Abstract
Aims
Soil nitrogen (N) mineralization-immobilization turnover (MIT) regulates the inorganic N supply in terrestrial ecosystems. Much research has been done to understand the factors that control the MIT in soils, but how plants-soil-microbe interactions influence the MIT remains to be further explored.
Methods
A series of 15N tracing experiments, including with and without maize (Zea mays L.) planting, maize shading, and maize root endophytes inoculation, were conducted to investigate the drivers of the change in MIT by the presence of plant.
Results
Soil gross N mineralization (M), especially the mineralization of recalcitrant organic-N to NH4+ (MNrec, 0.51 mg N kg−1 d−1) was significantly improved by maize compared with the control treatment (without maize) (0.07 mg N kg−1 d−1). MNrec significantly decreased after removing maize or covering maize with a black box (preventing photosynthesis). Soil dissolved organic carbon (DOC) concentration significantly decreased after preventing photosynthesis, and showed a significant positive relationship with MNrec, confirming that photosynthetic substrate supply was the dominating factor in stimulating MNrec. Simultaneously, the release of absorbed NH4+ on the cation exchange sites increased with decreasing MNrec when photosynthesis was prevented. Microbial N immobilization (I), especially NO3− immobilization rate (INO3), was significantly stimulated in all maize treatments compared to the control. The INO3 of unsterilized soil applied with unsterilized plant endophytes was significantly higher than unsterilized soil applied with sterilized endophytes, indicating a close relationship between endophytes and microorganisms on INO3.
Conclusions
M Nrec was stimulated by the photosynthetic substrate supply, and the increasing microbial N immobilization induced by plant significantly increased the ratio of I to M in the presence of maize, which was beneficial to soil N retention and reduced N loss. Our results provide new insights of the MIT for better understanding the productivity of agricultural systems and its driving factors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitrogen (N) is a crucial element for plant growth, as it is a constituent of organic compounds and regulates the synthesis of organic matter in plants. Soil N mineralization and immobilization are two key soil N transformation processes that occur simultaneously. Nitrogen is continually transferred within the soil from organic to inorganic forms and vice versa through N mineralization-immobilization turnover (MIT). Thus, the MIT determine the N availability and the productivity of terrestrial ecosystems (Keuper et al. 2017), and are important to maintain a suitable environmental and ecological N balance (Quan et al. 2021; Zhang et al. 2021; Zhu et al. 2013). The full understanding of the MIT and their controlling factors will shed light on the key drivers to maintain a suitable N balance and predict changes under changing environmental conditions. Nitrogen mineralization serves as the main pathway of inorganic N production, which can provide N nutrition for plants and microorganisms. Microbial N immobilization can temporarily store N in the microbial biomass, thereby reducing soil N loss. Although the immobilization would cause intense competition for inorganic N between microorganisms and plants, it is noteworthy that microbial N turnover is fast and about 70% assimilated N can be re-mineralized and promptly made available for plants (Spohn et al. 2016). Previous studies reported that the MIT was typically related to soil organic carbon (SOC), total N (TN), pH, soil microbial biomass, bulk density, temperature and precipitation, etc. (Elrys et al. 2021; Elrys et al. 2022; Romero et al. 2015). However, previous studies on the MIT are mostly carried out in the absence of plant or conducted by collecting rhizosphere soil and non-rhizosphere soil, thus, there is a dearth of information on MIT under plant-soil-microbe interaction.
Moreover, previous studies investigating the correlation between N processes and plant growth have primarily focused on net N mineralization rate rather than gross rate of N processes (Li et al. 2019; Mueller et al. 2013; Risch et al. 2019). While, the net mineralization rate which resulted from two opposite and concurrent microbial processes (i.e. gross N mineralization and N immobilization), failed to provide insights of the true inorganic N supply capacity. The 15N tracing tool, such as the 15N tracing model described by Müller et al. (2007), has an advantage to determine soil specific gross N transformations rates. Since Inselsbacher et al. (2013) initially attempted to introduce the effects of plants in the 15N tracing model, researches have gradually recognized the significant priming effects of plants on gross N transformation rates. He et al. (2021) observed that gross N mineralization rate was stimulated by the presence of plants compared to a control without plants. Additionally, NH4+ preferring plants induced a shift in microbial N uptake from NH4+ to NO3−, thereby alleviating the competition between plants and microorganism for available N (He et al. 2021). Plants can affect soil N process through N uptake or by rhizodeposition, including root litters and root exudates. On the one hand, plant NH4+ and NO3− uptake can alter the balance of the inorganic substrate, further promoting or inhibiting N processes (He et al. 2020). On the other hand, approximately 7–11% of carbon (C) from plant photosynthesis could be transferred to rhizosphere soil through root exudates (Pausch and Kuzyakov 2018; Van de Broek et al. 2020). Plants shading can limit the transfer of photosynthetically fixed C from plants to soil microorganisms (Pfennigwerth et al. 2018), and the humid conditions caused by shading are more conducive to the growth of pathogens (McCarthy-Neumann and Ibáñez 2013), thus leading to negative feedbacks in the plant-soil systems. The input of photosynthate provide C substrates via releasing dissolved organic matter (DOM), thus affecting the activity of microbes involved in inorganic N production (Zhao et al. 2022). It has been demonstrated that plant traits, such as root mass and C:N ratios, exert a greater influence on N mineralization than SOC and TN content (Fornara et al. 2011). Plant traits can directly influence root litters and the photosynthetic substrate supply, thus modifying the fractions of soil organic matter (SOM), e.g. DOM, particulate organic matter (POM). In addition, numerous SOM compounds are chemically recalcitrant or energetically ineffective for microorganisms (Kemmitt et al. 2008), whereas plants can secrete H+ ions or organic acids to promote the degradation of SOM (Masud et al. 2014), thereby affecting soil N conversion and enhancing N availability. In most studies, direct rhizosphere priming effects could be challenging in the measurement, and root induced changes on the N cycle were often studied by simulating rhizosphere inputs (Drake et al. 2011; Meier et al. 2017). There is little research to investigate the associations between the photosynthetic substrate supply and the MIT in the plant-soil systems.
Apart from the effect of photosynthetic substrate supply, plants might indirectly affect soil MIT via symbiotic microorganism, for example, endophytes. Endophytes, as plant symbiotic microorganisms, can benefit the host plant by facilitating nutrient absorption, disease resistance, insect resistance, and abiotic stress tolerance (Brader et al. 2017). For example, Elsharif (2023) observed the significant increases in the N mineralization rate and the available NH4+-N content in the soils inoculated with the endophytic fungus Phomopsis liquidambari. Chen et al. (2013) also found that the addition of Phomopsis liquidambari to soil could effectively alter the abundance and community structure of microbes in the rhizosphere and enhance the mineral N release in the soils. It seems that the plant endophytes may regulate the MIT in the plant-soil system to promote plant N absorption. However, compared to the previous studies, the effects of plant endophytes on these soil N transformation processes (not only net N mineralization) are not comprehensively and simultaneously studied in the plant-soil systems.
