Abstract
Background and aims
Human activities have increased the input of nitrogen (N) and phosphorus (P) into terrestrial ecosystems since the industrial revolution. These activities are expected to increase aboveground biomass (AGB) and further affect plants and soil microbial communities. Plant–microbe interactions play a significant role in shaping microbial communities. However, how soil microbial community respond to change in plant communities after N and P addition remains unclear, particularly in temperate steppe ecosystems.
Methods
A 12-year factorial combination experiment of N and P addition was conducted in a temperate steppe ecosystem to evaluate soil microbiomes in relation to plant communities and soil characteristics.
Results
Long-term N addition shifted the dominance of plant community from multiple species to sole dominance by Leymus chinensis. N addition did not significantly affect microbial α-diversity. However, P addition led to significantly increased bacterial richness, while NP addition led to significantly decreased arbuscular mycorrhizal fungal richness. Structural equation modeling indicated that available phosphorous (AP) significantly affected bacterial richness, while AP, dissolved inorganic nitrogen (DIN), and AGB significantly influenced arbuscular mycorrhizal fungal richness. Nutrient addition also significantly altered soil microbial community structures that can largely be explained by AGB and plant community compositions. Finally, network analysis revealed strong correlations between plant functional groups and dominant microbial taxa.
Conclusions
Microbial communities can be influenced by both N and P addition-induced changes in soil properties and plant communities. The significant associations between plant functional groups and dominant microbial taxa emphasize the important roles of plant-mediated effects on microbial communities after N and P addition.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The longstanding maxim in environmental microbiology that “everything is everywhere but the environment selects” (Baas Becking 1934) has been used to describe the global distributions of microorganisms. The concept suggests that although microorganisms are widely distributed globally (including in extreme environments) and possess exceptional diversity, substantial variation is observed in their spatial microbial diversity patterns that is primarily driven by environment heterogeneity (Fuhrman 2009; Green and Bohannan 2006; Pan et al. 2020; Shu and Huang 2022). Variation in climatic conditions (e.g., temperature and precipitation) among different regions occurs due to geographically variable parameters like latitude and altitude, resulting in biogeographical variation of microorganisms (Peay et al. 2017). Furthermore, the continuous impact of land use changes and global climate change caused by human activities on regional environments have been identified as key drivers underlying changes in soil microbial diversity. Intensification of human activities has not only caused global climate warming but also significantly impacted nitrogen (N) cycling within ecosystems (Peñuelas et al. 2013; Wen et al. 2022). Unprecedented input of N to ecosystems has occurred due to human activities, although minimal changes in phosphorus (P) deposition have been observed (Wen et al. 2022). This disproportional anthropogenic input of N relative to P can cause substantial changes in N and P balances, such that continual anthropogenic P input is needed to sustain stable ecosystem productivity (Fleischer et al. 2019; Peñuelas et al. 2013; Vitousek et al. 2010). These changes have significantly impacted grassland ecosystems that cover 40% of the global land area and particularly biodiversity.
The importance of bacteria and fungi in terrestrial soil habitats is evident in their control of biodiversity and their regulation of diverse critical soil biogeochemical processes (Bahram et al. 2018). Much research attention has been given toward understanding the impacts of N and P additions on soil microbial communities, but these studies have yielded mixed results. In addition to climatic factors, soil pH is a significant determinant of soil microbial community composition and functioning at global and regional scales (Fierer and Jackson 2006; Liu et al. 2020; Peay et al. 2017). Most bacteria generally exhibit optimal growth at neutral pH (Fierer and Jackson 2006; Zhou et al. 2020), and N-induced soil acidification often leads to reduced microbial diversity (Dai et al. 2018; Yang et al. 2021). However, some studies have suggested that bacterial diversity may not significantly decline until N additions reach a certain critical threshold (Liu et al. 2020). Soil fungi exhibit lower sensitivity to pH changes induced by N enrichment, and fungal diversity exhibits lesser responses to N addition (Wang et al. 2023). Moreover, increased soil nutrient effectiveness resulting from the addition of N and P may influence microbial community composition at the local scale (Kaspari et al. 2017; Philippot et al. 2013b; Yang et al. 2021; Zhu et al. 2023). These processes occur because changes in nutrient effectiveness affect the biodiversity of microorganisms with different life history strategies (Li et al. 2023b; Philippot et al. 2013a), thereby further impacting potential multi-trophic interactions among soil microbial communities (Zhu et al. 2023).
N and P are critical for increasing aboveground biomass in temperate grasslands. Their application consequently typically exerts diverse influences on biodiversity and species composition (Ling et al. 2017; Liu et al. 2021; Yang et al. 2015). Numerous studies of the impact of N addition on plant community diversity have yielded mixed results (Liu et al. 2020, 2021), but the responses of grassland ecosystems to P addition are poorly understood (van Dobben et al. 2017). Moreover, significant changes in grassland vegetation communities following nutrient addition should be accompanied by changes in associated soil microbial communities due to the strong interactions among plants, soil characteristics, and microorganisms. Plants primarily regulate the growth and activity of microbial communities through rhizosphere effects (i.e., influences of root exudates on microbial communities) and plant immune system functioning that shape specific soil microbial community structures (Bai et al. 2022; Trivedi et al. 2022). For example, increased input of root exudates with labile substrates into soils often induces rapid growth of microorganisms with higher metabolic efficiencies (Feng and Wang 2023; Su et al. 2023). In addition, the amount and quality of plant litter input into soils varies depending on the composition and functional traits of different plant communities (Chu et al. 2011; Yang et al. 2018). Fungi decompose more complex organic materials and the ratio of Fungi to Bacteria changes depending on plant litter input (Yao et al. 2018b). Plants subsequently shape soil microbial community compositions through competition for nutrients (especially N) (Feng and Wang 2023) and modification of abiotic soil environments (Guo et al. 2018).
The complex interactions between plants and microorganisms play a significant role in shaping soil microbial communities (Peay et al. 2017; Yang et al. 2018), yet the roles of plants are seldom considered when investigating the factors influencing the structures of grassland soil microbial communities (Liu et al. 2021). This is especially evident when evaluating significant changes in plant community composition after long-term nutrient additions (Chalcraft et al. 2008). Consequently, it is imperative to simultaneously consider both soil and plant factors to comprehensively understand the mechanisms by which nitrogen and phosphorus additions affect soil microbial communities. Here, the responses of plant and soil microbial (bacteria, total fungi, and arbuscular mycorrhizal fungi (AMF)) communities were evaluated after long-term N and P additions. The distinct response mechanisms of plant and soil microbial communities to N and P input were also investigated at the level of diversity changes. Specifically, we hypothesized that (1) higher nutrient availability induced by long-term addition of N and P alters plant diversity and community composition by changing the biomass of specific plant functional groups, and (2) microbial diversity and community composition are strongly influenced by soil properties (e.g., nutrient availability and pH) and plant community characteristics after long-term N and P addition.
Materials and methods
Study site
The study was conducted in a typical area of the Stipa Baicalansis steppe, within Hulunbeier of Inner Mongolia, China (119°42′ E and 48°30′ N). The area experiences a climate characterized by warm summers and cold winters that are influenced by a temperate continental monsoon climate. The mean annual precipitation and mean annual temperature are 329 mm and − 0.7 °C, respectively. The natural vegetation in the area comprises grasses dominated by species such as S. baicalensis and Leymus chinensi, and common species including Achnatherum sibiricum, Filifolium sibiricum, Cleistogenes squarrosa, Carex duriuscula, Astragalus melilotoides, Thaictrum petaloideum, and Klasea centauroides (Yu et al. 2015).
Sixteen 8 m × 8 m plots (4 replicates × 4 treatments) were initiated in the summer of 2010 for a full factorial N and P addition experiment, with the inclusion of a 2 m buffer strip between the plots. Nitrogen (as urea) and P (as triple superphosphate) were each fertilized yearly at a rate of 100 kg ha–1 year–1 either alone or together, consistent with the standard protocols of the Nutrient Network (Borer et al. 2014). Unfertilized plots served as controls. Four treatments were included overall, including the control, N fertilization (N), P fertilization (P), and N plus P fertilization (NP).
