Abstract
Aims
The relationships between nitrogen (N) and phosphorus (P) acquisition strategies among herbaceous legume species remain poorly understood, particularly in relation to how they are altered by N availability. This study aimed to investigate the relationships between N2 fixation, plant N concentration and P acquisition through two main strategies in temperate herbaceous legumes, and to demonstrate the influences of soil N availability on these relationships.
Methods
In a field pot experiment, eight temperate herbaceous legumes were grown with or without N addition. Plant growth, plant N and P concentrations, N2 fixation, arbuscular mycorrhizal (AM) fungal colonization and root phosphatase activity (RPA) were measured, and the relationships between N2 fixation, plant N concentration and each P acquisition strategy were assessed under contrasting N availability.
Results
N addition increased RPA. However, AM fungal colonization showed species-specific responses to N addition, and the ratio of AM fungal colonization to root phosphatase activity was decreased by N addition. Among eight legume species, AM fungal colonization increased with N2 fixation rate in the absence of N addition, but no relationship was observed with N addition. RPA increased with plant N concentration among legume species, regardless of N addition.
Conclusions
As two key P acquisition strategies, neither RPA nor AM fungal colonization of temperate herbaceous legumes were species-specific traits, since both were positively correlated with N2 fixation rate and plant N concentration. In addition, the correlation between N2 fixation and AM fungal colonization was regulated by N availability. While N enrichment intensifies the legume reliance on RPA for acquiring more P, it weakens the association of N2 fixation in driving across species AM colonization.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Phosphorus (P) is a key nutrient limiting plant distribution, growth and function (Augusto et al. 2013; Turner et al. 2018). P limitation can be considerable in ecosystems with low P availability, such as tropical rain forests where soils are highly weathered (Hedin et al. 2009; Nasto et al. 2014) and on ancient soil landscapes (Denton et al. 2007). Therefore, much effort has been made to understand feedbacks between growth, nutrient acquisition, and competition for P in these systems. Greater focus has been made in understanding feedbacks between growth, nutrient acquisition and competition of plants to P availability in these ecosystems. However, P limitations are likely to become increasingly widespread in terrestrial ecosystems (Vitousek et al. 2010; Zhang et al. 2014), due to long term soil management and land use history (Lambers et al. 2008; Png et al. 2017), and disproportionately greater anthropogenic nitrogen (N) inputs than P (Stevens et al. 2004; Vitousek et al. 2010).
Plant species have evolved strategies to overcome P limitations to growth, including modification of root morphology to increase root surface area for P absorption (Lambers et al. 2008; Wen et al. 2019), exudation of P-mobilizing compounds to activate mineral bound P in the rhizosphere (Pang et al. 2018; Wen et al. 2019), intensification of arbuscular mycorrhizal (AM)-plant interactions to extend root surface area for inorganic P acquisition (Nasto et al. 2017), and activation of root phosphatase to hydrolyze organic P (Png et al. 2017; Guilbeault-Mayers et al. 2020). Among the above mechanisms, the latter two mechanisms in particular may be influenced by plant N concentration (Nasto et al. 2014, 2019), since phosphatase enzyme production is N-expensive (Houlton et al. 2008), and AM symbiosis requires photosynthetically fixed carbon (C) investment that can be stimulated by greater plant N availability (Smith and Read 2008; Liang et al. 2020).
N-fixing legumes can assimilate atmospheric N as an additional N resource, and generally have 43 to 100% greater leaf N concentrations than non N-fixing plants (Li et al. 2015; Adams et al. 2016). Thus, N-fixing legumes are speculated to have some natural advantages related to P acquisition compared with non N-fixing plants, as confirmed through observation (Nasto et al. 2014; Png et al. 2017; Guilbeault-Mayers et al. 2020). An additional question is whether P acquisition strategies increase with N2 fixation capacity and/or plant N concentration among legume species (Nasto et al. 2014, 2017; Png et al. 2017; Batterman et al. 2018; Soper et al. 2019). Some studies confirmed that root phosphatase activity and AM fungal colonization of woody legumes tended to be somewhat phylogenetically conserved (Png et al. 2017), rather than directly linked to their N-fixation capacity and plant N concentration (Wurzburger and Hedin 2015; Batterman et al. 2018; Soper et al. 2019). Furthermore, root phosphatase activity of an N-fixing legume inhabiting tropical rainforest decreased slightly in response to increased plant N2 fixation in a glasshouse study (Batterman et al. 2013). In contrast, AM fungal colonization or root phosphatase activity can increase as a function of increased N2 fixation capacity for tropical woody legumes (Nasto et al. 2014, 2017). Thus, there still is great uncertainty about the feedback between P acquisition strategies and N nutrition of legumes. More research, in particular on herbaceous legumes, is necessary for a comprehensive understanding of the interactions between N and P acquisition in legumes (Olde Venterink 2011). Given the significant effect of plant N nutrition in driving P acquisition strategies of legume species, the interaction between N fixation or concentration and P acquisition strategies also is probably complex. First, there may not be a physiological relationship between N2 fixation capacity and plant N concentration (McKey 1994; Soper et al. 2019). Similarly, AM fungal colonization and root phosphatase activity may differ in their reliance on N2 fixation or plant N concentration, as root phosphatase activity may rely more on plant N concentration (Houlton et al. 2008), while AM fungal colonization may be more tightly correlated to root nodulation and N2 fixation (Oldroyd 2013). In addition, legume species may have a trade-off between AM fungal colonization and root phosphatase activity (Nasto et al. 2017; Soper et al. 2019). As a consequence, N concentration or N2 fixation may regulate two P acquisition strategies in inverse directions. Thus, further research should emphasize the distinction between specific P acquisition strategies and N2 fixation or plant N concentration.
