Abstract
Purpose
Hydroxyl ion release by maize (Zea mays L.) roots under acidic conditions was investigated with a view to develop a bioremediation method for ameliorating acid soils in tropical and subtropical regions.
Materials and methods
Two hydroponic culture experiments and one pot experiment were conducted: pH, nitrogen state, and rhizobox condition, which investigated the effects of different nitrogen forms on hydroxyl release by maize roots under acidic conditions.
Results and discussion
The pH of the culture solution increased as culture time rose. The gradient of change increased with rising NO3 −/NH4 + molar ratios. Maize roots released more hydroxyl ions at pH 4.0 than at pH 5.0. The amount of hydroxyl ions released by maize roots at a constant pH was greater than those at a nonconstant pH. Application of calcium nitrate reduced exchangeable acidity and increased the pH in an Ultisol rhizosphere, compared with bulk soil. The increasing magnitude of soil pH was greater at higher doses of N. The absorption of NO3 −–N increased as the NO3 −/NH4 + molar ratios rose, which was responsible for hydroxyl ion release and pH increases in culture solutions and rhizosphere.
Conclusions
Root-induced alkalization in the rhizosphere resulting from nitrate absorption by maize plants can be used to ameliorate acidic Ultisols.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Approximately 30 % of the world’s total land area consists of acid soils and it has been estimated that over 50 % of the world’s potential arable lands are acidic (von Uexküll and Mutert 1995). There are 203 million km2 of acid soils distributed in southern China and account for about 21 % of arable land in the country (Xiong and Li 1990). Soil acidification is accelerated by poor farming practices, increasingly serious acid rain, and excess application of ammonium-based fertilizers. Under high-intensity agricultural conditions, heavy applications of ammoniacal nitrogen fertilizers accelerate the acidification of agricultural soils greatly (Guo et al. 2010; Conyers et al. 1996; Xu et al. 2002; Sumner and Noble 2003). Ammonium-based fertilizers acidify soil in two ways: an excretion of H+ ions by plant roots due to absorption of ammonium and the acidification of the rhizosphere; and release of H+ ions from nitrification of ammonium and enhancement of soil acidification. In contrast, nitrate fertilizer results in alkalization of the rhizosphere (Jarvis and Robson 1983). Ammonium ions in the soil are oxidized to nitrate through nitrification, catalyzed by ammonium-oxidizing prokaryotes, and the release of protons during the process leads to a decrease in soil pH (Helyar et al. 1976). Especially when the nitrate ions migrate to deep soil layers or leach out of the rooting zone with rainfall, H+ ions remain in the soil, causing soil acidification (Cregan et al. 1998; Xu et al. 2002). If all or some of the nitrate transformed from NH4 + is absorbed by plants, soil acidification will be inhibited to some extent due to the release of hydroxyl ions during plant uptake of nitrate.
Nitrogen is absorbed more than any other mineral nutrients and is needed for the growth of plants, so nitrogen often becomes the main nutrient that limits plant growth. Plants have an ability to change the rhizosphere soil pH through ion uptake by their roots. Changes in rhizosphere pH are mainly based on the theory of anion and cation balance. In normal growth and development, plants release CO2 through root respiration, but release protons or hydroxyl ions and secrete organic acids through the active absorption of anions/cations and the elongation of root cells, thus changing the rhizosphere pH. When plant uptake of one type of charge exceeds the other, the plant maintains electro-neutrality by extrusion of H+ or OH−, which leads to acidification or alkalization of the rhizosphere soil (Tang and Rengel 2003). In order to maintain electro-neutrality at the soil–root interface, H+ or OH− ions excreted by the roots are stoichiometrically equal to the respective excess cation or anion uptake (Hedley et al. 1982; Van Beusichem et al. 1985, 1988). Durand et al. (2001) showed that when nitrogen, in the form of nitrate fertilizer, was present in the nutrient solution, the growth of wheat and oat would lead to a higher pH in the growth medium. Nitrate-based fertilizer application also increased the rhizosphere pH of wheat by up to 0.5 U (Tang et al. 2011). Although most plants can use ammonium (NH4 +) or nitrate (NO3 −) as a source of N, the effectiveness of these two N forms on tomato growth was affected by the NH4 +/NO3 − ratio (Errebhia and Wilcoxa 1990). Increases in the pH of the growth media, due to uptake of nitrate by plants, have been reported previously, but there have been few studies that have quantified hydroxyl ion release by plant roots in relation to nitrate uptake.
The use of nitrate to ameliorate soil acidity has been reported (Schubert and Yan 1997; Tang et al. 2000). Weligama et al. (2010a) conducted a 58-day culture experiment at four nitrogen levels using aluminum-tolerant wheat. They showed that rhizosphere alkalization has a defined pattern following changes in the NO3 − conditions. Applying nitrate to the soil promoted the growth of wheat shoots and roots, and increased rhizosphere pH, whereas application of urea and ammonium did not produce this effect (Weligama et al. 2008, 2010b). Experiments by Bravin et al. (2009) using two nitrogen forms and two N levels indicated that the absorption of nitrate can significantly increase rhizosphere pH.
