Abstract
New crop plants suited to grow in semiarid environments will be fundamental to the future of agriculture. The interactions between nitrogen supply and water availability that determine yield and quality in crops grown in semiarid environments are being elucidated. Tools for analyzing the metabolic changes associated with enhanced nitrogen assimilation under drought have been generated. Here is summarized the crop and other plants that have altered NUE, yield performances, and metabolic profiles caused by in planta expression of 31 different transgenes generated in the past two decades. The change in nitrogen concentration has profound effects on plant metabolisms. The metabolic changes resulted in phenotypic changes that included increases in mean plant biomass production in dry soils, tolerance to the herbicide phosphinothricin, tolerance to both severe and mild water deficit, and resistance to rotting necrotrophs. Leaves, and grain had higher nutritional value and higher yield, indicating improved NUE and WUE by some of the transgenes. Therefore, in view of global climate change, continued efforts to alter nitrogen fixation and assimilation by transgenesis and mutation should be pursued through technology stacking.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
11.1 Introduction
Nitrogen is an essential component in cellular physiology with only oxygen, carbon, and hydrogen being more abundant (Marchner 1995; Andrews et al. 2004). Nitrogen is present in numerous essential compounds including nucleoside phosphates and amino acids that form the building blocks of nucleic acids and proteins, respectively. In plants, nitrogen is used in large amounts in photosynthetic pigments, defense chemicals, and structural compounds. However, inorganic N is difficult to assimilate. Dinitrogen in the atmosphere is very inert. Reduction to ammonium requires the energy of a lightning bolt and two adenosine triphosphates (ATPs) or energy from petrochemicals or 12 ATP dephosphorylations per molecule within a nodule or other anaerobic environment. However, these ammonium fertilizers are prone to escape from the cell as ammonia gas. Photorespiration releases tenfold more ammonium than is assimilated from the environment and plants only re-assimilate 98 % of it. Consequently, a haze of ammonia gas is found floating above a photosynthetic canopy. That ammonia may be lost on the wind or returned to the plant or soil by rains or dew falls. Any improvements to these nitrogen cycles (Table 11.1; Tercé-Laforgue et al. 2004a,b; Seebauer et al. 2010; de Carvalho et al. 2011) can have a massive positive impact on the efficiency of agriculture, reduce its carbon footprint, and over geological time scales reverse some of the anthropogenic contributors to global warming.
The assimilation of ammonium has a second major problem associated with it. Ammonia is assimilated releasing one acidic proton per molecule (Marchner 1995). There is enough flux to reduce the pH of even well-buffered soils to concentrations that inhibit plant growth, both directly and by the release of toxic concentrations of micronutrients (Al and Mn in particular). Reduction within a nodule or other anaerobic environment compounds this problem by releasing two protons per ammonium produced (Indrasumunar et al. 2011, 2012). Soil acidification is a worldwide problem on a massive scale.
Nitrates and nitrites provide a solution to the acidification problem, as their reduction to ammonium absorbs 3–4 protons (Marchner 1995). So a pH-balanced fertilizer should theoretically be a 4 to 1 mixture of ammonium and nitrates. Nitrates and nitrites are the ions produced by those lightning bolts that provide about 10 % of the world’s reduced nitrogen a year. However, they are not without costs and problems. Nitrite is highly toxic to photosynthesis and respiration and so must be immediately reduced to ammonium. Plants produce massive amounts of nitrite reductase for this purpose. Nitrate is benign, easy to store and transport, and consequently is the major form of inorganic N found in plants. However, plants still produce tenfold more nitrate reductase than is absolutely needed for assimilation, growth, and yield (Kleinhofs et al. 1980; Wang et al. 2012). Why? That is still unclear.
The major problem with nitrates in the environment is that they are water soluble and so rapidly leached from soils (Lee and Nielsen 1987; David et al. 1997). So much is lost from agricultural soils, industrial activity, and human waste treatments that the world’s rivers, lakes, and oceans are significantly fertilized (Cherfas 1990; Burkholder et al. 1992). The algae are the microorganisms that benefit the most. Unfortunately, they run low on other nutrients (P, K) and so produce toxins to kill other organisms to obtain the limiting nutrients by their decomposition. In addition, they absorb much of the water’s oxygen (at night) killing even toxin-resistant aerobes. Finally, they bloom, blocking the light needed for photosynthesis by submerged organisms. Millions of acres of oceans are affected.
The major problem with nitrates in the human diet (water or food) is that they are metabolized to potent carcinogens (nitrosamines) in the acid of the human stomach (Tannenbaum et al. 1978; Moller et al. 1990; Mirvish 1985; Duncan et al. 1998). High nitrate and so nitrosamine amounts in human diets are associated with many different cancers as well as fertility problems. However, nitrates are naturally excreted in human and animal saliva for the purpose of producing some nitrosamines in the gut. This is because the combination of acid and nitrosamine effectively kills many human and animal pathogens resistant to one or the other. Helicobacter pylori is one example. This microbe causes stomach ulcers that left untreated often become cancerous. H. pylori is endemic and became more abundant as lifestyles became more stressful. Consequently, several epidemiological studies found diets high in nitrate to be healthful in the 1990s and beyond, whereas before that they were significantly unhealthful. Clearly, then the healthiest option is a low-nitrate diet and low-stress lifestyle. However where lifestyle change is not an option, for H. pylori and like pathogens, the lesions they cause are better treated with drugs than nitrosamines.
