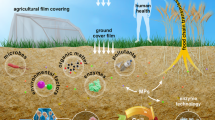
Abstract
Background and aims
Microplastics (MPs) are persistent pollutant distributes in sea, soil, and atmosphere widely. Nowadays, there has been an increasing awareness of negative impact of MPs on the environment, especially focusing on extensive research in the aquatic environment. However, there is still a significant research gap in the study of MPs in soil, despite the serious harm to soil.
Methods
This review conducts literature analysis by Citespace software, counting the distribution of MPs in different soils, analyzing the sources and types of MPs, integrating the accumulation and fate of MPs, and summarizing methods of separation and identification MPs in soil.
Results
The research on MPs in soil is sparse, with limited funding. Importantly, the annual average growth rate of MPs discharge into farmland exceeds into marine, and the comparability of detection results is poor. Research has found, synthetic textiles, tyres, and urban dust are the main sources, which mainly introduce through atmospheric sedimentation, sewage irrigation, sludge farming, and the use of agricultural film. What’s more, MPs in soil may have the risk of leaching from groundwater and contaminating it. Therefore, it is essential to establish a standard quantitative method for extracting and identifying microplastics in soil to better control their impact on the planting industry.
Conclusion
The MPs in soil seriously affect the growth of plants. Nutrient transfer and intergenerational transmission effects pose potential risks to human health. Consequently, the degradation of MPs by microorganisms in soil is an environmentally friendly and economically worthy topic for in-depth research.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
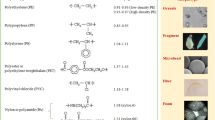
Plastics products are widely used in industry, agriculture, transportation, building materials, household appliances and other fields due to their low cost and price, light weight, electrical insulation, corrosion resistance, durability, and other characteristics. In the past 50 years, the global plastic output has been about 9.1 billion tons, with an annual production of more than 300 million tons of plastic, growing rapidly at a rate of 8.7% (Geyer et al. 2017). Because of the low reuse rate of plastic products, the huge production and inefficient management of plastic have become a serious environmental crisis. Most plastics include polyethylene (PE), polypropylene (PP), polystyrene (PS), polyvinyl chloride (PVC) and polyethylene terephthalate (PET) (Geyer et al. 2017), etc. Although the recycling rate of plastic products is increasing recently, only 6% ~ 26% of plastic waste has been recycled (Andrady 2011). Most of the rest are still released into the environment. The plastic waste in the environment can be transformed into smaller plastic fragments through long-term physical crushing, chemical decomposition, and biodegradation (Wright and Kelly 2017). These smaller particles of an astonishing number are difficult to be removed, thus causing grievous pollution in the environment. In 2004, British scholar Thompson first proposed the concept of microplastics (Thompson et al. 2004). In 2008, the National Oceanic and Atmospheric Administration (NOAA) of the United States defined microplastics (MPs) as plastic particles with a particle size less than 5 mm (Cózar et al. 2014). Later, some scholars continue to classify microplastic particles (MPs) with particle size less than 0.1 μm as nanoplastic particles (NPs) (Bouwmeester et al. 2015).
In recent years, researchers have continuously detected microplastics in oceans, lakes, rivers, sediments, beaches, soils and atmospheric environments (Liu et al. 2018; Wu et al. 2023; Eriksen et al. 2014; Zhang et al. 2018a).The existence of MPs is even found in the Arctic frozen area (Obbard et al. 2014). Microplastic pollution has been paid increasing attention and has been listed as the second largest scientific problem in the field of environment and ecology (Eriksen et al. 2014). Lots of studies on MPs have been carried out in the world, but they are mainly concentrated in ocean, estuary, lake and other water environments. The research on MPs in terrestrial environment, especially in soil, is still relatively scarce (Zhang et al. 2016).
There is currently limited research indicating the significant importance of studying MPs in soil. Microplastic pollution may affect soil properties by altering the pH, structure, fertility and nutrients of soil (Qi et al. 2020; Kumar et al. 2023). After 30 days of exposure to high-density PE, the pH of soil planted with perennial ryegrass is 0.62 units lower than that of the control group (de Souza Machado et al. 2019). The germination rate and height of ryegrass will decrease when there are biodegradable polylactic acid and high-density polyethylene (HDPE) MPs and fibrous MPs (Zubris and Richards 2005). MPs will also store (Bai et al. 2024), migrate (Klemmensen et al. 2024), erode (Li et al. 2022), degrade (Klemmensen et al. 2024), and infiltrate into groundwater after entering the soil (Brožová et al. 2023), thereby threatening animals, plants, and microorganisms. Changes in soil porosity and soil moisture caused by MPs may reduce the flow of oxygen in soil, thereby triggering alter in the relative distribution of anaerobic and aerobic microorganisms (Lehmann et al. 2019; Kumar et al. 2023), which leads to the loss of microbial habitat and the extinction of indigenous microorganisms (Rubol et al. 2013). For plants, the existence of MPs will cause mutations in the physical and chemical properties of soil, weakening the root system and nutrient status of plants, thus affecting plant growth (Judy et al. 2019). The presence of MPs in soil will also have an impact on small animals living in soil. It may directly hinder the mobility of animals if MPs adhesion to the outer surface (Kim and An 2019); In most cases, MPs may be accidentally ingested because animals mistake MPs for food. The intake of MPs will cause animals to feel false full, thus reducing the intake of biological carbon, further leading to energy consumption, growth decline and even death (Costa et al. 2016). Once ingested, MPs can also cause mechanical damage to the esophagus, intestinal obstruction, reproductive function decline and negative biochemical reactions, such as decreased immune response and metabolic disorder(Cole et al. 2013). On the other hand, soil biota can also affect the accumulation and fate of MPs. MPs can be ingested by soil animals and then turned into smaller particles even degraded completely. For example, digging and burrowing mammals, such as hamsters and moles, can further wear nanoplastics and MPs (Rillig et al. 2017). Additionally, the surface properties of NMPs such as tire and road wear particles, polymeric asphalt lead to the adsorption of heavy metals, endocrine disruptors, antibiotics, and other persistent organic pollutants, resulting in their co-migration in terrestrial environments (O’Kelly et al. 2022).
MPs degrade abnormally slowly in the natural environment, and they are likely to be ingested by organisms, accumulated, spread through the food chain, ultimately cause irreversible harm to the ecological environment and human health. At the European Society of Gastroenterology held in 2018, researchers reported that up to 9 kinds of MPs were detected in human feces for the first time (Liu et al. 2018). Xia Yankai and Luo Yongming found MPs in human thrombus samples for the first time (Wu et al. 2023). The impact of MPs on the environment and organisms cannot be underestimated. Scientists have been brainstorming and even interdisciplinary research in the past two years. Yang et al. pointed out the lagging monitoring of soil MPs and emphasized the importance and effectiveness of and legislation (Yang et al. 2021). Goli et al. suggest that artificial intelligence (AI) assist spectroscopy and hyperspectral imaging to improve MPs in soil detection, classification, and separation (Goli et al. 2022).In this paper, to improve the upcoming research, the research progress in analytical methods, pollution characteristics and source distribution of MPs in soil was reviewed, and the emphasis and key issues for future research were put forward.
