The work was supported by the In-house funding of ICAR-Indian Institute of Agricultural Biotechnology, Ranchi.
Author information
Authors and Affiliations
Contributions
SKL, SR, and PG conceived the outline and scope of the review. SKL, PG, SK, and SR prepared the contents of the manuscript. MGM, CV, VMMA, AP, and SM revised and edited the manuscript. The final manuscript has been approved for publication by all authors after reading it.
Corresponding author
Ethics declarations
Ethics Approval
Not applicable.
Competing Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lal, S.K., Gaggar, P., Kumar, S. et al. Recent Advancements in Nitrogen Use Efficiency in Crop Plants Achieved by Genomics and Targeted Genetic Engineering Approaches. Plant Mol Biol Rep (2024). https://doi.org/10.1007/s11105-024-01439-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11105-024-01439-4