Abstract
Background and aims
Root biomass is one of the most widely used parameters to characterise root growth and belowground carbon stock. Our aim was to define a standard method to estimate the root biomass of young and adult oil palm trees in commercial plantations.
Methods
Three methods based on the sampling excavation volume were compared using the same sampled tree. Work time and the number of workers required for each operation were recorded. We compared two large excavation volumes based on Voronoi tessellation and the standard root auger coring method in one 2-year-old and one 16-year-old commercial oil-palm plantation in Benin, West Africa.
Results
Oil palm total root biomass was estimated at 0.84 and 22.23 Mg ha−1 in the 2-year-old and 16-year-old plantation, respectively. Compared to the reference method, the simplified Voronoi trench method estimated slightly higher (+5%) and lower (−17%) total root biomass with no significant differences but required 2 and 3 times more labour time, respectively, while the auger method estimated significantly lower (−23% and − 53%) total root biomass in the 2-year-old and 16-year-old plantation, respectively. Coarse and fine root biomass were significantly higher under the windrow than under the footpath zones.
Conclusion
The simplified Voronoi trench method required twice as much labour time as the auger method but was most efficient way to estimate oil palm total root biomass, irrespective of the age of the plantation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Roots are the most essential organ for plants to access the water and minerals they need for their growth and development (Marschner et al. 1991; Tinker and Nye 2000) and to anchor the plant to its support, generally the soil (Ennos 2000). As (except for epiphytes) roots are hidden in the soil (Waisel et al. 1991), researchers always face methodological problems to access the root system. To date, numerous methods have been developed in order to characterize the root system architecture, or the size of the root system, or its distribution in the soil. The choice of method was mainly driven by the objective of the study and generally, a single methodology corresponds to each objective (Böhm 1979). There is consequently no single reliable methodology available (Vogt et al. 1998) to simultaneously sample different types of roots including coarse roots, depending on the tree species or plant age (Levillain et al. 2011). Thus, despite its importance for plant production and functioning, estimating belowground biomass and its distribution in the soil is still difficult and time consuming because of the large quantity of soil that has to be removed to access roots that are heterogeneously distributed in the soil (Böhm 1979; Waisel et al. 1991).
Different methods have been used to determine root biomass (Böhm 1979; Smit et al. 2000). The way they are set up generally depends on the ratio of their advantages to their disadvantages, and priority is usually given to those which are the least time consuming, cheapest and easiest to implement, sometimes jeopardising accuracy. Little information is available in the literature concerning the percentage of errors committed in the evaluation of root biomass using these different sampling methods as well as the time needed to achieve the result with each method (Levillain et al. 2011). The efficiency of the sampling method defined by Levillain et al. (2011) as the ratio between the error of the estimation using this method (compare to a reference method) and the time needed to apply the method (expressed in person day−1), is another key parameter for the classification of the root biomass estimation methods.
The easiest, cheapest, and quickest method to estimate root biomass is using allometric equations or models (Chave et al. 2005; Kenzo et al. 2009; Kralicek et al. 2017; Saint-André et al. 2005; Vieilledent et al. 2012; Yuen et al. 2013). But equations or models need to be calibrated with large datasets collected using reliable methods of excavation, the most common of which is the auger method (Böhm 1979). With this method, the volume of soil sampled is generally small (a few cm3), meaning the method is easy to apply, cheap and rapid. However, the reliability of this method is a major concern due to the very small volume of soil sampled, which leads to poor accuracy in estimating root biomass in the case of highly heterogeneous root distribution, and particularly when sampling coarse roots (Levillain et al. 2011). Comparable methods that sample a larger volume of soil than the auger, e.g. monolith methods, have been developed to avoid these drawbacks (Böhm 1979; Smit et al. 2000; Buczko and Kuchenbuch 2009). They consist in sampling monoliths or blocks of soil in a horizontal trench. According to Chopart and Siband (1999), this method requires removing a relatively large volume of soil, which means it is very labour consuming and causes considerable disturbance to the sampled area as a trench must be dug. This method is mostly used as complementary to the method of root impact cartography in a soil trench profile (Maurice et al. 2010).
Before applying any method, it is important to understand the architecture of the root system of the plant species concerned to position the excavation optimally relative to the plant concerned. This is particularly true for trees and shrubs which have different types of roots (e.g., vertical coarse taproots, plagiotropic, medium and fine roots) whose density varies with their distance from the plant (Atger 1992; Jourdan and Rey 1997a).
The Voronoi polygon or diagram method (Honda 1978; Snowdon et al. 2002; Saint-André et al. 2005) is hypothesised to be one of the most accurate methods because it samples a representative volume of soil (taking the distance to the plant into account) that is sufficiently large (a few m3) around the observed plant, including under the plant itself (Levillain et al. 2011). These authors compared four different sampling methods based on the soil sampling volume distributed around the sampled tree (Levillain et al. 2011). Using a dicotyledonous plant in a 6-year-old plantation of eucalypt trees with a taproot system, planted in a rectangular spacing design, these authors reported that the most efficient method to estimate fine root biomass was auger coring. According to the same authors, methods using larger sampling soil volumes (half and full Voronoi trenches) were shown to be the most appropriate methods to estimate medium and coarse root biomass, respectively. The aim of the present study is to assess the performance of the methods on a monocotyledonous tree with a fascicular and fibrous root system, such as the oil palm, which is traditionally planted in alternate rows based on an equilateral triangle planting design. As the coarse roots of the oil palm are not more than 1 cm in diameter and are rather soft to cut, we wanted to test the hypothesis (H1) that the auger method can efficiently sample all oil palm roots, including coarse roots.
Researchers interest in the development of the oil-palm root system is not recent (Yampolsky 1924; Ruer 1968; Tailliez 1971; Tinker 1976; Reis de Carvalho 1991; Cornaire et al. 1994; Henson and Chai 1997). The quantification of root biomass under and around oil palm trees were recurrent preoccupations up to recent times (Ruer 1969; Tailliez 1971; Henson and Chai 1997; Khalid et al. 1999; Nelson et al. 2006, 2014), often linked to the water and mineral nutrition of the trees. In previous studies, roots were sampled using different techniques based on small sampling volume such as flat extractor (Ruer 1969), “pala-draga” (Tailliez 1971), auger cores (Goh and Samsudin 1993; Henson and Chai 1997; Khalid et al. 1999; Mohd Haniff 1998) or monoliths (Dufrêne et al. 1993; Rey et al. 1998), but these methods did not sample the roots located directly under the oil palm trunk. Consequently, all these previous studies neglected a certain amount of root biomass, leading to underestimation of total root biomass. Some studies have shown that a large number of roots, mainly coarse primary roots, grow vertically downwards under the trunk (Jourdan and Rey 1997a), and that their contribution to total root biomass was consequently poorly estimated, at less than 2%, according to Harun et al. (2003). We hypothesise (H2) that the root biomass directly under the palm tree is larger than previously thought, especially in adult palms whose trunk diameters are bigger than the trunks of younger ones. We tested this hypothesis using the Voronoi trench method which makes it possible to collect roots under the trunk of the tree (Levillain et al. 2011; Nodichao et al. 2011).
The specific aim of this study was to test different sampling methods to assess the root biomass of the oil palm tree, chosen as a model of a fascicular and fibrous tree root system, growing in a plantation by comparing their efficiency, defined as the ratio of accuracy of the results to the labour time required to complete the experiment. We wished to identify a standard root system characterisation protocol to enable root type classification and root biomass assessment accurately and efficiently. This protocol will be useful in studies that aim to characterise the behaviours of different plant material in Genotype x Environment x mineral nutrition tests or other agronomic studies on date, coconut, and oil palms, worldwide.
Materials and methods
Planting material
The planting material used was the oil palm (Elaeis guineensis Jacq.), commercial variety (Tenera), C1001F seed category, vascular wilt tolerant (Ngando et al. 2013), extensively distributed in Africa, particularly in Benin. It came from Deli x La Me group, obtained by crossing Dura (DA 115D origin) and Pisifera (LM 2 T origin). The trees sampled in this study were selected in two contrasted plantations, one containing young, 2-year-old, unproductive palms, and the other adult 16-year-old productive palms.
