Abstract
Picrorhiza (Picrorhiza kurrooa) is an endangered medicinal plant with well-known hepatoprotective activity attributed to monoterpenoid picrosides. The present article details on regulatory genes of terpenoid metabolism, 3-hydroxy-3-methylglutaryl coenzyme A reductase (pkhmgr) and 1-deoxy-D-xylulose-5-phosphate synthase (pkdxs) from picrorhiza. Since no molecular information was available, these genes were cloned to full-length by degenerate primers and rapid amplification of cDNA ends, followed by cloning of the upstream sequences that showed the presence of core sequences for light and temperature responsiveness. Electrophoretic mobility shift assay confirmed binding of protein to these motifs. Expression of pkhmgr and pkdxs was up-regulated at 15°C as compared to at 25°C as well as under light as compared to dark conditions. Picrosides content exhibited the trend similar to gene expression. To rule out the possible limitation of carbon pool under dark condition, plantlets of picrorhiza were raised in vitro in Murashige and Skoog medium supplemented with 3% sucrose. Results showed similar up-regulation of both the genes and the higher picrosides content in in vitro raised plantlets in the presence of light. Data suggested the important roles played by light and temperature in regulating pkhmgr and pkdxs, and the picrosides level in picrorhiza.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
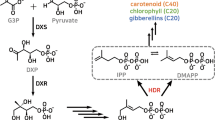
Terpenoids constitute the largest family of natural plant products with over 30,000 members possessing important biological and physiological functions (Sacchettini and Poulter 1997). Terpenoids are derived from geranyl diphosphate (GDP) which is synthesized by sequential head to tail addition of isopentenyl pyrophosphate and its allelic isomer dimethylallyl diphosphate (Wise and Croteau 1998). Synthesis of isopentenyl pyrophosphate and dimethylallyl diphosphate proceeds via cytosolic mevalonate (MVA) and plastid methylerythritol phosphate (MEP) pathways (Mahmoud and Croteau 2002; Hampel et al. 2006; Fig. 1). A cross-talk between these two pathways has also been reported (Hampel et al. 2005). Supply of GDP is critical in realizing the yield of terpenoids (Nogués et al. 2006), therefore, studies on regulation of genes in GDP biosynthesis assume central importance.
Schematic pathway for picrosides biosynthesis (adapted from Mahmoud and Croteau 2002). Geranyldiphosphate (GDP) can be derived from mevalonate (MVA) or methylerythritol phosphate (MEP) pathway. GDP yields iridoid, which is converted into picrosides in the presence of glucose and cinnamic acid or vanillic acid. Encircled numbers represent enzyme catalyzing the corresponding reaction step as follows: 1 1-deoxy-D-xylulose-5-phosphate synthase; 2 1-deoxy-D-xylulose-5-phosphate reductoisomerase; 3 2-C-methylerythritol 4-phosphate cytidyl transferase; 4 4-(cytidine-5′-diphospho)-2-C-methylerythritol kinase; 5 2-C-methylerythritol-2,4-cyclophosphate synthase; 6 1-hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate synthase; 7 1-hydroxy-2-methyl-2-(E)-butenyl-4-diphosphate reductase; 8 acetoacetyl CoA thiolase; 9 3-hydroxy-3-methylglutaryl coenzyme A synthase; 10 3-hydroxy-3-methylglutaryl coenzyme A reductase; 11 mevalonate kinase; 12 phosphomevalonate kinase; 13 mevalonate-5-pyrophosphate decarboxylase; 14 isopentenyl pyrophosphate isomerase; 15 geranyldiphosphate synthase. Solid arrows indicate known steps, whereas dotted arrows indicate unknown steps
Regulation of gene expression at the level of transcription is a major control point in many biological processes. Transcriptional regulation is achieved through binding of transcription factors to short consensus sequences of DNA known as cis-acting regulatory elements or motifs, usually located in the promoter regions, upstream of the coding sequences (Udvardi et al. 2007). It is generally believed that genes having similar expression patterns contain common motifs in their promoter (Klok et al. 2002). Thus, a common set of transcription factors co-regulate expression of genes and hence the pathway.
Picrosides, the medicinally important hepatoprotectants (Floersheim et al. 1990), are synthesized by picrorhiza (Picrorhiza kurrooa Royle ex Benth.), an endangered plant species of family Scrophulariaceae. The species is distributed between 3,000-5,000 m above mean sea level in Himalaya (Chettri et al. 2005). Indiscriminate and extensive harvesting and lack of organized cultivation of the plant has threatened its status in wild and listed as ‘endangered’ species by International Union for Conservation of Nature and Natural Resources (Nayar and Sastri 1990).
Picrosides are terpenoids with an iridane skeleton of monoterpene origin (Fig. 1). Previous studies have shown that 3-hydroxy-3-methylglutaryl coenzyme A reductase (hmgr) and 1-deoxy-D-xylulose-5-phosphate synthase (dxs) are regulatory genes in terpenoids biosynthesis (Korth et al. 2000; Hsieh and Goodman 2005) for which no information is available in picrorhiza. In the present work, we cloned these genes from picrorhiza, analyzed the cis-acting elements, and the motifs for light and temperature regulation were studied. Electrophoretic mobility shift assay (EMSA) confirmed binding of proteins to the identified motifs. Gene expression analysis supported the results on transcriptional regulation of hmgr and dxs by light and temperature. A positive relationship between expression of these genes and picrosides content was obtained.
Materials and methods
Plant material
Plants of picrorhiza (P. kurrooa Royle ex Benth.) were collected from its natural habitat at Rohtang pass (4,000 m altitude, 32°23′ N, 77°15′ E, India) and brought to the Institute at Palampur (1,300 m altitude; 32°06′ N, 76°33′ E, India). These were transplanted in plastic pots (20 cm height × 20 cm top diameter × 12 cm bottom diameter) containing soil, sand, and farm yard manure mixture in a ratio of 2:1:1, and were maintained in the experimental farm of the Institute. Plants were allowed to acclimatize for 3 months before start of the experiment. Experiments were performed on fifth leaf (position with respect to the top apical leaf designated as first leaf), which was harvested at the designated time, followed by freezing in liquid nitrogen and storage at −80°C for further use.