However produced and applied, microbes in the soil take up the bulk of all fertilizers before the plant can (Jahns and Kaltwasser 2000; Jahns et al. 1999; Trenkel 1997; Cabello et al. 2004; Garcia-Teijeiro et al. 2009; Koivunen et al. 2004). Microbes pass the N molecules through about seven microbial cells before plants absorb them. Ammonium can be assimilated or oxidized to nitrite, nitrate, nitrous oxide, or dinitrogen by microbial activities. Plants have to absorb N from microbes by force using highly efficient enzymes or by trade through symbiosis [reviewed by Ferguson and Indrasumunar (2011)]. In symbiosis the microbes are provided with sugars in return for ammonium. The microbes may be free living in the rhizosphere or housed in specialized structures like nodules. Symbiotic microbes produce a variety of chemical signals to elicit the delivery of sugars from the plants. These systems are ripe for manipulation by biotechnology approaches.
11.2 Plant Assimilations
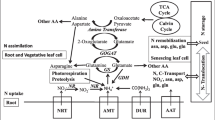
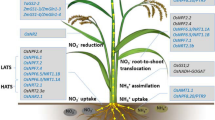
Because soil particles do not naturally have many N-containing minerals, and because N can be readily lost from the rooting environment, it is the nutrient element that most often limits plant growth and so agricultural yields (Marchner 1995; Specht et al. 1999; Duvick 2005). As noted above, nitrogen is found in the environment in many forms and comprises about 80 % of the earth’s atmosphere as triple-bonded nitrogen gas (N2). However, this large fraction of N is not directly accessible by plants and must be bonded to one or more of three other essential nutrient elements including oxygen and/or hydrogen through N-fixation processes and carbon through N-assimilation processes. Plants are able to absorb a little NH3 from the atmosphere through stomata in leaves, but this is dependent upon air concentrations. The ions \( \mathrm{ NO}_3^{-} \) and \( \mathrm{ NH}_4^{+} \) are the primary forms for uptake in by plants. The most abundant form that is available to the plant roots is \( \mathrm{ NO}_3^{-} \), and the most abundant form in leaves is \( \mathrm{ NH}_4^{+} \). The process of nitrification by soil bacteria readily converts fertilizer \( \mathrm{ NH}_4^{+} \) to \( \mathrm{ NO}_3^{-} \) (Trenkel 1997; Zhao et al. 2005). Relative nitrogen uptake is also dependent on soil conditions. Ammonium uptake is favored by a neutral pH and \( \mathrm{ NO}_3^{-} \) uptake is favored by low pH. Nitrate also does not bind to the negatively charged soil particles; therefore, it is more freely available to plant roots especially through mass flow of soil water than is \( \mathrm{ NH}_4^{+} \), which binds to negatively charged soil particles and so moves primarily by diffusion. As noted above, the assimilation of \( \mathrm{ NH}_4^{+} \) by roots causes the rhizosphere to become acidic, while \( \mathrm{ NO}_3^{-} \) causes the rhizosphere to become more basic.
11.2.1 Uptake of Nitrogen
Nitrogen uptake and assimilation summates a series of vital processes controlling plant growth and development (Godon et al 1996; Krouk et al 2010; Meyer et al. 2006). Nitrate, nitrite, and ammonium uptakes (and reuptakes following losses) occur against massive concentration gradients that require lots of energy to generate and maintain. Happily in agriculture, plants are spaced sufficiently that they have an excess of captured light energy relative to the N and C supplies. Transgenic plants overexpressing low-affinity nitrate uptake transporter Nrt1 increased the constitutive but not the induced nitrate uptake (Table 11.1; Liu et al. 1999). Equally, plants transgenic with Nrt2.1 the high-affinity nitrate transporter 2 increased nitrate influx under low-N conditions (Fraisier et al. 2000). Transgenic plants expressing an ammonium transporter increased nitrogen use efficiency (NUE; Gupta et al. 2008). Glutamate receptors in transgenic plants provided better growth. Equally the uptake of short peptides had positive effects. All these transport-associated phenotypes would be desirable in agricultural production systems directed toward greater efficiency and lower environmental impacts. A stack of the three transgenes would be of interest.
11.2.2 Nitrate Reduction
Nitrate acquired in the roots can be reduced in the shoot or the root or even stored in vacuoles in the root or shoot for later assimilation. However, nitrate must be reduced to a useable form. This occurs via a two-step process catalyzed by the enzymes nitrate reductase and nitrite reductase to form \( \mathrm{ NH}_4^{+} \). Both enzymes are produced in massive excess compared to flux needed through the pathway so that mutants that reduce their amounts by 90 % do not have phenotypes (Table 10.1; Kleinhofs et al. 1980). Equally some transgenic plants overexpressing nitrate reductase (NR) increased nitrate reduction but were not altered in phenotypes (Crete et al. 1997; Curtis et al. 1999; Djannane et al. 2002; Lea et al. 2004). However, two studies of NR overexpressing transgenic plants did record altered phenotypes including increased biomass, drought stress (Ferrario-Mery et al. 1998), and improved NUE and yield during N limitation (Loussaert et al. 2008). These phenotypes would be desirable in agricultural crops. Coupling of NR to photosynthesis should be possible by the transformation of plants with a ferredoxin-dependent NR from cyanobacteria.
11.2.3 Dinitrogen Fixation
The ability to fix dinitrogen is restricted to the bacterial world but widespread among microbes (Ferguson and Indrasumunar 2011; Valentine et al. 2011; Reid et al. 2011). Many different nif gene families exist suggesting selections for variation have been favorable for species. The need for an anaerobic environment for nif activity means that transferring the enzymes to plants will be difficult. To date, transgenics in this field are bacterial, as in hydrogenase-enhanced microbes, or if plant they are designed to improve the chances of nodule occupancy by improved bacterial strains. Strains that are most likely to set up nodule occupancy are rarely the most efficient nitrogen fixers. Plants also often fail to maintain effective nodules through flowering and pod set (Sinclair et al. 2007). Soybean and common bean, for example, have senescent nodules by flowering. Some species do have indeterminate nodules, and it would be a valid goal of biotechnology to transfer this trait to major legume crops.
