Abstract
Purpose
It is unknown how the Cd-mediated changes in the rhizospheric nitrifier community affect plant growth under different water management practices. This study examined the effect of Cd on microbial N transformation and its association with rice growth in two water regimes, continuous flooding (CF) and alternate wetting and drying (AWD), in a paddy soil.
Methods
The 16S rRNA, amoA genes in the rhizosphere and endosphere, and OsNTRs were determined using qPCR. Nitrifier community analysis was performed by sequencing the amoA genes. Concentrations of N and Cd in shoots and roots, and IAA in the roots were determined by flow injection analyzer, ICP-MS and enzyme-linked immunosorbent (ELISA), respectively.
Results
The direct and indirect negative effects of Cd bioavailability in the rhizosphere on plant growth and ultra-structure of roots and leaves were greater under AWD than CF after 56-day growth. Nitrifier community was changed by water and Cd treatments. Crenarchaeota, Nitrosopumilus, Nitrosopira, and Nitrosovibrio tenuis related species were the dominant nitrifier players. Increasing Cd level decreased plant N uptake, root-IAA concentration, and the abundance of 16S rRNA gene in the rhizosphere and endosphere to a greater extent under AWD than under CF. This in turn decreased the colonization of endophytes and the surface area of the roots, leading to decreased N uptake from the soil.
Conclusion
The results imply that appropriate water management and rhizospheric engineering in Cd-contaminated paddy fields may be important approaches to decreasing plant Cd uptake and rhizospheric nitrification.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One of the most important global environmental concerns in food security is the contamination of agricultural soils with heavy metals, such as cadmium (Cd) (Gallego et al. 2012), which enter the food chain. About half of the world’s population relies upon rice (Oryza sativa) as the staple food (Kosolsaksakul et al. 2014), while in contaminated paddy soils, rice plants take up Cd and store it in the grains that are consumed by humans or animals (Aziz et al. 2015). Annually, 30,000 tons of Cd is disposed to the environment, of which the major contribution (13,000 tons) comes from human activities (Gallego et al. 2012), such as atmospheric deposition, mining, urbanization, application of phosphate fertilizers and use of wastewater for irrigation of rice fields (Kosolsaksakul et al. 2014). High levels of Cd can decrease plant growth, grain yield and quality (Lee et al. 2013; Srivastava et al. 2014), causing alterations to plant tissues (Arao et al. 2009), disrupting photosynthesis (Arao et al. 2009), and regulating expressions of at least 36 proteins in rice (Lee et al. 2010).
Cadmium is considered a non-essential element for plant growth and physiology, its accumulation in plant has a series of harmful effects (Guo et al. 2016). The harmful effects of Cd include poor seed germination, inhibition of photosynthesis and the damage of photosystem II structure and function, poor CO2 fixation due to inactivation of CO2-fixing enzymes, breaking of chloroplast ultrastructure and inhibition of plant growth which my lead to death of plant (Khan et al. 2014; Li et al. 2020; Molina et al. 2020). Previous studies have shown that it can increase in ethylene level in Arabidopsis thaliana and Pisum sativum (Bücker-Neto et al. 2017). In addition, it increased the jasmonic acid (JA) level in P. sativum, and abscisic acid (ABA), salicylic acid (SA) and JA levels in rice (Kim et al. 2014; De et al. 2015). It was shown that Cd exposure decreased indole-3-acetic acid (IAA) content in A. thaliana and O. sativa (Ronzan et al. 2018).
The mobility in soil and plant uptake of Cd from soil is higher than those of other heavy metals (Song et al. 2015). Cd translocation from soil to shoots takes place via root uptake and as a result roots are likely to suffer from Cd damage first due to higher accumulation Cd than shoots (Uraguchi and Fujiwara 2013). It has been reported that root morphology and anatomy are considered as essential components of plants to study the Cd uptake and translocation in plant tissues (Huang et al. 2019). Different rice cultivars showed significant variation in Cd uptake, and water regimes also affect Cd accumulation in rice grains (Honma et al. 2016). Most of previous studies focused on membrane proteins involved in metal transport, root exudation of organic acids, formation of iron plaque on root surface, and different chemical forms of Cd in the rhizosphere (Fulda et al. 2013; Cheng et al. 2014). In addition, Cd toxicity can also cause negative effects on endogenous hormonal system, especially IAA (Ronzan et al. 2018).
Plant hormones are very important for growth regulation and play a key role in all developmental stages, along with abiotic stress (Spoel and Dong 2008). In addition, plants respond to stresses such as heavy metals, in terms of change in the level and distribution of plants hormones. Indole-3-acetic acid (IAA) in Arabidopsis is involved in abiotic stress responses (Hu et al. 2013). In accordance, IAA level was also reported to vary in response to heat, cold and drought stresses. Auxin plays a key role in the physiological processes of almost all plants, especially, lateral roots (LRs) formation and development. Cadmium inhibited lateral roots development, which plays an important role in water and nutrient absorption (Fattorini et al. 2017). Rice LRs were negatively affected by Cd via interfering with auxin homeostasis. However, it has been reported that Cd inhibit LRs in rice by disrupting auxin efflux transporter genes (OsPIN) mediated auxin distribution (Wang et al. 2021).
Water management practices influence the bioavailability of Cd in the paddy soils, which then affects its adsorption and accumulation by microbes and plants (Meng et al. 2018). It has been reported that alternate wetting and drying (AWD) water management increases whereas continuous flooding (CF) decreases Cd accumulation in rice grains (Afzal et al. 2019; Norton et al. 2017; Yang et al. 2017). The mentioned differences have been linked to impacts of redox conditions of soil on bioavailability and mobility of Cd. In addition, conversion of Cd from one oxidation state to another is also responsible for the difference in Cd availability in the soil and accumulation in plants under different water managements (Mlangeni et al. 2020). Furthermore, the application of N fertilizers increases the availability of Cd through affecting rhizosphere properties and soil chemical reactions, increasing Cd mobility and accumulation. Nitrogen application is well known to increase dry mass accumulation, leaf area expansion, and protein content through its impacts on Cd speciation, complexation, desorption, phytoavailability, and transportation by affecting soil properties and crop growth (Ata-Ul-Karim et al. 2020). A recent study reported that increasing N rate was strongly linked to a decrease in soil pH and increased soil redox potential (Eh), electric conductivity, cation exchange capacity (CEC), and wheat grain Cd concentration (Ata-Ul-Karim et al. 2020).
Previous studies have also reported that availability of Cd and low water content of soil have decreased the abundance of the N-transforming microbes, and toxicity to plants and soil-residing animals (Afzal et al. 2019; Liu et al. 2014, 2018). It has been reported that heavy metal pollution decreased microbial biomass, abundance, and ammonia oxidizing genes in paddy soil (Liu et al. 2018). In addition, decreased microbial abundances due to metal contamination might decrease the microbial mediated N transformation in paddy soil, particularly under alternate wetting and drying conditions (Afzal et al. 2019). About 80% nitrification rate was decreased by Cd-salt (200 mg kg−1) of soil (Smolders et al. 2001). Similarly, soil enzyme activities and nitrifier and denitrifier communities were reported to be sensitive to Cd contamination ranged from 2–10 mg kg−1 in paddy soil under AWD conditions (Afzal et al. 2019; Liu et al. 2014, 2018).