Plant and soil sampling and analyses
The aboveground vegetation was collected in mid-August 2021 when annual productivity peaked, and the species in each plot were enumerated using a quadrat measuring 1 m by 1 m. The collected plants were then dried at 65 °C for 48 h and weighed to estimate the aboveground plant biomass in each plot. Following live plant collection, 10 soil cores (3.5 cm diameter) were randomly collected at depths between 0 and 20 cm using a drill Then 10 cores were thoroughly mixed to construct a single soil sample for each plot, and every sample was sieved using a mesh size of 2 mm. The composite samples were then split into two portions, with one used for measuring soil physicochemical properties by natural drying and the second portion stored at − 20 °C for subsequent DNA extraction and microbial community analyses. Soil pH, organic carbon, total N, total P, inorganic N (NH4+–N and NO3––N), and available P were measured according to previously described methods (Bao 2000). The physicochemical measurements are shown in Table S2.
Soil microbial biomass
Phospholipid fatty acid (PLFA) profiles were analyzed by extracting the biomass of soil microbial functional groups from 3 g of freeze-dried soil samples, following methods described Zhang et al. (2019). PLFAs including 15:0iso, 15:0anteiso, 15:1isoω6c, 16:0iso, 17:0iso, and 17:0anteiso were used to calculate the biomass of Gram-positive (G +) bacteria, and the PLFAs 16:1ω7c, 16:1ω9c, 17:1ω8c, 18:1ω5c, 18:1ω7c, 21:1ω3c, 17:0cycloω7c, and 19:0cycloω7c were used to calculate the biomass of Gram-negative (G-) bacteria, as previously described (Yang et al. 2022b). The PLFA 16:1ω5 was used to calculate biomass of AMF biomass, and the PLFAs 18:1ω9c and 18:2ω6c were used to calculate fungal biomass, as previously described (Liang et al. 2016). The PLFAs 10Me16:0, 10Me17:0, 10MeC17:1ω7c, 10Me18:0, and 10MeC18:1ω7c were used to calculate actinomycete biomass (Li et al. 2023b). The PLFAs 14:0, 15:0, 15:0DMA, 16:0, 17:0, 18:0, and 20:0 were used to calculate the biomass of non-specific bacteria (Yang et al. 2022b). Total bacterial biomass was calculated by summing the biomass estimates of G + , G–, and non-specific bacteria. In addition, ratios of G + to G–, as well as fungi to bacteria, were calculated from their respective biomass levels (Yang et al. 2022b). PLFA concentrations were expressed as nmol g−1 dry soil.
DNA extraction and amplicon sequencing
DNA was extracted from soil samples using the FastDNA™ Spin Kit for Soil (116,560–200, MP Biomedicals, USA). DNA concentrations were determined using a NanoDrop 2000 UV–vis spectrophotometer (Thermo Scientific, Wilmington, USA), and DNA quality was examined by electrophoresis using a 1% agarose gel. The bacterial V3–V4 hypervariable regions of 16S rRNA genes were amplified using the primer pair 338F (5′-ACTCCTACGGGAGG CAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′), while fungal ITS1 genes were amplified using the primer pair ITS1F (5′-CTTGGTCATTTA GAGGAAGTAA-3′) and ITS2R (5′-GCTGCGTTCTTCATCGATGC-3′) (Chen et al. 2021). The V4–V5 hypervariable regions of fungal 18S rRNA genes were amplified using the primer pair AMV4.5NF (5′-AAGCTCGTAGTTGAATTTCG-3′) and AMDGR (5′-CCCAACTAT CCCTATTAATCAT-3′) (Ma et al. 2022). Detailed information of the PCR protocols and subsequent bioinformatics analysis are provided in the Supplementary material (Appendix A). The sequencing data generated in this study were submitted to the NCBI database under the accession numbers PRJNA928583, PRJNA1057071, and PRJNA1057077.
Network analysis
Ecological network analysis has been widely used to identify associations among taxa within microbial communities (Ma et al. 2022). Likewise, the exploration of interactions between plants and microorganisms using ecological networks has been increasingly used in recent years. Changes in soil properties resulting from the addition of N and P might modulate plant–microbiota interactions (in 't Zandt et al 2023).
Network analyses were conducted as follows. Prior to constructing the networks, AMF sequences (i.e., OTUs classified as Glomeromycota) were excluded from the fungal ITS representative sequence dataset (Ma et al. 2022). Bacterial genera, fungal genera, and AMF genera with relative abundances ≤ 0.05% were also removed. The plant community data were then filtered to retain species in over six sampling plots, considering the homogeneity of plant communities across the four nutrient addition treatments. Pearson correlation coefficients were calculated using the “corr.test” function of the R psych package to evaluate the significance of correlations among soil properties, microbial genera, and plant species. Correlations meeting the criteria of |R|≥ 0.8 and p < 0.05 were retained. The Cytoscape software program (version 3.9.1) was used to visualize the network.
Data analysis
Analysis of variance (ANOVA) tests were used to analyze differences in the distributions of soil, plant, and microbial variables. Tukey’s honest significant difference (HSD) tests were used to evaluate differences between different nutrient addition treatments. Principal coordinate analysis (PCoA) and PERMANOVA were conducted using the “vegan” package for R. The first two principal component axes were extracted to evaluate as variables related to plant composition. Moreover, linear and quadratic models were applied to evaluate the relationship direction between soil variables (DIN, pH, and AP) with plant and microbial diversity. In addition, a variance partitioning analysis (VPA) was conducted to assess the relative impacts of vegetation and soil characteristics on the composition of bacterial, fungal, and AMF communities. Mantel path analysis was conducted using the “ecodist” package that quantifies the direct and indirect effects of soil chemical properties and plant community composition on microbial community composition (Liu et al. 2020; Zhou et al. 2022). Models were developed from the measurements and evaluated using structural equation modeling (SEM) using regression analysis and previous insights to identify the effects of soil chemical properties, plant productivity, and plant richness on microbial richness after nutrient addition. The complete model including the hypothetical relationships considered a priori is shown in Fig. S1. The a priori model was constructed according to results from previous studies (Bai et al. 2010; Leff et al. 2015; Li et al. 2023b; Ma et al. 2021; Wang et al. 2023; Xia et al. 2023). Model adequacy was determined using the chi-square (χ2) test, the comparative fit index (CFI), and the root-mean-square error of approximation (RMSEA). The final model was improved by discarding non-significant pathways progressively from the a prior model based on the above indices. The SEM analysis was performed using the AMOS 22.0 software program (AMOS Development Corporation).
Results
Effects of N and P addition on plant aboveground biomass and microbial biomass
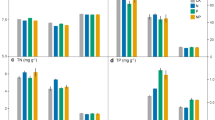
The biomass of plant and grass communities significantly increased after N and NP addition treatments when compared with the control, but there was no significant difference between the P addition treatment and the control (Fig. 1a, b; Table S3). In comparison with the control, plant community biomass was enhanced by 124.08% and 403.85% under N and NP addition treatments, respectively. Plant community biomass was closely related to grass biomass (Fig. S2a; R2 = 0.97, p < 0.001), and grass biomass contributed an average of 45.81–94.64% to the plant community biomass in different treatments. However, the N and NP addition treatments resulted in reduced legume and forb biomass. Furthermore, P addition treatment led to increased biomass of legumes (87.81%) and forbs (78.46%) (Fig. 1c, d). In addition, a negative correlation was observed between plant community biomass and that of legumes (Fig. S2b; R2 = 0.29, p < 0.05).
Effects of N and P addition on plant aboveground biomass. Biomass is shown for (a) plant communities, b grasses, c legumes, and (d) forbs. Error bars show standard deviations (n = 4). Different lowercase letters above each bar in the same panel indicate statistically significant differences between different treatments (Tukey’s HSD test, p < 0.05)
Bacterial biomass, fungal biomass, and total microbial biomass increased significantly after N addition compared with the control, but did not change in the P addition relative to the control (Fig. 2; Table S3). The addition of N and NP also resulted in significantly lower total microbial biomass compared with the addition of P (Fig. 2). Furthermore, the addition of P had a significant positive effect on AMF biomass (Fig. 2c). However, when N was added together with P, AMF biomass was significantly lowered (Fig. 2c). All nutrient addition treatments did not alter the G + /G– ratio, and the NP addition treatment led to a decreased fungi: bacteria ratio (Fig. 2i).