We hypothesized that (1) the growth of maize could potentially promote gross rates of N mineralization and immobilization; (2) photosynthetic substrate supply via root exudates could play a pivotal role in the priming effects of plants on the MIT; (3) plant symbiotic microorganism, e.g. endophytes, could promote gross rates of N mineralization and immobilization, thereby affecting the MIT. A series of 15N tracing pot experiments were established with maize (Zea mays L.) in an acidic soil to study the controlling mechanisms of the MIT in the presence of plants. In this study, three treatments, i.e. with and without maize planting, maize shading, and maize root endophytes inoculation, were set up and maize N uptake rates, soil gross N transformation rates, DOC and other soil properties were determined to test our hypotheses.
Materials and methods
Soil samples
An acidic soil (JX) was collected from Yingtan, Jiangxi Province, China (28°14′N, 117°13′E) in June 2022. The sample site was a typical subtropical humid monsoon climate with mean annual precipitation of 1750 mm and temperature of 17.5 °C, and the soil was classified as Ultisol according to soil taxonomy of the USDA. The maize fields have been established more than 50 years, and about 300 kg N ha−1 y−1 N fertilizer had been applied in the last 10 years. The surface soils (0–20 cm) of four sites were selected randomly, and the soils were mixed with equal amounts. Then the soil was passed through a 2 mm sieved after removing plant litters and stones. Part of JX soil was sterilized with 50 kGy γ-irradiation. The unsterilized soil pH was 4.90, and soil organic carbon (SOC) concentrations and the total N (TN) were 10.65 g kg−1 and 1.05 g kg−1, respectively. Soil sterilization did not alter pH, SOC and TN. The initial properties of sterilized and unsterilized soils were presented in Table S1.
Experimental design
Three experiments were conducted in this study.
Experiment 1 was conducted to test hypothesis 1 that plants play an essential role in regulating the MIT in maize-soil systems. 72 pots planted with maize (Zea mays L.) were prepared for this experiment. After 20 days of maize growth, 24 pots were selected randomly to conduct a 15N tracing experiment (treatment with maize plantation for 20 d, PM). For the remaining maize pots, 24 pots were selected randomly to conduct another 15N tracing experiment after removing maize plants for 3 days (treatment with maize removal for 3 d, RM3), or after 7 days (treatment with maize removal for 7 d, RM7). A control treatment without maize was set up as well.
Experiment 2 was conducted to test the effects of photosynthetic substrate supply via root exudates on the MIT. Except for PM and the control treatment, 48 pots planted with maize were covered with a black box and put in the dark room to prevent photosynthesis. Then 24 among 48 maize pots were selected randomly to conduct a 15N tracing experiment after 2 days in the dark (treatment with maize in the dark for 2 d, CM2), or 4 days in the dark (treatment with maize in the dark for 4 d, CM4), respectively.
Experiment 3 was conducted to test the potential role of plant endophytes in driving the MIT. Sterilized and unsterilized soils were mixed with or without endophytes, forming four samples: sterilized soil without endophyte (SS-E), sterilized soil inoculated with endophyte (SS + E), unsterilized soil without endophyte (US-E), unsterilized soil inoculated with endophyte (US+E). The endophytes were derived from maize roots. When maize grown in pots for 20 days, maize root was separated and cleaned up, then the root was divided into two parts. One part was sterilized in a high temperature autoclave (121 °C, 20 min) for the sterilized endophytes. For another part, the root surface was sterilized to avoid the interference of microorganisms on the root surface to the endophytes, and the unsterilized endophytes, i.e. the living endophytes, were obtained. The root surface sterilization method was as follows: after washing with tap water, the roots were surface-disinfected by ordered washing with 75% ethanol for 1 min, 2% sodium hypochlorite for 3 min, 75% ethanol for 1 min, followed by rinsing in sterile distilled water for 0.5 min (Ren et al. 2019). Then the sterilized and unsterilized endophytes were ground in the sterilized mortar, and prepared into endophyte solution. Then equal amounts of sterilized endophyte and unsterilized endophyte were added to the sterilized soil and unsterilized soil, respectively. The two +E treatments were mixed with endophytes of maize root, and the two -E treatments were mixed with an equivalent amount of sterilized endophytes of maize root. These four soil samples were used to conduct 15N tracing experiments.
15N tracing experiment
The experiments were conducted in September 2022 in Nanjing, Jiangsu Province, China (32°06′N, 118°54′E). Maize (Zea mays L.) seeds of ‘Zhengdan 958’ were pre-sterilized by 75% ethanol for 5 min and rinsed with the deionized water, then germinated on the moist gauze at 28 °C for 48 h. Maize seedlings were transplanted into 360 mL plastic jars which contained 200 g soil (oven-dry bases). After 3 days, Hoagland solution, urea and potassium dihydrogen phosphate was applied as base fertilizer. N, phosphorus (P), potassium (K) supply were 30, 15 and 24 mg N kg−1 soil, respectively. Maize pots were cultivated at 25 °C and 60% water holding capacity (WHC) in a greenhouse under artificial light intensity 5000 Lux (12 h light and 12 h dark cycle) for 20 days at the seeding stage.
The 15N tracing pot experiments of experiment 1 and experiment 2 were established according to He et al. (2020). 12 maize pots were labelled with NH415NO3 (10.12 atom%), and another 12 maize pots were labelled with15NH4NO3 (10.12 atom%) for each treatment. 2 mL volume of 15NH4NO3 or NH415NO3 solution, at a concentration of 40 mg NH4+-N kg−1 dry soil or 40 mg NO3−-N kg−1 dry soil, was applied with a 4-hole injection technique (Zhu et al. 2023). At 0.5, 24, 48, 72 h after 15N labelling, destructive sampling was carried out with three repetitions for every labeled treatment, and the soil was extracted with 1 M potassium chloride (KCl) (v/w, 5:1) for determining the concentrations and 15N enrichment of NO3− and NH4+. In the meantime, the collected maize plants were rinsed with 1 M KCl solution and deionized water, then denaturated all enzymes at 105 °C for 0.5 h and dried at 80 °C for measuring the weight. The dried plants were ground and passed through a sieve (0.15 mm) for determining the isotopic composition and concentrations of maize N. Soil pH and DOC were determined.