Furthermore, N concentration and N2 fixation, as well as P acquisition strategies of legumes may be changed by soil N availability more broadly in global terrestrial ecosystems (Vitousek et al. 1997; Stevens et al. 2004). With increasing soil N availability, the N concentration of legumes can generally be enhanced (Wolf et al. 2017), but their N2 fixation capacity can be largely inhibited by N enrichment (West et al. 2005; Barron et al. 2011; Zheng et al. 2019). Correspondingly, root phosphatase activity of legumes should be upregulated by increased soil N availability, as occurs in other terrestrial plants (Treseder and Vitousek 2001; Olde Venterink 2011; Marklein and Houlton 2012), while AM fungal colonization of roots may be downregulated under N enrichment. Additionally, following N enrichment, plant growth has less N limitation, which may greatly weaken the inter-specific differences in N2 fixation and plant N concentration in legumes. As a consequence, the power of N2 fixation and plant N concentration to drive the inter-specific variation in AM fungal colonization and root phosphatase activity may be reduced under N enrichment. To date, little research is available to directly expose the effects of N enrichment on these P acquisition strategies (Kafle et al. 2019), and in particular the relationships between N fixation or concentration and these phosphorus acquisition strategies for legumes.
Accordingly, we grew eight temperate herbaceous legumes under contrasting N availability in a field pot experiment, with an aim to investigate the relationships between N fixation or concentration and P acquisition strategies, and the relationships between soil N availability and P acquisition strategies. We hypothesized that a) AM fungal colonization and root phosphatase activity would increase as a function of N2 fixation and/or plant N concentration under low N availability; and b) that N enrichment would increase the root phosphatase activity, but decrease the AM fungal colonization of roots, and the positive correlation between N2 fixation and/or plant N concentration and P acquisition strategies would be absent under N enrichment.
Materials and methods
Study site
An outdoor pot experiment was conducted at the Songnen Grassland Research Station of Chinese Academy of Sciences (E123°31′, N44°33′; Elevation 145 m above sea level), in northeastern China. The mean annual temperature is 5.5 °C, and annual precipitation is 404 mm with more than 80% of rainfall occurring from May to September (2000–2016). The soil type is an alkaline meadow chernozem soil. Leymus chinensis, a perennial C3 rhizomatous grass is the dominant plant species at this site.
Pot experiment design
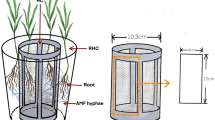
Eight species of herbaceous legumes that co-occur in Songnen grasslands, including four native species (Medicago ruthenica (L.) Sojak, Lespedeza daurica (Laxm.) Schindler, Melilotus officinalis (L.) Desr. and Glycine soja Sieb. et Zucc), and four cultivated species (Medicago sativa L. cv. Aohan, Medicago falcata L. cv. Hulunbeier, Medicago varia Martin. cv. Caoyuan No.3 and Lotus corniculatus L.) in addition to L. chinensis (as a reference plant for N fixation assessment) were individually grown in leak-free plastic pots (30 cm depth, 25 cm diameter) from May 2018 to August 2018. The cultivated soil in all pots was collected from a natural meadow at the 0 to 20 cm depth and passed through a 2 mm sieve, then evenly mixed. Five pre-experimental soil samples were collected for analyzing soil pH in a 1:5 soil-water solution using a PHS-25 pH meter (Leizi, Shanghai, China), soil organic matter concentration using the K2Cr2O7 method (Page 1982), total N concentration using the Kjeldahl method (Sparks et al. 1996) and total P concentration using persulfate oxidation followed by colorimetric analysis (Schade et al. 2003). Soil had a pH of 8.59 ± 0.05, 18.82 ± 0.22 mg g−1 organic matter, total N of 1.31 ± 0.03 mg g−1 and total P of 0.34 ± 0.02 mg g−1. An equal amount of soil was added into each pot with a bulk density of 1.21 g cm−3.
All seeds were collected in 2017 from a legume seed production field or, for L. chinensis, a natural meadow, at the Songnen Grassland Research Station. Before sowing, all seeds were surface-sterilized. Seeds were first sown in sand in a shallow seedling tray. Following germination, four seedlings were transferred to an individual pot for each species. Before transplanting, thirty additional seedlings were collected and measured to determine the averaged dry weight. Plants in each pot were inoculated using crushed nodules that were collected from the same leguminous seed production field.