Raising of in vitro shoot cultures of picrorhiza
Young leaves of picrorhiza were collected from plants grown in the experimental farm, washed with tween-20, and surface-disinfected with solution containing 0.05% (w/v) streptomycin sulfate and 0.05% (w/v) bavistin for 20 min. The explants were further washed with distilled water and surface sterilized with sequential treatment of 70% ethanol for 45 s and mercuric chloride 0.05% (w/v) for 10 min. Subsequently, these were washed with autoclaved distilled water and inoculated on Murashige and Skoog (MS) medium (Murashige and Skoog 1962) supplemented with 3% sucrose. Prior to autoclaving, pH of the medium was adjusted to 5.7 followed by addition of 0.8% agar. Unless indicated, regenerated plantlets were incubated at 25 ± 2°C with 16 h photoperiod (photosynthetic photon flux density, 70 µmol m−2 s−1).
Exposure of picrorhiza to temperature and light
Potted plants were shifted from the experimental farm to the plant growth chamber maintained at 25 ± 2°C (16 h photoperiod; photosynthetic photon flux density, 350 µmol m−2 s−1). After 5 days, a few pots were shifted to another growth chamber maintained at 15 ± 2°C. Data was collected on day 12th after shifting as detailed in the figure legends.
Our data on the effect of temperature showed poor expression of the targeted genes at 25°C, which led us to study the effect of light in plants maintained at 15°C. For dark treatment, plants were covered with a cardboard box to restrict entry of light and the samples were harvested at 12 h from start of the treatment. Any long-term experiment with the plants exposed to dark could lead to possible starvation thus limiting the carbon pool. In order to differentiate the impact of light through effect on carbon pool or the light per se, plants were raised in vitro through tissue culture as detailed above. Four-week-old plantlets raised at 25 ± 2°C were transferred to test tubes containing MS medium supplemented with 3% sucrose and maintained at 15 ± 2°C in light and complete darkness, separately. Samples were harvested on day 6 of the treatment for analysis of gene expression and picrosides content. Beyond day 6, plants kept in dark showed etiolation and yellowing.
Cloning of cDNAs of pkhmgr and pkdxs
RNA was isolated from leaf tissue using the method of Ghawana et al. (2007) and digested with DNase 1 (RNase-free) using Message Clean® Kit (GenHunter® Corporation, USA). Complementary DNA (cDNA) was synthesized as described by Singh et al. (2004). Degenerate primers for hmgr and dxs were designed from the conserved regions of corresponding genes reported for different plant sources and the partial gene sequences were amplified by PCR as detailed in Table 1. The amplicons were cloned in pGEM-T Easy Vector (Promega, USA), plasmids were isolated using Qiagen Plasmid Mini-isolation Kit (Qiagen, GmbH), and sequencing was performed using BigDye terminator cycle sequencing mix (Version 3.1; Applied Biosystems, USA) using an automated DNA sequencer (ABI PRISM™ 310 and 3130 xl Genetic Analyzer, Applied Biosystems, USA). Protocols were followed essentially as described by the respective manufacturers.
Full-length cDNAs were cloned by performing rapid amplification of cDNA ends (RACE; SMART™ RACE cDNA Amplification Kit; Clontech, USA) as per the manufacturer’s instructions using the gene specific primers (hmgr5′R, hmgr3′R, dxs5′R and dxs3′R; Table 1). These primers were designed based upon the partial sequences of the genes as cloned above. After aligning the sequences obtained by 5′ and 3′ RACE, full-length cDNA was amplified using the end sequences, cloned in pGEM-T Easy Vector (Promega, USA) and confirmed by sequencing.
Wherever needed, cDNA-sequences were analyzed using BLASTN, BLASTX, and BLASTP programs of National Center for Biotechnology Information (NCBI) with default parameters (http://www.ncbi.nlm.nih.gov/). Secondary structure of the deduced amino acid sequence was analyzed using Self-Optimized Prediction Method with Alignment (SOPMA; http://www.npsa-pbil.ibcp.fr/).
Construction of genome-walking libraries and cloning of promoters
DNA was isolated from leaf tissue using cetyl-trimethylammonium bromide-based procedure (Doyle and Doyle 1987), treated with RNase (DNase-free) and purified with phenol/choloform for constructing libraries for genome walking (GenomeWalker™ Universal Kit; BD Bioscience, Clontech, USA). Genomic DNA was digested for 16 h with restriction enzymes DraI, EcoRV, PvuII, and StuI, separately to create blunt-end fragments and purified using phenol/chloroform. The restricted fragments were ligated to adaptors supplied by the manufacturer to produce four separate libraries. Promoter sequences were amplified using the Advantage Genomic Polymerase Mix (BD Bioscience, Clontech, USA) in a thermocycler (i-cycler, Bio-Rad, USA). Primary PCR was performed with gene-specific primers (hmgrR-p and dxsR-p; Table 1) and the outer adaptor primer (AP1, supplied by the manufacturer) using all the four genomic libraries, followed by secondary PCR with nested gene-specific primers (hmgrR-s, dxsR-s; Table 1) and the nested adaptor primer (AP2, supplied by the manufacturer). The PCR fragments were analyzed on a 1.2% agarose gel. Amplicons were purified using QIAEX®II Gel Extraction Kit (Qiagen, GmbH), cloned into pGEM-T Easy Vector (Promega, USA), and sequenced. Sequence information was also used for subsequent rounds of genome walking (if needed).
