Abstract
Aims
Plants deploying a phosphorus (P)-mobilising strategy via carboxylate release have relatively high leaf manganese concentrations ([Mn]). Thus, leaf [Mn] is a proxy for the amount of rhizosheath carboxylates. Whether the concentrations of other leaf micronutrient, such as iron ([Fe]), zinc ([Zn]) and copper ([Cu]), show a similar signal for rhizosheath carboxylates is unclear.
Methods
We grew a large number of chickpea genotypes in two glasshouse studies with different growth media, P sources and P levels. Seven weeks after sowing, we determined concentrations of micronutrients in mature leaves, and the quantity and composition of rhizosheath carboxylates.
Results
For 100 genotypes grown in river sand with low P supply, leaf [Fe] (R2 = 0.36) and [Zn] (R2 = 0.22), like leaf [Mn] (R2 = 0.38), were positively correlated with the total amount of rhizosheath carboxylates. For 20 genotypes grown in a soil mixture, leaf [Fe], [Zn], [Cu] and [Mn] showed positive correlations with total rhizosheath carboxylates that were stronger under moderately low P (R2 = 0.59, 0.59, 0.54, 0.72) than severely low P (R2 = 0.39, 0.28, 0.20, 0.36) or sufficient P (R2 = 0.36, 0.00, 0.01, 0.50) supply. Malonate was the predominant carboxylate in the rhizosheath and was significantly correlated with leaf micronutrient concentrations in both experiments.
Conclusions
In addition to leaf [Mn], leaf [Fe] and [Zn] can be used as alternative and easily measurable proxies for belowground carboxylate-releasing processes in chickpea under low-P supply, particularly on moderately low-P soils.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The release of carboxylates by plant roots into the rhizosphere mobilises sparingly-available sources of inorganic and organic phosphorus (P) in soil, and is a well-known P-mining strategy for improving P acquisition, especially when soil P availability is low (Jones 1998; Dakora and Phillips 2002; Lambers et al. 2006; Shen et al. 2011). However, due to the technical difficulties and laboriousness of collecting and measuring rhizosphere carboxylates (Kidd et al. 2018; Oburger and Jones 2018; Wang and Lambers 2019), their characteristics and roles are frequently ignored by agronomists and plant breeders, especially under field conditions (Neumann et al. 2009; Lambers et al. 2015; Oburger and Schmidt 2016). Research under agronomically-relevant field conditions is crucial for assessing the importance of any trait considered a potential breeding target (Sadras et al. 2020). Hence, an easily measurable proxy for the assessment of belowground carboxylate-releasing processes in the field would be an invaluable tool for researchers and, potentially, crop breeders.
There is ample evidence that the release of carboxylates by plant species with cluster roots into the rhizosphere mobilises not only soil inorganic and organic P, but also a range of micronutrients (e.g., Fe, Mn, Zn and Cu) (Gardner et al. 1982a, b; Dinkelaker et al. 1989; Shane and Lambers 2005; Lambers et al. 2015; Oliveira et al. 2015; Delgado et al. 2021). For instance, Gardner et al. (1982a, b) found a positive correlation between shoot manganese concentration ([Mn]) in white lupin (Lupinus albus L.) and the dry weight of its cluster roots, the main site for the release of carboxylates and reduction of MnO2 in the rhizosphere. Similarly, Shane and Lambers (2005) reported that [Mn] and zinc concentration ([Zn]) in old leaves of Hakea prostrata increased under low P supply, and attributed this to the increased cluster-root formation and release of carboxylates. Across a coastal dune chronosequence in Jurien Bay, Western Australia, non-mycorrhizal species had greater leaf [Mn] than co-occurring mycorrhizal species which was associated with most non-mycorrhizal species on soils with very low P availability releasing relatively large amounts of rhizosheath carboxylates, expressing a typical P-mining strategy (Hayes et al. 2014; Lambers et al. 2015). Similar patterns have also been reported in Brazilian campos rupestres with acidic P-impoverished soils (Oliveira et al. 2015). By analysing a trait dataset for 727 species at 66 sites in Australia and New Zealand, Lambers et al. (2021) found that mycorrhizal plants had lower leaf [Mn] than plants with carboxylate-releasing roots. Likewise, when wheat (Triticum aestivum L.) was intercropped with white lupin, a higher leaf [Mn] was observed in wheat, implying that Mn uptake in wheat was facilitated by its cluster-rooted neighbour with greater carboxylate release (Gardner and Boundy 1983). Overall, these findings suggest that plants that release a relatively large amount of carboxylates tend to have relatively high leaf [Mn], especially under low-P conditions.
Further to the findings in plant species with cluster roots, the relationship between leaf [Mn] and belowground carboxylates has been confirmed in carboxylate-releasing species without cluster roots (Pang et al. 2018; Wen et al. 2020; Yu et al. 2020). Our recent studies revealed a positive correlation between mature leaf [Mn] and the total amount of rhizosheath carboxylates under low P supply among 100 chickpea (Cicer arietinum L.) genotypes (Pang et al. 2018), and under varying P levels among 20 genotypes (Wen et al. 2020). In 19 steppe species, Yu et al. (2020) also found that P-mobilising species, which release more carboxylates, always had greater Olsen-P concentrations in rhizosheath soil and leaf [Mn] than non-P-mobilising species. However, 1) carboxylates not only mobilise P and Mn, but also a range of other micronutrients (e.g., Fe, Zn and Cu) (Dinkelaker et al. 1989; Jones et al. 2003; Shane and Lambers 2005; Suriyagoda et al. 2012); 2) Fe2+, Mn2+ and Zn2+ have similar chemical properties, and share common transporters in root cells (Puig and Peñarrubia 2009; Socha and Guerinot 2014; Andresen et al. 2018); 3) the phloem mobility of Fe, Mn, Zn and Cu is relatively low, and hence these ions mainly accumulate in mature organs (such as old leaves) (White 2012a). Therefore, in addition to leaf [Mn], other leaf micronutrients, in particular [Fe], [Zn] and [Cu], might be proxies for rhizosheath carboxylates (Fig. 1).
Conceptual diagram illustrating how the concentration of specific leaf micronutrients can be used as an easily-measurable proxy for belowground carboxylate-releasing processes in chickpea (Hypothesis 1). Arrow 1: Carboxylates in the rhizosheath not only mobilise insoluble soil phosphorus (P), but also a range of micronutrients (e.g., Mn, Fe, Zn, Cu) adsorbed onto soil particles; arrow 2: increased root uptake of mobilised micronutrients in the rhizosheath; arrow 3: increased concentration of micronutrients in mature leaves
Increased root release of carboxylates is a typical response to P deficiency in many plant species (Shane et al. 2008; Pearse et al. 2007; Zhang et al. 2016; Zhou et al. 2016). Neumann and Römheld (1999) reported that a single genotype of chickpea increased carboxylate release under P deficiency, but this response was not observed in several later chickpea studies (Wouterlood et al. 2004a, b, 2005; Lyu et al. 2016; Wen et al. 2019). Our recent study of 20 chickpea genotypes showed that the total amount of rhizosheath carboxylates expressed per root dry weight strongly increased with increasing soil P availability, indicating that the release of total rhizosheath carboxylates in chickpea was not enhanced by low soil P availability (Wen et al. 2020). However, we still know little about whether and how chickpea adjusts the composition of rhizosheath carboxylates in response to increasing soil P availability. Moreover, with increasing soil P availability, the effects of growth dilution of nutrient accumulation became noticeable (Jarrell and Beverly 1981). For chickpea, given that leaf [Mn] was consistently correlated with rhizosheath carboxylates under contrasting soil P supply (Wen et al. 2020), whether leaf [Fe], [Zn] and [Cu] also show stable signals for the amount of rhizosheath carboxylates needs to be tested, as well as the relative contribution of the predominant rhizosheath carboxylates for the accumulation of leaf micronutrients.