A biological method was suggested to ameliorate soil acidity in a semi-arid region of Australia based on the root-induced alkalization of the rhizosphere due to nitrate uptake by wheat crops (Tang et al. 2011; Conyers et al. 2011). However, the applicability of the method for acid soils in humid tropical and subtropical regions needs to be examined. Therefore, the objectives of this study were (1) to investigate hydroxyl ion release by maize roots under acidic conditions in relation to the uptake of nitrate by plants using solution-culture experiments and (2) to examine the effect of nitrate-based fertilizer on soil pH in the rhizosphere of maize growing in acidic Ultisol using rhizobox experiments. The results will provide the basis for using biological resistance against soil acidification and for the bioremediation of acid soils in tropical and subtropical regions.
2 Materials and methods
2.1 Plant culture
Seeds of maize (Zea mays L.), cv. Jiangyu 403, were soaked overnight in deionized water, rinsed, placed on Petri dishes in a 25 °C thermostatic incubator for germination (3 days). The pregerminated seeds were sown to vermiculite (matrix) in a plastic box in the greenhouse. One week later, the plants were carefully removed without causing root damage. The roots and shoots were washed with tap water three times and then three times in distilled water. Uniform seedlings were selected, planted into plastic buckets filled with 1-L Hoagland’s nutrient solution (pH 6), and then left to grow in the buckets. The pH of the nutrient solution was adjusted with either dilute HCl or NaOH solutions. Before that, the maize plants were first exposed to one-third-strength nutrient solution for 1 week, half-strength nutrient solution for 1 week, and then full nutrient solution. When new white roots had grown, the plants were moved to the different treatment solutions.
In order to measure the effect of NO3 −/NH4 + molar ratios on hydroxyl release, the nitrogen in the nutrient solution was adjusted: NO3 −–N was replaced by NaNO3 and NH4 +–N was replaced by (NH4)2SO4. The experiment was conducted in a growth chamber. The day (16 h) temperature was 25 °C, relative humidity was 65 %, and light was 12,000 lx. The night (8 h) temperature was 20 °C, and relative humidity was 90 %. All nutrient solutions contained 30 mg/L dicyandiamide to inhibit nitrification of ammonium to nitrate.
2.2 Experiment 1: effects of initial solution pH on hydroxyl release from maize roots
The experiment was set up with four NO3 −/NH4 + molar ratios: 15:1, 3:1, 1:1 and 1:3. Total nitrogen concentration was 8 mM. The nutrient solution pH was adjusted to pH 4.0 and pH 5.0 with dilute HCl and NaOH solutions, respectively. The experiment was conducted in the greenhouse using a completely randomized design arrangement with three replicates. The plants were transferred to the nutrient solutions, and pH was monitored every 2 days. After 6 days, the shoots and roots of the plants were harvested. The collected samples were fixed in a 105 °C oven for 20 min and dried at 85 °C for 24 h. Then, their dry weights were recorded. After the culture experiments, the volume of the nutrient solutions was adjusted to the initial value with distilled water, and one part of the collected nutrient solution was used to measure hydroxyl ion release by the plant roots and the other part was analyzed for NO3 −–N and NH4 +–N content. The hydroxyl ions were titrated to the initial pH of the nutrient solution with 0.01 M H2SO4 using an automatic titration system (T-50 Titrator, Mettler Toledo, Switzerland). The hydroxyl release was deduced from the amount of H2SO4 delivered by the automated dispenser. The NH4 + and NO3 − in the solutions were determined using a continuous flow analytical system (Skalar San++, The Netherlands). The amount of NH4 + and NO3 − absorbed by maize was calculated from the difference in the concentrations of NH4 + and NO3 − in the solutions before and after the culture experiments.
2.3 Experiment 2: the release of hydroxyl from maize plant roots under constant pH
In order to stimulate the release of hydroxyl ions from maize plant roots under controlled conditions, constant pH experiments were conducted, in which the hydroxyl ions released from maize plant roots were automatically and continually neutralized by 0.01 M H2SO4 using an automatic titration system, as mentioned above. The nutrient solutions with the four NO3 −/NH4 + molar ratios were prepared, and then, the pH was adjusted to 4.0 or 5.0 with dilute HCl and NaOH, respectively. One maize plant was put in each of these nutrient solutions, and a constant solution pH was maintained using an automated titrator. The culture experiment took 10 h. The automated titration consisted of a pH electrode (double-junction electrode) that was immersed in the bucket and an automated dispenser with its pipette tip dipping into the nutrient solution. The amount of H2SO4 added over time was recorded on a computer connected to the pH-stat device. The hydroxyl release was deduced from the amounts of H+ delivered by the automated dispenser over the 10-h period. At the same time, a nonconstant pH experiment was conducted over the same period. After 10-h cultivation, the amount of hydroxyl ions released from the maize plant roots was determined. One part of the nutrient solution after culture was used to measure hydroxyl release, while the other part was used to measure NO3 −–N and NH4 +–N.