11.2.4 Ammonium Assimilation
The N acquired as \( \mathrm{ NH}_4^{+} \) does not require reduction upon uptake into the root, thus providing some energy savings to the plant over that of the \( \mathrm{ NO}_3^{-} \) form reviewed by Marchner (1995). However, it does require assimilation to avoid loss and at high concentrations (>10 mM) toxicity to the plant. Various studies have shown that under conditions of excessive \( \mathrm{ NH}_4^{+} \) uptake, most plant species will transport this N source to the shoot, which is more sensitive to ammonium ions (Marchner 1995).
One important process to build key macromolecules in any living organism is the acquisition and utilization of inorganic forms of nitrogen during metabolism (Lea and Miflin 2011). Plants use amino acids and their precursors and catabolic products for important metabolic activities. Various other roles of amino acids include nitrogen storage and transport and the production of a very large number of secondary compounds including structural lignin compounds, light-absorbing pigments, phenolics, and plant hormones. Plants convert the available inorganic nitrogen into organic compounds through the process of ammonium assimilation, which occurs in plants by two main pathways. The first and primary pathway involves a reaction with glutamate to form glutamine, which is catalyzed by glutamine synthetase (GS, EC 6.3.1.2) and requires an energy source of ATP. There are two isoenzymes of GS based on their location in the plant, either in the cytosol (GS1) or in the root plastids or shoot chloroplasts (GS2). Expressed in germinating seeds or in the vascular bundles of roots and shoots, the cytosolic form (GS1) produces glutamine for intracellular nitrogen transport. GS2 located in root plastids produces amide nitrogen for local consumption, while GS2 in the shoot chloroplasts re-assimilates photorespiratory ammonium (Lam et al. 1996). GS1 is encoded by a set of 3–6 paralogs in different crop species, so hetero-hexamers can form. However, the affinity for the substrates hardly differs. Amino acid identity is very high even to GS2. GS2 has a short peptide extension at the C terminus that might be involved in regulation by phosphorylation. Alleles of the GS1 and GS2 encoding genes do exist that differ in their regulation. Alleles of GS appear to underlie quantitative trait loci (QTL) determining NUE and seed yield (Cañas et al. 2009, 2010; Coque et al. 2008). Transgenic analyses have been made of GS2 but not GS1 (Table 11.1). Among the 12 studies in nine plant species, the phenotypes reported included enhanced accumulation of N, growth under N starvation, herbicide (PPT) tolerance, leaf-soluble protein, ammonia, amino acids, and chlorophyll. Some genes and constructs though decreased growth; salt, cold, and drought tolerance; seed yield; and amino acid content. Therefore, the use of GS transgenics in agriculture will be useful and desirable but only with careful attention to regulation and expression.
11.2.5 Transaminases
The glutamine molecules produced by GS are used by a whole series of transaminases to produce the 20 protein amino acids and some nonprotein amino acids. Cardinal among the transaminases is the reaction catalyzed by glutamate synthase (GOGAT, EC 1.4.14) to form glutamate. There are two common isoenzymes of GOGAT including a ferredoxin-dependent GOGAT (Fdx-GOGAT) and an NADH-dependent GOGAT (NADH-GOGAT). While both forms are plastidic, the Fdx-GOGAT enzyme is predominately found in photosynthetic organs, and the NADH-GOGAT enzyme is found more in non-photosynthetic tissues, such as in roots and the vascular bundles of developing leaves (Schoenbeck et al. 2000: Yamaya et al. 2002). An NADPH-dependent GOGAT can be found in certain organs and in many bacteria. Plants transgenic with the NADH-dependent plant GOGAT have been reported. Phenotypes included enhanced grain filling, grain weight, total C and N content, and dry weight (Table 10.1). Phenotypes were very similar to the benefits reported from alanine dehydrogenase and asparagines synthase suggesting that transaminases are acting on a common pathway.
11.2.6 Glutamate Dehydrogenases
The second pathway for ammonium assimilation also results in the formation of glutamate through a reversible reaction catalyzed by glutamate dehydrogenase (GDH, EC 1.4.1.2), with a lower energy requirement than GS/GOGAT. There are also at least two forms of GDH that occur in plants that include an NADH-dependent form found in the mitochondria and an NADPH-dependent form localized in the chloroplasts of photosynthetic organs. In addition, there are enzymes capable of aminating reactions that resemble GDH (Turano Personal communication). GDHs present in plants serve as a link between carbon and nitrogen metabolism due to the ability to assimilate ammonium into glutamate or deaminate glutamate into 2-oxoglutarate and ammonium (Forde and Lea 2007). However, due to the reversibility of this reaction, the assimilatory role of GDH is severely inhibited at low concentrations of ammonium. Additionally, GDH enzymes have a low affinity for ammonium compared with GS, which further limits their assimilatory effectiveness. It has been suggested that the NAD-requiring form of GDH may be involved in carbon rather than nitrogen metabolism, (Coruzzi and Brears 1999; Kisaki et al. 2007; Nadzan et al. 2007) with glutamate catabolism providing carbon skeletons both for the TCA cycle and energy production during carbon or energy deficit. Alternate functions for GDH have also been proposed in which it has been assigned the role of re-assimilating excess ammonium, due to the limited ability of the GS/GOGAT cycle, during specific developmental stages (Loulakakis et al. 2002), such as during germination, seed set, and leaf senescence (Coruzzi and Brears 1999; Kisaka et al. 2007).