Research and pollution status of MPs
Bibliometric analysis based on CiteSpace
A total of 16,876 literature information about MPs was collected from the Web of Science (2000–2024,Microplastics topic), which was used for bibliometric visual analysis by CiteSpace. Citespace (version 6.3.R1) as a software, using the Text Co-occurrence Network Analysis method, can be employed to visually explore the development trend in a certain period by means of keywords analysis, forming the evolution process of the frontier of research fields. It was possible to see the number of documents issued, contributing countries, institutions, authors, keywords, and directions of the future in the MPs sectors by the visual representation of the results.
Since 2014, the number of papers on MPs pollution has increased rapidly, among which there are relatively many papers on MPs in oceans and organisms (Fig. 1). As a new environmental problem, marine MPs pollution has attracted global attention and has been included in the regular monitoring scope of the marine environment. However, the research on MPs in soil is obviously lagging and lacking. The relevant research started in 2012, and the number of relevant publications accounted for about 24%, which indicates this has received widespread attention from 2022 (keywords: Publication Years).
The number of publications about MPs pollution in different types of environment matrices. a Increasing number of publications about MPs pollution from 2004 to 2024. b The percentages of publications about MPs pollution in sea, freshwater, biology, and soil in the total publications. Data are counted in papers published during January 2004 to May 2024 (basing on the database of Web of Science)
With the keyword Microplastics in soil, 2327 papers related to soil microplastics have been screened in web of science, downloaded and imported into Citespace, and visualized after removing duplicates. According to the frequency of keywords (Fig. 2), the research focuses on five aspects: biological toxicity, distribution and abundance, migration and identification methods, adsorption, and the fate and degradation behavior of MPs (keyword cluster: Keyword). The main research objects include ocean, fresh water and sediment, and the frequency of relevant keywords is higher than 1000 times. The key word "soil" only appeared 355 times, which imply that there is still a lack of significant progress on MPs in soil although there has been attention since 2022.
There are 417 international institutions (keyword cluster: Institution) and 461 authors participated in the field of soil MPs research (keyword cluster: Author)through CiteSpace visual analysis of the cooperation network (Fig. 3), of which 78 institutions have published more than 10 papers. Nine of the top ten production agencies are from China, a major agricultural producing country. However, the author with the largest number of articles is Rilingmathias C from Germany, who has 34 articles and works closely with Anderson Machado. Followed by Geissen Violette with 32 articles who frequently collaborates with Esperanza Lwanga. In addition, Luo Yongming, Yang An, Yang Xiaomei, and Jones David are all influential authors of this study. These authors are mainly from the United States, Germany, the Netherlands, China and other countries, which are also leaders in soil MPs research.
In general, the number of publications in a country is closely associated with the proportion of researchers, research institutions and the availability of research funds involved. Some countries pay the highest attention to MPs in soil, Germany, the United States, the Netherlands and China, etc.(keyword cluster: Country). It can be seen that there is close cooperation among authors and institutions, establishing an international platform for the research and communication of soil MPs, and global institutions are increasingly involved in the research of MPs in soil.
The amount of fund project investment is also one of the criteria to judge the degree of attention paid to research. As a major agricultural country, it is increasing year by year the investment of funds on MPs of soil in China (Fig. 4). The National Natural Science Foundation of China is the most important funded project in the field of soil MPs research. In addition, there are provincial and municipal fund projects, National S&T Planed Projects, funds of the Ministry of Education, national social science fund projects and other fund projects.
Current situation of microplastic pollution in soil
The terrestrial environment, as the gathering place of microplastic pollution, is considered as the main important source because of its transportation to marine plastics. Data shows that the number of MPs discharged into farmland in Europe and North America each year reaches 110,000 t and 730,000 t respectively (Nizzetto et al. 2016), both exceeding the annual average growth rate of MPs in marine surface water (Table 1).
According to the data in the published literature, MPs have almost been detected in densely populated cities, sparsely populated remote areas, and even Antarctica (Obbard et al. 2014). However, the existing data are not enough to analyze the overall pollution situation of soil MPs in the global or regional scope, and some data on the amounts, size, shape, and composition of MPs in the soil environment are lacking. In addition, there is no unified standard for the detection of MPs in soil, leading to differences in the methods used and poor comparability of relevant results.
Sources and distribution
Sources of MPs in soil
In a report, Boucher and Friot identified and evaluated seven major sources of MPs based on global literature information: tire, synthetic textile, marine coatings, road markings, personal care products, plastic pellets, and city dust (Boucher and Friot 2017), and their respective proportions are shown in Fig. 5. These different sources come from different family or business activities on land and at sea. Among them, synthetic textiles (35%), tire (28%) and city dust (24%) contribute the most to MPs.
Primary microplastic fibers are produced through fiber abrasion and shedding when synthetic textiles are washed in industrial laundries and households, and then discharged into wastewater. Using untreated wastewater directly for irrigation may eventually cause MPs to enter into the soil. It has been studied that MPs were detected in household wastewater up to 627,000 items/m3 (Majewsky et al. 2016). Correspondingly, irrigation wastewater contains enormous number of MPs particles with an average diameter of 164 to 327 μm (Hartline et al. 2016). Generally, the sewage will be treated by the wastewater plant. Some MPs will be filtered out in the sewage treatment system, up to 90% of the MPs removed from the sewage and retaining in the sludge,which is mostly used for agricultural fertilizer fields (Corradini et al. 2019). Accordingly, the concentration of MPs detected in the sludge is 1500 to 24,000 items/kg (Mahon et al. 2017). In Chile, the abundance of MPs in sewage sludge fluctuates between 18–41 items/g, with a median value of 34 items/g (He et al. 2019). In eastern Spain, the abundance of MPs detected in sewage sludge is 18,000 ± 15,940 items/kg (light density MPs) and 32,070 ± 19,080 items/kg (heavy density MPs) (Bai et al. 2017) Therefore, the application of sewage or sludge is the main source of MPs in soil.
The use of a large amount of plastic film in farmland is also a major source of MPs. It is a technology to obtain larger crop harvest and improve crop quality since plastic film has been widely used as the roof or mulching film of greenhouse, which can increase soil temperature and improve water use efficiency (Yang et al. 2020). In 2016, there were 4 million tons of plastic films on the global market, which is expected to grow at an annual growth rate of 5.6% by 2030 (Zhao et al. 2016). The most common type of polymer for plastic covering is polyethylene, including high-density, low-density, and linear low-density polyethylene. In China, Japan, and South Korea alone (accounting for 80% of the global plastic), 700,000 t of low-density PE are used every year (Li et al. 2016). It is a labor-intensive and time-consuming job to remove all mulch film from farmland. Therefore, mulch film is often left in farmland intentionally or unintentionally, forming MPs.
Tyre dust is also one of the important sources of MPs in soil. Tyres, brake and road will be worn during use, resulting in MPs. They may stay on the land, be blown into atmosphere by wind to become aerosols along with other city dust. Once it rains, they are washed away and become urban runoff, which flows through wetlandsand is intercepted into the soil. Sweden emits about 10,000 t of tire dust every year, and Germany even emits as much as 110,000 t (Blasing and Amelung 2018). City dust includes the abrasion of infrastructure (household dust, urban dust, tire dust, artificial turf, ports and docks, architectural coatings), as well as sand blasting and intentional dumping of abrasives (detergents). These MPs most often occur in urban environments, which can enter the soil through atmospheric sedimentation. Dris reported and estimated the deposition of fibrous MPs for the first time, in which the settling flux of outdoor atmospheric MPs can reach 0.3–1.5 fibers/m3 (Dris et al. 2015). In remote mountainous areas, 249 fragments, 73 films and 44 fibers can be deposited every day through atmospheric sedimentation (Zhang and Liu 2018). MPs carrying other air pollutants may be exchanged in different areas through air transport before they settle into the soil.