Plant-associated microbes are very important for plant growth directly or indirectly. Some of the rhizospheric and endospheric bacteria have the ability to produce IAA which is responsible for an increase in root surface via enhancement of root elongation, and growth of lateral roots and root hairs (Spaepen and Vanderleyden 2011). Hence the plant absorbs those nutrients which are not accessible to the plant, leading to better plant growth. In addition, some plant-associated bacteria are also responsible for elevated plant productivity by decreasing ethylene level in plants. Heavy metals can affect the abundance and composition of ammonia oxidizing archaea (AOA) and bacteria (AOB) (Ai et al. 2013; Hou et al. 2018). Previous studies reported that Cd stress altered microbial-mediated processes such as ammonification, nitrification, and denitrification in the soybean rhizosphere and bulk soil (Yang et al. 2007). However, it is unknown how altered AOA and AOB by Cd stress affects plant growth in different water regimes.
This study examined the effects of Cd level and water regime on rice growth and physiology, especially IAA level along with the N status in the roots. It also aimed to link the Cd-mediated changes in the AOA and AOB communities in the rhizosphere with bacterial abundance of the rhizosphere and endosphere under CF and AWD conditions. We hypothesized that Cd contamination would alter the microbial community of nitrifiers (AOA and AOB) in the rhizosphere by decreasing the abundances of ammonia monooxygenase (amoA) genes responsible for nitrification, and that the effects of Cd would be greater under AWD conditions as compared to CF conditions.
Material and methods
Experimental design
Soil samples were collected from the upper 10-cm layer at a paddy field site in Longyou County, Quzhou City (N 29˚ 0′ 00''; E 119˚ 5′ 24''), Zhejiang Province, China, before rice planting in spring 2017. The soil samples were air-dried and sieved to pass a 2-mm sieve for the determination of baseline soil properties. The soil had the following physicochemical characteristics: pH, 5.6; total Cd, 0.12 mg kg−1, organic C, 12.2 g kg−1; dissolved organic C, 68.6 mg kg−1; total N, 8.1 mg kg−1; sand, 57%; silt, 25%; and clay, 18%.
A greenhouse experiment was designed as completely factorial, consisting of two water regimes and four Cd levels in six replicates per treatment. The two water managements were 1) continuous flooding (CF) and 2) alternate wetting and drying (AWD) conditions. Cadmium was added as CdCl2·2.5H2O (99%, Tianjin Fuchen Chemical Co., Tianjin, China) at four levels: 1) Cd0, no Cd, 2) Cd2, 2 mg kg−1, 3) Cd5, 5 mg kg−1 and 4) Cd10, 10 mg kg−1 soil. Such a Cd range allowed to examine the N transformation in the conditions such as highly-contaminated mine sites and contaminated cropping fields, and has been used in previous studies (Liu et al. 2016; Zhou et al. 2018). A total 5 kg of soil was added to each pot. In the CF condition, the water level was continuously kept at approximately 3 cm above the soil surface while in the AWD conditions the water level was kept at 3 cm above the soil surface for 24 days, followed by dry period (no water was added) for the next 3 days. After this period, the pots were kept under flooding conditions for 3 days and dry period for the next 3 days. This cycle was continued for 56 days. Basal nutrients of equivalent to 120 kg N, 60 kg P and 50 kg K ha−1 as urea, calcium superphosphate and potassium chloride, respectively, at the transplanting stage. An additional dose of 60 kg N ha−1 was applied each at the tillering and panicle initiation stages.
Plant growth
Uniform seeds of rice [Oryza sativa (Indica) cultivar Yongyou 12] were surface-sterilized with 5% sodium hypochlorite solution for 10–12 min and then rinsed with autoclaved distilled water for 4 times. The seeds were germinated on sterile filter paper in Petri dishes for 3 days. The germinated seeds were then sown to seedbed. After further 21 days, four uniform seedlings were transplanted in each of the pots of various treatments, and were grown in a greenhouse at 70% humidity and day/night temperature cycle of 29/24 °C.
Plant growth and uptake of N and Cd
Plants were harvested after 24 and 56 days of treatment. Plant samples were washed with autoclaved distilled water and separated into roots and shoots. We did not treat the roots to remove iron plaque and so root Cd concentrations might be over-estimated. The plant samples were oven-dried at 80 °C for 2 days, and dry weights were recorded. The dry samples were finely ground to pass through a 0.15-mm sieve. The concentration of N was determined using the combustion procedure on a Vario MAX CNS elemental analyzer (Elementar Analysensysteme GmbH, Hanau, Germany). The concentration of Cd in the roots and shoots was determined using inductively coupled plasma mass spectrometry (ICP-MS) (PerkinElmer, NexION 300X) after digestion with concentrated HNO3-HF acids, using a microwave digestion system (MARS6, CEM). Briefly, oven-dried roots and shoots were ground to a fine powder and 0.5 g of each sample was digested in 5 mL HNO3:H2O2 (5:1, v/v) before subject to ICP-MS analysis.
Samples isolation from rhizosphere and endosphere for microbial analysis
Soil and plants were removed from each pot after 24 and 56 days of plant growth and the roots were carefully separated from the soil. In this study, we follow the definition of rhizosphere as the soil attached to roots at a distance of approximately 1 mm (Edwards et al. 2015). To collect rhizosphere soil, the root system was transferred to Falcon tubes containing 50 mL of sterile phosphate-buffered saline (PBS) solution. With the help of sterile forceps, the roots were stirred vigorously to clean the surface. The endophytic compartment was isolated following the previous protocol (Lebeis et al. 2015). Briefly, the roots were placed in the sterile distilled water and rinsed for 2–3 times and the debris was removed aseptically. Sonication was performed for 30 s at 50–60 Hz (output frequency 42 kHz, power 90 W, Branson Ultrasonics). All centrifuged fresh samples from the rhizosphere and sonicated roots endosphere were stored at -80 °C until microbial DNA isolation.
Soil chemical analysis
Fresh rhizosphere soil samples were also used for analyses of Cd, pH, soil organic C (SOC), NH4+_N, soil dissolved organic N (DON), total N, and bioavailable Cd and arsenic (As). Soil pH was measured after shaking the soil in water (1:2.5) for 30 min. To measure the soil Eh, a slurry of water-to-soil ratio of 1:2.5 was used (Afzal et al. 2019). Dichromate digestion was used to measure the SOC (Kalembasa and Jenkinson 1973). Both nitrate (NO3−) and ammonium (NH4+) were extracted with 1 M KCl, followed by analysis using a flow injection analyzer (SAN + + , Skalar, Holland). Fresh soil samples were used for extraction of available Cd with 0.01 M CaCl2 and determined using the ICP-MS. The DON was extracted in water, and analyzed using a Multi N/C TOC analyzer (Analytic Jena AG, Jena, Germany).
Plant sample collection, RNA extraction, cDNA and qRT-PCR
Root and shoots were collected and crashed in liquid nitrogen for RNA extraction. RNA was extracted by Trizol reagent according to manufacturer’s protocol and was then converted (reverse transcribed) to cDNA by Prime Script TMRT kit (Takara) according to manufacturer’s instructions. Then qPCR was performed for different samples with light cycler 96 well real-time PCR (Roche, Switzerland) using SYBER premix Ex Taq (Takara, Japan). Rice Actin 1 was used as an internal standard reference (Zhang et al. 2012) and data was analysed by the method described by Livak and Schmittgen (2001). Primer used in the current studied are in the Table S1.
Total DNA extraction, quantitative PCR, and microbial functional genes
Total DNA was extracted by FastDNA Spin Kit (MP Biomedicals, LLC., Solon, OH, USA), from frozen rhizospheric soil samples (0.5 g) following the manufacturer’s instructions. DNA purity and quantity were measured with the Nanodrop®ND-2000 UV–vis spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA), while DNA integrity and size were observed on a 0.7% agarose gel. DNA samples were stored at − 20 °C until analyses within 3 days after extraction.