2.4 Experiment 3: rhizobox experiment
The acidic Ultisol (U.S. Soil Taxonomy) (Haplic Acrisol in the WRB Taxonomy) used in this study was collected from Langxi, Anhui Province, China (31°6′ N, 119°8′ E). The soil is derived from Quarternary red earth. The field site had a history of canola-peanut cropping rotation for 20 years. The sample was taken from the topsoil (0–10 cm), air-dried and ground to pass a 2-mm sieve.
The experiment was carried out in a naturally lit glasshouse with five treatments. Three maize plants were grown in each rhizobox (120 × 140 × 170 mm, L × W × H) filled with 3 kg of air dried soil (Fig. 1). The rhizobox included bulk soil (a and e), transition (b and d) and rhizosphere sections (c), which were separated by a 0.15-mm nylon mesh. The nylon net can retain soil samples in respective sections, but allow water to flow through. Three levels (0, 100, and 200 mg N kg−1) of N fertilizer from two different sources (Ca(NO3)2∙4H2O and (NH4)2SO4), along with a basal dose 32 mg kg−1 P (equivalent to 150 mg kg−1 KH2PO4), were used in the study. No other nutrients were added apart from N, P, K, Ca, and S. The fertilizers were mixed with soil samples thoroughly, and then, exactly 1.0, 0.25, 0.5, 0.25, and 1.0 kg of air dried soil were added to the sections a–e (Fig. 1), respectively. The moisture was adjusted to 60 % of the water-holding capacity of the soil, and then, 50 mL water was added to each rhizobox every day until 3 days before harvest.
At the end of the rhizobox trial (90 days), the whole shoots and roots were harvested by removing them from the individual rhizoboxes. The plants were washed with deionized water, oven-dried at 85 °C to a constant weight, and then weighed in order to determine the dry matter yield.
Soil samples were collected from bulk soil and rhizosphere sections in the rhizobox separately, air-dried, and ground to pass a 0.3-mm sieve. Soil exchangeable H+ and Al3+ were extracted using 1.0 M KCl and then titrated with 0.25 M NaOH to pH 7.0 (Pansu and Gautheyrou 2006). The soil NH4 +–N and NO3 −–N were extracted with 2.0 M KCl using a 1:5 soil to solution ratio (Pansu and Gautheyrou 2006) and then determined by the continuous flow analytical system (Skalar San++, The Netherlands).
2.5 Statistical analysis
SPSS 20.0 for windows (Chicago, USA) was used for statistical analysis and data processing. A one-way analysis of variance (ANOVA) was undertaken for experiments 1, 2, and 3 to compare the significant differences between treatments. The dynamics of culture solution pH (experiment 1) and hydroxyl release from maize roots (experiment 2) were analyzed in two ways, first by using separate ANOVA for different NO3 −/NH4 + molar ratios at each time interval and then for different time intervals at each NO3 −/NH4 + molar ratio. For experiment 3, the data of soil pH, exchangeable acidity, and contents of NO3 − and NH4 + were analyzed for all treatments with bulk soils and rhizosphere soils combined together. The obtained results were used to compare the significant differences among the treatments for bulk soils or rhizosphere soils, and between bulk soil and rhizosphere soil for each treatment. The data for biomass yields in experiment 3 were analyzed for shoots and roots separately.
3 Results
3.1 Absorption of NO3 −–N and NH4 +–N by maize
The absorption of NO3 −–N increased but NH4 +–N declined when the NO3 −/NH4 + molar ratio rose for both initial pH values (Table 1). At initial pH 4.0, the highest NO3 −–N was absorbed by maize with a 15:1 NO3 −/NH4 + molar ratio, followed by the 3:1 ratio. Both of them were significantly higher than the 1:1 and 1:3 NO3 −/NH4 + molar ratios (P < 0.05). In contrast, the highest NH4 +–N was absorbed at a molar ratio of 1:3 NO3 −/NH4 +, which gradually and significantly decreased as the molar ratio rose (P < 0.05). At initial pH 5.0, the absorption of NO3 −–N decreased to a greater extent than at pH 4.0 but maintained an almost similar trend with regards to the different NO3 −/NH4 + molar ratios. The highest NH4 +–N absorption was found at a ratio of 1:3 followed by 1:1, 3:1, and 15:1.