Ecological parameters of the study area
The study was carried out in Pobè District, at the Agricultural Research Centre on Perennial Crops (CRAPP) of the National Institute of Agricultural Research of Benin (INRAB). The study area was located in Southeast Benin, longitude 3°55′E, latitude 6°50’N. The height above sea level was 120 m. Mean annual rainfall between 2000 and 2018 was 1200 mm, with a bimodal distribution. This distribution was uneven and variable, especially during the rainy seasons. The average annual temperature in the same period ranged between 25.8 °C and 30.0 °C. The average annual solar radiation in the same period was 14.91 MJ m−2 d−1.
The palms were planted 9 m apart in a staggered, equilateral triangle design at a planting density of 143 palms ha−1 on a ferrallitic soil (Ferralsols or Oxisols according to FAO or USDA soil classification, resp.). The soil was deep, unsaturated and characterised by its red colour, formed on clay-sandy sediments from the continental terminal. In the 0 to 30-cm layer, soils were sandy with only 5 to 15% of clay, then clay content suddenly increased to 35% between the depths of 30 and 60 cm, and reached 45% and even 50% below this depth (Azontondé 1991). Aholoukpè et al. (2016) proved that recycling fronds in windrows improved soil fertility parameters (C, N, K, CEC, Ca, Mg) in the 0–50 cm soil layer after 10 years. Leaf recycling significantly increased carbon and nitrogen contents in the 0–20 cm soil layer.
Experimental design
Three root sampling methods based on differential excavated soil volumes were tested: (M1) full Voronoi trench (total excavation of 1/12th of the Voronoi space around the sampled tree, Figs. 1 and 2c; Nodichao et al. 2011); (M2) simplified Voronoi trench (53% reduction in excavated volume with respect to the distance to the tree, Figs. 1b, c and 2b); (M3) auger method (Henson and Chai 1997) equally applied within the Voronoi trenches (Fig. 2a). These three methods were applied in the two contrasted oil palm plantations: one 2 years old, the other 16 years old. The three methods were applied successively to each oil palm tree, at the same place (inside the same Voronoi trench and respecting the same distance to sampled tree in a standard commercial plantation, Fig. 2a), at the same period of the season, starting with the least destructive method (auger method, M3) and ending with the full Voronoi method (M1), defined as the reference method due to the large and exhaustive soil volume to excavate. With the aim of assessing the effect of the windrow (zone where fronds are placed during bunch harvest or leaf pruning) on the root system biomass, the roots were sampled systematically both in the windrow and under the footpath between the rows (passage of the harvesters during bunch harvest where no fronds are piled) (Fig. 1a). Six representative palm trees were chosen in each of the two plantations; each with six neighbours of the same age and of comparable size (no replacement trees), i.e. a total of 72 experimental units considering the three sampling methods used on the 12 palms and the two sampling locations (windrow and footpath).
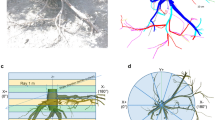
Voronoi space (hexagonal zone) of a sampled oil-palm tree within commercial planting design made up by 12 rectangular triangles (called Voronoi triangle) of same area (a). The Voronoi space was determined by the half distance between the observed palm and its six immediate neighbours (b). One of the 12 Voronoi triangles, inside the equilateral triangle formed by three neighbour palms, located in the planting row where frond leaves were parked in windrow (c). The triangle was divided into 6 different sampling zones according to distance to the centre of the tree: under the tree (Z0), until 1 m (Z1), from 1 m to 2.5 m (Z2c + Z2s) and from 2.5 to 4.5 m (Z3c + Z3s) from the centre of the tree
Position of the 15 root sampling locations for auger coring method (a), inside the different zones of the simplified (b) and complete Voronoi triangle (c). a = 30° arc portion; c = collar girth of the trunk; r = radius; Z0: 30° section of the trunk; Z1: zone where fertilizers were applied; Z2: transition zone between the windrow and the fertilization area, Z2 = Z2s + Z2c; Z3: zone where the fronds were parked into piles called windrow, Z3 = Z3s + Z3c.The figures in bold indicate the distance from the centre of the palm trunk
Method M1 (Figs. 1 and 2c)
The elementary Voronoi space around the sampling tree was designed by the half distance between the tree to be sampled and its neighbours (Levillain et al. 2011). In the oil palm plantation, this space was hexagonal and the trees were planted in alternate rows based on an equilateral triangle design (Nodichao et al. 2011). The area excavated for the full Voronoi method represented 1/12th of the Voronoi space, which was represented by a rectangular triangle with a base of 4.5 m and a hypotenuse of 5.2 m (Figs. 1 and 2). This rectangular triangle, which represented the full Voronoi trench, was divided into four zones based on different distances from the centre of the oil palm trunk, along the adjacent side of the rectangular triangle (Figs. 1b and 2): (i) Z0, directly under the trunk, between its centre and the edge of the trunk, its periphery made up by remaining basal part of leaves. This area was defined by the radius of the trunk and the angle of 1/12th of 360°, equal to 30°; (ii) Z1, from the periphery of the trunk to an average of 1 m away (considering the adjacent side of the rectangular triangle); (iii) Z2, between 1 and 2.5 m from the centre of the trunk; (iv) Z3, from 2.5 m until 4.5 m (half distance between two neighbouring trees) from the centre of the trunk. Z1 and Z2 zones represent the harvesting ring around the tree that was generally kept weeded and where fertilizers were applied. Z3 zone represents the windrows or footpath (depending on the sampling position). In each zone, the roots were sampled in 4 distinct soil depth horizons named H0 (roots above the surface of the soil, e.g. under the windrow), H1 (0–20 cm), H2 (20–50 cm), H3 (50–100 cm).
The rooting profile of the oil palm in Benin was not limited to a depth of 1 m (Nodichao et al. 2011). However, in this study, which aimed at comparing methods, we limited root sampling to a depth of 1 m for budgetary reasons to save labour time. The same soil depth layers were used for all three methods and at both positions (windrows and footpath).
Finally, for productive and mature oil palms, we assumed that all the roots of the sampled tree that grow outside the Voronoi trench are balanced by those of neighbouring trees growing inside the polygon (Honda 1978; Levillain et al. 2011; Snowdon et al. 2002).
Method M2 (Fig. 2b)
We aimed to reduce the volume of the full Voronoi trench by half, but only in the zones located farthest from the palm tree (Z2 and Z3) where a huge volume of soil should be excavated. The simplified Voronoi trench was limited to a strip of 70 cm in width along the base of the rectangular triangle. The reduced Z2 and Z3 zones were noted Z2s and Z3s while the complement zones were noted Z2c and Z3c, respectively. Thus, Z2 = Z2s + Z2c, and Z3 = Z3s + Z3c (Figs. 1b and 2).
Method M3 (Fig. 2a)
Three cores were sampled with a root auger (internal diameter 8 cm) in each zone of the Voronoi trench apart from under the palm tree itself (Z1, Z2s, Z2c, Z3s and Z3c). The three sample cores were located on one of the diagonals of each zone formed by the opposite angles from the point that lied at the centre of gravity of the zone. In two successive zones concerned by the same sampling point, two different corner angles were chosen to avoid sampling the same area (Fig. 2a; M3). On each sampled tree, a total of 9 and 15 cores were taken per simplified and full Voronoi trench, respectively (Fig. 2a). The Z0 zone was not sampled using this method as it was impossible to drill vertically under the tree without cutting it.
