Abstract
Background and aims
Global change drivers such as climate change influence decomposition by altering extrinsic site conditions and intrinsic litter traits. This study aimed to quantify the relative importance of these two pathways for litter decomposition in tropical forests.
Methods
The effects of soil nutrient availability, mean annual precipitation (MAP), and leaf litter chemistry on decomposition were isolated by measuring mass loss of leaf litter from 10 tropical tree species transplanted to 19 sites along independent gradients of soil fertility and precipitation in Panama. Across species, litter nitrogen (N) content ranged from 7.1 to 13 mg N g−1 and phosphorus (P) from 0.077 to 0.56 mg P g−1. Across sites, soil N content ranged from 1.7 to 5.5 g N kg−1, soil P from 77 to 1500 mg P kg−1, and MAP from 1900 to 2700 mm.
Results
Variation in leaf litter mass loss was explained largely by litter species identity (55 %). Site only explained a small, but significant, amount of variance (6.5 %); soil C:N ratio explained this response. Notably, neither litter nutrient content nor MAP were significant predictors of litter decomposition.
Conclusions
Changes in tree species composition may influence decomposition rates more than changes to site conditions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Huge amounts of carbon (C) are stored and cycled in tropical forests (Houghton 2005; Kauffman et al. 2009) and leaf litter decomposition represents a significant component of C and nutrient cycles (Schlesinger 1977; Schimel et al. 1994; Chen et al. 2011). Leaf litter decomposition is controlled by extrinsic (site condition) and intrinsic (litter trait) factors, both of which may be influenced by global change drivers. For example, climate change could alter the precipitation regime in tropical regions (Houghton and Prentice 2001; Malhi and Wright 2004), and rates of N fertilisation by deposition are increasing (Hietz et al. 2011). In addition to effects on soil water and N availability, global change drivers also affect vegetation community composition and litter traits. The most important extrinsic and intrinsic factor is much debated in tropical forests, and their relative importance is unresolved (Aerts 1997; Cox et al. 2000; Bardgett et al. 2008).
Precipitation influences the biomass, composition and activity of decomposer communities (Fierer et al. 2003; Drenovsky et al. 2010; Evans and Wallenstein 2011). For instance, microbial biomass is positively correlated with soil water availability in moist-evergreen and semi-evergreen forests in India (Pandey et al. 2009) and irrigation decreases the abundance of fungi relative to bacteria in leaf litter in a lowland tropical forest (Cornejo et al. 1994). The effects on decomposition however are inconclusive: mean annual precipitation (MAP) predicts decomposition rate of a common litter substrate at global (Powers et al. 2009) and regional scales (Austin and Vitousek 2000), but not locally (Vasconcelos et al. 2007; Wieder et al. 2009). It is possible that in wet tropical rainforests soil moisture is at optimal levels for decomposition thus other factors such as litter quality exert relatively greater influence (Wieder et al. 2009).
Fertilisation by atmospheric N deposition also affects site conditions, particularly N availability and soil pH, with implications for decomposition as shown in chronosequences, land use gradients, and fertilisation experiments (Arunachalam et al. 1996; Kaspari et al. 2008; Wardle et al. 2009). In tropical forests, experimental addition of N increased rates of decomposition in young subtropical montane forests (Hobbie and Vitousek 2000; Reed et al. 2011) but had no effect on leaf litter decomposition in lowland tropical forests in Panama (Kaspari et al. 2008) and Costa Rica (Cleveland et al. 2006). On the other hand, the Walker and Syers (1976) model, predicts that soil processes in tropical forests will be phosphorus (P) limited due to the strongly-weathered nature of the soils. Indeed, soil P concentration predicted decomposition rates among sites in a pan-tropical meta-analysis (Cleveland et al. 2011) and locally (Powers and Salute 2011; Kaspari et al. 2008). Crucially, the importance of soil nutrient status to decomposition is rarely considered independently of climate (Aerts 1997; Zhang et al. 2008), but is necessary to evaluate individual and synergistic impacts of global change drivers.
Precipitation change and N deposition affect vegetation composition, and thus the chemistry and structure of C inputs entering the soil via litter (Chapin 2003). In tropical forests, MAP is a strong predictor of vegetation composition along spatial (Gentry 1988; Pyke et al. 2001; Davidar et al. 2007) and temporal gradients (Laurance et al. 2004; Lewis et al. 2004); and strong soil-plant associations are also documented (Schreeg et al. 2010). Species-specific physiological adaptations to water stress and nutrient limitation lead to differences in leaf chemistry (Santiago et al. 2004; Kitajima and Poorter 2010; Santiago 2010). At the community level this results in a general negative relationship between MAP and leaf litter N, P and Mg concentration (Santiago et al. 2004; Fyllas et al. 2009), and a positive relationship between soil nutrient availaibilty and litter nutrient concentration.