To address the above questions, we analysed leaf micronutrient concentrations (i.e. Fe, Zn and Cu) and the amount and composition of rhizosheath carboxylates in 100 chickpea genotypes grown under low P supply from the experiment undertaken by Pang et al. (2018) and 20 chickpea genotypes grown at three P levels from the experiment undertaken by Wen et al. (2020). We tested three hypotheses: (1) in addition to leaf [Mn], the concentrations of other leaf micronutrients (i.e. Fe, Zn and Cu) can provide alternative and easily-measurable proxies for the assessment of belowground carboxylate-releasing processes under low-P conditions (see Fig. 1 for a conceptual framework); (2) soil P availability mediates the composition of rhizosheath carboxylates, and the dominant components of rhizosheath carboxylates play key roles in leaf micronutrient accumulation; (3) the indicative signals provided by leaf micronutrient concentrations are modified by increasing soil P availability, due to the incremental effects of growth dilution of the micronutrients.
Materials and methods
Experiment 1: River sand culture experiment
We explored genotypic variation in leaf micronutrient concentrations (Fe, Zn and Cu) and the potential relationship between leaf micronutrient concentrations and the amount of rhizosheath carboxylates (total or individual) under low P supply. A pot experiment was conducted in a controlled glasshouse with 100 chickpea genotypes grown in sterilised washed river sand with a growth-limiting P supply, 10 mg P kg−1 soil as FePO4; for a detailed description, see Pang et al. (2018). Notably, all micronutrients (Mn, Fe, Cu and Zn) were artificially supplemented: 3.90 mg Mn kg−1, as MnSO4·H2O; 4.38 mg Fe kg−1, as EDTA-FeNa and 18 mg Fe kg−1 accompanied by FePO4 addition; 2 mg Zn kg−1, as ZnSO4·7H2O; and 0.50 Cu mg kg−1, as CuSO4·5H2O. At harvest (49 days after sowing), mature (fully expanded) leaves on the main stem (including all shed leaves) were collected for leaf micronutrient analyses and the rhizosheath soil was collected for analysis of rhizosheath carboxylates (see details in ‘Measurements’ section).
Experiment 2: Soil mixture culture experiment
We investigated the responses of leaf micronutrient concentrations (Fe, Zn and Cu) and the amount of rhizosheath carboxylates (total or individual) to contrasting soil P availability and their potential relevance. According to the results of Experiment 1, 20 chickpea genotypes with different amounts of rhizosheath carboxylates (relative to root dry weight, RDW) were selected (18–104 μmol g−1 RDW). Three P treatments were applied: (1) FeP10, 10 mg P kg−1 soil with severely growth-limiting P supply, as insoluble FePO4 (extra pure, Acros Organics, Morris Plain, NJ, USA); (2) KP10, 10 mg P kg−1 soil with moderately growth-limiting P supply, as soluble KH2PO4; and (3) KP50, 50 mg P kg−1 soil with sufficient P supply, as soluble KH2PO4. Details of the experiment are included in Wen et al. (2020). Briefly, a sandy loam soil was obtained from the top layer (0-15 cm) of unfertilised native grass vegetation sites located at the Future Farm of the University of Western Australia, Pingelly, Western Australia (32.51°S, 116.99°E). Soil was air-dried and then passed through a 2-mm sieve. To decrease plant-available soil P levels and to facilitate the collection of rhizosheath carboxylates and root samples (Mimmo et al. 2011), we mixed the field soil with sterilised washed river sand (w/w = 1:9). The soil mixture contained: 2.5 mg kg−1 Colwell-P; 15.1 mg kg−1 DTPA-Mn; 13.1 mg kg−1 DTPA-Fe; 0.68 mg kg−1 DTPA-Zn and 0.27 mg kg−1 DTPA-Cu (Lindsay and Norvell 1978), except for an additional 18 mg Fe kg−1, accompanied with FePO4 addition, in the FeP10 treatment. Similar to Experiment 1, mature leaves on the main stem were collected at harvest (53 days after sowing) for leaf micronutrient analyses, as well as rhizosheath soil for analysis of rhizosheath carboxylates (see detailed procedures in ‘Measurements’ section).
Measurements
Leaf micronutrient analyses: at harvest, shoots were separated from roots; mature leaves (fully expanded) on the main stem (including shed leaves) were collected separately. All samples were dried at 70 °C for 72 h to constant weight, and ground separately to a fine powder using a Geno/Grinder 2010 (Spex SamplePrep, Metuchen, NJ, USA). Weighed subsamples, c. 100 mg, were digested using a concentrated HNO3–HClO4 (v/v = 3:1) mixture. The concentrations of Mn, Fe, Zn and Cu in mature leaves were determined by an inductively coupled plasma-optical emission spectrometer (ICP–OES, OPTIMA 5300 DV, Perkin–Elmer, Shelton, CT, USA).
Rhizosheath carboxylate analyses: at harvest, intact root systems were removed from the soil/sand and gently shaken to remove loosely adhering soil/sand (considered to be bulk soil /sand), leaving the tightly adhering soil/sand around the roots that was defined as rhizosheath soil/sand (Pang et al. 2017). Roots and rhizosheath soil were transferred to a beaker containing a known amount of 0.2 mM CaCl2 that varied depending on root volume (Pearse et al. 2007). Roots were repeatedly dunked into the solution until as much rhizosheath soil as possible was removed, taking care to minimise root damage. The rhizosheath extract was filtered through a 0.45-μm syringe filter into a 1-mL high-performance liquid chromatography (HPLC) vial. The HPLC samples were acidified with a drop of concentrated phosphoric acid and frozen at −20 °C until analysis. The analyses of carboxylates followed the method described by Cawthray (2003). The 11 carboxylic acid working standards included acetic, citric, cis-aconitic, fumaric, lactic, malic, malonic, maleic, shikimic, succinic and trans-aconitic acids.
Data analysis
In Experiment 1, to determine the genotypic variation in leaf micronutrient concentrations (i.e. [Fe], [Zn] and [Cu]) and the amount and composition of rhizosheath carboxylates in 100 chickpea genotypes, the coefficient of variation (CV) of leaf micronutrient concentrations and the amount of rhizosheath carboxylates (including total and individual) were calculated as the ratio of the standard deviation to the mean. The relationships between leaf micronutrient concentrations and the amount of rhizosheath carboxylates (total and individual) were assessed by linear regression analysis using the R package ‘AGRICOLAE’ (de Mendiburu 2017). Acetate was excluded from the total amount of rhizosheath carboxylates when assessing the relationship between leaf micronutrient concentrations and total amount of rhizosheath carboxylates because it is mainly produced by microbes in the rhizosheath (Saarnio et al. 2004) and, as a monocarboxylate, contributes little to mobilising soil P and micronutrients, unlike di- and tricarboxylates (Jones and Brassington 1998).