Employing a complete randomized block design, the experiment had two levels of N addition (0 and 14 g N m−2 as ammonium nitrate), with four replicates per species × N combination. The N addition rate corresponded to the level assumed to be sufficient to overcome plant N limitation in this grassland region (Zhan et al. 2017). In this experiment, plants were grown for 83 d on average, N fertilizer was applied four times at 0, 20, 40 and 60 d since planting with 3.5 g N m−2 each time, by dissolving the fertilizers into water. N additions significantly increased N concentrations and available N:P ratios in bulk soil, but not available P concentration during the sampling period (Table S1). During the experimental period, pots were protected from rainfall using plastic film that was applied prior to rain. Each pot was watered with 1 L of water if soil moisture declined by 15% (approaching 60% of soil field capacity) following an in-situ soil moisture examination at an interval of 3 to 4 days, which ensured that all pots were well-watered. Pot placements were re-randomized weekly in each replicated block.
Measurement and sampling
Each legume species was harvested once it approached first flower. Before collecting plant samples, leaf photosynthetic rate was determined on 2–3 mature and intact leaves per plant for each pot, using a CIRAS-2 portable photosynthetic measuring system (PP-Systems, Hitchin, UK). The intact soil core from each pot, together with plants, was carefully separated from the pot. The four individual plants were carefully separated from each other and their roots were lightly washed under tap water. Root tissue from one random individual was retained for analysis of root arbuscular mycorrhizal colonization and root phosphomonoesterase (hereafter, phosphatase) activity for each pot. In each pot, the remaining three legume individuals were separated into shoots, roots and nodules, respectively, subsequently dried at 60 °C and weighed for each plant tissue in each legume individual. Then, all tissues from one random individual in each pot were combined and finely ground for analysis of tissue 15N content. The shoots and roots in the remaining two legume individuals were independently combined and ground in each pot, and analyzed for N and P concentrations. In each pot, the mean of all measurements for each variable was calculated as a replicate for further data analysis. In total, four replicates were introduced into data analysis for each variable under each species × N combination.
Plant analysis
To measure mycorrhizal colonization, a fraction of fine roots (<2 mm diameter) was cut into eight 1-cm long segments, cleaned with 10% KOH solution for 72 h at room temperature, followed by 2 h at 90 °C, then rinsed with DI water and acidified in 3% HCl solution for 12 h, then stained with 0.05% trypan blue and destained in water for 12 h. Eight stained root segments were mounted on slides. The arbuscular mycorrhizal (AM) colonization rate of root were quantified according to the following formula described by Wu et al. (2015):
Root phosphatase activity (RPA) was measured using a 4-methylumbelliferone (MUB)-linked substrate method (Nasto et al. 2014, 2017). To do this, root subsamples were stored in 50 mM calcium sulphate (CaSO4) solution at 4 °C after harvest. Within 1 week of sampling, 20 to 30 mg of fine roots (<2 mm diameter) were immersed in 1 mL of 50 mM sodium acetate buffer (SAB; pH 5), 1 mL of 50 mM SAB (800 μL)/100 mM MUB solution (200 μL) and 1 mL of 50 mM SAB (800 μL)/100 mM MUB (200 μL)/200 mM 4-MUB PO43− (200 μL) solution in clear 12-well plates. The plates were shaken for 1 h (110 rpm) at room temperature and 200 μL subsamples from all wells were pipetted into a black 96-well microplate. Each sample included four analytical replicates and negative controls for sample and substrate fluorescence. Microplates were read at 365 nm excitation and 450 nm emission and enzyme activities were calculated as l mol 4-MUB-P g−1 root h−1.
The 15N natural abundance were analyzed for whole plant samples of L. chinensis and legumes using a MAT253 stable isotope mass spectrometer (ThermoFisher Scientific, Waltham, USA). For shoot and root samples, total nitrogen (TN) concentration was determined using the Kjeldahl method (Sparks et al., 1996), and the total phosphorus (TP) concentration was analyzed using persulfate oxidation followed by colorimetric analysis (Schade et al. 2003).
Data calculation and statistics
Relative growth rate (RGR) was calculated according to:
where Mf is the final plant dry weight (shoot + root) when harvested, Mi is the initial seedling dry weight (shoot + root) when planted, and dt is the length of the growth in days.
The percent of N derived from the atmosphere (%Ndfa) in legume tissue was estimated using the following formula (Unkovich et al. 2008; Li et al. 2016):
Where δ15N is the atom percent excess 15N relative to atmospheric N. The term ‘legume’ represents the whole legume plant including shoot, root and nodule tissues, the term ‘reference plant’ represents whole L. chinensis individual grown under different fertilizer conditions, the value of δ15N atmosphere is 0‰ (Unkovich et al. 2008).