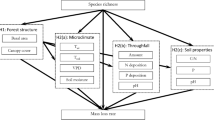
The relative importance of intrinsic and extrinsic factors for decomposition in tropical forests is unresolved (Zhang et al. 2008), but urgently required to improve predictions of future global climate and C cycle interactions (Schimel et al. 1994; Gardenas et al. 2011). Most studies to date have considered site and species factors as determinants of litter decomposition in isolation, and the exceptions to this have either used a small number of species (Austin and Vitousek 2000; Couteaux et al. 2002) or sites (Cusack et al. 2009; Wieder et al. 2009); thus the relative importance of these two pathways remains unresolved in tropical forests. The aims of this study were to determine: (1) the relative importance of site and species identity for leaf litter decomposition; and (2) the significance of site variables (MAP and soil nutrient availability) and species variables (litter quality) as predictors. To address these questions leaf litter from 10 species (representing a range of litter N and P content) was placed in 19 sites (along semi-independent gradients of MAP and soil nutrient availability) in a fully factorial reciprocal transplant, and mass loss was measured after one wet season.
Materials and methods
Study sites
This study was carried out in 19 1-ha plots in a 47 km × 48 km area in mature secondary forest (Pyke et al. 2001) in Panama, Central America (9° 10′ N, 79° 45′ W) (Fig. 1). The gradient of MAP arises from a North–south climate system, and the gradient of soil chemistry is due to geological variation (Pyke et al. 2001; Turner and Engelbrecht 2011). The study sites were selected from a network of tree census plots administered by the Centre for Tropical Forest Science of the Smithsonian Tropical Research Institute and previous studies in these sites report patterns of tree species composition correlated with regional precipitation trends (Pyke et al. 2001; Engelbrecht et al. 2007).
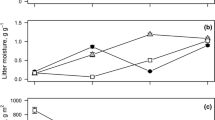
Location of 19 1- ha study sites (circles) along a north to south transect of mature secondary lowland tropical rainforest where leaf litter mass loss from 10 species was measured ,and location of 23 rainfall gauges (triangles) where total monthly precipitation data (TMP) was collected for 20 years from 1987 to 2007 and then used to model mean annual precipitation (MAP) at study sites
Using monthly precipitation data recorded from 1988 to 2007 at rainfall gauges within the study area (Fig. 1), mean monthly precipitation over 20 years was calculated for each gauge. Mean annual precipitation (MAP) at each gauge was calculated as the sum of months Jan - Dec. Mean wet season precipitation (MWP) at each gauge was calculated as the sum of months August to January. The two measures, MAP and MWP, were highly correlated (R2 = 0.98, p < 0.001) and so MAP was reported in this study in order to facilitate comparisons with other studies. MAP (mm) was significantly correlated distance north (m) (R2 = 0.77, p < 0.001), and the following linear relationship was used to predict MAP at study sites:
Thus predicted MAP at the study sites ranged from 1903 to 2816 mm (Table 2).
At each site, 13 systematically located soil cores were taken to 10 cm depth in August 2009 (except for total C and total N where n = 5). Soil chemistry, as opposed to litter chemistry, was measured to give a long term perspective on soil nutrient availability across sites Samples were analysed for pH and concentrations of total C, total N, total P, readily-exchangeable phosphate, and the extractable base cations calcium (Ca), potassium (K), and magnesium (Mg). Soil pH was determined in a 1:2 soil to solution ratio in deionized water using a glass electrode. Readily-exchangeable phosphate was determined by extraction with anion exchange membranes (AEM) using a method described in Turner and Romero (2009). Extractable base cations (Ca, K, Mg) were extracted with Mehlich-3 solution and analyzed for cations by inductively-coupled plasma optical-emission spectrometry (ICP–OES). Total extractable bases (TEB) was determined as the sum of the molar concentrations of Ca, K, and Mg. Sub-samples were air-dried, ground in a pestle and mortar and analysed for total C and N by combustion using a Carlo IRBA elemental analyser. Total P was determined on ground samples by ignition (550 °C, 1 h) and extraction in 1 M H2SO4 (1:50 soil to solution ratio, 16 h), with phosphate detection by automated molybdate colorimetry using a Lachat Quikchem 8500. Mean values of soil chemical values were calculated for each site.
Study species
The 10 species selected for this study (Table 1) were those for which sufficient litter had previously been collected. Previously published studies suggested that these species represent a broad spectrum of leaf traits (Wright et al. 2004; Kattge et al. 2011). Leaf litter was collected in traps in sites ‘SHER’ (7 species) and ‘BCI’ (3 species). Traps were emptied every 2 weeks during 2006 and contents were oven dried at 60 °C, sorted to species, and stored in airtight containers.