Root processing
After collection, the roots were cleaned and sorted into three classes of living roots according to their topological order and diameter (Jourdan and Rey 1997a): first order roots emerging on the root bole under the palm tree, named primary roots (R1) whose diameter was >5 mm; ramifications of R1 named secondary roots (R2), with a diameter ranging between 2 and 5 mm; all ramifications of R2 (tertiary and quaternary roots), named fine roots (Rf = R3+ R4), whose diameter was <2 mm. Dead roots were separated from living ones according to colour (young living roots were white or brown whereas dead roots were dark brown or black) and flexibility (dead roots were more flexible) and were discarded; only the living roots were included in the estimations of total root biomass. For each type of roots, the sampling zone, soil depth, and the total fresh weight of the samples were recorded and a sub-sample was weighed. The sub-samples were dried in the oven at 65 °C for 48 h to estimate their dry weights. The dry matter weights were then calculated for each root type, sampling zone and soil depth.
Total root biomass estimation according to each tree and per hectare
To assess the possible effect of windrows of fronds in the plantation on the distribution of oil palm roots in the soil, roots were sampled systematically according to the position of the windrows (W) and of the footpaths (P) without pruned fronds, and the footpath zone was hypothesised to be a homogenous zone with respect to soil organic matter (Aholoukpè et al. 2013).
Root biomass sampled using the auger method (M3) and the simplified Voronoi method (M2) were extrapolated to the theoretical soil volume sampled with the reference method (M1) in order to be able to compare the estimated root biomasses with the same sampled soil volume. According to the arrangement of the twelve rectangular triangles (Full Voronoi trenches, Fig. 1a, b) inside the hexagonal Voronoi space, four Voronoi trenches were under the frond windrows and the eight others were in the footpath zone, with no fronds. Then, for each method, to assess total root biomass (TRB) of each tree, we used Eq. 1:
For the total plantation, based on the tree planting density of 143 trees per hectare, we used Eq. 2:
For M1 and M2 methods, measurements of the zone actually excavated in the plantations were recorded in order to adjust our sampling excavations to the theoretical volumes of the different zones of soil around each tree. This was considered when processing for the total root biomass for each tree.
Comparison of methods and statistical analysis
As we assumed M1 sampled all the roots in the soil volume of the full Voronoi trench accurately, it was used as the reference method to which the two others (M2 and M3) were compared. The amount of root biomass obtained with method M3 was added to the amount obtained successively with M2 and M3 in the corresponding zones and soil depths.
The accuracy (AM) of methods M2 and M3 was determined as the relative difference in root biomass (RB) estimated by each method compared to that of the reference method (M1). The accuracy of the method (AM) is given by Eq. 3:
where x = {2; 3.}
Consequently, the error (EMx) of each method is given by Eq. 4:
The efficiency of the root sampling method was defined as the ratio between the percentage of error and the time required (in person day−1) to complete the work using the corresponding method. Thus, the most efficient method had the lowest absolute ratio.
For each root class (R1, R2 and Rf), soil depth (H0, H1, H2 and H3), soil zone (Z0, Z1, Z2 and Z3), sampling position (W or P), palm age (2 or 16 years old) and sampling method (M1, M2 and M3), the average root biomass was estimated for six trees located far from one another, considered as replicates. A total of 192 and 324 root samples were processed for the Voronoi and auger sampling methods, respectively. Total root biomass, percentage of error and efficiency were calculated for each method and the two palm tree ages. The differences between methods were statistically tested using the GLM (ANOVA Model) procedure in SAS (SAS® Institute Inc., Cary, NC, USA, 2004) combined with a Tukey’s test. In the Results section, mean root biomass is given with the standard deviation (s.d.) and expressed in terms of dry matter (Mg ha−1). The root biomass assessed per sampling zone and soil depth are expressed as a percentage (%) of total root biomass.
Results
Oil palm root biomass
Considering the reference method (M1) and 1 m of depth only, the total root biomass of the mature 16-year-old oil-palm plantation was 22.23 ± 0.81 Mg ha−1 while in the young 2-year-old plantation, it amounted to 0.84 ± 0.03 Mg ha−1 (Table 1). Root types were quantified in the mature oil-palm plantation at 17.02 ± 1.12 Mg ha−1 (representing 76.6% of TRB) for primary roots (R1); 3.11 ± 0.29 Mg ha−1 for secondary roots (R2) (14.0% of TRB) and 2.10 ± 0.25 Mg ha−1 (9.4% of TRB) for the fine roots (Rf). Using the same method and at the same depth in the 2-year-old oil-palm plantation, total root biomass was 0.36 ± 0.04 Mg ha−1 (42.8% of TRB); 0.26 ± 0.02 Mg ha−1 (31.0% of TRB) and 0.22 ± 0.02 Mg ha−1 (26.2% of TRB) for R1, R2 and Rf, respectively (Table 1). The relative proportion of the R2 and Rf were higher in the 2-year-old palms than in the matured palms.
More detailed results on root biomass according to the age of the palm tree, the type of root and soil depth for each sampled palm tree are given in Table S1; they showed little variation between trees.
Root biomass distribution according to the different sampling zones
Total root biomass differed significantly (p = 0.0001) in the different sampling zones, irrespective of the age of the plantation. At 16 years old, oil palm root biomass in Z0 (under the palm trunk) was 3.69 ± 0.53 Mg ha−1 which represented 16% of total root biomass (Fig. 3b). This root biomass increased progressively until Z3 where the biggest quantity of root biomass was recorded (8.33 ± 1.09 Mg ha−1 representing 37% of total root biomass) and more or less constant estimated root biomass in Z1 and Z2, at respectively, 5.44 ± 0.68 Mg ha−1 (24%) and 5.27 ± 0.64 Mg ha−1 (23%). Secondary (R2) and fine roots (Rf) were almost absent under the trunk (Z0, Fig. 3b). Close to the palm tree (Z1: up to 1 m from the stem), the amount of fine root biomass was higher than that of the secondary roots. By contrast, far from the palm tree (Z2: between 1 m and 2.5 m from the stem up to Z3: between 2.5 m and 4.5 m from the stem), R2 biomass was higher than that of fine roots (Fig. 3b). The primary roots also followed the same trend according to distance from the tree except in zone Z2, where a decrease in R1 biomass was observed.
Root biomass (Mg.ha−1) per sampling zones (a, b) and soil depth (c, d) for each root type of 2- (a, c) and 16- (b, d) year old oil-palm plantations. R1 (white bars): primary or coarse roots, R2 (grey bars): secondary or medium roots; Rf (black bars): fine or tertiary and quaternary roots. The different letters above bars indicate significant differences of root biomass for each root type according to distance to tree (Z0: under the palm Z1: up to 1 m, Z2: from 1 m to 2.5 m and Z3: up to 4.5 m from the centre of the sampled tree) and soil depth (p < 0.05, Tukey test, n = 6). The percentages (%) displayed account for the part of all root types per sampling zone regarding to distance to palm tree (a, b) and per soil depth horizons (c, d)
However, at 2 years old (Fig. 3a), the amount of oil palm root biomass increased with distance from the palm tree from 0.09 ± 0.02 Mg ha−1 in Z0, which represented 11% of total root biomass to 37% (0.32 ± 0.04 Mg ha−1) in Z1 and 41% (0.35 ± 0.05 Mg ha−1) in Z2; but decreased drastically to 11% (0.098 ± 0.03 Mg ha−1) in Z3 reflecting the progressive establishment of the juvenile root system.
Root biomass distribution according to soil depth
Irrespective of the age of the plantation, all root type biomasses differed significantly (p = 0.0001) between soil layers (Table 1). Root biomass in H0 (above the mineral soil) concerned only fine roots in the 16-y old plantation in zone Z3 (where pruned fronds were placed on the ground), it amounted to 0.05 ± 0.02 Mg ha−1. As this biomass concerned only 2 sampled trees out of 6 (Table S1, supplementary material), it was not included in our estimation of total root biomass.