Accumulation and fate of MPs in soil
Once the MPs are accumulated in soil, the soil surface provides a potential degradation environment for MPs due to the direct ultraviolet radiation, the increased availability of oxygen and relatively high temperature. What's more, the microorganisms and terrestrial organisms in soil may accelerate the biodegradation of plastics. In addition, agricultural processes such as tilling and crop rotation may transform plastic fragments into MPs. However, these non-human- interventions aids (driven by natural weathering and biological effects) are just a drop in the bucket for the long journey of microplastic degradation. The analysis results show that the weight loss rate of PE is only 0.1%—0.4% after soaking in soil for 800 days, and the weight loss rate of PP after soaking in soil for one year is only 0.4%, while PVC has not been degraded after 35 years of use in natural soil environment (Zhang and Liu 2018).It is urgent to accelerate the degradation of MPs by human intervention and the microorganisms and terrestrial organisms in soil may give a good ideas for this challenge.
MPs in the surface soil may enter the deeper soil through tilling, or they may migrate down along large cracks or fall by disturbance of biome in soil. All of the above may lead to the leaching of MPs in groundwater, polluting it. MPs or nanoplastics have not yet been analyzed in groundwater samples, but the transport through biological pores has been identified as a possible mechanism of groundwater pollution. MPs can be ingested by different types of terrestrial animals. Research shows that PE, PP, PVC, PS, PA, and other MPs can be ingested and accumulated in soil nematodes and Caenorhabditis elegans (Liu et al. 2018; Lei et al. 2018). PE MPs can be transported to the middle and bottom layers of the soil after 21 days by earthworms, and the smallest MPs can reach the deepest layer of the soil (Rillig et al. 2017). Lwanga evaluated and analyzed the micro and macro plastics in soil, earthworm manure, chicken manure, crops, and sandbags, and found that both micro and large plastics can enter the land food web (Huerta Lwanga et al. 2017). Therefore, the intake of soil organisms is an important factor affecting the accumulation of MPs in the soil system.
Analytical methods of MPs in soil
Undoubtedly, removing MPs from soil is the best remediation strategies to eliminate a range of MPs pollution. To bear the brunt is the ability to identify and separate MPs. However, they are so difficult that even the laboratory identification and screening of MPs is still in its infancy.
Overview of analytical methods
Although MPs are ubiquitous in marine, freshwater, soil and atmospheric environments, the analysis methods of MPs are varied in different environmental media (Zhang et al. 2016; Mai et al. 2018). At present, there are relatively fixed detection methods for micro plastics in the marine environment. However, due to the complexity of the soil, there is no standardized method to quantitatively analyze the MPs in soil. Soil is a mixture of solid (such as organic matter, clay, mineral, etc.) and liquid, which is the habitat of organisms and microorganisms. As animals and plants are often left in soil, the organic matter is rich, which can be further metabolized into relatively stable substances, namely humus. As a consequence, organic matter and other impurities may be firmly adhered to the MPs, affecting the flotation separation effect of MPs, and interfering with the identification of MPs through infrared microscopy (Peng et al. 2018). Therefore, the detection method of MPs in soil needs to be further improved based on the detection method of MPs in water environment. At present, it is urgent to develop an accurate and effective analysis method of MPs in soil samples.
For the collection of MPs in soil, it is necessary to determine the collection method according to the research purpose and actual environmental conditions, then select the appropriate sampling location to be studied, determine the sampling depth, and use tools to collect appropriate soil samples. The analysis method of MPs in soil is similar to that of sediment in water environment, mainly including separation, identification and quantification of test samples. Since there is no standardized method for the separation and extraction of MPs from soil, referring to the method for the separation of MPs from marine sediments, the separation of MPs from soil can be divided into two processes: extraction of MPs and removal of impurities. According to the proportion of clay and organic matter, soil samples are dried, screened, floated, filtered and density separated, and organic matter is extracted and digested. Finally, the potential MPs need to be visually identified under an optic microscope, followed by confirmation by micro-Fourier transformed infrared (m-FT-IR) or Raman spectroscopy (Liu et al. 2018; Peng et al. 2018). Theoretically, there is still much room for improvement in this method (Fig. 6).
Extraction of MPs
The structure of aggregates in soil is complex and restricted by many factors, so the operation of separating MPs from soil is usually more difficult than from water and sediment. The commonly used separation operations include density separation, screening filtration, pressurized fluid extraction, digestion, etc. Different extraction methods have their own advantages and disadvantages, and the extraction effects for microplastics are also different (Table 2).
The dry soil samples are sieved firstly to obtain MPs. In previous studies, there were varies in the size of sieves used for different media samples. For example, first, use 5mm sieve to remove larger impurities, and then use a 0.3mm sieve to screen and extract MPs from dried sediment beach samples (NOAA 2015). Different from water and sediment samples, it is generally recommended that soil samples pass through a 5 mm sieve initially, and then use density separation method to separate microplastic particles (Zhang and Liu 2018). In this process, the microplastic particles are floated out of the soil matrix by using the salt solution with high density(> 1.4 g/cm3). For example, 25% saturated solution of ZnBr2 (1.71g/cm3) cause sediment floatation with good recoveries (99%) for mixed microplastic sample that most closely represents environmental samples (Courtene-Jones et al. 2017). Pour soil sample into the high-density solution, and plastic particles float on the solution surface, while the denser soil materials remain at the bottom of solution. At present, using distilled water to extract light plastics (such as PE and PP) from soil is simple and economical. However, in most cases, saturated NaCl solution (density 1.18 g/cm3) is generally used to extract MPs in sediment; Nevertheless, it is now suspected that this method cannot separate many high-density MPs, such as PET or PVC (Zhang et al. 2018c).After all, the specific gravity of PET and PVC are 1.37 g/cm3 and 1.38 g/cm3, respectively. In a recent study, it was developed that a separation method of MPs in agricultural soil samples (Liu et al. 2018). Saturated NaCl solution (density 1.18 g/cm3) was still used for extraction, but the extraction times and ultrasonic treatment were increased (treated by ultrasonic for 2 min), and the flotation time was extended (Mixtures were stirred 30 min in order to float out supernatants, which were then settled for 24 h). Among the 9 common MPs added to the soil, 7 of PP, PE, polyamide (PA), polycarbonate (PC), acrylonitrile butadiene styrene (ABS), polymethyl methacrylate (PMMA) and PS particles were successfully extracted. Experimental results have shown that ultrasonic crushing has efficiency in extracting MPs, and increasing flotation time is beneficial for separating MPs, although not the longer the time, the better. Only PET and PVC particles were still not separated from the soil; Therefore, this method is not suitable for extracting PET and PVC.