In experiment 3, SS-E, SS + E, US-E and US+E treatments were labeled in 250 mL Erlenmeyer flasks, and each flask contained 20 g soil (oven-dry basis). For each treatment, 12 flasks were labelled with 15NH4NO3 (10.12 atom%), and another 12 flasks were labelled with NH415NO3 (10.12 atom%). 2 mL volume of NH415NO3 or 15NH4NO3 solution, at a concentration of 40 mg NO3−-N kg−1 dry soil or 40 mg NH4+-N kg−1 dry soil, was added with a pipette to ensure gas exchange while preventing excessive water evaporation, the flasks was sealed with a membrane featuring small holes. The soil was incubated for 72 h with 60% WHC and incubated at 25 °C incubator. At 0.5, 24, 48, and 72 h after 15N labelling, destructive sampling was carried out with three repetitions for every labeled treatment, and the soil was extracted with 1 M potassium chloride (KCl) (v/w, 5:1) for determining the concentrations and 15N enrichment of NO3− and NH4+. Soil pH and DOC were determined as well.
15N tracing analysis
The NtraceBasic model was employed to determine soil N transformation rates in the soil without maize (Müller et al. 2007) (Fig. S1), and the NtracePlant model was employed to determine the N transformation rates in plant-soil systems (He et al. 2020) (Fig. S2). A Markov Chain Monte Carlo (MCMC) algorithm was applied for parameters based on measured data. The method is well-suited to estimate a large quantity of parameters simultaneously and find the global minimum. By utilizing the probability density function (PDF) for all N processes, the average and standard deviations of each process were quantified. The misfit function of observed values and modeled values in the model took the variance of individual observed values into account. Furthermore, the kinetic parameters of zero-, first-, or Michaelis-Menten kinetics were set to minimize the misfit between the observed and modeled values in 15N analysis. The optimum model version was identified in Akaikes Information Criterion (AIC) based on Matlab (Version 7, The MathWorks Inc.) (Cox et al. 2006). Totally, the 15N excess and concentrations of soil NO3−, NH4+ and maize N (average ± standard deviation) were provided to the analysis tool. The optimization procedure resulted in a probability density function (PDF) for each parameter, from which parameter averages and standard deviations were calculated (Müller et al. 2007). Each analysis run was carried out with three parallel sequences to identify adequate iteration numbers. Based on the kinetic settings and the final parameters, average N transformation rates of each N processes in Fig. S1 and Fig. S2 were calculated over the 72-hour period and expressed in units of mg N kg−1 soil day−1. In Fig. S3-S5, the model and observed values of different treatments are presented.
Analytical methods
Soil pH was measured with the DMP-2 mV/pH (Quark Ltd., Nanjing, China) at a ratio of water to dry soil 2.5:1 (v:w). TN was analyzed with combustion method by a Finnigan FlashEA 1112 elemental analyzer (Thermo Finnigan, Germany). SOC was determined through digestion with H2SO4-K2Cr2O7. Soil DOC was measured in a water to soil ratio of 5:1 (v:w) by Analyzer Multi N/C (Analytik Jena, Jena, Germany). The concentrations of NO3− and NH4+ of soil extract were measured with a continuous flow analyser (SA1000, Skalar, Netherlands). Moreover, the NO3− and NH4+ in extracts were separated via MgO and Devarda’s alloy by micro-diffusion methods, then absorbed by oxalic acid, respectively (Zhu et al. 2019). Then, the 15N abundance of NO3− and NH4+ were measured with Isotope-Ratio Mass Spectrometry (IRMS) (Europa Scientific Integra, Crewe, UK). The 15N abundance of the maize N pools was determined with a Finnigan FlashEA 1112 elemental analyzer with IRMS system (Thermo Finnigan, Germany).
Statistical analyses and calculations
The following equations were used to calculate combined N transformation rates:
Gross N mineralization rate (M) = MNrec + MNlab.
Total NH4+ production rate = RNH4 + M.
Total maize N uptake rate (UTN) = UNH4 + UNO3.
where MNrec was the mineralization of recalcitrant organic-N to NH4+; MNlab was the mineralization of labile organic-N to NH4+; RNH4 was the release of absorbed NH4+; UNH4 was maize NH4+ uptake rate; UNO3 was maize NO3− uptake rate.
The least significant difference (LSD) of the 0.05 significance level was calculated for soil N transformation rates according to the three replicates (average and standard deviations) using SigmaStat 4.0 (Systat Software Inc., San Jose, CA, USA). One-way analysis of variance (ANOVA) was used to calculate significant differences of N transformation rates among the different treatments with or without maize plantation and maize shading treatments. A T-test was used to calculate significant difference between treatments with and without endophytes incubation in sterilized or unsterilized soils. Pearson correlation was used to test the relationships between N transformation rates, maize N uptake rates and soil properties in SPSS 23.0 (Inc., USA).
Results
Soil properties
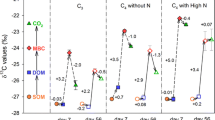
The initial soil pH (i.e., before conducting the maize pot experiments) was 4.90, and the initial SOC and TN were 10.65 and 1.05 g kg−1, respectively (Table S1). In general, during the growing period, maize did not change soil pH, SOC and TN compared to the control treatment (without maize) in different treatments. Compared with the initial DOC concentration of US-JX (196 mg kg−1), and soil sterilization significantly increased DOC concentrations (Table S1). After 20 days of maize planting, DOC concentration in PM (162 mg kg−1) were significantly higher than in the control treatment (137 mg kg−1) (P < 0.05) (Fig. 1a). DOC concentrations significantly declined after removing the maize, and DOC concentration in RM7 significantly decreased and showed no significant difference with the control treatment (Fig. 1a). Similarly, DOC concentrations significantly declined after the maize was covered with the black box in the dark room, and DOC concentrations of CM2 and CM4 were no significant difference from the control treatment (Fig. 1b). The DOC concentrations in SS-E and US-E were 335 and 179 mg kg−1, respectively, and the application of maize endophytes did not change DOC concentrations (SS-E vs. SS + E, US-E vs. US+E) (Fig. 1c).