In Experiment 2, to determine how leaf micronutrient concentrations and the amount of rhizosheath carboxylates among 20 chickpea genotypes responded to contrasting soil P availability, we used a two-way analysis of variance (ANOVA) with a randomised block to examine the effects of P treatments, genotypes, and their interaction on leaf micronutrient concentrations and the composition of rhizosheath carboxylates using the R package ‘AGRICOLAE’ (de Mendiburu 2017). Significant differences among means in each P treatment were based on Tukey’s HSD post-hoc analysis (P ≤ 0.05). The relationships between leaf micronutrient concentrations and the amount of rhizosheath carboxylates were also assessed by linear regression analysis as described above. All statistical analyses were performed using R Version 4.0.2 (R Development Core Team 2020).
Results
Leaf micronutrient concentrations and the amount of rhizosheath carboxylates in a large set of chickpea genotypes under low-P condition (experiment 1)
Across 100 chickpea genotypes, we observed significant intraspecific variation in leaf micronutrient concentrations (i.e. [Fe], [Zn] and [Cu]) and the quantity and composition of rhizosheath carboxylates under low-P condition (P < 0.001; Fig. 2). The predominant carboxylates in the rhizosheath were malonate, acetate, citrate and malate, which contributed to 99% of the amount of carboxylates detected in the rhizosheath (relative to RDW; Pang et al. 2018). Among the four predominant rhizosheath carboxylates, the concentration decreased in the order: malonate > acetate > citrate > malate (Fig. 2a-d), malonate ranged from 14.1 to 83 μmol g−1 RDW (CV = 33%); acetate ranged from 1.8 to 16.7 μmol g−1 RDW (CV = 29%); followed by citrate (2.0 to 13.3 μmol g−1 RDW, CV = 33%) and malate (0.4 to 4.9 μmol g−1 RDW, CV = 35%). The concentration of leaf micronutrients also varied among genotypes (Fig. 2e-g): compared with the variation of leaf [Mn] (743 to 3606 μg g−1, CV = 32%; Pang et al. 2018), leaf [Fe] ranged from 540 to 2982 μg g−1 (CV = 43%); leaf [Zn] ranged from 145 to 468 μg g−1 (CV = 23%); leaf [Cu] ranged from 8.2 to 25.7 μg g−1 (CV = 22%). The mean values of leaf [Mn], [Fe] and [Zn] were up to 37-fold, 13-fold and 14-fold greater than that considered adequate for crop growth, respectively (Fig. 2; Epstein and Bloom 2005), while leaf [Cu] was only 2.6-fold greater than the critical level (Fig. 2).
Boxplots showing the amount of four main rhizosheath carboxylates: a malonate, b acetate, c citrate and d malate; and the concentration of three micronutrients in mature leaves on the main stems: e iron ([Fe]), f zinc ([Zn]) and g copper ([Cu]) in Experiment 1. Data are for 100 chickpea genotypes grown in sterilised washed river sand with a growth-limiting supply of phosphorus (P), 10 mg P kg–1 dry soil as FePO4. The dashed line represents the average concentration of corresponding leaf micronutrient that is generally considered adequate for crop growth (Epstein and Bloom 2005). The boxplots show the median, and 25th and 75th percentiles. The whiskers extend to 1.5 times the interquartile range. Data presented beyond whiskers are outliers. Please note the different scales of the y-axes. RDW: root dry weight
Linking leaf micronutrient concentrations with belowground carboxylate-releasing processes, similar to leaf [Mn] (R2 = 0.38; Pang et al. 2018), leaf [Fe] and [Zn] had positive correlations with the total amount of rhizosheath carboxylates ([Fe]: R2 = 0.36, P < 0.001; [Zn]: R2 = 0.22, P < 0.001; Fig. 3); whereas no correlation was observed for leaf [Cu] and total rhizosheath carboxylates (P > 0.05; Fig. 3). Analogously, leaf [Fe] (R2 = 0.45) and [Zn] (R2 = 0.28), like leaf [Mn] (R2 = 0.48; Pang et al. 2018) were positively correlated with the amount of rhizosheath malonate (Fig. 3); conversely, leaf [Fe] and [Zn] had negative correlations with the amount of citrate and malate (Figs. S1, S2), and no correlation was found between leaf [Cu] and the amount of individual carboxylates in the rhizosheath (Figs. 3, S1, S2).
Correlation between the concentration of iron ([Fe]), copper ([Cu]) and zinc ([Zn]) in mature leaves on the main stem and total amount of rhizosheath carboxylates (excluding acetate) (a, c, e) or malonate only (b, d, f) in Experiment 1. Data are for 100 chickpea genotypes grown for seven weeks in sterilised washed river sand with a growth-limiting supply of phosphorus (P), 10 mg P kg–1 dry soil as FePO4. The shaded areas indicate the 95% confidence range, derived from the models. Total carboxylates (or malonate): the total amount of rhizosheath carboxylates (or malonate only) relative to root dry weight (RDW)
Leaf micronutrient concentrations in 20 chickpea genotypes with different amounts of rhizosheath carboxylates under contrasting soil P availability (experiment 2)
Rhizosheath carboxylates showed substantial genotypic variability in response to contrasting soil P availability among 20 chickpea genotypes (Fig. 4, Table S1). Similar to the observation in Experiment 1, four main carboxylates comprising malonate, acetate, citrate and malate, accounted for 99% of the amount of carboxylates detected in the rhizosheath in each P treatment (Fig. S3); all four predominant rhizosheath carboxylates showed significant genotypic variation in the amount and percentage in each P treatment (Figs. 4, S3; Table S1). With increasing soil P availability, the amount of malonate, citrate and malate increased significantly (P ≤ 0.05, Fig. 4), while the amount of acetate decreased (Fig. 4b). The percentage of malonate and malate also increased with increasing soil P availability, while that of acetate and citrate showed a significant decrease or was relatively stable, respectively (Fig. S3).
Boxplots showing the amount of four main rhizosheath carboxylates relative to root dry weight (RDW) of 20 chickpea genotypes with contrasting amounts of rhizosheath carboxylates in response to three phosphorus (P) treatments (Experiment 2). FeP10: 10 mg P kg−1 soil as FePO4; KP10 and KP50: 10 and 50 mg P kg−1 soil as KH2PO4, respectively. The boxplots show the median, and 25th and 75th percentiles. The whiskers extend to 1.5 times the interquartile range. Data presented beyond whiskers are outliers. Different upper letters denote significant differences among P treatments (based on Tukey’s post-hoc analysis, P ≤ 0.05). Please note the different scales of the y-axes
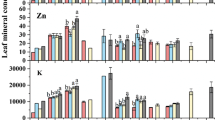
The concentrations of leaf micronutrients also expressed large intraspecific variability in the three P treatments (Fig. 5, Table S1). Across 20 genotypes, the average value of leaf micronutrient concentration decreased in the order: leaf [Mn] > [Fe] > [Zn] > [Cu] in each P treatment (Fig. 5). With increasing soil P availability, leaf [Mn] and [Fe] showed a significant increase (P ≤ 0.05, Fig. 5a, b), while leaf [Zn] and [Cu] increased from FeP10 to KP10 initially, and then decreased in KP50 (Fig. 5c, d). In contrast, the mean values of leaf [Mn], [Fe] and [Zn] in each P treatment were greater than the adequate levels (except for leaf [Fe] in FeP10), whereas the mean value of leaf [Cu] was lower than the adequate level in each P treatment (Fig. 5; Epstein and Bloom 2005).