In addition, CaCl2 solution (density 1.35 g/cm3) is also used to extract MPs from soil, which is relatively more efficient compared with NaCl solution, but divalent Ca2+ will agglomerate organic substances, affecting subsequent identification experiments. Van Cauwenberghe recommended using ZnCl2 or NaI with a solution density of 1.6 ~ 1.8 g/cm3 for experiments (Cauwenberghe et al. 2015). However, these substances are relatively expensive compared to sodium chloride. In addition, to achieve the maximum density of ZnCl2 solution, acid solution is usually added, which may change the morphology of MPs in soil samples. Except density flotation, other methods are also commonly used to separate MPs from soil matrix. Crichton proposed an oil extraction method to fully mix dry sediment samples with water and rapeseed oil until oil, water and minerals are completely separated. Once the MPs contact the rapeseed oil, they will be extracted to the oil layer, while other impurities will remain in the water, to realize the separation of MPs in the sediment (Crichton et al. 2017). Through this technology, the recovery efficiency of MPs is more than 90%, higher than that of NaI and CaCl2 density extraction. It can also extract weathered environmental MPs from sediment samples, and does not require long-term settlement and digestion, so the cost is low. Another study focused on the electrostatic behavior of MPs, which helps to separate MPs from a variety of environments, including water, sediment, and bleach sand; It is reported that the recovery rate of each plastic is close to 100% (Felsing et al. 2018). Nevertheless, it is uncertain whether these methods are suitable for large-scale separation of MPs from soil.
Removal of soil organic matter
The density of organic matter in soil is generally between 1.0 and 1.4 g · cm−3, which is similar to the density of plastics such as PET and nylon (Blasing et al. 2018). Therefore, simple density sorting is not enough, and steps to remove organic matter need to be added. For water, sediment and biological samples, different chemicals are used for digestion operations, including acid, alkaline or oxidation treatment, and enzymatic hydrolysis (Dehaut et al. 2016) (Table 3).
Furthermore, Hurley compared of 10 M NaOH, 10% KOH, 30% H2O2 solution and Fenton reagent in soil microplastic analysis, providing the key information on the removal efficiency of different reagents (Hurley et al. 2018). The use of alkaline solution (10 M NaOH, 10% KOH, 60 ℃) cannot effectively remove organic interfering substances from soil and sludge, and its effective removal rate of organic substances is less than 70%. 30% H2O2 pre-digestion at 70 ℃ can effectively remove organic matter and has no effect on most MPs. Fenton reagent is currently the most effective method for the removal of organics in sludge samples, with a removal rate of 86%. This shows that Fenton reagent combined with density separation is an effective extraction method of MPs. Another study showed that most organic matters were removed in a short time after HNO3 solution was digested; yet, under the effect of HNO3, the morphology of ABS, PA, PET, and other particles has changed (Scheurer and Bigalke 2018). Consequently, digestion may sometimes destroy MPs leading to erroneous results.
Identification and description
The identification of MPs is usually based on the physical and chemical characteristics of the particles separated from the mixture after the extraction and purification steps. The identification methods are also distinct in different studies, which includes on-site identification, visual identification method based on stereo microscope, chromatography, thermogravimetry, vibration spectrum and proton nuclear magnetic resonance spectrum, etc.
The visual recognition is the earliest and simplest analysis method of MPs (Möller et al. 2020). For MPs with large particle size (> 1 mm), it can be identified by naked eyes according to specific attributes (such as color, shape, or surface texture) (Löder et al. 2015; Liebezeit and Dubaish 2012). The shapes of MPs are generally divided into five categories: fiber, fragment, pellet, granule, and film. The common criteria for distinguishing MPs are as followings: 1) They cannot be easy to be torn apart; 2) Have distinguishable colors; 3) No visible cells or organic structures. Visual classification provides a simple and quick method for experts and nonprofessionals. To identify small MPs (i.e., < 1 mm) in soil, stereomicroscopes or dissecting microscopes with professional image software are widely used (Gouin et al. 2015), but there is a problem with visual classification. Various people observe different analysis results, which shows an error rate of 20–70% (Shim et al. 2017). For example, Eriksen described that about 20% of particles were wrongly identified. These particles were initially identified as MPs by visual observation, and later identified as aluminum silicate from coal ash by scanning electron microscopy (SEM) (Eriksen et al. 2013). In other studies, through visual counting, 32% of MPs with size less than 100 um were uncertain after Raman spectral analysis. Through FTIR analysis, up to 70% of particles were incorrectly identified as MPs (Lenz et al. 2015). Therefore, it is not enough to rely only on visual recognition, which must be determined by Raman spectroscopy or Fourier infrared spectroscopy.
Vibration spectra, such as Raman spectroscopy or Fourier transform infrared spectroscopy (FTIR), are widely used in the research of MPs, because they can accurately identify the type, abundance, shape, and size of polymers. U-FTIR spectroscopy can identify particles with a size of 10 to 500 um. Large particles (> 500 um) can be analyzed by ATR-FTIR spectrum with attenuated total reflection (Blasing and Amelung 2018), while Raman spectroscopy can be used for chemical imaging of samples with smaller size (< 10um). The running time of Raman imaging is significantly longer than that of FTIR imaging, but is independent of the shape, size or thickness of the measured particles. The shape, size or thickness of the measured particles may affect the results of u-FTIR imaging (Blasing and Amelung 2018). Due to the high absorption of infrared radiation by black particles, there are often unrecognized infrared spectra. In addition, Raman spectra are insensitive to water and atmospheric CO2. As a result, the background fluorescence of organic substances or pigments in the polymer may strongly interfere with the required spectrum, making them unrecognizable (Sperber et al. 2016). This may be a problem when dealing with soil samples of high organic content. For these two vibration spectroscopy methods, the samples need to be thoroughly purified before analysis. For solid samples, this operation significantly reduces the number of samples that can be processed. Fuller and Gautam used pressurized fluid extraction (PFE) to dissolve specific MPs in soil, evaporate the polymer solvent extract, and measure the solid residue through ATR-FTIR spectrometer (Fuller and Gautam 2016). This method is fast because it does not require sample purification and is not affected by particles size. However, like chromatographic method, it is also a destructive method, which only allows quantitative analysis and does not provide information about the number, size and shape of polymer particles. In addition, multiple polymer types in one sample will produce complex absorption spectra, which may hinder the identification of MPs.
Some researchers used chromatography to qualitatively and quantitatively identify various types of polymers. Size exclusion chromatography (SEC) liquid extraction is used for the identification and quantification of polystyrene (PS) and polyethylene terephthalate (PET) in soil samples (Eriksen et al. 2015). Pyr GC–MS is also a highly 0sensitive and effective method for characterizing and quantifying a variety of polymer types and their organic additives. It has been proved that Pyr GC–MS is very suitable for the detection of MPs in environmental samples (Elert et al. 2017). On the other hand, it also has several disadvantages. The size of the pyrolysis capsule and the number of samples in each run are very small, 1.5 mm and 0.5 mg, respectively. For this reason, heavy purification works have been required in matrix rich samples, making it very unsuitable for batch analysis (Elert et al. 2017). And that, it is easy to be polluted or even blocked. To overcome these shortcomings, Dimichen developed a thermal extraction desorption gas chromatography-mass spectrometer (TEDGC-MS) for the detection of MPs, which can hold up to 100 mg of samples in each operation. In addition to grinding and mixing to homogenize the samples, no pretreatment is required, and the processing time takes only 2 to 3 h (Fries et al. 2013).
