Abstract
Aims
Data about woody debris (WD) decomposition are very scarce for the Mediterranean basin. The specific aim of this work is to explore the relationships between WD traits with the decay rate.
Methods
We carried out a three-year litterbag decomposition experiment using ten WD types incubated in two plant communities (i.e. shrubland and woodland) and in laboratory conditions. WD was characterized for 31 chemical and anatomical traits, including macro- and micronutrients, lignin, and cellulose as well as organic chemistry by Solid-state Cross-Polarization Magic Angle Spinning Carbon-13 Nuclear Magnetic Resonance (13C CPMAS NMR) and Fourier transform infrared spectroscopy/ Attenuated Total Reflection (FT-IR/ATR spectroscopy).
Results
WD decay rate was negatively associated with di-O-alkyl, lignin/N and C/N ratios, but positively with N concentration. Less consistent but positive correlations were recored for K, Mn, and Na concentration. The alkyl C and carboxylic C regions, associated with aliphatic and amide compounds, was positively correlated with WD decomposition. Conversely, di-O-alkyl C and O-alkyl C fractions, largely associated with cellulose and hemicellulose, were negatively correlated with WD decay rate. Finally, the positive correlation between Na concentration and WD mass loss in field conditions suggest a role of this neglected micronutrient for wood decomposition. WD specific density and anatomical features, have a minor capability to explain decomposition rate.
Conclusions
Our findings demonstrate a major role of WD chemical traits in explaining the variability of decomposition in Mediterranean ecosystems.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The decomposition of plant debris is a pivotal process for biogeochemical cycles, affecting the formation of soil organic carbon (C) and soil nutrient stocks and, therefore, controlling ecosystem functioning as well as plant and microbial community composition. At both regional and global scales plant litter decomposition is largely influenced by environmental conditions i.e. water availability and temperature (Aerts 1997). Moreover, local-scale factors are also increasingly recognized as the primary control on the carbon dynamics of decomposing wood (Bradford et al. 2014). At a finer scale, where climatic conditions are relatively uniform, the chemical composition of plant tissue (Meentemeyer 1978; Cornwell et al. 2008) in interaction with soil microbiome (Bradford et al. 2017) is the most important factor determining the rate of organic matter decomposition.
A definition of plant litter quality based on its molecular composition is still challenging. Plant tissue contains a wide array of biomolecules having different susceptibility to microbial degradation e.g. sugars, complex carbohydrates, peptides, proteins, lipids, lignin, organic acids, and polyphenols. In this regard, a considerable research effort has been done in the last decades to identify effective indicators of plant molecular chemistry suitable as reliable predictors of plant debris decomposition rates (Swift et al. 1979; Makkonen et al. 2012). To date, most of the studies mainly focused on the decomposition rate on leaf litter, with far less attention paid to other plant tissues like root and woody debris (Berg and McClaugherty 2014).
Woody debris (WD) is a quantitatively important fraction of ecosystem C stock, accounting for up to 20% of total C in forests (Pan et al. 2011). WD usually decomposes at a slower rate than leaves and, for this reason, accumulates on the forest floor. Slow WD decay rate can be related to its low nitrogen (N) and nutrients concentration coupled with large lignin content (Weedon et al. 2009). N limitation becomes relevant for plant debris decomposition when the C/N ratio of the decomposing material lies above the threshold of ~ 30–35 (Taylor et al. 1989). When soil microbes attack WD having a high C/N ratio, usually above 100, N starvation dramatically limits their metabolic activity. The very low initial N content of WD can, indeed, create a microbial N limitation that can limit its decomposition. Under this assumption, exacerbated microbial N starvation and low decay rate should be expected in ecosystems where soil nutrient availability is low. However, decomposers can gain extra N from other external sources, i.e. the underlying soil. In this regard, several studies reported a significant transfer of N from the underlying soil to N poor litter during the decomposition process (Parton et al. 2007; Schimel and Hättenschwiler 2007; Bonanomi et al. 2017a). N can be transferred from soil to N poor litter through leaching and diffusion, or actively by the fungal mycelia networking and bridges (Frey et al. 2000; Lummer et al. 2012). In this event, if N transfer from the underlying soil is quantitatively relevant, enhances of WD decomposition rate should be expected in the ecosystem having nutrient-rich soil.
A meta-analysis (Weedon et al. 2009) reported that both chemical and anatomical WD traits exert control over their decomposition rates. In detail, the study found that N and phosphorus (P) correlate positively with decomposition rates, while C/N and lignin/N ratios negatively. Among anatomical traits, wood density, the most commonly measured trait, showed contrasting responses, with either positive (Chambers et al. 2000; Chave et al. 2009) and no significant correlations (Van Geffen et al. 2010) with decay rate. The extensive analysis of Weedon et al. (2009) also revealed many caveats about WD studies. First, there is a notable lack of studies concerning WD decomposition in Mediterranean ecosystems compared to boreal (Jonsson and Kruys 2001), temperate (Kahl et al. 2017), and also tropical forest biomes (Gora et al. 2019). Second, most of the experiments investigated the effect of macronutrients, i.e. N and P (Chen et al. 2015), on WD decomposition with few studies concerning other nutrients such as potassium (K), calcium (Ca), sodium (Na), magnesium (Mg), manganese (Mn), and iron (Fe) that play an important role in root and leaf litter decomposition (Silver and Miya 2001; Kaspari et al. 2008; Keiluweit et al. 2015). Finally, recent studies on leaf litter decomposition used chemical throughput methods as 13C-cross-polarization magic angle spinning (CPMAS) nuclear magnetic resonance (NMR) spectroscopy (Kögel-Knabner 2002), and FT-IR spectroscopy (Haberhauer et al. 1998), to characterize, at a molecular level, organic C based materials. In detail, studies based on 13C-CPMAS NMR have proved useful to describe the leaf litter C composition, resulting in an improved prediction capability of leaf litter decay rate compared to classic C/N and lignin/N ratio (Preston et al. 2009; Bonanomi et al. 2013; Cartenì et al. 2018). However, to date, no studies applied 13C-CPMAS NMR and FT-IR spectroscopy to WD decomposition to predict their decomposition rate.
To fill these gaps of knowledge, in this study we examine the importance of WD chemical and anatomical traits on their decomposition by a manipulative and long-term experiment carried out in Mediterranean ecosystems. In detail, the experiment includes WD of ten common plant species of the Mediterranean basin incubated in both laboratory and field conditions. Laboratory incubation was planned to estimate the value of maximum potential decomposition rates of WD, while field incubation was intended to provide information about decomposition at two sites representative of the major ecosystem types within the Mediterranean biome. WD was characterized for 33 anatomical and chemical traits including macro and micronutrients (i.e. P, K, Ca, Mg, Na, Fe, Zn, Mn, Cu), as well as C quality assessed by Solid-state Cross-Polarization Magic Angle Spinning Carbon-13 Nuclear Magnetic Resonance (13C CPMAS NMR) and Fourier transform infrared spectroscopy/Attenuated Total Reflection (FT-IR/ATR spectroscopy). The specific aims of this work were to: (i) describe WD features based on chemical and anatomical traits, (ii) assess the decomposition rate of ten WD types, and (iii) explore the relationships between WD chemical and anatomical traits with the decay rate. Based on the above considerations, two main hypotheses were tested: (1) decay rate of WD would be slower for high density, lignin-rich, and nutrient-poor tissues; (2) WD decay rate would be better predicted by an index that includes a descript of C quality, i.e. lignin/N or 13C CPMAS NMR parameters, compared to single nutrients or C/N ratio.