Sequences were analyzed in silico for detection of various motifs using “plant cis-acting regulatory DNA elements” (PLACE) database (http://www.dna.affrc.go.jp/PLACE ; Higo et al. 1999). PLACE has advantage over the other eukaryotic databases such as TFD and TRANSFAC in terms of containing larger numbers of eukaryotic cis-elements from vascular plants (Higo et al. 1999). Multiple Expectation-Maximization for Motif Elicitation was used for the detection of common motifs in both the promoters with motif width set to 6-9 and the number of motifs to be detected was set to 10 (http://meme.sdsc.edu/meme/meme). Since varying length of promoters was obtained for the two genes, the analysis of plus-strands was confined to 1,000 nucleotides upstream to the translation start site.
Reverse transcriptase-PCR analysis
DNA-free RNA was used to synthesize cDNA using Accuscript™ High Fidelity Ist strand cDNA Synthesis Kit (Stratagene, USA). This cDNA was to be used as template for Reverse transcriptase-PCR reaction using gene specific primers (hmgrF, hmgrR; dxsF, dxsR) as mentioned in Table 1. Cycling conditions were optimized to obtain amplification under the exponential phase. 26S rRNA based primer pair was used as internal control for expression studies (Singh et al. 2004). Amplicons were analyzed and quantified using the Alpha DigiDoc Gel Documentation and Image analysis system (Alpha Innotech, USA). Each experiment was repeated at least twice with three biological replicates each time and the representative figure of one experiment is shown in the manuscript.
Extraction and estimation of picrosides
Picrosides were estimated as described by Dutt et al. (2004). Leaf tissue was harvested, washed with distilled water, blotted dry, weighed, then frozen in liquid nitrogen and stored at −80°C. The frozen samples (20 mg) were ground to fine powder in liquid nitrogen using pestle and mortar followed by addition of 1.0 ml of 80% methanol with intermittent grinding for 1 min. Extract was transferred to a centrifuge tube and the pestle and mortar was rinsed with 1.0 ml of 80% methanol to recover the leftover sample. Extracts were pooled, centrifuged at 15,000×g for 15 min and the supernatant was used for picrosides estimation. Samples were filtered through 0.45 micron filter (Millipore, USA) for high pressure liquid chromatography analysis using LC 4000 module, 2487 dual λ absorbance detector (both from Waters, UK), and LiChrosorb® RP-18, 250 X 4.0 mm (Hibar) column (Merck & Co., Inc., USA). The mobile phase (flow rate, 1.0 ml/min) consisted of a mixture of trifluoroacetic acid (0.05%) and methanol-acetonitrile (1:1) in 70:30 ratio. Picrosides were monitored at 270 nm and quantified using pure picroside-I (P-I) and picroside-II (P-II) as standards.
Nuclear proteins extraction and electrophoretic mobility shift assay
Leaf tissue (20 g) was harvested from the plants growing at temperature of 15°C and a photoperiod of 16 h, and ground in pre-chilled pestle-mortar using extraction buffer [10 mM HEPES (pH 7.8), 10 mM KCl, 10 mM MgCl2, 5 mM EDTA, 1 mM DTT, 250 mM Sucrose, 0.2 mM PMSF and 0.5% Triton X-100] to isolate nuclear proteins essentially as described by Busk and Pages (1997). Oligonucleotides (30 mer) possessing low temperature responsive element (LTRE; CCGAA) and light responsive element (GATA), as present in promoters of pkhmgr and pkdxs, were designed having sequences: 5′-GAATTCAAAACTTTAACTGAAACCGAAAAATTGAGAAA-3′ (LTRE-1); 5′-GAATTCTTTCTCAATTTTTCGGTTTCAGTTAAAGTTTT-3′ (LTRE-2; complementary to LTRE-1); 5′-GTGCCAGTGTTGATATTATAAGTGCCAGTG-3′ (GATA-1); 5′-CACTGGCACTTATAATATCAACACTGGCAC-3′ (GATA-2; complementary to GATA-1). Equal moles of complementary oligonucleotides were mixed and annealed by incubating at 95°C for 5 min followed by slow cooling to room temperature. For preparation of probe for EMSA (Hennighausen and Lubon 1985), double-stranded oligonucleotides were radio-labeled with α-32P-dATP (3,000 Ci/mmol) using Klenow DNA polymerase I enzyme (USB, USA) that filled up the recessive ends. Recessive ends were created by incorporating GAATTC at 5′ end of the oligonucleotides. Binding of protein was carried out in 1× binding buffer [30 mM HEPES (pH 7.8), 60 mM KCl, 0.3 mM EDTA, 0.5 mM DTT and 10% glycerol] containing 4 µg of nuclear protein. For specific competition, unlabeled competitor DNA was added in excess to the binding mixture. After 10 min incubation at room temperature, probe (10,000 cpm) was added and incubated further for 20 min. Samples were loaded onto a 6% non-denaturing polyacrylamide gel and run in 0.5× TBE buffer [1× TBE is 50 mM Tris-borate (pH 8.2), 1 mM EDTA] at 15 V/cm. Gel was blotted onto 3MM filter paper (Whatman, Clifton, NJ), dried under vacuum at 80°C for 2 h, and subjected to autoradiography.
Results and discussion
Hepatoprotective activity of picrorhiza owes to the presence of monoterpenoid picrosides (P-I and P-II; Luper 1998). Since terpenoids are derived from GDP that can be synthesized both from cytoplasmic MVA and plastidic MEP pathways (Croteau et al. 2005), it is important to study the regulation of these two pathways as feeders of GDP. Enzyme 3-hydroxy-3-methylglutaryl coenzyme A reductase (encoded by hmgr) catalyzes reduction of 3-hydroxy-3-methylglutaryl coenzyme A into mevalonate and is regarded as rate-limiting step in the MVA pathway (Wang et al. 2007), for example in potato (Solanum tuberosum) and Arabidopsis (Yang et al. 1991; Leivar et al. 2005). Enzyme 1-deoxy-D-xylulose-5-phosphate synthase (encoded by dxs) catalyzes the first rate-limiting step involving condensation of pyruvate and glyceraldehyde-3-phosphate to produce 1-deoxy-D-xylulose-5-phosphate in the MEP pathway. The gene has been found to be regulatory (Muñoz-Bertomeu et al. 2006; Estevez et al. 2001), such as in tomato (Lycopersicon esculentum), periwinkle (Catharanthus roseus) and Arabidopsis (Lois et al. 2000; Chahed et al. 2000; Juan et al. 2001). Since hmgr and dxs are regarded as important genes of terpenoid biosynthesis pathway (Fig. 1), the present work studied these genes, their promoters, and the picrosides content to identify the gene regulation and its possible relationship with the picrosides content in picrorhiza.
