Abstract
Background and Aims
Leaf senescence is accelerated by soil moisture stress during reproductive growth in rainfed paddy rice under drought and in irrigated paddy rice under intermittent irrigation for saving water or mitigating methane emissions. Leaf senescence decreases leaf photosynthetic rate (An) and grain yield. We aimed to elucidate the mechanisms underlying the An decrease under soil moisture stress.
Methods
An, leaf content of ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), synthesis and degradation of Rubisco and cytokinin flux from roots were compared between plants grown in moisture-deficient soil (DR-plants) and flooded or wet soil (FL- or WE-plants, respectively) during senescence in pot-grown rice (Oryza sativa L.).
Results
The decreases in An and Rubisco content were larger in the DR-plants than in the FL-plants. An was closely correlated with Rubisco content during moisture stress treatment. The larger decrease of Rubisco content in the DR-plants was from increased Rubisco degradation rather than decreased synthesis. The amount of cytokinins transported from roots to shoots was smaller in the DR-plants. The application of 6-benzylaminopurine to leaves of the DR-plants suppressed Rubisco degradation. In a wilty mutant with impaired leaf hydraulic conductance, leaf senescence was significantly higher in the DR-plants than in the FL-plants, although leaf water potential of both groups decreased similarly under sunny conditions.
Conclusion
The main cause of an An decrease with senescence in rice under soil moisture stress was the decrease of cytokinin flux from roots to shoots and enhanced Rubisco degradation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rice is one of the most important food crops in the world. Since rice is much more susceptible to drought than many upland crops (Angus et al. 1983; Hirasawa 1999; Win et al. 2022), drought is the largest constraint to rice production in rainfed systems (Fukai et al. 1998; Sign et al. 2021; Venuprasad et al. 2007). Rainfed rice occupies approximately half of the world rice cultivation area (McLean et al. 2002; Salmon et al. 2015) but contributes only a quarter of worldwide rice production (McLean et al. 2002). Because of the scarcity of water resources under global warming (IPCC 2021), improving growth and yield of rainfed rice is a great challenge to meet the growing demand for rice (Long et al. 2015; MAFF 2019; Singh et al. 2021).
Paddy field flooding requires large amounts of irrigation water, but water availability for agriculture is decreasing (Carrijo et al. 2017; Tuong et al. 2005). Rice paddies are a major source (approximately 9% of all anthropogenic emissions) of the atmospheric greenhouse gas methane (CH4) (IPCC 2021). Intermittent irrigation (alternating wetting and drying, AWD) is being considered for saving irrigation water (Sign et al. 2021; Tuong et al. 2005) and decreasing CH4 emissions from irrigated paddy fields (Carrijo et al. 2017; Itoh et al. 2011). However, this irrigation management may lead to mild to severe water stress and decrease grain yield (Carrijo et al. 2017; Itoh et al. 2011; Tuong et al. 2005).
Water stress markedly decreases shoot growth and leaf photosynthesis by the decrease of water potential and by the acceleration of leaf senescence (Kramer and Boyer 1995; Munns and Millar 2023; Sade et al. 2018; Tardieu et al. 2018). Inhibition of leaf growth by water stress decreases the interception of solar radiation by the canopy, and this decreases canopy photosynthesis (Hirasawa 2014; Tardieu et al. 2018). The suppression of leaf expansion limits yield in the aerobic rice systems of upland fields (Kato and Katsura 2014). Acceleration of leaf senescence by water stress decrease canopy photosynthesis by decrease of individual leaf photosynthesis and leaf area of the canopy (Brevedan and Egli 2003; Chen et al. 2015; De Souza et al. 1997; Hirasawa 1999; Kramer and Boyer 1995; Zhang and Zhou 2013; Zhao et al. 2022). Drought develops during the ripening stage of rainfed paddy rice in many regions of Southeast Asia (Fukai et al. 1998; Miyagawa et al. 2006; Salmon et al. 2015). AWD is widely used in irrigated paddy rice during reproductive growth after almost all leaves have expanded (Carrijo et al. 2017). Leaf senescence during reproductive growth was accelerated in rice when the soil water potential at 5 cm depth was controlled in the range of 0 to − 20 or − 30 kPa, and this reduced dry matter production during reproductive growth and grain yield by 10% to 20% (Lu et al. 2000). These data indicate that the accelerated senescence is a major cause of yield reduction in rainfed and irrigated paddy rice under soil moisture stress. However, physiological and genetic traits that render paddy rice resilient to senescence under soil moisture stress have not been fully elucidated.
Much data have been accumulated on the physiology of leaf senescence (Lee and Masclaux-Daubresse 2021; Lim et al. 2007; Woo et al. 2019; Zhao et al. 2022). Leaf senescence is characterized as nutrient relocation from leaves to other organs. Abscisic acid, jasmonic acid, ethylene, salicylic acid and strigolactone promote leaf senescence, whereas gibberellic acid, cytokinins and auxin delay it (Guo et al. 2021). Cytokinins have been studied intensively as plant hormones delaying leaf senescence (Noodén et al. 1997; Sade et al. 2018). Cytokinin flux from roots to shoots is larger in stay-green cultivars than in normally senescing cultivars (He et al. 2005; Soejima et al. 1992, 1995). Exogenous application of cytokinins and expression of genes for cytokinin synthesis in transgenic plants delay leaf senescence and maintain high leaf photosynthesis in plants not only under wet conditions (Gan and Amasino 1995; Ookawa et al. 2004) but also under drought conditions (Peleg et al. 2011; Raineri et al. 2015; Reguera et al. 2013; Rivero et al. 2007; Sade et al. 2018). However, the mechanism underlying the accelerated decrease in leaf photosynthesis with senescence under water stress has not been fully understood.
Based on above findings, we hypothesize that cytokinins are involved in the accelerated decrease of leaf photosynthesis with senescence under water stress in rice, and here, we aimed to understand the mechanisms by which soil moisture stress accelerates leaf senescence and decreases leaf photosynthesis in rice. We analyzed (1) the effects of a decrease in leaf water potential and soil moisture on leaf senescence (Exp. 1), (2) the cause of a stronger decrease in leaf photosynthetic rate with senescence in plants grown in moisture-deficient soil than in plants grown in wet soil (Exp. 2), (3) the effects of soil moisture decrease on the inflow and outflow of nitrogen, synthesis and degradation of ribulose 1,5-bisphosphate carboxylase/oxygenase (Rubisco) and the expression of rbcS and rbcL in leaves (Exp. 3), (4) the effects of soil moisture decrease on cytokinin flux from roots to the shoot (Exp. 4) and (5) the effects of exogenous cytokinin on the inflow and outflow of nitrogen and the synthesis and degradation of Rubisco in leaves under soil moisture stress (Exp. 5). The results of this study would provide insight into breeding drought-tolerant rice varieties in the rainfed field and in the AWD system using molecular breeding.
Materials and methods
Plant materials
Rice (Oryza sativa L.) cv. Nipponbare, cv. Kinmaze and CM2088 mutant were used. CM2088 was derived from Kinmaze by mutagenesis of fertilized egg cells with N-metyl-N-nitroso urea (NMU) at Kyushu University (Sato et al. 2010); its wilty phenotype, CM2088 is caused by dramatically impaired hydraulic conductance in the nodes and leaf sheaths and blades but not in the roots or stems (Koizumi et al. 2007).
Cultivation and moisture treatment
Exp. 1
Nipponbare, Kinmaze and CM2088 were grown in 13.5 L plastic pots (24 cm diameter, 30 cm height) filled with a 1:1 (v/v) mixture of paddy field soil (alluvial soil from the Tama River) and upland field soil (Kanto diluvial soil) at a density of four or five plants per hill, with three hills per pot. They were sown in early April or late June and were grown outdoors in the University Farm at Fuchu, Tokyo, Japan (35˚41’N, 139˚12’E). Each pot was individually flooded with water so that the soil surface was always below the water surface. Fertilizer was applied at 1.0 g N, 0.44 g P and 0.83 g K per pot as a basal dressing, and 1.0 g N per pot as a topdressing at the tillering or panicle formation stage. Soil moisture treatments were conducted in a naturally ventilated greenhouse: irrigation was withheld from some plants (DR-plants), and the other plants were grown under flooded conditions (FL-plants). Each pot individually weighed every morning with a 0.5 g precision balance. After daily evapotranspiration of the DR-plants, estimated from the decrease of pot weight, had decreased to approximately 50% to 60% of that of the FL-plants, the DR-plants were irrigated daily with the reduced amount of pot weight to keep soil moisture content at the same level (Fig. 1a). The experiments were arranged in a completely randomized design with three to four replications with one pot per replicate unless otherwise noted.
Changes of (a) pot weight and (b–e) SPAD values of (b) the flag leaf and (c) 2nd, (d) 3rd and (e) 4th leaves from the flag leaf in cv. Nipponbare plants grown in field soil under flooded (FL) or moisture-deficient (DR) conditions (Exp. 1). The treatment started at full heading. Inserted table in (a) shows soil water content (dry weight basis) and xylem water potential of the 3rd leaf at predawn (PD Ψxylem) (n = 3). DAT: days after the start of treatment. RW: DR plants re-flooded at 17 DAT. Values are means ± SE (n = 3). The same letters in a graph represent no significant difference at the 5% level (Student’s t-test at 10 and 17 DAT or Tukey HSD test at 31 DAT)
Exps. 2–5
Nipponbare was grown in 3.8 L plastic pots (16 cm diameter, 19 cm height) filled with vermiculite (Fukushima Vermiculite Co., Fukushima, Japan) sieved through a 2-mm mesh at a density of five or six plants per hill, with four hills per pot in a growth chamber (KG-50HLA; Koito Manufacturing Co., Ltd., Tokyo, Japan; 12/12 h light/dark cycle, air temperature 28/23 °C, relative humidity 60% to 80%, ~ 1000 μmol photons m−2 s−1 photosynthetically active radiation at the top of the canopy). Vermiculite in pots was flooded with Kimura B solution, which contained 0.36 mM (NH4)2SO4, 0.09 mM K2SO4, 0.55 mM MgSO4, 0.18 mM KNO3, 0.37 mM Ca(NO3)2, 0.18 mM KH2PO4 and 0.04 mM Fe-EDTA. Approximately 1 month before heading, the concentration of the solution was doubled. The solution was replenished daily with the solution. Every 6 days, the solution was completely drained from the bottom of the pot and replaced with fresh solution.