Materials and methods
Woody debris collection
Ten Mediterranean species were selected for this study belonging to different growth forms (Duckworth et al 2000) (Supplementary Figure S1): i.e. evergreen shrub (Cistus monspeliensis L., Erica arborea L., Myrtus communis L., Pistacia lentiscus L., Spartium junceum L.), deciduous shrub (Crataegus monogyna Jacq.), evergreen tree (Olea europaea L., Quercus ilex L.), deciduous tree (Ficus carica L., Quercus pubescens Willd.). The ten woody debris are expected to cover a wide range of N content, C/N ratio, and lignin content. Woody branches hereafter indicate as WD, with the diameter ranging from 1.5 to 3 cm were cut from randomly selected individuals (N > 20) in Southern Italy (Cicerale 40°19’ N 15°07’ E, 186 m a.s.l.). We selected this relatively small WD diameter because it represents the most abundant fraction at the study sites. WD was air-dried at room temperature in a ventilated chamber for 50 days until reaching constant weight and stored afterward.
Study sites and decomposition experiment
Plant litter (leaf, root, woody debris) decomposition in field conditions largely depends on litter chemistry, water availability, and temperature (Aerts 1997). In this study, we decomposed WD both in laboratory and field conditions. The laboratory experiment was included to focus on the importance of WD chemistry and anatomy and to reduce the influence of local climatic factors, e.g. water availability and temperature, on decay rate.
The decomposition experiment was carried out according to the litterbag method (Berg and McClaugherty 2014). WD was cut with scissors to obtain pieces of 3 g each. Litterbags (25 × 25 cm2, mesh size 2 mm) were filled with 5 dry WD pieces of each 10 woody species placed in four different environmental conditions: i. laboratory, with soil collected from the shrubland; ii. laboratory, with soil collected from the woodland; iii. over litter layer of shrubland field, and; iv. over litter layer of woodland field. The shrubland (Cicerale) and woodland (Portici, Supplementary Figure S2) fields share a similar Mediterranean climate but with different soil properties (Table 1, Supplementary Table S1). These ecosystem types were selected because there, the 10 selected species naturally coexist. The main characteristics of the soil, vegetation, and climate of the two ecosystem types are reported in Table 1 and Supplementary Table S1. According to the Standard US Department of Agriculture Soil Textural Classification Triangle (Brady and Weil 2001), studied soils can be classified as sand (Portici) and sandy loam (Cicerale). The pH in H2O suspensions of both soils is neutral although differences in carbonate abundance. The Portici soil has a higher content of organic Carbon and total Nitrogen than Cicerale. Cation Exchange Capacity (CEC) values indicate that both soils have a good capacity to hold cations, mostly calcium. Micronutrient availability is generally low, especially that of Zn in Cicerale and Cu in Portici soils.
In the laboratory conditions, litterbags were placed in a growth chamber with controlled and not limiting temperature (24 ± 2 °C day and 18 ± 2 °C night) and water - watered with distilled water every week to reach the soil water holding capacity, previously determined equal to 18% in volume for shrubland and 24% for woodland soils using the methods reported in Bonanomi et al. (2017c). In the field condition, litterbags were placed on the soil surface in permanent plots of 30 × 30 m in shrubland and woodland.
The full experimental design included four incubation environments, ten WD types, with five replicated litterbags for each species and each of the five dates of retrieval from the beginning of the experiment (i.e. 30, 90, 180, 360, 720, and 1,080 days). At each date, WD was collected, oven-dried (35 °C until a constant weight was reached), cleaned from soil debris, and weighed afterward to the nearest 0.001 g.
Chemical analyses
Undecomposed WD was ground to get a powder (< 1 mm) and submitted to the chemical determination that was carried out in triplicate. Total C and N content by flash combustion of micro samples (5 mg each) by a CN-elemental analyzer (Flash EA2000 Thermo). Macro and micronutrients (P, K, Ca, Mg, Na, Fe, Zn, Mn, Cu) were determined after acid digestion (in 6 ml of HNO3 65% and 2 ml of H2O2 35%) in a microwave digestion system (Ethos 900, Milestone). P concentration was determined by the molybdenum blue assay method (Murphy and Riley 1962) after neutralization of the solution with NaOH. The concentration of the other macro and micronutrients was determined by flame atomic absorption spectrometry (AAnalyst 700, Perkin Elmer). The acid-detergent hydrolysable fraction (indicated as labile C), cellulose, and lignin were determined with the method described by Gessner (2005). Briefly, labile C was assessed by mild acid hydrolysis with 0.5 M H2SO4 and the use of the detergent cetyltrimethylammonium (20 g l− 1). Cellulose was quantified as hydrolysable fraction after the sulphuric acid digestion (loss after treatment with 72% H2SO4 for 4 hours), while proximate lignin was determined as unhydrolyzable fraction (loss upon ignition after the H2SO4 treatment). All fractions are reported as ash-free dry mass. It is important to note that the lignin assessed with this method does not correspond to pure lignin, rather it includes several other hydrolysis-resistant organic compounds, i.e. waxes, suberin, cutin, and condensed tannins at varying proportion (Berg and McClaugherty 2014).
WD was also characterized by FT-IR/ATR and 13C-CPMAS NMR obtained in solid-state. For the 13C-CPMAS NMR analysis, the spectrometer used was a Bruker AV-300 equipped with a 4 mm wide-bore MAS probe (for further details see Bonanomi et al. 2013). Spectral regions and corresponding C types were identified following Kögel-Knabner (2002) and Bonanomi et al. (2019a, b): 0–45 ppm = alkyl C; 46–60 ppm = methoxyl and N-alkyl C; 61–90 ppm = O-alkyl C; 91–110 ppm = di-O-alkyl C; 111–140 ppm = H- and C- substituted aromatic C; 141–160 ppm O-substituted aromatic C (phenolic and O-aryl C); 161–190 ppm carboxyl C. Concerning the 161–190 ppm region, the carboxylic C term was used to indicate the absorption of carboxylic acids and their ester and amide derivatives. The relative contribution of each spectral region was assessed by the integration of MestreNova 6.2.0 software (Mestre-lab Research 2010) and expressed as a percentage of the total area. The alkyl C / O-alkyl C ratio (0– 45 / 61–110) and the O-alkyl C / methoxyl and N-alkyl C ratio (61–90 / 46–60; thereafter indicated as CC / MC), which are considered indicators of the degree of organic matter decomposition, were calculated after Almendros et al. (2000).
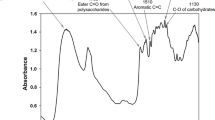
For FT-IR/ATR analysis the spectrometer used was a Frontier (Perkin Elmer) equipped with an ATR unit. The spectra were recorded on WD powder of each woody species at a resolution of 4 cm− 1 for 32 scans in the range from 4,000 to 650 cm− 1. The powdered samples were pressed against the diamond crystal of the ATR device, and the same pressure was applied for all measurements. A background spectrum of the clear window was recorded before to the acquisition of sample spectra. To compare the wood spectra, the spectra were normalized on the peak with maximum intensity (at 1,027 cm− 1) using software Spectrum (v. 10.03.09.0139, Perkin Elmer). The assignment in terms of peak intensity and shift was: intra-molecular hydrogen bonds in cellulose at 3350 cm− 1; C-H in aromatic methoxyl groups and in methyl and methylene groups of side chains at 2850 and 2919 cm− 1; C1-H of cellulose and hemicelluloses at 895 cm− 1; CH2 in crystalline cellulose at 1315 cm− 1, C-H in cellulose and hemicelluloses at 1369 cm− 1, C = O in hemicelluloses at 1730 and 1739 cm− 1; principally C-O and C-O-C in cellulose and hemicelluloses at 988, 1027, 1053, 1107 and 1158 cm− 1; principally C-O, C = O, C-C, C = C and C-H in lignin at 1230, 1421, 1453, 1507, 1593–1620 cm− 1 8.