To quantify the abundance of amoA and 16S rRNA genes, quantitative PCR (qPCR) assays in triplicate for each sample were used. Each reaction consisted of 20 μL of the reaction mixture containing 10 μL SYBR Premix Ex Taq (Takara, Dalian, China), 0.1 μL of 100 mM of each primer and 1 μL of 10 × diluted DNA template (10 ng) and 8.8 μL Milli Q-water. The reaction was performed in the LightCycler 480 (Roche Applied Sciences, Mannheim, Germany) with a melting curve analysis carried out at the end of each run to confirm PCR product specificity. A standard curve for qPCR was generated as previously demonstrated by Diet al. (2009). Briefly, amoA and 16S rRNA genes were PCR-amplified with primers and PCR conditions listed in Table S1. PCR products were purified with NucleoSpin®Gel and PCR Clean-up (Nucleospin, Macherey–Nagel) prior to cloning into the pGEM-T Easy Vector (Promega, Madison, WI, USA), followed by transformation into Escherichia coli JM109 competent cells (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Confirmed positive clones were purified and sequenced using the ABI PRISM ® 3730 Genetic ABI PRISM® 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) with the BigDye ® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems) being used for sequencing reactions. For standard curves, a known number of copies of the plasmid DNA (102–1013) containing the target gene was subjected to qPCR assay in triplicate. For amplification of the amoA and 16S rRNA genes, high efficiencies of 92.3–105.2% were obtained with R values of 0.996–0.999.
The same procedure was used for cloning of AOA and AOB amoA genes under different treatments and 25 positive clones were randomly selected for sequencing. For the obtained sequencing via ABI PRISM ® 3730 Genetic ABI PRISM® 3730 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) with the BigDye ® Terminator v3.1 Cycle Sequencing Kit, the evolutionary history was inferred using the Neighbor-Joining method (Saitou and Nei 1987). The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (1000 replicates) is shown below the branches (Felsenstein 1985). The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were computed using the Maximum Composite Likelihood method (Tamura et al. 2004). Evolutionary analyses were conducted in MEGA6 (Tamura et al. 2013).
Transmission electron microscopy
Leaf segments and root tips were selected from control and 10 mg kg−1 Cd treated plants. Total three specimens per treatment were prepared for TEM. Leaf segments without veins (1 mm2) of the fifth newly-grown leaves and the root tips of 2–3 mm were fixed in a 2.5% (v/v) glutaraldehyde in 0.1 M phosphate-buffered saline solution (PBS) (pH 7.4) overnight and then washed three times with PBS for electron microscopy. After post-fixation of samples in 1% (m/v) in OsO4 for 1 h, the samples were washed with PBS for three consecutive times and dehydrated for 15–20 min in each of graded ethanol (50, 60, 70, 80, 90, 95, and 100%, v/v) with a final immersion in absolute acetone for 20 min. The samples were embedded in Surr’s resin overnight and kept at 70 °C for 9 h. The ultra-thin sections (about 80 nm) were cut using the LEICA EM UC7 ultratome and mounted on copper grids for the transmission electron microscope (TEM 1230EX, JEOL, Japan) at 60 kV.
Indole acetic acid (IAA) measurement
An enzyme-linked immunosorbent (ELISA) procedure (Rapid bio, USA) was used to determine the concentration of IAA in the root. Fresh root tissues were ground in liquid N2 and extracted in 100% methanol. The extract was centrifuged at 4,000 × g for 10 min at room temperature. The supernatant was vacuum-evaporated to 1/10 of the initial volume and acetic acid (1%) was used to dissolve the residue, followed by filtration through 0.20-µm filters as suggested by Nakurte et al. (2012). All standards and samples were mixed with horseradish peroxide-conjugated reagent, and were incubated at 37 °C for 30 min according to the manufacturer’s protocol. The Synergy™ HT Multi-detection micro plate reader (BioTek, Vermont USA) was used for optical density (OD) measurements.
Statistical analysis
Data Procession System (DPS) was used for analysis of variance (ANOVA). One-way ANOVA was used for 24-day treatments while two-way ANOVA analyses were conducted to test the effect of Cd levels and water regimes and their interactions after 56-day treatments, followed by comparison between means using the least significant difference (LSD). Correlation analysis, principal component analysis (PCA), and presentation were performed using R version (3.4.2). The PCA analysis was used to identify the relationships between soil and plant measurements under Cd stress in both CF and AWD. The first principal component has the largest variance due to the orthogonal transformation. Figures were constructed using software Origin (Origin Pro 9.0 for Windows).
Results
Bioavailability of Cd, pH and Eh
The bioavailability of Cd was higher at Day 24 and then decreased significantly (P ≤ 0.01) until Day 56 with the decrease being greater in the CF than in the AWD. For example, it decreased by 80% and 90% at 5 and 10 mg Cd kg−1, respectively, under CF, as compared to 10% and 27%, respectively, under AWD (Fig. 1A). By Day 56, Cd bioavailability in the rhizosphere was significantly (P ≤ 0.01) higher in the AWD as compared to CF (Table S2). Compared with the initial pH, soil pH decreased by 0.3 units at day 24, and 1.1–1.4 units at day 56 under AWD irrespective of Cd treatment. In comparison, the pH increased at 0–5 µM Cd at day 24, and at 0 Cd at day 56 but decreased at 2–10 µM Cd at day 56 under CF (Table S3). In general, Eh was higher under AWD than under CF and increased with increasing Cd level (Table S3).
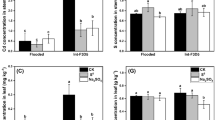
Concentrations of CaCl2-extractable Cd (A), dissolved organic N (DON) (B), and concentrations of NH4+-N (C) and NO3−-N (D) in the rhizosphere of rice plants grown under continuous flooding for 24 (24d) and 56 days (56d-CF) and under alternate wetting and drying (AWD) conditions for 56 days (56d-AWD) at Cd levels of 0, 2, 5 and 10 mg kg−1. Error bars indicate ± standard error of the mean of three replicates
Concentrations of dissolved organic N (DON), NH4 + and NO3 −
The concentration of DON in the no-Cd control under CF was higher at Day 24 than at Day 56. It decreased with increasing Cd addition and decreased more at Day 56 than Day 24. For example, DON decreased by 26, 49 and 74% after 24 days at Cd levels 2, 5, and 10 mg kg−1 of soil, respectively. After 56 days, the DON concentration decreased more under AWD than CF with decreases being 32, 66 and 71% for AWD, and 18, 46, and 58% for CF at Cd level 2, 5, and 10 mg kg−1, respectively (Fig. 1B), leading to a significant water × Cd interaction (Table S2).
Increasing Cd levels significantly (P ≤ 0.01) increased the NH4+_N concentration. Compared with the control, NH4+_N concentration increased by 30, 50 and 74% at Cd levels 2, 5, and 10 mg kg−1, respectively. The highest concentration of NH4+_N (35 mg kg−1) was noted at Cd level of 10 mg kg−1 after 24 days (Fig. 1C, Table S2). By Day56, the NH4+_N concentration (29 mg kg−1) was 38% higher in the AWD condition as compared to the CF condition (21 mg kg−1) (Table S2). The NH4+_N concentration increased by 23, 90, and 161% under AWD and by 0, 37 and 82% under CF at 2, 5, and 10 mg Cd kg−1 of soil, respectively (Table S2).