Soil moisture treatment was started at full heading stage for 7 days (Exp. 2) or 12 days (Exps. 3 to 5). The solution was drained from the bottom of the pots overnight, and the plants together with vermiculite were transferred into 13.5 L plastic pots containing vermiculite with a water content of 170% on the dry weight basis (water potential, − 0.04 MPa; WE-plants) or of 25% (− 0.4 MPa; DR-plants). The water potential of the vermiculite was determined with an isopiestic psychrometer (Boyer 1995). Each pot individually weighed every morning before the light was turned on with a 0.1 g precision balance. Deionized water was added daily with the reduced amount of pot weight in the WE-plants to compensate for the decrease in pot weight. After daily evapotranspiration of the DR-plants had decreased to approximately 40% to 50% of that of the WE-plants, the DR-plants were irrigated daily to compensate for a further decrease in pot weight. The experiments were arranged in a completely randomized design with four or five replications with one pot per replicate.
Water potential
Leaf water potential (Ψleaf) was measured during the middle of the day with a thermocouple psychrometer by the dew-point method (HR33T microvoltmeter and C-51 or C-52 sample chamber, Wescor Inc., Logan, UT, USA) according to Koizumi et al. (2007). In brief, a leaf disc of 0.28 cm2, excluding the midrib, was excised with a leaf punch and installed in a sample chamber. The chamber was placed in a box of foam polystyrene for 3 h in a room at 25 °C, and water potential was measured.
Leaf xylem water potential (Ψxylem) was determined at the end of night with a pressure chamber instrument (model 3005, Soil Moisture Equipment Inc., Santa Barbara, CA, USA). A leaf was covered with a moistened plastic bag, quickly excised from the plant and installed in the pressure chamber. The inner wall of the pressure chamber was covered with wet filter paper. The chamber was pressurized with compressed air at a rate of approximately 0.003 MPa s−1. Ψleaf and Ψxylem values of one or two leaves per replicate were measured.
SPAD value, photosynthetic rate, stomatal conductance and chlorophyll, Rubisco and nitrogen contents of a leaf (Exps. 1 and 2)
Relative values of leaf chlorophyll content were measured with a chlorophyll meter (SPAD-502, Konica Minolta, Osaka, Japan) and expressed as SPAD value. Measurements were taken at the central part of four to six leaves per replicate, not including the midrib, and used the mean as the measured value.
Net photosynthetic rate (An) and stomatal conductance (gs) in a leaf attached on the main stem were measured with a portable photosynthetic system (LI-6400; LI-COR Inc., Lincoln, NE, USA) and LED light source (LI-6400-02B; LI-COR Inc.) during the day. Leaf temperature in the leaf chamber was maintained at 30 °C unless otherwise noted, and the photosynthetically active radiation at the leaf surface was maintained at 2000 μmol photons m−2 s−1 (at which photosynthesis is sufficiently light-saturated). Ambient CO2 concentration in the assimilation chamber (Ca) was 350 ± 1 μmol mol−1 and the leaf-to-air vapor pressure difference was 1.2–1.5 kPa. Measurements for the plants grown in the growth chamber were started 1 h after light was turned on in the day. Each measurement was taken after the leaf gas exchange rate reached a steady state in the assimilation chamber (usually within 30 min after installing a leaf in the chamber). We measured An and gs of two leaves with an averaged SPAD value per replicate and used the mean as the measured value.
After An and gs measurements, the leaves were collected on ice, frozen in a − 80 °C freezer as quick as possible after the fresh weight and area of each leaf were measured, and stored at − 80 °C prior to chlorophyll, Rubisco and nitrogen analysis. Leaves were homogenized on ice with a mortar and pestle in a solution containing 50 mM Tris–HCl (pH 7.5), 1 mM EDTA, 10 mM β-mercaptoethanol and 10% (w/w) insoluble polyvinylpyrrolidone (Polyclar VT; Wako Chemical Industries, Tokyo, Japan). Chlorophyll was extracted from the homogenate with 96% ethanol and the concentration of chlorophyll a + b was calculated from the absorbance of the extract at 649 nm and 665 nm measured with a spectrophotometer (U-3210, Hitachi Co., Tokyo, Japan) according to Wintermans and De Mots (1965).
The homogenate was centrifuged at 10,000 × g for 10 min at 4 °C; Rubisco was quantitated in the supernatant by the single radial immunodiffusion method of Sugiyama and Hirayama (1983) with rabbit polyclonal antibodies raised against purified rice Rubisco. In brief, 2 μl of purified rice Rubisco standard solutions with serial concentration or leaf extracts was placed in wells in agar gel plates and incubated at 25 ℃ for 48 h to allow radial diffusion. After removing the soluble protein, the gels were stained with Coomassie Brilliant Blue R-250 and the areas of the precipitin rings were measured.
Leaf nitrogen was quantified with a carbon and nitrogen analyzer (MT-600, Yanaco Inc., Kyoto, Japan) in leaves that had been oven-dried at 90 °C for 3 days.
Dry mass and nitrogen content of a plant (Exps. 2 and 5)
Plants were separated into leaf blades, leaf sheaths plus stems, panicles, roots and dead parts and dried in a ventilated oven at 90 °C for at least 3 days. Nitrogen content of each sample was calculated as the product of dry weight and nitrogen concentration determined with the MT-600 carbon and nitrogen analyzer. We measured dry mass and nitrogen content of all plants in a pot per replicate and used the mean as measured value.
Root exudate collection and cytokinin bioassay (Exps. 1 and 4)
The procedures were conducted according to Soejima et al. (1992, 1995) and San-oh et al. (2005). We selected a hill with an average number of stems in a pot per replicate in the exudate collection. Stems were cut 10 cm above the soil surface; an absorbent cotton was placed on the cut end and covered with a polyethylene bag. Exudates were collected for 12 h during night in the dark at 25 °C unless noted otherwise. Cottons with the exudates were stored at –80 °C until cytokinin determination. Exudates in the cotton were extracted in 80% methanol and each extract was evaporated under vacuum to an aqueous residue. The residue was dissolved in water and loaded onto a Sep-pak C18 cartridge (Waters, Milford, MA, USA). The cartridge was eluted with 40% methanol. The eluate was evaporated to dryness and dissolved in 1.0 mL of water for the bioassay. Cytokinin activities of xylem exudates were determined using a betacyanin bioassay (Biddington and Thomas 1973) using hypocotyls plus cotyledons of Amaranthus tricolor. Concentrations of cytokinin-induced betacyanins extracted from the explants were determined as the difference between the absorbance values at 542 nm and 620 nm measured with the U-3210 spectrophotometer. The cytokinin activity (concentration) was estimated by comparing a standard curve made by a serial dilution of a solution of 6-benzylaminopurine (BA), and the results were expressed as “6-benzylaminopurine (BA) equivalent quantities” by multiplying the estimated concentration by the exudation rate.
Mass spectrometric analysis of cytokinins in xylem exudates (Exp. 4)
The main cytokinins in xylem exudates of rice roots—zeatin (Z), zeatin riboside (ZR), zeatin-O-glucoside ((OZ)Z), N6(Δ2-isopentenyl) adenine (iP) and N6(Δ2-isopentenyl) adenosine (iPA) (Soejima et al. 1992, 1995) were determined using deuterium-label standards (Hashizume et al. 1979; Sugiyama and Hashizume 1982) according to Soejima et al. (1992, 1995). Deuterium-label preparations of Z and ZR consisted of the trans-isomer (92%) and cis-isomer (8%). Exudate samples were purified using Sep-pak C18 cartridges and were separated by HPLC on a reverse phase column (Shim-pack CLC-ODS, 150 × 6.0 mm i.d., Shimadzu Co.; detection at 270 nm; flow rate 1.0 mL min−1). The mobile phase was 40% methanol, and the eluate was separated into six fractions (retention time [Rt, min]: fraction A, 0–4; B, 4–9; C, 9–13; D, 13–18; E, 18–23; F, 23–27). Fraction F was separated in 23% acetonitrile as the mobile phase, with each of these subsequently fractionated as single peaks of iP (Rt: 12–14) and iPA (Rt: 10–12). Fraction B was separated in 11% acetonitrile as the mobile phase, and the fractions corresponding to Z (Rt: 9–14) and trans- and cis-ZR (Rt: 14–18) were collected. The fraction containing Z was separated into trans-Z (Rt: 9–11) and cis-Z (Rt: 11–14) in 30% methanol as the mobile phase.
Fraction A is expected to contain conjugated cytokinins (Hashizume et al. 1979). Samples were dissolved in 0.3 mL of β-glucosidase solution (1 mg mL−1; Sigma Co., St. Louis, MO, USA) and incubated at 37 °C for 15 h, and the reaction was stopped by adding 3 mL of 100% methanol. The mixture was centrifuged (3,000 × g, 20 min), purified using Sep-pak C18 cartridges and separated by HPLC into a highly polar fraction and the Z fraction. After adding 2H-labeled standards, the Z fraction was processed as described previously for (OG)Z.