In general, due to the complexity of soil environment and its rich organic properties, the analysis of MPs in soil is still a great challenge. At present, the methods used to determine MPs in soil are inconsistent, including spatial and temporal change patterns, effects of environmental factors, pollution control, etc. These have led to the inability to compare data among different studies and to confirm whether these results represent the true level of MPs pollution. To effectively monitor and compare, it is necessary for the scientific community to standardize the analysis and evaluation methods and procedures of MPs in soil.
The remediation strategies of MPs in soil
Undoubtedly, the best remediation strategy of MPs in soil is nothing more than removing MPs from soil. Identifying and descripting MPs is only the first and most important step in manually removing MPs, which has been done this well from beginning to end in this paper to review the research by scientists around the world. This is not enough, in addition, many new ways need to be explored, which is necessary and possible—nature has its own laws of operation. Some organisms have been found the ability of degradation MPs – invertebrates (Rhyzopertha Dominica), microorganisms (Aspergillus flavus, Bacillus) and algae, etc. (Zhang et al. 2020, 2022; Yang et al. 2014; Sanniyasi et al. 2021; Khan et al. 2023; Eydi et al. 2024). Most studies in this regard occur in aquatic environments (Especially sediments in oceans and lakes), but rarely in soil. Discussing more methods to eliminate existing MPs has become the focus of many scientists.
MPs are abundant in soil, seriously damaging the interaction between plants and soil. The interaction is of great significance for ecosystem stability and plant survival and development. Plants promote the healthy development of soil by improving soil structure, secreting root substances, and preventing soil erosion. Correspondently, soil provides nutrients and water for plants, regulates soil microbial communities and affects plant root development and physiological processes. This interaction creates a good synergistic effect between plants and soil, promoting the healthy operation of the ecosystem, in which the roots play an important role. As is well known, the presence of a large amount of MPs in soil has the greatest impact on disrupting the rhizosphere nutrient exchange of plants, preventing them from obtaining sufficient nutrients through root absorption, thereby seriously affecting plant growth and resulting in the crops cannot achieve high yields.
There are abundant microorganisms in soil, which are different from aquatic environments and have their own characteristics. Recently, an increasing number of studies suggest that microorganisms in soil are involved in the rhizosphere trophic uptake. For example, the fungi also play vital roles in soil structure, plant performance, and plant biodiversity and productivity (He et al. 2009). Arbuscular mycorrhizas (AM) are formed between arbuscular mycorrhizal fungi (AMF) and roots of ~ 70% of ~ 391,000 higher plant species. AMF acquires soil nutrients, such as nitrogen (N), phosphorus (P) and other mineral nutrients, and transport them to their host plant (Luo et al. 2023; He et al. 2009; Selosse et al. 2006). In an arbuscular mycorrhiza, the intraradical mycelium (IRM) often penetrates root cortical cells to form arbuscules, while the extraradical mycelium (ERM) extends into soil, far beyond the root zone. The ERM is extensive enabling plant access to nutrient resources well beyond the root. If AM can absorb nutrients outside the root depletion zone while degrading MPs, it would be the best situation. Of course, this requires further experimental verification. Undoubtedly, there are so many microorganisms in soil that may be waiting to discover their additional functions. If an unlimited amount of soil microorganisms are used to treat a large amount of MPs, it is definitely an environmentally and friendly way.
Conclusion: the main challenges and prospects of future research
To sum up, current research shows that the understanding of MPs in soil is increasing. However, there are still many deficiencies in the understanding of MPs pollution and its impact on the environment (Zhang et al. 2016, 2018c).There are still many problems in the analysis method, environmental concentration, source, fate and ecological consequences of MPs in soil.
As is known to all, there are MPs in soil, much more than in atmospheric and aquatic environments. Therefore, it is of great significance to study the potential toxicity of MPs to organisms in soil and this seriously affects the growth of plants on soil. The effect of nutrient transfer and intergenerational transfer should not be underestimated. The MPs accumulated by plants can be transferred through the food chain, posing potential risks to human health. As a new persistent pollutant, it is urgent to investigate the distribution, transportation, and degradation of MPs in terrestrial environment to quantify environmental behavior and their impacts. For example, MPs in soil can be transported horizontally by wind and water or vertically by water or soil organisms, or even degraded gradually driven by microorganisms or physicochemistry, by which the source and degradation rate of MPs can be mastered.
Through bibliometric analysis based on CiteSpace, it is found that, compared to aquatic environments such as the ocean, there is a significant lack of research although soil MPs started early. The United States, Germany, the Netherlands, China and other countries have initially established an international platform for research and exchange. Nonetheless, global research on MPs in soil is only in its infancy due to insufficient investment in fund projects. The current study largely lacks some data on the concentration, volume, type, and composition of MPs in soil environment, and the data are not sufficient to analyze the overall contamination of soil MPs on a global or regional scale. The analysis of MPs and plastic usage patterns in different types of soil are also needed to gain more quantitative data, and even to participate in the development of simulation experiments. MPs in the study of normalized is imperative, to obtain the real condition of MPs in soil, in addition to establish a standardized method about collection, separation and analysis of MPs samples in soil, which is suitable for different source, composition, size and shape of MPs. That is, establish accurate, simple and efficient detection method of MPs in soil, and take MPs of soil into the scope of conventional environmental monitoring, quantify the risk of plastic pollution.
MPs removal technology needs further development. The addition of MPs removal process in wastewater treatment will help reduce the amount of MPs from access to soil ecosystems via sewage irrigation. At present, among the removal technologies of MPs in soil, the use of bioremediation technology to remove MPs has attracted wide attention due to its potential for energy conservation and environmental protection. However, there are also potential risks such as the release of adsorbed pollutants and the formation of toxic secondary metabolites of plastics. Secondary metabolites were detected in almost all sludges. For example, di-2-ethylhexyl phthalate (DEHP) and di-ethylhexyl adipate (DEHA) as the common plasticizer, can generate toxic metabolites including 2-ethylhexanoic acid, 2-ethylhexanol, and 2-ethylhexanal (Beauchesne et al. 2008). However, not all of the metabolites are toxic, and the environmental impacts and fate of the degradation products arising from plasticizers are unclear. Therefore, the removal of MPs in the environment remains to be further studied. In this regard, it is necessary to increase the awareness of MPs and fund investment to reduce the use and emission of plastics at the source. For example, changing the behavior of manufacturers and consumers, and guiding people to minimize the plastic waste left in soil environment. In addition, people need to rely on legal means to clarify the responsibilities and related penalties of government departments and enterprises in the production, use, recycling and disposal of plastic in order to control plastic pollution in soil. For the huge amount of preexisting MPs, microbial degradation of MPs in soil is a subject of environmental protection and economy, which is worth investment funds in in-deep research.
Data Availability
The datasets analysed in the current study are available in the text.