The shoot and root dry weights of maize varied significantly as the NO3 −/NH4 + molar ratio increased (Table 1). The highest shoot dry weight was obtained at the 15:1 NO3 −/NH4 + molar ratio, which was significantly higher than the 3:1 and 1:3 ratios (P < 0.05) but identical to 1:1. With roots, the highest dry weight was also obtained at a ratio of 15:1 and was statistically similar to 3:1 and 1:1, which were significantly higher than the 1:3 NO3 −/NH4 + molar ratio (P < 0.05).
3.2 Release of hydroxyl ions due to different NO3 −/NH4 + molar ratios
The release of hydroxyl ions by maize roots during the 6-day culture period was influenced by the different molar ratios of NO3 −/NH4 + (Table 1). At initial pH 4.0, hydroxyl release from maize roots gradually and significantly (P < 0.05) increased as the NO3 −/NH4 + molar ratio rose and reached its maximum at the molar ratio of 15:1. The amount of hydroxyl ions released at the NO3 −/NH4 + molar ratio of 15:1 was significantly higher than that at 1:1, but identical to 3:1. Similarly, the amount of released hydroxyl ions decreased at initial pH 5.0 when the NO3 −/NH4 + molar ratio declined from 15:1 to 1:1. However, no hydroxyl ions were released at the molar ratio of 1:3. The amounts of OH− ions released at ratio 1:1 were significantly lower than these at the 3:1 and 15:1 ratios (P < 0.05), but the difference between the ratios 3:1 and 15:1 was not significant. At the same molar ratio, the amounts of hydroxyl ions released from roots were significantly greater at initial pH 4.0 than at initial pH 5.0.
3.3 The pH of nutrient solutions when affected by different NO3 −/NH4 + molar ratios
Different NO3 −/NH4 + molar ratios changed the pH of the nutrient solutions during hydroponic culturing (Fig. 2). The results showed that at initial pH 4.0, the pH of the culture solutions for all treatments gradually increased as culture time rose. The pH increased significantly with culture time at NO3 −/NH4 + molar ratios of 15:1, 3:1, and 1:1 during the whole culture period (P < 0.05) (Fig. 2a). At the ratio of 1:3, the pH increased significantly from 2 to 4 days (P < 0.05), but the increase in the pH from 4 to 6 days was not significant. When different treatments were compared, the differences of the pH among the molar ratios of 15:1, 3:1, and 1:1 were not significant at 2 and 4 days, but the pH for these treatments was significantly higher than that for the molar ratio of 1:3. The pH increased significantly as the molar ratio rose at 6 days (P < 0.05). Result also showed that the gradient of pH change increased at the higher NO3 −/NH4 + molar ratios (Fig. 2a). After 6 days of culture, the magnitude of the pH increase was 1.21, 1.00, 0.80, and 0.26 U for 15:1, 3:1, 1:1, and 1:3 NO3 −/NH4 + molar ratios, respectively.
In the solution with an initial pH 5.0, the culture medium pH increased significantly as culture time rose for NO3 −/NH4 + molar ratios of 15:1 and 3:1 (P < 0.05) (Fig. 2b). However, the rate of increase was lower than the corresponding NO3 −/NH4 + molar ratios at pH 4.0. The increase in solution pH with culture time was not significant at the molar ratio of 1:1. The pH decreased with the increasing culture time at the molar ratio of 1:3 due to proton release from maize roots. At 2 days, the pH at the molar ratio of 15:1 was significantly higher than that at the molar ratios of 3:1 and 1:1 (P < 0.05), but the difference of the solution pH between the molar ratios of 3:1 and 1:1 was not significant. At 4 and 6 days, the differences of the solution pH among the molar ratios of 15:1, 3:1, and 1:1 were significant (P < 0.05). At the end of the 6-day culture period, 0.52, 0.38, and 0.15 U pH increases were observed for the 15:1, 3:1, and 1:1 NO3 −/NH4 + molar ratios, respectively, while it decreased by 0.22 U for the 1:3 NO3 −/NH4 + molar ratio.
3.4 Hydroxyl release under different pH-state conditions
The hydroxyl (OH−) release trend at constant pH 4.0 by maize roots during the 10-h incubation period is shown in Fig. 3. The OH− release increased significantly with time at the molar ratios of 15:1, 3:1, 1:1, and 1:3 (P < 0.05). Up to 2 h of growth, the maize roots released a similar amount of OH− ions for the different NO3 −/NH4 + molar ratios. After 2 h, the release of OH− increased significantly as the NO3 −/NH4 + molar ratio rose (P < 0.05). Higher concentrations of nitrate resulted in greater hydroxyl ion release. This was similar to the hydroxyl ion release from maize roots under the nonconstant pH condition (Table 1). The amount of hydroxyl ions released from maize roots under the constant pH condition was much higher than that under the nonconstant pH condition (Table 2). At the molar ratios of 1:1 and 3:1, the amount of OH− ions released from maize roots under the constant pH condition was 6.5 and 6 times greater than that under the nonconstant pH condition, respectively (Table 2).
