Abstract
Aims
The effect of flooding on carbohydrate and nitrogen metabolism in leaves of peach (Prunus persica cv. UFSun) trees grafted onto six different Prunus spp. rootstock cultivars with either peach or plum parentage (peach: ‘Flordaguard’, ‘Guardian’, ‘Nemaguard’, ‘P-22’; plum: ‘R5064–5’, and ‘MP-29’) were evaluated.
Methods
Young peach trees were divided into two treatments: 1) non-flooded (control) and 2) flooded trees. Trees in the flooded treatment were subjected to 8 days of flooding (the root zone was submerged in containers filled with tap water) before leaves were harvested for biochemical analyses. Carbohydrate contents, enzymatic activity related to sucrose metabolism, glycolysis, nitrogen assimilation, and nitrogen metabolism of the scions were sampled from 40 leaves from each replication of each treatment.
Results
Enzymatic activities of acid invertase were lower, and neutral invertase, sucrose synthase, and sucrose phosphosynthase were higher in scions of flooded trees compared to those of non-flooded trees. Trees in the flooded treatment had lower activity of enzymes involved in glycolysis. Carbohydrate contents (glucose, sucrose, and fructose) were higher in flooded than control plants for scions on all rootstocks except ‘Flordaguard’, in which the scion glucose level was not different between treatments. Scions in the flooded treatment had lower activity of enzymes involved in nitrogen assimilation and metabolism, and consequently lower NO3− and NO2− content than scions of trees in the non-flooded treatment.
Conclusions
Flooding of P. persica cv. UFSun on six different Prunus spp. rootstocks caused enzymes in leaves of the scion involved in sucrose metabolism to be more active compared to those in the non-flooded control treatment. However, most enzymes involved in glycolysis were less active in flooded than non-flooded trees, except in scions on plum rootstocks, whose hexokinase (HK) and fructokinase (FK) enzymes were more active in the flooded than the non-flooded treatment. This suggests that scions on plum rootstocks could be more efficient at generating hexose sugars during glycolysis. The higher contents of sugars present in scions on flooded compared to non-flooded rootstocks could be indicative of translocation being hindered by flooding. Compared to non-flooded trees, flooding generally resulted in lower enzymatic activity related to nitrogen assimilation and metabolism (scions on plum rootstocks were largely unaffected), which consequently led to lower levels of nitrate and nitrite, while ammonium levels were higher in flooded scions. The lower HK and FK activities in scions on peach rootstocks compared to plum rootstocks may have made photosynthesis hexose limited, which would disrupt the electron transport chain and lead to less energy being available for nitrogen assimilation and metabolism as these processes are connected.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate change is leading to increased incidences of flooding, resulting in significant crop losses in many parts of the world (Bailey-Serres et al. 2012; Arora 2019). Fruit growers in subtropical regions often experience a rainy summer season which can result in transient flooding. Peach production has expanded from temperate regions into these more flood-prone subtropical regions due to the success of subtropical stone fruit breeding programs. Peaches are typically grown on Prunus spp. rootstocks, which are mildly sensitive to flooding (Morales-Olmedo et al. 2021). However, the wide genetic diversity of this genus allows for varying levels of resistance/susceptibility, and thus there is a need for characterization of flood tolerance among peach rootstocks. Flooding reduces soil oxygen content which limits root respiration, ultimately hindering plant growth and production by blocking the translocation of nutrients between the aerial and below-ground portions of the plant (Morales-Olmedo et al. 2021). Both shoots and roots are affected by oxygen-deficient soil conditions (Araki et al. 2012), with roots affected first by these conditions. Although examining the response of roots to flooding is useful for assessing flooding stress, characterizing the early flooding response of the root system of perennial plants can prove difficult (Crocker et al. 2003) as much of the root system is often damaged very soon after flooding starts. Examining the responses of the above-ground portion of the plant to flooding, particularly concerning plant metabolism, can provide significant insights into the responses of plants to flooding.
Flooding significantly hinders plant growth and development (Schaffer et al. 1992), partially as a result of a shift from aerobic to anaerobic respiration in the roots, which reduces energy produced in the form of ATP. Additionally, anaerobic respiration in the roots can lead to production and translocation to the shoots of phytotoxic metabolites that can kill the plant (Schaffer et al. 1992). Flooding generally results in the generation of reactive oxygen species (ROS) that can exacerbate plant stress (Gries et al. 1990). Reactive oxygen species can destroy molecules including lipids, nucleic acids, and proteins which could hinder several physiological processes (Bandyopadhyay et al. 1999). In numerous fruit crop species including peach, stomatal conductance (gs) is adversely affected by flooding, which leads to a reduction in net CO2 assimilation (A) (Schaffer et al. 1992). In a study of flooding effects of peach scions on six different Prunus spp. rootstocks with either peach (Prunus persica (L.) Batsch) or plum (Prunus domestica.) parentage (peach: ‘Flordaguard’, ‘Guardian’, ‘Nemaguard’, ‘P-22’; plum: ‘R5064–5’, and ‘MP-29’), McGee et al. (2021) observed that gs on all but two rootstocks decreased as early as two days after trees were first flooded, and gs decreased in the remaining rootstocks within four to eight days of flooding. In that study, scions on rootstocks ‘R5064–5’ and ‘MP-29’ had the highest gs 2 to 6 days after the flooding began. The reduction in A in the flooded trees was likely linked to reduced intercellular CO2 concentrations (Ci) as a result of changes in stomatal aperture (Pezeshki 2001) because a simultaneous decrease in Ci and gs indicates that decreased A is due to stomatal factors (Ploetz and Schaffer 1989; Schaffer and Ploetz 1989). The decline in Ci results in adverse effects on the electron transport chain resulting in an upregulation of ROS (Mahajan and Tuteja 2005), which was also observed in the study of McGee et al. (2021). Morales-Olmedo et al. (2021) reported that Prunus rootstocks that retain their photosynthetic rates during flooding are deemed tolerant to flooding, suggesting that the ability of scions on flooded rootstocks ‘MP-29’ and ‘R5064–5’ to maintain higher A than the scions on flooded peach rootstocks observed by McGee et al. (2021) is an indication of their flood tolerance. In addition to stomatal limitations to A caused by flooding, flooding stress can result in biochemical limitations to A, such as reduced carboxylation efficiency (Morales-Olmedo et al. 2021). Morales-Olmedo et al. (2021) determined that responses to flooding in Prunus rootstocks included reduced A and gs, usually accompanied by reduced root growth. McGee et al. (2021) compared peach, plum, and plum x peach rootstocks, all grafted with the peach scion ‘UFSun’, a low-chill cultivar adapted for subtropical climates (Sarkhosh 2018), and determined that ‘UFSun’ on plum and/or plum x peach rootstocks is more tolerant of flooding than ‘UFSun’ on peach rootstocks. To the authors’ knowledge, the effects of flooding on carbon (C) and nitrogen (N) metabolism in Prunus rootstocks have not been elucidated.