References
Andrady AL (2011) MPs in the marine environment. Mar Pollut Bull 62:1596–1605
Avio CG, Gorbi S, Regoli F (2015) Experimental development of a new protocol for extraction and characterization of microplastics in fish tissues: first observations in commercial species from Adriatic Sea. Mar Environ Res 111:18–26
Bai R, Liu H, Cui J, Wu Y, Guo X, Liu Q, Liu Q, Gao H, Yan C, He W (2024) The characteristics and influencing factors of farmland soil microplastic in Hetao Irrigation District. China J Hazard Mater 465:133472. https://doi.org/10.1016/j.jhazmat.2024.133472
Bai Y, Zang C, Gu M, Gu C, Shao H, Guan Y, Wang X, Zhou X, Shan Y, Feng K (2017) Sewage sludge as an initial fertility driver for rapid improvement of mudflat salt-soils. Sci Total Environ 578:47–55. https://doi.org/10.1016/j.scitotenv.2016.06.083
Beauchesne I, Barnabé S, Cooper DG, Nicell JA (2008) Plasticizers and related toxic degradation products in wastewater sludges. Water Sci Technol 57(3):367–374. https://doi.org/10.2166/wst.2008.001
Blasing M, Amelung W (2018) Plastics in soil: analytical methods and possible sources. Sci Total Environ 612:422–435
Boucher J, Friot D (2017) Primary microplastics in the oceans: a global evaluation of sources. Iucn, Gland, pp 227–229
Bouwmeester H, Hollman PC, Peters RJ (2015) Potential health impacts of environmental released micro- and nanoplastics in the human food chain production chain: experiences from nanotoxicity. Environ Sci Technol 49:8932–8947
Brožová K, Halfar J, Čabanová K, Motyka O, Drabinová S, Hanus P, Heviánková S (2023) The first evidence of microplastic occurrence in mine water: the largest black coal mining area in the Czech Republic. Water Res 244:120538. https://doi.org/10.1016/j.watres.2023.120538
Cauwenberghe LV, Devriese L, Galgani F, Robbens J, Janssen CR (2015) MPs in sediments: a review of techniques, occurrence and effects. Mar Environ Res 111:5–17
Chai B, Wei Q, She Y, Lu G, Dang Z, Yin H (2020) Soil microplastic pollution in an e-waste dismantling zone of China. Waste Manag 118:291–301. https://doi.org/10.1016/j.wasman.2020.08.048
Claessens M, van Cauwenberghe L, Vandegehuchte MB, Janssen CR (2013) New techniques for the detection of MPs in sediments and field collected organisms. Mar Pollut Bull 70(1/2):227–233. https://doi.org/10.1016/j.marpolbul.2013.03.009
Cole M, Lindeque P, Fileman E, Halsband C, Goodhead R, Moger J, Galloway TS (2013) Microplastic ingestion by zooplankton. Environ Sci Technol 47(12):6646–6655
Cole M, Webb H, Lindeque PK, Fileman ES, Halsband C, Galloway TS (2014) Isolation of MPs in biota-rich seawater samples and marine organisms. Sci Rep 4:4528
Corradini F, Meza P, Eguiluz R, Casado F, Huerta-Lwanga E, Geissen V (2019) Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci Total Environ 671:411–420
Courtene-Jones W, Quinn B, Gary SF, Mogg AOM, Narayanaswamy BE (2017) Microplastic pollution identified in deep-sea water and ingested by benthic invertebrates in the Rockall Trough, North Atlantic Ocean. Environ Pollut 231(Pt 1):271–280. https://doi.org/10.1016/j.envpol.2017
Cózar A, Echevarría F, González-Gordillo JI, Irigoien X, Ubeda B, Hernández-León S, Palma AT, Navarro S, García-de-Lomas J, Ruiz A, Fernández-de-Puelles ML, Duarte CM (2014) Plastic debris in the open ocean. Proc Natl Acad Sci USA 111(28):10239–10244
Crichton EM, Noel M, Gies EA, Ross PA (2017) A novel, density-independent and FTIR-compatible approach for the rapid extraction of MPs from aquatic sediments. Anal Method 9:1419–1428
da Costa JP, Santos PSM, Duarte AC, Rocha-Santos T (2016) (Nano) plastics in the environment-sources, fates and effects. Sci Total Environ 566:15–26. https://doi.org/10.1016/j.scitotenv.2016.05.041
Davidson K, Dudas SE (2016) Microplastic ingestion by wild and cultured Manila clams (Venerupis philippinarum) from baynes soun, British Columbia. Arch Environ Contam Toxicol 71(2):147–156
de Souza Machado AA, Lau CW, Kloas W, Bergmann J, Bachelier JB, Faltin E, Becker R, Görlich AS, Rillig MC (2019) Microplastics can change soil properties and affect plant performance. Environ Sci Technol 53(10):6044–6052. https://doi.org/10.1021/acs.est.9b01339
De Witte B, Devriese L, Bekaert K, Hoffman S, Vandermeersch G, Cooreman K, Robbens J (2014) Quality assessment of the blue mussel (Mytilus edulis): comparison between commercial and wild types. Mar Pollut Bull 85(1):146–155. https://doi.org/10.1016/j.marpolbul.2014.06.006
Dehaut A, Cassone AL, Frere L, Hermabessiere L, Himber C, Rinnert E, Riviere G, Lambert C, Soudant P, Huvet A, Duflos G, PaulePont I (2016) MPs in seafood: benchmark protocol for their extraction and characterization. Environ Pollut 215:223–233
Dris R, Gasperi J, Rocher V, Saad M, Renault N, Tassin B (2015) Microplastic contamination in an urban areaea case study in greater Paris. Environ Chem 12:592–599
Elert AM, Becker R, Duemichen E, Eisentraut P, Falkenhagen J, Sturm H, Braun U (2017) Comparison of different methods for MP detection: what can we learn from them, and why asking the right question before measurements matters? Environ Pollut 231:1256–1264. https://doi.org/10.1016/j.envpol.2017.08.074
Enders K, Lenz R, Beer S (2017) Extraction of microplastic from biota: recommended acidic digestion destroys common plastic polymers. ICES J Mar Sci 74(1):326–331
Eriksen M, Lebreton LC, Carson HS, Thiel M, Moore CJ, Borerro JC, Galgani F, Ryan PG, Reisser J (2014) Plastic pollution in the world’s oceans: more than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 9:e111913. https://doi.org/10.1371/journal.pone.0111913
Eriksen M, Mason S, Wilson S, Box C, Zellers A, Edwards W, Farley H, Amato S (2013) Microplastic pollution in the surface waters of the Laurentian great Lakes. Mar Pollut Bull 77:177–182. https://doi.org/10.1016/j.marpolbul.2013.10.007
Eydi Gabrabad M, Yari M, Bonyadi Z (2024) Using Spirulina platensis as a natural biocoagulant for polystyrene removal from aqueous medium: performance, optimization, and modeling. Sci Rep 14(1):2506. https://doi.org/10.1038/s41598-024-53123-y
Felsing S, Kochleus C, Buchinger S, Brennholt N, Stock F, Reifferscheid G (2018) A new approach in separating MPs from environmental samples based on their electrostatic behavior. Environ Pollut 234:20–28. https://doi.org/10.1016/j.envpol.2017.11.013
Foekema EM, De Gruijter C, Mergia MT, van Franeker JA, Murk AJ, Koelmans AA (2013) Plastic in north sea fish. Environ Sci Technol 47(15):8818–8824. https://doi.org/10.1021/es400931b