3.5 Effect of N fertilizer on pH and the exchangeable acidity of an Ultisol
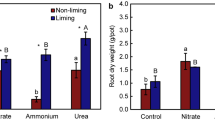
Rhizobox experiments were used to investigate the effect of ammonium- and nitrate-based fertilizer applications on soil acidity of an Ultisol. Figure 4a shows that the application of ammonium sulfate as a source of N fertilizer at 200 mg N kg−1 decreased the pH in both the bulk soil and the rhizosphere soil (P < 0.05), whereas calcium nitrate caused an increase in pH of rhizosphere soil at two addition levels of N (P < 0.05). However, application of calcium nitrate did not change bulk soil pH significantly. Calcium nitrate at 100 and 200 mg N kg−1 increased rhizosphere pH by 0.24 and 0.36 U, respectively, compared to the bulk soil. However, application of ammonium sulfate decreased soil pH by 0.11 and 0.12 U in the rhizosphere soil compared to the bulk soil at 100 and 200 mg N kg−1, respectively.
Changes in soil pH (a) and soil exchangeable acidity (b) induced by maize growth in the rhizobox experiment under different sources and levels of N fertilizers after 90 days (vertical bars represent ±SE). Statistical analysis was conducted with bulk and rhizosphere soils combined together, different letters on pillars indicate significant differences among treatments (P < 0.05) (Experiment 3)
Application of calcium nitrate as a source of N resulted in a decrease in exchangeable acidity of rhizosphere soil (P < 0.05), but did not change the exchangeabe acidity of bulk soil significantly (Fig. 4b). The decrease in exchangeable acidity of rhizosphere soil after Ca(NO3)2 application increased (P < 0.05) as the N application rate rose. The application of 200 mg N kg−1 Ca(NO3)2 decreased the exchangeable acidity of rhizosphere soil by 56 % compared to control and by 44 % when compared to that of bulk soil of the treatment. In contrast, ammonium sulfate significantly enhanced exchangeable acidity of both the bulk soil and rhizosphere soil compared to the control soil (P < 0.05) (Fig. 4b).
3.6 NO3 −–N and NH4 +–N contents in the soils
The soil NO3 − contents after the 90-day rhizobox experiment are shown in Fig. 5a. The NO3 − content in the control soil was negligible, but increased in the bulk soil after the application of Ca(NO3)2. The NO3 − residue in the rhizosphere soil was significantly lower than the bulk soil, but the pots subjected to the higher Ca(NO3)2 application rate (200 mg N kg−1) retained significantly more NO3 − for both the bulk and the rhizosphere soils (P < 0.05). The NO3 − residues in the (NH4)2SO4-treated rhizosphere soil were negligible. However, the higher NO3 − content in the (NH4)2SO4 treated bulk soil, compared to its corresponding control, remained a matter of interest. The difference in NO3 − residues between the bulk soil and rhizosphere soil represents NO3 − absorption. The NH4 +–N content increased due to the application of (NH4)2SO4 and it jumped to nearly 100 mg kg−1 in the bulk soil when 200 mg N kg−1 of (NH4)2SO4 was applied (Fig. 5b).
NO3 −–N (a) and NH4 +–N (b) concentrations in bulk and rhizosphere soils of rhizobox experiment under different sources and application rates of N fertilizers after 90 days (vertical bars represent ±SE). Statistical analysis was conducted with bulk and rhizosphere soils combined together, different letters on pillars indicate significant differences among treatments (P < 0.05) (Experiment 3)
3.7 Biomass yield in rhizobox experiment
The root and shoot dry biomass yield of the maize increased significantly (P < 0.05) after the application of N fertilizer with regards to both the application rate and sources of N fertilizer. The nitrate form brought superior results of maize shoots over the corresponding ammonium form, but the difference between two N resources at the same application rate was not significant (Fig. 6). The highest root dry matter yield also occurred when the maize received Ca(NO3)2 at the higher application rate of 200 mg N kg−1, which was statistically identical to the lower dose (100 mg N kg−1) of N from the same source, but significantly higher than the treatment of 100 mg N kg−1 (NH4)2SO4.