Prior to statistical analyses, all data were tested for normality using the Shapiro-Wilk test and homoscedasticity using the Levene test. General linear models (GLM) were applied to determine the main and interaction effects of N addition and species on all measured variables, with block as a random factor. However, no significant block effects nor interactions between block and other factors were found. The least significant difference (LSD) test was performed to compare the means of variables among species, following one-way ANOVA. Paired-samples T tests were used to analyze the variable difference between control and N addition treatment. Regression analysis was used to examine the relationships between biological N fixation rate, leaf photosynthetic rate, plant N concentration and P acquisition strategies (e.g., AM fungal colonization of root, RPA, AM fungal colonization: root phosphatase activity ratio). Significance for all statistical tests was evaluated at P = 0.05. All data were analyzed using SPSS17.0 software (Chicago, IL, USA).
Results
Plant growth and plant nutrients
The relative growth rate (RGR; F = 418.44, P < 0.001), root biomass (F = 189.10, P < 0.001) and leaf photosynthetic rate (F = 9.18, P < 0.001) of legumes all showed significant inter-specific variation. N addition increased the RGR (F = 73.33, P < 0.001) and leaf photosynthetic rate (F = 237.37, P < 0.001), but decreased the root biomass of legume species (F = 6.94, P = 0.01; Table 1). Regardless of N addition, plant N (F = 16.64, P < 0.001) and P (F = 19.67, P < 0.001) concentrations showed significant inter-specific variation. Averaged across eight legume species, N addition significantly increased the plant N concentration by 18.4% (F = 275.33, P < 0.001), but had negligible effects on plant P concentration (F = 2.21, P = 0.14; Table 1).
Root nodulation and biological N fixation rate
Legume species showed significant inter-specific differences in root nodule dry weight (F = 176.30, P < 0.001) and biological N2 fixation rate (%Ndfa; F = 29.18, P < 0.001). N addition decreased root nodule dry weight (F = 528.60, P < 0.001) and %Ndfa (F = 436.52, P < 0.001), and reduced the inter-specific variation of root nodule dry weight (F = 18.91, P < 0.001) and %Ndfa (F = 9.00, P < 0.001; Table 2). Without N addition, there was a significant positive correlation between %Ndfa and leaf photosynthetic rate across eight legume species (R2 = 0.92, P < 0.001; Fig. 1a). Regardless of N addition, the %Ndfa had no significant relationship with plant N concentrations across eight legume species (P > 0.05; Fig. 1b).
Correlation relationships between leaf photosynthetic rate (a), plant N concentration (b) and biological N2 fixation rate (%Ndfa) across eight herbaceous legume species under control and N addition. Control, no nitrogen addition; values are means±standard errors (n = 4); the regression line represents significant correlation relationship (P ≤ 0.05)
P acquisition strategies
There was a significant interaction between N addition and species on arbuscular mycorrhizal (AM) colonization of legume roots (F = 6.74, P < 0.001), but the main effect of N addition was insignificant (F = 1.90, P = 0.17; Table 3). Root phosphatase activity (RPA) showed significant inter-specific difference (F = 37.18, P < 0.001), and N addition increased RPA (F = 965.71, P < 0.001; Table 3). Legume species were significantly different in AM fungal colonization and RPA (F = 16.85, P < 0.001), and N addition decreased the value and inter-specific variation of AM fungal colonization: RPA (F = 181.94, P < 0.001; Table 3). Among legume species, AM fungal colonization and RPA showed no significant correlation without N addition (R2 = 0.12, P = 0.39), but significant positive correlations when N was added (R2 = 0.68, P = 0.01; Fig. 2).
Correlation relationships between arbuscular mycorrhizal fungal (AMF) colonization and root phosphatase activity (PRA) across eight herbaceous legume species under control and N addition. Control, no nitrogen addition; values are means±standard errors (n = 4); the regression line represents significant correlation relationship (P ≤ 0.05)
Among eight legume species, AM fungal colonization (R2 = 0.50, P = 0.045) and the ratio of AM fungal colonization to root phosphatase activity (R2 = 0.53, P = 0.04) increased with %Ndfa without N addition, but these significant correlations were absent if N was added (P > 0.05; Fig. 3a, e). RPA of legumes did not change as a function of %Ndfa, regardless of N addition (P > 0.05; Fig. 3c). Plant N concentration was not correlated with AM fungal colonization across legume species under contrasting N addition (P > 0.05; Fig. 3b). Regardless of N addition, RPA increased with plant N concentration among eight legume species (P < 0.01; Fig. 3d). The ratio of AM fungal colonization to root phosphatase activity declined with plant N concentration under N addition (R2 = 0.72, P = 0.008; Fig. 3f).