The Z fraction was tert-butyldimethylsilylated with a mixture of acetonitrile and tert-butyldimethylsilyl (Tokyo Kasei Co., Tokyo, Japan) at 80 °C for 20 min. The ZR and iPA fractions were trimethylsilylated with TMS-HT (hexamethyldisilazane and trimethylchlorosilane in pyridine; Tokyo Kasei Co.) at 120 °C for 1 h. The iP fraction was silylated with a mixture of acetonitrile, bis(trimethylsilyl)-acetamide (Tokyo Kasei Co.) and trimethyl-chlorosilane (Tokyo Kasei Co.) at 80 °C for 20 min.
Each cytokinin fraction was injected into a fused silica capillary column (DB-1; 15 m × 0.25 mm i.d., 0.25 μm film thickness; J&W Scientific, Folsom, CA, USA) installed in a gas chromatograph (5890 series II; Hewlett Packard, Rockville, MD, USA). Helium gas with a head pressure set at 100 kPa was used as the GC carrier gas. The injection part and the GC–MS interface temperatures were 250 °C. The column temperature was maintained isothermally at 70 °C (Z, iP) or 100 °C (ZR, iPA) for 2 min, then raised to 280 °C at a rate of 30 °C min−1 and maintained isothermally at 280 °C for 4 min. An Automass 50A mass spectrometer (JEOL, Tokyo, Japan) was used; ion source temperature was 250 °C and ionization energy was 70 eV. SIM was set up according to Soejima et al. (1992).
Nitrogen (N) inflow and outflow, Rubisco synthesis and degradation, and rbcS and rbcL expression (Exps. 3 and 5)
15 N-labelling
When the flag leaf had just emerged from the leaf sheath of the leaf below 18 days before full heading, the nutrient solution was drained from the bottom of the pot. The pot was filled with the nutrient solution without nitrogen, and the solution was completely drained again. This procedure was performed three times, and 1 L of double-strength nutrient solution containing 2 mM (15NH4)2SO4 (30.3 atom % excess) was added to each pot containing nutrient solution without nitrogen, and plants were grown for 4 days. Then the plants were grown again in a non-labeled double-strength Kimura B solution until soil moisture treatment at full heading. One flag leaf attached on the main stem with average SPAD value was used per replicate in the following each analysis. Leaves were collected between 1 and 3 h after light was turned on.
Leaf N content and 15N analysis
Dried and milled individual leaves were digested by Kjeldahl method. The total leaf N content (LNC) was determined by the indophenol method.
15N analysis was carried out according to Mae et al. (1983). Nitrogen in the digest was absorbed with 0.1 M HCl by the micro-diffusion method (Yoneyama et al. 1975). Part of the HCl solution was put into a glass micro-capillary tube with 1 mm inner diameter and dried in an oven. This 15N-containing tube was transferred into a glass tube with 2 mm inner diameter, and the 15N discharge tube was prepared according to Kano et al. (1974). The amount of 15N in total leaf N was determined by emission spectrography with a 15N-analyzer (N-151, JASCO Co., Tokyo, Japan).
Leaf Rubisco content and 15N analysis of isolated Rubisco
The collected leaves were frozen and stored at − 80 °C until analysis. They were homogenized using a chilled pestle and mortar in 50 mM sodium phosphate buffer (pH 7.5) containing 2 mM iodoacetic acid, 0.8% (v/v) 2-mercaptoethanol and 5% glycerol, and Triton X-100 (final concentration 0.1%; Bio-Rad, Hercules, CA, USA) was added to the homogenate (Suzuki et al. 2001b). The mixture was centrifuged at 15,000 × g for 10 min at 4 °C. The supernatant was separated in polyacrylamide gels containing sodium dodecyl sulfate (SDS). The gels were stained with Coomassie Brilliant Blue R-250, and the bands of Rubisco subunits were excised, extracted with formamide, and Rubisco content was determined spectrophotometrically according to Makino et al. (1986). Calibration curves were obtained by using Rubisco purified from rice leaves.
For 15N analysis, Rubisco was isolated according to Suzuki et al. (2001b) with some modifications. An aliquot of the supernatant from the centrifuged homogenate was mixed with an equal volume of glycerol and stored at − 20 °C. This mixture was separated in a polyacrylamide minislab gel without SDS (3.0% stacking gel, 5.0% separation gel). The part of the gel corresponding to Rubisco was cut out and homogenized in 50 mM sodium phosphate (pH 7.5). The homogenate was supplemented with Triton X-100 (final concentration 0.2%) and shaken at 180 times per minute at 37 °C for 14–18 h. The mixture was centrifuged at 15,000 × g at room temperature for 5 min, and protein in the supernatant was precipitated by the addition of trichloroacetic acid (final concentration 10%). The precipitate was collected by centrifugation at 15,000 × g at 4 °C for 15 min, washed with 80% (v/v) ethanol and dissolved in 0.1 M NaOH. Aliquots were placed in microtubes and the amount of 15N was determined by emission spectrography with the N-151 15N-analyzer.
Estimation of N flux and Rubisco synthesis and degradation in the leaf blade
The influx and efflux of N during the period (g N m−2 leaf area day.−1) from time t1 to t2 (t1, t2: days after start of the soil moisture treatment) were calculated according to Mae et al. (1983). \({\mathrm{N}}_{\mathrm{efflux t}1-\mathrm{t}2}=\left(\left({}^{15}{\mathrm{N}}_{\mathrm{t}1}-{}^{15}{\mathrm{N}}_{\mathrm{t}2}\right)/{}^{15}{\mathrm{N}}_{\mathrm{t}1}\right)\times {\mathrm{N}}_{\mathrm{t}1}/\left({\mathrm{t}}_{2}-{\mathrm{t}}_{1}\right)\)
where 15Nt1 and 15Nt2 are labeled N contents at t1 and t2, respectively, calculated as follows:
Labeled N = (15N atom% excess of the leaf) / (15N atom% excess of [15NH4]2SO4 fed to the plants) × leaf N content.
The amount of Rubisco synthesized (RubSyn t1-t2) and degraded (RubDeg t1-t2) during the period from t1 to t2 (g Rubisco m−2 leaf area day−1) was calculated according to Mae et al. (1983).
where 15NRubt1 and 15NRubt2 are labeled Rubisco contents at t1 and t2, respectively, and NRubt1, and NRubt2 are the total Rubisco contents at t1 and t2, respectively.
Levels of rbcS and rbcL mRNAs
The collected leaves were frozen in liquid nitrogen immediately then stored at − 80 °C until analysis. Total leaf RNA was extracted according to Suzuki et al. (2001a). Part of the total RNA was blotted onto a positively charged nylon membrane (Hybond-N+, Amersham Pharmacia Biotech, Piscataway, NJ, USA) with a slot-blot apparatus (Bio-Dot; Bio-Rad) according to Suzuki et al. (2001b). The hybridization and washing of the membranes were carried out by following the digoxigenin (DIG) system user’s guide (Roche Diagnostics, Indianapolis, IN, USA). The DIG-labelled DNA probes for rbcS and rbcL were prepared from fragments of rice rbcS (Matsuoka et al. 1988) and rbcL (Hirai et al. 1985), respectively, with the PCR DIG synthesis kit (Roche Diagnostics). Signals were detected with a DIG luminescent detection kit (Roche Diagnostics). Signal intensities were determined with a densitometer (GS-700, Bio-Rad).
Application of exogenous cytokinin (Exp. 5)
The entire aboveground parts of two hills were sprayed every day during the soil moisture treatment with 30 mL of a 10−4 M solution of BA that contained 0.05% (v/v) Tween 20 as a surfactant (BA-plants) or 30 mL of water containing 0.05% Tween 20 (control); all these plants were growing in the same pot. During spraying, to prevent the solution from splashing onto adjacent hills, a 95 cm high plastic board was placed between the hills and an absorbent cotton was placed on the vermiculite to prevent the solution from spreading onto the vermiculite.
Statistical analysis
The mean of the measurements of a replicate was used as the raw datum for analysis, and differences were tested by Student’s t-test or Tukey HSD test, as appropriate, in Exps. 1 through 4, and by paired t-test in Exp. 5. Pearson’s correlation coefficient was calculated to test the significance of the relationship between An or LNC and Rubisco contents. All statistical analyses were performed in JMP v.13 software (SAS Institute, Cary, NC, USA).
Results
Effects of soil moisture stress on SPAD , A n , LNC and Ψ leaf (Exp. 1)
As a readout for leaf senescence, we compared the above parameters in the flag leaf (youngest) and in the 2nd, 3rd and 4th (oldest) leaves from the flag leaf using Nipponbare (Fig. 1). The SPAD value decreased more in the 2nd, 3rd and 4th leaves in the DR-plants than in the FL-plants at 10 days after the start of soil moisture treatment (DAT), and in all leaves at 17 DAT and 31 DAT (Figs. 1b–e, S1). Re-flooding of the DR-plants at 17 DAT slightly but significantly recovered SPAD values at 31 DAT in flag leaves only (Fig. 1b–e). The An and LNC values of the 3rd leaf also decreased and were significantly lower in the DR-plants than in the FL-plants at 20 and 38 DAT (Fig. 2). Re-flooding of the DR-plants at 17 DAT tended to partially recover LNC but not An at 38 DAT (Fig. 2). We examined the effects of different intensities of soil moisture stress on SPAD value, An and LNC using Kinmaze (Fig. S2). The SPAD value and An decreased more under severe soil moisture stress than under mild soil moisture stress.