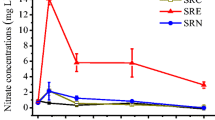
The addition of Cd decreased the concentration of NO3−-N in the rhizosphere with the concentration being fourfold lower at 10 mg Cd kg−1 (0.8 mg kg−1) compared to the control (3.6 mg kg−1) after 24 days. By Day56, under CF, the concentration of NO3−-N in soil decreased slightly with increasing Cd levels (Fig. 1D). Under AWD, NO3−-N concentration remained significantly (P ≤ 0.01) lower (1.7 mg kg−1) at 10 mg Cd kg−1 compared to the control (3.2 mg kg−1). In term of percentage decreased, after 24 days, 4, 57 and 79% decreased was observed in the concentration of NO3−-N than the control at Cd levels 2, 5, and 10 mg kg−1, respectively. However, a lower concentration of NO3−-N was observed after 56 days during AWD and CF. For example, 14, 19, and 49% decreased in concentration of NO3−-N during AWD and 37, 18 and 38% decreased were noted for CF at Cd levels 2, 5, and 10 mg kg−1 of soil, respectively.
Overall, the negative effect of Cd on nitrification, as indicated by NO3−_N concentration, was significantly greater (P ≤ 0.01) in the AWD as compared to the CF (Table S2).
Effect of Cd on plant growth
Plants grown under AWD accumulated more Cd in shoots and roots as compared to CF after 56 days. The addition of 10 mg Cd kg−1 increased shoot Cd concentration by threefold after 56 days of AWD as compared to CF (Table 1).
A higher concentration of Cd was observed in the roots grown under AWD (56-fold) than under CF (52-fold) after 56 days at 10 mg Cd kg−1 compared to the nil-Cd control. During AWD, the Cd accumulation was significantly (P ≤ 0.01) higher at 10 mg Cd kg−1, which was a2-fold increase compared to the respective level Cd of CF after 56 days. In addition, the root Cd accumulation was twofold higher in AWD after 56 days as compared to the 24 days at 10 mg Cd kg−1. Under CF, the concentration of Cd in the root was onefold higher after 56 days compared to 24 days (Table 1).
The addition of 10 mg Cd kg−1 decreased dry weights of roots and shoot by 68% and 27% under CF, and 82% and 41% under AWD, respectively (P ≤ 0.01). The effect of Cd on plant growth at Day 56 was greater under AWD than under CF (Table S2). The addition of 10 mg Cd kg−1 decreased plant height by 19% under AWD and 11% under CF (Table 1).
Changes in the ultra-structure of leaf and root
Transmission electron micrographs of root tips under both CF and AWD without Cd addition showed well-structured cytoplasmic matrix with smooth and continuous cell walls and bigger nucleus as compared to those with Cd addition (Fig. S1A-B). The ultra-structure of roots tips of plants grown under CF with Cd addition showed a number of changes including swelling of mitochondria, distorted cristae, the disappearance of vacuoles and damaged nuclear membrane (Fig. S1C). The Cd addition showed pronounced negative effects on the ultra-structure of root tips under AWD; the nucleus, nucleolus, mitochondria, and other cell organelles were completely disappeared in the cytoplasmic matrix (Fig. S1D).
Endogenous indole acetic acid (IAA) level in roots
Increasing Cd levels decreased the concentration of IAA in roots significantly (P ≤ 0.05) (Table 1). After 24 days of flooding, the concentration of IAA in roots decreased by 4, 42, and 54% at 2, 5, and 10 mg Cd kg−1, respectively, as compared to the nil-Cd control. After 56 days, the root IAA concentration was significantly lower under AWD as compared to CF. For example, root IAA decreased by 32, 43, and 66% under AWD and by 24, 25, and 45% under CF at 2, 5, and 10 mg Cd kg−1, respectively, leading to a significant Cd × water interaction (P ≤ 0.01) (Table 1).
The concentration of N in shoots and roots
The Cd contamination significantly (P ≤ 0.01) decreased the concentration of N in shoots and roots with the decrease being greater in the AWD than CF after 56 days of plant growth. Shoot N concentration of the plants grown at 10 mg Cd kg−1 was 2.03% and 2.68% after 56 days of AWD and CF, respectively (Table 1). Similarly, the concentration of N in the root at 10 mg Cd kg−1was also lower under AWD (0.54%) than under CF (0.89%) after 56 days (Table 1).
Effect of Cd on the abundances of the amoA and 16S rRNA genes
The addition of Cd significantly decreased the amoA gene number in rhizosphere (Table S2). The copy number of archaeal amoA genes was 60% higher than bacterial amoA in rhizosphere under both CF and AWD conditions, and was significantly (P ≤ 0.01) decreased with increasing Cd levels, and slightly decreased over time (Fig. 2). The abundance of archaeal amoA was significantly decreased (P ≤ 0.01) by Cd as compared to bacterial amoA in rhizosphere (Fig. 2A-B). In comparison, the copy number of bacterial amoA genes was generally higher under CF than under AWD. Under CF condition for 56 days, there was no significant difference in the abundance of bacterial amoA gene when Cd addition increased from 2 to 5 mg kg−1, but significantly (P ≤ 0.01) decreased when Cd level further increased to 10 mg kg−1 (Fig. 2B). However, bacterial amoA gene abundances were significantly decreased at all Cd levels than the control after 56 days of AWD (Fig. 2B). In addition, the copy number of archaeal amoA genes in rhizosphere decreased with time but was still higher than that of bacterial amoA genes by Day 56. In our experiment, the copy number of l amoA gene was significantly (P ≤ 0.01) decreased in rhizosphere under AWD as compared to CF with all Cd levels as compared to the control (Fig. 2A).
The abundances of archaeal (A) and bacterial (B) amoA genes in the rhizosphere of rice plants grown for 24 days of flooding (24d), and 56 days of continuous flooding (56d-CF) and alternate wetting and drying (56d-AWD) at Cd levels of 0 (Cd0), 2 (Cd2), 5 (Cd5) and 10 mg kg−1 (Cd10). Different lower-case letters indicate significant difference (P ≤ 0.01) among the different Cd levels, and Cd × water interaction. Error bars indicate ± standard error of the mean of three replicates
The addition of Cd (5 and 10 mg kg−1) decreased (P ≤ 0.01) the abundance of the 16S rRNA gene in the endosphere of the plants grown for 56 days under CF and ADW as compared to the respective controls (Fig. 3A). Overall, the abundances of 16S rRNA gene in the endosphere and in rhizosphere were significantly (P ≤ 0.01) lower under AWD than under CF (Fig. 3). The effect of Cd was more prominent in the AWD condition as compared to CF conditions.
The abundance of bacterial 16S rRNA genes in the endosphere (A) and rhizosphere (B) of rice plants grown for 24 days of flooding (24d), and 56 days of continuous flooding (56d-CF) and alternate wetting and drying (56d-AWD) at Cd levels of 0 (Cd0), 2 (Cd2), 5 (Cd5) and 10 mg kg−1 (Cd10). Different lower-case letters indicate significant difference (P ≤ 0.01) among the different Cd levels, and Cd × water interaction. Error bars indicate ± standard error of the mean of three replicates
After 24 days, increasing Cd levels decreased the copy number of the 16S rRNA gene observed in rhizosphere (Fig. 3B). There was no significant difference after 56 days in the abundance of 16S rRNA gene in the rhizosphere at Cd levels of 2, 5 and 10 mg kg−1 under CF (Fig. 3B). However, a significant (P ≤ 0.01) difference among the Cd levels of 2, 5 and 10 mg kg−1 in the abundance of 16S rRNA gene in rhizosphere after 56 days of AWD condition were observed (Fig. 3B).
Two-way ANOVA results showed that Cd addition significantly decreased copy numbers of 16S rRNA, AOA and AOB amoA genes in rhizosphere and endosphere after 24 days of flooding treatment. After 56 days, the abundances of AOA and AOB amoA genes were significantly affected by combined water and Cd treatments but AOA amoA genes were more affected by AWD (P ≤ 0.01) and Cd together (P ≤ 0.05) (Table S2). There was a significant interaction between water regime and Cd on root-IAA and Cd concentrations. Increasing Cd level decreased the concentrations of root-IAA and increased Cd concentrations in roots and shoots with the effects being greater under AWD than under CF (Table 1, Fig. 4). Positive correlations were recorded among of OsNTRs, NH4+-N, rhizosphere soil Cd, and root Cd accumulation under both water regimes. Furthermore, microbial gene abundances were correlated positively with root-IAA concentration while negatively with the concentrations of bioavailable Cd in the rhizosphere and Cd in the roots (Fig. 5).