Quantitative anatomy
Thin undecomposed WD sections (18–22 µm) of each plant species were obtained using a sliding microtome (HM 400, Microm International GmbH, Walldorf, Germany). To obtain permanent histological preparations, following the method of Schweingruber and Poschlod (2005), sections were stained with astrablue (2%) and safranin (safranin 1%, water-soluble), which resulted in unlignified cells appearing blue and lignified cells appearing red. Afterward, sections were dehydrated using a series of ethanol solutions of increasing concentrations, washed with xylol, and then permanently preserved by embedding them into Canada balsam.
Images of cross-sections were captured at a magnification of 100×, using a reflected light microscope (AxioPhot, Carl Zeiss, Jena, Germany) equipped with a CCD camera (DCM300, ScopeTek). Sequential images were first stitched (ICE, Microsoft) and then analysed for xylem measurement using the image-analysis software ImageJ v.1.40 (National Institute of Health, Bethesda, MD, USA). In particular, within the measuring frame, the number of conducting vessels (N), the vessel density (VD, n/total area), the average vessel size (AV, mm2), the lumen Feret diameter (D, mm2) as well as the area fraction (A, % of total area) of conducting cells were measured.
For the determination of specific density, three air-dried WD pieces including bark were weighed and then measured for volume via Archimedes’ Principle. WD specific density was expressed as the ratio of the dry weight of WD to its dry volume (g/cm3).
Data analysis
Analysis of variance (ANOVA) and Tukey post-hoc pair-wise comparisons of chemical and anatomical traits of undecomposed WD was used to assess differences among species means. Significance was evaluated in all cases at P < 0.05. The WD negative exponential decay constant (k) was calculated according to Berg and McClaugherty (2014). The model equation was Mt = M0∙e− kt, where M0 is the initial WD mass, Mt is the WD mass remaining after a certain time t, and k is the decay rate constant.
We used general linear models (GLMs) to test main and second-order interactive effects of WD type (10 species), environmental conditions (shrubland, woodland and field and laboratory), and decomposition time (treated as a continuous covariate) on mass remaining percentage. Statistical differences in slope coefficients of linear models were tested by means of the Tukey HSD multiple comparison post-hoc test at α = 0.05. Percentage mass remaining data were log-transformed before applying both GLM and Tukey HSD post-hoc tests. Data were managed and analysed in R (R Core Team 2020) by means of both “emmeans” and “multcomp” packages (Lenth 2020; Hothorn et al. 2008).
To address the relationship between WD decay rate (k) with its chemistry and anatomy we calculated correlation according to non-parametric Spearman correlation. In detail, correlations were calculated between k and nutrient concentrations, cellulose and lignin content, 13C NMR regions and index for the tested plant materials. To control for multiple comparisons, correlation was tested for statistical significance at α = 0.05/N, with N being the number of performed correlation tests, by applying the Bonferroni’s correction.
Two different multivariate approaches were used for chemical and anatomical data of undecomposed WD. Cluster Analyses (CA) based on Unweighted Pair Group Method with Arithmetic mean (UPGMA) aggregation rule and 1 - Pearson’s correlation coefficient as a distance measure (Podani 2000) were used to test the similarity in the chemical composition of wood debris from different species. Cluster analysis has been performed on 13C NMR and FT-IR/ATR data (Bonanomi et al. 2019a, b). Two Principal Component Analysis (PCA) was carried out to provide a synthetic representation of variation in wood anatomical or chemical traits occurring in different species. The association between chemical and anatomical features of WD from different species and decay rate (k) was visualized by the method of the supplementary variables following the approach proposed by Legendre and Legendre (2012). The method permits to plot the decay rate (k) value in the ordination space without that it affect its eigenfactor and eigenvalues, offering to estimate the dependence of decay rates (k) with chemical and anatomical traits via samples ordination. In addition, we correlated WD wood decay rate with coordinate of plant species on the first three components from the principal component analysis. The software STATISTICA 10 (StatSoft Inc., USA) and R was used for statistical analyses (R Core Team 2020).
Results
Chemical and anatomical WD traits
Chemistry of undecomposed WD largely varied among species (Table 2, Supplementary Table S2). C/N ratio showed a large variation, ranging between 74.2 of F. carica and 251.8 for C. monspeliensis. Lignin content varied between 8.8% of F. carica and 25.8% for E. arborea. Concerning other elements, chemistry varied among species showing a very high concentration of Ca in both the Quercus species, with Q. pubescens having also the highest concentration of Fe and Q. ilex the highest concentration of Mn. It is also notable the high concentration of Mg, Na and Zn showed by F. carica. P. lentiscus had the highest concentration of Cu, ⁓2 time higher than the following Q. pubescens, and ⁓4 time higher of the lowest Cu content of C. monspeliensis.
Considering 13C-CPMAS NMR data (Table 2; Fig. 1a), all the spectra showed high predominance of O-alkyl C, mainly associated with sugars and polysaccharides, accounting for most of the total (from 51.6% in E. arborea up to 59.3% in C. monspeliensis), followed by di-O-alkyl ranging between 13.5% in F. carica and 15.6 in C. monspeliensis. Methoxyl C accounted for percentages between 9.1% in P. lentiscus and 11.5% in E. arborea. H- C- aromatic C reached the high relative abundance (8.2%) in E. arborea woody debris, being much higher than the other species ranging between a minimum of 4.6% in C. monspeliensis and 6.9% in C. monogyna.
Chemical differences among organic of the 10 woody debris species. (a) 13C-CPMAS NMR and (c) FT-IR spectra of the materials. For (a), values are relative abundance standardized for each spectra. (b-d) Hierarchical clustering(CA) of spectral data based on Unweighted Pair Group Method with Arithmetic mean (UPGMA) aggregation rule and 1 - Pearson’s correlation coefficient as a linkage distance measure
Dendrogram of WD (Fig. 1b), based on 13C-CPMAS NMR, shows that S. junceum and Q. ilex as well M. communis and O. europaea are very similar each other, with F. carica having the larger distance from the others WD. FT-IR/ATR spectra of the ten WD (Fig. 1c) show bands attributable to the major wood components such as cellulose, hemicelluloses, and lignin (Popescu et al. 2007; Rowell 2012; Emmanuel et al. 2015; Traoré et al. 2018). When the FT-IR spectra were analysed, the obtained dendrogram (Fig. 1d) showed a high dissimilarity between M. communis and other species. In addition, WDs of Q. pubescens with C. monogyna and E. arborea with S. junceum make two separated clusters from other species (Fig. 1d).
Concerning WD anatomy traits, F. carica and Q. ilex showed the highest vessel density (VD), ⁓4 time higher than Q. pubescens that have the lowest value (Table 2, Table S2). The average vessel size (AV) and the lumen Feret diameter (D) had rather an opposite trend to VD with Q. pubescens and Q. ilex respectively having the highest and the lowest values. The evergreen shrub M. communis had the highest area fraction (% of total area) of conducting cells, while both the Quercus species closely followed by C. monspeliensis and S. junceum showed the lowest values. WD density ranged between 0.7 g/cm3 for F. carica and 1.0 g/cm3 for P. lentiscus.