Changes of (a) leaf photosynthetic rate (An), and (b) leaf nitrogen content (LNC) of the 3rd leaf from the flag leaf in cv. Nipponbare grown in field soil under flooded (FL) or moisture-deficient conditions (DR) (Exp. 1). The treatment was started at full heading. DAT: days after the start of treatment. RW: re-flooded at 17 DAT. Values are means ± SE (n = 3). The same letters in a graph represent no significant difference at the 5% level (Student’s t-test at 20 DAT or Tukey HSD test at 38 DAT)
The Ψleaf values were significantly lower in the DR-plants than in the FL-plants regardless of cloudiness in Kinmaze (Fig. 3a). The same pattern was observed under cloudy sky, but the Ψleaf values did not differ significantly between the DR- and FL-plants under sunny sky in CM2088. Compared with Kinmaze, Ψleaf decreased significantly and leaves wilted remarkably in CM2088 under sunny sky in the FL plants (Figs. 3a, S3). The SPAD value of the 2nd leaves from the upper most fully expanded leaf did not differ significantly among the samples at 1 DAT (Fig. 3b) and 12 DAT (Fig. 3c). The SPAD value of the 4th leaf did not differ at 1 DAT, but it was significantly lower in both Kinmaze and CM2088 in the DR-plants than in the FL-plants at 12 DAT (Fig. 3c). Thus, the CM2088 leaves maintained a high SPAD value similar to that of Kinmaze under FL conditions, even though their Ψleaf decreased markedly in the sun. The exudation rate did not differ significantly between Kinmaze and CM2088 FL-plants (Table S1).
(a) Leaf water potential (Ψleaf) under cloudy (Cloud) and sunny sky (Sun), and (b, c) SPAD values in Kinmaze (K) and CM2088 (CM) grown in flooded (FL) or moisture-deficient (DR) soil (Exp. 1). All measurements were performed at panicle formation to heading. 2nd and 4th are leaf numbers from the upper most fully expanded leaf. The 2nd leaf was used to measure Ψleaf. Time points of the measurements: Ψleaf (cloud), 12 days after the start of treatment (DAT); Ψleaf (sun), 16 DAT; SPAD values, (b) 1 DAT and (c) 12 DAT. Values are means ± SE (n = 3 for Ψleaf and n = 4 for SPAD value). The same letters in a graph represent no significant difference at the 5% level (Tukey HSD test)
Cause of the enhanced A n decrease with senescence in moisture-deficient soil (Exp. 2)
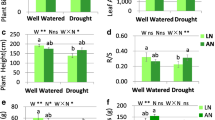
Daily evapotranspiration in the DR-plants decreased to 40%–50% of that of the WE-plants a few days after soil moisture treatment (Fig. S4) and the DR-plants had 0.2 to 0.6 MPa lower Ψleaf than the WE-plants during the day (Fig. 4). Several days after the start of moisture treatment, An decreased with senescence in both the WE- and DR-plants, but this decrease was larger in the DR-plants than in the WE-plants (Fig. 5a). Similar decreases were observed in gs (Fig. 5b), LNC (Fig. 5d), chlorophyll content (Fig. 5e) and Rubisco content (Fig. 5f) except for LNC and chlorophyll content of the flag leaf. An/Ci (intercellular CO2 concentration) was lower in the DR-plants than in the WE-plants although the difference was significant at 8% level in the flag leaf (Fig. 5c), indicating that a larger biochemical limitation of photosynthesis was involved in the larger reduction of An in the DR-plants than in the WE-plants (Cornic et al. 1989; Farquhar et al. 1980; Hirasawa 2018; Sage et al. 2017). Close correlations were found between Rubisco content and An (Fig. 6a) or LNC (Fig. 6b).
(a) Photosynthetic rate (An), (b) stomatal conductance (gs), (c) An/Ci (intercellular CO2 concentration), (d) nitrogen content (LNC), (e) chlorophyll (Chl) content and (f) Rubisco content of the flag leaf and the 2nd and 3rd leaves from the flag leaf in plants grown in sufficiently wet (WE) or moisture-deficient (DR) vermiculite (Exp. 2). Leaf temperature in the leaf chamber was maintained at 25 °C. BT: plants before treatment. WE, DR: values at 7 days after the start of treatment (DAT) for the flag leaf, at 6 DAT for the 2nd leaf, and at 5 DAT for the 3rd leaf. Values are means ± SE (n = 4). The same letters in a graph represent no significant difference at the 5% level (Tukey HSD test)
Relationships (a) between photosynthetic rate (An) and Rubisco content and (b) between Rubisco content and nitrogen content (LNC) of the flag leaf and the 2nd and 3rd leaves from the flag leaf (Exp. 2). BT: plants before treatment. WE, DR: plants grown in sufficiently wet or moisture-deficient vermiculite, respectively
Nitrogen content and nitrogen partitioning were significantly lower in the leaf blade and significantly higher in roots of the DR-plants than in those of the WE-plants at 7 DAT, with no significant differences in total nitrogen content (Table 1). Dry weight and dry matter partitioning was significantly lower in the leaf blade and dry matter partitioning to roots was significantly higher in the DR-plants than in the WE-plants (Table S2).
Nitrogen inflow and outflow and Rubisco synthesis and degradation in the flag leaf in moisture-deficient soil (Exp. 3)
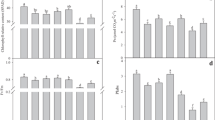
During moisture treatment for 12 days, the daily evapotranspiration of the DR-plants was approximately 60% of that of the WE-plants at 4 DAT and 40% to 50% thereafter, and Ψxylem at the end of night decreased to − 0.3 to − 0.4 MPa within a few DAT and was maintained between − 0.5 and − 0.8 MPa thereafter (Fig. S5). LNC of the flag leaf decreased more in the DR-plants than in the WE-plants (Fig. 7a). The N inflow was significantly larger in the WE- than in the DR-plants at 0–3 DAT and decreased in both groups at 3–12 DAT (Fig. 7b). On the contrary, the N outflow was significantly larger in the DR-plants than in the WE-plants at 0–3 DAT (Fig. 7c). At 3–12 DAT, the N outflow decreased in both groups and was slightly but significantly larger in the WE-plants than in the DR-plants.
Changes in (a) nitrogen content (LNC), (b) nitrogen (N) inflow and (c) N outflow in the flag leaf of plants grown in sufficiently wet (WE) or moisture-deficient (DR) vermiculite (Exp. 3). DAT: days after the start of treatment. Values are means ± SE (n = 4). Differences between the WE and DR plants: significant at the *5%, **1% and ***0.1% α level (Student’s t-test)
Rubisco content of the flag leaf decreased more in the DR-plants than in the WE-plants, although the difference was significant at 3 DAT but not at 12 DAT (Fig. 8a). Rubisco synthesis was very low in both groups except at 0–3 DAT in the WE-plants (Fig. 8b). Rubisco degradation was significantly larger in the DR-plants than in the WE-plants at 0–3 DAT and did not differ between the groups at 3–12 DAT (Fig. 8c). At 3 DAT, the level of rbcS mRNA was significantly higher in the WE-plants than in DR-plants, with no significant difference in the level of rbcL mRNA (Fig. 9).
Changes in Rubisco (a) content, (b) synthesis and (c) degradation in the flag leaf of plants grown in sufficiently wet (WE) or moisture-deficient (DR) vermiculite (Exp. 3). DAT: days after the start of treatment. Values are means ± SE (n = 4). Differences between the WE and DR plants: significant at the *5%, **1% and ***0.1% α level; ns, not significant at the 5% level (Student’s t-test)
Relative levels of (a) rbcS mRNA and (b) rbcL mRNA in the flag leaf of plants grown in sufficiently wet (WE) or moisture-deficient (DR) vermiculite (Exp. 3). Values at 3 days after the start of treatment (DAT) were normalized to the average value at 0 DAT. Values are means ± SE (n = 5). Differences between the WE and DR plants: significant at the *5% α level; ns, not significant at the 5% level (Student’s t-test)
Cytokinin levels in exudates in moisture-deficient soil (Exp. 4)
Since the exudation rate was smaller, the cytokinin concentration in the exudates determined by the Amaranthus bioassay was higher in the DR-plants than in the WE-plants (Table S3). The cytokinin flux to the shoot was smaller in the DR-plants than in the WE-plants (Table 2). The concentrations of all cytokinins determined by the mass spectrometric analysis of exudates tended to be higher or were significantly higher (for (OG)Z and iPA) in the DR-plants than in the WE-plants (Table S3). The total cytokinin flux to the shoot and those of t-Z, (OG)Z and iPA were significantly smaller in the DR-plants than in the WE-plants (Table 2).
Effects of exogenous cytokinin application on nitrogen inflow and outflow and Rubisco synthesis and degradation (Exp. 5)
During moisture treatment for 12 days, the LNC decrease in the flag leaf was smaller in the BA-plants than in the control plants, and the difference was significant at 12 DAT (Fig. 10a). The N inflow was extremely small, but it was significantly larger in the control plants than in the BA-plants (Fig. 10b). The N outflow was larger at 0–3 DAT than at 3–12 DAT and was significantly smaller in the BA-plants than in the control plants at 0–3 DAT (Fig. 10c).
Changes in (a) nitrogen content (LNC), (b) nitrogen (N) inflow and (c) N outflow in the flag leaf of plants supplied with solution supplemented with 6-benzylaminopurine (BA) or containing no BA (control) (Exp. 5). DAT: days after the start of moisture treatment. Values are means ± SE (n = 4). Differences between the control and BA plants: significant at the *5%, **1% and ***0.1% α level; ns, not significant at the 5% level (paired t-test)
During moisture treatment, the Rubisco content of the flag leaf decreased to a smaller extent and its values remained higher in the BA-plants than in the control plants although they were not significant at 12 DAT (Fig. 11a). Rubisco synthesis was very low in both groups (Fig. 11b). Rubisco degradation was much smaller in the BA-plants than in the control plants at 0–3 DAT but did not differ significantly between the groups at 3–12 DAT (Fig. 11c).