The effects of N and P addition on microbial biomass (a–g), the G + /G– PLFA ratio (h), and the F/B PLFA ratio (i). Error bars indicate standard deviations (n = 4). Different lowercase letters above each bar in the same panel indicate statistically significant differences between treatments (Tukey’s HSD test, p < 0.05)
Effects of N and P addition on plant and soil microorganism α-diversity and community composition
The addition of N and NP significantly decreased plant Shannon and richness indices (Fig. 3a; Table S3), but did not impact microbial diversity, including that of bacterial, fungal, and AMF communities alone after N addition (Fig. 3b, c, d; Table S3). NP addition also led to a significantly increased bacterial community Shannon index, while P addition alone significantly increased bacterial richness (Fig. 3b). In comparison, the Shannon and richness indices of fungal and AMF communities did not exhibit a significant response to N and P addition but did exhibit such a response to NP addition wherein AMF communities exhibited significantly reduced richness (Fig. 3d; p < 0.05). PERMANOVA tests revealed that all nutrient addition treatments (N, P, and NP) altered plant and soil microbial (bacteria, AMF, and fungi) community structures, with the exception of non-significant changes in AMF communities after P addition (Fig. 4; Table S4).
The effects of N and P addition on plant and soil microbial diversity. a Plant, b bacterial, c fungal, and (d) AMF diversity levels are shown. Species richness is represented by the Patrick index for plants and the Chao1 index for microbial communities. The box plots show the medians as solid line within boxes and means as hollow diamond within boxes, in addition to the 25th and 75th percentiles as vertical bars. The upper and lower whiskers denote the maximum and minimum values, respectively. Different lowercase letters above each box in the same panel indicate statistically significant differences between treatments (Tukey’s HSD test, p < 0.05)
Beta diversity (community composition) of plant (a), soil bacterial (b), soil fungal (c), and soil AMF communities (d) in response to N, P, and NP addition treatments. Community structural differences were assessed by principal coordinates analysis (PCoA) based on Bray–Curtis dissimilarity distances. The effects of N, P, and NP addition treatments on community structures were assessed using permutational analysis of variance (PERMANOVA) tests. Asterisks (*, **, and ***) following r2 values indicate statistical significance at p ≤ 0.05, ≤ 0.01, and ≤ 0.001, respectively
Association of soil microbial diversity and community structures with plant communities and soil properties
The relationships among microbial genera, plants, and soil properties were evaluated (Fig. 5; Table S6). Soil pH was the largest node (degree: 20) among analyzed soil properties and exhibited a significantly negative correlation with multiple bacterial genera, most of which have been identified as acidophilic taxa. L. chinensis was the largest node (degree: 10) among all plant species, with half of the genera exhibiting significant correlations being Proteobacteria, and all of them exhibiting a positive correlation with L. chinensis presence. Overall, positive correlations (85.15%) dominated the network of associations among microbial genera, plants, and soil properties.
Network analysis describing the relationships among the distribution of microbial (bacterial, non-mycorrhizal fungal and AMF) genera, plants species, and soil properties. The overall network was visualized significant associations (R2 > 0.80, p < 0.05) of AMF, bacterial, and non-mycorrhizal fungal operational taxonomic units (genera), plant species, and soil properties. Each node represents a microbial genus or soil property, and the color indicates different microbial taxa, while the node size is proportional to node connectivity. Red lines indicate positive correlations and purple lines indicate negative correlations
The contributions of soil properties and vegetation variables to variation in bacterial, fungal, and AMF community composition were quantified by Mantel and partial-Mantel tests (Fig. 6a). Mantel analysis indicated that AGB exerted the most significant influence on plant (rM = 0.778, p = 0.001), fungal (rM = 0.547, p = 0.001), and AMF (rM = 0.498, p = 0.001) communities. Soil pH (rM = 0.770, p = 0.001) was the best predictor of bacterial community composition. In addition, bacterial and fungal community composition were significantly associated with plant community composition after accounting for AGB and all soil properties. VPA demonstrated that the amount of variation captured by environmental and vegetation variables was 46.9%, 34.9%, and 21.6% for bacterial, fungal and AMF communities, respectively (Fig. 6b). Interactions between soil properties and vegetation variables were significantly associated with bacterial and fungal communities, but not AMF communities (Fig. 6b). In addition, a higher percentage of vegetation (9.39%) than environmental factors (5.42%) explained fungal community variation, while vegetation factors (10.38% and 10.10%) were equally as important as soil properties (11.39% and 11.43%) for bacterial and AMF communities, respectively.
The effects of environmental factors and plant properties on microbial richness and community structure after nutrient addition. a Mantel path analysis linking taxonomic composition of microbial (bacterial, fungal, and AMF) communities to soil chemical attributes and plant communities. b VPA was used to identify the relative contributions of soil factors and vegetation properties to microbial (bacterial, fungal, and AMF) communities. c Structural equation modeling (SEM) analysis of the effects of N and P addition on soil microbial (bacterial, fungal, and AMF) richness via pathways related to soil chemical attributes and plant richness. Red and blue solid arrows connecting the boxes represent significant positive and negative effects ((p < < 0.05), respectively. Pathways without a significant effect are indicated by broken lines (p > 0.05). Values close to variables refer to the variance accounted for by the model (as R2 values). Values associated with the arrows represent standardized path coefficients
SEM demonstrated that changes in plant and microbial richness caused by N and/or P additions were primarily attributed to changes in nutrient availability and aboveground biomass (Fig. 6c). AGB significantly increased with nutrient availability. Variation in AMF richness was roughly equally associated with AP, DIN and AGB. The addition of P positively influenced bacterial richness and led to increased soil P availability. In addition, soil fungal richness was primarily associated with DIN.
Discussion
The effect of N and P addition on plant biomass, diversity, and community structures
Here, N and P addition were shown to shape plant diversity and community structure by leading to increased biomass of specific plant functional groups, consistent with our hypothesis. Long-term N and NP addition over 11 years led to enhanced plant aboveground biomass via increased biomass of grasses like L. chinensis, but decreased plant species diversity, leading to reduced biomass of legumes and forbs (Fig. 1; Table S1). These results are consistent with multiple previous studies of temperate steppe ecosystems (Bai et al. 2010; Zhao et al. 2019). Mechanisms underlying the loss of plant diversity induced by N addition have been widely investigated and include soil acidification (Horswill et al. 2008), interspecific differences in resource-use strategies, and shoot competition for light among different plant species (Borer et al. 2014; He et al. 2016; Li et al. 2023a). In a previous experiment conducted in the same grassland that involved N and P addition, N addition over three years led to significantly increased biomass of multiple grasses (e.g., L. chinensis and S. baicalensis), while the responses of other species to N addition were mixed (Yu et al. 2015). In this study, the biomass of L. chinensis accounted for over 80% of the total aboveground biomass after 11 years of N addition, while many legume and forb species even disappeared (Table S1). Long-term nutrient addition shifted the dominance of the plant community by several plants to single dominance by L. chinensis. L. chinensis can quickly absorb added N due to its strong root system and high plasticity of N acquisition strategies (Li et al. 2023a) that accelerates the growth of L. chinensis during the early stages of N addition (Zhao et al. 2019). However, the addition of N alleviates nutrient limitation in grassland soils, potentially shifting the limiting factors for plant growth to other resources (e.g., mineral P and light). Consequently, L. chinensis was the dominant canopy species within plant communities (Li et al. 2023a) and exhibited significantly increased biomass and cover due to long-term N addition that reduced light availability for low-lying plants and resulted in further decreases in species diversity (Borer et al. 2014; Hautier et al. 2009; Suding et al. 2005). Moreover, decreased plant species richness may be attributed to acidification induced in the early stages of N addition (Horswill et al. 2008).