Concentrations of indole acetic acid (IAA) (A) and Cd (B) in the roots of rice plants grown at Cd levels of 0 (Cd0), 2 (Cd2), 5 (Cd5) and 10 mg kg−1 (Cd10) after 24 days of flooding (24d), and 56 days of continuous flooding (56d-CF) and alternate wetting and drying (56d-AWD) conditions. Different lower-case letters indicate significant difference (P ≤ 0.01) among the different Cd levels, and Cd × water interaction. Error bars indicate ± standard error of the mean of three replicates
Phylogenetic analysis of AOA and AOB amoA genes
In the present study, the archaeal and bacterial nitrifiers were changed under different treatments. The AOA community at Cd10 under CF adopted the same node at the tree and classified as uncultured Thaurmarchaeote and Archaeon. However, at the same Cd level (Cd10), under AWD, the AOA community was closely related to Crenarchaeote. Overall, AOA was related to Nitrosopumilus sp, Candidatus and Nitrosomarinus (Fig. 6). Similarly, the AOB community was also changed under different Cd and water treatment. The AOB under AWD at Cd10 was related to Nitrosopira and Nitrosovibrio tenuis. In addition, AOB is closely related to Xanthomonas citri and Nitrosopira under CF at Cd10.
Phylogenetic analysis of AOA community under continuous flooding (circles) and alternate wetting and drying (inverted triangles) conditions after 56 days. Note; empty circle: Cd0, black filled circles: Cd10 under continuous flooding, and black filed inverted triangles Cd10 under alternate wetting and drying
Correlations of Cd with nitrification, rhizospheric, endospheric and rice nitrate translocation genes
Under the CF condition, soil Cd bioavailability correlated negatively (P ≤ 0.01) with concentrations of DON, NO3−-N, AOA, AOB amoA and 16S rRNA gene abundances in the rhizosphere and endosphere, and root-IAA level, but positively with NH4+-N after 24 days. Rice OsNTR2.1 and OsNTR2.2 (Fig. S2) were significantly negatively correlated with NO3−-N after 24 and 56 days of treatment under the CF condition. After 56 days, the available Cd in soil was positively correlated with NH4+-N and negatively with NO3−-N concentration. NO3−-N was positively correlated with the abundance of AOA and AOB amoA genes after 24 and 56 days under the CF condition. Endospheric (R2 = -0.99) and rhizospheric (R2 = -0.94) bacterial abundances were negatively correlated with nitrate transporter gene in plants (Fig. S3). Root-IAA concentration correlated positively and root Cd concentration negatively with the abundances of endospheric and rhizospheric 16S rRNA genes under both CF and AWD conditions while root-IAA concentration negatively correlated with root Cd concentration.
After 56 days of AWD conditions, the concentrations of DON, NO3−-N and the abundances of 16S rRNA, AOA and AOB amoA genes in rhizosphere showed significant negative correlations with the concentrations of bioavailable Cd in soil and plant Cd uptake. In addition, 16S rRNA in the rhizosphere and root-IAA also showed significant negative correlation with the concentrations of bioavailable Cd in soil and plant accumulated Cd. The abundance of microbial 16S rRNA gene was positively correlated with DON (P ≤ 0.01). The concentrations of plant accumulated Cd, correlated significantly positive with the expression of OsNTR2.1 but not OsNTR2.2. In addition, a strong negative correlation (P ≤ 0.01) was observed between OsNTRs and soil NO3−-N after 24 and 56 days of treatment under the AWD condition. A positive correlation was observed between concentrations of NH4+-N and available Cd in soil. The endospheric bacterial abundance showed a strong positive correlation (P = 0.01, R2 = 0.93) with root-IAA concentration but a negative correlation (P = 0.01, R2 = -0.84) with root-Cd concentration (Fig. S4).
Discussion
Water treatment effects on Cd availability and Cd toxicity to plants
This study showed that the concentration of CaCl2-extractable Cd was lower under CF than under AWD. Similarly, Vink et al. (2010) also reported that Cd solubility in paddy soil decreased under CF conditions. Under water-saturated conditions, reduced organic substances (i.e., acetate, formate and other organic acids) produced which increased metal–organic complexes and the surface charges of soil, and hence increased the adsorption of metal ions in soil (Hernandez-Soriano and Jimenez-Lopez 2012; Kinniburgh et al. 1999). Another reason for the low availability of Cd under the CF condition was the low redox potential in soil which promoted the conversion of sulfates to S2−, leading to the formation of insoluble CdS. Furthermore, reduced soil conditions also favored the formation of Cd carbonate and increase in soil pH which is associated with more sorption sites (de Livera et al. 2011; Kosolsaksakul et al. 2014; Wang et al. 2019).
In our present study, Cd addition altered the ultra-structure of rice roots with the effect of Cd being larger under AWD than CF condition (Fig. S1). The addition of Cd significantly affected the ultra-structure of chloroplast and roots under both CF and AWD conditions. Previous studies have reported that Cd accumulation dropped in rice tissues under CF conditions due to the formation of non-toxic and non-bioavailable CdS (Afzal et al. 2019). As a result, the AWD had a greater effect on the plant ultra-structure than CF due to the increased accumulation of Cd in roots of rice under AWD (Fig. S1C-D). Our results are consistent with previous observations that plants displayed a response to Cd stress by changes in the ultra-structure (Liu et al. 2003; Rizwan et al. 2016).
The main effect on plant growth, physiology, biochemistry, and biomass could be assigned to Cd toxicity to the plant. Increasing Cd addition decreased the N uptake and plant biomass production (Table 1 and Fig. S2) with the effects being more pronounced under AWD than under CF, which is associated with the higher bioavailability of Cd. The toxicity of Cd to rice plants resulted in leaf chlorosis and stunted growth (Srivastava et al. 2014). The uptake of Cd by rice plants could alter the leaf and root ultra-structure, which damaged the rice photosynthetic apparatus in a previous study (Wang et al. 2014) and our present study. In addition, the death of plants was also sometimes reported due to severe Cd toxicity (Srivastava et al. 2014). However, along with direct Cd toxicity, other factors such as the lower abundances of amoA and 16S rRNA genes under AWD (Table S2) might also contribute to the decreasing plant growth and biomass indirectly. These decreased microbial abundances might decrease the recruitment of soil bacteria in the rhizosphere and colonization of endophytic bacteria inside the roots. A decrease in plant IAA might cause the decrease in surface areas of the roots to a greater extent under AWD than under CF. The role of endophytic IAA in increasing root surface areas to absorb nutrients from soil by over-production of root hairs and lateral roots has been reported (Davies 2010). Abundances of endophytic bacteria in the present study decreased, which further supported this proposition that Cd decreased the endophytic IAA decreasing uptake of nutrients. Most of the endospheric and rhizospheric microbial communities play their roles in nutrient acquisition by plants through increasing the availability of nutrients such as N, P and Fe in the rhizosphere (Marschner et al. 2011). The decreased copy number of endospheric and rhizospheric 16S rRNA along with a decreased abundance of amoA genes might be another possible reason along with direct Cd toxicity for the decreased N uptake of rice in our study.