Degenerate primers (Table 1) followed by RACE led to amplification of 2,245 bp and 2,317 bp of pkhmgr (accession no. DQ347962) and pkdxs (accession no. EU561005), respectively. The cDNAs consisted of 106 and 50 bp of 5′ untranslated region; 453 bp, and 203 bp of 3′ untranslated regions; and open reading frames of 1,686 and 2,064 bp for pkhmgr and pkdxs, respectively. Deduced proteins (hereinafter referred to as pkHMGR and pkDXS, respectively) had molecular masses/isoelectric points of 60.65 kDa/7.24 and 73.63 kDa/8.60, respectively. BLASTX analysis showed extensive identity (>70%) of the deduced proteins with those from other plant sources (Electronic Supplementary Fig. S1).
In silico analysis showed the presence of corresponding conserved domains in both of the deduced proteins (Electronic Supplementary Fig. S2). HMG coenzyme A reductase class I domain was found to be present between amino acid (aa) positions 162 and 551 in pkHMGR. In pkDXS, three characteristic domains were recorded. These were: thiamine pyrophosphate binding domain (between aa positions 77 and 334), transketolase pyrimidine binding domain (between aa positions 364 and 527), and transketolase–c terminal domain (between aa positions 545 and 667).
SOPMA analysis revealed 46.52% α-helices, 4.99% β-turns, 14.62% extended strands and 33.87% random coils in pkHMGR. In pkDXS, α-helices, β-turns, extended strands, and random coils were 36.97%, 6.84%, 14.56%, 41.63%, respectively (Electronic Supplementary Table S1, Fig. S3). Deduced secondary structures were in agreement with those reported for functional genes in other plant systems (Electronic Supplementary Table S1). Various in silico analyses suggested the cloned genes to be functional as has been reported by the other studies (Walter et al. 2002; Wang et al. 2007).
Cloning and analysis of promoters of pkhmgr and pkdxs
The promoters of pkhmgr (Upkhmgr, 1,065 bp; accession no. FJ228691) and pkdxs (Upkdxs,1,146 bp; accession no. FJ228692) were cloned by genome walking. In silico analysis was limited to 1 kb sequences upstream of translation start site for consistency in comparative analysis of the two promoters under study. Table 2 lists various motifs along with their function and location in the two promoters analyzed. A total of 73 types of motifs were identified in Upkhmgr and Upkdxs, out of which 24 motifs were common to both, whereas 27 and 22 motifs were specific to Upkhmgr and Upkdxs, respectively (Table 2). In Upkhmgr, number of motifs identified for basal transcription, light responsiveness, low temperature responsiveness, tissue specificity (leaf, seed, root, flower, meristems), hormone (gibberellins, cytokinins, abscisic acid, and auxin) responsiveness and biotic factors were 11, 17, 7, 61, 19, and 11, respectively. These figures for Upkdxs were 9, 16, 8, 28, 23, and 7 for the above motifs in the same order.
Ecological niche of picrorhiza is characterized by an environment of low temperature and high light intensity (Chandra 2004). Light responsive motifs in both the promoters were spread throughout the promoter (Fig. 2) and these include: GATA box (WGATAR; Gilmartin et al. 1990), GT-1 (GRWAAW; Villain et al. 1996), TATA box (TTATTT; Tjaden et al. 1995), and I box (GATAA; Terzaghi and Cashmore 1995). GATA factors were first identified as proteins that interact with conserved WGATAR (W = T or A; R = G or A) motifs involved in erythroid-specific gene expression in vertebrates (Evans et al. 1988) and have been mostly implicated in light-dependent gene regulation in plants (Reyes et al. 2004). In Arabidopsis, Teakle et al. (2002) established that different families of GATA-binding factors bind to DNA sequence elements containing GATA or GAT sequence. It was suggested that GATA core sequence in the GATA motif was important for binding relevant transcription factors and the flanking sequences are non-consequential.
Nucleotide sequences of Upkhmgr and Upkdxs promoters cloned from picrorhiza. One thousand nucleotides upstream to translation start site were analyzed. The nucleotide just upstream to the first nucleotide of translation start codon is numbered as −1 and subsequent upstream numbering is in descending order. Light and temperature responsive motifs are boxed and underlined, respectively
GT-1 box is a regulatory motif usually found in tandem repeats in the promoter region of many different plant genes regulated by light (Villain et al. 1996). Apart from light, GT-1 is also shown to be regulating salt and pathogen induced gene expression (Park et al. 2004) and hence is not considered very specific in relation to regulation by light.
Apart from the above motifs common in the two promoters, promoter-specific light responsive motifs were also detected. In Upkhmgr, GCCAC (SORLIP) box was identified which is found to be over-represented in the phytochrome A-induced promoters (Hudson and Quail 2003). Upkdxs possessed CGGATA (REbeta) box, which is shown to be required for phytochrome regulation in Lemna gibba Lhcb21 gene promoter (Degenhardt and Tobin 1996). Yet another light responsive box GGTTAA was identified in Upkdxs, which has been reported to be present in upstream sequences of the light-responsive pea rbcS-3A gene (Green et al. 1988).
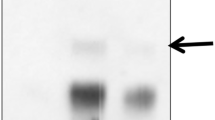
Among the various light responsive motifs analyzed in silico, GATA core sequence was specific to light and was present in Upkhmgr and Upkdxs. Therefore, GATA core was selected to study its interaction with putative transcription factors by EMSA. Results showed prominent shifts for GATA motif (Fig. 3 lane 5) suggesting binding of putative transcription factors to the motif in vitro.