Changes in Rubisco (a) content, (b) synthesis and (c) degradation in the flag leaf of the plants supplied with solution supplemented with 6-benzylaminopurine (BA) or containing no BA (control) (Exp. 5). DAT: days after the start of treatment. Values are means ± SE (n = 4). Differences between the control and BA plants: significant at the *5% and ***0.1% α level; ns, not significant at the 5% level (paired t-test)
Total nitrogen content did not differ, whereas the nitrogen content of the leaf blade was higher and that of the stem + leaf sheath was lower in the BA-plants than in the control plants (Table 3). Nitrogen partitioning to the leaf blade was significantly larger and that to the panicle and stem + leaf sheath was significantly smaller in the BA-plants (Table 3). Dry weight of the leaf blade and dry matter partitioning to the leaf blade were significantly higher in the BA-plants than in the control plants (Table S4).
Discussion
Decrease in A n with senescence is accelerated by the decrease in soil moisture rather than by that in leaf water potential
Chlorophyll content and An decreased with senescence more rapidly in plants grown in moisture-deficient soil than in flooded or wet soil (Figs. 1–3, 5, S1), as is commonly observed in many crop plants (Brevedan and Egli 2003; Chen et al. 2015; De Souza et al. 1997; Kramer and Boyer 1995; Zhang and Zou 2013). These decreases were mostly irreversible (Figs. 1 and 2), as in soybean (Brevedan and Egli 2003; De Souza et al. 1997). Ψleaf decrease and leaf senescence progressed similarly in CM2088 and Kinmaze grown in moisture-deficient soil (Fig. 3). However, leaf senescence did not progress in CM2088 grown in flooded soil even though the Ψleaf decrease under sunny sky was similar to that in moisture-deficient soil (Fig. 3). In the flooded soil, the gs of CM2088 was similar to that of Kinmaze in the morning, but it decreased markedly compared with Kinmaze in the midday of a clear day because of the remarkable reduction of Ψleaf (Koizumi et al. 2007). This suggests that the high content of leaf chlorophyll in CM2088 in flooded soil is not associated with high gs (and An). The capacity for root water uptake does not differ between CM2088 and Kinmaze in flooded soil (Koizumi et al. 2007). Accordingly, we observed no difference in root exudation rate between CM2088 and Kinmaze grown in flooded soil (Table S1). Overall, we attribute the accelerated leaf senescence in plants grown in moisture-deficient soil to the soil moisture decrease per se, but not to the Ψleaf decrease.
The decrease in A n with senescence under soil moisture stress is accelerated more by the enhanced degradation of Rubisco
The decrease of CO2 diffusion (through stomata and in mesophyll) is a main cause of the An reduction under mild water stress in newly developed leaves or in leaves not under the senescence process (Cornic et al. 1989; Flexas et al. 2004; Hirasawa et al. 1989). In contrast, water stress accelerated nitrogen relocation from leaves to other organs in the leaves under senescence (Figs. 7 and 8, Table 1; Brevendan and Egli 2003), and the biochemical limitation of photosynthesis was a major cause of the An decrease (Fig. 5). In the biochemical limitation of photosynthesis, An is limited either by RuBP carboxylation or by RuBP regeneration in response to CO2 concentration according to C3 photosynthesis model (Farquhar et al. 1980). In the various genotypes of rice, An was limited by the rate of RuBP carboxylation at Ci ≦ 350 μmol mol−1, in which the Ci estimated at the present ambient CO2 concentration was well included (Adachi et al. 2013). Rubisco activity per leaf area decreased with senescence due to the decrease in the level of Rubisco protein, not due to the decrease of its specific activity (Makino et al. 1983). On the contrary, Rubisco activity per leaf area did not decrease under water stress in the leaves not under senescence (Brestic et al. 1995). Rubisco content was closely correlated with An in all samples in wet and moisture-deficient soil (Fig. 6a). Taken together, we conclude that the level of Rubisco was a major limiting factor for An in the leaves under senescence in the current study.
Rubisco is actively synthesized during leaf expansion, and its degradation starts soon after full leaf expansion and continues during senescence (Mae et al. 1983). The levels of rbcS and rbcL mRNAs during the life span of a leaf are coordinated with those in the amount of Rubisco synthesized (Suzuki et al. 2001b). We started soil moisture treatment at full heading, i.e. approximately 2 weeks after the full expansion of the flag leaf. This coincides with the highest content of N and Rubisco in the flag leaf (Mae et al. 1983; Makino et al. 1983). In the first 3 days of soil moisture treatment, N inflow into the flag leaf and Rubisco synthesis were lower, and N outflow from the flag leaf and Rubisco degradation were higher in the DR-plants than in the WE-plants (Figs. 7, 8). Rubisco content decreased more because its degradation exceeded synthesis much more in the DR-plants than in the WE-plants (Fig. 8). We conclude that the main cause of the An decrease with senescence after full heading under soil moisture stress was the enhanced Rubisco degradation.
We attributed the decrease in Rubisco synthesis in the DR-plants to the decreased level of rbcS mRNA (Fig. 9). The level of rbcS mRNA can be affected by the N inflow into the leaf (Imai et al. 2008) and by leaf water content (Zhang et al. 2013). The decrease in leaf water content or Ψleaf could not be attributed to the accelerated decrease in SPAD value (or chlorophyll content) with senescence (Fig. 3). The decrease in the N inflow into the leaf might be the main cause of the decreases in the rbcS mRNA level and Rubisco synthesis (Figs. 7, 8, 9). We cannot distinguish whether the decrease in the N inflow into the leaf in the DR-plants was due to a decrease in root N uptake and assimilation (Gonzalez-Dugo et al. 2010; O’Toole and Baldia 1982) or to a decrease in N remobilization from other organs to the leaf (Zhang et al. 2022) in the current study.
Decrease in cytokinin transport from roots to shoots accelerates the A n decrease under soil moisture stress
Cytokinin delays leaf senescence in well-watered plants (Gan and Amasino 1995; Richmond and Lang 1957; Singh et al. 1992) and plants under water stress (Peleg et al. 2011; Reguera et al. 2013; Rivero et al. 2007; Sade et al. 2018). Exogenous cytokinin application delayed the decrease in Rubisco with senescence in rice plants grown in flooded soil (Ookawa et al. 2004) and in moisture-deficient soil (Fig. 11).
Cytokinins are synthesized in leaves and roots and are transported to roots or the shoot (Ko et al. 2014; Sakakibara 2006). Here we showed that the accelerated leaf senescence under soil moisture stress was caused mainly by root water stress, not by leaf water stress (Fig. 3). Plants that transport larger cytokinin amounts from roots to shoots show delayed leaf senescence (Sato et al. 2007; Sitton et al. 1967) and plants with the stay-green phenotype transport large amounts of cytokinins from roots to shoots (He et al. 2005; Ookawa et al. 2004; Soejima et al. 1992, 1995). Soil moisture stress decreased cytokinin flux from roots to shoots in rice (Table 2), as reported previously (Bano et al. 1993; Jogawat et al. 2021; Kudoyarova et al. 2007). Decreased cytokinin flux from roots would decrease the cytokinin concentration in leaves despite the high cytokinin concentration in xylem sap (Table S3) (Kudoyarova et al. 2007). Overall, we attribute the accelerated decease in An with senescence under soil moisture stress to the decrease in cytokinin flux from roots to shoots.
There are reports showing that Z and ZR are mainly synthesized in roots while iP and iPA are dominantly synthesized in shoots (Ko et al. 2004; Sakakibara 2006; Zhang et al. 2022). However, the large flux of iP and iPA from roots to shoots were measured in rice (Table 2) (Soejima et al. 1992, 1995), and root flux of t-Z and (OG)Z as well as iPA decreased significantly and iP flux tended to decrease in the moisture-deficient soil (Table 2), indicating that not only root flux of Z and ZR but also that of iP and iPA are involved in delaying An decrease under soil moisture stress.
We did not examine associations of cytokinins with other plant hormones on leaf senescence under soil moisture stress. Levels of abscisic acid and auxin also changed in the transgenic plants for cytokinin production (Ghanem et al. 2011; Havlová et al. 2008). Crosstalk between cytokinins and other plant hormones is now investigated intensively (El-Showk et al. 2013; Nishiyama et al. 2011; Zubo and Schaller 2020). Their crosstalk under soil moisture stress remains in a future study.
Association of the decreased cytokinin flux from roots to shoots with Rubisco degradation and nitrogen partitioning under soil moisture stress
During senescence, leaf photosynthetic apparatus is degraded, An decreases and nutrients are remobilized (Hӧrtensteiner and Feller 2002; Sade et al. 2018). In the current study, the accelerated An decrease under soil moisture stress was caused mainly by Rubisco degradation (Figs. 5, 6, 8). The Rubisco degradation under soil moisture stress was mitigated by exogenous cytokinin application (Fig. 11). A large proportion of total leaf nitrogen (approximately 30%) is found in Rubisco (Makino et al. 1992), and the decrease in An and leaf Rubisco content with senescence was larger in the DR-plants than in the WE-plants (Figs. 5, 6). As a consequence, nitrogen partitioning to the leaf blade was significantly lower in the DR-plants than in the WE-plants (Table 1).
Rubisco is degraded via autophagy (Ishida et al. 2014) and autophagy is linked to cytokinins (Acheampong et al. 2020; Gou et al. 2019). Under water stress, cytokinins preserve cell structure and the photosynthetic machinery in transgenic plants (Rivero et al. 2009, 2010) or when applied exogenously (Chernyad’ev 2005). Taken together, we attribute the decreased cytokinin flux from roots to shoots to the enhanced degradation of Rubisco and the enhanced nitrogen outflow in leaves. The overall mechanism by which cytokinins suppress the inhibition of leaf photosynthesis under soil moisture stress is a topic for a future study.