Nitrogen is an essential element critical to plant growth and development, which requires energy and C skeletons to be assimilated into the leaf (Foyer et al. 1998). Thus, C metabolism is linked to N metabolism as they share energy supplied directly from photosynthetic electron transport, CO2 fixation, or from the respiration of fixed C, all of which depend on carbohydrates such as glucose, sucrose, and fructose (Huppe and Turpin 1994). Nitrogen is also linked to amino acid production and is involved in many complex metabolic interactions (Kusano et al. 2011). Measuring metabolite levels provides basic information about biological and physiological changes triggered by N status (Tschoep et al. 2009). Plant stress tolerance has been correlated with soluble sugar content, and may also be affected by N status, which is dependent upon soluble sugar content (Gao et al. 2018; Redillas et al. 2012; Tarkowski and Van den Ende 2015). Soluble sugars are utilized for respiration and other biological processes essential to plant growth and development, which are often depleted as a result of inhibition of physiological processes such as reduced A, or via the production of ROS. Flooding stress also results in the degradation of starch, especially when abscisic acid (ABA) is upregulated, as it further degrades starch (Thalmann and Santelia 2017). González et al. (2009) reported that in quinoa (Chenopodium quinoa Willd.), waterlogging resulted in higher leaf total soluble sugars compared to non-flooded conditions. Bailey-Serres and Voesenek (2008) indicated that flood-tolerant species exhibit higher nitrate reductase activity than species that are susceptible to flooding stress. Lamami et al. (2014) further supported this notion and reported that nitrate reduction regenerating NAD+ from NADH could be linked to the adaption of plants to low oxygen stress. Ren et al. (2017) reported that waterlogging of maize significantly reduced N uptake, translocation, and accumulation and decreased activity of enzymes involved in N metabolism. In that study, enzymatic activity related to N metabolism caused a reduction in photosynthetic capacity and dry matter accumulation, ultimately leading to reduced yield. Overall higher levels of N were considered beneficial during hypoxia stress. Kusano et al. (2011) reviewed the relationship between N content and C metabolism in rice, maize, and various dicots and concluded that nitrate levels were positively correlated with amino acid and organic acid levels and that carbohydrate levels were affected by N status. The effects of abiotic stress on C and N metabolism have been documented in several forest tree and tree fruit crops and generally follow similar trends among species. Ferner et al. (2012) reported that short-term flooding (5 days) did not change carbohydrate levels in beech (Fagus sylvatica L.) or oak (Quercus robur L.) trees, but long-term flooding (14 days) caused a two-fold increase in soluble sugars. They determined that changes in glucose and fructose contents are better indicators of flooding stress than sucrose because glucose and fructose levels increased whereas sucrose levels remained the same in both species after flooding. Root hypoxia reduced leaf nitrate content and dry matter production, but increased nitrate reductase activity in tomatoes (Solanum Lycopersicum L.) (Horchani and Aschi-Smiti 2010). The authors postulated that this response could indicate an adaptation of roots to transient hypoxia. McGee et al. (2021) reported a reduction in scion N content in leaves of P. persica L. cv. UFSun budded onto five of six Prunus rootstock genotypes tested. In that study, ‘MP-29’ was the only rootstock with leaf N levels of the scion unchanged after eight days of flooding. Although the researchers found the rootstocks to possess various levels of flood tolerance, it was unclear what could be causing the reduced A and nitrogen contents (McGee et al. 2021).
Flooding sensitivity of Prunus spp. is commonly associated with reduced gs and A, followed by a reduction in root and shoot growth (Morales-Olmedo et al. 2021). The Prunus rootstock, ‘Guardian’, has been determined to be very sensitive to flooding (Schaffer et al. 1992) and thus serves as a useful point of comparison for evaluating flooding sensitivity of other Prunus rootstocks. McGee et al. (2021) reported that A of peach scions on ‘MP-29’ and ‘R5064–5’ had higher A than scions on ‘Flordaguard’ after 4 and 6 days of flooding, but there were no differences among scions on six different Prunus rootstocks in the flooded treatment after 8 days. Stomatal conductance was reduced in scions on peach rootstocks under the flooded compared to the non-flooded treatment after two days, but in plum rootstocks this decrease was delayed until the fourth day of flooding (McGee et al. 2021). The less negative effect of flooding on A and gs in scions on plum rootstocks than peach rootstocks suggests greater transient flooding tolerance of scions on rootstocks ‘MP-29’ and ‘R5064–5’ than on peach rootstocks. It has been widely reported that plum-based rootstocks are more tolerant than peach-based rootstocks (Salvatierra et al. 2020).