In contrast to plant GDHs, those found in microbes are very active in the assimilation of ammonium (Lightfoot et al. 1999; 2001). Plants did not have the opportunity to incorporate this type of NADPH-dependent GDH because the bacterial lines that gave rise to chloroplasts do not contain gdhA genes. The few cyanobacteria with GDH activity have acquired genes by transgenesis or cellular fusions. Transgenic plants in six crop species have been produced that express gdhA genes from three microbes (Ameziane et al. 2000; Lightfoot et al. 2007). Phenotypes in plants include increased biomass, water deficit tolerance, nutritional value, herbicide resistance, N assimilation, NUE, water use efficiency (WUE), amino acid, and sugar content (Mungur et al. 2005, 2006; Lightfoot 2008; Lightfoot and Fakhoury 2010; Nolte et al. 2004; Nolte 2009; Table 11.1). GDH genes used in this way are being evaluated for commercialization.
One problem faced by this and the alanine dehydrogenase transgenics (Good et al. 2005; Shrawat et al. 2008) is a dependence on soil type for some of the beneficial effects. GDH seems to provide a growth advantage on silty-loam clay soils common in the southern Midwest. In contrast, the alanine dehydrogenase transgenics seem to work best on sandy soils. Combining the technologies or altering their regulation might provide stable beneficial effects in many soil types and locations.
11.2.7 Other Aminases
A variety of other enzymes exist that are capable of aminating reactions. Each will be a candidate for overexpression in transgenic plants. Phenylalanine ammonia lyase has been used in many transgenic plants. Equally, the enzymes of cyanide assimilations (cysteine metabolism) might be more active than previously thought and could be manipulated. Alteration of the enzymes of heme and chlorophyll biosynthesis might be tried again. The Escherichia coli hemA gene was functional, but hemB became insoluble in plant chloroplasts (Denhart, Lightfoot and Gupta Unpublished). In this same pathway, the protoporphyrinogen oxidases are targets of increasingly used and useful selective herbicides. Another major sink of amines are the lignins and lignols. Emerging research suggests that transgenic manipulation of these pathways will alter NUE and therefore WUE (Castiglioni et al. 2008; Century et al. 2008; Goldman 2009; Vidal 2010; Jung et al. 2011). These enzymes might also be usefully manipulated in stacks with transgenes to improve NUE, WUE, and other traits including herbicide tolerance.
11.3 Conclusions
The assimilation of inorganic nitrogen is a key process in the productivity of crop plants. There are many steps at which metabolic improvements can be made. In the future, the chance to provide active nodules to nonlegumes will provide an impetus for biotechnology. In addition, the combination of existing transgenes and new promoter for their regulation will provide for new avenues in crop improvement.
References
Abiko T, Wakayama M, Kawakami A, Obara M, Kisaka H, Miwa T, Aoki N, Ohsugi R (2010) Changes in nitrogen assimilation, metabolism, and growth in transgenic rice plants expressing a fungal NADP(H)-dependent glutamate dehydrogenase (gdhA). Planta 232(2):299–311
Ameziane R, Bernhard K, Lightfoot DA (2000) Expression of the bacterial gdhA encoding glutamate dehydrogenase in tobacco affects plant growth and development. Plant Soil 221:47–57
Andrews M, Lea PJ, Raven JA, Lindsey K (2004) Can genetic manipulation of plant nitrogen assimilation enzymes result in increased crop yield and greater N-use efficiency? An assessment. Ann Appl Biol 145:25–35
Basra A, Edgerton M, Lee GJ, Lu M, Lutfiyya LL, Wu W, Wu X (2007) Plants containing a heterologous flavohemoglobin gene and methods of use thereof. US Patent 20,070,074,312
Burkholder JM, Noga EJ, Hobbs CH, Glasgow HB Jr (1992) New ‘phantom’ dinoflagellate is the causative agent of major estuarine fish kills. Nature 358:407–410
Cabello P, Roldán MD, Moreno-Vivián C (2004) Nitrate reduction and the nitrogen cycle in archaea? Microbiology 150:3527–3546
Cai H, Zhou Y, Xiao J, Li X, Zhang Q, Lian X (2009) Overexpressed glutamine synthetase gene modifies nitrogen metabolism and abiotic stress responses in rice. Plant Cell Rep 28(3):527–537
Cañas RA, Quilleré I, Christ A, Hirel B (2009) Nitrogen metabolism in the developing ear of maize (Zea mays): analysis of two lines contrasting in their mode of nitrogen management. New Phytol 184(2):340–352
Cañas RA, Quilleré I, Lea PJ, Hirel B (2010) Analysis of amino acid metabolism in the ear of maize mutants deficient in two cytosolic glutamine synthetase isoenzymes highlights the importance of asparagine for nitrogen translocation within sink organs. Plant Biotechnol J 8:966–978