Soil dissolved organic carbon (DOC) concentration in the different treatments. PM, the treatment that 15N tracing experiment was conducted at the time after 20 days maize growth; RM3, the treatment that 15N tracing experiment was conducted at the time when the maize (20 days growth) was removed for 3 days; RM7, the treatment that 15N tracing experiment was conducted at the time when the maize (20 days growth) was removed for 7 days; CM2, the treatment that 15N tracing experiment was conducted at the time when the maize (20 days growth) was covered with the black box for 2 days; CM4, the treatment that 15N tracing experiment was conducted at the time when the maize (20 days growth) was covered with the black box for 4 days; SS-E, the sterilized soil without endophytes; SS + E, the sterilized soil inoculated with endophytes; US-E, the unsterilized soil without endophytes; US+E, the unsterilized soil inoculated with endophytes. Error bar was standard deviation (n = 3). The different lowercase letters above bars indicated significant differences among the treatments with or without maize planting and the treatments with maize shading or not, and the asterisks above bars indicated significant differences between treatments with and without endophytes incubation in sterilized or unsterilized soil (P < 0.05)
NH4 + production rates
Soil gross N mineralization rate (M) in the presence of maize (PM) was significantly higher than that in the control treatment. Specifically, the mineralization of recalcitrant organic-N to NH4+ (MNrec) of PM (0.51 mg N kg−1 d−1) was significantly higher than of the control treatment (0.07 mg N kg−1 d−1) (Fig. 2a). Compared with PM, MNrec significantly decreased after maize removal, and MNrec in RM3 (0.17 mg N kg−1 d−1) and RM7 (0.14 mg N kg−1 d−1) was significantly lower than PM and showing no significant difference to the control treatment. MNrec in CM2 and CM4 was also significantly lower (< 0.21 mg N kg−1 d−1) compared to PM (Fig. 2b), showing no significant difference to the control treatment (Fig. 2b). In the maize planting and shading treatments, MNrec showed significant positive relationship with soil DOC (P < 0.01) (Fig. 5a). Besides, there was no significant difference of the mineralization of labile organic-N to NH4+ (MNlab) between PM, RM3, RM7, CM2, CM4 and the control treatment (0.08–0.23 mg N kg−1 d−1) (Fig. 2a, b). The application of plant endophytes did not change MNrec and MNlab both in sterilized and unsterilized soil (SS-E vs. SS + E, US-E vs. US+E) (Fig. 2c).
NH4+ production rates in the different treatments. MNlab, the mineralization of labile organic-N to NH4+; MNrec, the mineralization of recalcitrant organic-N to NH4+; RNH4, the release rate of NH4+ on cation exchange sites. The meaning of other abbreviation and symbol are same as Fig. 1
The release rate of adsorbed NH4+ on cation exchange sites (RNH4) in PM was 0.06 mg N kg−1 d−1, showing no significant difference with the control treatment, and maize removal (RM3 and RM7) did not significantly alter RNH4 (Fig. 2d). The RNH4 in CM2 was 1.16 mg N kg−1 d−1, significantly higher than that in PM (Fig. 2e). RNH4 in CM4 (0.47 mg N kg−1 d−1) decreased compared to CM2, and showed no significant difference with PM and the control treatment. The application of endophytes did not alter RNH4 in sterilized or unsterilized soil (< 0.14 mg N kg−1 d−1) (Fig. 2f).
Mineral N consumption rates
Generally, compared with the control treatment without maize, soil gross N immobilization rate (I, both immobilization of NO3−, INO3, and, immobilization of NH4+, INH4) was significantly stimulated in the presence of maize, especially INO3. The INO3 of PM was 5.82 mg N kg−1 d−1, which was significantly higher than in the control treatment (0.26 mg N kg−1 d−1) (Fig. 3a). Compared with PM, INO3 significantly decreased in RM3 and RM7 (< 2.67 mg N kg−1 d−1). However, INO3 in CM2 and CM4 showed no significant difference to PM (Fig. 3b). Additionally, in unsterilized soil, INO3 of US+E (2.06 mg N kg−1 d−1) was significantly higher than that of US-E (0.75 mg N kg−1 d−1), and INO3 was negligible in sterilized soil whether endophytes were applied or not (Fig. 3c).
Microbial N immobilization rates and the ratio of I/M of the different treatments. INH4, microbial NH4+ immobilization rate; INO3, microbial NO3− immobilization rate; I/M, the ratio of total microbial N immobilization rates to gross N mineralization rates. The meaning of other abbreviation and symbol are same as Fig. 1
The INH4 of PM was 1.40 mg N kg−1 d−1, which was significantly higher than that in the control treatment (0.39 mg N kg−1 d−1), and INH4 in RM3 showed no significant difference with PM. However, INH4 significantly decreased in RM7 (0.81 mg N kg−1 d−1), and showed no significant difference with the control treatment (Fig. 3a). INH4 of CM2 and CM4 showed no significant difference with PM (Fig. 3b). In maize removal and in the dark, INH4 increased with MNrec except for RM3 (P = 0.06, figure not shown). INH4 of US-E reached 1.19 mg N kg−1 d−1, which was significantly higher than US+E (0.67 mg N kg−1 d−1), and INH4 in sterilized soil was negligible (Fig. 3c).
The ratio of microbial N immobilization rate (INO3 + INH4) to gross N mineralization rate (I/M) in PM was 9.98, which was significantly higher than that in the control treatment (4.20) (Fig. 3d). The I/M in RM3 and RM7 showed no significant difference with PM, however, the I/M in CM2 (20.36) and CM4 (26.32) was significantly higher that PM (Fig. 3e). I/M of US+E reached 1.67, which was significantly higher than US-E (1.10 mg N kg−1 d−1), and there was no significant difference between SS-E and SS + E (Fig. 3f).
Maize NO3− uptake rate (UNO3) was 9.55 mg N kg−1 d−1 in PM, which was significantly higher than UNO3 in CM2 and CM4 (3.38 and 2.39 mg N kg−1 d−1, respectively) (Fig. 4a). Similarly, maize NH4+ uptake rate (UNH4) in PM was 4.14 mg N kg−1 d−1, and it was significantly higher than that in CM2 and CM4 (1.37 and 0.68 mg N kg−1 d−1, respectively) (Fig. 4a). The UNH4 increased with M (Fig. 5b) and maize total N uptake rate (UTN) decreased with microbial N immobilization rate (INH4 + INO3) (Fig. 5c).
Maize N uptake rate. UNH4, maize NH4+ uptake rate; and UNO3, maize NO3− uptake rate. PM, CM2 andCM4 as Fig. 1. Error bar was standard deviation (n = 3), and different lowercase letters above bars indicated significant differences among different maize treatments (P < 0.05)
Relationships among maize N uptake rate, soil gross N transformation rate and soil properties. DOC, soil dissolved organic carbon; MNrec, mineralization of recalcitrant organic-N to NH4+; M, soil gross N mineralization rate; INH4, microbial NH4+ immobilization rate; INO3, microbial NO3− immobilization rate; UNH4, maize NH4+ uptake rate; UTN, UNH4 + UNO3. The meaning of other abbreviation is same as Fig. 1
Discussion
Soil gross N mineralization (M) and microbial N immobilization (I) rates were altered with the presence of maize. The mineralization of recalcitrant organic-N to NH4+ (MNrec), the immobilization of NO3− and NH4+ (INO3 and INH4) were all promoted by maize plantation compared to the control, possibly owing to the photosynthetic substrate supply via root exudates and the interactions between plant endophytes and soil microorganisms. These results indicate that plant activities can affect gross N mineralization and immobilization rates and have an influence on the MIT, which is in line with our first hypothesis.