Correlation relationships between biological N2 fixation rate (%Ndfa), plant N concentration and arbuscular mycorrhizal fungal (AMF) colonization (a, b), root phosphatase activity (RPA; c, d), ratio of arbuscular mycorrhizal fungal (AMF) colonization to root phosphatase activity (RPA) (e, f) across eight legume species under control and N addition. Control, no nitrogen addition; values are means±standard errors (n = 4); the regression line represents significant correlation relationship (P ≤ 0.05)
Discussion
The effects of N addition on root phosphatase activity and AM fungal colonization of temperate herbaceous legumes
In this study, root phosphatase activity was, on average, upregulated by 38% under N addition, to a similar extent as identified in previous research (Treseder and Vitousek 2001; Olde Venterink 2011; Marklein and Houlton 2012). As a further factor regulating total root phosphatase activity per plant, root biomass of legume plants decreased by 4%. Thus, the upregulated root phosphatase activity has primarily driven an increase in total amount of root phosphatase activity per plant. Greater root phosphatase activity may reflect a greater P demand of legume plants under N enrichment (Elser et al. 2007; Sulieman and Tran 2015). N addition approximately doubled the N availability in soil (Table S1), and thus enhanced the growth of legumes. As a consequence, the P demand and uptake were enhanced. However, N addition failed to improve the external P availability for legumes, which intensified P acquisition in legume roots to balance P demand. Increasing biomass allocation to roots is considered a physical strategy to improve P acquisition (Soper et al. 2019; Wen et al. 2019), but was not supported by our results where the root biomass fraction declined marginally with N addition. Therefore, a physiological strategy, such as an increase in root phosphatase activity, may become increasingly important to enhance root P acquisition. In addition, regardless of P demand, the excretion of root phosphatase enzymes may have directly been stimulated by an increase in N supply (Olander and Vitousek 2000; Houlton et al. 2008; Marklein and Houlton 2012).
We observed that N addition had no significant effects on AM fungal colonization in most of the eight legume species, but enhanced the AM fungal colonization of L. daurica, and reduced the AM fungal colonization of G. soja. This result was not completely in line with our hypothesis, but can be supported by wider experimental evidence suggesting that N addition could have neutral (Yang et al. 2018), positive (Porras-Alfaro et al. 2007; Zheng et al. 2014), or negative effects on AM fungal colonization of roots (Treseder 2004; Lu et al. 2020). In this study, all plants were grown in the same soil medium, suggesting that species specific responses likely drive those different responses of L. daurica, G. soja and other legumes in regard to N addition, in terms of AM fungal colonization. On the one hand, legume species can differ in N/P demands and acquisition capacity with N supply, consequently altering their reliance on symbiosis with AM fungi, since AM fungi contribute to nutrient uptake by plants (Oldroyd 2013; Kalfe et al. 2019). On the other hand, AM fungal colonization can be host specific (Montesinos-Navarro et al. 2012), which could lead to the species-specific responses in AM fungal colonization to N supply (Lu et al. 2020). Finally, N-fixing legumes may balance carbon allocation to photosynthetic organs, nodulated roots or AM root systems differently according to N availability (Johnson et al. 2003; Kalfe et al. 2019), and the species-specific responses to AM fungal colonization may reflect this balance.
N addition decreased the ratio of AM fungal colonization to root phosphatase activity in legumes, which reflects a legume trade-off between resource cost and nutrient acquisition benefit in response to varying resource availability (Nasto et al. 2014). For example, it is estimated that mineral nutrient uptake, including P via AM fungal colonization had relatively higher C vs N costs than the production of phosphatase enzymes (Treseder and Vitousek 2001; Nasto et al. 2014). With N addition, as the N limitation for plant growth is relieved, legumes can invest N resources in a lower C:N ratio strategy to acquire P (e.g. production of phosphatase enzymes), rather than the higher C:N ratio strategy of AM fungal colonization and N2 fixation (West et al. 2005; Nasto et al. 2014). It is well known that AM fungal colonization and root phosphatase activity increase plant access to different soil P forms (inorganic vs. organic; Nasto et al. 2017), thus the N-driven increase in root phosphatase activity may intensify the competition among legumes and coexisting species for soil organic P in the future (Nasto et al. 2017; Soper et al. 2019).
Relationship between nitrogen and phosphorus acquisition and its regulation by N availability
Overall, we observed the non-coordinated effects of N2 fixation and plant N concentration on specific P acquisition strategy at the species level, because legume species achieving a greater N2 fixation rate did not result in a higher plant N concentration. Equally, it has been argued that higher N in legumes is a result of genetics, rather than fixation, as many non-fixing legumes also have high foliar N (McKey 1994; Soper et al. 2019). This is also supported in part by the finding that tropical tree species with the same N fixation capacity showed considerable differences in plant N concentration (Png et al. 2017). Additionally, in contrast to previous observations (Nasto et al. 2017; Soper et al. 2019), the current study did not find any significant intra-specific trade-off between AM fungal colonization and root phosphatase activity across eight herbaceous legumes. As a consequence, N2 fixation or plant N concentration did not significantly regulate the two P acquisition strategies in inverse patterns. On the contrary, a positive correlation between these two P acquisition strategies was observed under N addition. This result means that legume species with greater capacity to exploit organic P can also have a greater capacity to acquire inorganic P, suggesting the potential competitive advantage relative to other legume species under high N and low P environments.