Woody debris decay rate
WD decay rate was significantly affected by the plant species, incubation time, and incubation conditions (Table S3). M. communis showed the lowest decay rate, with S. junceum, P. lentiscus and F. carica having the highest with other species showing intermediate values (Fig. 2a).
Decay rate (k) of woody debris from 10 plant species decomposed in laboratory and field conditions for 1,080 days. a Decay rate for each species averaged across the four experimental conditions, values are ordered by ascending k, different letters indicate statistically significant differences (P ≤ 0.05); b Decay rate in the four experimental condition, values are the average of the ten species in each experimental site; c Decay rate of each species in the four experimental condition, values are average of five replications. Error bars indicate standard deviations
GLM analysis revealed a highly significant interaction between WD species and incubation conditions (Table S3, Figure S3). Three species showed significant difference in decay rate between shrubland and woodland soils when decomposed in controlled condition, with C. monogyna, and C. monspeliensis that decompose faster in shrubland soil and F. carica in woodland soil (Fig. 2c). In addition, M. communis showed a very low decay rate in all conditions except for incubation in woodland field (Fig. 2c). Noteworthy, Q. ilex WD showed a low decay rate in both field sites, but a notable high mass loss was recorded in laboratory conditions with both the woodland and shrubland soils (Fig. 2c).
Linking decay rate with woody debris traits
Considering the chemical parameters of undecomposed WD, the decay rate showed a strong positive correlation with N concentration in all incubation conditions (Fig. 3). Noteworthy, P concentration was not correlated with mass loss at both study sites. Labile C, cellulose, lignin, and N/P ratio showed not significant correlation with decay rate.
Heat-plot of correlation calculated through Spearman rank between woody debris decay rate (k) with chemical and anatomical traits in the four experimental conditions. Asterisks indicate statistically significant correlations for r (P < 0.01, after controlling for multiple comparisons according to the Bonferroni’s correction)
C/N and Lignin/N ratios were negatively and consistently correlated with the decay rate. Among cations, Ca and Mg showed inconsistent correlations with decay rate. K and Na showed positive correlations with wood mass loss in field condition, while Mn concentration was positive correlated with decay rate of WD incubated in laboratory. The concentration of micronutrients i.e. Fe, Cu, and Zn, was not significantly correlated with WD decomposition.
Among 13C-CPMAS NMR, the decay rate was negatively correlated with di-O-alky C region. On the contrary, the decay rate was positively correlated with alkyl C and carboxylic C regions, as well as with the alkyl C/O-alkyl ratio in all conditions with the exception of woodland field (Fig. 3).
Considering anatomical features of WD, a general trend of weak correlations was found. A positive correlation with decay rate were found only for vessel density in laboratory with woodland soil (Fig. 3). Negative correlations with decay rate were found for lumen area in laboratory conditions but not in the field.
The principal component analysis (PCA) of chemical traits provided a satisfactory ordination of the WD, with the first two eigenvalues accounting for 57.32% (33.12% and 24.16%) of the total variance (Fig. 4a). PCA synthesized the relationships between WD chemistry with the decay rate, highlighting a cluster of parameters negatively associated with decay rate (i.e. C/N ratio, lignin/N ratio, O-alkyl C and di-O-alkyl C regions). PCA also clarifies the strong positive association between WD decay rate and N content and alkyl C and carboxyl C region of 13C CPMAS NMR spectra. We found that the first PCA axis was negatively correlated with WD decay rate (Supplementary Figure S5). Concerning the PCA of anatomical traits, the first two eigenvalues account for 75.07% (51.69% and 23.38%, respectively) of the total variance (Fig. 4b). We found that only the second axe was positively correlated with WD decay rate (Supplementary Figure S6).
Principal component analysis (PCA) loading plots generated from woody debris elemental chemistry and 13C-CPMAS NMR traits (a) and anatomical traits (b) based on values recorded in the 10 litter materials. Woody debris decay rate (kd) is also plotted as supplementary variables (red vector) following Legendre and Legendre (2012)
Discussion
This study, for the first time, presents WD decay rates of ten tree and shrub species from the Mediterranean basin. The use of 33 chemical and anatomical traits highlights the importance of WD type in explaining the large interspecific variation of decomposition rate. N concentration showed the strongest positive correlation with mass loss, with positive but less consistent correlations recorded for K, Mn, Ca, and Na. C/N and lignin N ratios being the most robust predictors of decay, with useful information provided by 13C CPMAS NMR that highlight the potential role of alkyl C and carboxylic C regions associated with aliphatic and amide compounds that promote WD decomposition.
Woody debris decay rate in Mediterranean ecosystems
Decomposition rates of leaf litter in Mediterranean conditions are usually slower than in temperate and tropical biomes because of limiting abiotic constraints; i.e. summer drought coupled with a poor chemical quality that includes high lignin and low N content. Here, we found a large overlap of WD decomposition rates with data from tropical, temperate, and boreal regions (Weedon et al. 2009). The overlap of decomposition rates with other ecosystems is due to the large variability observed among the ten species, ranging from 0.033 yr− 1 of C. monspeliensis decomposed in the woodland field to 0.495 yr− 1 for S. junceum decomposed in the laboratory, with soil collected from the shrubland. The large variability of decomposition rate among species, even in the homogeneous environmental conditions of the laboratory, highlight the importance of WD traits over environmental conditions, in controlling the decomposition process.
However, in Mediterranean ecosystems, the specific dynamics of climatic conditions critically affects soil organic matter and litter decomposition. In summer, the high temperature and the low soil moisture caused by the drought may strongly limit microbial and fungal activity (Fioretto et al. 2005) whereas, the milder and wetter autumn and spring seasons induce very high metabolic rates of microbiome temporarily promoting litter decomposition (Coûteaux et al. 1995; Incerti et al. 2011). In Mediterranean summer drought conditions, drying and rewetting soil episodes may affect mineralization and microbial activity (Jarvis et al. 2007). In addition, shrublands are more prone to soil water depletion, despite the higher annual rainfall compared to woodland, because in this plant community the canopy is more open compared to Quercus ilex woodland (Llorens and Domingo 2007). Several studies highlighted that the Mediterranean summer drought establishes microclimatic conditions particularly severe and, in such conditions, the forest canopy can buffer the microclimatic extremes by promoting a decrease of air temperature and a simultaneous increase of air relative humidity and soil moisture (Bonanomi et al. 2018). Anyway, our experimental design, cannot disentangle the limiting role of drought, temperature, and local microbiome as independent factors on WD decay rate. Further studies that combine detailed microclimatic assessment are needed to assess the importance of water shortage in limiting WD decomposition.
Nutrient and lignin
Correlation and multivariate PCA analyses highlight the potential role of some nutrients (N, K, Mn, Ca, and Na) and lignin in controlling WD decay rate. Rates of mass loss were negatively associated with lignin and aromatic C types and positively to N concentration. This finding, indeed, is largely confirmative of well-established previous knowledge reported for both leaf litter and large woody debris (Meentemeyer 1978; Taylor et al. 1989). In general, wood is characterized by low N concentration coupled with high lignin content, conditions that induce a strong N limitation for decomposer microbes. C/N ratio of all decomposing material lies above the threshold of ~ 30–35 (Taylor et al. 1989), also for the nitrogen-fixing shrub S. junceum having the lowest C/N ratio after F. carica.