Leaf litter chemistry of each species was measured on a subsample of three replicate leaves per species. Litter was ground in a ball mill (Retsch 8000 mixer mill). Total C and N were measured using a Flash HT elemental analyzer connected through a ConfloIII interface to a Delta V Advantage isotope ratio mass spectrometer (Thermo, Bremen, Germany). Total Ca, K, Mg, and P were determined by digestion under pressure at 180 °C in concentrated nitric acid, with detection by ICP–OES. Litter pH was determined in a 1:20 weight ratio of oven dried chopped leaves to deionized water.
Leaf litter mass loss
Leaf litter mass loss was quantified during the wet-season (August – January) 2009, using the litterbag method. Litterbags were constructed from 17 × 17 cm squares of 1 mm nylon mesh and filled with approximately 2 g (one to three leaves) of single-species litter. The litterbag method excludes meso- and macro-fauna, but allows rapid assessment and is comparable with other studies. Litterbags of each species were replicated five times within each site. Litterbags were deployed at the start of the wet season and pinned to the litter surface at intervals of 1 m between species, and 50 m between replicates. Litterbags were recollected at the end of the wet season (180 days later), returned to the lab, and stored at 4 °C for a maximum of 1 month until processing. Litterbags were carefully opened and all material was removed. Decomposing leaf litter samples were gently rinsed on a 400 μm sieve with distilled water. Samples were dried at 60 °C for 48 h, allowed to equilibrate with laboratory humidity for 60 min, and then mass remaining was recorded. Mass remaining was subtracted from initial mass to give mass loss (ML), and divided by initial mass to give relative mass loss (RML) expressed as a percentage. In order to account for loss of litter fragments during sampling, transport and processing, “travel” litterbags were constructed (three replicates per species). Travel litterbags were transported to study sites but immediately returned to the laboratory. They were then processed in the same way as sample litterbags, and travel RML calculated. Initial mass of sample litterbags was corrected for species mean travel RML (<1.7 %).
Statistical modelling
To determine the relative importance of species and site identity, and to identify predictors of variation in RML among sites and among species, a linear mixed effects (LME) modelling approach was used. An LME approach was necessary to avoid pseudo-replication because for each explanatory variable only one value (the site or species mean) was used to model five replicated measures of RML.
Explanatory variables were checked for normality, and data were transformed to normal distribution if necessary. All variables were standardised by subtracting the mean and dividing by the standard deviation.
Pearson correlation coefficients (r) revealed several pairs of explanatory variables that were highly correlated and so variance inflation factor (VIF) analysis was used to select explanatory variables and thus reduce co-linearity (Zuur et al. 2009). VIF quantifies the severity of multi-colinearity in an ordinary least squares regression analysis, and is an index of how much the variance of a model coefficient is increased by the colinearity. VIFs were calculated, the explanatory variable with greatest VIF was removed, VIFs were recalculated, and this process was repeated until VIFs were < 3.0 and r was < 0.6. The remaining explanatory variables were used to model RML.
A model was constructed for each possible covariance structure (i.e., all possible combinations of site slope, site intercept, species slope, and species intercept), leading to 16 models in total. All 16 models had the same fixed component containing all explanatory variables. The models were compared using Akaike information criterion (AIC), which is an index of goodness of fit and reflects how well the model agrees with the data given the number of parameters used.
The best-fit model (i.e., that with lowest AIC score) was used to investigate the role of the fixed site and species explanatory variables. The significance of explanatory variables was then assessed using a likelhood ratio test (LRT) on a chi-squared distribution procedure. This procedure tests the likelihood that changes in AIC when a term is removed from the model occur by chance. The estimates and standard deviations of fixed effect beta coefficients, and the variance associated with random effects, are reported. Model residuals were checked for normal distribution, homogeneity of variance and independence. All statistical analyses were produced using the R language and environment for statistical computing and the following additional packages; AED 1.0, stats 2.14.1, lme4 0.999375-42, and nlme 3.1–102.
Results
Six site variables (soil P, C:P, Ca, K, TEB and readily-extractable phosphate) and one species variable (litter C:P) were negatively skewed and were log base 10 transformed (Tables 2 and 3).
Pearson correlation coefficients (r) revealed several pairs of explanatory variables that were highly correlated (Tables 4 and 5), and VIF analysis resulted in omission of the following seven site variables prior to modelling: soil TEB, Ca, N, Ca, Mg, pH, and readily-extractable phosphate and the following four species variables: litter C:P, Ca, pH, and P.
The remaining four site variables were: MAP (which ranged from 1906 to 2816 mm), C:N (which ranged from 9.64 to 15.2), total C (which ranged from 20.9 to 60.6 mg g−1, and was correlated with total N), C:P (which ranged from 39.3 to 352, and was correlated with P and readily-exchangeable phosphate), and K (which ranged from 26.2 to 256 μg g−1, and was correlated with Ca, Mg, TEB and pH).