Biomass dry weight of roots (a) and shoots (b) of maize under different sources and application rates of N fertilizers after the 90-day rhizobox experiment (vertical bars represent ±SE). Statistical analysis was conducted for roots and shoots separately, different letters on pillars indicate significant differences among treatments (P < 0.05) (Experiment 3)
4 Discussion
The nutrient solution pH changes under hydroponic culture with different NO3 −/NH4 + molar ratios were due to the absorption of nitrate or NH4 + and the concomitant release of OH− or H+ ions by the maize plants. Plants generally extrude net excess H+ when cation uptake exceeds anion uptake and, conversely, extrude net excess of OH−/HCO3 − or consume H+ when anion uptake exceeds cation uptake (Paul et al. 2003; Tang and Rengel 2003; Hinsinger et al. 2003). This is the major reason why the amount of hydroxyl released by maize roots, and subsequently, the pH in the medium increased as the molar ratios of NO3 −/NH4 + rose (Fig. 2) and why the protons were released by the plant roots at 1:3 NO3 −/NH4 + at initial pH 5.0 (Fig. 2). The more nitrate absorption by maize roots at the higher molar ratio of NO3 −/NH4 + led to greater release of OH− ions from the roots (Table 1), while the more NH4 + absorption by maize roots at the molar ratio of 1:3 led to greater release of H+ and thus the decrease in solution pH at initial pH5.0 (Table 1 and Fig. 2). These observations were also consistent with previous reports (Römheld et al. 1984; Klotz and Horst 1988; Arnold 1992; Sas et al. 2001, 2002). Therefore, the form of nitrogen supplied plays a key role in the overall cation–anion relationship in plants and hence in alkali or acid production. Additionally, the uptake of other nutrients in solution, such as SO4 2−, Cl−, Na+, K+, Ca2+, and Mg2+, can also contribute to the release of OH− or H+ from maize roots. This is why the amount of OH− released at the higher NO3 −/NH4 + molar ratios (15:1 and 3:1) was much lower than the differences in the amounts of NO3 − and NH4 + absorbed by maize (Table 1). At pH 4.0 and at a NO3 −/NH4 + molar ratio of 1:3, the larger quantities of SO4 2− absorbed by the maize plants induced the release of more OH− by the plant and led to the net release of OH− (Table 1) (Durand et al. 2001), even though the amount of NO3 − absorbed by the maize plants was lower than the amount of NH4 + (Table 2).
The pH is an important factor influencing the uptake of NO3 − by maize plants and the subsequent release of OH− by their roots. This study showed that maize plants absorbed more NO3 − at pH 4, which led to a greater increase in the amount of OH− being released from their roots compared with pH 5.0 at the same NO3 −/NH4 + molar ratio. The release of OH− ions from maize roots was observed for the NO3 −/NH4 + molar ratios of 15:1, 3:1, and 1:1 at initial pH 5.0. If the solution had a NO3 −/NH4 + molar ratio of 1:3 at initial pH 5.0, then the plant released protons and made the solution acidic. It is generally considered that media with a lower pH are more conducive to the absorption of NO3 −, since the low pH can increase the enzyme activity of ATP (Yan et al. 1998). Therefore, the relatively low pH and high NO3 −/NH4 + molar ratio enhanced the absorption of nitrate by maize roots and the release of OH− ions from roots of the plant, which provided the good conditions for developing the bioamelioration method of acid soils.
The amount of OH− released from maize plant roots under constant pH conditions was much greater than under nonconstant pH conditions. This suggested that more OH− was continuously being released from maize plant roots in order to maintain the alkaline environment needed for maize growth under constant pH conditions because the OH− released from the roots was neutralized over time. This is similar to field conditions, in which the OH− released from maize plant roots can be consumed by soils due to the neutralization of soil acidity by these hydroxyl ions. Highly acidic soil in maize fields increases the amount of OH− ions released from maize plant roots and soil pH would be expected to increase with increasing time of cultivation. Maize plants may release hydroxyl ions in order to maintain a suitable growth environment because the hydroxyl ions released from the maize roots would be neutralized in time due to the strong buffering capacity of soils to acid or alkali. Since the soil becomes increasingly alkaline as the number of years of maize cropping increases, the amount of hydroxyl ions released from the maize roots should decrease. Soils have a stronger buffering capacity to acid or alkali than the nutrient solutions and thus the same amount of hydroxyl ions led to a less increase in soil pH than in the nutrient solution pH. The rhizobox experiments provided evidence for this. After 90-day growth, the soil pH was 0.24–0.36 U higher in the rhizosphere than in the bulk soil, which was much lower than the pH change in the solution culture experiments (Fig. 2).
The rhizobox experiments indicated that the application of nitrate-based fertilizer led to a higher soil pH in the rhizosphere of maize plants than in the bulk soil. An opposite trend was observed when ammonium-based fertilizer was used. These results were consistent with the previous observation that soil alkalization was associated with NO3 − nutrition and acidification was associated with NH4 + nutrition and N2 fixation (Jarvis and Robson 1983; Khonje et al. 1989; Bolan et al. 1991; Tang and Rengel 2003).