In this study, we investigated the effects of flooding on carbohydrate contents, sucrose metabolism, and glycolysis, the accumulation of different forms of N, enzymatic activity related to N assimilation and metabolism, and how these changes could influence flooding tolerance. The objectives were to determine how the carbon and nitrogen metabolism of the P. persica scion cultivar UFSun on six Prunus rootstocks is affected by flooding and if these processes differ among tolerant and sensitive rootstocks. We hypothesized that the scion on flood-tolerant rootstocks would have higher leaf soluble sugar content, N contents, and enzymatic activity (enzymes related to sucrose metabolism, glycolysis, and N metabolism) than on flooding sensitive rootstocks.
Materials and methods
Experimental design and plant material
Prunus spp. rootstocks, each grafted with the P. persica cultivar ‘UFSun’ as the scion, were obtained from the United States Department of Agriculture, Agricultural Research Service (USDA-ARS) in Byron, Georgia, USA. Trees were one year old (from the time of grafting). The rootstocks used in this study were ‘Flordaguard’, ‘Nemaguard’, ‘Guardian’, ‘P-22’, ‘R5064–5’, and ‘MP-29’. ‘Flordaguard’. ‘Nemaguard’, and ‘Guardian’ are peach rootstocks that have been successfully released and are being used for commercial production in the southeastern USA, while ‘P-22’ is a peach rootstock resulting from a cross of ‘Guardian’ and ‘Flordaguard’ that is under consideration for release. ‘MP-29’ is an interspecific hybrid selected from a cross of Prunus umbellata and P. persica (Beckman et al. 2012). ‘R5064–5’ is a complex plum hybrid with no peach parentage; although the specific plum species are not known, it has been determined that neither parent was P. domestica (Thomas Beckman, USDA-ARS, personal communication). The levels of flooding tolerance for these rootstocks were previously determined by Schaffer et al. (1992) and McGee et al. (2021): ‘Flordaguard’ - very sensitive, ‘Guardian’ - very sensitive, ‘Nemaguard’ – sensitive, ‘P-22’ - sensitive, ‘R5064–5’ - tolerant, ‘MP-29’ - tolerant.
Trees were transplanted into 38-L polyester/polypropylene bags (247Garden, Montebello, CA, USA) containing a potting medium of 60% Jolly Gardner Pro-line c/25 mix (Old Castle Lawn and Garden, Atlanta, GA, USA) and 40% Griffis Lumber Garden Soil (Griffis Lumber and Sawmill, Gainesville, FL, USA). The experiment was conducted in a greenhouse at the University of Florida in Gainesville, Florida. The plants were divided into two treatments: 1) flooded and 2) non-flooded (control). Each treatment consisted of 5 single-tree replicates of each of the 5 rootstocks, for a total of 60 plants arranged in a complete block design. In the flooded treatment, the root system of each tree was submerged to 5 cm above the graft union by placing the tree and planting bag in a 75.7-L container filled with tap water. Each tree was flooded in a separate container. Trees in the control treatment were not flooded and irrigated for 5 min twice daily at a rate of 12.1 L/h via a microjet sprinkler system. After 8 days (>50% wilting of some flooded plants), trees and planting bags in the flooded treatment were removed from the larger container of tap water (unflooded).
Leaf sample collection
Leaf samples used for the biochemical analyses were collected from trees in the flooded and non-flooded treatments 8 days after treatments were initiated. Forty fully expanded mature leaves were collected from each tree. The leaves were randomly collected from all sides of the canopy of each tree and placed in a Ziploc® bag. Samples were immediately stored in a freezer at −80 °C until they were ready to be shipped overnight, in a Styrofoam cooler with dry ice, to the University of New Hampshire (Durham, NH, USA) for analyses.
Sugars, starch, and enzymatic activities
The method described by Jones et al. (1977) was used for the determination of glucose. The method of Buysse and Merckx (1993) was used for the determination of sucrose, fructose, and total soluble sugar (TSS) content, which was not limited to glucose, sucrose, and fructose and thus may reflect values higher than the total of these respective sugars. Enzymatic activity was determined in frozen leaf tissue (0.5 g) using the procedure described by Shahid et al. (2018). The Bradford (1976) assay was used for protein determination. Total acid invertase (AI) was estimated using a mixture (1 mL) comprised of a 0.2 mL extract of sodium acetate (0.1 M, pH 4.8) and sucrose (0.1 M). Neutral invertase (NI) was estimated using a mixture of dipotassium phosphate (0.1 M) with pH 7.2, sodium citrate (0.1 M), and sucrose (0.1 M) as described by Shahid et al. (2018). For the determination of AI/NI, 0.2 mL of enzyme extract was incubated for 40 min at 37 °C, the reaction was then stopped by adding dinitrosalicylic (1 mL), and the extract was boiled for 5 min. The absorbance of the reaction mixture was measured at 520 nm with a spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). The AI or NI activities are expressed as the units of reducing sugars produced from sucrose per minute. Reducing sugars were determined using the method described by Buysse and Merckx (1993). Sucrose synthase (SS) and sucrose phosphate synthase (SPS) were extracted and quantified as described by Rufty and Huber (1983). The SPS activity for SS (Bird et al. 1974), SS activity for sucrose cleavage (Lowell et al. 1989), and fructokinase (Zrenner et al. 1995) were also determined. Hexokinase (HK), Pyruvate kinase (PK), and phosphofructokinase (PFK) were measured using commercial enzyme kits (Sigma-Aldrich, St. Louis, MO, USA).
Nitrate, nitrite, and ammonium content
Leaf tissue was boiled in distilled water (1:10 w/v) for 20 min then filtered through Whatman No. 1 filter paper to determine leaf nitrate (NO3−), nitrite (NO2−), and ammonium (NH4+) contents. Nitrate content was determined using the protocol of Cataldo et al. (1975), NO2− was assayed according to the methodology of Snell and Snell (1949), and NH4+ was estimated by the procedure of Molins-Legua et al. (2006). Reaction mixtures for N analysis were prepared following the protocol of Shahid et al. (2018).