The remaining four species variables were: litter C (which ranged from 357 to 509 mg C g−1), C:N (which ranged from 36.9 to 64.4, and was correlated with N), K (which ranged from 1.91 to 6.07 mg K g−1, and was correlated with P and C:P), and Mg (which ranged from 1.18 to 4.11 mg Mg g−1, and was correlated with Ca and pH).
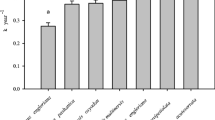
Mean leaf litter RML at the end of the experiment was 50.3 %. Mean RML varied over 2-fold among species, from 29.4 % in Vochysia ferruginea (VOCF) to 80.9 % in Aspidiosperma spruceanum (ASPC), while site means less than doubled, from 41.2 % at ALBR to 62.4 % at BCI (Table 6).
An ANOVA model confirmed that both species and site explained significant proportion of variation in RML (p < 0.001) (Table 7). Species explained 54.5 % of variation in RML; site explained 6.54 %. The optimal variance-covariance model included a random species slope, species intercept, and site intercept (Table 8). Thus both site and species had significant effect on mean RML, and species responded differently to main effects.
Removal of soil C:N from the model decreased model fit (Table 9). The standardised beta coefficient of soil C:N was −0.16, i.e., decreased soil C:N ratio was linked to increased RML (Table 9, Fig. 2). No other site or species variables improved model fit.
Discussion
The aim of this study was to determine: (1) the relative importance of site and species identity for leaf litter mass loss, and (2) and the significance of site variables (MAP and soil nutrient availability) and species variables (litter quality) as predictors. The main findings of this study were: (1) both species and site identity influenced mean RML but species had greater effect than site, (2) soil C:N was a significant predictor of RML, and (3) leaf litter nutrient concentrations and ratios did not predict RML among species.
Species identity as the predominant driver of decomposition
The main driver of decomposition rate was litter species identity, rather than site, which supports the view that plant traits are important determinants of ecosystem processes within tropical forests (Aerts 1997), as in other biomes (Cornwell et al. 2008; De Deyn et al. 2008; Zhang et al. 2008), and suggests that vegetation may act as a pathway between global change drivers and the C cycle. The relatively few studies that have explicitly examined the relative effects of site and species identity for decomposition generally support the conclusion that species identity plays a more significant role than site factors in explaining decomposition. For example, species identity had greater influence on leaf litter decomposition than did precipitation in a tropical forest in Costa Rica (Wieder et al. 2009). These findings support the view that plant traits play a significant role, relative to climatic and other abiotic factors, in driving decomposition, and presumably other ecosystem processes at the landscape scale (Cornwell et al. 2008; De Deyn et al. 2008).
Soil C:N ratio as a robust predictor of decomposition
Decomposition rate was negatively related to soil C:N ratio across sites. An explanation for increased decomposition with increased soil N is via its impact on decomposer growth and activity (Fog 1988). This finding of a landscape-scale control of decomposition by soil C:N has not been reported in other tropical forest studies. However, Cleveland et al. (2011) found that soil P best predicted decomposition in a pan-tropical meta-analysis, and soil P was correlated with soil N in their dataset. In a recent meta-analysis of N addition effects on microbial biomass and soil CO2 efflux, Janssens et al. (2010) noted that studies of N addition in areas with severely weathered soils, such as the tropics, are too rare to allow statistical meta-analysis. Across a range of ecosystems, Allison and Martiny (2008) report microbial community compositional changes in response to nutrient fertilisation in 84 % of 38 studies. The functional significance of such change was reported by Treseder (2008), whereby a meta-analysis of 82 field studies found that addition of N was associated with a 15 % reduction in microbial biomass, and a reduction in soil CO2 emissions. However, no studies in tropical forests met the criteria required for the meta-analysis. Despite theoretical and empirical evidence of P limitation to decomposition in tropical forests (Walker and Syers 1976; Hobbie and Vitousek 2000; Kaspari et al. 2008) there was no effect of soil C:P in this study.
The significant relationship between soil C:N and leaf litter mass loss reported in this study suggests that there is a potential for anthropogenic disturbances other than climate change, such as N enrichment, to influence soil processes and the C cycle in tropical forests. Also, soil C:N is widely used as an index of the extent to which organic matter is decomposed, and these findings suggest that this measure could be a useful proxy metric for estimating decomposition rate at a given site over a range of climate and litter substrates.