Boxplots showing the concentration of a manganese ([Mn]), b iron ([Fe]), c zinc ([Zn]) and d copper ([Cu]) in mature leaves on the main stem of 20 chickpea genotypes with contrasting amounts of rhizosheath carboxylates in response to three phosphorus (P) treatments (Experiment 2). FeP10: 10 mg P kg−1 soil as FePO4; KP10 and KP50: 10 and 50 mg P kg−1 soil as KH2PO4, respectively. The dashed line represents the average concentration of corresponding leaf micronutrient that is generally considered adequate for crop growth (Epstein and Bloom 2005). The boxplots show the median, and 25th and 75th percentiles. The whiskers extend to 1.5 times the interquartile range. Data presented beyond whiskers are outliers. Different upper letters denote significant differences among P treatments (based on Tukey’s post-hoc analysis, P ≤ 0.05). Please note the different scales of the y-axes
The correlations between the concentrations of leaf micronutrients and the amount of rhizosheath carboxylates (total and individual) were analysed for each P treatment (Figs. 6, 7, S4, S5). For FeP10 and KP10, leaf [Fe], [Zn] and [Cu] (R2 = 0.28–0.64), like leaf [Mn] (R2 = 0.36–0.72; Wen et al. 2020), were positively correlated with the total amount of rhizosheath carboxylates or that of malonate (all P ≤ 0.01, Figs. 6, 7). In KP50, only leaf [Fe] (R2 = 0.36, P < 0.01) and leaf [Mn] (R2 = 0.50, P < 0.01; Wen et al. 2020) were correlated with the total rhizosheath carboxylates or malonate, while leaf [Zn] and [Cu] had no correlation (all P > 0.05, Figs. 6, 7). In contrast, the correlations between leaf micronutrient concentrations and total rhizosheath carboxylates or malonate in KP10 (e.g., total carboxylates: R2 = 0.54–0.59) was consistently stronger than that in FeP10 (e.g., total carboxylates: R2 = 0.28–0.39) and KP50 (e.g., total carboxylates: R2 = 0–0.36; Figs. 6, 7). Regardless of P treatment, the concentrations of all four leaf micronutrients (i.e. [Mn], [Fe], [Zn] and [Cu]) had no correlation with the amount of rhizosheath citrate or malate (Figs. S4, S5).
Correlations between the concentration of a iron ([Fe]), b zinc ([Zn]) and c copper ([Cu]) in mature leaves on the main stem and the total amount of rhizosheath carboxylates (exclude acetate) for 20 chickpea genotypes under three phosphorus (P) treatments (n = 20) in Experiment 2. FeP10: 10 mg P kg−1 soil as FePO4; KP10 and KP50: 10 and 50 mg P kg−1 soil as KH2PO4, respectively. The shaded areas indicate the 95% confidence range, derived from the models. Total carboxylates: total amount of rhizosheath di- and tricarboxylates relative to root dry weight (RDW)
Correlation between the concentration of a manganese ([Mn]), b iron ([Fe]), c zinc ([Zn]) and d copper ([Cu]) in mature leaves on the main stem and the amount of rhizosheath malonate for 20 chickpea genotypes under three phosphorus (P) treatments in Experiment 2 (n = 20). FeP10: 10 mg P kg−1 soil as FePO4; KP10 and KP50: 10 or 50 mg P kg−1 soil as KH2PO4, respectively. The shaded areas indicate the 95% confidence range, derived from the models. Malonate: amount of malonate in the rhizosheath relative to root dry weight (RDW)
Discussion
Leaf micronutrient concentrations provide proxies for rhizosheath carboxylates in chickpea under low-P conditions
Carboxylates released from plant roots not only mobilise insoluble soil P, but also solubilise a range of micronutrients in the rhizosphere, and subsequently increase the uptake of micronutrients by plants, especially that of Mn (Gardner et al. 1982a, b; Dinkelaker et al. 1989, 1995; Shane and Lambers 2005; Oliveira et al. 2015). Consistent with this effect, under low-P conditions, we found that not only leaf [Mn], but also leaf [Fe] and [Zn] provided proxies for the assessment of belowground carboxylate-releasing processes in chickpea. Specifically, similar to leaf [Mn] (Pang et al. 2018; Wen et al. 2020), leaf [Fe] and [Zn] showed a positive correlation with the total amount of rhizosheath carboxylates (relative to RDW) in both experiments under low P supply; conversely, the correlation for leaf [Cu] was not consistent, and only found in Experiment 2 (under FeP10 and KP10 treatments). Thus, our first hypothesis (Fig. 1) was partly supported. Remarkably, we noted that the signals provided by different leaf micronutrients (i.e. Mn, Fe, Zn and Cu) were not uniform, as further discussed below.
The strong correlation of leaf [Fe] with the total amount of rhizosheath carboxylates across a wide range of leaf [Fe] was unexpected, because plant Fe uptake is generally considered to be more tightly controlled than that of Mn and Zn, and thus Fe toxicity is avoided in most plant species (Baxter et al. 2008; Thomine and Vert 2013; Jeong et al. 2017). In the present study, we found that a large quantity of both Mn and Fe accumulated in mature chickpea leaves (similar concentration ranges) and that their concentrations were strongly correlated with the total amount of rhizosheath carboxylates. In contrast, leaf [Mn] in Hakea prostrata was tightly correlated with biomass investment in cluster roots, while leaf [Fe] was relatively stable (Shane and Lambers 2005). In white lupin, Dinkelaker et al. (1989) showed that low P significantly increased the availability of soil micronutrients around the cluster root zones, i.e. DTPA-Fe, DTPA-Mn, and DTPA-Zn. Manganese mainly accumulated in shoots of white lupin (shoot [Mn]: 720 μg g−1, root [Mn]: 160 μg g−1), while Fe was intercepted in roots (shoot [Fe]: 62 μg g−1; root [Fe]: 2167 μg g−1), particularly in intercellular spaces (i.e. in the apoplast) of the epidermis and outer cortex (Gardner et al. 1983; Dinkelaker et al. 1989). The divergences in Fe allocation between chickpea and white lupin suggest that different mechanisms operate in the two species. Accumulation of Fe in the apoplast of lupin roots may trigger the formation of cluster roots (Zhou et al. 2020). Interestingly, chickpea synthesises and exudes a large amount of carboxylates from its leaves, stems and pods (Koundal and Sinha 1983; Stevenson et al. 2010; Devi et al. 2014; Gross et al. 2021). This unusual characteristic may help chickpea to detoxify and tolerate high Fe concentrations in mature leaves, as there is considerable evidence showing that carboxylates play a key role in the complexation of metal ions in leaves (Sun et al. 2006; Tian et al. 2011; Leitenmaier and Küpper 2013). To date, the mechanisms underlying the contrasting patterns in distribution of micronutrients (e.g., Mn and Fe) between shoots and roots are not fully understood, and these merit further study through consideration of more species.