Cd effect on microbial genes, nitrification, and N uptake
Ammonia oxidizing archaea (AOA) and bacteria (AOB) communities were changed in the rhizosphere of plant (Figs. 6 and 7). Similar results have also been reported in paddy soil under different water and different levels of Cd (Afzal et al. 2019). Crenarchaeota was the dominated species during AWD. Due to drop in rhizospheric pH, and rhizospheric effect increased Cd availability which led to the domination of Crenarchaeota in the AWD. Previous study has indicated a significantly positive correlation of Crenarchaeota with the environmental factors such as pH and heavy metals (Cu, Pb, and Zn) (Wang et al. 2018a). However, the Nitrosopumilus representatives were also dominated under CF, because of its dominancy in flooding and slightly alkaline pH condition (Nacke et al. 2017). In the present study, pH was slightly lower under AWD than CF condition. Nitrosopira and Nitrosovibrio tenuis which were responsible for nitrification process were more active with lower pH (Gieseke et al. 2006). Therefore, Nitrosopira and Nitrosovibrio tenuis were the dominated AOB in the rhizosphere under AWD.
Phylogenetic analysis of AOB community under continuous flooding (circles) and alternate wetting and drying (inverted triangles) conditions after 56 days. Note; empty rhombus: Cd0, black filled circles: Cd10 under continuous flooding, empty inverted triangles: Cd0, and black filed inverted triangles Cd10 under alternate wetting and drying
The concentration of Cd in soil was inversely proportional to nitrification in the rhizosphere of rice plants via the effect on the abundances of AOA and AOB which actively participated in nitrification (Fig. 2). Recent studies reported that Cd contamination decreased the taxonomic species and altered the composition of the microbial community in soil (Afzal et al. 2019; Feng et al. 2018). A similar effect of Cd on the abundance of amoA genes was previously reported (Liu et al. 2014; Wang et al. 2018b). The effect of Cd on AOA was greater under AWD as compared to CF (Table S2) with a direct effect on ammonium oxidation that led to low conversion of NH4+ to NO3− under AWD (Fig. 1C-D). In addition, we also observed high level of expression of plant nitrate transporter genes OsNTRs due to low level of NO3− in the soil. Cerezo et al. (2001) and Sorgonà et al. (2011) reported that OsNRT2.1 was briefly expressed in response to nitrate deficiency. Despite its low expression level, OsNRT2.2 expression seemed to follow the same regulation in response to nitrate availability (Noguero and Lacombe 2016). We assume that during treatments the bioavailability of other heavy metals such as As was not significantly changed (Table S4) and the toxicity was assigned to Cd in the rhizosphere for microbes.
The higher bioavailability of Cd under AWD than CF might result in the greater decrease in the abundance of amoA genes under Cd contamination. As a result, both the physiological and biochemical changes in plant and microbes led to decreases in the concentration of N in roots and shoots under AWD when the plants were grown at 10 mg Cd kg−1 (Table 1). In other studies, irrigation practices in paddy fields changed soil redox potential and microbial activities (Afzal et al. 2019). Aeration in soil occurred during drainage of water which in turn regenerated the electron acceptors like ferric ions and sulfate for the respiration of microorganisms (Ratering and Conrad 1998), which could explain the lower copy number of amoA genes under AWD as compared to CF.
Endophytes and rhizospheric bacteria are beneficial for normal plant growth along with the nitrifier community in the rhizosphere. In the present study, the addition of Cd decreased rhizospheric and endospheric bacterial abundances. The copy number of the 16S rRNA genes was lower under AWD condition as compared to CF condition (Fig. 3, Table S2). This low copy number of 16S rRNA in the rhizosphere and endosphere reflected the 27% higher amount of bioavailable Cd in the rhizosphere, and a sixfold increase in Cd concentration in plant roots under AWD than CF (Fig. 1A, Table 1).
About 25% lower concentration of IAA in the roots was observed at 10 mg Cd kg−1 under AWD than under CF (Fig. 4). Most of the endophytes possess the ability to produce IAA which has multiple functions during plant growth cycle from plant growth hormone to microbial invasion and subsiding the plant defense system during bacterial colonization inside the roots (Spaepen et al. 2007). The recruitments of rhizospheric microbes also depend upon the root exudation which increases when endophytes release IAA. The increased concentration of endophytic IAA favors cell-wall loosening and hence the growth of plant roots, which increases the total amounts of root exudates and rhizospheric bacteria (Chi et al. 2005). A recent study reported that the inhibition of IAA synthesis in maize roots was associated with 66% decrease in the colonization of endophytic bacteria while the combined application of endophytes and exogenous IAA increased the endophyte colonization in roots by up to 90% (Mehmood et al. 2018). Our present study showed that Cd contamination decreased the level of IAA in roots more under AWD than CF, which could be another reason for the decreased abundances of the endospheric and rhizospheric bacteria under AWD.
Our results demonstrated that water management practices for rice cultivation showed different effects on Cd bioavailability and nitrification in the rhizosphere along with the bacterial abundance in the rhizosphere and endosphere. The addition of Cd affected the plant growth more under AWD than under CF condition, which was associated with the higher Cd availability, the decreased abundances of endophytes, rhizospheric microbes, AOA and AOB, and root-IAA level, and with the decreased nutrient uptake and increased Cd uptake under AWD condition. The decreased uptake of N under AWD was mainly due to a high bioavailability of Cd which altered the ultra-structure of plant roots and leaves, causing poor plant growth (Fig. S1). The results imply that appropriate water management and rhizospheric engineering in Cd-contaminated paddy fields may be important approaches to decreasing plant Cd uptake and improve rice growth in Cd-contaminated paddy fields.