That there was no relationship between MAP and leaf litter mass loss is consistent with other studies in tropical forests at similar precipitation ranges. Nepstad et al. (2002) found no effect of throughfall exclusion from 5000 to 2500 mm year−1 on leaf litter decomposition rate, and although Powers et al. (2009) observed a positive relationship between MAP and decomposition among tropical forests globally, the relationship was driven by sites experiencing MAP outside the range of this study. The lack of significant effect of MAP on leaf litter mass loss observed here supports the notion that, at optimal soil moisture conditions for microbial processes (Prescott 2010), factors other than rainfall, such as litter or soil chemistry, will limit decomposition. On the other hand, it is also possible that other climatic variables correlate with decomposition rate in these sites. For example, Anaya et al. (2012) found that the magnitude of large events during the wet season influenced decomposition rates; probably through physical displacement of litter fragments from mesh bags. This study only measured decomposition rate during the wet season, but soil water deficit likely inhibits decomposition during the dry season, when evapotranspiration is less than precipitation. Thus a measure of decomposition throughout the year may reveal that decomposition is controlled by dry season length in these forests.
Leaf litter nutrient concentrations
We observed no significant effects of leaf litter nutrient content on decomposition in this study. The importance of leaf litter nutrient concentrations for decomposition is reported in several meta-analyses in tropical forest (Aerts 1997; Waring 2012) and other biomes (Cornwell et al. 2008; Zhang et al. 2008) but similar to Haettenschwiler et al. (2011) in French Guiana, there was no effect of initial litter nutrients on mass loss in this study. The litterbags were deployed for only 6 months, and around 50 % of the initial mass remained at the termination of the study. Given that bags were deployed during the rainy season, litter solubility may have been the dominant driver of interspecific variation in decay rates.
Haettenschwiler et al. (2011) found no correlations among decomposition and litter N or P in French Guiana, and instead suggested that litter C accessibility acted as a significant control. In other studies several metrics of accessible carbon such as non-lignin C (Haettenschwiler and Jorgensen 2010), water soluble C (Wieder et al. 2009), labile C (Fanin et al 2011) and the ratio of non structural carbohydrates and phenolics to condensed tannins and lignin (Haettenschwiler and Jorgensen 2010) correlate positively with litter mass loss, while measures of inaccessible carbon such as lignin concentration (Austin and Vitousek 2000), lignin:P (Wieder et al. 2009) and lignin:N (Aerts 1997) correlate negatively with litter mass loss.
Conclusions
This study provides support for a dominant effect of species identity, relative to other factors such as MAP and soil nutrient content, in regulating decomposition in a lowland tropical forest. This suggests that changes in tree species composition due to global change drivers, such as increased atmospheric CO2 concentration (Laurance et al. 2004) or drought intensity (Nepstad et al. 2007), will have important consequences for belowground processes and the C cycle. Furthermore, soil C:N explained variation in decomposition among sites, but MAP did not. Overall, these findings support the notion that global change drivers which influence soil nutrient availability and species composition will influence decomposition processes in tropical forests, and hence the C cycle (Aerts 1997; Bardgett et al. 2008; Fortunel et al. 2009).
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: A triangular relationship. Oikos 79:439–449
Allison SD, Martiny JBH (2008) Resistance, resilience, and redundancy in microbial communities. Proc Natl Acad Sci U S A 105:11512–11519
Anaya CA, Jaramillo VJ, Martínez-Yrízar A, García-Oliva F (2012) Large rainfall pulses control litter decomposition in a tropical dry forest: evidence from an 8-year study. Ecosystems 15:652–663
Arunachalam A, Pandey H, Tripathi R, Maithani K (1996) Fine root decomposition and nutrient mineralization patterns in a subtropical humid forest following tree cutting. For Ecol Manag 86:141–150
Austin AT, Vitousek P (2000) Precipitation, decomposition and litter decomposability of Metrosideros polymorpha in native forests on Hawai’i. J Ecol 88:129–138