Castiglioni P, Warner D, Bensen RJ, Anstrom DC, Harrison J, Stoeker M et al (2008) Bacterial RNA chaperones confer abiotic stress tolerance in plants and improved grain yield in maize under water-limited conditions. Plant Physiol 147:446–455
Century K, Reuber TL, Ratcliffe OJ (2008) Regulating the regulators: the future prospects for transcription-factor-based agricultural biotechnology products. Plant Physiol 147(1):20–29
Cherfas J (1990) The fringe of the ocean – under siege from land. Science 248:163–165
Coque M, Martin A, Veyrieras JB, Hirel B, Gallais A (2008) Genetic variation for N-remobilization and post-silking N-uptake in a set of maize recombinant inbred lines. 3. QTL detection and coincidences. Theor Appl Genet 117(5):729–747
Coruzzi G, Brears M (1999) Transgenic plants that exhibit enhanced nitrogen assimilation. US Patent 5,955,651
Coruzzi G, Oliveira I, Lam HM, Hsieh MH (1998) Plant glutamate receptors. US Patent 5, 824,867
Creelman RA, Ratcliffe O, Reuber TL, Zhang J, Nadzan G (2009) Polynucleotides and polypeptides in plants. US Patent 7,511,190
Crete P, Caboche M, Meyer C (1997) Nitrite reductase expression is regulated at the post-transcriptional level by the nitrogen source in Nicotiana plumbaginifolia and Arabidopsis thaliana. Plant J 11:625–634
Curtis IS, Power JB, de Llat AAM (1999) Expression of chimeric nitrate reductase gene in transgenic lettuce reduces nitrate in leaves. Plant Cell Rep 18:889–896
David MB, Gentry LE, Kovacic DA, Smith KM (1997) Nitrogen balance in and export from an agricultural watershed. J Environ Qual 26:1038–1048
de Carvalho JF, Madgwick PJ, Powers SJ, Keys AJ, Lea PJ, Parry MA (2011) An engineered pathway for glyoxylate metabolism in tobacco plants aimed to avoid the release of ammonia in photorespiration. BMC Biotechnol 11:111–121
Djannane S, Chauvin JE, Meyer C (2002) Glasshouse behavior of eight transgenic potato clones with a modified nitrate reductase expression under two fertilization regimes. J Exp Bot 53:1037–1045
Donn G, Tischer J, Smith A, Goodman HM (1984) Herbicide-resistant alfalfa cells: an example of gene amplification in plants. J Mol Appl Genet 2:621–635
Dotson SB, Edgerton MD, Wu J, Xie Z, Lee GJ, Adams TR, Nelson, DE (2009) Transgenic plants with enhanced agronomic traits. US Patent 20,090,049,573, Feb 19
Duncan D, Dykhuisen R, Frazer A, MacKenzie H, Golden M, Benjamin N, Leifert C (1998) Human health effects of nitrate. Gut 42:334–340
Duvick DN (2005) The contribution of breeding to yield advances in maize (Zea mays L.). Adv Agron 86:83–145
Fei H, Chaillou S, Mahon JD, Vessey JK (2003) Overexpression of a soyabean cytosolic glutamine synthetase gene linked to organ - specific promoters in pea plants grown in different concentrations of nitrate. Planta 216:467–474
Ferrario-Méry S, Valadier MH, Foyer C (1998) Overexpression of nitrate reductase in tobacco delays drought-induced decreases in nitrate reductase activity and mRNA. Plant Physiol 117:293–302
Ferrario-Méry S, Valadier MH, Godefroy N, Miallier D, Hirel B, Foyer CH, Suzuki A (2002) Diurnal changes in ammonia assimilation in transformed tobacco plants expressing ferredoxin-dependent glutamate synthase mRNA in the antisense orientation. Plant Sci 163:59–67
Ferguson BJ, Indrasumunar A (2011) Soybean nodulation and nitrogen fixation, Chap 4. In: Hendricks BP (ed) Agricultural research updates. Nova Science, Hauppauge, NY, pp 103–119
Forde BG, Lea PJ (2007) Glutamate in plants: metabolism, regulation, and signaling. J Exp Bot 58(9):2339–2358
Fraisier V, Gojon A, Tillard P, Daniel-Vedele F (2000) Constitutive expression of a putative high affinity nitrate transporter in Nicotiana plumbaginifolia: evidence for post transcriptional regulation by a reduced nitrogen source. Plant J 23:489–496
Fuentes SI, Allen DJ, Ortiz-Lopez A, Hernández G (2001) Over-expression of cytosolic glutamine synthetase increases photosynthesis and growth at low nitrogen concentrations. J Exp Bot 52:1071–1081
Gallardo F, Fu J, Canton FR, Garcia-Gutierrez A, Canovas FM, Kirby EG (1999) Expression of a conifer glutamine synthetase in transgenic poplar. Planta 210:19–26
Garcia-Teijeiro R, Lightfoot DA, Hernandez JD (2009) Effect of a modified urea fertilizer on soil microbial populations around maize (Zea mays L.) roots. Commun Soil Sci Plant Anal 40:2152–2168
Godon C, Krapp A, Leydecker MT, Daniel-Vedele F, Caboche M (1996) Methylammonium-resistant mutants of Nicotiana plumbaginifolia are affected in nitrate transport. Mol Gen Genet 250(3):357–366
Goldman BS, Darveaux, B, Cleveland J, Abad MS, Sayed M (2009) Genes and uses for plant improvement. US Patent Application 2009/0,070,897
Good AG, Johnson SJ, DePauw MD, Carroll RT, Savidov N, Vidamir J, Lu Z, Taylor G, Stroeher V (2007) Engineering nitrogen use efficiency with alanine aminotransferase. Can J Bot 85:252–262