Nitrate and ammonium are the usual nitrogen forms absorbed by plants and their selective absorption is related to the plant species. Different plants have different assimilation parts (Smiley et al. 1974). The nitrate was assimilated by the roots of monocots, leading to a significant increase in the rhizosphere pH around the plants (Smiley et al. 1974). Wheat absorbed nitrate, and this led to an increase in rhizosphere pH when nitrate-based fertilizer was applied to an acid soil (Conyers et al. 2011; Tang et al. 2011). A biological method has been suggested to ameliorate soil acidity in semi-arid regions of Australia based on the root-induced alkalization of the rhizosphere resulting from nitrate uptake by wheat crops (Tang et al. 2011; Conyers et al. 2011). The results from the hydroponic experiment and rhizobox experiment in this paper suggested that root-induced alkalization in the rhizosphere resulting from nitrate uptake by maize plants can also be used to ameliorate soil acidity in Ultisols from subtropical regions. The high precipitation in tropical and subtropical regions may accelerate leaching loss of nitrate from rhizosphere, which inhibits the application of the method under field conditions. However, some techniques can be developed to reduce leaching loss of nitrate from soils, such as slow release fertilizers. In addition, soils in tropical and subtropiacl regions carry positive charge on their surfaces and thus the adsorption of nitrate by positively charged soil particles will also reduce the leaching loss of nitrate. The field trails need to be conducted in future to evaluate the ameliorating efficiency of the method on soil acidity in tropical and subtropical regions.
The application of Ca(NO3)2 did not increase biomass yield of maize plants significantly compared with the (NH4)2SO4 treatments in short-term pot experiments (Fig. 6). The beneficial effect of rhizosphere alkalization on crop yield needs to be evaluated under field conditions in the future.
5 Conclusions
Results from the solution culture experiments indicated that absorption of nitrate by maize plants led to a release of hydroxyl ions from their roots and an increase in the pH of the medium. The amount of hydroxyl ions released from maize roots increased with rising NO3 −/NH4 + molar ratios and more hydroxyl ions were released from maize roots at initial pH 4.0. The amount of hydroxyl ions released under the constant pH condition was much higher than that under the nonconstant pH condition. The rhizobox experiment under natural conditions suggested that the root-induced alkalization in the rhizosphere resulting from nitrate uptake by maize plants can be used to ameliorate soil acidity in Ultisols from subtropical regions.
References
Arnold G (1992) Soil acidification as caused by the nitrogen uptake pattern of Scots pine (Pinus sylvestris). Plant Soil 142:41–51
Bolan NS, Hedley MJ, White RE (1991) Processes of soil acidification during nitrogen cycling with emphasis on legume based pastures. Plant Soil 134:53–63
Bravin MN, Marti AL, Clairotte M, Hinsinger P (2009) Rhizosphere alkalisation—a major driver of copper bioavailability over a broad pH range in an acidic copper-contaminators soil. Plant Soil 318:257–268
Conyers MK, Heenan DP, Poile GJ, Cullis BR, Helyar KR (1996) Influence of dryland agricultural management practices on the acidification of soil profile. Soil Till Res 37:127–141
Conyers MK, Tang C, Poile GJ, Liu DL, Chen DL, Nuruzzaman Z (2011) A combination of biological activity and the nitrate form of nitrogen can be used to ameliorate subsurface soil acidity under dryland wheat farming. Plant Soil 348:155–166
Cregan P, Scott B (1998) Soil acidification—an agricultural and environmental problem. In: Pratley J, Robertson A (eds) Agriculture and the environmental imperative. CSIRO, Collingwood, pp 98–128
Durand R, Bellon N, Jaillard B (2001) Determining the net flux of charge released by maize roots by directly measuring variations of the alkalinity in the nutrient solution. Plant Soil 229:305–318
Errebhia M, Wilcoxa GE (1990) Tomato growth and nutrient uptake pattern as influenced by nitrogen form ratio. J Plant Nutr 13:1031–1043
Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KWT, Vitousek PM, Zhang FS (2010) Significant acidification in major Chinese croplands. Science 327:1008–1010
Hedley MJ, Nye PH, White RE (1982) Plant induced changes in the rhizosphere of rape (Brassica napus var. Emerald) seedlings: I. The origin of the pH change. New Phytol 91:31–44
Helyar KR (1976) Nitrogen cycling and soil acidification. J Aust Institute Agri Sci 42:217–221
Hinsinger P, Plassard C, Tang C, Jaillard B (2003) Origins of root mediated pH changes in rhizosphere and their responses to environmental constrains: a review. Plant Soil 248:43–59