For N poor materials (i.e. small and large WD, coarse root tissues) mass loss is strongly dependent by exogenous N sources (Bonanomi et al. 2017a). In fact, saprotroph microbes can gain extra N from the underlying soil to sustain their growth and activity. In this regard, several studies focusing on leaf litter reported a significant transfer of N from the underlying soil to N poor litter during the decomposition process (Schimel and Hättenschwiler 2007). Nutrient poor soils may exacerbate microbial N starvation and, thus, further slow decay rate. Accordingly, we found that the positive correlations between decay rate with N concentration and the negative one with C/N and lignin/N ratios were higher in shrubland compared to woodland, suggesting a stronger N limitation in the shrubland. Further studies, including our data from this ongoing long-term experiment, could provide useful insights to quantify and assess the dynamics and impact of N limitation and transfer from soil to WD at later decomposition stages (Berglund and Ågren 2012).
Recent studies criticized the C/N ratio, questioning its capability to predict leaf (Hättenschwiler et al. 2011; Bonanomi et al. 2013) and fine root decomposition (Goebel et al. 2011), as well as its performances in describing other soil functions like soil aggregation stability, soil water repellences, and organic matter phytotoxicity (Cartenì et al. 2018). However, this does not seem the case for WD decomposition where C/N achieved very high and significant correlations with the decay rate. We suspect that the very ample range of C/N ratio and their general high value determines an extreme N limitation that would override the inherent limitation of this parameter: i.e. lack a description of C chemistry.
P is considered an important macronutrient, controlling large WD decomposition in different ecosystems (Weedon et al. 2009). Here, we found no consistent correlations between WD decay rate and their P concentration. A similar finding was reported for fifteen WD types in tropical forest (Van Geffen et al. 2010). Among macronutrients, Ca is quantitatively the most abundant after N, in WD. Silver and Miya (2001) identified Ca as the most important limiting factor for the decomposition of large root debris on a global scale. Here, we found a negative correlation between Ca and WD decay rate in woodland. This result is due to the exceptional high Ca concentration of Q. ilex and Q. pubescens WD that recorded a low decay rate in field conditions.
Notably, we found strong positive correlations between K concentrations in WD and decay rate in field conditions. Previously studies reported a rapid K leaching during decomposition of leaf litter (Osono and Takeda 2004; Bonanomi et al. 2010). Abbott and Crossley (1982), instead, reported that K accumulates in decomposing Quercus prinus wood litter in xeric sites, while K was rapidly released in mesic one. These results suggest that K would be a limiting factor for WD decomposition in dry climates.
Most of the literature about WD decomposition limits the attention to macro-elements, while microelements such as Na, Mn, Fe, Zn, and Cu, have been less considered (but see Chen et al. 2015; Keiluweit et al. 2015). In this regard, we found strong and positive correlations between Na and Mn concentration in WD and their decay rate. Previous studies reported that the mass loss of leaf litter and wood debris is positively correlated with their Mn concentration (Stendahl et al. 2017). Mn is considered important in promoting the turnover of the stable litter fraction because litter-decomposing fungi use manganese peroxidase enzyme to decompose wood (Fujii et al. 2013). Here, however, Mn was positively correlated with the decay rate only in laboratory conditions. Further studies are needed to clarify the role of Mn in controlling WD decomposition in the Mediterranean climate. Na is often considered toxic for plants and, at high concentrations, even for insects and animals (Findlay and Kelly 2011), Kaspari et al. (2017) found a positive role of Na for terrestrial plant productivity by catalyzing the use of N and P by soil invertebrates. Concerning wood decomposition, recent studies revealed a positive role of Na in influencing the decomposition of coarse woody debris in tropical forests (Gora et al. 2018). The study of Kaspari et al. (2014) also revealed that in tropical forest Na exogenous application boost fungal and insect activity, with a strong promoting effect on leaf and wood decomposition. The authors suggest that Na limitation is more likely to occur in the inland area, far from ocean spray. Our data suggest that Na could be a limiting factor for WD decomposition also in Mediterranean conditions. Notably, we found that Na was limiting in the field but not in laboratory conditions. In fact, invertebrates are absent in the laboratory and this may explain the lack of correlation with decay rate. Alternatively, in the laboratory conditions, mineral Na and also other nutrients (K, Mg, N in mineral forms) would accumulate because root uptake is lacking, and mineralization is faster because of the higher soil temperature and non-limiting moisture conditions. Further studies that manipulate Na availability on the forest floor are necessary to assess if Na shortage constraint ecosystem carbon cycle outside tropical forests.
13C CPMAS NMR and FT-IR parameters
The extensive characterization of WD chemical traits carried out in this study, combining 13C CPMAS NMR and FT-IR data provide a unique data-set to shed light on the link between wood chemistry and decomposition. First, 13C CPMAS NMR revealed that WD spectra are dominated by O-alkyl C and di O-alkyl C fractions, associated with cellulose and hemicellulose, that together account for a percentage ranging from 67.1% and 74.9% of the whole spectrum. As a consequence, other fractions like aromatic C, carboxylic C, and alkyl C represent only a minor fraction of the spectra compared to other leaf litter (Almendros et al. 2000; Preston et al. 2009; Bonanomi et al. 2013). In addition, we found less variability in organic C type composition among WD types, compared to was previously reported for Mediterranean leaf litter (Incerti et al. 2018) or organic amendment used in agriculture (Sarker et al. 2018).
Concerning decomposition rate, it can be highlighted that WD contains many types of biomolecules with different susceptibility to microbial attack: simple carbohydrates, cellulose, hemicellulose, lipids, lignin, organic acids, and polyphenols. We found that WD decay rate was negatively correlated with di O-Alkyl C region. This is surprising considering that these fractions are associated with cellulose and hemicellulose, the biopolymer more susceptible to microbial attack compared to lignin. In fact, previous studies about leaf litter reported that decomposition rate is positively correlated with the relative abundance of O-Alkyl C (Bonanomi et al. 2013). In addition, a recent study demonstrates that bacterial and fungi preferentially feed on leaf litter rich in O-Alkyl C (Bonanomi et al. 2017b). This contradictory result could be partially explained by considering that in WD a large proportion of plant cellulose is not accessible to microbes because entrapped with lignin. Thereafter, decomposition of the more labile C fraction cannot proceed independently of lignin degradation (Adair et al. 2008). Another explanation calls into question the overall composition of WD in terms of organic C chemistry. We found that a strong positive correlation between WD decay rate and the aliphatic alkyl C fraction, characteristic of lipid waxes, fatty acids, cutins, and suberin. In addition, a strong positive correlation with the decay rate was found with the alkyl C/O-alkyl ratio, highlighting the positive role of the aliphatic fraction.
Highly significant positive correlations with the decay rate were also found with the carboxylic C fraction associated with organic acids and the amide carbon types. Indeed, alkyl C and carboxyl C represent a minor fraction of WD 13C CPMAS NMR spectra but seem to play a crucial role in decomposition. Previous studies reported that a high relative abundance of carboxyl C in organic amendments promotes N mineralization (Bonanomi et al. 2019a, b). In this context, we propose that a larger relative abundance of alkyl C and carboxyl C types promotes WD decomposition because would provide a more diversified and complete substrate for microbial decomposers. In this regard, the presence of amide and fatty acids within the wood tissue would provide to decomposer bacterial and fungi a more complete and complementary carbon sources that would promote the decomposition of the recalcitrant organic fractions. Further studies are needed to test this hypothesis.