Fries E, Dekiff JH, Willmeyer J, Nuelle MT, Ebert M, Remy D (2013) Identification of polymer types and additives in marine microplastic particles using pyrolysis-GC/MS and scanning electron microscopy. Environ Sci Process Impacts 15(10):1949–1956
Fuller S, Gautam AA (2016) Procedure for measuring MPs using pressur-ized fluid extraction. Environ Sci Technol 11:5774–5780
Geyer R, Jambeck JR, Law KL (2017) Law, Production, use, and fate of all plastics ever made. Sci Adv 3:e1700782. https://doi.org/10.1126/sciadv.1700782
Goli VN, Paleologos EK, Farid A, Mohamed AMO, O’Kelly BC, El Gamal MM, Singh DN (2022) Extraction, characterisation and remediation of microplastics from organic solid matrices. Environmental Geotechnics 40:1–34. https://doi.org/10.1680/jenge.21.00072
Gouin T, Avalos J, Brunning I (2015) Use of micro-plastic beads in cosmetic products in Europe and their estimated emissions to the North Sea environment. SOFW J 141(4):40–46
Hartline NL, Bruce NJ, Karba SN, Ruff EO, Sonar SU, Holden PA (2016) Micro-fiber masses recovered from conventional machine washing of new or aged garments. Environ Sci Technol 21:11532–11538. https://doi.org/10.1021/acs.est.6b03045
He P, Chen L, Shao L, Zhang H, Lü F (2019) Municipal solid waste (MSW) landfill: a source of microplastics? Evidence of microplastics in landfill leachate. Water Res 159:38–45. https://doi.org/10.1016/j.watres.2019.04.060
He X, Xu M, Guo Y, Zhou J (2009) Use of 15N stable isotope to quantify nitrogen transfer between mycorrhizal plants. J Plant Ecol 2:107–118. https://doi.org/10.1093/jpe/rtp015
Herrera A, Garrido-Amador P, Martínez I, Samper MD, López-Martínez J, Gómez M, Packard TT (2018) Novel methodology to isolate microplastics from vegetal-rich samples. Mar Pollut Bull 129(1):61–69. https://doi.org/10.1016/j.marpolbul
Huerta Lwanga E, Mendoza Vega J, Ku Quej V, Chi JLA, Sanchez Del Cid L, Chi C, Escalona Segura G, Gertsen H, Salánki T, van der Ploeg M, Koelmans AA, Geissen V (2017) Field evidence for transfer of plastic debris along a terrestrial food chain. Sci Rep 7:14071. https://doi.org/10.1038/s41598-017-14588-2
Hurley RR, Lusher AL, Olsen M, Nizzetto L (2018) Validation of a method for extracting mps from complex, organic-rich. Environ Matrices Environ Sci Technol 52(13):7409–7417. https://doi.org/10.1021/acs.est.8b01517
Judy JD, Williams M, Gregg A, Oliver D, Kumar A, Kookana R, Kirby JK (2019) Microplastics in municipal mixed-waste organic outputs induce minimal short to long-term toxicity in key terrestrial biota. Environ Pollut 252:522–531. https://doi.org/10.1016/j.envpol.2019.05.027
Khan S, Ali SA, Ali AS (2023) Biodegradation of low density polyethylene (LDPE) by mesophilic fungus ‘Penicillium citrinum’isolated from soils of plastic waste dump yard, Bhopal. India Environ Technol 44(15):2300–2314. https://doi.org/10.1080/09593330.2022.2027025
Kim SW, An YJ (2019) Soil microplastics inhibit the movement of springtail species. Environ Int 126:699–706
Klemmensen NDR, Chand R, Blanco MS, Vollertsen J (2024) Microplastic abundance in sludge-treated fields: variance and estimated half-life. Sci Total Environ 922:171394. https://doi.org/10.1016/j.scitotenv.2024.171394
Kumar A, Mishra S, Pandey R, Yu ZG, Kumar M, Khoo KS, Show PL (2023) Microplastics in terrestrial ecosystems: Un-ignorable impacts on soil characterises, nutrient storage and its cycling. TrAC, Trends Anal Chem 158:116869. https://doi.org/10.1016/j.trac.2022.116869
Lehmann A, Fitschen K, Rillig MC (2019) Abiotic and biotic factors influencing the effect of microplastic on soil aggregation. Soil Systems 3(1):21
Lei L, Wu S, Lu S, Liu M, Song Y, Fu Z, Shi H, Raley-Susman KM, He D (2018) Microplastic particles cause intestinal damage and other adverse effects in zebrafish Danio rerio and nematode Caenorhabditis elegans. Sci Total Environ 619–620:1–8. https://doi.org/10.1016/j.scitotenv.2017.11.103
Lenz R, Enders K, Stedmon CA, Mackenzie DMA, Nielsen TG (2015) A critical assessment of visual identification of marine microplastic using Raman spectroscopy for analysis improvement. Mar Pollut Bull 100:82–91. https://doi.org/10.1016/j.marpolbul.2015.09.026
Li K, Ma D, Wu J, Chai C, Shi Y (2016) Distribution of phthalate esters in agricultural soil with plastic film mulching in Shandong Peninsula, East China. Chemosphere 164:314–321. https://doi.org/10.1016/j.chemosphere.2016.08.068
Li XY, Liu HT, Wang LX, Guo HN, Zhang J, Gao D (2022) Effects of typical sludge treatment on microplastics in China-characteristics, abundance and micro-morphological evidence. Sci Total Environ 826:154206. https://doi.org/10.1016/j.scitotenv.2022.154206
Liebezeit G, Dubaish F (2012) Microplastics in beaches of the East Frisian islands Spiekeroog and Kachelotplate. Bull Environ Contam Toxicol 89(1):213–217
Liu M, Lu S, Song Y, Lei L, Hu J, Lv W, Zhou W, Cao C, Shi H, Yang X, He D (2018) Microplastic and mesoplastic pollution in farmland soils in suburbs of Shanghai, China. Environ Pollut 242:855–862. https://doi.org/10.1016/j.envpol.2018.07.051
Löder MGJ, Kuczera M, Mintenig S (2015) Focal plane array detector-based micro-Fourier-transform infrared imaging for the analysis of microplastics in environmental samples. Environ Chem 12(5):563–581
Luo X, Liu Y, Li S, He X (2023) Interplant carbon and nitrogen transfers mediated by common arbuscular mycorrhizal networks: beneficial pathways for system functionality. Front Plant Sci 14:1169310. https://doi.org/10.3389/fpls.2023.1169310
Mahon AM, O’Connell B, Healy MG, O’Connor I, Officer R, Nash R, Morrison L (2017) Microplastics in sewage sludge: effects of treatment. Environ Sci Technol 51(2):810–818. https://doi.org/10.1021/acs.est.6b04048
Mai L, Bao LJ, Shi L, Wong CS, Zeng EY (2018) A review of methods for measuring MPs in aquatic environments. Environ Sci Pollut Res 25:11319–11332. https://doi.org/10.1007/s11356-018-1692-0
Majewsky M, Bitter H, Eiche E, Horn H (2016) Determination of microplastic polyethylene (PE) and polypropylene (PP) in environmental samples using thermal analysis (TGA-DSC). Sci Total Environ 568:507–511. https://doi.org/10.1016/j.scitotenv.2016.06.017
Möller JN, Löder MGJ, Laforsch C (2020) Finding microplastics in soils: a review of analytical methods. Environ Sci Technol 54(4):2078–2090
Nizzetto L, Futter M, Langaas S (2016) Are agricultural soils dumps for MPs of urban origin? Environ Sci Technol 50(20):10777–10779. https://doi.org/10.1021/acs.est.6b04140