Bardgett RD, Freeman C, Ostle NJ (2008) Microbial contributions to climate change through carbon cycle feedbacks. ISME J 2:805–814
Chapin FS (2003) Effects of plant traits on ecosystem and regional processes: a conceptual framework for predicting the consequences of global change. Ann Bot 91:455–463
Chen G-s, Yang Y-s, Guo J-f, Xie J-s, Yang Z-j (2011) Relationships between carbon allocation and partitioning of soil respiration across world mature forests. Plant Ecol 212:195–206
Cleveland C, Reed S, Townsend A (2006) Nutrient regulation of organic matter decomposition in a tropical rain forest. Ecology 87:492–503
Cleveland CC, Townsend AR, Taylor P, Alvarez-Clare S, Bustamante MMC, Chuyong G, Dobrowski SZ, Grierson P, Harms KE, Houlton BZ, Marklein A, Parton W, Porder S, Reed SC, Sierra CA, Silver WL, Tanner EVJ, Wieder WR (2011) Relationships among net primary productivity, nutrients and climate in tropical rain forest: a pan-tropical analysis. Ecol Lett 14:1313–1317
Cornejo F, Varela A, Wright S J (1994) Tropical forest litter decomposition under seasonal drought: nutrient release, fungi and bacteria. Oikos 70:183–192
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Perez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, Van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Diaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Victoria Vaieretti M, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Couteaux M, Sarmiento L, Bottner P, Acevedo D, Thiery J (2002) Decomposition of standard plant material along an altitudinal transect (65-3968 m) in the tropical Andes. Soil Biol Biochem 34:69–78
Cox P, Betts R, Jones C, Spall S, Totterdell I (2000) Acceleration of global warming due to carbon-cycle feedbacks in a coupled climate model. Nature 408:184–187
Cusack DF, Chou WW, Yang WH, Harmon ME, Silver WL (2009) Controls on long-term root and leaf litter decomposition in neotropical forests. Glob Chang Biol 15:1339–1355
Davidar P, Rajagopal B, Mohandass D, Puyravaud J-P, Condit R, Wright SJ, Leigh EG (2007) The effect of climatic gradients, topographic variation and species traits on the beta diversity of rain forest trees. Glob Ecol Biogeogr 16:510–518
De Deyn GB, Cornelissen JHC, Bardgett RD (2008) Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol Lett 11:516–531
Drenovsky RE, Steenwerth KL, Jackson LE, Scow KM (2010) Land use and climatic factors structure regional patterns in soil microbial communities. Glob Ecol Biogeogr 19:27–39
Engelbrecht BMJ, Comita L, Condit R, Kursar T, Tyree M, Turner B, Hubbell S (2007) Drought sensitivity shapes species distribution patterns in tropical forests. Nature 447:80–82
Evans SE, Wallenstein MD (2011) Soil microbial community response to drying and rewetting stress: does historical precipitation regime matter? Biogeochemistry 1–16
Fanin N, Haettenschwiler S, Barantal S, Schimann H, Fromin N (2011) Does variability in litter quality determine soil microbial respiration in an Amazonian rainforest? Soil Biol Biochem 43:1014–1022
Fierer N, Schimel J, Holden P (2003) Influence of drying-rewetting frequency on soil bacterial community structure. Microb Ecol 45:63–71
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic-matter. Biol Rev Camb Philos Soc 63:433–462
Fortunel C, Garnier E, Joffre R, Kazakou E, Quested H, Grigulis K, Lavorel S, Ansquer P, Castro H, Cruz P, Dolezal J, Eriksson O, Freitas H, Golodets C, Jouany C, Kigel J, Kleyer M, Lehsten V, Leps J, Meier T, Pakeman R, Papadimitriou M, Papanastasis VP, Quetier F, Robson M, Sternberg M, Theau J-P, Thebault A, Zarovali M (2009) Leaf traits capture the effects of land use changes and climate on litter decomposability of grasslands across Europe. Ecology 90:598–611
Fyllas NM, Patino S, Baker TR, Nardoto GB, Martinelli LA, Quesada CA, Paiva R, Schwarz M, Horna V, Mercado LM, Santos A, Arroyo L, Jimenez EM, Luizao FJ, Neill DA, Silva N, Prieto A, Rudas A, Silviera M, Vieira ICG, Lopez-Gonzalez G, Malhi Y, Phillips OL, Lloyd J (2009) Basin-wide variations in foliar properties of Amazonian forest: phylogeny, soils and climate. Biogeosciences 6:2677–2708
Gardenas AI, Agren GI, Bird JA, Clarholm M, Hallin S, Ineson P, Katterer T, Knicker H, Nilsson SI, Nasholm T, Ogle S, Paustian K, Persson T, Stendahl J (2011) Knowledge gaps in soil carbon and nitrogen interactions - From molecular to global scale. Soil Biol Biochem 43:702–717