Without N addition, our results indicated that root phosphatase activity was positively correlated with plant N concentration. It seems straightforward that legumes with a higher tissue N concentration have greater root phosphatase activity, because the production of root phosphatase enzymes is N dependent (Olander and Vitousek 2000; Houlton et al. 2008). However, previous research rarely examined and identified this positive relationship in a multi-species experiment (Soper et al. 2019), although many studies have confirmed that N-fixing legumes possess a greater root phosphatase activity than non N-fixing plants (Nasto et al. 2014; Guilbeault-Mayers et al. 2020). Previous studies have found that rhizobial symbiosis and AM fungal colonization can faciliate each other by nutrient complementarity (Püschel et al. 2017), and signal molecular regulation (Xie et al. 1998; Oldroyd 2013); both symbiotic interactions can also act synergistically to enhance the growth response of the host plant (Kalfe et al. 2019). However, negative feedbacks between rhizobial symbiosis and AM fungi colonization have also been observed (Valentine et al. 2013; Kalfe et al. 2019). Our result showed intense inter-species correlations between N2 fixation and AM fungal colonization without N addition. It is likely that more root nodulation has concurrently increased P demand, increasing AM fungal colonization of legume species (Oldroyd 2013). In addition, it was found that photosynthetic rates of legume species increased with N2 fixation rates under non N addition, which was likely attributed to increased C costs and C fixation by legume species with higher N fixation capacity (Fisher et al. 2010). As a consequence, higher leaf photosynthetic rates possibly allowed greater investment in AM fungi via C allocation (Smith and Read 2008).
However, N addition disrupted the intra-specific link between N2 fixation and AM fungal colonization. In our study, the supply of N fertilizer increased legume access to exogenous N that is a metabolically low-cost N resource compared with symbiotic N2 fixation (West et al. 2005; Fisher et al. 2010), potentially weakening the significance of N2 fixation in driving plant growth (e.g., via photosynthetic C fixation), and consequently reducing the legume N2 fixation rate and its inter-specific variation, as well as its positive association with leaf photosynthetic rate. Overall, N2 fixation in the legume failed to regulate AM fungal colonization under N enrichment. This result is similar to a previous estimation that N2 fixation had little function in driving AM fungal colonization in N-rich rain forest ecosystems (Nasto et al. 2017). In our initial hypothesis, the positive relationship between root phosphatase activity and plant N concentration of legumes also would be reduced through N addition, provided that the increase in N availability weakened the inter-specific difference in plant N concentrations. However, the observation that N addition did not alter the inter-specific variation of plant N concentration suggests that cellular N assimilation is different for these herbaceous legumes, regardless of the N availability. This result was in partial agreement with the hypothesis by McKey (1994) that tissue N concentration of legumes reflects evolutionary N acquisition strategies. As a consequence, plant N concentration showed similar control to root phosphatase activity with or without N addition.
Conclusions
In this area of investigation, most research has focused on tropical woody legumes. Our results provide new evidence characterizing the relationships between N and P acquisition in temperate herbaceous legumes. According to our results, AM fungal colonization and root phosphatase activity of pasture legumes are not species specific traits, because they can be positively regulated by N2 fixation rate or plant N concentration. However, the correlation between N2 fixation and AM fungal colonization depends on N availability. Generally, N enrichment increases the root phosphatase activity and reduces the ratio of AM fungal colonization to root phosphatase activity of temperate herbaceous legumes. Additionally, N enrichment will reduce the positive correlation between N2 fixation rate and AM fungal colonization. As more root phosphatase activity increases plant access to soil organic P, it can be hypothesised that N enrichment in grassland ecosystems will intensify the competition among legumes and coexisting species for soil organic P in the future, and legumes with high N concentration will have a competitive advantage for acquiring organic P.
References
Adams MA, Turnbull TL, Sprent JI, Buchmann N (2016) Legumes are different: leaf nitrogen, photosynthesis, and water use efficiency. Proc Natl Acad Sci U S A 113:4098–4103
Augusto L, Delerue F, Gallet-Budynek A, Achat DL (2013) Global assessment of limitation to symbiotic nitrogen fixation by phosphorus availability in terrestrial ecosystems using a meta-analysis approach. Glob Biogeochem Cycles 27:804–815