Abbreviations
- CF:
-
Continuous flooding
- AWD:
-
Alternate wetting and drying
- qPCR:
-
Quantitative PCR
- IAA:
-
Indole Acetic Acid
- ICP-MS:
-
Inductively coupled plasma mass spectrometry
- ELISA:
-
Enzyme-Linked Immunosorbent
- Cd:
-
Cadmium
- N:
-
Nitrogen
- ROS:
-
Reactive Oxygen Species
- CO2 :
-
Carbon Dioxide
- JA:
-
Jasmonic Acid
- ABA:
-
Abscisic Acid
- SA:
-
Salicylic acid
- P:
-
Phosphorus
- Fe:
-
Iron
- amoA :
-
Ammonia monooxygenase
- AOA:
-
Ammonia-Oxidizing Archaea
- AOB:
-
Ammonia-Oxidizing Bacteria
- PBS:
-
Phosphate-buffered saline
- SOC:
-
Soil organic C
- DON:
-
Soil organic N
- ANOVA:
-
Analysis of variance
- LSD:
-
Least significant difference
- OsNTRs :
-
Oryza sativa Nitrate transporters
References
Afzal M, Yu M, Tang C, Zhang L, Muhammad N, Zhao H, Feng J, Yu L, Xu J (2019) The negative impact of cadmium on nitrogen transformation processes in a paddy soil is greater under non-flooding than flooding conditions. Environ Int 129:451–460
Ai C, Liang G, Sun J, Wang X, He P, Zhou W (2013) Different roles of rhizosphere effect and long-term fertilization in the activity and community structure of ammonia oxidizers in a calcareous fluvo-aquic soil. Soil Biol Biochem 57:30–42
Arao T, Kawasaki A, Baba K, Mori S, Matsumoto S (2009) Effects of water management on cadmium and arsenic accumulation and dimethylarsinic acid concentrations in Japanese rice. Environ Sci Technol 43:9361–9367
Ata-Ul-Karim ST, Cang LY, Zhou, Wang D (2020) Interactions between nitrogen application and soil properties and their impacts on the transfer of cadmium from soil to wheat (Triticum aestivum L.) grain. Geoderma 357:113923
Aziz R, Rafiq MT, Li T, Liu D, He Z, Stoffella P, Sun K, Xiaoe Y (2015) Uptake of cadmium by rice grown on contaminated soils and its bioavailability/toxicity in human cell lines (Caco-2/HL-7702). J Agric Food Chem 63:3599–3608
Bücker-Neto L, Paiva ALS, Machado RD, Arenhart RA, Margis-Pinheiro M (2017) Interactions between plant hormones and heavy metals responses. Genet Mol Biol 40:373–386
Cerezo M, Tillard P, Filleur S, Munos S, Daniel-Vedele F, Gojon A (2001) Major alterations of the regulation of root NO3- uptake are associated with the mutation of Nrt2.1 and Nrt2.2 genes in Arabidopsis. Plant Physiol 127:262–271
Cheng H, Wang M, Wong M, Ye HZ (2014) Does radial oxygen loss and iron plaque formation on roots alter Cd and Pb uptake and distribution in rice plant tissues? Plant Soil 375:137–148
Chi F, Shen SH, Cheng HP, Jing YX, Yanni YG, Dazzo FB (2005) Ascending migration of endophytic rhizobia, from roots to leaves, inside rice plants and assessment of benefits to rice growth physiology. Appl Environ Microbiol 71:7271–7278
Davies PJ (2010) The plant hormones: their nature, occurrence, and functions. In: Davies PJ (ed) Plant Hormones. Springer, Dordrecht, pp 1–15
de Livera J, McLaughlin MJ, Hettiarachchi GM, Kirby JK, Beak DG (2011) Cadmium solubility in paddy soils: effects of soil oxidation, metal sulfides and competitive ions. Sci Total Environ 409:1489–1497
De SS, Cuypers A, Vangronsveld J, Remans T (2015) Gene networks involved in hormonal control of root development in Arabidopsis thaliana: a framework for studying its disturbance by metal stress. Int J Mol Sci 16:19195–19224
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2009) Nitrification driven by bacteria and not archaea in nitrogen-rich grassland soils. Nat Geosci 2:621–624
Edwards J, Johnson C, Santos-Medellín C, Lurie E, Podishetty NK, Bhatnagar S, Eisen JA, Sundaresan V (2015) Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Natl Acad Sci USA 112:E911–E920
Feng G, Xie T, Wang X, Bai J, Tang L, Zhao H, Wei W, Wang M, Zhao Y (2018) Metagenomic analysis of microbial community and function involved in Cd-contaminated soil. BMC Microbiol 18:11
Gallego SM, Pena LB, Barcia RA, Azpilicueta CE, Iannone MF, Rosales EP, Zawoznik MS, Groppa MD, Benavides MP (2012) Unravelling cadmium toxicity and tolerance in plants: insight into regulatory mechanisms. Environ Exp Bot 83:33–46
Fattorini L, Ronzan M, Piacentini D, Rovere FD, Virgilio CD, Sofo A, Altamura MM, Falasca G (2017) Cadmium and arsenic affect quiescent centre formation and maintenance in Arabidopsis thaliana post-embryonic roots disrupting auxin biosynthesis and transport. Environ Exp Bot 144:37–48
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evol 39:783–791
Fulda B, Voegelin A, Kretzschmar R (2013) Redox-controlled changes in cadmium solubility and solid-phase speciation in a paddy soil as affected by reducible sulfate and copper. Environ Sci Technol 47:12775–12783
Gieseke A, Tarre S, Green M, de Beer D (2006) Nitrification in a biofilm at low pH values: role of in situ microenvironments and acid tolerance. Appl Environ Microbiol 72:4283–4292
Guo H, Hong C, Chen X, Xu Y, Liu Y, Jiang D, Zheng B (2016) Different growth and physiological responses to cadmium of the three Miscanthus species. PLoS ONE 11(1):16
Hernandez-Soriano MC, Jimenez-Lopez JC (2012) Effects of soil water content and organic matter addition on the speciation and bioavailability of heavy metals. Sci Total Environ 423:55–61
Honma T, Ohba H, Kaneko-Kadokura A, Makino T, Nakamura K, Katou H (2016) Optimal soil Eh, pH, and water management for simultaneously minimizing arsenic and cadmium concentrations in rice grains. Environ Sci Technol 50:4178–4185
Hou D, Wang R, Gao X, Wang K, Lin Z, Ge J, Liu T, Wei S, Chen W, Xie R (2018) Cultivar-specific response of bacterial community to cadmium contamination in the rhizosphere of rice (Oryza sativa L.). Environ Pollut 241:63–73
Hu YF, Zhou G, Na XF, Yang L, Nan WB, Liu X, Zhang YQ, Li JL, Bia YR (2013) Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings. J Plant Physiol 170:965–975
Huang L, Li WC, Tam NFY, Ye Z (2019) Effects of root morphology and anatomy on cadmium uptake and translocation in rice (Oryza sativa L.) J Environ Sci. 75:296–306
Kalembasa SJ, Jenkinson DS (1973) Comparative study of titrimetric and gravimetric methods for determination of organic carbon in soil. J Sci Food Agric 24:1085–1090
Khan AL, Waqas M, Hussain J, Al-Harrasi A, Lee IJ (2014) Fungal endophyte Penicillium janthinellum LK5 can reduce cadmium toxicity in Solanum lycopersicum (Sitiens and Rhe). Biol Fertil Soils 50:75–85
Kim YH, Khan AL, Kim DH, Lee SY, Kim KM, Waqas M, Jung HY, Shin JH, Kim JG, Lee IJ (2014) Silicon mitigates heavy metal stress by regulating P-type heavy metal ATPases, Oryza sativa low silicon genes, and endogenous phytohormones. BMC Plant Biol 14:13
Kinniburgh DG, van Riemsdijk WH, Koopal LK, Borkovec M, Benedetti MF, Avena MJ (1999) Ion binding to natural organic matter: competition, heterogeneity, stoichiometry and thermodynamic consistency. Colloids Surf A Physicochem Eng Asp 151:147–166
Kosolsaksakul P, Farmer JG, Oliver IW, Graham MC (2014) Geochemical associations and availability of cadmium (Cd) in a paddy field system, northwestern Thailand. Environ Pollut 187:153–161
Lebeis SL, Paredes SH, Lundberg DS, Breakfield N, Gehring J, McDonald M, Malfatti S, Glavina del Rio T, Jones CD, Tringe SG (2015) Plant Microbiome. Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science 349:860–864
Lee K, Bae DW, Kim SH, Han HJ, Liu X, Park HC, Lim CO, Lee SY, Chung WS (2010) Comparative proteomic analysis of the short-term responses of rice roots and leaves to cadmium. J Plant Physiol 167:161–168
Lee HJ, Abdula SE, Jang DW, Park SH, Yoon UH, Jung YJ, Kang KK, Nou IS, Cho YG (2013) Over expression of the glutamine synthetase gene modulates oxidative stress response in rice after exposure to cadmium stress. Plant Cell Rep 32:1521–1529