The concentration ranges of four micronutrients (Mn, Fe, Zn and Cu) in chickpea leaves differed significantly, with leaf [Mn] and [Fe] remarkably higher in magnitude than leaf [Zn] and [Cu] in both experiments, indicating that the signals provided by leaf [Mn] and [Fe] were stronger than those of leaf [Zn] and [Cu]. Similar results were obtained for other species, especially when plants were cultivated on acid soils rich in available Mn and Fe, e.g., durum wheat (Triticum turgidum L. var. durum, Karagiannidis and Hadjisavva-Zinoviadi 1998), upland rice (Oryza sativa L., Fageria et al. 2002), and 12 forage species (Lindström et al. 2013). This reflects inherent differences in the demand or tolerance of leaf micronutrients (Mn, Fe, Zn and Cu) among plant species. Compared with the average concentrations of leaf micronutrients considered adequate for crop growth (Epstein and Bloom 2005), leaf [Mn], [Fe] and [Zn] were significantly higher than these adequate levels ([Mn] > 50 μg g−1; [Fe] > 100 μg g−1; [Zn] > 20 μg g−1), leaf [Cu] in Experiment 1 was slightly higher than the critical level ([Cu] > 6 μg g−1), but lower in Experiment 2. These contrasting variation ranges in leaf [Cu] may account for the different correlations between leaf [Cu] and the total amount of rhizosheath carboxylates observed in the two experiments. Contrary to the other three leaf micronutrients ([Mn], [Fe] and [Zn]), we surmise that leaf [Cu] is only correlated with rhizosheath carboxylates when leaf [Cu] is below sufficiency level in Experiment 2 (< 6 μg g−1; Fig. 5c), and hence there is no correlation when leaf [Cu] is adequate for plant growth in Experiment 1 (> 6 μg g−1; Fig. 2g). Overall, the narrow range and unstable signals of leaf [Cu] discount it as a proxy for the assessment of belowground carboxylate-releasing processes under low-P conditions.
Responses of leaf micronutrient concentrations to rhizosheath carboxylates in chickpea under contrasting soil P availability
As shown in Wen et al. (2020), chickpea strongly increases the total amount of rhizosheath carboxylates with increasing soil P availability, suggesting carboxylate exudation may be a mechanism to dispose of excess carbon in chickpea (Prescott et al. 2020). In this study, we found that malonate, acetate, citrate and malate comprised the main carboxylates in the rhizosheath of chickpea, in accordance with previous studies, which reported malonate, citrate and malate as the predominant rhizosheath carboxylates (Veneklaas et al. 2003; Wouterlood et al. 2004a, b, 2005). Acetate (possibly as a microbial product in the rhizosheath; Saarnio et al. 2004) also accounted for a moderate proportion of carboxylates detected in both experiments, but as a monocarboxylate, it will contribute little to the mobilisation of soil P and micronutrients (Jones and Brassington 1998). Among the four main rhizosheath carboxylates, the amount and percentage of malonate and malate increased with increasing soil P availability, while the percentage of acetate and citrate showed a significant decrease or was relatively stable. These results validate our second hypothesis that soil P availability mediates the composition of rhizosheath carboxylates. Furthermore, our results show that only malonate was tightly and positively correlated with both total rhizosheath carboxylates and the concentrations of leaf micronutrients (i.e. Mn and Fe in both experiments), implying that malonate as the predominant carboxylate in the rhizosheath of chickpea, plays the key role in the accumulation of leaf micronutrients. Therefore, our second hypothesis was fully supported.
With increasing soil P availability, the greater amount of total rhizosheath carboxylates under KP10 and KP50, relative to the FeP10 treatment, was also reflected in increased [Mn] and [Fe] in mature leaves. Similar results have been reported by Huang et al. (2017), who found that the seedlings of Western Australian peppermint tree (Agonis flexuosa L.) showed an increase in the total amount of rhizosheath carboxylates and leaf [Mn] concurrently in response to increasing soil P availability. In the present study, leaf [Zn] and [Cu] in chickpea responded differently from that of leaf [Mn] and [Fe] to increasing soil P availability: leaf [Zn] and [Cu] initially increased when supplied with 10 mg P kg−1 as KH2PO4, then decreased when supplied with 50 mg P kg−1 as KH2PO4, suggesting that the signals of leaf [Zn] and [Cu] are modified by increasing soil P availability (hypothesis 3). There are three possible explanations. First, this may result from a balance between the dilution by growth (Jarrell and Beverly 1981) and the mobilisation effect of carboxylates: under KP50, total rhizosheath carboxylates increased relative to those in KP10, but leaf [Zn] and [Cu] decreased, indicating that the biomass dilution effect was greater than the mobilisation effect of carboxylates; moreover, the concentrations of both Zn and Cu in soil and leaves were inherently lower in magnitude than those of Mn and Fe, thus, the signals provided by leaf [Zn] and [Cu] were more affected by biomass dilution than those by leaf [Mn] and [Fe]. Second, a decrease in leaf [Zn] and [Cu] may be related to the interactions between P and micronutrients (Tsai and Schmidt 2017). A high soil P supply inhibited plant Zn and Cu uptake, as have been well documented (Touchton et al. 1980; Haldar and Mandal 1981; Zhang et al. 2017, 2020). We have no explanation for the mechanism of chickpea selectively maintaining substantial Mn and Fe uptake under KP50, but note that similar results have been reported for other species (Huang et al. 2017; Zhang et al. 2020). Third, antagonistic interactions between micronutrients may also contribute to a decrease in leaf [Zn] and [Cu] under KP50. Competition for root uptake between ions carrying the same charge has been shown in most experimental conditions (White 2012b; He et al. 2017; Andresen et al. 2018); thus, we assume that the enhanced absorption of Mn and Fe under KP50 may decrease Zn and Cu uptake in chickpea plants.
Remarkably, we found that the correlations between leaf micronutrient concentrations (i.e. leaf [Mn], [Fe] and [Zn]) and rhizosheath carboxylates in KP10 (R2 > 0.5) were consistently stronger than those in FeP10 and KP50 (R2 < 0.5; Figs. 6, 7), suggesting the proxies work best under moderate soil P availability. This can also be explained by soil P availability affecting the balance between the dilution effect through growth (Jarrell and Beverly 1981) and the mobilisation effect of carboxylates as discussed above. Under severely low soil P availability (FeP10), the lower amount of rhizosheath carboxylates may be the key factor limiting soil micronutrient mobilisation (Fig. 4). In contrast, under sufficient P supply (KP50), while total rhizosheath carboxylates increased, the shoot biomass increased much faster (Wen et al. 2020), and thus the effect of biomass dilution presumably dominated over the signals provided by leaf micronutrients. Therefore, the correlations under moderately low-P supply remained consistently tighter than those under either severely low- or sufficient soil P availability.
Conclusions and perspectives
We demonstrated that, in addition to leaf [Mn], leaf [Fe] and [Zn] can be used as easily measurable proxies for the amount of rhizosheath carboxylates in a range of chickpea genotypes under low-P supply, especially on moderately low-P soils. Furthermore, the composition of rhizosheath carboxylates was affected by soil P availability. Malonate, as the predominant carboxylate in the rhizosheath of chickpea, consistently played a key role in the accumulation of leaf micronutrients. Finally, our results show that the signals provided by leaf [Mn] and [Fe] were stronger than those of leaf [Zn] and [Cu], and less influenced by high soil P availability.