Electrophoretic mobility shift assay showing DNA-protein interaction. Lane 1 radio-labeled low temperature responsive sequence (LTRE; 5′-GAATTCAAAACTTTAACTGAAACCGAAAAATTGAGAAA-3′); lane 2 radio-labeled LTRE sequence incubated with nuclear protein; lane 3 radio-labeled LTRE sequence incubated with nuclear protein and unlabeled (functioning as specific competitor) LTRE sequence; lane 4 radio-labeled light responsive (GATA) sequence (5′-GTGCCAGTGTTGATATTATAAGTGCCAGTG-3′); lane 5 radio-labeled GATA sequence incubated with nuclear protein; and lane 6 radio-labeled GATA sequence incubated with nuclear protein and unlabeled (functioning as specific competitor) GATA sequence. Core LTRE and GATA sequences are underlined
Low temperature responsive motifs were investigated to examine various cis-acting regulatory elements and trans-acting factors that have been reported to be involved in plants responses to temperature. Presence of several motifs involved in low temperature responses were detected in both the promoters analyzed (Table 2, Fig. 2). These included low temperature responsive elements (LTRE, CCGAC/CCGAA) and dehydration responsive elements [DREs: CANNTG (MYC), WAACCA, CNGTTR, TAACTG, YAACKG (MYB), ACGT, and MACGYGB (ABRE)]. LTREs containing pentanucleotide CCGAC/CCGAA have been identified in a number of promoters of a low-temperature-responsive gene such as in Brassica napus (BN115) and Hordeum vulgare (ABA-regulated barley gene, HVA1; Dunn et al. 1998). EMSA showed the importance of CCGA core sequence within the pentanucleotide LTRE for DNA protein interaction. Motifs related to water stress/drought viz; MYC, MYB, and ABRE like boxes are involved in ABA-mediated responses to various adverse environmental cues including cold (Agarwal et al. 2006; Choi et al. 2000).
LTRE core sequence CCGA was present in Upkhmgr and Upkdxs and was used in DNA-protein interaction studies. Results showed prominent shifts for CCGA motif (Fig. 3 lane 2) suggesting binding of putative transcription factors to the motif in vitro.
Data on in silico analysis and EMSA suggested the possible functionality of light and temperature responsive motifs in promoters of both the genes. The next logical question was if these genes are also regulated by light and temperature? And how does it correlate with the picrosides content under these conditions.
Effect of light and temperature on expression of pkhmgr and pkdxs, and picrosides content
Expression of pkhmgr and pkdxs was higher by 216% and 286%, respectively, at 15°C as compared to those at 25°C (Fig. 4a, b). Also, total picrosides increased by 22% at 15°C as compared to the plants maintained at 25°C (Fig. 4c). Since expression of pkhmgr and pkdxs was very poor at 25°C, experiment on the effect of dark was performed at 15°C. In a span of 12 h, expression of pkhmgr and pkdxs was higher by 70% and 112% in light as compared to that observed under dark (Fig. 5a, b). Picrosides content in light was higher by 163% of that observed under dark conditions (Fig. 5c).
Effect of temperature on expression of pkhmgr and pkdxs, and picrosides content in fifth leaf of picrorhiza. Panel a shows gene expression, wherein 26S rRNA was used as a marker for equal loading. Panel b shows integrated density value (IDV) of each amplicon as obtained in panel a. Panel c shows change in picrosides content. Values in panel b and c are average of three separate biological replicates; error bar represents standard error of the mean
Effects of light and dark on expression of pkhmgr and pkdxs, and picrosides content in fifth leaf of picrorhiza plants (a, b, c) and plantlets raised through tissue culture (d, e, f). Panels a and d show gene expression, wherein 26S rRNA was used as a marker for equal loading. Panels b and e show integrated density value (IDV) of each amplicon as obtained in panels a and d. Panels c and f show changes in picrosides content. Values in panel b, c, e, and f are average of three separate biological replicates; error bar represents standard error of the mean
Dark conditions might starve the plants and it is likely that gene expression and picrosides content in dark could be a reflection of the effect of carbon limitation. Light would affect carbon pool through photosynthesis and the role of carbon pool in regulating secondary metabolites has been shown in Hypericum perforatum L. (Mosaleeyanon et al. 2005). Also, the possibility exists that light modulated gene expression independent of carbon pool (Fey et al. 2005). Hence, the experiment was carried out using in vitro raised plantlets with the medium fortified with sucrose (3%). In this system also, expression of pkhmgr and pkdxs was higher by 173% and 112% in light as compared to that recorded under dark (Fig. 5d, e). Also, the picrosides content in light was higher by 389% as compared to those in dark (Fig. 5f). Gene expression was in agreement with the promoter data wherein the motifs for light (e.g., GATA) and low temperature (e.g., LTRE) were present. EMSA also showed binding of the putative transcription factors to these motifs (Fig. 3).
The role of light and temperature in modulating a range of terpenoids and the corresponding transcripts has been documented, but there is no universal behavior and it varies depending upon the metabolite under study and the plant species. The same gene, hmgr for example, is stimulated by light in Triticum aestivum (Aoyagi et al. 1993), pea (Wong et al. 1982) and potato (Korth et al. 2000) but is downregulated by light in Lithospermum erythrorhizon (Lange et al. 1998). Effect of light on the activity of hmgr promoter has been reported that explained the light mediated alteration in hmgr transcripts (Learned and Connolly 1997). Gene dxs has also been reported to be regulated by light in Arabidopsis (Hsieh and Goodman 2005; Cordoba et al. 2009).