Li J, Wu F, Fang Q, Wu Z, Duan Q, Li X, Ye W (2020) The mutual effects of graphene oxide nanosheets and cadmium on the growth, cadmium uptake and accumulation in rice. Plant Physiol Biochem 147:289–294
Liu J, Li K, Xu J, Liang J, Lu X, Yang J, Zhu Q (2003) Interaction of Cd and five mineral nutrients for uptake and accumulation in different rice cultivars and genotypes. Field Crops Res 83:271–281
Liu Y, Liu Y, Ding Y, Zheng J, Zhou T, Pan G, Crowley D, Li L, Zheng J, Zhang X (2014) Abundance, composition and activity of ammonia oxidizer and denitrifier communities in metal polluted rice paddies from South China. PLoS ONE 9:e102000
Liu XG, Tian D, Jiang C, Zhang L, Kong (2016) Cadmium (Cd) distribution and contamination in Chinese paddy soils on national scale. Environ Sci Pollut Res 23:17941–17952
Liu Y, Shen K, Wu Y, Wang G (2018) Abundance and structure composition of nirK and nosZ genes as well as denitrifying activity in heavy metal-polluted paddy soils. Geomicrobiol J 35:100–107
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods 25:402–408
Marschner P, Crowley D, Rengel Z (2011) Rhizosphere interactions between microorganisms and plants govern iron and phosphorus acquisition along the root axis–model and research methods. Soil Biol Biochem 43:883–894
Mehmood A, Hussain A, Irshad M, Khan N, Hamayun M, Ismail ASG, Lee IJ (2018) IAA and flavonoids modulates the association between maize roots and phyto stimulant endophytic Aspergillus famigatus greenish. J Plant Interact 13:532–542
Meng D, Li J, Liu T, Liu Y, Yan M, Hu J, Li X, Liu X, Liang Y, Liu H, Yin H (2018) Effects of redox potential on soil cadmium solubility: Insight into microbial community. J Environ Sci 75:224–232
Mlangeni AT, Perez M, Raab A, Krupp EM, Norton GJ, Feldmann J (2020) Simultaneous stimulation of arsenic methylation and inhibition of cadmium bioaccumulation in rice grain using zero valent iron and alternate wetting and drying water management. Sci Total Environ 711:134696
Molina AS, Lugo MA, Pérez CMV, Vargas GS, Zirulnik F, Leporati J, Ferrol N, Azcón-Aguilar C (2020) Effect of arbuscular mycorrhizal colonization on cadmium-mediated oxidative stress in Glycine max (L.). Merr Plants 9:108
Nakurte I, Keisa A, Rostoks N (2012) Development and validation of a reversed-phase liquid chromatography method for the simultaneous determination of indole-3-acetic acid, indole-3-pyruvic acid, and abscisic acid in barley (Hordeum vulgare L.). J Anal Methods Chem 2012:1–6
Nacke H, Schöning I, Schindler M, Schrumpf M, Daniel R, Nicol GW, Prosser JI (2017) Links between seawater flooding, soil ammonia oxidiser communities and their response to changes in salinity. FEMS Microbiol Ecol 93(11). https://doi.org/10.1093/femsec/fix144
Noguero M, Lacombe B (2016) Transporters involved in root nitrate uptake and sensing by arabidopsis. Front Plant Sci 7:1391
Norton GJ, Shafaei M, Travis AJ, Deacon CM, Danku J, Pond D, Cochrane N, Lockhart K, Salt D, Zhang H (2017) Impact of alternate wetting and drying on rice physiology, grain production, and grain quality. Field Crops Res 205:1–13
Ratering S, Conrad R (1998) Effects of short-term drainage and aeration on the production of methane in submerged rice soil. Global Change Biol 4:397–407
Rizwan M, Ali S, Adrees M, Rizvi H, Zia-ur-Rehman M, Hannan F, Qayyum MF, Hafeez F, Ok YS (2016) Cadmium stress in rice: toxic effects, tolerance mechanisms, and management: a critical review. Environ Sci Pollut Re 23:17859–17879
Ronzan M, Piacentini D, Fattorini L, Della RF, Eiche E, Riemann M, Altamura M, Falasca G (2018) Cadmium and arsenic affect root development in Oryza sativa L. negatively interacting with auxin. Environ Exp Bot 151:64–75
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Smolders E, Brans K, Coppens F, Merckx R (2001) Potential nitrification rate as a tool for screening toxicity in metal-contaminated soils. Environ Toxicol Chem 20:2469–2474
Song W, Chen S, Liu J, Li C, Song N, Ning L, Bin L (2015) Variation of Cd concentration in various rice cultivars and derivation of cadmium toxicity thresholds for paddy soil by species-sensitivity distribution. J Integr Agri 14:1845–1854
Sorgonà A, Lupini A, Mercati F, Di Dio L, Sunseri F, Abenavoli MR (2011) Nitrate uptake along the maize primary root: an integrated physiological and molecular approach. Plant Cell Environ 34(7):1127–1140
Spaepen S, Vanderleyden J (2011) Auxin and plant-microbial interactins. Cold Spring Harb Perspect Biol 3:1–11
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Spoel SH, Dong X (2008) Making sense of hormone crosstalk during plant immune responses. Cell Host Microbe 3:348–351
Srivastava RK, Pandey P, Rajpoot R, Rani A, Dubey R (2014) Cadmium and lead interactive effects on oxidative stress and antioxidative responses in rice seedlings. Protoplasma 251:1047–1065
Tamura K, Nei M, Kumar S (2004) Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101:11030–11035
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Uraguchi S, Fujiwara T (2013) Rice breaks ground for cadmium-free cereals. Curr Opin Plant Biol 16:328–334
Vink JP, Harmsen J, Rijnaarts H (2010) Delayed immobilization of heavy metals in soils and sediments under reducing and anaerobic conditions; consequences for flooding and storage. J Soils Sediment 10:1633–1645
Wang Y, Jiang X, Li K, Wu M, Zhang R, Zhang L, Chen G (2014) Photosynthetic responses of Oryza sativa L. seedlings to cadmium stress: physiological, biochemical and ultrastructural analyses. Biometals 27:389–401
Wang H, Zeng Y, Guo C, Bao Y, Lu G, Reinfelder JR, Dang Z (2018a) Bacterial, archaeal, and fungal community responses to acid mine drainage-laden pollution in a rice paddy soil ecosystem. Sci Total Environ 616–617:107–116
Wang J, Wang L, Zhu L, Wang J (2018b) Individual and combined effects of enrofloxacin and cadmium on soil microbial biomass and the ammonia-oxidizing functional gene. Sci Total Environ 624:900–907
Wang J, Wang PM, Gu Y, Kopittke PM, Zhao FJ, Wang P (2019) Iron–manganese (Oxyhydro) oxides, rather than oxidation of sulfides, determine mobilization of Cd during soil drainage in paddy soil systems. Environ Sci Technol 53:2500–2508
Wang HQ, Xuan W, Huang XY, Mao C, Zhao FJ (2021) Cadmium inhibits lateral root emergence in rice by disrupting OsPIN-mediated auxin distribution and the protective effect of OsHMA3. Plant Cell Physiol 62:166–177
Yang J, Zhou Q, Zhang J (2017) Moderate wetting and drying increases rice yield and reduces water use, grain arsenic level, and methane emission. Crop J 5:151–158
Yang Y, Chen YX, Tian GM, Zhang ZJ (2007) Dynamics of microbial activity related to N cycling in Cd-contaminated soil during growth of soybean. Pedosphere 17:383–388
Zhang CC, Yuan WY, Zhang QF (2012) RPL1, a gene involved in epigenetic processes regulates phenotypic plasticity in rice. Mol Plant 5:482–493
Zhou Z, Chen Z, Pan H, Sun B, Zeng D, He L, Yang R, Zhou G (2018) Cadmium contamination in soils and crops in four mining areas, China. J Geochem Explor 192:72–84
Acknowledgements
This work was supported by the National Natural Science Foundation of China (41991334, 41721001), the 111 Project (B17039), and Agriculture Research System of China (CARS-01-31).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Additional information
Responsible Editor: Fangjie Zhao.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1977 KB)
Rights and permissions
About this article
Cite this article
Afzal, M., Tang, C., Yu, M. et al. Water regime is important to determine cadmium toxicity on rice growth and rhizospheric nitrifier communities in contaminated paddy soils. Plant Soil 472, 609–628 (2022). https://doi.org/10.1007/s11104-021-05272-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-05272-6