Nitrogen assimilation enzymes
The protocol of Sym (1984) was used for the determination of nitrate reductase (NR) activity. Leaf tissue (0.5 g) was treated with phosphate buffer (pH 7.0), 0.5 mL of 0.02 M KNO3, 0.5 mL of sulphanilamide, and 0.5 mL of N-(1-naphthyl)-ethylene diamine dihydrochloride and then centrifuged at 4°C at 15,000 g. The activity was calculated as the amount of NO2 released g−1 FW h−1. The methods of Ramarao et al. (1983) were used to determine nitrite reductase activity (NiRA); the reaction mixture was created following the protocols of Shahid et al. (2018). Glutamine synthase (GS) activity was determined using the procedures of Lillo (1984). Frozen leaf tissue (0.5 g) was blended with Tris-hydrochloric acid (50 mM, pH 7.8), 15% glycerol, 2-mercaptoethanol (14 mM), ethylenediaminetetraacetic acid (1 mM) and triton x-100 (0.1%). The mixture was centrifuged for 20 min at 4°C at 15,000 g to produce an enzyme extract. The mixture was composed as described by Shahid et al. (2018). Glutamine-2-oxoglutarate aminotransferase (GOGAT) activity was estimated as described by Singh and Srivastava (1986). Leaf tissue (0.5 g) was blended in sodium phosphate buffer (0.2 M, pH 7.5) supplemented with thylenediaminetetraacetic acid (2 mM), potassium chloride (50 mM), 2-mercaptoethanol (0.1% v/v) and triton x-100 (0.1%), then centrifuged for 15 min at 4°C at 10,000 g. Procedures described by Singh and Srivastava (1983) were used to determine glutamate dehydrogenase (GDH) activity from 0.5 g of leaf tissue, ground in sodium phosphate buffer (0.5 M, pH 7.4), sucrose (0.4 M), and ethylenediaminetetraacetic acid (2 mM). Each variable was calculated in the following units GS (A540 g−1 FW h−1), GOGAT (1 μmol NADH oxidized h−1), and GDH (μmol NADH oxidized per hour).
Statistical analyses
A two-way analysis of variance (ANOVA) was used for all response variables to test for main effects and interactions between rootstock and flooding treatments. A one-way ANOVA and Waller-Duncan K-Ratio test were used to compare means among rootstocks and a non-paired T-test was used to compare means between flooding treatments. All data were analyzed with SAS Statistical Software (Version 9.4, SAS Institute, Cary, NC, USA).
Results
Enzymes for sucrose metabolism and glycolysis
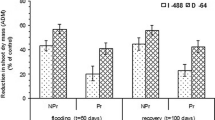
The activity of AI was lower in the flooded treatment, whereas activities of NI, SS, and SPS were higher in the flooded treatment than in the non-flooded treatment for scions on all rootstocks (Fig. 1A, B, C, and D respectively). The largest treatment difference in AI activity (41% lower in flooded than non-flooded plants) was in scions on ‘Flordaguard’ (Fig. 1A), while the largest difference in activity of NI, SS, and SPS (300%, 214%, and 218% higher in flooded than non-flooded plants) was in scions on ‘MP-29’ (Fig. 1B, C, and D respectively). In the flooded treatment, scions on ‘MP-29’ and ‘R5064–5’ had the highest enzyme activities of AI, NI, SS, and SPS (except for scions on ‘P-22’) (Fig. 1A, B, C, and D respectively).
Effect of rootstock and flooding treatment on acid invertase (A), neutral invertase (B), sucrose synthase (C), and sucrose phosphate synthase (D) activities in leaves of Prunus persica cv. UFSun budded on Flordaguard, Guardian, MP-29, Nemaguard, P-22, and R5064-5 rootstocks. Different lowercase letters indicate significant differences among rootstocks in the non-flooded treatment, different uppercase letters indicate significant difference among rootstocks in the flooded treatment according to a Waller-Duncan K-Ratio Test (P < 0.05). Single (*) or double (**) asterisks indicate a significant difference at P < 0.05 or 0.01, respectively and ns indicates no significant difference between flooding treatments of each rootstock according to a non-paired T-test
Scions on rootstocks ‘MP-29’ and ‘R5064–5’responded differently to flooding in terms of FK and HK activities, then scions on the peach rootstocks (Fig. 2A and B). Flooding reduced activities of these sugar sensing molecules in scions on peach rootstocks (25% to 38% lower in flooded than non-flooded plants) except for those on ‘P-22′ whose HK activity was unchanged (Fig. 2B). Flooded plants had lower activities of PK (13% to 59% lower in flooded than non-flooded plants) and PFK (4% to 31% lower in flooded than non-flooded plants) than non-flooded plants in scions on the peach rootstocks (Fig. 2C and D). In scions on ‘MP-29′ and ‘R5064–5′, PFK activity was unaffected by flooding (Fig. 2C), whereas PK activity was not significantly different between flooding treatments in scions on ‘R5064–5′ (Fig. 2D). Scions of flooded plants on ‘MP-29′ and ‘R5064–5′ had higher activities of FK (83% and 36% higher in flooded than non-flooded plants, respectively) and HK (17% and 9% higher in flooded than non-flooded plants, respectively) and the smallest differences in activities of PFK (4% and 10% lower in flooded than non-flooded plants, respectively) and PK (13% and 16% lower in flooded than non-flooded plants, respectively) than scions of non-flooded plants.