Soitamo et al. (2008) showed that light at low temperature induced expression of genes involved in synthesis of phenylpropanoids, carotenoids, and terpenoids. In Arabidopsis, light-dependent flavonoid (Fuglevand et al. 1996) and phenylpropanoid (Hemm et al. 2004) biosynthesis has been attributed to: (1) upregulation of relevant genes at transcriptional level, and (2) the involvement of the primary photoreceptors- phytochrome B and cryptochrome. Light-mediated accumulation of reducing equivalents in the stroma and reactive oxygen species has been reported to function as signals from chloroplasts to the nucleus leading to altered gene expression (Fey et al. 2005). Low temperature mediated increase in secondary metabolites in Arabidopsis has mainly been attributed to the transcriptional upregulation of genes of secondary metabolism, which in turn, has been suggested due to the over-expression of relevant transcription factors at low temperature (Hannah et al. 2005). It is also possible that upregulation of the two regulatory genes of the picrosides biosynthesis pathway by light and low temperature increased carbon partitioning towards terpenoid metabolism resulting in higher picrosides content in picrorhiza.
Our results that light and low temperature favor picrosides accumulation are in agreement with the picrosides levels in natural population of picrorhiza, wherein it has been reported that picrosides content increased by 135% in the plants growing at high (4,145 m) as compared to the low (1,350 m) altitude (Singh et al. 2005). Increase in altitude accompanies decrease in temperature and increase in light quanta (Streb et al. 1998). Molecular data on promoter analysis, gene expression, and picrosides content validated the importance of light and temperature in regulating picrosides content in picrorhiza.
Abbreviations
- aa:
-
amino acid
- DXS:
-
1-deoxy-D-xylulose-5-phosphate synthase
- EMSA:
-
electrophoretic mobility shift assay
- GDP:
-
geranyl diphosphate
- HMGR:
-
3-hydroxy-3-methylglutaryl coenzyme A reductase
- MEP:
-
methylerythritol phosphate
- MVA:
-
mevalonate
- PLACE:
-
plant cis-acting regulatory elements
References
Agarwal M, Hao Y, Kapoor A, Dong CH, Fujii H, Zheng X, Zhu JK (2006) A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J Biol Chem 28:37636–37645
Aoyagi K, Beyou A, Moon K, Fang L, Ulrich T (1993) Isolation and characterization of cDNAs encoding wheat 3-hydroxy-3-methylglutaryl coenzyme A reductase. Plant Physiol 102:623–628
Busk PK, Pages M (1997) Microextraction of nuclear proteins from single maize embryos. Plant Mol Biol Rep 15:371–376
Chahed K, Oudin A, Guivarc’h N, Hamdi S, Che’nieux J-H, Rideau M, Clastre M (2000) 1-Deoxy-d-xylulose 5-phosphate synthase from periwinkle: cDNA identification and induced gene expression in terpenoidindole alkaloid-producing cells. Plant Physiol Bioch 38:559–566
Chandra S (2004) Effect of altitude on energy exchange characteristics of some alpine medicinal crops from central Himalayas. J Agron Crop Sci 190:13–20
Chettri N, Sharma E, Lama SD (2005) Non-timber forest produces utilization, distribution and status in a trekking corridor of Sikkim, India. Lyonia 8:89–101
Choi H, Hong J, Ha J, Kang J, Kim SY (2000) ABRE/ACGT binding sites ABFs, a family of ABA-responsive element binding factors. J Biol Chem 275:1723–1730
Cordoba E, Salmi M, León P (2009) Unravelling the regulatory mechanisms that modulate the MEP pathway in higher plants. J Exp Bot 60:2933–2943
Croteau RB, Davis EM, Ringer KL, Wildung MR (2005) (-)-Menthol biosynthesis and molecular genetics. Naturwissenschaften 92:562–577
Degenhardt J, Tobin EM (1996) A DNA binding activity for one of two closely defined phytochrome regulatory elements in an Lhcb promoter is more abundant in etiolated than in green plants. Plant Cell 8:31–41
Doyle JJ, Doyle JL (1987) A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19:11–15
Dunn MA, White AJ, Vural S, Hughes MA (1998) Identification of promoter elements in a low-temperature-responsive gene (blt4.9) from barley (Hordeum vulgare L.). Plant Mol Biol 38:551–564
Dutt S, Kiddle G, Singh B, Khambay B, Foyer CH (2004) Differential accumulation of picrosides in Picrorhiza kurrooa Royle ex Benth plants. http://www.rothamsted-international.org/publications_posters.shtml
Estevez JM, Cantero A, Reindl A, Reichler S, Leon P (2001) 1-Deoxy-D-xylulose-5-phosphate synthase, a limiting enzyme for plastid isoprenoid biosynthesis in plants. J Biol Chem 25:22901–22909
Evans T, Reitman M, Felsenfeld G (1988) An erythrocyte specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci USA 85:5976–5980
Fey V, Wagner R, Brautigam K, Wirtz M, Hell R, Dietzmann A, Leister D, Oelmüller R, Pfannschmidt T (2005) Retrograde plastid redox signals in the expression of nuclear genes for chloroplast proteins of Arabidopsis thaliana. J Biol Chem 280:5318–5328
Floersheim GL, Bieri A, Koenig R, Pletscher A (1990) Protection against Amanita phalloides by the iridoid glycoside mixture of Picrorhiza kurrooa (kutkin). Agents Actions 29:386–387
Fuglevand G, Jackson JA, Jenkins GI (1996) UV-B, UV-A, and blue light signal transduction pathways interact synergistically to regulate chalcone synthase gene expression in Arabidopsis. Plant Cell 8:2347–2357
Ghawana S, Singh K, Raizada J, Rani A, Bhardwaj PK, Kumar S (2007) A method for rapid isolation of RNA and kit thereof. WO 2007/113614