In the 16-year-old plantation, total root biomass increased with the rooting depth of the palm tree (Table 1, Fig. 3d). This distribution represented 21% (4.70 ± 0.60 Mg ha−1), 38% (8.57 ± 0.90 Mg ha−1) and 41% (8.96 ± 1.20 Mg ha−1) in the 0–20 cm, 20–50 cm and 50–100 cm depth layers, respectively (Fig. 3d). Considering the different root types, R1 followed the same trend while R2 were more abundant between 20 and 50 cm (Fig. 3d, Table 1). By contrast, fine root biomass was higher in the top soil (0–20 cm payer) and decreased drastically with soil depth.
In the 2-year-old plantation, root biomass increased between topsoil and 50 cm of depth from 39% in the 0–20 cm layer to 47% in 20–50 cm layer, then decreased to 14% below a depth of 50 cm (Fig. 3c, Table 1). R1 and R2 followed the same trend as in the old palm trees, whereas Rf decreased with soil depth (Table 1).
Irrespective of the age of the oil palms, the amount of fine root biomass was always highest in the 0–20 cm soil layer and decreased with soil depth. By contrast, R1 and R2 biomass were higher between the 20 and 50 cm soil layers than at other soil depths (Figs. 3c, d).
Root biomass distribution in the windrow and footpath sampling zones
In the 2-year-old plantation with the reference method (M1), no significant difference (p > 0.05) was found between total root biomass excavated from the windrow (W), where the pruned palm fronds are placed during harvest, and from the footpath (P), where workers walked during harvest and for cultural management practices (0.89 ± 0.028 t ha−1 under the windrow and 0.83 ± 0.029 Mg ha−1 under the pathway). But the difference was significant (p = 0.020) in the 16-year-old plantation (23.86 ± 0.88 Mg ha−1 under the windrow and 21.41 ± 0.74 Mg ha−1 under the pathway).
Likewise, there was no significant difference in secondary root biomass (p > 0.05) between the windrow and the footpath, irrespective of age of the palm trees (Fig. 4). On the other hand, the Rf (W: 2.86 ± 1.49 Mg ha−1 and P: 1.72 ± 0.68 Mg ha−1) and R1 (W: 18 ± 1.27 Mg ha−1 and P: 16.59 ± 0.16 Mg ha−1) biomasses differed significantly (p = 0.042) between the windrows and the footpath in the 16-year-old oil palm tree plantation (Fig. 4b), but were not significant in the young 2-year-old plantation (Fig. 4a). The difference in fine root biomass between the two locations in mature plantation amounted to 1.14 Mg ha−1 whereas it was only 1.41 Mg ha−1 for R1 (Fig. 4b).
Root biomass (Mg.ha−1) of each root type located in footpath zones (white bars) and in windrow (black bars) in 2- (a) and 16- (b) year old oil-palm plantations. R1: primary or coarse roots, R2: secondary or medium roots; Rf: fine or tertiary and quaternary roots. The different letters above bars indicate significant differences of root biomass between windrow and footpath zones (p < 0.05, Tukey test, n = 6). The percentage values in the bars represent the part of root biomass of each root type (%)
With the simplified Voronoi trench method, there was a significant difference in oil palm root biomass under the windrow and under the footpath (p = 0.030), irrespective of the age of the oil palm plantation. The auger method revealed no difference between the windrow and footpath zones in the 16-year-old plantation (p > 0.05). By contrast, in the 2-year-old oil-palm plantation, the same method revealed a highly significant difference (p < 0.001), indicating a high heterogeneity in root distribution around young palm trees.
Accuracy of the root sampling method in estimating root biomass
There was no significant difference between the simplified Voronoi (M2) method and full Voronoi method (M1) based on the estimation of total root biomass (p > 0.05), irrespective of the age of the palms. However, we found a significant difference between the full Voronoi method (M1) and auger method (M3) for the estimation of root biomass, irrespective of the age of the palm trees (p < 0.01). In the 16-year-old oil palms and the 0 to 1 m soil layer, total root dry matter was estimated at 22.23 ± 0.81 Mg ha−1, 18.41 ± 1.26 Mg ha−1 and 10.45 ± 0.34 Mg ha−1 with the reference (M1), simplified Voronoi (M2) and auger method (M3), respectively. In the young, 2-year-old oil palm plantation, total root biomass was estimated at 0.84 ± 0.03 Mg ha−1, 0.90 ± 0.03 Mg ha−1 and 0.66 ± 0.026 Mg ha−1 with the reference (M1), simplified Voronoi (M2) and auger methods (M3), respectively. The percentage of error of each method (M2 and M3 compared to the reference method M1) according to the different root types (R1, R2 and Rf), the sampling positions (W and P), the sampling zones (Z1, Z2, Z2s, Z2c, Z3, Z3s and Z3c) and the three soil depths (H1, H2 and H3), refer to the plantation ages (2 and 16 years old) are given in Supplementary material, Tables S2 and S3, respectively. The results we present here (Table 2) is for the whole soil profile sampled (0–1 m).
In the 2-year-old plantation (Table 2), the auger method underestimated total root biomass by 23%, the simplified Voronoi method overestimated slightly by 4.7%. This result confirmed the significant difference (p < 0.01) found between the auger method and the reference method. However, the simplified Voronoi method and the reference method made similar errors (p > 0.05). The percentages of errors presented below according to the different root types and the sampling zones concern the auger method compared to the reference method for the whole soil profile (0–100 cm). With the auger method, R1 was badly sampled with a large underestimation by 56% in Z1, by 42% in Z2 and a large overestimation by 68% in Z3 of total root biomass compared to the reference method (Table S2). However, R2 was well sampled with the auger method except in Z3 (Z3s + Z3c) where 44% higher estimate of total root biomass was observed. But specifically considering the Z3s zone, total root biomass was estimated to be 17% higher with the auger method than with the full Voronoi trench method. By contrast, with still the auger method, the percentages of error were 10% and 15% lower in Z1 and Z2, respectively (Table S2). The fine roots were quite well sampled with the auger method except in Z3. The percentage of error in Z1, Z2 and Z3 was −13%, −26% and + 30%, respectively. However, considering the Z3s zone, the auger method estimated lower total root biomass (−18%) than the full Voronoi trench method (Table S2). Because we were unable to sample the Z0 zone with the auger method, this method underestimated by −11% the total root biomass of juvenile plantations.
In the 16-year-old plantation and considering the whole soil profile (i.e. 0 to 100 cm), the auger method produced a significantly lower estimation (−53.1%) of total root biomass, whereas the error was 17.1% less with the simplified Voronoi trench method compared to the reference method (Table 2). The difference between the auger method and the full Voronoi method was significant (p < 0.01) while the simplified Voronoi method did not differ significantly from the full Voronoi trench method (p > 0.05). The percentages of errors presented below according to the different root types and the sampling zones only compare the auger method to the reference method. In the 16-year-old palm plantation, R1 was poorly sampled with the auger method that produced huge errors −78%, −41% and − 29% in estimating root biomass for the Z1, Z2 and Z3 zones, respectively, compared to the full Voronoi trench method. The R2 were also badly sampled by the auger method with −26%, −32% and − 23% errors in Z1, Z2 and Z3 zones respectively, compared to the full Voronoi trench method. However, estimates of root biomass using the auger method were reduced to −16% in the Z3s zone (Table S3). Surprisingly, the fine roots were also poorly sampled by the auger method with −44% and − 21% lower estimates of fine root biomass and + 34% higher estimation in Z1, Z2 and Z3 zones, respectively, than the full Voronoi trench method (Table S3). Furthermore, the auger method was not able to sample the Z0 zone, which led to a − 16% lower estimation of the total root biomass compared to full Voronoi trench method.
Considering the previous results, the simplified Voronoi method led to smaller errors when compared to reference method than the auger method, irrespective of the age of the oil palm trees. The auger method produced more errors, and was less accurate in the 16-year-old plantation than in the 2-year-old one.