To date, the accurate quantification of carboxylates in soil, particularly a real-time monitoring, is still a major technical challenge, as carboxylates are easily absorbed by soil particles and decomposed by soil microbes. Though the correlation coefficient between leaf micronutrient concentrations and the amount of rhizosheath carboxylates is not extremely high in our study, as carboxylates could be affected by many factors including soil properties, plant characteristics and the interplay between the uptake of micronutrients and macronutrients in plants. Our study has obviously opened a possibility of using easily measurable aboveground indices to indicate a belowground carboxylate-releasing P-acquisition strategy. The significant correlation between the concentration of leaf micronutrients (especially leaf [Mn] and [Fe]) and the amount of rhizosheath carboxylates observed in chickpea may also be applicable to other crops, especially grain legumes, providing a valuable screening tool for rapidly identifying genotypes with high P-acquisition efficiency on low-P soils. To take advantage of our findings further, we propose to explore the correlation between leaf micronutrients and rhizosheath carboxylates in a wider range of crop species and under different growth conditions.
References
Andresen E, Peiter E, Kupper H (2018) Trace metal metabolism in plants. J Exp Bot 69:909–954. https://doi.org/10.1093/jxb/erx465
Baxter IR, Vitek O, Lahner B, Muthukumar B, Borghi M, Morrissey J, Guerinot ML, Salt DE (2008) The leaf ionome as a multivariable system to detect a plant's physiological status. Proc Natl Acad Sci U S A 105:12081–12086. https://doi.org/10.1073/pnas.0804175105
Cawthray GR (2003) An improved reversed-phase liquid chromatographic method for the analysis of low-molecular mass organic acids in plant root exudates. J Chromatogr A 1011:233–240. https://doi.org/10.1016/s0021-9673(03)01129-4
Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245:35–47. https://doi.org/10.1023/A:1020809400075
de Mendiburu F (2017) Agricolae: Statistical procedures for agricultural research. R package version 1:2–8. http://CRAN.Rproject.org/packages/agricolae. Accessed 18 Jan 2020
Delgado M, Zúñiga-Feest A, Reyes-Díaz M, Barra PJ, Ruiz S, Bertin-Benavides A, Valle S, Pereira M, Lambers H (2021) Ecophysiological performance of proteaceae species from southern South America growing on substrates derived from young volcanic materials. Front Plant Sci 12:636056. https://doi.org/10.3389/fpls.2021.636056
Devi VS, Rao PA, Sharma SP, Sharma HC (2014) Interaction of acid exudates in chickpea with biological activity of Bacillus thuringiensis towards Helicoverpa armigera. J Appl Entomol 138:289–296. https://doi.org/10.1111/jen.12056
Dinkelaker B, Römheld V, Marschner H (1989) Citric acid excretion and precipitation of calcium citrate in the rhizosphere of white lupin (Lupinus albus L.). Plant Cell Environ 12:285–292. https://doi.org/10.1111/j.1365-3040.1989.tb01942.x
Dinkelaker B, Hengeler C, Marschner H (1995) Distribution and function of proteoid roots and other root clusters. Plant Biol 108:183–200. https://doi.org/10.1111/j.1438-8677.1995.tb00850.x
Epstein E, Bloom AJ (2005) Mineralnutrition of plants: principles andperspectives. Sinauer Associates, Sunderland, p 50
Fageria NK, Baligar VC, Clark RB (2002) Micronutrients in crop production. Adv Agron 77:185–268. https://doi.org/10.1016/S0065-2113(02)77015-6
Gardner WK, Boundy KA (1983) The acquisition of phosphorus by Lupinus albus L. IV. The effect of interplanting wheat and white lupin on the growth and mineral composition of the two species. Plant Soil 70:391–402. https://doi.org/10.1007/bf02374894
Gardner W, Parbery D, Barber D (1982a) The acquisition of phosphorus by Lupinus albus L. I. some characteristics of the soil/root interface. Plant Soil 68:19–32. https://doi.org/10.1007/bf02374724
Gardner W, Parbery D, Barber D (1982b) The acquisition of phosphorus by Lupinus albus L. II. The effect of varying phosphorus supply and soil type on some characteristics of the soil/root interface. Plant Soil 68:33–41. https://doi.org/10.1007/BF02374894
Gardner W, Barber D, Parbery D (1983) The acquisition of phosphorus by Lupinus albus L. III. The probable mechanism by which phosphorus movement in the soil/root interface is enhanced. Plant Soil 70:107–124. https://doi.org/10.1007/BF02374754
Gross A, Tiwari S, Shtein I, Erel R (2021) Direct foliar uptake of phosphorus from desert dust. New Phytol. https://doi.org/10.1111/nph.17344
Haldar M, Mandal LN (1981) Effect of phosphorus and zinc on the growth and phosphorus, zinc, copper, iron and manganese nutrition of rice. Plant Soil 59:415–425. https://doi.org/10.1007/BF02184546
Hayes P, Turner BL, Lambers H, Laliberté E (2014) Foliar nutrient concentrations and resorption efficiency in plants of contrasting nutrient-acquisition strategies along a 2-million-year dune chronosequence. J Ecol 102:396–410. https://doi.org/10.1111/1365-2745.12196
He XL, Fan SK, Zhu J, Guan MY, Liu XX, Zhang YS, Jin CW (2017) Iron supply prevents cd uptake in Arabidopsis by inhibiting IRT1 expression and favoring competition between Fe and cd uptake. Plant Soil 416:453–462. https://doi.org/10.1007/s11104-017-3232-y
Huang G, Hayes PE, Ryan MH, Pang J, Lambers H (2017) Peppermint trees shift their phosphorus-acquisition strategy along a strong gradient of plant-available phosphorus by increasing their transpiration at very low phosphorus availability. Oecologia 185:387–400. https://doi.org/10.1007/s00442-017-3961-x
Jarrell WM, Beverly RB (1981) The dilution effect in plant nutrition studies. Adv Agron 34:197–224. https://doi.org/10.1016/S0065-2113(08)60887-1
Jeong J, Merkovich A, Clyne M, Connolly EL (2017) Directing iron transport in dicots: regulation of iron acquisition and translocation. Curr Opin Plant Biol 39:106–113. https://doi.org/10.1016/j.pbi.2017.06.014
Jones DL (1998) Organic acids in the rhizosphere – a critical review. Plant Soil 205:25–44. https://doi.org/10.1023/A:1004356007312
Jones DL, Brassington DS (1998) Sorption of organic acids in acid soils and its implications in the rhizosphere. Eur J Soil Sci 49:447–455. https://doi.org/10.1046/j.1365-2389.1998.4930447.x
Jones DL, Dennis PG, Owen AG, van Hees PAW (2003) Organic acid behavior in soils – misconceptions and knowledge gaps. Plant Soil 248:31–41. https://doi.org/10.1023/a:1022304332313