Maize photosynthetic substrate supply regulates rhizosphere NH4 + production
There was a comprehensive effect of soil physicochemical properties in the literature (Cheng et al. 2019; Wang et al. 2016), and their interactions with soil microorganism (Ribbons et al. 2016; Zeng et al. 2014) on soil N mineralization. However, in our study, the short-time (20 days) growth of maize in the greenhouse (temperature at 25 °C, soil moisture at 60%WHC) did not significantly alter pH, SOC and TN. This suggests a direct effect of maize plants activity on MNrec. This is consistent with the result that MNrec sharply decreased after maize was covered with a black box for 2 days (preventing plant photosynthesis), which confirmed our second hypothesis. Furthermore, there was a significantly positive correlation between soil DOC concentration and MNrec (P < 0.01, Fig. 5a). These results revealed that plant photosynthate input via root exudates may play a crucial role in the priming effects of plants on the MIT, which confirmed our second hypothesis. Generally, 30% even more carbon (C) from plant photosynthesis would be transferred to rhizosphere soil as the C investment for plants nutrients foraging (Van de Broek et al. 2020), thus altering the quantity and quality of soil dissolved organic matters (DOMs). The DOMs can further induce changes of soil biological and physicochemical properties, thus potentially affecting the soil N cycle, e.g. N mineralization (Zhu et al. 2014). As Zhao et al. (2022) reported, plant can recruit N transforming functional microorganisms and assemble the core microbial community though secreting root exudates (sugars, amino acids and organic acid) to produce more mineral N for plant growth. Conversely, microorganisms would release secondary metabolite to stimulate root exudates (amino acids, organic acids) to accelerate the decomposition of organic matters (Phillips et al. 2004). In general, our result revealed the role of photosynthetic substrate supply via root exudates on stimulating MNrec. Previous studies also reported that plants with strong photosynthesis can exhibit a strong stimulation on mineralization of soil native SOM (Henneron et al. 2020a; Henneron et al. 2020b), possibly owing providing carbon substrates (i.e., root exudates). However, maize removal and maize in the dark did not significantly alter soil mineralization of labile organic-N (MNlab). So, we further conclude that the plant mainly stimulated soil mineralization of recalcitrant organic-N for improving NH4+ availability, which was in line with maize NH4+ uptake rates increasing with soil gross N mineralization rates.
The release rate of absorbed NH4+ (RNH4) was significantly stimulated with decreasing MNrec when photosynthesis was prevented. Nitrogen is a crucial element for plant growth, thus during the dark, the plants still need to ingest N for survival. So, the increase of RNH4 could make up for the N deficiency caused by the decreased MNrec. However, the input of photosynthate decreased rapidly under dark condition, suggesting that there might be other factors in stimulating RNH4 except the input of maize photosynthate.
Maize root endophytes regulate rhizosphere microbial N immobilization
Our results suggest that maize growth is the main stimulant for microbial N immobilization. Generally, large quantities of native SOM compounds in soil are energetically unviable or chemically recalcitrant as microbial substrates (Kemmitt et al. 2008). Plant growth enhances the soil C availability, and it has been shown that microbes in rhizosphere soil derived more than 50% of biomass C from root exudates (van Hees et al. 2005). Maize root exudates can provide the energy for microorganism growth, but also can act as signal substances to enhance microorganism activities (Escudero-Martinez and Bulgarelli 2019; Maurer et al. 2021; van der Putten et al. 2016). This is in line with previous studies showing that the activity of microorganism in rhizosphere soil was more than 10 times great higher than that in non-rhizosphere soil (Kuzyakov 2010), and the high specific surface area of microorganisms enabled them to capture inorganic N very quickly (Fischer and Edmeades 2010), which may explain the increasing INO3 and INH4. Root exudates were originated from photosynthesis, but interestingly microbial N immobilization did not decrease when maize did not undergo further photosynthesis (plant in the dark room). This suggests that the effect of photosynthate input via root exudates might have a hysteresis effect on microbial N immobilization compared with plant photosynthesis. However, microbial N immobilization was significantly inhibited after removing maize, which proved that maize itself, e.g. N uptake from soil, also had an effect on microbial N immobilization. It is noteworthy that soil microbial N immobilization rates were significant accelerated with the application of maize endophytes in unsterilized soil. Meanwhile, there was no priming effect on microbial N immobilization when endophytes applied in sterilized soil. These results further indicated that the activation of gross microbial N immobilization by maize was possibly due to the synergistic effect of maize endophytes and soil microorganisms. However, there was no direct response of the release of absorbed NH4+ and soil N mineralization with endophytes applied alone. In our study, the added endophytes were derived from maize, but did not test the effect in the living plant body, so we cannot prove a direct influence of endophytes on soil NH4+ production processes with plants. Therefore, more details of endophytes in the living plant should be investigated in future studies for exploring the role of endophytes on soil gross N transformation processes.
The addition of 15N in the experiment may also be a potential reason for the stimulation of N immobilization, especially when the N transformation rates were calculated by the 15N isotopic pool dilution method. The main reason is that the N transformation rates were calculated by zero-order kinetic in the 15N isotopic pool dilution method. In this study, gross N transformation rates were calculated by the 15N tracing tool with zero-, first-order or Michaelis-Menten kinetics. Thus, the stimulating effect by N addition can be greatly weakened. In the control of this study, 15N was also added, but microbial N immobilization and organic N mineralization rates were both low and comparable (0.39 mg N kg−1 d−1 vs.0.16 mg N kg−1 d−1). Therefore, the excitation of microbial N immobilization with maize planting was not caused by the addition of 15N, but by maize growth.
Our findings show that soil microorganisms showed a greater preference for NO3− than for NH4+ in the presence of maize, and the gross N immobilization was governed by INO3. This contradicts previous studies that microbes preferentially utilize NH4+ over NO3− because NH4+ can be immobilized directly and it is a more energy efficient process (Romero et al. 2015; Wang et al. 2019). Moreover, in this study, the higher INO3 may be limited by maize NH4+ demand. Although maize NO3− uptake rates were significantly higher than maize NH4+ uptake rates, confirming maize preference for NO3− (Wang et al. 2018), there is still be an NH4+ demand for maize growth. There was a synergistic effect of maize NH4+ uptake and NO3− uptake (He et al. 2022; Subbarao and Searchinger 2021), furthermore, maize NH4+ uptake is an important nutrient strategy to absorb sufficient N for growth and increasing the yield (George et al. 2016). He et al. (2022) found that even though there was a high NO3− production capacity (high autotrophic nitrification) in alkaline soil, maize NO3− uptake was limited by low availability of NH4+, resulting in low NO3− uptake rates. There is a strong competitive relationship between plant NH4+ uptake and microbial NH4+ immobilization. The rates of NH4+ uptake by maize were higher than microbial NH4+ immobilization rates, suggesting that plants outcompeted microbial NH4+ acquisition. This result in microorganisms can only turn to assimilate another effective N, i.e. NO3−, to meet the microbial N demand. This should be one of N acquisition strategies between plant and microorganisms. Most microorganisms switch towards uptake NO3− from uptake NH4+, saving NH4+ is used to meet plant NH4+ demand. In addition, our previous studies have shown that maize growth could significantly stimulate the direct oxidation of organic N to NO3−, which may provide substrate for microbial NO3− immobilization (He et al. 2022).