Gentry A (1988) Changed in plant community diversity and floristic composition on environmental and geographical gradients. Ann Mo Bot Gard 75:1–34
Haettenschwiler S, Coq S, Barantal S, Handa IT (2011) Leaf traits and decomposition in tropical rainforests: revisiting some commonly held views and towards a new hypothesis. New Phytol 189:950–965
Haettenschwiler S, Jorgensen HB (2010) Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J Ecol 98:754–763
Hietz P, Turner BL, Wanek W, Richter A, Nock CA, Wright SJ (2011) Long-term change in the nitrogen cycle of tropical forests. Science 334:664–666
Hobbie S, Vitousek P (2000) Nutrient limitation of decomposition in Hawaiian forests. Ecology 81:1867–1877
Houghton and Prentice (2001) Climate change: the science basis:1–94
Houghton R (2005) Aboveground forest biomass and the global carbon balance. Glob Chang Biol 11:945–958
Janssens IA, Dieleman W, Luyssaert S, Subke JA, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G, Papale D, Piao SL, Schulze ED, Tang J, Law BE (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322
Kaspari M, Garcia MN, Harms KE, Santana M, Wright SJ, Yavitt JB (2008) Multiple nutrients limit litterfall and decomposition in a tropical forest. Ecol Lett 11:35–43
Kattge J, Diaz S, Lavorel S, Prentice C, Leadley P, Boenisch G, Garnier E, Westoby M, Reich PB, Wright IJ, Cornelissen JHC, Violle C, Harrison SP, van Bodegom PM, Reichstein M, Enquist BJ, Soudzilovskaia NA, Ackerly DD, Anand M, Atkin O, Bahn M, Baker TR, Baldocchi D, Bekker R, Blanco CC, Blonder B, Bond WJ, Bradstock R, Bunker DE, Casanoves F, Cavender-Bares J, Chambers JQ, Chapin FS, Chave J, Coomes D, Cornwell WK, Craine JM, Dobrin BH, Duarte L, Durka W, Elser J, Esser G, Estiarte M, Fagan WF, Fang J, Fernandez-Mendez F, Fidelis A, Finegan B, Flores O, Ford H, Frank D, Freschet GT, Fyllas NM, Gallagher RV, Green WA, Gutierrez AG, Hickler T, Higgins SI, Hodgson JG, Jalili A, Jansen S, Joly CA, Kerkhoff AJ, Kirkup D, Kitajima K, Kleyer M, Klotz S, Knops JMH, Kramer K, Kuehn I, Kurokawa H, Laughlin D, Lee TD, Leishman M, Lens F, Lenz T, Lewis SL, Lloyd J, Llusia J, Louault F, Ma S, Mahecha MD, Manning P, Massad T, Medlyn BE, Messier J, Moles AT, Mueller SC, Nadrowski K, Naeem S, Niinemets U, Noellert S, Nueske A, Ogaya R, Oleksyn J, Onipchenko VG, Onoda Y, Ordonez J, Overbeck G, Ozinga WA, Patino S, Paula S, Pausas JG, Penuelas J, Phillips OL, Pillar V, Poorter H, Poorter L, Poschlod P, Prinzing A, Proulx R, Rammig A, Reinsch S, Reu B, Sack L, Salgado-Negre B, Sardans J, Shiodera S, Shipley B, Siefert A, Sosinski E, Soussana J-F, Swaine E, Swenson N, Thompson K, Thornton P, Waldram M, Weiher E, White M, White S, Wright SJ, Yguel B, Zaehle S, Zanne AE, Wirth C (2011) TRY - a global database of plant traits. Glob Chang Biol 17:2905–2935
Kauffman JB, Hughes RF, Heider C (2009) Carbon pool and biomass dynamics associated with deforestation, land use, and agricultural abandonment in the neotropics. Ecol Appl 19:1211–1222
Kitajima K, Poorter L (2010) Tissue‐level leaf toughness, but not lamina thickness, predicts sapling leaf lifespan and shade tolerance of tropical tree species. New Phytologist
Laurance W, Oliveira A, Laurance S, Condit R, Nascimento H, Sanchez-Thorin A, Lovejoy T, Andrade A, D’Angelo S, Ribeiro J, Dick C (2004) Pervasive alteration of tree communities in undisturbed Amazonian forests. Nature 428:171–175
Lewis S, Phillips O, Baker TR, Lloyd J, Malhi Y, Almeida S, Higuchi N, Laurance W, Neill D, Silva J, Terborgh J, Lezama A, Martinez R, Brown S, Chave J, Kuebler C, Vargas P, Vinceti B (2004) Concerted changes in tropical forest structure and dynamics: evidence from 50 South American long-term plots. Philos Trans: Biol Sci 359:421–436
Malhi Y, Wright SJ (2004) Spatial patterns and recent trends in the climate of tropical rainforest regions. Philos Trans: Biol Sci 359:311–329
Nepstad D, Moutinho P, Dias M, Davidson E, Cardinot G, Markewitz D, Figueiredo R, Vianna N, Chambers J, Ray D, Guerreiros J, Lefebvre P, Sternberg L, Moreira M, Barros L, Ishida F, Tohlver I, Belk E, Kalif K, Schwalbe K (2002) The effects of partial throughfall exclusion on canopy processes, aboveground production, and biogeochemistry of an Amazon forest. J Geophys Res 107:8085
Nepstad DC, Tohver IM, Ray D, Moutinho P, Cardinot G (2007) Mortality of large trees and lianas following experimental drought in an amazon forest. Ecology 88:2259–2269