Jarvis SC, Robson AD (1983) The effect of nitrogen nutrition of plants on the development of acidity in West Australian soils II. Effects of differences in cation/anion balance between plant species grown under non leaching conditions. Aust J Agri Res 34:355–365
Khonje DJ, Varsa EC, Klubek B (1989) The acidulation effects of nitrogenous fertilizers on selected chemical and microbiological properties of soil. Commun Soil Sci Plant Analy 20:1377–1395
KIotz F, Hoist WJ (1988) Genotypic differences in aluminium tolerance of soybean (Glycine max L.) as affected by ammonium and nitrate-nitrogen nutrition. J Plant Physiol 132:702–707
Pansu M, Gautheyrou J (2006) Handbook of soil analysis—mineralogical, organic and inorganic methods. Springer-Verlag, Heidelberg
Paul KI, Black AS, Conyers MK (2003) Development of acidic subsurface layers of soil under various management systems. Adv Agron 78:187–214
Römheld V, Mailer C, Marsehner H (1984) Localization and capacity of proton pumps in roots of intact sunflower plants. Plant Physiol 76:603–606
Sas L, Rengel Z, Tang C (2001) Excess cation uptake and extrusion of proton and organic acid anions in Lupinus albus under P deficiency. Plant Soil 160:1191–1198
Sas L, Rengel Z, Tang C (2002) The effect of nitrogen nutrition on cluster root formation and proton extrusion by Lupinus albus. Ann Bot 89:435–442
Schubert S, Yan F (1997) Nitrate and ammonium nutrition of plants effects on acid/base balance and adaptation of root cell plasma lemma H+ ATP-pase. Z Pflanzenernahr Bodenk 160:175–281
Smiley RW (1974) Rhizosphere pH as influenced by plants, soils and nitrogen fertilizers. Soil Sci Soc Am Proc 38:795–799
Sumner ME, Noble AD (2003) Soil acidification: the world story. In: Rengel Z (ed) Handbook of soil acidity. Marcel Dekker, New York, pp 1–28
Tang C, Rengel Z (2003) Role of plant cation/anion uptake ratio in soil acidification. In: Rengel Z (ed) Handbook of soil acidity. Marcel Dekker, New York, pp 57–81
Tang C, Raphael C, Rengel Z, Bowden JW (2000) Understanding subsoil acidification: effect of nitrogen transformation and nitrate leaching. Aust J Soil Res 38:837–849
Tang C, Conyers MK, Nuruzzaman M, Poile GJ, Liu DL (2011) Biological amelioration of subsoil acidity through managing nitrate uptake by wheat crops. Plant Soil 338:383–397
Van Beusichem ML, Baas R, Kirkby EA, Nelemans JA (1985) Intracellular pH regulation during NO3 − assimilation in shoot and roots of Ricinus communis. Plant Physiol 78:768–773
Van Beusichem ML, Kirkby EA, Baas R (1988) Influence of nitrate and ammonium nutrition on the uptake assimilation, and distribution of nutrients in Ricinus cornmunis. Plant Physiol 86:914–921
Von Uexküll HR, Mutert E (1995) Global extent, development and economic impact of acid soils. In: Date RA, Grundon NJ, Raymet GE, Probert ME (eds) Plant–soil interaction at low pH: principles and management. Kluwer , Dordrecht, pp 5–19
Weligama C, Tang C, Sale PWG, Conyers MK, Liu DL (2008) Localised nitrate application together with phosphorus enhances root proliferation of wheat and maximizes rhizosphere alkalisation in acid subsoil. Plant Soil 312:101–115
Weligama C, Sale PWG, Conyers MK, Liu DL, Tang C (2010) Nitrate leaching stimulates subsurface root growth of wheat and increases rhizosphere alkalisation in a highly acidic soil. Plant Soil 328:119–132
Wellgama C, Tang C, Sate PWG, Conyers MK, Liu DL (2010) Application of nitrogen in NO3 − form increases rhizophere alkalisation in the subsurface soil layer in an acid soil. Plant Soil 333:403–416
Xiong Y, Li QK (1990) Soils of China. Science , Beijing, pp 67–79
Xu RK, Coventry DR, Farhoodi A, Schultz JE (2002) Soil acidification as influenced by crop rotations, stubble management, and application of nitrogenous fertilizers, Tarlee, South Australia. Aust J Soil Res 40:483–496
Yan F, Feuerle R, Schaffer S (1998) Adaptation of active proton pumping and plasma lemma ATPase activity of corn roots to low root medium pH. Plant Physiol l17:311–319
Acknowledgments
This study was supported by the Knowledge Innovation Program Foundation of the Chinese Academy of Sciences (KZCX2-EW-405) and the National Natural Science Foundation of China (41230855).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Caixian Tang
Rights and permissions
About this article
Cite this article
Masud, M.M., Guo, D., Li, Jy. et al. Hydroxyl release by maize (Zea mays L.) roots under acidic conditions due to nitrate absorption and its potential to ameliorate an acidic Ultisol. J Soils Sediments 14, 845–853 (2014). https://doi.org/10.1007/s11368-013-0837-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11368-013-0837-5