Gilmartin PM, Sarokin L, Memelink J, Chua NH (1990) Molecular light switches for plant genes. Plant Cell 2:369–378
Green PJ, Yong MH, Cuouo M, Kano-Murakami Y, Silverstein P, Chua NH (1988) Binding site requirements for the pea GT-1 correlate with sequences required for light dependent transcriptional activation of the rbcS-3A gene. EMBO J 7:4035–4044
Hampel D, Mosandl A, Wiist M (2005) Biosynthesis of mono- and sesquiterpenes in carrot roots and leaves (Daucus carota L.): metabolic cross talk of cytosolic mevalonate and plastidial methylerythritol phosphate pathways. Phytochemistry 66:305–311
Hampel D, Mosandl A, Wiist M (2006) Biosynthesis of mono- and sesquiterpenes in strawberry fruits and foliage: 2H labeling studies. J Agric Food Chem 54:1473–1478
Hannah MA, Heyer AG, Hincha DK (2005) A global survey of gene regulation during cold acclimation in Arabidopsis thaliana. PLoS Genet 1:e26s
Hemm MR, Rider SD, Ogas J, Murry DJ, Chapple C (2004) Light induces phenylpropanoid metabolism in Arabidopsis roots. Plant J 38:765–778
Hennighausen L, Lubon H (1985) Interaction of protein with DNA in vitro. Method Enzymol 152:721–735
Higo K, Ugawa Y, Iwamoto M, Korenaga T (1999) Plant cis-acting regulatory DNA elements (PLACE) database. Nucleic Acids Res 27:297–300
Hsieh MH, Goodman HM (2005) The arabidopsis IspH homolog is involved in the plastid nonmevalonate pathway of isoprenoid biosynthesis. Plant Physiol 138:641–653
Hudson ME, Quail PH (2003) Identification of promoter motifs involved in the network of phytochrome A-regulated gene expression by combined analysis of genomic sequence and microarray data. Plant Physiol 133:1605–1616
Juan ME, Araceli C, Andreas R, Stuart R, Patricia L (2001) 1-Deoxy-D-xylulose-5-phosphate synthase, a limiting enzyme for plastidic isoprenoid biosynthesis in plants. J Biol Chem 276:22901–22909
Klok EJ, Wilson IW, Wilson D, Chapman SC, Ewing RM, Somerville SC, Peacock WJ, Dolferus R, Dennis ES (2002) Expression profile analysis of the low-oxygen response in Arabidopsis root cultures. Plant Cell 14:2481–2494
Korth KL, Jaggard DAW, Dixon RA (2000) Developmental and light-regulated post-translational control of 3-hydroxy-3-methylglutaryl-CoA reductase levels in potato. Plant J 23:507–516
Lange BM, Severin K, Bechthold A, Heide L (1998) Regulatory role of microsomal 3-hydroxy-3-methylglutaryl-coenzyme A reductase for shikonin biosynthesis in Lithospermum erythrorhizon cell suspension cultures. Planta 204:234–241
Learned RM, Connolly EL (1997) Light modulates the spatial patterns of 3-hydroxy-3-methylglutaryl coenzyme A reductase gene expression in Arabidopsis thaliana. Plant J 11:499–511
Leivar P, Gonzalez VM, Castel S, Trelease RN, Lesias C, Arro M, Boronat A, Campos N, Ferrer A, Busquets X (2005) Subcellular localization of arabidopsis 3-hydroxy-3-methylglutaryl-coenzyme a reductase. Plant Physiol 137:57–69
Lois LM, Rodriguez-Concepcion M, Gallego F, Campos N, Boronat A (2000) Carotenoid biosynthesis during tomato fruit development: regulatory role of 1-deoxy-D-xylulose 5-phosphate synthase. Plant J 22:503–513
Luper S (1998) A review of plants used in the treatment of liver disease: part 1. Altern Med Rev 3:410–421
Mahmoud SS, Croteau RB (2002) Strategies for transgenic manipulation of monoterpene biosynthesis in plants. Trends Plant Sci 7:366–373
Mosaleeyanon K, Zobayed SMA, Afreen F, Kozai T (2005) Relationships between net photosynthetic rate and secondary metabolite contents in St. John’s wort. Plant Sci 169:523–531
Munoz-Bertomeu J, Arrillaga I, Ros R, Segura J (2006) Up-regulation of 1-deoxy-D-xylulose-5-phosphate synthase enhances production of essential oils in transgenic spike lavender. Plant Physiol 142:890–900
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue culture. Physiol Plant 15:473–497
Nayar MP, Sastri ARK (1990) Red data plants of India. CSIR Publication, New Delhi, p 271
Nogués I, Brilli F, Loreto F (2006) Dimethylallyl diphosphate and geranyl diphosphate pools of plant species characterized by different isoprenoid emissions. Plant Physiol 141:721–730
Park HC, Kim ML, Kang YH, Jeon JM, Yoo JH, Kim MC, Park CY, Jeong JC, Moon BC, Lee JH, Yoon HW, Lee SH, Chung WS, Lim CO, Lee SY, Hong JC, Cho MJ (2004) Pathogen- and NaCl-induced expression of the SCaM-4 promoter is mediated in part by a GT-1 box that interacts with a GT-1-like transcription factor. Plant Physiol 135:2150–2161
Reyes JC, Muro-Pastor MI, Florencio FJ (2004) The GATA family of transcription factors in Arabidopsis and rice. Plant Physiol 134:1718–1732
Sacchettini JC, Poulter CD (1997) Creating isoprenoid diversity. Science 277:1788–1789
Singh K, Raizada J, Bhardwaj P, Ghawana S, Rani A, Singh H, Kaul K, Kumar S (2004) 26S rRNA-based internal control gene primer pair for reverse transcription-polymerase chain reaction-based quantitative expression studies in diverse plant species. Anal Biochem 335:330–333
Singh N, Gupta AP, Singh B, Kaul VK (2005) Quantification of picroside-I and picroside-II in Picrorhiza kurrooa by HPTLC. J Liq Chromatogr & Rel Technol 28:1679–1691
Soitamo AJ, Piippo M, Allahverdiyeva Y, Battchikova N, Aro EM (2008) Light has a specific role in modulating Arabidopsis gene expression at low temperature. BMC Plant Biol 8:13
Streb P, Shang W, Feierabend J, Bligny R (1998) Divergent strategies of photoprotection in high-mountain plants. Planta 207:313–324