Soluble sugars and starch
There were significantly higher contents of glucose, sucrose, fructose, and TSS (Fig. 3A, B, C, and E respectively) in scions in the flooded treatment compared to those in the non-flooded treatment, except for sucrose content in scions on ‘Flordaguard’, which was not affected by flooding (Fig. 3B). The largest changes in glucose, fructose, and sucrose content were in scions on ‘MP-29’ (119%, 110%, and 323% higher in flooded than non-flooded plants). Leaf starch content was higher in scions on all rootstocks in the non-flooded compared to the flooded treatment and the smallest differences were observed in scions on ‘MP-29’ (27% lower in flooded than non-flooded plants) (Fig. 3D). Total soluble sugars were higher in scions of flooded trees than non-flooded trees, and the largest differences occurred in scions on ‘MP-29’ and ‘R5064–5’ (375% and 281% higher in flooded than non-flooded plants) (Fig. 3E).
Nitrate, nitrite, and ammonium content
Leaf nitrate content in the scions was lower in the flooded than non-flooded treatment for scions on all rootstocks except ‘MP-29’ and ‘R5064–5’ (Fig. 4A). Leaf nitrite content was also lower in the flooded than non-flooded treatment for scions on all rootstocks except ‘R5064–5’ (Fig. 4B), but ammonium content in the scions was higher in the flooded than non-flooded treatment on all rootstocks (Fig. 4C). Scions on ‘Flordaguard’ exhibited the largest difference in leaf NO3− content between treatments (87% lower in flooded than non-flooded plants) (Fig. 4A), while scions on ‘MP-29’ or ‘R5064–5’ exhibited no difference. Scions on ‘Flordaguard’ had the largest difference in NO2− content between treatments (32% lower in flooded than non-flooded plants) whereas scions on ‘R5064–5’ exhibited no differences (Fig. 4B). Scions on ‘Flordaguard’ and ‘P-22’ (which has ‘Flordaguard’ parentage) had the largest differences of the toxic compound NH4− between treatments (48% and 109% higher in flooded than non-flooded plants, respectively) compared to scions on the other rootstocks (Fig. 4C).
Enzymes for nitrogen metabolism
Scions on most peach rootstocks exhibited lower levels of enzyme activity of NR, NiR, GS, and GOGAT (Fig. 5A, B, C, and D respectively) in the flooded treatment than the non-flooded treatment, except for GDH activity which was higher in the flooded treatment (Fig. 5E). Activities of NR and GS were not different between the flooded and non-flooded treatments in scions on ‘Guardian’ (Fig. A and C, respectively). Activities of NR, NiR, GS, and GDH in scions on ‘MP-29’ did not differ between treatments (Fig. 5A,B,C, and E, respectively), whereas NiR, GS, or GDH activities in scions on ‘R5064–5’ did not differ between treatments (Fig. 5B,C, and E). Enzymatic activity of GOGAT was lower in scions on both plum rootstocks in the flooded treatment compared to the non-flooded treatment (Fig. 5D). Scions on ‘R5064–5’ had lower activity of NR in the flooded treatment than non-flooded treatment (Fig. 5A). The largest differences between NR, NiR, GS, and GOGAT (Fig. 5A, B, C, and D respectively) between the flooded and non-flooded treatments occurred in scions on ‘Flordagaurd’ (38%, 22%, 28%, and 22% lower in flooded than non-flooded plants, respectively) and ‘Nemagaurd’ (30%, 16%, 29%, and 26% lower in flooded than non-flooded plants). The largest treatment difference in GDH activity was seen in ‘Flordaguard (46% higher in flooded than non-flooded plants) (Fig. 5E).
Discussion
Carbon metabolism
The increases in leaf carbohydrate contents in scions on all flooded rootstocks suggests that sugar translocation to the root system was impaired, contrary to the response observed in wheat where glucose, sucrose, and fructose were lower in flooded than non-flooded plants (Huang and Johnson 1995). Similar to our observations of Prunus rootstocks, a few studies have shown that flooding reduced sugar translocation in the phloem of oak trees and castor oil plants, resulting in higher sugar content in flooded than non-flooded plants (Ferner et al. 2012; Peuke et al. 2015). A study of forest trees found that carbohydrate levels and efficient use of the glycolytic pathway are major aspects in determining tolerance to flooding (Kreuzwieser et al. 2004). Enzymatic activity associated with glycolysis, in the current study, indicated that rootstocks with plum parentage possess some genetic difference which allows for more efficient phosphorylation of sugars. Hexokinase and FK have been linked to the phosphorylation of glucose and fructose, and FK has been found to regulate starch synthesis (Granot et al. 2014). The activities in scions on peach rootstocks in the current study suggest that scions on peach rootstocks have diminished efficiency for phosphorylating glucose and fructose under flooded conditions, while glucose phosphorylation efficiency is improved for scions on rootstocks with plum parentage. This could explain why scions on flooded plum rootstocks have greater efficiency of the glycolytic pathway under flooding conditions than those on peach rootstocks. Ultimately, having more active HK and FK molecules increases the hexose pool and improves sucrose metabolism (Fukao and Bailey-Serres 2004; Salvatierra et al. 2020). However, with the low levels of enzymatic activity related to sucrose metabolism and glycolysis, the decrease of HK and FK activities, and the large negative differences in starch content in scions on peach rootstocks, it could be possible that increases in soluble sugars in scions on peach rootstocks are, at least partially, the result of starch degradation. Phosphofructokinase is another important enzyme in glycolysis as it is responsible for the conversion of fructose 6-bisphosphate and ATP to fructose 1,6-biphosphate and ADP (Granot et al. 2014). The activity of PFK in scions on plum rootstocks was unaffected by flooding, whereas PFK activity in scions on peach rootstocks was lower in the flooded than non-flooded treatment which could result in further reduced efficiency of the glycolytic pathway in scions on peach rootstocks. Furthermore, lower AI activity in flooded plants suggests a strain on the photosynthetic process, as AI is important for biological processes related to sucrose metabolism and hydrolysis for growth (Kulshrestha et al. 2013). Sucrose molecules are important for various metabolic activities and signaling pathways for different genes involved in photosynthesis (Rosa et al. 2009). Neutral invertase has similar activity and function as AI, but its functions are less well elucidated (Shen et al. 2018). The response of AI and NI in scions on peach rootstocks suggests that rootstocks with peach parentage are less efficient at sucrose metabolism than those with plum parentage. The activities of SS and SPS further support this idea as scions in the flooded treatment on peach rootstocks had lower SS and SPS activities compared to those on plum rootstocks. Sucrose synthase and SPS have similar roles in synthesizing sucrose. Santaniello et al. (2014) reported that corn and potato with lower SS activity exhibited more sensitivity to hypoxia stress compared to those with higher SS activity, which can be seen in the current study as scions on the more flood-sensitive peach rootstocks experienced lower SS activity in the flooded treatment than scions on plum rootstocks, causing them to synthesize and hydrolyze sucrose less efficiently than scions on plum rootstocks. Carbohydrate levels have been correlated with flooding tolerance; crops with limited carbohydrate availability only survived transient flooding (Fukao and Bailey-Serres 2004). Thus, the ability of scions on plum rootstocks to exhibit higher levels of carbohydrate may explain, at least in part, their greater degree of flood tolerance.