Towards improving rice productivity in rainfed and AWD systems
Decreased leaf water content (or water potential) under mild water stress usually recovers fully after rewatering, but the decreased An does not (Chaves et al. 2009; Kramer and Boyer 1995). The causes of the not-full recovery differ between young and senescent leaves. In newly developed young leaves or in leaves not under the senescence process, the main cause is an incomplete recovery of CO2 diffusion (stomatal and mesophyll conductance) and the biochemical limitation of photosynthesis is not the cause (Flexas et al. 2009). In the senescent leaves, the decreased LNC did not recover completely after rewatering (Fig. 2; Brevendan and Egli 2002). This would be an important cause of the not-full recovery of An after rewatering as the biochemical limitation of photosynthesis.
Soil drought develops during the ripening stage in rainfed paddy rice (Fukai et al. 1998; Miyagawa et al. 2006). The AWD systems are widely used during reproductive growth (Carrijo et al. 2017). Soil moisture stress accelerates the decrease in An and leaf area with senescence in rice after heading (Lu et al. 2000). The effects of leaf senescence on dry matter production and grain yield should be large because the decreased An does not recover fully after re-irrigation and new leaves no longer expand after heading. Thus, delaying the decrease of An with senescence would be important during soil moisture stress in rice in rainfed and AWD systems for avoiding a decrease in grain yield. Results of the current study suggest that cytokinins are effective in delaying the An decrease with senescence in rice under rainfed and AWD systems. Cytokinin effects on biomass and grain production in rice remain to be examined in the rainfed field and the AWD field.
The exogenous cytokinin application may affect the carbon–nitrogen ratio of grain and, therefore, grain quality because it does not increase the nitrogen accumulation in the plants (Table 3; Ookawa et al. 2004). Cytokinin effects on grain quality may also need to be considered in rice production. If high leaf nitrogen content can be achieved before soil moisture decreases, the leaves would maintain high An even under soil moisture stress (Fig. S6). Compost application before planting and/or nitrogen topdressing before soil moisture stress would efficiently increase leaf nitrogen content (Sriphirom et al. 2020). A stay green rice cultivar, Akenohoshi shows high roots capacities of cytokinin transport and nitrogen uptake during ripening in flooding soil (Ookawa et al. 2003). Improving the capacity of root nitrogen uptake as well as the management of nitrogen fertilizer application for delaying senescence under water stress would also be an important strategy of the research. Quantitative trait loci (QTLs) for leaf senescence have been detected and a near-isogenic line that maintains high leaf chlorophyll and high root nitrogen transport during ripening in flooded soil has been developed (Yamamoto et al. 2017). Identification of QTLs and genes for maintaining high capacities of cytokinin biosynthesis and nitrogen transport in roots under soil moisture stress and their effects on dry matter production and grain yield and quality are the topic for future studies.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Change history
05 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s11104-023-06402-y
References
Acheampong AK, Shanks C, Cheng C-Y, Schaller GE, Dagdas Y, Kieber JJ (2020) EXO70D isoforms mediate selective autophagic degradation of type-A ARR proteins to regulate cytokinin sensitivity. Pro Natl Acad Sci USA 117:27034–27043. https://doi.org/10.1073/pnas.2013161117
Adachi S, Nakae T, Uchida M, Soda K, Takai T, Oi T, Yamamoto T, Ookaw T, Miyake H, Yano M, Hirasawa T (2013) The mesophyll anatomy enhancing CO2 diffusion is a key trait for improving rice photosynthesis. J Exp Bot 64:1061–1072. https://doi.org/10.1093/jxb/ers382
Angus JF, Hasegawa S, Hsiao TC, Liboon SP, Zandstrta HG (1983) The water balance of post-monsoonal dryland crops. J Agric Sci 101:699–710. https://doi.org/10.1017/S0021859600038739
Bano A, Dӧrffling K, Bettin D, Hahn H (1993) Abscisic acid and cytokinins as possible root-to-shoot signals in xylem sap of rice plants in drying soil. Aust J Plant Physiol 20:109–115. https://doi.org/10.1071/PP9930109
Biddington NL, Thomas TH (1973) A modified Amaranthus betacyanin bioassay for the rapid determination of cytokinins in plant extracts. Planta 111:183–186
Boyer JS (1995) Measuring the water status of plants and soils. Academic Press, San Diego
Brestic M, Cornic G, Fryer MJ, Baker NR (1995) Does photorespiration protect the photosynthetic apparatus in french bean leaves from photoinhibition during drought stress? Planta 196:450–457
Brevedan RE, Egli DB (2003) Short periods of water stress during seed filling, leaf senescence, and yield of soybean. Crop Sci 43:2083–2088. https://doi.org/10.2135/cropsci2003.2083
Carrijo DR, Lund ME, Linquist BA (2017) Rice yield and water use under alternate wetting and drying irrigation: A meta-analysis. Field Crop Res 203:173–180. https://doi.org/10.1016/j.fcr.2016.12.002
Chaves MM, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560. https://doi.org/10.1093/aob/mcn125
Chen D, Wong S, Xiong B, Cao B, Deng X (2015) Carbon/nitrogen imbalance associated with drought-induced leaf senescence in Sorghum bicolor. Plos One. https://doi.org/10.1371/journal.pone.0137026
Chernyad’ev II (2005) Effect of water stress on the photosynthetic apparatus of plants and the protective role of cytokinins: A review. Appl Biochem Micro 41:115–128
Cornic G, Le Gouallec JL, Briantais JM, Hodges M (1989) Effect of dehydration and high light on photosynthesis of two C3 plants (Phaseolus vulgaris L. and Elatostema repens (Lour.) Hall f.). Planta 177:84–90
De Souza PI, Egli DB, Bruening WP (1997) Water stress during seed filling and leaf senescence in soybean. Agron J 89:807–812. https://doi.org/10.2134/agronj1997.00021962008900050015x
El-Showk S, Ruonala R, Helariutta Y (2013) Crossing paths: cytokinin signaling and crosstalk. Development 140:1373–1383. https://doi.org/10.1242/dev.086371
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90
Flexas J, Bota J, Loreto F, Cornic G, Sharkey TD (2004) Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3 plants. Plant Biol 6:269–279. https://doi.org/10.1055/s-2004-820867
Flexas J, Barόn M, Bota J, Ducruet J-M, Gallé A, Galḿs J, Jiménez M, Pou A, Ribas-Carbό M, Sajinani C, Tomás M, Medrano H (2009) Photosynthesis limitations during water stress acclimation and recovery in the drought-adapted Vitis hybrid Richter-110 (V. berlandieri × V. rupestris). J Exp Bot 60:2361–2377. https://doi.org/10.1093/jxb/erp069
Fukai S, Sittisuang P, Chanphengsay M (1998) Increasing production of rainfed lowland rice in drought prone environments – A case study in Thailand and Laos. Plant Prod Sci 1:75–82. https://doi.org/10.1626/pps.1.75
Gan S, Amasino RM (1995) Inhibition of leaf senescence by autoregulated production of cytokinin. Science 270:1986–1988. https://doi.org/10.1126/science.270.5244.1986
Ghanem ME, Albacete A, Smgocki AC, Frébort I, Pospíšilová H, Martínez-Andu’jar C, Acosta M, Sánchez-Bravo J, Lutts S, Dodd IC, Pérez-Alfocea F (2011) Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot 62:125–140. https://doi.org/10.1093/jxb/erq266
Gonzalez-Dugo V, Durand J-L, Gastal F (2010) Water deficit and nitrogen nutrition of crops. A Review Agron. Sustain Dev 30:529–544. https://doi.org/10.1051/agro/2009059
Gou W, Li X, Guo S, Liu Y, Li F, Xie Q (2019) Autophagy in plant: A new orchestrator in the regulation of the phytohormones homeostasis. Int J Mol Sci 20:2900. https://doi.org/10.3390/ijms20122900
Guo Y, Ren G, Zhang K, Miao Y, Guo H (2021) Leaf senescence: progression, regulation, and application. Mol Hort 1:5. https://doi.org/10.1186/s43897-021-00006-9
Hashizume T, Sugiyama T, Imura M, Cory HT, Scott HF, McCloskey JA (1979) Determination of cytokinins by mass spectrometry based on stable isotope dilution. Anal Biochem 92:111–122. https://doi.org/10.1016/0003-2697(79)90631-6
Havlová M, Dobrev PI, Motyka V, Štorchova H, Libus J, Dobrá J, Malbeck J, Gaudinová A, Vanková R (2008) The role of cytokinins in responses to water deficit in tobacco plants over-expressing trans-zeatin O-glucosyltransferase gene under 35S or SAG12 promoters. Plant Cell Environ 31:341–353. https://doi.org/10.1111/j.1365-3040.2007.01766.x
He P, Osaki M, Takabe M, Shinano T, Wasaki J (2005) Endogenous hormones and expression of senescence-related genes in different senescent types of maize. J Exp Bot 56:1117–1128. https://doi.org/10.1093/jxb/eri103