Karagiannidis N, Hadjisavva-Zinoviadi S (1998) The mycorrhizal fungus Glomus mosseae enhances growth, yield and chemical composition of a durum wheat variety in 10 different soils. Nutr Cycl Agroecosyst 52:1–7. https://doi.org/10.1023/a:1016311118034
Kidd DR, Ryan MH, Hahne D, Haling RE, Lambers H, Sandral GA, Simpson RJ, Cawthray GR (2018) The carboxylate composition of rhizosheath and root exudates from twelve species of grassland and crop legumes with special reference to the occurrence of citramalate. Plant Soil 424:389–403. https://doi.org/10.1007/s11104-017-3534-0
Koundal KR, Sinha SK (1983) Evaluation of the significance of malic-acid secretion in chickpea. Physiol Plant 58:189–192. https://doi.org/10.1111/j.1399-3054.1983.tb04167.x
Lambers H, Shane MW, Cramer MD, Pearse SJ, Veneklaas EJ (2006) Root structure and functioning for efficient acquisition of phosphorus: matching morphological and physiological traits. Ann Bot 98:693–713. https://doi.org/10.1093/aob/mcl114
Lambers H, Hayes PE, Laliberte E, Oliveira RS, Turner BL (2015) Leaf manganese accumulation and phosphorus-acquisition efficiency. Trends Plant Sci 20:83–90. https://doi.org/10.1016/j.tplants.2014.10.007
Lambers H, Wright IJ, Guilherme Pereira C, Bellingham PJ, Bentley LP, Boonman A, Cernusak LA, Foulds W, Gleason SM, Gray EF, Hayes PE, Kooyman RM, Malhi Y, Richardson SJ, Shane MW, Staudinger C, Stock WD, Swarts ND, Turner BL, Turner J, Veneklaas EJ, Wasaki J, Westoby M, Xu Y (2021) Leaf manganese concentrations as a tool to assess belowground plant functioning in phosphorus-impoverished environments. Plant Soil. https://doi.org/10.1007/s11104-020-04690-2
Leitenmaier B, Küpper H (2013) Compartmentation and complexation of metals in hyperaccumulator plants. Front Plant Sci 4:374. https://doi.org/10.3389/fpls.2013.00374
Lindsay WL, Norvell WA (1978) Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci Soc Am J 42:421–428. https://doi.org/10.2136/sssaj1978.03615995004200030009x
Lindström BEM, Frankow-Lindberg BE, Dahlin AS, Wivstad M, Watson CA (2013) Micronutrient concentrations in common and novel forage species and varieties grown on two contrasting soils. Grass Forage Sci 68:427–436. https://doi.org/10.1111/gfs.12006
Lyu Y, Tang H, Li H, Zhang F, Rengel Z, Whalley WR, Shen J (2016) Major crop species show differential balance between root morphological and physiological responses to variable phosphorus supply. Front Plant Sci 7:1939. https://doi.org/10.3389/fpls.2016.01939
Mimmo T, Hann S, Jaitz L, Cesco S, Gessa CE, Puschenreiter M (2011) Time and substrate dependent exudation of carboxylates by Lupinus albus L. and Brassica napus L. Plant Physiol Biochem 49:1272–1278. https://doi.org/10.1016/j.plaphy.2011.08.012
Neumann G, Römheld V (1999) Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 211:121–130. https://doi.org/10.1023/A:1004380832118
Neumann G, George TS, Plassard C (2009) Strategies and methods for studying the rhizosphere-the plant science toolbox. Plant Soil 321:431–456. https://doi.org/10.1007/s11104-009-9953-9
Oburger E, Jones DL (2018) Sampling root exudates – mission impossible? Rhizosphere 6:116–133. https://doi.org/10.1016/j.rhisph.2018.06.004
Oburger E, Schmidt H (2016) New methods to unravel rhizosphere processes. Trends Plant Sci 21:243–255. https://doi.org/10.1016/j.tplants.2015.12.005
Oliveira RS, Galvao HC, de Campos MC, Eller CB, Pearse SJ, Lambers H (2015) Mineral nutrition of campos rupestres plant species on contrasting nutrient-impoverished soil types. New Phytol 205:1183–1194. https://doi.org/10.1111/nph.13175
Pang JY, Ryan MH, Siddique KHM, Simpson RJ (2017) Unwrapping the rhizosheath. Plant Soil 418:129–139. https://doi.org/10.1007/s11104-017-3358-y
Pang J, Bansal R, Zhao H, Bohuon E, Lambers H, Ryan MH, Ranathunge K, Siddique KHM (2018) The carboxylate-releasing phosphorus-mobilizing strategy can be proxied by foliar manganese concentration in a large set of chickpea germplasm under low phosphorus supply. New Phytol 219:518–529. https://doi.org/10.1111/nph.15200
Pearse SJ, Veneklaas EJ, Cawthray G, Bolland MD, Lambers H (2007) Carboxylate composition of root exudates does not relate consistently to a crop species' ability to use phosphorus from aluminium, iron or calcium phosphate sources. New Phytol 173:181–190. https://doi.org/10.1111/j.1469-8137.2006.01897.x
Prescott CE, Grayston SJ, Helmisaari HS, Kaštovská E, Körner C, Lambers H, Meier IC, Millard P, Ostonen I (2020) Surplus carbon drives allocation and plant-soil interactions. Trends Ecol Evol 35:1110–1118. https://doi.org/10.1016/j.tree.2020.08.007
Puig S, Peñarrubia L (2009) Placing metal micronutrients in context: transport and distribution in plants. Curr Opin Plant Biol 12:299–306. https://doi.org/10.1016/j.pbi.2009.04.008
R Development Core Team (2020) R: a language and environment for statistical computing. Vienna, Austria: R foundation for statistical computing. http://www.R-project.org/. Accessed 18 Jan 2020
Saarnio S, Wittenmayer L, Merbach W (2004) Rhizospheric exudation of Eriophorum vaginatum L. – potential link to methanogenesis. Plant Soil 267:343–355. https://doi.org/10.1007/s11104-005-0140-3
Sadras V, Alston J, Aphalo P, Connor D, Denison RF, Fischer T, Gray R, Hayman P, Kirkegaard J, Kirchmann H, Kropff M, Lafitte HR, Langridge P, Lenne J, Mínguez MI, Passioura J, Porter JR, Reeves T, Rodriguez D, Ryan M, Villalobos FJ, Wood D (2020) Making science more effective for agriculture. Adv Agron 163:153–177. https://doi.org/10.1016/bs.agron.2020.05.003
Shane MW, Lambers H (2005) Manganese accumulation in leaves of Hakea prostrata (Proteaceae) and the significance of cluster roots for micronutrient uptake as dependent on phosphorus supply. Physiol Plant 124:441–450. https://doi.org/10.1111/j.1399-3054.2005.00527.x
Shane MW, Lambers H, Cawthray GR, Kuhn AJ, Schurr U (2008) Impact of phosphorus mineral source (Al–P or Fe–P) and pH on cluster-root formation and carboxylate exudation in Lupinus albus L. Plant Soil 304:169–178. https://doi.org/10.1007/s11104-007-9535-7
Shen J, Yuan L, Zhang J, Li H, Bai Z, Chen X, Zhang W, Zhang F (2011) Phosphorus dynamics: from soil to plant. Plant Physiol 156:997–1005. https://doi.org/10.1104/pp.111.175232
Socha AL, Guerinot ML (2014) Mn-euvering manganese: the role of transporter gene family members in manganese uptake and mobilization in plants. Front Plant Sci 5:106. https://doi.org/10.3389/fpls.2014.00106
Stevenson PC, D'Cunha RF, Grzywacz D (2010) Inactivation of baculovirus by isoflavonoids on chickpea (Cicer arietinum) leaf surfaces reduces the efficacy of nucleopolyhedrovirus against Helicoverpa armigera. J Chem Ecol 36:227–235. https://doi.org/10.1007/s10886-010-9748-8