Nitrogen metabolism
Plant demand for N is closely associated with growth, and thus lower contents of nitrogen and less active enzymes related to nitrogen assimilation and metabolism are indicative of reduced growth. A study of popular trees (Populus x canescens) by Liu et al. (2015) found that NO3− uptake was reduced by hypoxia whereas NH4+ uptake was not. They also postulate that NO3− was preferentially assimilated by developing tissues to ensure growth and survival under hypoxic or waterlogged conditions. Horchani and Aschi-Smiti (2010) found that root hypoxia in tomato also reduced leaf NO3− content. Similar results were observed in the current study, though only scions on peach rootstocks exhibited reduced uptake of NO3− under flooded conditions, whereas plum rootstocks maintained similar levels of NO3− in both flooded and non-flooded treatments. Results of the current study agree with studies of coniferous species, where NH4+ uptake limited uptake of NO3− (Liu et al. 2015), whereas scions on peach rootstocks exhibited high levels of NH4+ and consequently lower levels of NO3− and those on plum rootstocks had low levels of NH4+ and high levels of NO3−. Ammonium can be toxic to plants due to the depletion of carbon, damage to cellular structures, and disruption of a multitude of biological processes including photosynthesis (Bittsánszky et al. 2015). This would result in less growth of scions on peach rootstocks and exacerbated stress in these trees due to the elevated levels of NH4+, which could explain the greater sensitivity exhibited by scions on peach rootstocks.
Glutamate dehydrogenase is an important enzyme that links C and N metabolism (catalyzes the NADP+ linked deamination of glutamate to ammonia) and is used by cereal crops under low-oxygen stress to supply carbon (from amino acids) to the tricarboxylic acid cycle (Miflin and Habash 2002). Flooding resulted in an increase in GDH contents for all rootstocks except ‘MP-29’ and ‘R5064–5’ which suggests that scions on peach rootstocks are compensating for diminished carbon supply by cleaving carbon from amino acids, while those on plum rootstocks can continue producing carbohydrates during short-term flooding. Several studies have shown that reduced activity of enzymes involved in N metabolism is common in flood-sensitive plants during and after periods of hypoxia (Bailey-Serres and Voesenek 2008; Limami et al. 2014; Ren et al. 2017). Scions on plum rootstocks exhibited the smallest difference in NH4+ (slightly higher in the flooded compared to non-flooded trees), no differences in NO3-, and largely no differences in enzymatic activity related to N assimilation or metabolism between the flooded and non-flooded treatments, while N levels and enzymatic activity for scions on peach rootstocks were largely lower in the flooded than non-flooded treatments. This suggests that scions on rootstocks with plum parentage can maintain higher amounts of N and enzymatic activity related to N metabolism, ultimately being able to continue growth while under flooded conditions which contribute to their greater degree of flooding tolerance.
Conclusions
This study advances the understanding of how short-term flooding stress affects carbon and nitrogen metabolism of low-chill peach scions on various Prunus rootstocks. The results of the current study suggest that a low-chill peach cultivar when grown on plum and peach rootstocks respond similarly to flooding stress when examining carbon metabolism (except for HK and FK), while nitrogen metabolism in scions on peach is largely negatively affected (lower values in flooded treatment than non-flooded) and those on plum are mostly unaffected. The increased activities of HK and FK imply that the scions on plum rootstocks have more efficient phosphorylation of glucose and fructose than those on the peach rootstocks. This suggests that the hexose-phosphate pool is diminished in scions on peach rootstocks causing the metabolic pathways to be hexose limited, ultimately reducing the energy available for essential functions such as carbon assimilation, which is linked to N metabolism, which in turn is negatively affected. Thus, in flood-prone, subtropical areas where low-chill peach trees are planted, consideration should be given to replacing ‘Flordaguard’, the most common rootstock for low-chill peaches, with plum rootstocks such as ‘MP-29’ or another flood-tolerant rootstock.
Data availability
Any data related to this study are available upon request.