Labour time and efficiency of root sampling methods
There was a major difference in the amount of labour time needed for root sampling depending on the method and irrespective of the age of the plantations at the 1% level. Otherwise, the volumes of soil handled differed with the method at the 5% level, but did not differ with the age of the plantation. The average labour time needed for the full Voronoi trench method for 6 sampled trees was 113.6 ± 16.9 person day−1 in the 16-year-old plantation and 42.1 ± 8.8 person day−1 in the 2-year-old plantation (Table 2). The shortest labour time was required with the auger method (6.8 ± 1.1 person day−1 and 13.4 ± 6.1 for the 2-year-old and 16-year-old plantation, respectively). The full Voronoi method required handling 5.85 m3 of soil while it was more than 1000 times less (5.10−3 m3) using the auger method. The efficiency of the simplified Voronoi method was 0.24 and 0.46 for 2-year-old and 16-year-old plantations, respectively. With the auger method, efficiency was 3.42 and 3.96 for the 2-year-old and 16-year-old plantations, respectively (Table 2). The simplified Voronoi method was thus more efficient than the auger method compared to the reference method, irrespective of the age of the plantation (Table 2).
Discussion
Estimating oil-palm root biomass
Total root biomass estimated using the full Voronoi trench method for young 2-year-old oil palms in our study (0.84 ± 0.03 Mg ha−1) was slightly lower than that estimated in previous studies conducted in Benin using the same method but on 4-year-old palms (Nodichao 2008; Nodichao et al. 2011). These authors estimated total root biomass ranging from 2.41 ± 0.3 to 3.22 ± 0.25 Mg ha−1 depending on the oil palm progenies. For the progeny C1001F studied here, Nodichao et al. (2011) estimated total root biomass at 2.87 ± 0.17 Mg ha−1. This amount represents almost 3.3 times our estimation in the 2-year-old plantation. This difference might be due to the exponential root growth after the plantation reached the age of 2, but was probably due to soil variability and different local weather conditions in the two sites that are located 50 km apart. Nevertheless, in both situations, root biomass was highly variable, as pointed out in many studies (e.g. Vogt et al. 1998; Jackson et al. 1997) particularly in young plantations when the root system is not yet well established (Jourdan 1995; Jourdan and Rey 1997a).
The quite low root biomass observed in young oil palms showed that their root system was still in establishing itself with only 6 root types, whereas the adult oil palm has 8 root types with the same number (4) of topological root order (Jourdan and Rey 1997a), indicating an exponential increase in root production with age by young oil palms. Indeed, 2 years after planting, the R2 biomass increased suddenly and reached 0.57 Mg ha−1 at 3 years old, while the fine root biomass amounted to 0.215 Mg ha−1 at the same stage (Jourdan 1995). Subsequently, total root biomass increased exponentially during the young stage due to the increasing number of new R1 emitted following the 8th month after germination. At the end of the young stage, R1 biomass represents more than 40% of total biomass and this amount increases thereafter to stabilize at around 80% in adult phase (Jourdan 1995), which was confirmed in our study (Table 1).
Using the auger coring method, Haniff et al. (2014) estimated similar total root biomass in young oil palms in the northern region of Malaysia (dry site): 1.07 ± 0.24 Mg ha−1 for 2.5-year-old oil palms (Table 3) compared to 0.84 Mg ha−1 in the 2-year-old trees in our study. The slight differences might be due to the difference in the genetic origin of the palms, the soil environments and maybe the rainfall regime in the two sites. In the west coast region of Malaysian peninsular (more humid) on mineral soil, using a root auger in the 0–100 cm soil profile, Henson and Chai (1997) estimated total root biomass at 4.4 Mg ha−1 in 4-year-old palms (Table 3) which is significantly higher (about 2.2 times higher) than that estimated by Haniff et al. (2014) (2.03 Mg ha−1 at 4.5-y old), about 5.2 times higher than our estimation in the 2-year-old oil-palm plantation, and about 1.5 times higher than that estimated by Nodichao et al. (2011) in 4-year-old C1001F progeny but using the full Voronoi trench method.
All the differences observed in the young oil-palm plantations around the world could be explained by the different methods used, which involved different sampled soil volumes, different sampled soil locations around the trees, and finally, a poor consideration of the spatial heterogeneity of the root distribution, which is particularly high at that growth stage. Moreover, different ecological and soil conditions may influence the young oil-palm root dynamics and root distribution according to soil depth. When comparing the wetter Malaysian and Indonesian sites with the drier sites in Ivory Coast and Benin and if we include the differences in oil palm ages and the variability in the genetic material, comparisons are really difficult to accurately assess oil-palm root biomass (Table 3).
Using the auger method in adult palms, Henson and Chai (1997) found a higher total root biomass in a coastal alluvial soil (15.9 Mg ha−1 at 10-years) than in inland mineral soils (7.0–10.8 Mg ha−1), both of which are less than our observations in 16-year-old oil palms (22.23 Mg ha−1). Using the auger method in a 9-year-old oil-palm plantation in the “Llanos Orientales” savannahs in Colombia, Rüegg et al. (2019) estimated total root biomass to be 16.9 ± 1.3 Mg ha−1, with 8.6 ± 0.4 Mg ha−1 of fine roots in the top 30-cm soil layer. The same authors estimated the total root biomass of a 15-year-old oil-palm plantation at 23.05 Mg ha−1 (Rüegg 2017) similar to our value of 22.23 Mg ha−1 in our 16-year-old plantation, even if ecology conditions in Benin are less favourable for oil palm productivity (Table 3).
Using the auger method in Sumatra, Syahrinudin (2005) estimated oil palm root biomass to be 16.1 Mg ha−1 in a 10-year-old oil-palm plantation, which is higher than in Benin conditions where Nodichao (2008) estimated the total root biomass of an 11-y old oil-palm plantation to be 11 Mg ha−1 using the full Voronoi trench method, considered as the reference method in the present study. Here again, the differences in root biomass production between oil palm trees growing in different ecological conditions could also be explained by root growth dynamics. Indeed, according to Jourdan and Rey (1997a), at the adult stage, more primary roots were produced than the other types of roots because of their regular speed of growth which is about 0.3 cm day−1, corresponding to a 1-m increase per year and with no mortality in those roots, However, oil palm roots stop elongating in the 3- to 4-month dry season in Benin (Nodichao et al. 2011) whereas this phenomenon was not observed in Ivory Coast (Jourdan and Rey 1997a) or at wetter sites like in Indonesia (Jourdan, Unpublished data).
Effect of distance from the tree on root biomass distribution
According to the distance from the tree in Columbia, Rüegg (2017) found no significant effects in oil-palm total root biomass between zones in the shallow rooting profile (0–30 cm). But, when the weeding circle (fertilizer application zone) was included in the analysis, the effect of the distance from the tree was only significant in the fine root biomass in the topsoil (0–10 cm) (Rüegg et al. 2019), whereas total root biomass was evenly distributed in the three sampled layers (0–10 cm, 10–20 cm and 20–30 cm) (Rüegg 2017). These observations only resemble our results for primary roots in the 16-year-old plantation (Fig. 3b) but not those of the other root types at that age nor for any root types in the 2-year-old plantation (Fig. 3a). Syahrinudin (2005) uprooted 3, 10, 20 and 30-year-old palms, and found that the so-called trunk base, made up by the base of the trunk itself and the roots located below down to a depth of 1 m (equivalent to Z0 in our study, but we exclude the trunk base) added another 10, 55, 45 and 46% to total root biomass, respectively. These results are similar to our observations but only in the 2-year-old plantation (11%) where the weight of the trunk base was not included at 2 years old. According to preliminary observations in Benin using the simplified Voronoi trench method, the primary root biomass in Z0 amounted to 20% of total root biomass in 4-year-old oil palms (Nodichao 2008) and reached 17% in 11-year-old oil palms in Sumatra, Indonesia (Jourdan, Unpublished data).