Large immobilization of available N can be interpreted as a mechanism to protect the applied N fertilizer from being lost from the soil via volatilization, denitrification, leaching processes and surface runoff (Kuzyakov and Xu 2013). The N immobilized by microbial biomass will eventually turn into microbial necromass, which provides a SOM stock, and any change of the SOM pool can possibly be related to global change effects (Yang et al. 2022). Although there was competition for N between microorganism and plant, as shown by the negative relationship between maize N uptake rates and microbial N immobilization rates (Fig. 5c), the high microbial biomass turnover rate still supports the N being assimilated and re-mineralized to be available again in supporting the N demand of plant and other biological processes, thus ultimately reducing the risk for N loss (Romero et al. 2015). Therefore, maize has a competitive advantage N competition in the long run owing to the unidirectional flow of N nutrients from soil to plant roots. Plant-microorganism interactions is a strategy to maintain ecosystem stability, and the temporal niche differentiation reflecting plant and microbe generation times lead to mutualistic relationships in rhizosphere. This tight connection between plants and microbes has already been postulated by Hiltner (1904) in his seminal paper when the term rhizosphere was coined for the first time.
Conclusions
Mineral N production and consumption in plant-soil system is controlled by a diverse array of processes that interact with each other. In particular, plants can significantly stimulate the mineralization of recalcitrant organic-N (MNrec) and microbial N immobilization rates (Fig. 6). The photosynthetic substrate supply via root exudates were identified to be the controlling factors of stimulating MNrec, thereby producing soil NH4+ availability. The increasing microbial N immobilization rate may be accelerated by maize owing to the tight interactions between plant endophytes and soil microorganisms. We conclude that increasing microbial N immobilization can benefit soil N retention and reduce N loss. The results pave the way towards a better understanding of the priming effects on soil N mineralization-immobilization turnover in plant-soil systems.
References
Brader G, Compant S, Vescio K, Mitter B, Trognitz F, Ma LJ, Sessitsch A (2017) Ecology and genomic insights into plant-pathogenic and plant-nonpathogenic endophytes. Annu Rev Phytopathol 55:61–83. https://doi.org/10.1146/annurev-phyto-080516-035641
Chen XP, Cui ZL, Fan MS, Vitousek P, Zhao M, Ma W, Wang ZL, Zhang WJ, Yan XY, Yang JC, Deng XP, Gao Q, Zhang Q, Guo SW, Ren J, Li SQ, Ye YL, Wang ZH, Huang JL et al (2014) Producing more grain with lower environmental costs. Nature 514:486–489. https://doi.org/10.1038/nature13609
Chen Y, Ren CG, Yang B, Peng Y, Dai CC (2013) Priming effects of the endophytic fungus Phomopsis liquidambari on soil mineral N transformation. Microb Ecol 65:161–170. https://doi.org/10.1007/s00248-012-0093-z
Cheng Y, Wang J, Chang SX, Cai ZC, Müller C, Zhang JB (2019) Nitrogen deposition affects both net and gross soil nitrogen transformations in forest ecosystems: a review. Environ Pollut 244:608–616. https://doi.org/10.1016/j.envpol.2018.10.054
Cox GM, Gibbons JM, Wood ATA, Craigon J, Ramsden SJ, Crout NMJ (2006) Towards the systematic simplification of mechanistic models. Ecol Model 198:240–246. https://doi.org/10.1016/j.ecolmodel.2006.04.016
Drake JE, Gallet-Budynek A, Hofmockel KS, Bernhardt ES, Billings SA, Jackson RB, Johnsen KS, Lichter J, McCarthy HR, McCormack ML, Moore DJP, Oren R, Palmroth S, Phillips RP, Pippen JS, Pritchard SG, Treseder KK, Schlesinger WH, DeLucia EH, Finzi AC (2011) Increases in the flux of carbon belowground stimulate nitrogen uptake and sustain the long-term enhancement of forest productivity under elevated CO2. Ecol Lett 14:349–357. https://doi.org/10.1111/j.1461-0248.2011.01593.x
Elrys AS, Ali A, Zhang HM, Cheng Y, Zhang JB, Cai ZC, Müller C, Chang SX (2021) Patterns and drivers of global gross nitrogen mineralization in soils. Glob Chang Biol 27:5950–5962. https://doi.org/10.1111/gcb.15851
Elrys AS, Chen Z, Wang J, Uwiragiye Y, Helmy AM, Desoky EM, Cheng Y, Zhang JB, Cai ZC, Müller C (2022) Global patterns of soil gross immobilization of ammonium and nitrate in terrestrial ecosystems. Glob Chang Biol 28:4472–4488. https://doi.org/10.1111/gcb.16202
Elsharif NA, El awamie MW, Matuoog N (2023) Will the endophytic fungus Phomopsis liquidambari increase N-mineralization in maize soil? PLoS One 18(11): e0293281. https://doi.org/10.1371/journal.pone.0293281
Escudero-Martinez C, Bulgarelli D (2019) Tracing the evolutionary routes of plant-microbiota interactions. Curr Opin Microbiol 49:34–40. https://doi.org/10.1016/j.mib.2019.09.013
Fischer RA, Edmeades GO (2010) Breeding and cereal yield progress. Crop Sci 50:S-85–S-98. https://doi.org/10.2135/cropsci2009.10.0564
Fornara DA, Bardgett R, Steinbeiss S, Zak DR, Gleixner G, Tilman D (2011) Plant effects on soil N mineralization are mediated by the composition of multiple soil organic fractions. Ecol Res 26:201–208. https://doi.org/10.1007/s11284-010-0777-0
George J, Holtham L, Sabermanesh K, Heuer S, Tester M, Plett D, Garnett T (2016) Small amounts of ammonium (NH4+) can increase growth of maize (Zea mays). J Plant Nutr Soil Sci 79:717–725. https://doi.org/10.1002/jpln.201500625
Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KWT, Vitousek PM, Zhang FS (2010) Significant acidification in major Chinese croplands. Science 327:1008. https://doi.org/10.1126/science.1182570
He MQ, Meng L, Chen SD, Dan XQ, Zhao C, He XX, Cai ZC, Zhang JB, Müller C (2022) Maize seedlings prefer NO3− over NH4+ independent of pH changes. J Soil Sci Plant Nut 22:2847–2856. https://doi.org/10.1007/s42729-022-00850-8