The addition of P in this study did not affect grass biomass. A possible reason for this could be that N is the primary limiting nutrient in temperate steppe environments and adding P alone minimally affects grass species, especially L. chinensis. P addition also largely increased legume and forb biomass. This effect can be attributed to the improved availability of P resources and enhanced nutrient use efficiencies in legumes and forbs, thereby indirectly increasing their competitive advantage over grasses species (Bi et al. 2019; Ren et al. 2017; Zhao et al. 2019). In addition, this indicates that P is a limiting factor in the temperate steppe and P addition might alleviate P limitation of plant growth (Zhao et al. 2019). The addition of P alone did not exert any influence on plant diversity (Fig. 3a) but resulted in significant shifts of plant community structures (Fig. 4a) that are consistent with an earlier study of 7-year nutrient additions (Yan et al. 2022). P addition alone may shape plant community structure by increasing legume and forb biomass, without altering plant diversity, which is partially consistent with our first hypothesis. Significant changes in plant community structure resulting from P addition alone may be attributed to changes in the biomass of non-dominant plant species like Astragalus laxmannii, Artemisia stechmanniana, and Thermopsis lanceolata. However, the addition of P alone did not alter grass biomass that serves as the canopy species within the communities (Li et al. 2023a). Consequently, changes in height asymmetry would not be generated among plant functional groups (Guo et al. 2022), and influences on species diversity would not be apparent.
Furthermore, the results from this study suggest that above-ground biomass under long-term NP addition treatment was significantly higher than in the N addition alone. L. chinensis was the only gramineous plant in the NP addition treatment that contributed to over 90% of the total above-ground biomass. The NP addition treatment also led to decreased biomass of legumes and forbs at a magnitude comparable to that of N addition alone (Fig. 1). The responses of aboveground biomass to N and P addition were consistent with previous studies, indicating a synergistic effect of NP addition on aboveground biomass in temperate steppe ecosystems (Schleuss et al. 2020; Vázquez et al. 2023). Thus, NP addition enables plants to overcome growth limitations caused by N and P deficiency in temperate steppe ecosystems, further increasing aboveground biomass compared with when only N is added (Vázquez et al. 2023).
The effect of N and P addition on microbial diversity
Unlike plants, long-term N addition reduced soil microbial biomass (Fig. 2), although the diversity of bacteria, fungi, and AMF did not decrease (Fig. 3). These results are consistent with those reported by Wang et al. (2023). The latter study proposed a pattern comprising an initial decrease and subsequently increasing responses of microbial richness to N addition over an experimental duration exceeding 10 years, indicating microbial adaptation to long-term N additions.
The addition of phosphorus significantly increased the Shannon and richness indices of soil bacteria in this study (Fig. 3b). This could be due to the crucial role of P in the growth and metabolism of microorganisms, including the synthesis of nucleotides and regulation of enzyme activity (Elser et al. 2003; Xia et al. 2023). Consequently, bacteria with relatively higher growth rates may enhance their competitive advantage over other microorganisms in P-amended grassland systems when facing environmental stress. This supposition is also supported by microbial biomass observations that demonstrate significant decreases in the biomass of both bacteria and fungi after N additions, while the fungi: bacteria ratio significantly decreased after NP addition treatment (Fig. 2). Almost no significant response in soil fungal richness to the addition of nutrients was observed in this study (Fig. 3c), in contrast to results from a previous experiment in a temperate meadow, wherein N and P addition caused increased soil fungal richness (Yan et al. 2022). These trends can be attributed to the temporal variability of fungal richness in response to nutrient additions. An alternative explanation is that the moderate rate of nutrient addition employed in this study, in contrast to others with high nutrient application rates (Kim et al. 2015; Porras-Alfaro et al. 2007), may allow fungal communities to react to long-term nutrient additions without impacting fungal richness. Moreover, multiple studies have indicated that fungal diversity is less sensitive to nutrient addition compared with soil bacterial diversity (Wang et al. 2023).
Although the total fungal diversity did not significantly change with nutrient addition in this study, AMF diversity was significantly and positively associated with nutrient addition. Multiple different responses of AMF to nutrient additions have been observed, with N and P addition potentially increasing (Camenzind et al. 2016), decreasing (Leff et al. 2015), or having no effect (Duenas et al. 2020) on AMF diversity. The various impacts of N and P addition on AMF diversity may be jointly regulated by soil and plant properties in different ecosystems (Ma et al. 2021; Van Geel et al. 2018). In this study, NP addition significantly reduced AMF richness compared with P addition alone (Fig. 3d), consistent with many previous studies (Egerton-Warburton et al. 2007; Johnson 2010; Lekberg et al. 2021; Ma et al. 2021). The mutualistic symbiotic associations formed by AMF with most plants allow them to uptake carbon (C) from host plants and also facilitate nutrient uptake in host plants (Kiers et al. 2011). In particular, the C-for-P trade is a key aspect of symbiosis between plants and mycorrhizal fungi (Johnson 2010). In this study, the improvement in soil nutrient effectiveness in P-rich soils following NP addition usually led to increased plant biomass. The direct absorption of P from the soil by roots is preferential for plants due to its lower cost compared with AMF uptake, supporting the trade balance model prediction (Johnson 2010; Zheng et al. 2022). In addition, AMF are critical subterranean symbionts associated with plants, and the decrease in diversity and increase in plant biomass under NP addition treatment could result in the “homogenization” of mycorrhizal environments (Hooper et al. 2000; Ma et al. 2021), potentially leading to lower AMF diversity. As suggested by the SEM, AGB was the most important predictor of AMF richness (Fig. 6c).
The linkages between plant and microbial communities under N and P addition treatments
The responses of plants and soil microbial communities to soil nutrient availability are diverse and complex (Liu et al. 2021). Most studies investigating the impacts of nutrient enrichment on species community composition have primarily focused on aboveground organisms and neglected belowground microbial communities (Chalcraft et al. 2008; Conradi et al. 2017; Seabloom et al. 2021). Here, the impacts of N and P additions on plant and soil microbial community structures were evaluated in addition to assessing the contributions of vegetation and soil properties on changes in soil microbial communities under long-term nutrient addition conditions in temperate steppes for the first time.
Nutrient addition significantly altered the vegetation community structures of grassland. Long-term N and NP addition shifted the dominance of the plant community by multiple species to sole dominance by L. chinensis, while long-term P addition increased the importance of legumes and forbs in the grasslands (Bi et al. 2019; Ren et al. 2017). Nutrient addition significantly altered the soil microbial communities in this study, except for when P was added alone, which did not significantly affect soil AMF communities (Fig. 4). Differences in the dominant microbial taxa also serve as an indicator of microbial community changes caused by nutrient additions. Significant shifts have been observed for multiple dominant bacterial taxa after long-term N addition due to the greater sensitivity of bacteria to nutrient enrichment relative to fungi (Leff et al. 2015; Rousk et al. 2010; Wang et al. 2023). For example, N addition promoted the growth of copiotrophic bacteria (e.g., Proteobacteria, Firmicutes, and Bacteroidota), but inhibited the growth of oligotrophic bacteria (e.g., Acidobacteria, Chloroflexi, and Verrucomicrobiota) (Table S5). However, N addition did not significantly affect dominant fungal taxa in soils. In addition, many soil microbial taxa were oppositely effected by P and N additions alone (Leff et al. 2015), although the divergent changes caused by single additions can be alleviated or counterbalanced when N and P are added together.