Effect of soil depth on root biomass distribution
According to soil depth, our results clearly show that the oil-palm roots were mostly unevenly distributed at both young and adult stages (Figs. 3c, d). Indeed, primary roots, which accounted for the majority of total root biomass (Table 1, Nodichao et al. 2011), were mainly located in the 20–50 cm and 20–100 cm soil layer, while fine roots preferentially colonized the topsoil (0–20 cm) layer in our 2- and 16-year-old plantations, respectively. Secondary roots were mostly evenly distributed throughout the whole root profile observed at both ages. These results are typical of those in the oil palm root literature (Henson and Chai 1997; Khalid et al. 1999; Nelson et al. 2006; Nodichao et al. 2011; Syahrinudin 2005) despite the different ecotypes. This is probably due to the regular topology and geometry of the root system architecture of this monocotyledonous tree (Jourdan and Rey 1997a) where the growth direction of R1 and R2 is fixed according to the perception of gravitropism (Jourdan et al. 2000). Primary roots were found growing either vertically (under the palms) or horizontally (around the palms). The R2 branched on the horizontal R1 were mostly grew vertically either upwards or downwards (Jourdan and Rey 1997a), due to the specific perception of gravitropism in the root cap of these roots (Jourdan et al. 2000) and this phenomenon is observed worldwide.
The oil palm root system is not limited to a depth of 1 m. As many authors have reported, it can go down to a depth of at least 6 m in deep ferrallitic sandy soil in Ivory Coast (Jourdan and Rey 1997a) or in the loamy-sandy soils of the Eastern Amazon region of Brazil (Sommer et al. 2000) or in the mineral soil of western Kalimantan and Sumatra, Indonesia (Syahrinudin 2005). However, most root biomass is concentrated in the top 60 cm (Syahrinudin 2005), mainly due to R1 roots located in that soil layer. For that reason, in our study, we chose to stop the excavations at a depth of 1 m in order to compare the sampling methods in a rooting profile that includes the maximum root biomass.
Effect of windrow on root biomass distribution
Using the full Voronoi trench method, no difference in total root biomass was found (p = 0.606) between the windrow (W) and the footpath (P) zones in the 2-year-old plantation. This is quite obvious because no fronds are pruned and placed on the ground in the young development stages of the oil palm, in order to maximise photosynthetic activity for carbon production, storage and allocation to roots before fruit production, which starts at the beginning of the third year. Thus, no windrows could add heterogeneity to soil organic matter (Aholoukpè et al. 2016) leading to spatial heterogeneity of root distribution. According to Aholoukpè et al. (2016), recycled oil palm fronds that are placed in windrows after pruning only improve soil fertility after 10 years. This could explain why the difference in root biomass between windrow and footpath zones was only observed in the 16-year oil-palm plantation.
The lack of any difference in root biomass between the windrow and footpath zones in the 16-year-old plantation with the auger method questions the accuracy of the method itself when used to sample oil-palm roots because differences were clearly observed with both the reference and the simplified method. The marked difference between the windrow and footpath zones in the 2-year-old plantation using auger method despite of the lack of pruned fronds, shows that this difference is not related to the windrow but more likely to the inability of the auger method to capture even highly heterogenous root distribution.
The difference in primary root biomass between windrow and footpath zones could be explained by the lower soil density observed under the windrow zones (Aholoukpè et al. 2016), leading to favourable R1 growth dynamics. Indeed, in Eastern plains of Colombia, Rüegg (Rüegg 2017; Rüegg et al. 2019) found that shallow (0–10 cm) root biomass increased significantly under the piles of fronds due to more R1 and Rf proliferation compared to in deeper soil layers, whereas this phenomenon was not observed under the footpath zone.
Fine roots are considered as the most important organs for water and nutrient uptake (Marschner et al. 1991; Nelson et al. 2006; Tinker and Nye 2000) and they proliferate in presence of organic matter or humidity (Aholoukpè et al. 2016). According to Jourdan (1995) and confirmed by Nodichao (2008), oil palm fine roots are preferentially located under the windrow because of the higher soil organic matter and humidity content. Windrows usually contain the empty fruit bunches and frond leaves, rachis, spikes during pruning management, and their application in oil palm plantations should improve local soil fertility and hence improve local root proliferation (Aholoukpè et al. 2016; Kheong et al. 2010). This would be in agreement with Lopèz-Bucio et al. (2002), who reported that plants developed more roots in response to nutrient hotspots that stimulate root growth.
Comparison of root sampling methods
Our results clearly show that the auger method produced lower estimates of oil palm root biomass than the reference method. A technical reason could be simply the relatively limited volume of soil sampled. Indeed, when an auger with a small diameter is used, the probability of sampling heterogeneously (horizontally and vertically) distributed roots is rather low, particularly in young plantations. A (horizontally and vertically) homogeneously distributed root system in a more suitable environment for oil palm growth such as in Asia or South America would probably minimise the percentage of error committed with the auger method. What is more, it is almost impossible to efficiently collect coarse roots (Ø ≥ 1 cm) using the auger method. Given these results, our first hypothesis that the root auger method is efficient for sampling primary roots, is invalidated.
According to Kirby and Rackham (1971), root biomass is underestimated using the auger method if the number of cores is not sufficiently representative of the distribution of the rooting system in the different soil layers (Uwe et al. 2008). This has been confirmed in cereal crops with no coarse roots (Kumar et al. 1993). Thus, increasing the number of auger sampling points would probably reduce the error but a systematic reduction in the percentage of errors is not guaranteed. The main challenge is to be able to capture roots in the soil (which can be considered as a black box), given the heterogeneous root distribution (considering all the zones apart from the tree and all soil layers). Then, sampling roots using small volume auger cores must tackle the problem of the representativeness of root distribution in the soil, which is quite difficult to assess prior to root sampling. However, in a well root-colonized soil volume (suitable environment, very old plantation), the percentage of error would decrease substantially with auger sampling, regardless of the number of sampling points. The auger method cannot properly sample roots located very close to and immediately under the trunk of the palm tree (Z0 zone) without cutting the palm and drilling across the trunk base. Z0 is the zone where primary roots are denser and mainly grow vertically (Jourdan and Rey 1997a), sampling them in a small volume of soil with a standard root auger (4–10 cm in diameter) is thus difficult or impossible.
In contrast, the full Voronoi trench method estimates root biomass using a huge sampled volume 1/12th of which is made up of the soil around the selected palm surrounded by 6 neighbours. As the reference method, it was supposed to sample all the roots located under the palm tree, integrating all variability as a function of soil depth and distance from the tree. Between these two methods, the simplified Voronoi method also samples a big volume of soil. It is long and wide enough to be representative of the spatial heterogeneity of root distribution according to distance from the tree. The width of the trench in Z2s and Z3s is the minimum width that can be obtained by a single worker down to a depth of 1 m or more. This size also makes it possible to sample all the roots located under the trunk of the tree, as the reference method does (same Z0 and Z1) which confirms our second hypothesis. The lack of difference in the estimation of root biomass between these last two methods makes the simplified Voronoi method as accurate as the reference method and is thus recommended for users.
Efficiency of the different sampling methods
It took about 3 times as long labour time (person day−1) to sample the roots of the 16-year-old palms than those of the 2-year-old palms using the full Voronoi method and about twice as much time using the simplified Voronoi method than the auger method (Table 2). Using the auger instead of a full excavation (full Voronoi trench method), reduces the sampling time and therefore the related costs 8-fold. But, as the accuracy of the auger method was not demonstrated (compared to the reference method, the auger method underestimated by 23.3% and 53.1% the total root biomass of the 2- and 16-year-old plantations, respectively), this argument should not be discriminating. The full Voronoi method requires handling 5.85 m3 of soil per replicated zone (windrow or footpath in adult plantations) or per replicated tree (in young plantations) and down to a depth of 1 m, justifying the high labour requirements for root processing (42 and 114 person day−1 for the 2- and 16-year-old plantations, respectively, Table 2). Compared to the reference method, the main advantage of the simplified Voronoi method is the significant reduction in the volume of soil sampled (2.2 times less) without losing too much accuracy in the estimated total root biomass (+5 and − 17% of errors in the 2- and 16-year-old plantation, respectively, Table 2). Moreover, the simplified Voronoi method properly accounts for the heterogeneity of the root distribution in the plantation according to distance from the tree, the windrows and soil depth. Finally, the simplified Voronoi trench method reduced the labour time by 2.1 (20 person day−1) and 3.0 (38 person day−1) in the 2- and 16-year-old plantations, respectively, (Table 2) and accordingly also the cost. The efficiency, calculated here as the ratio of the error obtained by the auger or simplified Voronoi method compared to the reference method and the time required to perform the method, were clearly in favour of the simplified Voronoi method. This method was 14 and 9 times more efficient in estimating root biomass than the auger method in the 2- and 16-year-old oil-palm plantations, respectively (Table 2).