Pandey CB, Srivastava RC, Singh RK (2009) Soil nitrogen mineralization and microbial biomass relation, and nitrogen conservation in humid-tropics. Soil Sci Soc Am J 73:1142–1149
Powers JS, Montgomery RA, Adair EC, Brearley FQ, Dewalt SJ, Castanho CT, Chave J, Deinert E, Ganzhorn JU, Gilbert ME, Antonio Gonzalez-Iturbe J, Bunyavejchewin S, Grau HR, Harms KE, Hiremath A, Iriarte-Vivar S, Manzane E, De Oliveira AA, Poorter L, Ramanamanjato J-B, Salk C, Varela A, Weiblen GD, Lerdau MT (2009) Decomposition in tropical forests: a pan-tropical study of the effects of litter type, litter placement and mesofaunal exclusion across a precipitation gradient. J Ecol 97:801–811
Powers JS, Salute S (2011) Macro- and micronutrient effects on decomposition of leaf litter from two tropical tree species: inferences from a short-term laboratory incubation. Plant Soil 346:245–257
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149
Pyke C, Condit R, Aguilar S, Lao S (2001) Floristic composition across a climatic gradient in a neotropical lowland forest. J Veg Sci 12:553–566
Reed SC, Vitousek PM, Cleveland CC (2011) Are patterns in nutrient limitation belowground consistent with those aboveground: results from a 4 million year chronosequence. Biogeochemistry 106:323–336
Santiago LS (2010) Can growth form classification predict litter nutrient dynamics and decomposition rates in lowland wet forest? Biotropica 42:72–79
Santiago LS, Kitajima K, Wright SJ, Mulkey S (2004) Coordinated changes in photosynthesis, water relations and leaf nutritional traits of canopy trees along a precipitation gradient in lowland tropical forest. Oecologia 139:495–502
Schimel D, Braswell B, Holland E, McKeown R, Ojima D, Painter T, Parton W, Townsend A (1994) Climatic, edaphis, and biotic controls over storage and turnover of carbon in soils. Glob Biogeochem Cycles 8:279–293
Schlesinger W (1977) Carbon balance in terrestrial detritus. Annu Rev Ecol Syst 8:51–81
Schreeg LA, Kress WJ, Erickson DL, Swenson NG (2010) Phylogenetic analysis of local-scale tree soil associations in a lowland moist tropical forest. PLoS One 5, e13685
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Turner BL, Engelbrecht BMJ (2011) Soil organic phosphorus in lowland tropical rain forests. Biogeochemistry 103:297–315
Turner BL, Romero TE (2009) Short-term changes in extractable inorganic nutrients during storage of tropical rain forest soils. Soil Sci Soc Am J 73:1972
Vasconcelos SS, Zarin DJ, da Rosa Silva MB, Oliveira FA (2007) Leaf decomposition in a dry season irrigation experiment in Eastern Amazonian forest regrowth. Biotropica 39:593–600
Walker T, Syers J (1976) The fate of phosphorus during pedogenesis. Geoderma 15:1–19
Wardle DA, Bardgett RD, Walker LR, Bonner KI (2009) Among- and within-species variation in plant litter decomposition in contrasting long-term chronosequences. Funct Ecol 23:442–453
Waring BG (2012) A meta-analysis of climatic and chemical controls on leaf litter decay rates in tropical forests. Ecosystems 15:999–1009
Wieder WR, Cleveland CC, Townsend AR (2009) Controls over leaf litter decomposition in wet tropical forests. Ecology 90:3333–3341
Wright I, Reich P, Westoby M, Ackerly D, Baruch Z, Bongers F, Cavender-Bares J, Chapin T, Cornelissen JHC, Diemer M, Flexas J, Garnier E, Groom P, Gulias J, Hikosaka K, Lamont B, Lee T, Lee W, Lusk C, Midgley J, Navas M, Niinemets U, Oleksyn J, Osada N, Poorter H, Poot P, Prior L, Pyankov V, Roumet C, Thomas S, Tjoelker M, Veneklaas E, Villar R (2004) The worldwide leaf economics spectrum. Nature 428:821–827
Zhang D, Hui D, Luo Y, Zhou G (2008) Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J Plant Ecol 1:85–93
Zuur AF, Ieno EN, Elphick CS (2009) A protocol for data exploration to avoid common statistical problems. Methods Ecol Evol 1:3–14
Acknowledgments
The authors thank Dayana Agudo, Helen Quirk, Tania Romero, Didimo Urena, and Joe Wright for their contribution to the study. Laura Schreeg provided critical comments on the manuscript. This research was funded through a NERC algorithm grant awarded to Sarah Dale.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Alfonso Escudero.
Rights and permissions
About this article
Cite this article
Dale, S.E., Turner, B.L. & Bardgett, R.D. Isolating the effects of precipitation, soil conditions, and litter quality on leaf litter decomposition in lowland tropical forests. Plant Soil 394, 225–238 (2015). https://doi.org/10.1007/s11104-015-2511-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2511-8