The compositions of microbial communities can be influenced by both nutrient addition-induced changes in environmental factors and plant communities. Soil nutrient availability and soil pH were significantly associated with the functional and taxonomic groups of both plant and microbial communities. Soil pH is frequently reported to affect microbial community composition, because it directly limits or constrains the physiological activities of microorganisms (Zeng et al. 2016; Zhou et al. 2020). However, lowering soil pH could potentially elevate the abundance of acidophilic bacteria. This process was supported by the network analysis in this study that demonstrated a significant negative correlation between soil pH and multiple bacterial genera (Table S6), with the majority of these genera being acidophilic bacteria (Högfors-Rönnholm et al. 2023; Pankratov and Dedysh 2010; Shu et al. 2023; van den Heuvel et al. 2010; Wolinska et al. 2018; Yang et al. 2022a). Concomitantly, only two fungal genera exhibited significant correlations of their relative abundance with soil pH, suggesting that soil bacteria are more sensitive to changes in pH caused by nutrient additions relative to soil fungi. Partial Mantel tests were also used in this study to evaluate the importance of plant communities in shaping microbial communities. The results suggested that bacterial and non-mycorrhizal fungal communities were still strongly associated with plant community compositions, even when considering the influences of AGB and soil properties (Fig. 6a). This indicates that plant and microbial communities are closely synchronized under long-term nutrient addition (Liu et al. 2020; Zhang et al. 2017). Numerous significant correlations were also observed between plant species and microbial genera based on the network analysis of this study. These results suggest that microbial diversity and community composition may be strongly altered by soil properties (e.g., nutrient availability and pH) and plant community characteristics under long-term N and P addition, which supports our second hypothesis. For example, the distributions of multiple microbial genera were significantly correlated with L. chinensis distributions, with genera belonging to the phylum Proteobacteria exhibiting significant positive correlations (Fig. 5; Table S6). These results are consistent with a previous study that indicated that the restoration of degraded L. chinensis steppe increased the abundance of soil Proteobacteria (Yao et al. 2018a). In addition, multiple plant species, including legumes, forbs, and non-dominant grasses, exhibited significant positive correlations solely with fungi (Table S6). These results potentially reflect that plant functional groups and traits mediate changes in microbial microenvironments and carbon resources (Chen et al. 2021; Liu et al. 2020). AMF communities exhibited higher sensitivity to aboveground biomass than to plant community composition. This could be attributed to long-term nutrient addition enhancing soil nutrient availability, thereby leading to the allocation of more plant biomass aboveground leading to light competition and resulting in changes in the C-for-P trade between plants and AMF, which is the main factor that shapes AMF community structures (Johnson 2010; Zheng et al. 2022). In addition, plant–mycorrhizal interactions were suggested to have crucial roles in plant–microbe correlations, and this impact has been also demonstrated by field-based investigations (Huang et al. 2023; Lastovetsky et al. 2022; Liao et al. 2023).
Overall, plants may mediate soil microbial communities in at least three ways. (1) First, regulation may occur via the synchrony between plant communities and microbial communities (Liu et al. 2020; Zhang et al. 2017). (2) Second, regulation may occur by providing microhabitats and regulating carbon resources (Chen et al. 2021; Liu et al. 2020). (3) Lastly, these interactions may occur via plant–mycorrhizal interactions (Lastovetsky et al. 2022; Li et al. 2020). To rigorously differentiate the effects of plant communities and environmental factors on soil microbial communities, further empirical experiments involving the manipulation of specific host plant species (Schlatter et al. 2015) or plant community assemblies (Liu et al. 2020) under standardized environmental conditions are necessary. Such studies will further highlight the importance of plant–microbe interactions.
Conclusions
In this study, N addition to a steppe system experimental plot induced changes in plant community structures and diversity by significantly increasing grass biomass, while P addition alone only changed plant community structures primarily due to increases in legume and forb biomass. Meanwhile, NP addition further promoted L. chinensis growth but severely undermined the growth advantage of legumes and forbs, thereby exacerbating negative impacts on species diversity. Consequently, long-term N addition shifted the dominance of the plant community by multiple species to a sole L. chinensis dominated community. Furthermore, these results revealed a positive relationship between increased bacterial diversity and improved soil P availability. However, changes in bacterial community composition could be largely explained by aboveground biomass and plant community composition. Despite total fungal diversity was insensitive to N and P addition, increased nutrient availability led to increased aboveground biomass that then influenced the C-for-P trade interactions between plants and mycorrhizal fungi, consequently inducing changes in AMF communities. In addition, the co-occurrence patterns of soil properties, microbial genera distributions, and plants provides insights into the potential relationships between plant functional groups and dominant microbial taxa. Overall, the close synchrony between plant communities and soil microbial communities, as well as the significant relationships between plant functional groups and dominant microbial taxa, emphasizes the important roles of plant-mediated effects on microbial communities and provide new insights into the responses of plant–microbial interactions to long-term N and P deposition.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Baas Becking LGM (1934) Geobiologie of inleiding tot de milieukunde. Van Stockum & Zoon, Hague
Bahram M, Hildebrand F, Forslund SK, Anderson JL, Soudzilovskaia NA, Bodegom PM, Bengtsson-Palme J, Anslan S, Coelho LP, Harend H, Huerta-Cepas J, Medema MH, Maltz MR, Mundra S, Olsson PA, Pent M, Polme S, Sunagawa S, Ryberg M, Tedersoo L, Bork P (2018) Structure and function of the global topsoil microbiome. Nature 560:233–237. https://doi.org/10.1038/s41586-018-0386-6
Bai YF, Wu JG, Clark CM, Naeem S, Pan QM, Huang JH, Zhang LX, Han XG (2010) Tradeoffs and thresholds in the effects of nitrogen addition on biodiversity and ecosystem functioning: evidence from inner Mongolia Grasslands. Glob Chang Biol 16:358–372. https://doi.org/10.1111/j.1365-2486.2009.01950.x
Bai B, Liu WD, Qiu XY, Zhang J, Zhang JY, Bai Y (2022) The root microbiome: community assembly and its contributions to plant fitness. J Integr Plant Biol 64:230–243. https://doi.org/10.1111/jipb.13226
Bao SD (2000) Soil and agricultural chemistry analysis. Agriculture Press of China, Beijing