Hirai A, Ishibashi T, Morikami A, Iwatsuki N, Shinozaki K, Sugiura M (1985) Rice chloroplast DNA: a physical map and the location of the genes for the large subunit of ribulose 1,5-bisphosphate carboxylase and the 32 kD photosystem II reaction center protein. Theor Appl Genet 70:117–122
Hirasawa T (2014) Photosynthesis and biomass production in energy crops. In: Tojo S, Hirasawa T (eds) Research approaches to sustainable biomass systems. Elsevier, Oxford, pp 52–61
Hirasawa T, Iida Y, Ishihara K (1989) Dominant factors in reduction of photosynthetic rate affected by air humidity and leaf water potential in rice plants. Japan Jour Crop Sci 58:383–389. https://doi.org/10.1626/jcs.58.383
Hirasawa T (1999) Physiological characterization of the rice plant for tolerance of water deficit. In: Ito O, O’Toole J, Hardy B (eds) Genetic Improvement of Rice for Water-Limited Environments. IRRI, Los Baños, pp 89–98
Hirasawa T (2018) Leaf photosynthesis of upland and lowland crops grown under moisture-rich conditions. In: Adams WW III, Terashima I (eds) The leaf: A platform for performing photosynthesis. Springer International Publishing AG, Cham, pp 345–369
Hӧrtensteiner S, Feller U (2002) Nitrogen metabolism and remobilization during senescence. J Exp Bot 53:927–937. https://doi.org/10.1093/jexbot/53.370.927
Imai K, Suzuki Y, Mae T, Makino A (2008) Changes in the synthesis of Rubisco in rice leaves in relation to senescence and N influx. Ann Bot 101:135–144. https://doi.org/10.1093/aob/mcm270
IPCC (2021) Climate change 2021: the physical science basis. In: Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis M, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (eds) Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press, Cambridge and New York. https://report.ipcc.ch/ar6/wg1/IPCC_AR6_WGI_FullReport.pdf
Ishida H, Izumi M, Wada S, Makino A (2014) Roles of autophagy in chloroplast recycling. Biochim Biophys Acta 1837:512–521. https://doi.org/10.1016/j.bbabio.2013.11.009
Itoh M, Sudo S, Mori S, Saito H, Yoshida T, Shiratori Y, Suga S, Yoshikawa N, Suzue Y, Mizukami H, Mochida T, Yagi K (2011) Mitigation of methane emissions from paddy fields by prolonging midseason drainage. Agr Ecosyst Environ 141:359–372. https://doi.org/10.1016/j.agee.2011.03.019
Jogawat A, Yadav B, Chhaya LN, Singh AK, Narayan OP (2021) Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: A review. Physiol Plant 172:1106–1132. https://doi.org/10.1111/ppl.13328
Kano H, Yoneyama T, Kumazawa K (1974) Determination of 15N by emission spectrography. Jpn J Soil Sci Plant Nutr 45:549–559. https://doi.org/10.20710/dojo.45.11_549
Kato Y, Katsura K (2014) Rice adaptation to aerobic soils: Physiological considerations and implications for agronomy. Plant Prod Sci 17:1–12. https://doi.org/10.1626/pps.17.1
Ko D, Kang L, Kiba T, Park J, Kojima M, Do J, Kim KY, Kwon M, Endler A, Song W-Y, Martinoia E, Sakakibara H, Lee Y (2014) Arabidopsis ABCG14 is essential for the root-to-shoot translocation of cytokinin. Pro Natl Acad Sci USA 111:7150–7155. https://doi.org/10.1073/pnas.1321519111
Koizumi K, Ookawa T, Satoh H, Hirasawa T (2007) A wilty mutant of rice impaired hydraulic conductance. Plant Cell Physiol 48:1219–1228. https://doi.org/10.1093/pcp/pcm092
Kramer PJ, Boyer JS (1995) Water relations of plants and soils. Academic Press, San Diego
Kudoyarova GR, Vysotskaya LB, Cherkozyanova A, Dodd IC (2007) Effect of partial rootzone drying on the concentration of zeatin-type cytokinins in tomato (Solanum lycopersicum L.) xylem sap and leaves. J Exp Bot 58:161–168. https://doi.org/10.1093/jxb/erl116
Lee S, Masclaux-Daubresse C (2021) Current understanding of leaf senescence in rice. Int J Mol Sci 22:4515. https://doi.org/10.3390/ijms22094515
Lim PK, Kim HJ, Nam HG (2007) Leaf senescence. Ann Rev. Plant Biol 58:115–136. https://doi.org/10.1146/annurev.arplant.57.032905.105316
Long SP, Marshall-Colon A, Zhu X-G (2015) Meeting the global food demand of the future by engineering crop photosynthesis and yield potential. Cell 161:56–66. https://doi.org/10.1016/j.cell.2015.03.019
Lu J, Ookawa T, Hirasawa T (2000) The effects of irrigation regimes on the water use, dry matter production and physiological responses of paddy rice. Plant Soil 223:207–216
Mae T, Makino A, Ohira K (1983) Changes in the amount of ribulose bisphosphate carboxylase synthesized and degraded during the life span of rice leaf (Oryza sativa L.). Plant Cell Physiol 24:1079–1086. https://doi.org/10.1093/oxfordjournals.pcp.a076611
MAFF (Ministry of Agriculture, Forestry and Fisheries of Japan) (2019) Prospect of the world food supply and demand in 2050. (In Japanese) https://www.maff.go.jp/j/zyukyu/jki/j_zyukyu_mitosi/attach/pdf/index-12.pdf. Accessed 9 Feb 2022
Makino A, Mae T, Ohira K (1983) Photosynthesis and ribulose 1,5-bisphoaphate carboxylase in rice leaves. Plant Physiol 73:1002–1007. https://doi.org/10.1104/pp.73.4.1002
Makino A, Mae T, Ohira K (1986) Colorimetric measurement of protein stained with Coomassie Brilliant Blue R on sodium dodecyl sulfate-polyacrylamide gel electrophoresis by eluting with formamide. Agric Biol Chem 50:1911–1912. https://doi.org/10.1080/00021369.1986.10867672
Makino A, Sakashita H, Hidema J, Mae T, Ojima K, Osmond B (1992) Distinctive responses of ribulose-1,5-bisphosphate carboxylase and carbonic anhydrase in wheat leaves to nitrogen nutrition and their possible relationships to CO2-transfer resistance. Plant Physiol 100:1737–1743. https://doi.org/10.1104/pp.100.4.1737
Matsuoka M, Kano-Murakami Y, Tanaka Y, Ozeki Y, Yamamoto N (1988) Classification and nucleotide sequence of cDNA encoding the small subunit of ribulose-1,5-bisphosphate carboxylase from rice. Plant Cell Physiol 29:1015–1022. https://doi.org/10.1093/oxfordjournals.pcp.a07758110.1104/pp.96.2.355
McLean JL, Dawe DC, Hardy B, Hettel GP (2002) Rice almanac. WARDA, Bouakē; CIAT, Cali; FAO, Rome, IRRI, Los Baňos
Miyagawa S, Tsuji T, Watanabe K, Hoshikawa, (2006) Long-term and spacial evaluation of rice crop performance of rain-fed paddy field in a village of Northeast Thailand. Tropics 15:39–49. https://doi.org/10.3759/tropics.15.39
Munns R, Millar AH (2023) Seven plant capacities to adapt to abiotic stress. J Exp Bot 74:4308–4323. https://doi.org/10.1093/jxb/erad179
Nishiyama R, Watanabe Y, Fujita Y, Le DT, Kojima M, Werner T, Vankova R, Yamaguchi-Shinozaki K, Shinozaki K, Kakimoito T, Sakakibara H, Schmülling T, Tran L-SP (2011) Analysis of cytokinin mutants and regulation of cytokinin metabolic genes reveals important regulatory roles of cytokinins in drought, salt and abscisic acid responses, and abscisic acid biosynthesis. Plant Cell 23:2169–2183
Noodén LD, Guiamét JJ, John I (1997) Senescence mechanism. Physiol Plant 101:746–753. https://doi.org/10.1111/j.1399-3054.1997.tb01059.x
O’Toole JC, Baldia EP (1982) Water deficit and mineral uptake in rice. Crop Sci 22:1144–1150. https://doi.org/10.2135/cropsci1982.0011183X002200060014x
Ookawa T, Naruoka Y, Yamazaki T, Suga J, Hirasawa T (2003) A comparison of the accumulation and partitioning of nitrogen in plants between two rice cultivars, Akenohoshi and Nipponbare, at the ripening stage. Plant Prod Sci 6:172–178. https://doi.org/10.1626/pps.6.172
Ookawa T, Naruoka Y, Sayama A, Hirasawa T (2004) Cytokinin effects on riburose-1,5-bisphoshate carboxylase/oxygenase and nitrogen partitioning in rice during ripening. Crop Sci 44:2107–2115. https://doi.org/10.2135/cropsci2004.2107
Peleg Z, Reguera M, Tumimbang E, Walia H, Blumwald E (2011) Cytokinin-mediated source ⁄sink modifications improve drought tolerance and increase grain yield in rice under water-stress. Plant Biotechnol J 9:747–758. https://doi.org/10.1111/j.1467-7652.2010.00584.x
Raineri J, Wang S, Peleg Z, Blumwald E, Chan RL (2015) The rice transcription factor OsWRKY47 is a positive regulator of the response to water deficit stress. Plant Mol Biol 88:401–413. https://doi.org/10.1007/s11103-015-0329-7
Reguera M, Peleg Z, Abdel-Tawab YM, Tumimbang EB, Delatorre CA, Blumwald E (2013) Stress-induced cytokinin synthesis increases drought tolerance through the coordinated regulation of carbon and nitrogen assimilation in rice. Plant Physiol 163:1609–1622. https://doi.org/10.1104/pp.113.227702
Richmond AE, Lang A (1957) Effect of kinetin on protein content and survival of detached Xanthium leaves. Science 125:650–651. https://doi.org/10.1126/science.125.3249.650.b
Rivero RM, Kojima M, Gepstein A, Sakakibara H, Mittler R, Gepstein S, Blumwald E (2007) Delayed leaf senescence induces extreme drought tolerance in a flowering plant. Pro Natl Acad Sci USA 104:19631–19636. https://doi.org/10.1073/pnas.0709453104
Rivero RM, Shulaev V, Blumwald E (2009) Cytokinin-dependent photorespiration and the protection of photosynthesis during water deficit. Plant Physiol 150:1530–1540. https://doi.org/10.1104/pp.109.139378