A similar study which compared methods, including root auger, simplified and full Voronoi trenches, performed on a dicotyledonous tree (eucalypt) in a Congolese forest plantation, led to contrasted results (Levillain et al. 2011). Eucalypt fine roots were homogeneously distributed within the plantation and therefore were most efficiently sampled using the auger method, whereas the full Voronoi trench method was recommended to sample the coarse and medium roots, which were distributed heterogeneously.
Conclusion
Total root biomass of 2-year-old oil-palm was estimated at 0.84 ± 0.03 Mg ha−1 whereas that of 16-year-old palms was estimated at 22.23 ± 0.81 Mg ha−1. The simplified Voronoi method was the most efficient method to estimate root biomass, especially at 2-year old. The comparison of this method with the reference method revealed a higher (+4.7%) estimation of root biomass in a 2-year-old plantation and lower (−17.2%) estimation in a 16-year-old oil palm plantation. Our first hypothesis was not validated: the auger method did not enable efficient sampling of the primary roots and produced a twice 2 lower (−53.1%) estimation of total root biomass stock of 16-year-old oil palms and even a − 23.3% lower estimation of 2-year-old palms. In particular, the root auger method did not show differences in fine root biomass between the windrow and the footpath in adult plantations whereas these differences exist (more than 30% more root biomass under the windrow at the adult stage compared to under the footpath). On the other hand, although with serious drawbacks in accuracy, the auger method was the fastest.
Our study suggests that, compared to the reference method, the simplified Voronoi trench method, with a reduced volume of 2.72 m3 (compared to 5.85 m3 with the full Voronoi trench method), required 2 and 3 times fewer person day−1 to sort out and process root samples, and with a reliable accuracy of +4.7% and − 17.2% of errors, and is therefore the most efficient method to assess the root biomass of 2- and 16-year-old oil-palm plantations, respectively. This method is highly recommended to estimate the root biomass of oil palm trees with respect to soil depth, distance from the tree, and including the roots located directly under the tree, thereby validating our second hypothesis, and accounting for soil heterogeneity in commercial plantations and as expected, all monocot trees in such standard planting design.
References
Aholoukpè NSH, Amadji LG, Blavet D, Chotte J-L, Deleporte P, Dubos B, Flori A, Jourdan C (2016) Effet de la gestion des feuilles d’élagage du palmier à huile sur le stock de carbone et les propriétés physico-chimiques du sol dans les palmeraies villageoises du Bénin. Biotechnol Agron Soc Environ 20(2):171–182
Aholoukpè H, Dubos B, Flori A, Deleporte P, Amadji LG, Chotte J-L, Blavet D (2013) Estimating aboveground biomass of oil palm: Allometric equations for estimating frond biomass. For Ecol Manag 292:122–129
Atger C. 1992. Essai sur l’architecture racinaire des arbres. Thèse de Doctorat, Uni. Montpellier II
Azontondé HA (1991) Propriétés physiques et hydrauliques des sols au Bénin. Soil water balance in the Sudano-Sahelian zone. Proceedings of the Niamey work shop; February 1991. IA HS Publ.no.199, 1991. pp 253–256
Böhm W (1979) Methods of studying root systems. Springer, Berlin
Buczko U, Kuchenbuch RO (2009) Evaluation of a core sampling scheme to characterize root length density of maize. Plant Soil 316:205–215
Chave J, Andalo C, Brown S, Cairns MA, Chambers JQ, Eamus D, Folster H, Fromard F, Higuchi N, Kira T, Lescure JP, Nelson BW, Ogawa H, Puig H, Rier B, Yamakura T (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145:87–99
Chopart JL, Siband P (1999) Development and validation of a model to describe root lenght at maize from root counts on soil profiles. Plant Soil 214:61–74
Cornaire B, Daniel C, Zuily-Fodil Y, Lamade E (1994) Le comportement du palmier à huile sous stress hydrique. Données du problème, premiers résultats et voies de recherche. Oléagineux 49:1–12
Dufrêne E, Dubos B, Rey H, Quencez P, Saugier B (1993) Changes in evapotranspiration from an oil palm stand (Elaeis guineensis Jacq.) exposed to seasonal soil water deficit. Oléagineux 48:105–120
Ennos AR (2000) The mechanics of root anchorage. Adv Bot Res 33:123–128 Rû
Goh KJ, Samsudin A (1993) The root system of the oil palm (Elaeis guineensis, Jacq.). I: a modified soil core method for root study. Elaeis 5:1–11
Haniff MH, Zuraidah Y, Roslan MMN (2014) Oil palm root study at a northern region in peninsula Malaysia. Int J Agric Innov Res 3(3):797–801 ISSN (Online) 2319–1473
Harun MH, Henson IE, Noor MR (2003) Estimating root biomass beneath the oil palm trunk. In: Proceedings of PIPOC 2003 Agricultural Conference. Malaysian Palm Oil Board, Kuala Lumpur, pp 953–959
Henson IE, Chai SH (1997) Analysis of oil palm productivity. II. Biomass, distribution, productivity and turn-over of the root system. Elaeis 9:78–92
Honda M (1978) Description of cellular pattern by Dirichlet domains: the two dimensional case. J Theor Biol 72:523–543
Jackson RB, Mooney HA, Schulze ED (1997) A global budget for fine root biomass, surface area, and nutrient contents. Proc Natl Acad Sci U S A 94:7362–7366
Jourdan C (1995) Modélisation de l'architecture et du développement du système racinaire du palmier à huile (Elaeis guineensis Jacq.). Thèse de doctorat. Université des Sciences et Techniques du Languedoc, Montpellier II, p 243
Jourdan C, Rey H (1997a) Architecture and development of the oil-palm (Elaeis guineensis Jacq.) root system. Plant Soil 189:33–48
Jourdan C, Rey H (1997b) Modelling and simulation of the architecture and development of the oil-palm (Elaeis guineensis Jacq.) root system. II. Estimation of root parameters using the RACINES postprocessor. Plant Soil 190:235–246
Jourdan C, Michaux-Ferrière N, Perbal G (2000) Root system architecture and gravitropism in the oil palm. Ann Bot 85:861–868
Kheong LV, Rahman ZA, Musa MH, Hussein A (2010) Empty fruit bunch application and oil palm root proliferation. J Oil Palm Res 22:750–757
Kenzo T, Ichie T, Hattori D, Itioka T, Handa C, Ohkubo T, Kendawang JJ, Nakamura M, Sakaguchi M, Takahashi N, Okamoto M (2009) Development of allometric relationships for accurate estimation of above-and below-ground biomass in tropical secondary forests in Sarawak, Malaysia. J Trop Ecol 25(04):371–386. https://doi.org/10.1017/S0266467409006129
Khalid H, Zin ZZ, Anderson JM (1999) Quantification of oil palm biomass and nutrient value in a mature plantation. II Below-ground biomass. J Oil Palm Res 11:63–71
Kirby EJM, Rackham O (1971) A note on the root growth of barley. J Appl Ecol 8:919–924
Kralicek K, Huy B, Poudel KP, Temesgen H, Salas C (2017) Simultaneous estimation of above- and below-ground biomass in tropical forests of Viet Nam. For Ecol Manag 390:147–156. https://doi.org/10.1016/j.foreco.2017.01.030