Barron AR, Purves DW, Hedin LO (2011) Facultative nitrogen fixation by canopy legumes in a lowland tropical forest. Oecologia 165:511–520
Batterman SA, Hall JS, Turner BL, Hedin LO, Walter JKL, Sheldon P, Breugel MV (2018) Phosphatase activity and nitrogen fixation reflect species differences, not nutrient trading or nutrient balance, across tropical rainforest trees. Ecol Lett 21:1486–1495
Batterman SA, Wurzburger N, Hedin LO (2013) Nitrogen and phosphorus interact to control tropical symbiotic N2 fixation: a test in Inga punctata. J Ecol 101:1400–1408
Denton MD, Veneklaas EJ, Freimoser FM, Lambers H (2007) Banksia species (Proteaceae) from severely phosphorus-impoverished soils exhibit extreme efficiency in the use and re-mobilisation of phosphorus. Plant Cell Environ 30:1557–1565
Elser JJ, Bracken MES, Cleland EE, Gruner DS, Harpole WS, Hillebrand H, Ngai JT, Seabloom EW, Shurin JB, Smith JE (2007) Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecol Lett 10:1135–1142
Fisher JB, Sitch S, Malhi Y, Huntingford C, Tan SY (2010) Carbon cost of plant nitrogen acquisition: a mechanistic, globally applicable model of plant nitrogen uptake, retranslocation, and fixation. Glob Biogeochem Cycles 24:GB1014
Guilbeault-Mayers X, Turner BL, Laliberté E (2020) Greater root phosphatase activity of tropical trees at low phosphorus despite strong variation among species. Ecology:e03090
Hedin LO, Brookshire ENJ, Menge DNL, Barron AR (2009) The nitrogen paradox in tropical forest ecosystems. Annu Rev Ecol Evol S 40:613–635
Houlton BZ, Wang YP, Vitousek PM, Field CB (2008) A unifying framework for dinitrogen fixation in the terrestrial biosphere. Nature 454:327–330
Johnson NC, Rowland DL, Corkidi L, Egerton-Warburton LM, Allen EB (2003) Nitrogen enrichment alters mycorrhizal allocation at five Mesic to semiarid grasslands. Ecology 84:1895–1908
Kafle A, Garcia K, Wang XR, Pfeffer PE, Strahan GD, Bücking H (2019) Nutrient demand and fungal access to resources control the carbon allocation to the symbiotic partners in tripartite interactions of Medicago truncatula. Plant Cell Environ 42:270–284
Lambers H, Raven JA, Shaver GR, Smith SE (2008) Plant nutrient-acquisition strategies change with soil age. Trends Ecol Evol 23:95–103
Li Q, Song YT, Li GD, Yu PJ, Wang P, Zhou DW (2015) Grass-legume mixtures impact soil N, species recruitment, and productivity in temperate steppe grassland. Plant Soil 394:271–285
Li Q, Yu PJ, Li GD, Zhou DW (2016) Grass-legume ratio can change soil carbon and nitrogen storage in a temperate steppe grassland. Soil Till Res 157:23–31
Liang X, Zhang T, Lu X, Ellsworth DS, BassiriRad H, You CM, Wang D, He PC, Deng Q, Liu H, Mo JM, Ye Q (2020) Global response patterns of plant photosynthesis to nitrogen addition: a meta-analysis. Glob Chang Biol 26:3585–3600
Lu Y, Liu X, Chen F, Zhou SR (2020) Shifts in plant community composition weaken the negative effect of nitrogen addition on community-level arbuscular mycorrhizal fungi colonization. P Roy Soc B: Biol Sci 287:20200483
Marklein AR, Houlton BZ (2012) Nitrogen inputs accelerate phosphorus cycling rates across a wide variety of terrestrial ecosystems. New Phytol 193:696–704
McKey D (1994) Legumes and nitrogen-the evolutionary ecology of a nitrogen demanding lifestyle. In: Sprent JI, McKey D (eds) Advances in legume systematics, part 5: the nitrogen factor. Kew, UK, Royal Botanic Gardens, pp 211–228
Montesinos-Navarro A, Segarra-Moragues JG, Valiente-Banuet A, Verdú M (2012) The network structure of plant-arbuscular mycorrhizal fungi. New Phytol 194:536–547
Nasto MK, Alvarez-Clare S, Lekberg Y, Sullivan BW, Townsend AR, Cleveland CC (2014) Interactions among nitrogen fixation and soil phosphorus acquisition strategies in lowland tropical rain forests. Ecol Lett 17:1282–1289
Nasto MK, Osborne BB, Lekberg Y, Asner GP, Balzotti CS, Porder S, Taylor PG, Townsend AR, Cleveland CC (2017) Nutrient acquisition, soil phosphorus partitioning and competition among trees in a lowland tropical rain forest. New Phytol 214:1506–1517
Nasto MK, Winter K, Turner BL, Cleveland CC (2019) Nutrient acquisition strategies augment growth in tropical N2-fixing trees in nutrient-poor soil and under elevated CO2. Ecology 100:e02646
Olander LP, Vitousek PM (2000) Regulation of soil phosphatase and chitinase activity by N and P availability. Biogeochem 49:175–190
Olde Venterink H (2011) Legumes have a higher root phosphatase activity than other forbs, particularly under low inorganic P and N supply. Plant Soil 347:137–146
Oldroyd GED (2013) Speak, friend, and enter: signalling systems that promote beneficial symbiotic associations in plants. Nat Rev Microbiol 11:252–263
Page AL (1982) Methods of soil analysis, part 2. Chemical and Microbiological Properties. American Society of Agronomy, Soil Science Society of America, Masison, WI
Pang J, Ruchi B, Zhao R, Bohuon E, Lambers H, Ryan MH, Ranathunge K, Siddique KHM (2018) The carboxylate-releasing phosphorus-mobilising strategy can be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytol 219:518–529