He XX, Chi QD, Cai ZC, Cheng Y, Zhang JB, Müller C (2020) 15N tracing studies including plant N uptake processes provide new insights on gross N transformations in soil-plant systems. Soil Biol Biochem 141:107666. https://doi.org/10.1016/j.soilbio.2019.107666
He XX, Chi QD, Zhao C, Cheng Y, Huang XQ, Zhao J, Cai ZC, Zhang JB, Müller C (2021) Plants with an ammonium preference affect soil N transformations to optimize their N acquisition. Soil Biol Biochem 155:108158. https://doi.org/10.1016/j.soilbio.2021.108158
Henneron L, Cros C, Picon-Cochard C, Rahimian V, Fontaine S (2020a) Plant economic strategies of grassland species control soil carbon dynamics through rhizodeposition. J Ecol 108:528–545. https://doi.org/10.1111/1365-2745.13276
Henneron L, Kardol P, Wardle DA, Cros C, Fontaine S (2020b) Rhizosphere control of soil nitrogen cycling: a key component of plant economic strategies. New Phytol 228:1269–1282. https://doi.org/10.1111/nph.16760
Hiltner L (1904) Über neuere Erfahrungen und Probleme auf dem Gebiete der Bodenbakteriologie unter besonderer Berücksichtigung der Gründüngung und der Brache, Bodenpflege und Pflanzenbau. V. Lehrgang der Deutschen Landwirtschafts-Gesellschaft für Wanderlehrer. Deutsche Landwirtschafts-Gesellschaft, Eisenach, pp 59–78
Inselsbacher E, Wanek W, Strauss J, Zechmeister-Boltenstern S, Müller C (2013) A novel 15N tracer model reveals: plant nitrate uptake governs nitrogen transformation rates in agricultural soils. Soil Biol Biochem 57:301–310. https://doi.org/10.1016/j.soilbio.2012.10.010
Kemmitt SJ, Lanyon CV, Waite IS, Wen Q, Addiscott TM, Bird NRA, O’Donnell AG, Brookes PC (2008) Mineralization of native soil organic matter is not regulated by the size, activity or composition of the soil microbial biomass-a new perspective. Soil Biol Biochem 40:61–73. https://doi.org/10.1016/j.soilbio.2007.06.021
Keuper F, Dorrepaal E, van Bodegom PM, van Logtestijn R, Venhuizen G, van Hal J, Aerts R (2017) Experimentally increased nutrient availability at the permafrost thaw front selectively enhances biomass production of deep-rooting subarctic peatland species. Glob Chang Biol 23:4257–4266. https://doi.org/10.1111/gcb.13804
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371. https://doi.org/10.1016/j.soilbio.2010.04.003
Kuzyakov Y, Xu X (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198:656–669. https://doi.org/10.1111/nph.12235
Li ZL, Tian DS, Wang BX, Wang JS, Wang S, Chen HYH, Xu XF, Wang CH, He NP, Niu SL (2019) Microbes drive global soil nitrogen mineralization and availability. Glob Chang Biol 25:1078–1088. https://doi.org/10.1111/gcb.14557
Mao SH, Zhang HH, Zhuang GC, Li XJ, Liu Q, Zhou Z, Wang WL, Li CY, Lu KY, Liu XT, Montgomery A, Joye SB, Zhang YZ, Yang GP (2022) Aerobic oxidation of methane significantly reduces global diffusive methane emissions from shallow marine waters. Nat Commun 13:7309. https://doi.org/10.1038/s41467-022-35082-y
Masud MM, Guo D, Li JY, Xu RK (2014) Hydroxyl release by maize (Zea mays L.) roots under acidic conditions due to nitrate absorption and its potential to ameliorate an acidic Ultisol. J Soils Sediments 14:845–853. https://doi.org/10.1007/s11368-013-0837-5
Maurer D, Malique F, Alfarraj S, Albasher G, Horn MA, Butterbach-Bahl K, Dannenmann M, Rennenberg H (2021) Interactive regulation of root exudation and rhizosphere denitrification by plant metabolite content and soil properties. Plant Soil 467:107–127. https://doi.org/10.1007/s11104-021-05069-7
McCarthy-Neumann S, Ibáñez I (2013) Plant-soil feedback links negative distance dependence and light gradient partitioning during seedling establishment. Ecology 94:780–786. https://doi.org/10.1890/12-1338.1
Meier IC, Finzi AC, Phillips RP (2017) Root exudates increase N availability by stimulating microbial turnover of fast-cycling N pools. Soil Biol Biochem 106:119–128. https://doi.org/10.1016/j.soilbio.2016.12.004
Mueller KE, Hobbie SE, Tilman D, Reich PB (2013) Effects of plant diversity, N fertilization, and elevated carbon dioxide on grassland soil N cycling in a long-term experiment. Glob Chang Biol 19:1249–1261. https://doi.org/10.1111/gcb.12096
Müller C, Rütting T, Kattge J, Laughlin RJ, Stevens RJ (2007) Estimation of parameters in complex 15N tracing models by Monte Carlo sampling. Soil Biol Biochem 39:715–726. https://doi.org/10.1016/j.soilbio.2006.09.021
Pausch J, Kuzyakov Y (2018) Carbon input by roots into the soil: quantification of rhizodeposition from root to ecosystem scale. Glob Chang Biol 24:1–12. https://doi.org/10.1111/gcb.13850
Pfennigwerth AA, Van Nuland ME, Bailey JK, Schweitzer JA (2018) Plant-soil feedbacks mediate shrub expansion in declining forests, but only in the right light. J Ecol 106:179–194. https://doi.org/10.1111/1365-2745.12833
Phillips DA, Fox TC, King MD, Bhuvaneswari TV, Teuber LR (2004) Microbial products trigger amino acid exudation from plant roots. Plant Physiol 136:2887–2894. https://doi.org/10.1104/pp.104.044222
Quan Z, Zhang X, Fang Y (2021) The undefined N source might be overestimated by 15N tracer trials. Glob Chang Biol 27:467–468. https://doi.org/10.1111/gcb.15371
Ren F, Dong W, Yan DH (2019) Organs, cultivars, soil, and fruit properties affect structure of endophytic mycobiota of Pinggu peach trees. Microorganisms 7:322. https://doi.org/10.3390/microorganisms7090322
Ribbons RR, Levy-Booth DJ, Masse J, Grayston SJ, McDonald MA, Vesterdal L, Prescott CE (2016) Linking microbial communities, functional genes and nitrogen-cycling processes in forest floors under four tree species. Soil Biol Biochem 103:181–191. https://doi.org/10.1016/j.soilbio.2016.07.024
Risch AC, Zimmermann S, Ochoa-Hueso R, Schütz M, Frey B, Firn JL, Fay PA, Hagedorn F, Borer ET, Seabloom EW, Harpole WS, Knops JMH, McCulley RL, Broadbent AAD, Stevens CJ, Silveira ML, Adler PB, Báez S, Biederman LA et al (2019) Soil net nitrogen mineralisation across global grasslands. Nat Commun 10:4981. https://doi.org/10.1038/s41467-019-12948-2