Bi YX, Zhou P, Li SJ, Wei YQ, Xiong X, Shi YH, Liu N, Zhang YJ (2019) Interspecific interactions contribute to higher forage yield and are affected by phosphorus application in a fully-mixed perennial legume and grass intercropping system. Field Crop Res 244:107636. https://doi.org/10.1016/j.fcr.2019.107636
Borer ET, Seabloom EW, Gruner DS, Harpole WS, Hillebrand H, Lind EM, Adler PB, Alberti J, Anderson TM, Bakker JD, Biederman L, Blumenthal D, Brown CS, Brudvig LA, Buckley YM, Cadotte M, Chu CJ, Cleland EE, Crawley MJ, Daleo P, Damschen EI, Davies KF, DeCrappeo NM, Du GZ, Firn J, Hautier Y, Heckman RW, Hector A, HilleRisLambers J, Iribarne O, Klein JA, Knops JMH, La Pierre KJ, Leakey ADB, Li W, MacDougall AS, McCulley RL, Melbourne BA, Mitchell CE, Moore JL, Mortensen B, O’Halloran LR, Orrock JL, Pascual J, Prober SM, Pyke DA, Risch AC, Schuetz M, Smith MD, Stevens CJ, Sullivan LL, Williams RJ, Wragg PD, Wright JP, Yang LH (2014) Herbivores and nutrients control grassland plant diversity via light limitation. Nature 508:517–520. https://doi.org/10.1038/nature13144
Camenzind T, Homeier J, Dietrich K, Hempel S, Hertel D, Krohn A, Leuschner C, Oelmann Y, Olsson PA, Suárez JP, Rillig MC (2016) Opposing effects of nitrogen versus phosphorus additions on mycorrhizal fungal abundance along an elevational gradient in tropical montane forests. Soil Biol Biochem 94:37–47. https://doi.org/10.1016/j.soilbio.2015.11.011
Chalcraft DR, Cox SB, Clark C, Cleland EE, Suding KN, Weiher E, Pennington D (2008) Scale-dependent responses of plant biodiversity to nitrogen enrichment. Ecology 89:2165–2171. https://doi.org/10.1890/07-0971.1
Chen WJ, Zhou HK, Wu Y, Li YZ, Qiao LL, Wang J, Zhai JY, Song YH, Zhao ZW, Zhang ZH, Liu GB, Zhao XQ, You QM, Xue S (2021) Plant-mediated effects of long-term warming on soil microorganisms on the Qinghai-Tibet Plateau. CATENA 204:105391. https://doi.org/10.1016/j.catena.2021.105391
Chu HY, Neufeld JD, Walker VK, Grogan P (2011) The influence of vegetation type on the dominant soil bacteria, archaea, and fungi in a low arctic tundra landscape. Soil Sci Soc Am J 75:1756–1765. https://doi.org/10.2136/sssaj2011.0057
Conradi T, Temperton VM, Kollmann J (2017) Resource availability determines the importance of niche-based versus stochastic community assembly in grasslands. Oikos 126:1134–1141. https://doi.org/10.1111/oik.03969
Dai ZM, Su WQ, Chen HH, Barberán A, Zhao HC, Yu MJ, Yu L, Brookes PC, Schadt CW, Chang SX, Xu JM (2018) Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of and in agro-ecosystems across the globe. Glob Chang Biol 24:3452–3461. https://doi.org/10.1111/gcb.14163
Duenas JF, Camenzind T, Roy J, Hempel S, Homeier J, Suárez JP, Rillig MC (2020) Moderate phosphorus additions consistently affect community composition of arbuscular mycorrhizal fungi in tropical montane forests in southern Ecuador. New Phytol 227:1505–1518. https://doi.org/10.1111/nph.16641
Egerton-Warburton LM, Johnson NC, Allen EB (2007) Mycorrhizal community dynamics following nitrogen fertilization: a cross-site test in five grasslands. Ecol Monogr 77:527–544. https://doi.org/10.1890/06-1772.1
Elser JJ, Acharya K, Kyle M, Cotner J, Makino W, Markow T, Watts T, Hobbie S, Fagan W, Schade J, Hood J, Sterner RW (2003) Growth rate-stoichiometry couplings in diverse biota. Ecol Lett 6:936–943. https://doi.org/10.1046/j.1461-0248.2003.00518.x
Feng XJ, Wang SM (2023) Plant influences on soil microbial carbon pump efficiency. Glob Chang Biol 29:3854–3856. https://doi.org/10.1111/gcb.16728
Fierer N, Jackson RB (2006) The diversity and biogeography of soil bacterial communities. P Natl Acad Sci USA 103:626–631. https://doi.org/10.1073/pnas.0507535103
Fleischer K, Dolman AJ, van der Molen MK, Rebel KT, Erisman JW, Wassen MJ, Pak B, Lu XJ, Rammig A, Wang YP (2019) Nitrogen deposition maintains a positive effect on terrestrial carbon sequestration in the 21st century eespite growing phosphorus limitation at regional scales. Glob Biogeochem Cycles 33:810–824. https://doi.org/10.1029/2018gb005952
Fuhrman JA (2009) Microbial community structure and its functional implications. Nature 459:193–199. https://doi.org/10.1038/nature08058
Green J, Bohannan BJM (2006) Spatial scaling of microbial biodiversity. Trends Ecol Evol 21:501–507. https://doi.org/10.1016/j.tree.2006.06.012
Guo YQ, Chen XT, Wu YY, Zhang L, Cheng JM, Wei GH, Lin YB (2018) Natural revegetation of a semiarid habitat alters taxonomic and functional diversity of soil microbial communities. Sci Total Environ 635:598–606. https://doi.org/10.1016/j.scitotenv.2018.04.171
Guo N, Xie MY, Fang Z, Jiao F, Han XY (2022) Divergent responses of plant biomass and diversity to short-term nitrogen and phosphorus addition in three types of steppe in Inner Mongolia, China. Ecol Process 11:32. https://doi.org/10.1186/s13717-022-00376-y
Hautier Y, Niklaus PA, Hector A (2009) Competition for light causes plant biodiversity loss after eutrophication. Science 324:636–638. https://doi.org/10.1126/science.1169640
He KJ, Qi Y, Huang YM, Chen HY, Sheng ZL, Xu X, Duan L (2016) Response of aboveground biomass and diversity to nitrogen addition a five-year experiment in semi-arid grassland of Inner Mongolia. China Sci Rep-Uk 6:31919. https://doi.org/10.1038/srep31919
Högfors-Rönnholm E, Sten P, Christel S, Frojdo S, Lillhonga T, Nowak P, Osterholm P, Dopson M, Engblom S (2023) Targeting oxidation sites on boreal acid sulfate soil macropore surfaces mitigates acid and metal release to recipient water streams. Appl Geochem 158:105779. https://doi.org/10.1016/j.apgeochem.2023.105779
Hooper DU, Bignell DE, Brown VK, Brussaard L, Dangerfield JM, Wall DH, Wardle DA, Coleman DC, Giller KE, Lavelle P, Van der Putten WH, De Ruiter PC, Rusek J, Silver WL, Tiedje JM, Wolters V (2000) Interactions between aboveground and belowground biodiversity in terrestrial ecosystems: patterns, mechanisms, and feedbacks. Bioscience 50:1049–1061. https://doi.org/10.1641/0006-3568(2000)050[1049:Ibaabb]2.0.Co;2
Horswill P, O’Sullivan O, Phoenix GK, Lee JA, Leake JR (2008) Base cation depletion, eutrophication and acidification of species-rich grasslands in response to long-term simulated nitrogen deposition. Environ Pollut 155:336–349. https://doi.org/10.1016/j.envpol.2007.11.006
Huang H, Liu SJ, Du Y, Tang JJ, Hu LL, Chen X (2023) Carbon allocation mediated by arbuscular mycorrhizal fungi alters the soil microbial community under various phosphorus levels. Fungal Ecol 62:101227. https://doi.org/10.1016/j.funeco.2023.101227
in ’t Zandt D, Kolaríková Z, Cajthaml T, Münzbergová Z (2023) Plant community stability is associated with a decoupling of prokaryote and fungal soil networks. Nat Commun 14:3736. https://doi.org/10.1038/s41467-023-39464-8
Johnson NC (2010) Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647. https://doi.org/10.1111/j.1469-8137.2009.03110.x
Kaspari M, Bujan J, Weiser MD, Ning D, Michaletz ST, He ZL, Enquist BJ, Waide RB, Zhou JZ, Turner BL, Wright SJ (2017) Biogeochemistry drives diversity in the prokaryotes, fungi, and invertebrates of a Panama forest. Ecology 98:2019–2028. https://doi.org/10.1002/ecy.1895
Kiers ET, Duhamel M, Beesetty Y, Mensah JA, Franken O, Verbruggen E, Fellbaum CR, Kowalchuk GA, Hart MM, Bago A, Palmer TM, West SA, Vandenkoornhuyse P, Jansa J, Bücking H (2011) Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 333:880–882. https://doi.org/10.1126/science.1208473