Gupta R, Liu J, Dhugga KS, Simmons CR (2008) Manipulation of ammonium transporters (AMTs) to improve nitrogen use efficiency in higher plants. US Patent Application 12/045,098
Gupta R, Liu J, Dhugga KS, Simmons CR (2011) Manipulation of ammonium transporters (AMTs) to improve nitrogen use efficiency in higher plants. US Patent Application 13/043,109
Habash DZ, Massiah AJ, Rong HL, Wallsgrove RM, Leigh RA (2001) The role of cytosolic glutamine synthetase in wheat. Ann Appl Biol 2001(138):83–89
Harrigan GG, Ridley WP, Miller KD, Sorbet R, Riordan SG, Nemeth MA, Reeves W, Pester TA (2010) The forage and grain of MON 87460, a drought-tolerant corn hybrid, are compositionally equivalent to that of conventional corn. J Agric Food Chem 57:9754–9763
Harrison J, Crescenzo MP, Hirel B (2003) Does lowering glutamine synthetase activity in nodules modify nitrogen metabolism and growth of Lotus japonicus L. Plant Physiol 133:253–262
Hemon P, Robbins MP, Cullimore JV (1990) Targeting of glutamine synthetase to the mitochondria of transgenic tobacco. Plant Mol Biol 15:895–904
Hoshida H, Tanaka Y, Hibino T, Hayashi Y, Tanaka A (2000) Enhanced tolerance to salt stress in transgenic rice that overexpresses chloroplast glutamine synthetase. Plant Mol Biol 43:103–111
Indrasumunar A, Searle I, Lin M-H, Kereszt A, Men A, Carroll BJ, Gresshoff PM (2011) Nodulation factor receptor kinase 1a controls nodule organ number in soybean (Glycine max L. Merr). Plant J 59:61–68
Indrasumunar A, Kereszt A, Gresshoff PM (2012) Soybean nodulation factor receptor proteins, encoding nucleic acids and uses therefore. US Patent Application 2011/0,231,952
Jahns T, Kaltwasser H (2000) Mechanism of microbial degradation of slow release fertilizers. J Polym Environ 8:11–16
Jahns T, Schepp R, Siersdorfer C, Kaltwasser H (1999) Biodegradation of slow-release fertilizers (methyleneureas) in soil. J Environ Polym Degrad 7:75–82
Jung HG, Mertens DR, Phillips RL (2011) Effect of reduced ferulate-mediated lignin/arabinoxylan cross-linking in corn silage on feed intake, digestibility, and milk production. J Dairy Sci 94:5124–5137
Kisaka H, Kida T (2005) Method of producing transgenic plants having improved amino acid composition. US Patent 6, 969, 782
Kisaka H, Kida T, Miwa T (2007) Transgenic tomato plants that overexpress a gene for NADH-dependent glutamate dehydrogenase (legdh1). Breed Sci 57:101–106
Kleinhofs A, Kuo T, Warner RL (1980) Characterization of nitrate reductase-deficient barley mutants. Mol Gen Genet 177:421–425
Koivunen M, Morisseau C, Horwarth WR, Hammock BD (2004) Isolation of a strain of Agrobacterium tumefaciens (Rhizobium radiobacter) utilizing methylene urea (ureaformaldehyde) as nitrogen source. Can J Microbiol 50(3):167–174
Krouk G, Crawford NM, Coruzzi GM, Tsay YF (2010) Nitrate signaling: adaptation to fluctuating environments. Curr Opin Plant Biol 13:266–273
Kozaki A, Takeba G (1996) Photorespiration protects C3 plants from photooxidation. Nature 384:557–560
Kurai T, Wakayama M, Abiko T, Yanagisawa S, Aoki N, Ohsugi R (2011) Introduction of the ZmDof1 gene into rice enhances carbon and nitrogen assimilation under low-nitrogen conditions. Plant Biotechnol J 9:826–837
Lam HM, Wong P, Chan HK, Yam KM, Chow CM, Corruzi GM (2003) Overexpression of the ASN1 gene enhances nitrogen status in seeds of Arabidopsis. Plant Physiol 132:926–935
Lea PJ, Miflin BJ (2011) Nitrogen assimilation and its relevance to crop improvement, Chapter 1. In: Foyer C, Zhang H (eds) Nitrogen metabolism in plants in the post-genomic era. Annu Plant Rev 42:1–40
Lea US, ten Hoopen F, Provan F, Kaiser WM, Meyer C, Lillo C (2004) Mutation of the regulatory phosphorylation site of tobacco nitrate reductase results in constitutive activation of the enzyme in vivo and nitrite accumulation. Plant J 35:566–573
Lee LK, Nielsen EG (1987) The extent and costs of groundwater contamination by agriculture. J Soil Water Conserv 42:243–248
Lightfoot DA (2008) Blue revolution brings risks and rewards. Science 321:771–772
Lightfoot DA (2009) Genes for use in improving nitrate use efficiency in crops. In: Wood AJ, Jenks MA (eds) Genes for plant abiotic stress. Wiley Blackwell, New York, pp 167–182
Lightfoot DA, Fakhoury A (2010) Methods of using plants containing the gdhA gene: aflatoxin reduction and fungal rot resistance. US Patent 8,383,887
Lightfoot DA, Long LM, Vidal ME (1999) Plants containing the gdhA gene and methods of use thereof. US Patent 5,998,700
Lightfoot DA, Long LM, Vidal ME (2001) Plants containing the gdhA gene and methods of use thereof. US Patent 6,329,573
Lightfoot DA, Bernhardt K, Mungur R, Nolte S, Ameziane R, Colter A, Jones K, Iqbal MJ, Varsa EC, Young BG (2007) Improved drought tolerance of transgenic Zea mays plants that express the glutamate dehydrogenase gene (gdhA) of E. coli. Euphytica 156:106–115