Romero CM, Engel R, Chen CC, Wallander R (2015) Microbial immobilization of nitrogen-15 labelled ammonium and nitrate in an agricultural soil. Soil Sci Soc Am J 79:595–602. https://doi.org/10.2136/sssaj2014.08.0332
Spohn M, Klaus K, Wanek W, Richter A (2016) Microbial carbon use efficiency and biomass turnover times depending on soil depth - implications for carbon cycling. Soil Biol Biochem 96:74–81. https://doi.org/10.1016/j.soilbio.2016.01.016
Subbarao GV, Searchinger TD (2021) Opinion: a "more ammonium solution" to mitigate nitrogen pollution and boost crop yields. Proc Natl Acad Sci 118:e2107576118. https://doi.org/10.1073/pnas.2107576118
Van de Broek M, Ghiasi S, Decock C, Hund A, Abiven S, Friedli C, Werner RA, Six J (2020) The soil organic carbon stabilization potential of old and new wheat cultivars: a 13CO2-labeling study. Biogeosciences 17:2971–2986. https://doi.org/10.5194/bg-17-2971-2020
van der Putten WH, Bradford MA, Pernilla Brinkman E, van de Voorde TFJ, Veen GF (2016) Where, when and how plant-soil feedback matters in a changing world. Funct Ecol 30:1109–1121. https://doi.org/10.1111/1365-2435.12657
van Hees PAW, Jones DL, Finlay R, Godbold DL, Lundström US (2005) The carbon we do not see - the impact of low molecular weight compounds on carbon dynamics and respiration in forest soils: a review. Soil Biol Biochem 37:1–13. https://doi.org/10.1016/j.soilbio.2004.06.010
Wang C, Zheng MM, Hu AY, Zhu CQ, Shen RF (2018) Diazotroph abundance and community composition in an acidic soil in response to aluminum-tolerant and aluminum-sensitive maize (Zea mays L.) cultivars under two nitrogen fertilizer forms. Plant Soil 424:463–478. https://doi.org/10.1007/s11104-017-3550-0
Wang J, Cheng Y, Zhang JB, Müller C, Cai ZC (2016) Soil gross nitrogen transformations along a secondary succession transect in the north subtropical forest ecosystem of Southwest China. Geoderma 280:88–95. https://doi.org/10.1016/j.geoderma.2016.06.016
Wang J, Sun N, Xu MG, Wang SQ, Zhang JB, Cai ZC, Cheng Y (2019) The influence of long-term animal manure and crop residue application on abiotic and biotic N immobilization in an acidified agricultural soil. Geoderma 337:710–717. https://doi.org/10.1016/j.geoderma.2018.10.022
Waqas M, Khan AL, Kamran M, Hamayun M, Kang SM, Kim YH, Lee IJ (2012) Endophytic fungi produce gibberellins and indoleacetic acid and promotes host-plant growth during stress. Molecules 17:10754–10773. https://doi.org/10.3390/molecules170910754
Wisniewski-Dyé F, Borziak K, Khalsa-Moyers G, Alexandre G, Sukharnikov LO, Wuichet K, Hurst GB, McDonald WH, Robertson JS, Barbe V, Calteau A, Rouy Z, Mangenot S, Prigent-Combaret C, Normand P, Boyer M, Siguier P, Dessaux Y, Elmerich C et al (2011) Azospirillum genomes reveal transition of bacteria from aquatic to terrestrial environments. PLoS Genet 7:e1002430. https://doi.org/10.1371/journal.pgen.1002430
Yang YL, Xie HT, Mao Z, Bao XL, He HB, Zhang XD, Liang C (2022) Fungi determine increased soil organic carbon more than bacteria through their necromass inputs in conservation tillage croplands. Soil Biol Biochem 167:108587. https://doi.org/10.1016/j.soilbio.2022.108587
Zeng YL, Xiang WH, Deng XW, Fang X, Liu C, Peng CH (2014) Soil N forms and gross transformation rates in Chinese subtropical forests dominated by different tree species. Plant Soil 384:231–242. https://doi.org/10.1007/s11104-014-2206-6
Zhang YS, Pan BB, Lam SK, Bai E, Hou PF, Chen DL (2021) Predicting the ratio of nitrification to immobilization to reflect the potential risk of nitrogen loss worldwide. Environ Sci Technol 55:7721–7730. https://doi.org/10.1021/acs.est.0c08514
Zhao C, He XX, Dan XQ, He MQ, Zhao J, Meng H, Cai ZC, Zhang JB (2022) Soil dissolved organic matters mediate bacterial taxa to enhance nitrification rates under wheat cultivation. Sci Total Environ 828:154418. https://doi.org/10.1016/j.scitotenv.2022.154418
Zhu B, Gutknecht JLM, Herman DJ, Keck DC, Firestone MK, Cheng WX (2014) Rhizosphere priming effects on soil carbon and nitrogen mineralization. Soil Biol Biochem 76:183–192. https://doi.org/10.1016/j.soilbio.2014.04.033
Zhu GD, Song XT, Ju XT, Zhang JB, Müller C, Sylvester-Bradley R, Thorman RE, Bingham I, Rees RM (2019) Gross N transformation rates and related N2O emissions in Chinese and UK agricultural soils. Sci Total Environ 666:176–186. https://doi.org/10.1016/j.scitotenv.2019.02.241
Zhu Q, Wang W, Dai S, Meng L, He M, Chen S, Dan X, Cai Z, Zhang J, Müller C (2023) Effects of the number of 15N-injection needles on the estimation of gross N transformation rates using 15N tracing tool including plant. Biol Fertil Soils. https://doi.org/10.1007/s00374-023-01697-6
Zhu TB, Meng TZ, Zhang JB, Yin YF, Cai ZC, Yang WY, Zhong WH (2013) Nitrogen mineralization, immobilization turnover, heterotrophic nitrification, and microbial groups in acid forest soils of subtropical China. Biol Fertil Soils 49:323–331. https://doi.org/10.1007/s00374-012-0725-y
Acknowledgments
This work was supported by the Postgraduate Research & Practice Innovation Program of Jiangsu Province [KYCX23_1700], the National Natural Science Foundation of China [grant number 41830642], and the “Double World-Classes” Development in Geography project. The study was carried out as part of the IAEA funded coordinated research project “Minimizing farming impacts on climate change by enhancing carbon and nitrogen capture and storage in Agro-Ecosystems (D1.50.16)” and was carried out in close collaboration with the German Science Foundation research unit DASIM (FOR 2337).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Feike A. Dijkstra.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 855 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, M., Chen, S., Yang, W. et al. Priming effects of maize growth and photosynthetic substrate supply on soil N mineralization-immobilization turnover. Plant Soil (2024). https://doi.org/10.1007/s11104-024-06815-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11104-024-06815-3