Kumar K, Prihar S, Gajri RP (1993) Determination of root distribution of wheat by auger sampling. Plant Soil 149:245–253
Levillain J, Thongo M’Bou A, Deleporte P, Saint-André L, Jourdan C (2011) Is the simple auger coring method reliable for belowground standing biomass estimation in Eucalyptus forest plantations? Ann Bot 108:221–230. https://doi.org/10.1093/aob/mcr102
Lopèz-Bucio J, Hernandez-Abreu E, Sanchez-Calderon L, Nieto-Jacobo MF, Simpson J, Herrera-Estrella L (2002) Phosphate availability alters architecture and causes change in hormone sensitivity in the Arabidopsis root system. Plant Physiol 129:244–256
Marschner H, Waisel Y, Eshel A, Kafkafi U (1991) Root-induced changes in the availability of micronutrients in the rhizosphere. In: Waisel Y, Eshel A, Kafkafi U (eds) Plant-roots:-the-hidden-half. Book in Series Soils, Plants, and the Environment. Marcel Dekker, Inc., pp 503–528
Maurice J, Laclau J-P, Scorzoni RD, Gonçalves JLM, Nouvellon Y, Bouillet J-P, Stape JL, Ranger J, Behling M, Chopart J-L (2010) Fine root isotropy in Eucalyptus grandis plantations. Towards the prediction of root length densities from root counts on trench walls. Plant Soil 334:261–275. https://doi.org/10.1007/s11104-010-0380-8
Mohd Haniff H (1998) Techniques for sampling oil palm root. II. Auger for peat soils. J Oil Palm Res 10(2):57–60
Nelson PN, Banabas M, Scotter DR, Webb MJ (2006) Using soil water depletion to measure spatial distribution of root activity in oil palm (Elaeis guineensis Jacq.) plantations. Plant Soil 286:109–121
Nelson PN, Webb MJ, Banabas M, Nake S, Goodrick I, Gordon J, O’Grady D, Dubos B (2014) Methods to account for tree-scale variability in soil- and plant-related parameters in oil palm plantations. Plant Soil 374:459–471
Ngando EGF, Etta CE, Ntsomboh GN, Oben TT (2013) Breeding oil palm (Elaeis guineensis Jacq.) for fusarium wilt tolerance: an overview of research programmes and seed production potentialities in Cameroon. Int J Agric Sci 3(5):513–520
Nodichao L, Chopart J-L, Roupsard O, Vauclin M, Ake S, Jourdan C (2011) Genotypic variability of oil palm root system distribution in the field. Consequences for water uptake. Plant Soil 341(1–2):505–520. https://doi.org/10.1007/s11104-010-0663-0
Nodichao L (2008) Biodiversité racinaire, absorption potassique et résistance à la sécheresse chez le palmier à huile au Bénin. Thèse Université Cocody Abidjan, Côte d’Ivoire, p 346
Ouvrier M (1995) Etude du système racinaire, Rapport interne, IDE-FOR-DPO, p 20
Reis de Carvalho CJ (1991) Mécanismes de résistance à la sécheresse chez des plantes jeunes et adultes de palmier à huile (Elaeis guineensis Jacq.). Thèse de doctorat. Paris-Sud, Orsay, p 203
Rey H, Quencez P, Dufrêne E, Dubos B (1998) Profils hydriques et alimentation en eau du palmier à huile en côte d’Ivoire. Plantation Recherche Développement 5:47–53
Rüegg J (2017) Oil palm plantations in savannas: impact on biomass carbon stocks and soil organic carbon (SOC) dynamics, Master Thesis in Environmental Sciences at ETH Zürich. Ecole Polytechnique Fédérale de Lausanne, p 64
Rüegg J, Quezada JC, Santonja M, Ghazoul J, Kuzyakov Y, Buttler A, Guillaume T (2019) Drivers of soil carbon stabilization in oil palm plantations. Land Degrad Dev 30:1904–1915. https://doi.org/10.1002/ldr.3380
Ruer P (1968) Contribution à l’étude du système racinaire du palmier à huile. These de docteur Ingénieur. Université de Paris, Paris, p 117
Ruer P (1969) Système racinaire du palmier à huile et alimentation hydrique. Oléagineux 24:327–330
Saint-André L, Thongo M'Bou A, Mabiala A, Mouvondy WJ, Jourdan C, Roupsard O, Deleporte P, Hamel O, Nouvellon Y (2005) Age-related equations for above- and below-ground biomass of a Eucalyptus hybrid in Congo. For Ecol Manag 205(1–3):199–214. https://doi.org/10.1016/j.foreco.2004.10.006
Smit AL, Bengough AG, Engels C, Van Noordwijk M, Pellerin S, Van de Geijn SC (2000) Root methods. A handbook. Springer Verlag, Berlin
Snowdon P, Raison J, Keith H, Ritson P, Grierson P, Adams M, Montagu K, Bi HQ, Burrows W, Eamus D (2002) Protocol for sampling tree and stand biomass. NCAS technical report N° 31. Australian Greenhouse Office, Canberra, p 77
Sommer R, Denich M, Vlek PLG (2000) Carbon storage and root penetration in deep soils under small-farmer land-use systems in the eastern Amazon region, Brazil. Plant Soil 219:231–241
Syahrinudin (2005) The potential of oil palm and forest plantations for carbon sequestration on degraded land in Indonesia. In: Ecology and Development Series No. 28. Cuvillier Verlag, Göttingen
Tailliez B (1971) Le système racinaire du palmier à huile sur la plantation de San Alberto (Colombie). Oléagineux 26:435–447
Tinker PB (1976) Soil requirements of the oil palm. In: Corley RHV, Hardon JJ, Wood RJ (eds) Developments in crop science. 1: Oil Palm Research. Elsevier, Amsterdam, pp 165–174
Tinker PB, Nye PH (2000) Solute movement in the rhizosphere. Oxford University Press, New York, p 444
Uwe B, Rolf OK, Horst HG (2008) Evaluation of a core sampling scheme to characterize root length density of maize. Plant Soil 316:205–215. https://doi.org/10.1007/s11104-008-9771-5
Vieilledent G, Vaudry R, Andriamanohisoa SFD, Rakotonarivo OS, Randrianasolo HZ, Razafindrabe HN, Bidaud Rakotoarivony C, Ebeling J, Rasamoelina M (2012) A universal approach to estimate biomass and carbon stock in tropical forests using generic allometric models. Ecol Appl 22:572–583
Vogt KA, Vogt DJ, Bloomfield J (1998) Analysis of some direct and indirect methods for estimating root biomass and production of forests at an ecosystem level. Plant Soil 200:71–89
Waisel Y, Eshel A, Kafkafi U (1991) Plant roots - the hidden half. Marcel Dekker, Inc., New York, p 512
Yampolsky C (1924) The pneumathodes on the roots of the oil palm (Elaeis guineensis Jacq.). Amer J Bot 11:502–512
Yuen JQ, Ziegler AD, Webb EL, Ryan CM (2013) Uncertainty in below-ground carbon biomass for major land covers in Southeast Asia. For Ecol Manag 310:915–926. https://doi.org/10.1016/j.foreco.2013.09.042
Acknowledgements
Authors are grateful to INRAB and CIRAD for providing financial support for this study. The authors warmly thank the technical staff of the Ecophysiology laboratory of CRAPP for their help particularly with root sampling in the field and processing in the lab. We thank Daphne Goodfellow for correcting the English and the two anonymous reviewers for their insightful comments and suggestions on how to improve our manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Peter J. Gregory.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 44.8 kb)
Rights and permissions
About this article
Cite this article
Dassou, O., Nodichao, L., Aholoukpè, H. et al. Improving the methodology for root biomass estimation in monocotyledonous tree plantations: case of oil palm (Elaeis guineensis. Jacq) in West Africa. Plant Soil 465, 593–611 (2021). https://doi.org/10.1007/s11104-021-04939-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-021-04939-4