Limami A, Phillipson B, Ameziane R, Pernollet N, Jiang Q, Roy R, Deleens E, Chaumont-Bonnet M. Gresshoff PM, Hirel B (1999) Does root glutamine synthetase control plant biomass production in lotus japonicus L.? Planta. 209, 495–502
Liu KH, Huang CY, Tsay YF (1999) CHL1 is a dual-affinity nitrate transporter of Arabidopsis involved in multiple phases of nitrate uptake. Plant Cell 11:865–874
Loulakakis KA, Primikirios NI, Nikolantonakis MA, Roubelakis-Angelakis KA (2002) Immunocharacterization of Vitis vinifera L. ferredoxin-dependent glutamate synthase, and its spatial and temporal changes during leaf development. Planta 215:630–638
Loussaert DF, O’ Neill D, Simmons CR, Wang H (2008) Nitrate reductases from red algae, compositions and methods of use thereof. US Patent 20,080,313,775, Dec 18
Marchner H (1995) Mineral nutrition of higher plants. Academic, London, 889 p
Meyer R, Yuan Z, Afzal J, Iqbal J, Zhu M, Garvey G, Lightfoot d (2006) Identification of Gsr1: a locus inferred to regulate gene expression in response to exogenous glutamine. Euphytica 151:291–302
Migge A, Carrayol E, Hirel B, Becker TW (2000) Leaf specific overexpression of plastidic glutamine synthetase stimulates the growth of transgenic tobacco seedlings. Planta 2:252–260
Mirvish SS (1985) Gastric cancer and salivary nitrate and nitrite. Nature 315:461–462
Moller H, Landt J, Pederson E, Jensen P, Autrup H, Jensen OM (1990) Endogenous nitrosation in relation to nitrate exposure from drinking water and diet in a danish rural population. Cancer Res 49:3117–3121
Mungur R, Glass ADM, Goodenow DB, Lightfoot DA (2005) Metabolic fingerprinting in transgenic Nicotiana tabacum altered by the Escherichia coli glutamate dehydrogenase gene. J Biomed Biotechnol 2:198–214
Mungur R, Wood AJ, Lightfoot DA (2006) Water potential during water deficit in transgenic plants: Nicotiana tabacum altered by the Escherichia coli glutamate dehydrogenase gene. Plant Growth Regul 50:231–238
Nadzan G, Schneeberger R, Feldmann K (2007) Nucleotide sequences and corresponding polypeptides conferring improved nitrogen use efficiency characteristics in plants. US Patent 20,070,169,219
Nelson DE, Repetti PP, Adams TR, Creelman RA, Wu J, Warner DC et al (2007) Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc Natl Acad Sci USA 104:16450–16455
Nolte SA (2009) Metabolic analysis of resistance to glufosinate in gdhA transgenic tobacco. PhD Dissertation, Plant Biology, Southern Illinois University, Carbondale, IL, 244 p
Nolte SA, Young BG, Mungur R, Lightfoot DA (2004) The glutamate dehydrogenase gene gdhA increased the resistance of tobacco to glufosinate. Weed Res 44:335–339
Oh JY, Warner RL, Kleinhofs A (1980) Effect of nitrate reductase-deficiency upon growth, yield and protein in barley. Crop Sci 20:487–490
Oliveira IC, Brears T, Knight TJ, Clark A, Coruzzi GM (2002) Overexpression of cytosolic glutamate synthetase. Relation to nitrogen, light, and photorespiration. Plant Physiol 129:1170–1180
Ortega JL, Temple SJ, Sengupta-Gopalan C (2001) Constitutive overexpression of cytosolic glutamine synthetase (GS1) gene in transgenic alfalfa demonstrates that GS1 be regulated at the level of RNA stability and protein turnover. Plant Physiol 126:109–121
Pathak RR, Ahmad A, Lochab S, Raghuram N (2008) Molecular physiology of plant nitrogen use efficiency and biotechnological options for its enhancement. Curr Sci 94:1394–1403
Pracharoenwattana I, Zhou W, Keech O, Francisco PB, Udomchalothorn T, Tschoep H, Stitt M, Gibon Y, Smith SM (2010) Arabidopsis has a cytosolic fumarase required for the massive allocation of photosynthate into fumaric acid and for rapid plant growth on high nitrogen. Plant J 62:785–795
Reid DE, Ferguson BJ, Hayashi S, Lin Y-H, Gresshoff PM (2011) Molecular mechanisms controlling legume autoregulation of nodulation. Ann Bot 108:789–795
Rubio-Wilhelmi Mdel M, Sanchez-Rodriguez E, Rosales MA, Blasco B, Rios JJ, Romero L, Blumwald E, Ruiz JM (2012) Ammonium formation and assimilation in P(SARK):IPT tobacco transgenic plants under low N. J Plant Physiol 169:157–162
Schmidt RR, Miller P (2009) Polypeptides and polynucleotides relating to the α- and β-subunits of glutamate dehydrogenases and methods of use. US Patent 7,485,771
Schneeberger R, Margolles-Clark E, Park J-H, Jankowski B, Bobzin SC (2008) Modulating plant nitrogen levels. US Patent 7335510-A 1, 26 Feb 2008
Schoenbeck MA et al (2000) Decreased NADH-glutamate synthase activity in nodules and flowers of alfalfa (Medicago sativa L.) transformed with an antisense glutamate synthase transgene. J Exp Bot 51:29–39
Seebauer JR, Singletary GW, Krumpelman PM, Ruffo ML, Below FE (2010) Relationship of source and sink in determining kernel composition of maize. J Exp Bot 61:511–519
Sentoku N, Tanignchi M, Sugiyama T, Ishimaru K, Ohsugi R, Takaiwa F, Toki S (2000) Analysis of the transgenic tobacco plants expressing Panicum miliaceum aspartate aminotransferase genes. Plant Cell Rep 19:598–603