Masura J, Baker J, Foster G, Arthur C (2015) Laboratory methods for the analysis of microplastics in the marine environment: recommendations for quantifying synthetic particles in waters and sediments. NOAA Technical Memorandum NOSOR&R-48
Nuelle MT, Dekiff JH, Remy D, Fries E (2014) A new analytical approach for monitoring microplastics in marine sediments. Environ Pollut 184:161–169. https://doi.org/10.1016/j.envpol.2013.07.027
O’Kelly BC, Pantos O, Weaver L, Sarris TS, Goli VSNS, Mohammad A, Singh P, Singh DN (2022) Fate and impact of nano/microplastic in the geoenvironment-ecotoxicological perspective. Environ Geotech 40:1–14. https://doi.org/10.1680/jenge.22.00053
Obbard RW, Sadri S, Wong YQ (2014) Global warming releases microplastic legacy frozen in Arctic Sea ice. Earth’s Future 2:315–320
Peng G, Xu P, Zhu B, Bai M, Li D (2018) MPs in freshwater river sediments in Shanghai, China: a case study of risk assessment in megaecities. Environ Pollut 234:448–456. https://doi.org/10.1016/j.envpol.2017.11.034
Qi R, Jones DL, Li Z, Liu Q, Yan C (2020) Behavior of microplastics and plastic film residues in the soil environment: a critical review. Sci Total Environ 703:134722. https://doi.org/10.1016/j.scitotenv.2019.134722
Rillig MC, Ziersch L, Hempel S (2017) Microplastic transport in soil by earthworms. Sci Rep 7:1362
Rubol S, Manzoni S, Bellin A (2013) Modeling soil moisture and oxygen effects on soil biogeochemical cycles including dissimilatory nitrate reduction to ammonium (DNRA). Adv Water Resour 62:106–124
Sanniyasi E, Gopal RK, Gunasekar DK, Raj PP (2021) Biodegradation of low-density polyethylene (LDPE) sheet by Microalga, Uronema Africanum Borge. Sci Rep 11(1):17233. https://doi.org/10.1038/s41598-021-96315-6
Scheurer M, Bigalke M (2018) MPs in Swiss floodplain soils. Environ Sci Technol 52:3591–3598
Selosse MA, Richard F, He X, Simard SW (2006) Mycorrhizal networks: des liaisons dangereuses? Trends Ecol Evol 21(11):621–628. https://doi.org/10.1016/j.tree.2006.07.003
Shim WJ, Hong SH, Eo SE (2017) Identification methods in microplastic analysis: a review. Anal Methods 9(9):1384–1391
Soursou V, Campo J, Picó Y (2024) Spatio-temporal variation and ecological risk assessment of microplastics along the touristic beaches of a mediterranean coast transect (Valencia province, East Spain). J Environ Manage 354:120315. https://doi.org/10.1016/j.jenvman.2024.120315
Sperber CV, Lewandowski H, Tamburini F, Bernasconi SM, Amelung W, Frossard E (2016) Kinetics of enzymeecatalysed oxygen isotope exchange between phosphateand water revealed by Raman spectroscopy. Raman Spectrosc 48:368–373
Thompson RC, Olsen Y, Mitchell RP, Davis A, Rowland SJ, John AW, McGonigle D, Russell AE (2004) Lost at sea: where is all the plastic? Science 304(5672):838. https://doi.org/10.1126/science.1094559
Wright SL, Kelly FJ (2017) Plastic and human health: a micro issue? Environ Sci Technol 51:6634–6647. https://doi.org/10.1021/acs.est.7b00423
Wu D, Feng Y, Wang R, Jiang J, Guan Q, Yang X, Wei H, Xia Y, Luo Y (2023) Pigment microparticles and microplastics found in human thrombi based on Raman spectral evidence. J Adv Res 49:141–150. https://doi.org/10.1016/j.jare.2022.09.004
Yang L, Zhang Y, Kang S, Wang Z, Wu C (2020) Microplastics in freshwater sediment: a review on methods, occurrence, and sources. Sci Total Environ 754:141948. https://doi.org/10.1016/j.scitotenv.2020.141948
Yang J, Yang Y, Wu WM, Zhao J, Jiang L (2014) Evidence of polyethylene biodegradation by bacterial strains from the guts of plastic-eating waxworms. Environ Sci Technol 48(23):13776–13784. https://doi.org/10.1021/es504038a
Yang L, Zhang Y, Kang S, Wang Z, Wu C (2021) Microplastics in soil: a review on methods, occurrence, sources, and potential risk. Sci Total Environ 780:146546. https://doi.org/10.1016/j.scitotenv.2021.146546
Zhang G, Liu Y (2018) The distribution of MPs in soil aggregate fractions in southwestern China. Sci Total Environ 642:12–20
Zhang H, Zhou Q, Xie Z, Zhou Y, Tu C, Fu C, Mi W, Ebinghaus R, Christie P, Luo Y (2018a) Occurrences of organophosphorus esters and phthalates in the MPs from the coastal beaches in north China. Sci Total Environ 616–617:1505–1512
Zhang K, Shi H, Peng J, Wang Y, Xiong X, Wu C, Lam PKS (2018b) Microplastic pollution in China’s inland water systems: a review of findings, methods, characteristics, effects, and management. Sci Total Environ 630:1641–1653. https://doi.org/10.1016/j.scitotenv.2018.02.300
Zhang S, Yang X, Gertsen H, Peters P, Salánki T, Geissen V (2018c) A simple method for the extraction and identification of light density MPs from soil. Sci Total Environ 616–617:1056–1065. https://doi.org/10.1016/j.scitotenv.2017.10.213
Zhang K, Su J, Xiong X, Wu X, Wu C, Liu J (2016) Microplastic pollution of lakeshore sediments from remote lakes in Tibet plateau, China. Environ Pollut 219:450–455. https://doi.org/10.1016/j.envpol.2016.05.048
Zhang J, Gao D, Li Q, Zhao Y, Li L, Lin H, Bi Q, Zhao Y (2020) Biodegradation of polyethylene microplastic particles by the fungus Aspergillus flavus from the guts of wax moth Galleria mellonella. Sci Total Environ 704:135931. https://doi.org/10.1016/j.scitotenv.2019.135931
Zhang Y, Li Y, Gou H, Xu F, Zhang X, Yang L (2022) Screening of polyethylene-degrading bacteria from Rhyzopertha dominica and evaluation of its key enzymes degrading polyethylene. Polymers 14(23). https://doi.org/10.3390/polym14235127
Zhao Y, Li Y, Wang J (2016) Buried straw layer plus plastic mulching reduces soil salinity and increases sunflower yield in saline soils. Soil Tillage Res 155:363–370
Zhou Q, Zhang H, Zhou Y, Li Y, Xue Y, Fu C, Tu C, Luo Y (2016) Separation of MPs from a coastal soil and their surface microscopic features, China. Sci Bull 61:1604–1611
Zubris KAV, Richards BK (2005) Synthetic fibers as an indicator of land application of sludge. Environ Pollut 138(2):201–211
Funding
This work was funded by a China Scholarship Council grant (202006995017).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Tim S. George.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Yuanqin Zeng contributed as the first author.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, L., Chen, Y., Li, Q. et al. Microplastics in soil: a review on research status, sources, methods, and remediation strategies. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06858-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06858-6