References
Araki H, Hossain MA, Takahashi T (2012) Waterlogging and hypoxia have permanent effects on wheat root growth and respiration. J Agron Crop Sci 198:264–275
Arora NK (2019) Impact of climate change on agriculture production and its sustainable solutions. Environ Sustainability 2:5–6
Bailey-Serres J, Voesenek L (2008) Flooding stress: acclimations and genetic diversity. Ann Rev Plant Biol 59:313–339
Bailey-Serres, J, Lee SC, E. Brinton, E (2012) Waterproofing crops: effective flooding survival strategies. Plant Physiol 160:1698–1709
Bandyopadhyay U, Das D, Banerjee RK (1999) Reactive oxygen species: oxidative damage and pathogenesis. Curr Sci 77:658–666
Beckman TG, Chaparro JX, Sherman WB (2012) ‘MP-29’, a clonal interspecific hybrid rootstock for peach. HortScience 47:128–131
Bird IF, Cornelius MJ, Keys AJ, Whittingham CP (1974) Intracellular site of sucrose synthesis in leaves. Phytochemistry 13:59–64
Bittsánszky AK, Pilinszky G, Gyulai G, Komives T (2015) Overcoming ammonium toxicity. Plant Sci 231:184–190
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Buysse JAN, Merckx R (1993) An improved colorimetric method to quantify sugar content of plant tissue. J Exp Bot 44:1627–1629
Cataldo DA, Maroon M, Schrader LE, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Comm Soil Sci Plant Anal 6:71–80
Crocker TL, Hendrick RL, Ruess RW, Pregitzer KS, Burton AJ, Allen MF, Shan J, Morris LA (2003) Substituting root numbers for length: improving the use of minirhizotrons to study fine root dynamics. Appl Soil Ecol 23:127–135
Ferner E, Rennenberg H, Kreuzwieser J (2012) Effect of flooding on C metabolism of flood-tolerant (Quercus robur) and non-tolerant (Fagus sylvatica) tree species. Tree Physiol 32:135–145
Foyer CH, Valadier MH, Migge A, Becker TW (1998) Drought-induced effects on nitrate reductase activity and mRNA and on the coordination of nitrogen and carbon metabolism in maize leaves. Plant Physiology 117(1):283–292
Fukao T, Bailey-Serres J (2004) Plant responses to hypoxia–is survival a balancing act. Trends Plant Sci 9:449–456
Gao Y, Long R, Kang J, Wang Z, Zhang T, Sun H, Li X, Yang Q (2018) Comparative proteomic analysis reveals that antioxidant system and soluble sugar metabolism contribute to salt tolerance in alfalfa (Medicago sativa L.) leaves. J Proteome Res 18:191–203
González JA, Gallardo M, Hilal MB, Rosa MD, Prado FE (2009) Physiological responses of quinoa (Chenopodium quinoa) to drought and waterlogging stresses: dry matter partitioning. Bot Studies 50:35–42
Granot D, Kelly G, Stein O, David-Schwartz R (2014) Substantial roles of hexokinase and fructokinase in the effects of sugars on plant physiology and development. J Exp Bot 65:809–819
Gries C, Kappen L, Lösch R (1990) Mechanism of flood tolerance in reed, Phragmites australis (Cav.) Trin. ex Steudel. New Phytol. 114:589–593
Horchani F, Aschi-Smiti S (2010) Prolonged root hypoxia effects on enzymes involved in nitrogen assimilation pathway in tomato plants. Plant Signal Behav 5:1583–1589
Huang B, Johnson JW (1995) Root respiration and carbohydrate status of two wheat genotypes in response to hypoxia. Ann Bot 75:427–432
Huppe HC, Turpin DH (1994) Integration of carbon and nitrogen metabolism in plant and algal cells. Ann Rev Plant Physiol Plant Mol Biol 45:577–607
Jones MGK, Outlaw WH, Lowry OH (1977) Enzymic assay of 10−7 to 10−14 moles of sucrose in plant tissues. Plant Physiol 60:379–383
Kreuzwieser J, Papadopoulou E, Rennenberg H (2004) Interaction of flooding with carbon metabolism of forest trees. Plant Biol 6:299–306
Kulshrestha S, Tyagi P, Sindhi V, Yadavilli KS (2013) Invertase and its applications–a brief review. J Pharm Res 7:792–797
Kusano M, Fukushima A, Redestig H, Saito K (2011) Metabolomic approaches toward understanding nitrogen metabolism in plants. J Exp Bot 62:1439–1453
Lillo C (1984) Diurnal variations of nitrite reductase, glutamine synthetase, glutamate synthase, alanine aminotransferase and aspartate aminotransferase in barley leaves. Physiologia Plant 61:214–218
Limami AM, Diab H, Lothier J (2014) Nitrogen metabolism in plants under low oxygen stress. Planta 239:531–541
Liu B, Rennenberg H, Kreuzwieser J (2015) Hypoxia affects nitrogen uptake and distribution in young poplar (Populus× canescens) trees. PLoS One 26:10
Lowell CA, Tomlinson PT, Koch KE (1989) Sucrose-metabolizing enzymes in transport tissues and adjacent sink structures in developing citrus fruit. Plant Physiol 90:1394–1402
Mahajan S, Tuteja N (2005) Cold, salinity and drought stresses: an overview. Archives Biochem Biophys 444:139–158
McGee T, Shahid MA, Beckman TG, Chaparro JX, Schaffer B, Sarkhosh A (2021) Physiological and biochemical characterization of six Prunus rootstocks in response to flooding. Environ Exp Bot 183:104368
Miflin BJ, Habash DZ (2002) The role of glutamine synthetase and glutamate dehydrogenase in nitrogen assimilation and possibilities for improvement in the nitrogen utilization of crops. J Exp Bot 53:979–987
Molins-Legua C, Meseguer-Lloret S, Moliner-Martinez Y, Campíns-Falcó P (2006) A guide for selecting the most appropriate method for ammonium determination in water analysis. Trends Analytic Chemy 25:282–290
Morales-Olmedo MG, Sellés G, Pinto M, Ortiz M (2021) Full-field characterization of sweet cherry rootstocks: responses to soil with different air-filled porosities. Plant Soil:1–17