Kim YC, Gao C, Zheng Y, He XH, Yang W, Chen L, Wan SQ, Guo LD (2015) Arbuscular mycorrhizal fungal community response to warming and nitrogen addition in a semiarid steppe ecosystem. Mycorrhiza 25:267–276. https://doi.org/10.1007/s00572-014-0608-1
Lastovetsky OA, Caruso T, Brennan FP, Wall DP, McMahon S, Doyle E (2022) Evidence of a selective and bi-directional relationship between arbuscular mycorrhizal fungal and bacterial communities co-inhabiting plant roots. Environ Microbiol 24:5378–5391. https://doi.org/10.1111/1462-2920.16227
Leff JW, Jones SE, Prober SM, Barberán A, Borer ET, Firn JL, Harpole WS, Hobbie SE, Hofmockel KS, Knops JMH, McCulley RL, La Pierre K, Risch AC, Seabloom EW, Schütz M, Steenbock C, Stevens CJ, Fierer N (2015) Consistent responses of soil microbial communities to elevated nutrient inputs in grasslands across the globe. P Natl Acad Sci USA 112:10967–10972. https://doi.org/10.1073/pnas.1508382112
Lekberg Y, Arnillas CA, Borer ET, Bullington LS, Fierer N, Kennedy PG, Leff JW, Luis AD, Seabloom EW, Henning JA (2021) Nitrogen and phosphorus fertilization consistently favor pathogenic over mutualistic fungi in grassland soils. Nat Commun 12:3484. https://doi.org/10.1038/s41467-021-23605-y
Li JH, Xie S, Wilson GWT, Cobb AB, Tang SM, Guo LZ, Wang K, Deng B (2020) Plant-microbial interactions facilitate grassland species coexistence at the community level. Oikos 129:533–543. https://doi.org/10.1111/oik.06609
Li FS, Minggagud H, Jarvie S, Wang YH, Yan YZ, Gong XQ, Han P, Zhang Q (2023a) Mowing mitigates the adverse effects of fertilization on plant diversity and changes soil bacterial and fungal community structure in the Inner Mongolia grassland. Agr Ecosyst Environ 346:108358. https://doi.org/10.1016/j.agee.2023.108358
Li N, Du HB, Li MH, Na RS, Dong RK, He HS, Zong SW, Huang LR, Wu ZF (2023b) Deyeuxia angustifolia upward migration and nitrogen deposition change soil microbial community structure in an alpine tundra. Soil Biol Biochem 180:109009. https://doi.org/10.1016/j.soilbio.2023.109009
Liang C, Kao-Kniffin J, Sanford GR, Wickings K, Balser TC, Jackson RD (2016) Microorganisms and their residues under restored perennial grassland communities of varying diversity. Soil Biol Biochem 103:192–200. https://doi.org/10.1016/j.soilbio.2016.08.002
Liao XH, Zhao J, Xu L, Tang L, Li JN, Zhang W, Xiao J, Xiao D, Nie YP, Zou DS, Wang KL (2023) Arbuscular mycorrhizal fungi increase the interspecific competition between two forage plant species and stabilize the soil microbial network during a drought event: evidence from the field. Appl Soil Ecol 185:104805. https://doi.org/10.1016/j.apsoil.2023.104805
Ling N, Chen DM, Guo H, Wei JX, Bai YF, Shen QR, Hu SJ (2017) Differential responses of soil bacterial communities to long-term N and P inputs in a semi-arid steppe. Geoderma 292:25–33. https://doi.org/10.1016/j.geoderma.2017.01.013
Liu WX, Jiang L, Yang S, Wang Z, Tian R, Peng ZY, Chen YL, Zhang XX, Kuang JL, Ling N, Wang SP, Liu LL (2020) Critical transition of soil bacterial diversity and composition triggered by nitrogen enrichment. Ecology 101:e03053. https://doi.org/10.1002/ecy.3053
Liu WX, Liu LL, Yang X, Deng MF, Wang Z, Wang PD, Yang S, Li P, Peng ZY, Yang L, Jiang L (2021) Long-term nitrogen input alters plant and soil bacterial, but not fungal beta diversity in a semiarid grassland. Glob Chang Biol 27:3939–3950. https://doi.org/10.1111/gcb.15681
Ma XC, Geng QH, Zhang HG, Bian CY, Chen HYH, Jiang DL, Xu X (2021) Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multifunctionality. New Phytol 229:2957–2969. https://doi.org/10.1111/nph.17077
Ma YY, Wang DZ, Guo XS, Zhu YG, Delgado-Baquerizo M, Chu HY (2022) Root stoichiometry explains wheat endophytes and their link with crop production after four decades of fertilization. Sci Total Environ 846:157407. https://doi.org/10.1016/j.scitotenv.2022.157407
Pan S, Wang Y, Qiu YP, Chen DM, Zhang L, Ye CL, Guo H, Zhu WX, Chen AQ, Xu GH, Zhang Y, Bai YF, Hu SJ (2020) Nitrogen-induced acidification, not N-nutrient, dominates suppressive N effects on arbuscular mycorrhizal fungi. Glob Chang Biol 26:6568–6580. https://doi.org/10.1111/gcb.15311
Pankratov TA, Dedysh SN (2010) gen. nov., sp nov., sp nov., sp nov and sp nov., acidophilic, polymer-degrading acidobacteria from peat bogs. Int J Syst Evol Micr 60:2951–2959. https://doi.org/10.1099/ijs.0.021824-0
Peay KG, von Sperber C, Cardarelli E, Toju H, Francis CA, Chadwick OA, Vitousek PM (2017) Convergence and contrast in the community structure of Bacteria, Fungi and Archaea along a tropical elevation-climate gradient. Fems Microbiol Ecol 93:fix045. https://doi.org/10.1093/femsec/fix045
Peñuelas J, Poulter B, Sardans J, Ciais P, van der Velde M, Bopp L, Boucher O, Godderis Y, Hinsinger P, Llusia J, Nardin E, Vicca S, Obersteiner M, Janssens IA (2013) Human-induced nitrogen-phosphorus imbalances alter natural and managed ecosystems across the globe. Nat Commun 4:2934. https://doi.org/10.1038/ncomms3934
Philippot L, Raaijmakers JM, Lemanceau P, van der Putten WH (2013a) Going back to the roots: the microbial ecology of the rhizosphere. Nat Rev Microbiol 11:789–799. https://doi.org/10.1038/nrmicro3109
Philippot L, Spor A, Hénault C, Bru D, Bizouard F, Jones CM, Sarr A, Maron PA (2013b) Loss in microbial diversity affects nitrogen cycling in soil. Isme J 7:1609–1619. https://doi.org/10.1038/ismej.2013.34
Porras-Alfaro A, Herrera J, Natvig DO, Sinsabaugh RL (2007) Effect of long-term nitrogen fertilization on mycorrhizal fungi associated with a dominant grass in a semiarid grassland. Plant Soil 296:65–75. https://doi.org/10.1007/s11104-007-9290-9
Ren F, Song WM, Chen LT, Mi ZR, Zhang ZH, Zhu WY, Zhou HK, Cao GM, He JS (2017) Phosphorus does not alleviate the negative effect of nitrogen enrichment on legume performance in an alpine grassland. J Plant Ecol 10:822–830. https://doi.org/10.1093/jpe/rtw089
Rousk J, Bååth E, Brookes PC, Lauber CL, Lozupone C, Caporaso JG, Knight R, Fierer N (2010) Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J 4:1340–1351. https://doi.org/10.1038/ismej.2010.58
Schlatter DC, Bakker MG, Bradeen JM, Kinkel LL (2015) Plant community richness and microbial interactions structure bacterial communities in soil. Ecology 96:134–142. https://doi.org/10.1890/13-1648.1
Schleuss PM, Widdig M, Heintz-Buschart A, Kirkman K, Spohn M (2020) Interactions of nitrogen and phosphorus cycling promote P acquisition and explain synergistic plant-growth responses. Ecology 101:e03003. https://doi.org/10.1002/ecy.3003
Seabloom EW, Adler PB, Alberti J, Biederman L, Buckley YM, Cadotte MW, Collins SL, Dee L, Fay PA, Firn J, Hagenah N, Harpole WS, Hautier Y, Hector A, Hobbie SE, Isbell F, Knops JMH, Komatsu KJ, Laungani R, MacDougall A, McCulley RL, Moore JL, Morgan JW, Ohlert T, Prober SM, Risch AC, Schuetz M, Stevens CJ, Borer ET (2021) Increasing effects of chronic nutrient enrichment on plant diversity loss and ecosystem productivity over time. Ecology 102:e03218. https://doi.org/10.1002/ecy.3218
Shu WS, Huang LN (2022) Microbial diversity in extreme environments. Nat Rev Microbiol 20:219–235. https://doi.org/10.1038/s41579-021-00648-y
Shu X, Zhang KR, Zhang QF, Wang WB (2023) Changes in the composition of rhizosphere bacterial communities in response to soil types and acid rain. J Environ Manage 325:116493. https://doi.org/10.1016/j.jenvman.2022.116493
Su J, Zhang HY, Han XG, Lv RF, Liu L, Jiang Y, Li H, Kuzyakov Y, Wei CZ (2023) 5300-Year-old soil carbon is less primed than young soil organic matter. Glob Chang Biol 29:260–275. https://doi.org/10.1111/gcb.16463