Anatomical traits
Correlation and multivariate analyses revealed that anatomical features of WD showed a general trend of weak correlations with the decay rate. WD density is commonly believed to be among the primary controls over wood decomposition rates (Chao et al. 2009; Chave et al. 2009; Mori et al. 2014). However, we found that this parameter was poorly correlated with mass loss in all conditions, a result consistent with both a global meta-analysis of wood decomposition rates (Weedon et al. 2009), and with a study carried out in the tropical forest on fifteen species (Van Geffen et al. 2010).
Both vessel size and maximum lumen Feret’s diameter had a minor role in decomposition rate, thus disagreeing with the hypothesis that large vessel elements provide favourable microsites for fungal activity in terms of moisture and oxygen conditions, as well as increased physical access for fungal hyphae to a larger proportion of wood fragment volume (Dix and Webster 1995). While, vessel density exerted a positive influence on the decomposition rate especially when the WD was incubated in laboratory conditions with woodland soil. In this regard we should hypothesize that, as decomposition proceeds, the closely spaced vessels may easily connect to each other, resulting in a gradual increase in the decomposition surface inside the wood. In this context, the negative correlation with lumen area percent i.e. the percentage of xylem cross-section occupied by vessels depending on both vessel density and size, in laboratory condition deserve further exploration.
Conclusions
To our knowledge, this is the first multi-species study that compares WD decomposition in the Mediterranean basin. The use of a wide array of WD traits highlights that chemical features and anatomical parameters contribute to explaining the observed interspecific variation of decomposition rate. In agreement with previous studies, N concentration seems the most limiting nutrients, suggesting that WD decomposition is N rather than P limited. Noteworthy, we found that decomposition was positively correlated with K, Mn, and Na concentration in WD. The positive effect of Na support recent findings from tropical forests (Kaspari et al. 2014). C/N and lignin N ratios were the best predictors of decay, but 13C CPMAS NMR revealed that the presence of aliphatic and amide compounds in WD strongly promoted their decay rate. Our results highlight key variables associated with rates of decomposition but the descriptive nature of this study potentially caused spurious relationships with decomposition caused by associations among multiple predictors. Regardless, these findings represent multiple important avenues for future research and experimental confirmation of hypothesized processes. In fact, the present study fills those gaps concerning WD decomposition in Mediterranean ecosystems providing data for a more accurate C modelling at ecosystem scale. We acknowledge that the presented decay rates are based on 3 years of experimental study, a time frame rather short in terms of deadwood decomposition in natural ecosystems. In addition, the use of WD with the diameter < 3 cm limits the result extrapolation to wood debris with a larger diameter (Oberle et al. 2018). Finally, no data are reported about soil microbiome that plays a crucial role in decomposing recalcitrant organic substrate like woody debris (Gora et al. 2019), and organic polymers (Bonanomi et al. 2020). An additional challenge for future researches will be to translate an explicit representation of WD biomolecular composition and transformations, the mutual interactions between chemical pools and microbiome, and their cascade effects on the decay rate, as recently proposed in the OMDY model for leaf litter (Incerti et al. 2017). Our on-going long-term (10 years) litter-bag experiment will shed light on other aspects of WD decomposition including chemical and anatomical changes of wood, the successional trajectories of bacterial and fungal communities, and on the role of nutrient transfer from soil to wood in the process of decomposition.
References
Abbott DT, Crossley DA Jr (1982) Woody litter decomposition following clear-cutting. Ecology 63:35–42. https://doi.org/10.2307/1937028
Adair EC, Parton WJ, Del Grosso SJ, Silver WL, Harmon ME, Hall SA, Burke IC, Hart SC (2008) Simple three-pool model accurately describes patterns of long-term litter decomposition in diverse climates. Glob Chang Biol 14:2636–2660. https://doi.org/10.1111/j.1365-2486.2008.01674.x
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449. https://doi.org/10.2307/3546886
Almendros G, Dorado J, González-Vila FJ, Blanco MJ, Lankes U (2000) 13C NMR assessment of decomposition patterns during composting of forest and shrub biomass. Soil Biol Biochem 32:793–804. https://doi.org/10.1016/S0038-0717(99)00202-3
Berg B, McClaugherty C (2014) Plant litter: Decomposition, humus formation and carbon sequestration. Springer-Verlag, Berlin
Berglund SL, Ågren GI (2012) When will litter mixtures decompose faster or slower than individual litters? A model for two litters. Oikos 121:1112–1120. https://doi.org/10.1111/j.1600-0706.2011.19787.x
Bonanomi G, Incerti G, Antignani V, Capodilupo M, Mazzoleni S (2010) Decomposition and nutrient dynamics in mixed litter of Mediterranean species. Plant Soil 331:481–496. https://doi.org/10.1007/s11104-009-0269-6
Bonanomi G, Incerti G, Giannino F, Mingo A, Lanzotti V, Mazzoleni S (2013) Litter quality assessed by solid state 13C NMR spectroscopy predicts decay rate better than C/N and Lignin/N ratios. Soil Biol Biochem 56:40–48. https://doi.org/10.1016/j.soilbio.2012.03.003
Bonanomi G, Cesarano G, Gaglione SA, Ippolito F, Sarker TC, Rao MA (2017a) Soil fertility promotes decomposition rate of nutrient poor, but not nutrient rich litter through nitrogen transfer. Plant Soil 412:397–411. https://doi.org/10.1007/s11104-016-3072-1
Bonanomi G, Cesarano G, Lombardi N, Motti R, Scala F, Mazzoleni S, Incerti G (2017b) Litter chemistry explains contrasting feeding preferences of bacteria, fungi, and higher plants. Sci Rep 7:9208. https://doi.org/10.1038/s41598-017-09145-w
Bonanomi G, Chirico GB, Palladino M, Gaglione SA, Crispo DG, Lazzaro U, Sica B, Cesarano G, Ippolito F, Sarker TC, Rippa M, Scala F (2017c) Combined application of photo-selective mulching films and beneficial microbes affects crop yield and irrigation water productivity in intensive farming systems. Agric Water Manage 184:104–113. https://doi.org/10.1016/j.agwat.2017.01.011
Bonanomi G, Incerti G, Abd El-gawad AM, Sarker TC, Stinca A, Motti R, Cesarano G, Teobaldelli M, Saulino L, Cona F, Chirico GB et al (2018) Windstorm disturbance triggers multiple species invasion in a Mediterranean forest. iForest 11:64–71. https://doi.org/10.3832/ifor2374-010
Bonanomi G, De Filippis F, Cesarano G, La Storia A, Zotti M, Mazzoleni S, Incerti G (2019a) Linking bacterial and eukaryotic microbiota to litter chemistry: Combining next generation sequencing with 13C CPMAS NMR spectroscopy. Soil Biol Biochem 129:110–121. https://doi.org/10.1016/j.soilbio.2018.11.013