Sun RL, Zhou QX, Jin CX (2006) Cadmium accumulation in relation to organic acids in leaves of Solanum nigrum L. as a newly found cadmium hyperaccumulator. Plant Soil 285:125–134. https://doi.org/10.1007/s11104-006-0064-6
Suriyagoda LDB, Lambers H, Renton M, Ryan MH (2012) Growth, carboxylate exudates and nutrient dynamics in three herbaceous perennial plant species under low, moderate and high phosphorus supply. Plant Soil 358:100–112. https://doi.org/10.1007/s11104-012-1311-7
Thomine S, Vert G (2013) Iron transport in plants: better be safe than sorry. Curr Opin Plant Biol 16:322–327. https://doi.org/10.1016/j.pbi.2013.01.003
Tian S, Lu L, Labavitch J, Yang X, He Z, Hu H, Sarangi R, Newville M, Commisso J, Brown P (2011) Cellular sequestration of cadmium in the hyperaccumulator plant species Sedum alfredii. Plant Physiol 157:1914–1925. https://doi.org/10.1104/pp.111.183947
Touchton JT, Johnson JW, Cunfer BM (1980) The relationship between phosphorus and copper concentrations in wheat. Commun Soil Sci Plant Anal 11:1051–1066. https://doi.org/10.1080/00103628009367104
Tsai H-H, Schmidt W (2017) One way. Or another? Iron uptake in plants. New Phytol 214:500–505. https://doi.org/10.1111/nph.14477
Veneklaas EJ, Stevens J, Cawthray GR, Turner S, Grigg AM, Lambers H (2003) Chickpea and white lupin rhizosphere carboxylates vary with soil properties and enhance phosphorus uptake. Plant Soil 248:187–197. https://doi.org/10.1023/A:1022367312851
Wang Y, Lambers H (2019) Root-released organic anions in response to low phosphorus availability: recent progress, challenges and future perspectives. Plant Soil 447:135–156. https://doi.org/10.1007/s11104-019-03972-8
Wen Z, Li H, Shen Q, Tang X, Xiong C, Li H, Pang J, Ryan MH, Lambers H, Shen J (2019) Tradeoffs among root morphology, exudation and mycorrhizal symbioses for phosphorus-acquisition strategies of 16 crop species. New Phytol 223:882–895. https://doi.org/10.1111/nph.15833
Wen Z, Pang J, Tueux G, Liu Y, Shen J, Ryan MH, Lambers H, Siddique KHM (2020) Contrasting patterns in biomass allocation, root morphology and mycorrhizal symbiosis for phosphorus acquisition among 20 chickpea genotypes with different amounts of rhizosheath carboxylates. Funct Ecol 34:1311–1324. https://doi.org/10.1111/1365-2435.13562
White PJ (2012a) Long-distance transport in the xylem and phloem. In: Marschner P (ed) Marschner's mineral nutrition of higher plants, 3rd edn. Academic Press, San Diego, pp 49–70
White PJ (2012b) Ion uptake mechanisms of individual cells and roots: short-distance transport. In: Marschner P (ed) Marschner's mineral nutrition of higher plants, 3rd edn. Academic Press, San Diego, pp 7–44
Wouterlood M, Cawthray GR, Scanlon TT, Lambers H, Veneklaas EJ (2004a) Carboxylate concentrations in the rhizosphere of lateral roots of chickpea (Cicer arietinum) increase during plant development, but are not correlated with phosphorus status of soil or plants. New Phytol 162:745–753. https://doi.org/10.1111/j.1469-8137.2004.01070.x
Wouterlood M, Cawthray GR, Turner S, Lambers H, Veneklaas EJ (2004b) Rhizosphere carboxylate concentrations of chickpea are affected by genotype and soil type. Plant Soil 261:1–10. https://doi.org/10.1023/B:PLSO.0000035568.28893.f6
Wouterlood M, Lambers H, Veneklaas EJ (2005) Plant phosphorus status has a limited influence on the concentration of phosphorus-mobilising carboxylates in the rhizosphere of chickpea. Funct Plant Biol 32:153–159. https://doi.org/10.1071/Fp04084
Yu RP, Li XX, Xiao ZH, Lambers H, Li L (2020) Phosphorus facilitation and covariation of root traits in steppe species. New Phytol 226:1285–1298. https://doi.org/10.1111/nph.16499
Zhang D, Zhang C, Tang X, Li H, Zhang F, Rengel Z, Whalley WR, Davies WJ, Shen J (2016) Increased soil phosphorus availability induced by faba bean root exudation stimulates root growth and phosphorus uptake in neighbouring maize. New Phytol 209:823–831. https://doi.org/10.1111/nph.13613
Zhang W, Chen X-X, Liu Y-M, Liu D-Y, Chen X-P, Zou C-Q (2017) Zinc uptake by roots and accumulation in maize plants as affected by phosphorus application and arbuscular mycorrhizal colonization. Plant Soil 413:59–71. https://doi.org/10.1007/s11104-017-3213-1
Zhang W, Zou C, Chen X, Liu Y, Liu D, Yang H, Deng Y, Chen X (2020) Phosphorus application decreased copper concentration but not iron in maize grain. Agronomy 10:1716. https://doi.org/10.3390/agronomy10111716
Zhou T, Du Y, Ahmed S, Liu T, Ren M, Liu W, Yang W (2016) Genotypic differences in phosphorus efficiency and the performance of physiological characteristics in response to low phosphorus stress of soybean in southwest of China. Front Plant Sci 7:1776. https://doi.org/10.3389/fpls.2016.01776
Zhou Y, Neuhauser B, Neumann G, Ludewig U (2020) LaALMT1 mediates malate release from phosphorus-deficient white lupin root tips and metal root to shoot translocation. Plant Cell Environ 43:1691–1706. https://doi.org/10.1111/pce.13762
Acknowledgments
This work was funded by the Australian Research Council Linkage Projects (LP200100341), the National Natural Science Foundation of China (31772402, 31330070), and National Key Research and Development Program of China (2016YFE0101100, 2017YFD0200200). We thank Rob Creasy and Bill Piasini for help with maintaining the plants in the glasshouse, Greg Cawthray for help with the analysis of carboxylates, Michael Smirk for help with the micronutrient analyses, and Dr. Jing Dai for the constructive comments on results discussion. We also greatly appreciate the support from the Chinese Scholarship Council (CSC) providing a Ph.D. student visiting scholarship to Zhihui Wen.
Author information
Authors and Affiliations
Contributions
Z.W., J.P., M.H.R., J.S., K.H.M.S. and H.L. designed the study; Z.W. and J.P. performed the experiments and collected the data; Z.W., J.P., M.H.R., J.S., K.H.M.S. and H.L. analysed and interpreted the data; Z.W. led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding authors
Additional information
Responsible Editor: Enzo Lombi
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 1228 kb)
Rights and permissions
About this article
Cite this article
Wen, Z., Pang, J., Ryan, M.H. et al. In addition to foliar manganese concentration, both iron and zinc provide proxies for rhizosheath carboxylates in chickpea under low phosphorus supply. Plant Soil 465, 31–46 (2021). https://doi.org/10.1007/s11104-021-04988-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-04988-9