Peuke AD, Gessler A, Trumbore S, Windt CW, Homan N, Gerkema E, Van As H (2015) Phloem flow and sugar transport in Ricinus communis L. is inhibited under anoxic conditions of shoot or roots. Plant Cell Environ 38:433–447
Pezeshki SR (2001) Wetland plant responses to soil flooding. Environ. Exp. Bot. 46:299–312
Ploetz RC, Schaffer B (1989) Effects of flooding and Phytophthora root rot on net gas exchange and growth of avocado. Phytopathology 79:204–208
Ramarao CS, Patil VH, Dhak BD, Kadrekar SB (1983) A simple in vivo method for the determination of nitrite reductase activity in rice roots. Z Pflanzenphysiol 109:81–85
Redillas MCFR, Park S-H, Lee JW, Kim YS, Jeong JS, Jung H, Bang SW, Hahn T-R, Kim J-K (2012) Accumulation of trehalose increases soluble sugar contents in rice plants conferring tolerance to drought and salt stress. Plant Biotech Rep 6:89–96
Ren B, Dong S, Zhao B, Liu P, Zhang J (2017) Responses of nitrogen metabolism, uptake and translocation of maize to waterlogging at different growth stages. Front Plant Sci 8:1216
Rosa M, Prado C, Podazza G, Interdonato R, González JA, Hilal M, Prado FE (2009) Soluble sugars: metabolism, sensing and abiotic stress: a complex network in the life of plants. Plant Signal Behav 4:388–393
Rufty TW, Huber SC (1983) Changes in starch formation and activities of sucrose phosphate synthase and cytoplasmic fructose-1, 6-bisphosphatase in response to source-sink alterations. Plant Physiol 72:474–480
Salvatierra A, Toro G, Mateluna P, Opazo I, Ortiz M, Pimentel P (2020) Keep Calm and survive: adaptation strategies to energy crisis in fruit trees under root hypoxia. Plants (Basel). 9(9):1108. https://doi.org/10.3390/plants9091108
Santaniello A, Loreti E, Gonzali S, Novi G, Perata P (2014) A reassessment of the role of sucrose synthase in the hypoxic sucrose-ethanol transition in Arabidopsis. Plant Cell Env 37:294–302
Sarkhosh A, Olmstead M, Chaparro J, Andersen P, Williamson J (2018) Florida peach and nectarine varieties. Horticultural sciences department, Florida cooperative extension service, Institute of food and agricultural sciences, University of Florida EDIS Publication Cir1159. p 17. https://edis.ifas.ufl.edu/mg374
Schaffer B, Andersen PC, Ploetz RC (1992) Responses of fruit crops to flooding. Hort Rev 13:257–313
Schaffer B, Ploetz RC (1989) Net gas exchange as damage indicator forPhytophthora root rot of flooded and non-flooded avocado. HortScience. 24:653–655
Shahid MA, Balal RM, Khan M, Zotarelli L, Liu G, Ghazanfar MU, Rathinasabapathi B, Mattson NS, Martínez-Nicolas JJ, Garcia-Sanchez F (2018) Ploidy level of citrus rootstocks affect the carbon and nitrogen metabolism in the leaves of chromium-stressed Kinnow mandarin plants. Env Exp Bot 149:70–80
Shen LB, Yao Y, He H, Qin YL, Liu ZJ, Liu WX, Qi ZQ, Yang LJ, Cao ZM, Yang Y (2018) Genome-wide identification, expression, and functional analysis of the alkaline/neutral invertase gene family in pepper. Int J Mol Sci 19:224
Singh RP, Srivastava HS (1983) Regulation of glutamate dehydrogenase activity by amino acids in maize seedlings. Physiol Plant 57:549–554
Singh RP, Srivastava HS (1986) Increase in glutamate synthase (NADH) activity in maize seedlings in response to nitrate and ammonium nitrogen. Physiol Plant 66:413–416
Snell FD, Snell FT (1949) Colorimetric methods of analysis. D. Van Norstrand Co. Inc., New York
Sym GJ (1984) Optimisation of the in-vivo assay conditions for nitrate reductase in barley (Hordeum vulgare L. cv. Igri). J Sci Food Agricul 35:725–730
Tarkowski ŁP, Van den Ende W (2015) Cold tolerance triggered by soluble sugars: a multifaceted countermeasure. Front Plant Sci 6:203
Thalmann M, Santelia D (2017) Starch as a determinant of plant fitness under abiotic stress. New Phytol 214:943–951
Tschoep H, Gibon Y, Carillo P, Armengaud P, Szecowka M, Nunes-Nesi A, Fernie AR, Koehl K, Stitt M (2009) Adjustment of growth and central metabolism to a mild but sustained nitrogen-limitation in Arabidopsis. Plant Cell Env 32:300–318
Zrenner R, Salanoubat M, Willmitzer L, Sonnewald U (1995) Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). Plant J 7:97–107
Acknowledgements
The USDA National Institute of Food and Agriculture (Hatch project) and the University of Florida Institute of Food and Agricultural Sciences are acknowledged for financial and material support of this study. Special thanks go to Dr. Tom Beckman, Dustin Huff and the stone fruit laboratories in the Horticultural Sciences Department at the University of Florida for their assistance during the experiment.
Funding
This work was supported by early career seed fund awarded University of Florida Institute of Food and Agricultural Sciences.
Author information
Authors and Affiliations
Contributions
Trequan McGee: Formal analysis, Investigation, Writing - original.
draft. Bruce Schaffer: Writing - review & editing, Resources. Muhammad Adnan Shahid: Formal analysis, Investigation, Visualization. Jose X. Chaparro: Writing - review &.
editing, Resources. Ali Sarkhosh: Supervision, Writing - review & editing, Project administration, Resources, Validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Responsible Editor: Gustavo Gabriel Striker.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
McGee, T., Schaffer, B., Shahid, M.A. et al. Carbon and nitrogen metabolism in peach trees on different Prunus rootstocks in response to flooding. Plant Soil 475, 427–441 (2022). https://doi.org/10.1007/s11104-022-05377-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-022-05377-6