Teakle GR, Manfield IW, Graham JF, Philip M, Gilmartin PM (2002) Arabidopsis thaliana GATA factors: organisation, expression and DNA-binding characteristics. Plant Mol Biol 50:43–57
Terzaghi WB, Cashmore AR (1995) Light-regulated transcription. Annu Rev Plant Physiol Plant Mol Biol 46:445–474
Tjaden G, Edwards JW, Coruzzi GM (1995) Cis elements and trans-acting factors affecting regulation of a non-photosynthetic light-regulated gene for chloroplast glutamine synthetase. Plant Physiol 108:1109–1117
Udvardi MK, Kakar K, Wandrey M, Montanari O, Murray J, Andriankaja A, Zhang JY, Benedito V, Hofer JM, Chueng F, Town CD (2007) Legume transcription factors: global regulators of plant development and response to the environment. Plant Physiol 144:538–549
Villain P, Mache R, Zhou DX (1996) The mechanism of GT element-mediated cell type-specific transcriptional control. J Biol Chem 271:32593–32598
Walter MH, Hans J, Strack D (2002) Two distantly related genes encoding 1-deoxy-d-xylulose 5-phosphate synthases: differential regulation in shoots and apocarotenoid-accumulating mycorrhizal roots. Plant J 31:243–254
Wang Y, Guo B, Zhang F, Yao H, Miao Z, Tang K (2007) Molecular cloning and functional analysis of the gene encoding 3-hydroxy-3-methylglutaryl coenzyme A reductase from hazel (Corylus avellana L. Gasaway). J Biochem Mol Biol 40:861–869
Wise ML, Croteau R (1998) Comprehensive natural products chemistry: isoprenoid biosynthesis. In: Cane DE (ed) Pergamon, Oxford
Wong RJ, McCormack DK, Russell DW (1982) Plastid 3-hydroxy-3-methylglutaryl coenzyme A reductase has distinctive kinetic and regulatory features: properties of the enzyme and positive phytochrome control of activity in pea seedlings. Arch Biochem Biophys 216:631–638
Yang Z, Park H, Lacy GH, Cramer CL (1991) Differential activation of potato 3-hydroxy-3-methylglutaryl coenzyme A reductase genes by wounding and pathogen challenge. Plant Cell 3:397–405
Acknowledgments
We acknowledge financial support from Council of Scientific and Industrial Research (CSIR) for work on gene cloning through Mission Mode program entitled “Development of medicinal plant chemotypes for enhanced marker and value added compounds (COR002)”. Financial support from the Department of Biotechnology (DBT), Govt. of India is duly acknowledged for funding the project entitled “Molecular cloning and characterization of regulatory genes involved in picrosides metabolism in Picrorhiza kurrooa” sanctioned vide sanction order number BT/PR6614/PBD/17/425/2005 to work on promoters. TK thanks CSIR, India, for awarding CSIR Diamond Jubilee Research Intern Award; HS thanks CSIR for awarding junior and senior research fellowships. AK, KD, and SKS gratefully acknowledge DBT for providing assistantship. Technical help provided by Mr. Digvijay Singh in gene sequencing is duly acknowledged. The manuscript represents IHBT communication number 0812.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Electronic Supplementary Table S1
Comparison of predicted secondary structures of HMGR and DXS of picrorhiza with those from other plants. Protein sequences were retrieved from the NCBI databank and secondary structural prediction was carried out using ExPASy server tool SOPMA (http://www.npsa-pbil.ibcp.fr/) by keeping similarity threshold 8 and conformational states 4. (DOC 74 kb)
Electronic Supplementary Fig. S1
Multiple sequence alignment of deduced amino acids encoded by pkhmgr (3-hydroxy-3-methylglutaryl coenzyme A reductase; S1 a) and pkdxs (1-deoxy-D-xylulose-5-phosphate synthase; S1 b) of P. kurrooa. Alignment was performed by Accelrys software tool (Accelrys Gene software, version 2.5 of Accelrys software Inc., USA). Sequences denoted are: P. kurrooa -PK (ABC74565), Nicotiana tabacum -NT (AAB87727), Eucommia ulmoides -EU (AAV54051), Andrographis paniculata -AP (AAP14352), Litchi chinensis -LC (ABF56181), Mentha x piperita -MP (AAC33513), Antirrhinum majus -AM (AAW28999), Tagetes erecta -TE (AAG10432), Catharanthus roseus -CR (CAA09804). Accession number of the deduced amino acid sequences is shown in the respective parenthesis. (PPT 394 kb)
Electronic Supplementary Fig. S2
Conserved domains of deduced proteins pkHMGR (3-hydroxy-3-methylglutaryl coenzyme A reductase) and pkDXS (1-deoxy-D-xylulose-5-phosphate synthase) of picrorhiza. Deduced amino acid sequences were analyzed for the location of conserved domains using Conserved Domain Database available at NCBI (http://www.ncbi.nlm.nih.gov/ Structure/cdd/wrpsb.cgi). (PPT 39 kb)
Electronic Supplementary Fig. S3
Predicted secondary structure of deduced proteins, pkHMGR (3-hydroxy-3-methylglutaryl coenzyme A reductase) and pkDXS (1-deoxy-D-xylulose-5-phosphate synthase) using ExPASy server tool, SOPMA (http://www.npsa-pbil.ibcp.fr/) by keeping similarity threshold 8 and conformational states 4. Helices, sheets, turns, and coils are indicated with the longest, the second longest, the second shortest and the shortest vertical lines, respectively. (PPT 104 kb)
Rights and permissions
About this article
Cite this article
Kawoosa, T., Singh, H., Kumar, A. et al. Light and temperature regulated terpene biosynthesis: hepatoprotective monoterpene picroside accumulation in Picrorhiza kurrooa . Funct Integr Genomics 10, 393–404 (2010). https://doi.org/10.1007/s10142-009-0152-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10142-009-0152-9