Rivero RM, Gimeno J, Deynze AV, Walia H, Blumwald E (2010) Enhanced cytokinin synthesis in tobacco plants expressing PSARK::IPT prevents the degradation of photosynthetic protein complexes during drought. Plant Cell Physiol 51:1929–1941. https://doi.org/10.1093/pcp/pcq143
Sade N, Umnajkitikorn K, del Wilhelmi MMR, Wright M, Wang S, Blumwald E (2018) Delaying chloroplast turnover increases water-deficit stress tolerance through the enhancement of nitrogen assimilation in rice. J Exp Bot 69:867–878. https://doi.org/10.1093/jxb/erx235
Sage RF, Adachi S, Hirasawa T (2017) Improving photosynthesis in rice: from small steps to giant leaps. In: Sasaki T (ed) Achieving sustainable cultivation of rice, Vol. 1. Breeding for higher yield and quality, 1st edn. Burleigh Dodds Science Publishing Limitted, Cambridge, pp 77–107
Sakakibara H (2006) Cytokinins: Activity, biosynthesis, and translocation. Annu Rev Plant Biol 57:431–449. https://doi.org/10.1146/annurev.arplant.57.032905.105231
Salmon JM, Friedl MA, Frolking S, Wisser D (2015) Global rain-fed, irrigated, and paddy cropland: A new high resolution map derived from remote sensing, crop inventories and climate data. Int J Appl Earth Obs 38:321–334. https://doi.org/10.1016/j.jag.2015.01.014
San-oh Y, Sugiyama T, Yoshita D, Ookawa T, Hirasawa T (2005) The effect of planting pattern on the rate of photosynthesis and related processes during ripening in rice plants. Field Crops Res 96:113–124. https://doi.org/10.1016/j.fcr.2005.06.002
Sato J, Shiraiwa T, Sakashita M, Tsujimoto Y, Yoshida R (2007) The occurrence of delayed stem senescence in relation to trans-zeatin riboside level in the xylem exudate in soybeans grown under excess-wet and drought soil conditions. Plant Prod Sci 10:460–467. https://doi.org/10.1626/pps.10.460
Sato H, Matsusaka H, Kumamaru T (2010) Use of N-methyl-N-nitrosourea treatment of fertilized egg cells for saturation mutagenesis of rice. Breed Sci 60:475–483. https://doi.org/10.1270/jsbbs.60.475
Singh S, Letham DS, Palni LMS (1992) Cytokinin biochemistry in relation to leaf senescence. VII. Endogenous cytokinin levels and exogenous applications of cytokinins in relation to sequential leaf senescence of tobacco. Physiol Plant 86:388–397. https://doi.org/10.1111/j.1399-3054.1992.tb01334.x
Singh B, Mishra S, Bisht DS, Joshi R (2021) Growing rice with less water: improving productivity by decreasing water demand. In: Ali J, Wani SH (eds) Rice improvement: physiological, molecular breeding and genetic perspectives. Springer Nature, Switzerland AG, Cham, pp 147–170. https://doi.org/10.1007/978-3-030-66530-2
Sitton D, Itai C, Kende H (1967) Decreased cytokinin production in the roots as a factor in shoot senescence. Planta 73:296–300
Soejima H, Sugiyama T, Ishihara K (1992) Changes in cytokinin activities and mass spectrometric analysis of cytokinins in root exudates of rice plant (Oryza sativa L.). Plant Physiol 100:1724–1729. https://doi.org/10.1104/pp.100.4.1724
Soejima H, Sugiyama T, Ishihara K (1995) Changes in the chlorophyll contents of leaves and in levels of cytokinins in root exudates during ripening of rice cultivars Nipponbare and Akenohoshi. Plant Cell Physiol 36:1105–1114. https://doi.org/10.1093/oxfordjournals.pcp.a078854
Sriphirom P, Chidthaisong A, Yagi K, Tripetchkul S, Towprayoon S (2020) Evaluation of biochar applications combined with alternate wetting and drying (AWD) water management in rice field as a methane mitigation option for farmers’ adoption. Soil Sci Plant Nutr 66:37–49. https://doi.org/10.1080/00380768.2019.1706431
Sugiyama T, Hashizume T (1982) Mass spectrometric determination of cytokinins in the top of tobacco plants using deuterium-labeled standards. Nucleic Acids Res 11:61–64
Sugiyama T, Hirayama Y (1983) Correlation of the activities of phosphoenolpyruvate carboxylase and pyruvate, orthophosphate dikinase with biomass in maize seedlings. Plant Cell Physiol 24:783–787. https://doi.org/10.1093/oxfordjournals.pcp.a076578
Suzuki Y, Makino A, Mae T (2001a) An efficient method for extraction of RNA from rice leaves at different ages using benzyl chloride. J Exp Bot 52:1775–1579. https://doi.org/10.1093/jexbot/52.360.1575
Suzuki Y, Makino A, Mae T (2001b) Changes in the turnover of Rubisco and leaves of mRNAs of rbcL and rbcS in rice leaves from emergence to senescence. Plant, Cell Environ 24:1353–1360. https://doi.org/10.1046/j.0016-8025.2001.00789.x
Tardieu F, Simonneau T, Mullar B (2018) The physiological basis of drought tolerance in crop plants: a scenario-dependent probabilistic approach. Annu Rev Plant Biol 69:733–759. https://doi.org/10.1146/annurev-arplant-042817-040218
Tuong TP, Bouman BA, Mortimer M (2005) More rice, less water−Integrated approaches for increasing water productivity in irrigated rice-based systems in Asia. Plant Prod Sci 8:231–241. https://doi.org/10.1626/pps.8.231
Venuprasad R, Lafitte HR, Atlin GN (2007) Response to direct selection for grain yield under drought stress in rice. Crop Sci 47:285–293. https://doi.org/10.2135/cropsci2006.03.0181
Win TN, Oo TTTS, Shwe KK, Htwe NM, Oo HH, Thuzar M, Yamamoto Y, Omori H, Hirasawa T (2022) Large leaf modulus of elasticity is associated with the distinct midday reduction of photosynthesis in rice: a comparison with green gram. Plant Prod Sci 25:224–231. https://doi.org/10.1080/1343943X.2022.2047077
Wintermans JFGI, De Mots A (1965) Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta 109:448–453. https://doi.org/10.1016/0926-6585(65)90170-6
Woo HR, Kim HJ, Lim PO, Nam HG (2019) Leaf senescence: Systems and dynamics aspects. Annu Rev Plant Biol 70:347–376. https://doi.org/10.1146/annurev-arplant-050718-095859
Yamamoto T, Suzuki T, Suzuki K, Adachi S, Sun J, Yano M, Ookawa T, Hirasawa T (2017) Characterization of a genomic region that maintains chlorophyll and nitrogen contents during ripening in a high-yielding stay-green rice cultivar. Field Crops Res 206:54–64. https://doi.org/10.1016/j.fcr.2017.03.001
Yoneyama T, Arima K, Kumazawa K (1975) Sample preparation from dilute ammonium solution for emission spectrographic analysis of heavy nitrogen. Jpn J Soil Sci Plant Nutr 46:146–147. https://doi.org/10.20710/dojo.46.4_146
Zhang H, Zhou C (2013) Signal transduction in leaf senescence. Plant Mol Biol 82:539–545. https://doi.org/10.1007/s11103-013-0040-5
Zhang L, Zhang L, Sun J, Zhang Z, Ren H, Sui X (2013) Rubisco gene expression and photosynthetic characteristics of cucumber seedlings in response to water deficit. Sci Hortic 161:81–87. https://doi.org/10.1016/j.scienta.2013.06.029
Zhang W, Zhou Y, Li C, Zhu K, Xu Y, Wang W, Liu L, Zhang H, Gu J, Wang Z, Zhang J, Yang J (2022) Post-anthesis moderate soil-drying facilitates source-to-sink remobilization of nitrogen via redistributing cytokinins in rice. Field Crops Res 282:108692. https://doi.org/10.1016/j.fcr.2022.108692
Zhao W, Zhao H, Wang H, He Y (2022) Research progress on the relationship between leaf senescence and quality, yield and stress resistance in horticultural plants. Frot Plant Sci 13:1044500. https://doi.org/10.3389/fpls.2022.1044500
Zubo YO, Schaller GE (2020) Role of the cytokinin-activated type-B response regulators in hormone crosstalk. Plants 9:166. https://doi.org/10.3390/plants9020166
Acknowledgements
The authors are grateful to Professor Tadahiko Mae (Graduate School of Agricultural Science, Tohoku University) for helpful suggestions on the analysis using 15N. We also thank Dr. Shunsuke Adachi (Graduate School of Agriculture and Technology, Tokyo University of Agriculture and Technology) for critical review of the manuscript. Seeds of CM2088 mutant were provided through Institute of Genetic Resources, Faculty of Agriculture, Kyushu University.
Funding
This work was supported in part by JSPS KAKENHI Grant Number 14360010 and by the Ministry of Agriculture, Forestry and Fisheries, Japan (Bio Cosmos Program).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design, material preparation, and data collection and analysis. The first draft of the manuscript was written by Koji Nakanishi, Hiroko Fujiki, Koichi Ozaki, Satoko Yanahara, Naoko Takeuchi and Tadashi Hirasawa, and all authors reviewed and commented on the previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Responsible Editor: Stefan K. Arndt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakanishi, K., Fujiki, H., Ozaki, K. et al. Decrease of cytokinin flux from roots enhances degradation of ribulose-1,5-bisphosphate carboxylase/oxygenase: a mechanism of the accelerated decrease of leaf photosynthesis with senescence under soil moisture stress in rice (Oryza sativa L.). Plant Soil 496, 391–411 (2024). https://doi.org/10.1007/s11104-023-06367-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-023-06367-y