Png GK, Turner BL, Albornoz FE, Hayes PE, Lambers H, Laliberté E (2017) Greater root phosphatase activity in nitrogen-fixing rhizobial but not actinorhizal plants with declining phosphorus availability. J Ecol 105:1246–1255
Porras-Alfaro A, Herrera J, Natvig DO, Sinsabaugh RL (2007) Effect of long-term nitrogen fertilization on mycorrhizal fungi associated with a dominant grass in a semiarid grassland. Plant Soil 296:65–75
Püschel D, Janoušková M, Voříšková A, Gryndlerová H, Vosátka M, Jansa J (2017) Arbuscular mycorrhiza stimulates biological nitrogen fixation in two Medicago spp through improved phosphorus acquisition. Front Plant Sci 8:390
Schade JD, Kyle M, Hobbie SE, Fagan WF, Elser JJ (2003) Stoichiometric tracking of soil nutrients by a desert insect herbivore. Ecol Lett 6:96–101
Smith SE, Read D (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, London, UK
Soper FM, Nasto MK, Osborne BB, Cleveland CC (2019) Nitrogen fixation and foliar nitrogen do not predict phosphorus acquisition strategies in tropical trees. J Ecol 107:118–126
Sparks DL, Page A, Helmke P, Loeppert R, Soltanpour P, Tabatabai M, Johnston C, Sumner M (1996) Methods of soil analysis. Part 3-chemical methods. Soil science Society of America, Masison, WI
Stevens CJ, Dise NB, Mountford JO, Gowing DJ (2004) Impact of nitrogen deposition on the species richness of grasslands. Science 303:1876–1879
Sulieman S, Tran LSP (2015) Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci 239:36–43
Treseder KK (2004) A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO2 in field studies. New Phytol 164:347–355
Treseder KK, Vitousek PM (2001) Effects of soil nutrient availability on investment in acquisition of N and P in Hawaiian rain forests. Ecology 82:1–9
Turner BL, Brenes-Arguedas T, Condit R (2018) Pervasive phosphorus limitation of tree species but not communities in tropical forests. Nature 555:367–370
Unkovich M, Herridge D, Peoples M, Cadisch G, Boddey B, Giller K, Alves B, Chalk P (2008) Measuring plant-associated nitrogen fixation in agricultural systems. Australian Centre for International Agricultural Research (ACIAR)
Vitousek PM, Aber JD, Howarth RW, Likens GE, Matson PA, Schindler DW, Schlesinger WH, Tilman DG (1997) Human alteration of the global nitrogen cycle: sources and consequences. Ecol Appl 7:737–750
Vitousek PM, Porder S, Houlton BZ, Chadwick OA (2010) Terrestrial phosphorus limitation: mechanisms, implications, and nitrogen-phosphorus interactions. Ecol Appl 20:5–15
Wen Z, Li H, Shen Q, Tang XM, Xiong CY, Li HG, Pang JY, Ryan MH, Lambers H, Shen JB (2019) Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol 223:882–895
West JB, HilleRisLambers J, Lee TD, Hobbie SE, Reich PB (2005) Legume species identity and soil nitrogen supply determine symbiotic nitrogen-fixation responses to elevated atmospheric [CO2]. New Phytol 167:523–530
Wolf AA, Funk JL, Menge DN (2017) The symbionts made me do it: legumes are not hardwired for high nitrogen concentrations but incorporate more nitrogen when inoculated. New Phytol 213:690–699
Wu N, Li Z, Liu H, Tang M (2015) Influence of arbuscular mycorrhiza on photosynthesis and water status of Populus cathayana Rehder males and females under salt stress. Acta Physiol Plant 37:183
Wurzburger N, Hedin LO (2015) Taxonomic identity determines N2 fixation by canopy trees across lowland forests. Ecol Lett 19:62–70
Zhan S, Wang Y, Zhu Z, Li W, Bai Y (2017) Nitrogen enrichment alters plant N: P stoichiometry and intensifies phosphorus limitation in a steppe ecosystem. Environ Exp Bot 134:21–32
Zheng M, Zhou Z, Luo Y, Zhao P, Mo J (2019) Global pattern and controls of biological nitrogen fixation under nutrient enrichment: a meta-analysis. Glob Change Biol 25:3018–3030
Zheng Y, Kim YC, Tian XF, Chen L, Yang W, Gao C, Song MH, Xu XL, Guo LD (2014) Differential responses of arbuscular mycorrhizal fungi to nitrogen addition in a near pristine Tibetan alpine meadow. FEMS Microbiol Ecol 89:594–605
Acknowledgements
This study was supported by Strategic Science and Technology Guide Project of CAS (XDA23060403) and National Key Research and Development Program of China (2016YFC0500606) and Natural Science Foundation of China (31600318) and Jilin Province Science and Technology Development Plan (20190303066SF) and The grant of Youth Innovation Promotion Association of CAS (2016210).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Maarja Öpik.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Supplementary data consist of the following. Table S1: Available nitrogen (NH4++NO3−) and available P concentrations and available N:P ratio in bulk soil.
ESM 1
(DOCX 13 kb)
Rights and permissions
About this article
Cite this article
Li, Q., Denton, M.D., Huang, Y. et al. Nitrogen enrichment intensifies legume reliance on root phosphatase activity but weakens inter-specific correlations between N2 fixation and mycorrhizal colonization. Plant Soil 465, 503–514 (2021). https://doi.org/10.1007/s11104-021-04989-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-04989-8