Suding KN, Collins SL, Gough L, Clark C, Cleland EE, Gross KL, Milchunas DG, Pennings S (2005) Functional- and abundance-based mechanisms explain diversity loss due to N fertilization. Proc Natl Acad Sci USA 102:4387–4392. https://doi.org/10.1073/pnas.0408648102
Trivedi P, Batista BD, Bazany KE, Singh BK (2022) Plant-microbiome interactions under a changing world: responses, consequences and perspectives. New Phytol 234:1951–1959. https://doi.org/10.1111/nph.18016
van den Heuvel RN, van der Biezen E, Jetten MSM, Hefting MM, Kartal B (2010) Denitrification at pH 4 by a soil-derived-dominated community. Environ Microbiol 12:3264–3271. https://doi.org/10.1111/j.1462-2920.2010.02301.x
van Dobben HF, Wamelink GWW, Slim PA, Kaminski J, Piorkowski H (2017) Species-rich grassland can persist under nitrogen-rich but phosphorus-limited conditions. Plant Soil 411:451–466. https://doi.org/10.1007/s11104-016-3021-z
Van Geel M, Jacquemyn H, Plue J, Saar L, Kasari L, Peeters G, van Acker K, Honnay O, Ceulemans T (2018) Abiotic rather than biotic filtering shapes the arbuscular mycorrhizal fungal communities of European seminatural grasslands. New Phytol 220:1262–1272. https://doi.org/10.1111/nph.14947
Vázquez E, Borer ET, Bugalho MN, Caldeira MC, McCulley RL, Risch AC, Seabloom EW, Wheeler GR, Spohn M (2023) The synergistic response of primary production in grasslands to combined nitrogen and phosphorus addition is caused by increased nutrient uptake and retention. Plant Soil:371–385. https://doi.org/10.1007/s11104-023-06083-7
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20:5–15. https://doi.org/10.1890/08-0127.1
Wang XD, Feng JG, Ao GKL, Qin WK, Han MG, Shen YW, Liu ML, Chen Y, Zhu B (2023) Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil Biol Biochem 179:108982. https://doi.org/10.1016/j.soilbio.2023.108982
Wen Z, Wang RY, Li Q, Liu JN, Ma X, Xu W, Tang AH, Collett JL, Li HG, Liu XJ (2022) Spatiotemporal variations of nitrogen and phosphorus deposition across China. Sci Total Environ 830:154740. https://doi.org/10.1016/j.scitotenv.2022.154740
Wolinska A, Kuzniar A, Zielenkiewicz U, Banach A, Blaszczyk M (2018) Indicators of arable soils fatigue Bacterial - families and genera: a metagenomic approach. Ecol Indic 93:490–500. https://doi.org/10.1016/j.ecolind.2018.05.033
Xia ST, Jiang J, Liu FC, Chang ZB, Yu MX, Liu CY, Wang YP, Yan JH (2023) Phosphorus addition promotes plant nitrogen uptake mainly via enhancing microbial activities: a global meta-analysis. Appl Soil Ecol 188:104927. https://doi.org/10.1016/j.apsoil.2023.104927
Yan Y, Sun XT, Sun FW, Zhao YA, Sun W, Guo JX, Zhang T (2022) Sensitivity of soil fungal and bacterial community compositions to nitrogen and phosphorus additions in a temperate meadow. Plant Soil 471:477–490. https://doi.org/10.1007/s11104-021-05237-9
Yang A, Liu NN, Tian QY, Bai WM, Williams M, Wang QB, Li LH, Zhang WH (2015) Rhizosphere bacterial communities of dominant steppe plants shift in response to a gradient of simulated nitrogen deposition. Front Microbiol 6:789. https://doi.org/10.3389/fmicb.2015.00789
Yang F, Wu JJ, Zhang DD, Chen Q, Zhang Q, Cheng XL (2018) Soil bacterial community composition and diversity in relation to edaphic properties and plant traits in grasslands of southern China. Appl Soil Ecol 128:43–53. https://doi.org/10.1016/j.apsoil.2018.04.001
Yang Y, Li T, Wang YQ, Cheng H, Chang SX, Liang C, An SS (2021) Negative effects of multiple global change factors on soil microbial diversity. Soil Biol Biochem 156:108229. https://doi.org/10.1016/j.soilbio.2021.108229
Yang CD, Liu JJ, Ying HC, Lu SG (2022a) Soil pore structure changes induced by biochar affect microbial diversity and community structure in an Ultisol. Soil till Res 224:105505. https://doi.org/10.1016/j.still.2022.105505
Yang YL, Xie HT, Mao Z, Bao XL, He HB, Zhang XD, Liang C (2022b) Fungi determine increased soil organic carbon more than bacteria through their necromass inputs in conservation tillage croplands. Soil Biol Biochem 167:108587. https://doi.org/10.1016/j.soilbio.2022.108587
Yao MJ, Rui JP, Li JB, Wang JM, Cao WD, Li XZ (2018a) Soil bacterial community shifts driven by restoration time and steppe types in the degraded steppe of Inner Mongolia. CATENA 165:228–236. https://doi.org/10.1016/j.catena.2018.02.006
Yao XD, Zhang NL, Zeng H, Wang W (2018b) Effects of soil depth and plant-soil interaction onmicrobial community in temperate grasslands of northern China. Sci Total Environ 630:96–102. https://doi.org/10.1016/j.scitotenv.2018.02.155
Yu L, Song XL, Zhao JN, Wang H, Bai L, Yang DL (2015) Responses of plant diversity and primary productivity to nutrient addition in a grassland, China. J Integr Agr 14:2099–2108. https://doi.org/10.1016/S2095-3119(14)61001-7
Zeng J, Liu XJ, Song L, Lin XG, Zhang HY, Shen CC, Chu HY (2016) Nitrogen fertilization directly affects soil bacterial diversity and indirectly affects bacterial community composition. Soil Biol Biochem 92:41–49. https://doi.org/10.1016/j.soilbio.2015.09.018
Zhang XM, Johnston ER, Li LH, Konstantinidis KT, Han XG (2017) Experimental warming reveals positive feedbacks to climate change in the Eurasian Steppe. ISME J 11:885–895. https://doi.org/10.1038/ismej.2016.180
Zhang YY, Zheng NG, Wang J, Yao HY, Qiu QF, Chapman SJ (2019) High turnover rate of free phospholipids in soil confirms the classic hypothesis of PLFA methodology. Soil Biol Biochem 135:323–330. https://doi.org/10.1016/j.soilbio.2019.05.023
Zhao YA, Yang B, Li MX, Xiao RQ, Rao KY, Wang JQ, Zhang T, Guo JX (2019) Community composition, structure and productivity in response to nitrogen and phosphorus additions in a temperate meadow. Sci Total Environ 654:863–871. https://doi.org/10.1016/j.scitotenv.2018.11.155
Zheng Z, Ma XY, Zhang Y, Liu YJ, Zhang SH (2022) Soil properties and plant community-level traits mediate arbuscular mycorrhizal fungal response to nitrogen enrichment and altered precipitation. Appl Soil Ecol 169:104245. https://doi.org/10.1016/j.apsoil.2021.104245
Zhou ZH, Wang CK, Luo YQ (2020) Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat Commun 11:3072. https://doi.org/10.1038/s41467-020-16881-7
Zhou ZB, Zhang YJ, Zhang FG (2022) Abundant and rare bacteria possess different diversity and function in crop monoculture and rotation systems across regional farmland. Soil Biol Biochem 171:108742. https://doi.org/10.1016/j.soilbio.2022.108742
Zhu LY, Chen Y, Sun RB, Zhang JB, Hale L, Dumack K, Geisen S, Deng Y, Duan YH, Zhu B, Li Y, Liu WZ, Wang XY, Griffiths BS, Bonkowski M, Zhou JZ, Sun B (2023) Resource-dependent biodiversity and potential multi-trophic interactions determine belowground functional trait stability. Microbiome 11:95. https://doi.org/10.1186/s40168-023-01539-5
Acknowledgements
We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2023YFF1304102) and National Natural Science Foundation of China (42007046; 41877343).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Raúl Ochoa-Hueso.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, H., Jiang, N., Wang, H. et al. Importance of plant community composition and aboveground biomass in shaping microbial communities following long-term nitrogen and phosphorus addition in a temperate steppe ecosystem. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06881-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06881-7