Bonanomi G, Sarker TC, Zotti M, Cesarano G, Allevato E, Mazzoleni S (2019b) Predicting nitrogen mineralization from organic amendments: beyond C/N ratio by 13 C-CPMAS NMR approach. Plant Soil 441:129–146. https://doi.org/10.1007/s11104-019-04099-6
Bonanomi G, Maisto G, De Marco A, Cesarano G, Zotti M, Mazzei P, Libralato G, Staropoli A, Siciliano A, De Filippis F, La Storia A, Piccolo A, Vinale F, Crasto A, Guida M, Ercolini G, Incerti G (2020) The fate of cigarette butts in different environments: Decay rate, chemical changes and ecotoxicity revealed by a 5-years decomposition experiment. Environ Pollut 261:114108. https://doi.org/10.1016/j.envpol.2020.114108
Bradford MA, Warren Ii RJ, Baldrian P, Crowther TW, Maynard DS, Oldfield EE, Wieder WR, Wood SA, King JR (2014) Climate fails to predict wood decomposition at regional scales. Nat Clim Chang 4:625. https://doi.org/10.1038/nclimate2251
Bradford MA, Veen GC, Bonis A, Bradford EM, Classen AT, Cornelissen JHC, Crowther TW, De Long JR, Freschet GT, Kardol P, Manrubia-Freixa M, Maynard DS, Newman GS, Logtestijn RSP, Viketoft M, Wardle DA, Wieder WR, Wood SA, van der Putten WH (2017) A test of the hierarchical model of litter decomposition. Nat Ecol Evol 1:1836–1845. https://doi.org/10.1038/s41559-017-0367-4
Brady NC, Weil RR (2001) The nature and properties of soils, 13th edn. Prentice Hall, New York
Cartenì F, Sarker TC, Bonanomi G, Cesarano G, Esposito A, Incerti G, Mazzoleni S, Lanzotti V, Giannino F (2018) Linking plant phytochemistry to soil processes and functions: the usefulness of 13C NMR spectroscopy. Phytochem Rev 17:815–832. https://doi.org/10.1007/s11101-018-9560-6
Chambers JQ, Higuchi N, Schimel JP, Ferreira LV, Melack JM (2000) Decomposition and carbon cycling of dead trees in tropical forests of the central Amazon. Oecologia 122:380–388. https://doi.org/10.1007/s004420050044
Chao KJ, Phillips OL, Baker TL, Peacock J, Lopez-Gonzalez G, Vásquez Martínez R, Monteagudo A, Torres-Lezama A (2009) After trees die: quantities and determinants of necromass across Amazonia. Biogeosciences 6:1615–1626. https://doi.org/10.5194/bg-6-1615-2009
Chave J, Coomes D, Jansen S, Lewis SL, Swenson NG, Zanne AE (2009) Towards a worldwide wood economics spectrum. Ecol Lett 12:351–366. https://doi.org/10.1111/j.1461-0248.2009.01285.x
Chen Y, Sayer EJ, Li Z, Mo Q, Li Y, Ding Y, Wang J, Lu X, Tang J, Wang F (2015) Nutrient limitation of woody debris decomposition in a tropical forest: contrasting effects of N and P addition. Funct Ecol 30:295–304. https://doi.org/10.1111/1365-2435.12471
Cornwell WK, Cornelissen JH, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Pérez-Harguindeguy N et al (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071. https://doi.org/10.1111/j.1461-0248.2008.01219.x
Coûteaux MM, Bottner P, Berg B (1995) Litter decomposition, climate and litter quality. Tree 10:63–66. https://doi.org/10.1016/S0169-5347(00)88978-8
Dix NJ, Webster J (1995) Fungal Ecology. Chapman & Hall, London
Duckworth JC, Kent M, Ramsay PM (2000) Plant functional types: an alternative to taxonomic plant community description in biogeography? Prog Phys Geogr 24:515–542. https://doi.org/10.1177/030913330002400403
Emmanuel V, Odile B, Céline R (2015) FTIR spectroscopy of woods: A new approach to study the weathering of the carving face of a sculpture. Spectrochim Acta A Mol Biomol Spectrosc 136:1255–1259. https://doi.org/10.1016/j.saa.2014.10.011
Findlay SE, Kelly VR (2011) Emerging indirect and long-term road salt effects on ecosystems. Ann N Y Acad Sci 1223:58–68. https://doi.org/10.1111/j.1749-6632.2010.05942.x
Fioretto A, Di Nardo C, Papa S, Fuggi A (2005) Lignin and cellulose degradation and nitrogen dynamics during decomposition of three leaf litter species in a Mediterranean ecosystem. Soil Biol Biochem 37:1083–1091. https://doi.org/10.1016/j.soilbio.2004.11.007
Frey SD, Elliott ET, Paustian K, Peterson GA (2000) Fungal translocation as a mechanism for soil nitrogen inputs to surface residue decomposition in a no-tillage agroecosystem. Soil Biol Biochem 32:689–698. https://doi.org/10.1016/S0038-0717(99)00205-9
Fujii K, Uemura M, Hayakawa C, Funakawa S, Kosaki T (2013) Environmental control of lignin peroxidase, manganese peroxidase, and laccase activities in forest floor layers in humid Asia. Soil Biol Biochem 57:109–115
Gessner MO (2005) Proximate lignin and cellulose. In: Graca MAS, Bärlocher F, Gessner MO (eds) Methods to study litter decomposition. A practical guide. Springer Verlag, Dordrecht, pp 115–120
Goebel M, Hobbie SE, Bulaj B, Zadworny M, Archibald DD, Oleksyn J, Reich PB, Eissenstat DM (2011) Decomposition of the finest root branching orders: linking belowground dynamics to fine-root function and structure. Ecol Monogr 81:89–102. https://doi.org/10.1890/09-2390.1
Gora EM, Sayer EJ, Turner BL, Tanner EVJ (2018) Decomposition of coarse woody debris in a long-term litter manipulation experiment: A focus on nutrient availability. Funct Ecol 32:1128–1138. https://doi.org/10.1111/1365-2435.13047
Gora EM, Lucas JM, Yanoviak SP (2019) Microbial composition and wood decomposition rates vary with microclimate from the ground to the canopy in a tropical forest. Ecosystems 22:1206. https://doi.org/10.1007/s10021-019-00359-9
Haberhauer G, Rafferty B, Strebl F, Gerzabek MH (1998) Comparison of the composition of forest soil litter derived from three different sites at various decompositional stages using FTIR spectroscopy. Geoderma 83:331–342. https://doi.org/10.1016/S0016-7061(98)00008-1
Hättenschwiler S, Coq S, Barantal S, Tanya I (2011) Leaf traits and decomposition in tropical rainforests: revisiting some commonly held views and towards a new hypothesis. New Phytol 189:950–965. https://doi.org/10.1111/j.1469-8137.2010.03483.x
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50:346–363. https://doi.org/10.1002/bimj.200810425
Incerti G, Bonanomi G, Giannino F, Piermatteo D, Castaldi S, Fioretto A, Papa S, De Marco A, Fierro A, Maggi O et al (2011) Litter decomposition in Mediterranean ecosystems: modelling the controlling role of climatic conditions and litter quality. Appl Soil Ecol 49:148–157. https://doi.org/10.1016/j.apsoil.2011.06.004
Incerti G, Bonanomi G, Giannino F, Cartenì F, Spaccini R, Mazzei P, Piccolo A, Mazzoleni S (2017) OMDY: a new model of organic matter decomposition based on biomolecular content as assessed by 13C-CPMAS-NMR. Plant Soil 411:377–394. https://doi.org/10.1007/s11104-016-3039-2
Incerti G, Cartenì F, Cesarano G, Sarker TC, Abd El-Gawad AM, D’Ascoli R, Bonanomi G, Giannino F (2018) Faster N release, but not C loss, from leaf litter of invasives compared to native species in Mediterranean ecosystems. Front Plant Sci 9:534. https://doi.org/10.3389/fpls.2018.00534