Shrawat AK, Carroll RT, DePauw M, Taylor GJ, Good AG (2008) Genetic engineering of improved nitrogen use efficiency in rice by the tissue-specific expression of alanine aminotransferase. Plant Biotechnol J 6:722–732
Sinclair TR, Purcell LC, King CA, Sneller CH, Chen P, Vadez V (2007) Drought tolerance and yield increase of soybean resulting from improved symbiotic N fixation. Field Crops Res 101:68–71
Sobolev AP, Testone G, Santoro F, Nicolodi C, Iannelli MA, Amato ME, Ianniello A, Brosio E, Giannino D, Mannina L (2010) Quality traits of conventional and transgenic lettuce (Lactuca sativa L.) at harvesting by NMR metabolic profiling. J Agric Food Chem 58(11):6928–6936
Specht JE, Hume DJ, Kumudini SV (1999) Soybean yield potential – a genetic and physiological perspective. Crop Sci 39:1560–1571
Suarez R, Márquez J, Shishkova S, Hernández G (2003) Overexpression of alfalfa cytosolic glutamine synthetase in nodules and flowers of transgenic Lotus japonicus plants. Physiol Plant 117:326–336
Takahashi M, Sasaki Y, Ida S, Morikawa H (2001) Nitrite reductase gene enrichment improves assimilation of NO2 in Arabidopsis. Plant Physiol 126:731–741
Tan Q, Zhang L, Grant J, Cooper P, Tegeder M (2010) Increased phloem transport of S-methyl-methionine positively affects sulfur and nitrogen metabolism and seed development in pea plants. Plant Physiol 154(4):1886–1896
Tannenbaum SR, Fett D, Young VR, Land PD, Bruce WR (1978) Nitrite and nitrate are formed by endogenous synthesis in the human intestine. Science 200:1487–1491
Tercé-Laforgue T, Dubois F, Ferrario-Méry S, Pou de Crecenzo M-A, Sangwan R, Hirel B (2004a) Glutamate dehydrogenase of tobacco is mainly induced in the cytosol of phloem companion cells when ammonia is provided either externally or released during photorespiration. Plant Physiol 136:4308–4317
Tercé-Laforgue T, Mäck G, Hirel B (2004b) New insights towards the function of glutamate dehydrogenase revealed during source-sink transition of tobacco (Nicotiana tabacum) plants grown under different nitrogen regimes. Physiol Plant 120:220–228
Trenkel ME (1997) Improving fertilizer use efficiency. Controlled-release and stabilized fertilizers in agriculture. International Fertilizer Industry Association, Paris, 100 p
Valentine V, Benedito VA, Kang Y (2011) Legume nitrogen fixation and soil abiotic stress, Chap 9. In: Foyer C, Zhang H (eds) Nitrogen metabolism in plants in the post-genomic era. Annu Plant Rev 42:207–248
Vidal EA, Araus V, Lu C, Parry G, Green PJ, Coruzzi GM, Gutiérrez RA (2010) Nitrate-responsive miR393/AFB3 regulatory module controls root system architecture in Arabidopsis thaliana. Proc Natl Acad Sci USA 107:4477–4482
Vincent R et al (1997) Over expression of a soybean gene encoding cytosolic glutamine synthetase in shoots of transgenic Lotus cornicultus L. plants triggers changes in ammonium and plant development. Planta 201:424–433
Vincentz M, Caboche M (1991) Constitutive expression of nitrate reductase allows normal growth and development of Nicotiana plumbaginifolia plants. EMBO J 10:1027–1035
Wang X, Peng F, Li M, Yang L, Li GJ (2012) Expression of a heterologous SnRK1 in tomato increases carbon assimilation, nitrogen uptake and modifies fruit development. Plant Physiol 169:1173–1182
Yamaya T, Obara M, Nakajima H, Sasaki S, Hayakawa T, Sato T (2002) Genetic manipulation and quantitative-trait loci mapping for nitrogen recycling in rice. J Exp Bot 53:917–925
Yanagisawa S, Akiyama A, Kisaka H, Uchimiya H, Miwa T (2004) Metabolic engineering with Dof1 transcription factor in plants: improved nitrogen assimilation and growth under low-nitrogen conditions. Proc Natl Acad Sci USA 101:7833–7838
Zhang H, Forde BG (1998) An Arabidopsis MADS box gene that controls nutrient-induced changes in root architecture. Science 279:407–409
Zhang Y, Dickinson JR, Paul MJ, Halford NJ (2003) Molecular cloning of an Arabidopsis homologue of GCN2, a protein kinase involved in co-ordinated response to amino acid starvation. Planta 217:668–675
Zhao Y, Li W, Zhou Z, Wang L, Pan Y, Zhao L (2005) Dynamics of microbial community structure and cellulolytic activity in agricultural soil amended with two biofertilizers. Eur J Soil Biol 41:21–29
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2013 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Lightfoot, D.A. (2013). Nitrogen Fixation and Assimilation. In: Kole, C. (eds) Genomics and Breeding for Climate-Resilient Crops. Springer, Berlin, Heidelberg. https://doi.org/10.1007/978-3-642-37048-9_11
Download citation
DOI: https://doi.org/10.1007/978-3-642-37048-9_11
Published:
Publisher Name: Springer, Berlin, Heidelberg
Print ISBN: 978-3-642-37047-2
Online ISBN: 978-3-642-37048-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)