Jarvis P, Rey A, Petsikos C, Wingate L, Rayment M, Pereira J, Banza J, David J, Miglietta F, Borghetti M, Manca G, Valentini R (2007) Drying and wetting of Mediterranean soils stimulates decomposition and carbon dioxide emission: the “Birch effect”. Tree Physiol 27:929–940. https://doi.org/10.1093/treephys/27.7.929
Jonsson BG, Kruys N (2001) Ecology of woody debris in boreal forest. Blackwell Publishing, Oxford
Kahl T, Arnstadt T, Baber K, Bässler C, Bauhus J, Borken W, Buscot F, Floren A, Heibl C, Hessenmöller D (2017) Wood decay rates of 13 temperate tree species in relation to wood properties, enzyme activities and organismic diversities. For Ecol Manage 391:86–95. https://doi.org/10.1016/j.foreco.2017.02.012
Kaspari M, Garcia MN, Harms KE, Santana M, Wright SJ, Yavitt JB (2008) Multiple nutrients limit litterfall and decomposition in a tropical forest. Ecol Lett 11:35–43. https://doi.org/10.1111/j.1461-0248.2007.01124.x
Kaspari M, Clay NA, Donoso DA, Yanoviak SP (2014) Sodium fertilization increases termites and enhances decomposition in an Amazonian forest. Ecology 95:795–800. https://doi.org/10.1890/13-1274.1
Kaspari M, Roeder KA, Benson B, Weiser MD, Sanders NJ (2017) Sodium co-limits and catalyzes macronutrients in a prairie food web. Ecology 98:315–320. https://doi.org/10.1002/ecy.1677
Keiluweit M, Peter N, Harmon ME, Jingdong Mark E, M, Pett-Ridge J, Kleber M, (2015) Long-term litter decomposition controlled by manganese redox cycling. Proc Natl Acad Sci 112:E5253. https://doi.org/10.1073/pnas.1508945112
Kögel-Knabner I (2002) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34:139–162. https://doi.org/10.1016/S0038-0717(01)00158-4
Legendre P, Legendre LF (eds) (2012) Numerical ecology, 2nd English edn. Elsevier Science, Amsterdam
Lenth R (2020) Emmeans: estimated marginal means, aka Least-Squares Means. R package version 1.4.7. https://CRAN.R-project.org/package=emmeans. Accessed 10 Jan 2020
Llorens P, Domingo F (2007) Rainfall partitioning by vegetation under Mediterranean conditions. A review of studies in Europe. J Hydrol 335:37–54. https://doi.org/10.1016/j.jhydrol.2006.10.032
Lummer D, Scheu S, Butenschoen O (2012) Connecting litter quality, microbial community and nitrogen transfer mechanisms in decomposing litter mixtures. Oikos 121:1649–1655. https://doi.org/10.1111/j.1600-0706.2011.20073.x
Makkonen M, Berg MP, Handa IT, Hättenschwiler S, van Ruijven J, van Bodegom PM, Aerts R (2012) Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol Lett 15:1033–1041. https://doi.org/10.1111/j.1461-0248.2012.01826.x
Meentemeyer V (1978) Macroclimate and lignin control of litter decomposition rates. Ecology 59:465–472. https://doi.org/10.2307/1936576
Mori S, Itoh A, Nanami S, Tan S, Chong L, Yamakura T (2014) Effect of wood density and water permeability on wood decomposition rates of 32 Bornean rainforest trees. J Plant Ecol. https://doi.org/10.1093/jpe/rtt041
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Oberle B, Covey KR, Dunham KM, Hernandez EJ, Walton ML, Young DF, Zanne AE (2018) Dissecting the effects of diameter on wood decay emphasizes the importance of cross-stem conductivity in Fraxinus americana. Ecosystems 21(1):85–97
Osono T, Takeda H (2004) Potassium, calcium, and magnesium dynamics during litter decomposition in a cool temperate forest. J For Res 9:23–31. https://doi.org/10.1007/s10310-003-0047-x
Pan Y, Birdsey RA, Fang J, Houghton R, Kauppi PE, Kurz WA, Phillips OL, Shvidenko A, Lewis SL, Canadell JG et al (2011) A large and persistent carbon sink in the world’s forests. Science 333:988–993. https://doi.org/10.1126/science.1201609
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC, Fasth B (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364. https://doi.org/10.1126/science.1134853
Podani J (2000) Introduction to the exploration of multivariate biological data. Backhuys Publishers, Leiden
Popescu CM, Popescu MC, Singurel G, Vasile C, Argyropoulos DS, Willfor S (2007) Spectral characterization of eucalyptus wood. Appl Spectrosc 61:1168–1177. https://doi.org/10.1366/000370207782597076
Preston CM, Nault JR, Trofymow J (2009) Chemical changes during 6 years of decomposition of 11 litters in some Canadian forest sites. Part 2. 13C abundance, solid-state 13C NMR spectroscopy and the meaning of “lignin”. Ecosystems 12:1078–1102. https://doi.org/10.1007/s10021-009-9267-z
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. https://www.R-project.org/. Accessed 10 Jan 2020
Rowell RM (2012) Handbook of wood chemistry and wood composites. CRC Press, Boca Raton
Sarker TC, Incerti G, Spaccini R, Piccolo A, Mazzoleni S, Bonanomi G (2018) Linking organic matter chemistry with soil aggregate stability: Insight from 13C NMR spectroscopy. Soil Biol Biochem 117:175–184. https://doi.org/10.1016/j.soilbio.2017.11.011
Schimel JP, Hättenschwiler S (2007) Nitrogen transfer between decomposing leaves of different N status. Soil Biol Biochem 39:1428–1436. https://doi.org/10.1016/j.soilbio.2006.12.037
Schweingruber FH, Poschlod P (2005) Growth rings in herbs and shrubs: life span, age determination and stem anatomy. For Snow Landsc Res 79:195–415
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419. https://doi.org/10.1007/s004420100740
Stendahl J, Berg B, Lindahl BD (2017) Manganese availability is negatively associated with carbon storage in northern coniferous forest humus layers. Sci Rep 7(1):1–6
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in Terrestrial Ecosystems. California University Press, Berkeley
Taylor BR, Parkinson D, Parsons WF (1989) Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70:97–104. https://doi.org/10.2307/1938416
Traoré M, Kaal J, Cortizas AM (2018) Differentiation between pine woods according to species and growing location using FTIR-ATR. Wood Sci Technol 52:487–504. https://doi.org/10.1007/s00226-017-0967-9
Van Geffen KG, Poorter L, Sass-Klaassen U, Van Logtestijn RS, Cornelissen JH (2010) The trait contribution to wood decomposition rates of 15 Neotropical tree species. Ecology 91:3686–3697. https://doi.org/10.1890/09-2224.1
Weedon JT, Cornwell WK, Cornelissen JH, Zanne AE, Wirth, Coomes DA (2009) Global meta-analysis of wood decomposition rates: a role for trait variation among tree species? Ecol Lett 12:45–56. https://doi.org/10.1111/j.1461-0248.2008.01259.x
Acknowledgements
The 13C-CPMAS NMR measurements were performed at the CERMANU-Interdepartmental Research Centre for Nuclear Magnetic Resonance, University of Napoli Federico II. The assistance of the staff is gratefully acknowledged. The authors would like to thank the referees for their valuable comments which helped to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Feike A. Dijkstra.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
ESM 1
(DOCX 9.00 MB)
Rights and permissions
About this article
Cite this article
Bonanomi, G., Zotti, M., Cesarano, G. et al. Decomposition of woody debris in Mediterranean ecosystems: the role of wood chemical and anatomical traits. Plant Soil 460, 263–280 (2021). https://doi.org/10.1007/s11104-020-04799-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04799-4