Abstract
Aims
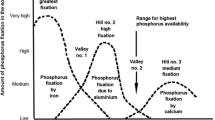
Many soil scientists think that soil phosphate availability is highest at near-neutral pH and decreases with decreasing pH. This belief does not appear to have ever been subjected to experimental test.
Methods
In a pot trial, we measured response curves to phosphate at seven pH(CaCl2) values ranging from 3.99 to 7.26 using three plant species: mustard (Brassica campestris), lucerne – also known as alfalfa – (Medicago sativa) and rice (Oryza sativa). We used a form of the Mitscherlich equation that allowed us to estimate the phosphate contribution from the soil as well as the slope of the response curve and the maximum yield.
Results
Plants grew best near pH 5.5 and worst at near-neutral pH. We think the large decrease in growth with increasing pH was caused by decreasing rate of P uptake by plants. There was a smaller decrease in growth as pH decreased below 5.5. We think this was caused by aluminium toxicity.
Conclusions
The conventional belief that phosphate availability is greatest near neutral pH is wrong.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Most textbooks teach that phosphate availability increases with increasing pH reaching a maximum at near neutral pH. This is based on the idea that phosphate reaction with soil is controlled by precipitation of iron phosphate at low pH, aluminium phosphate at medium pH and calcium phosphate at high pH leaving a minimum at near neutral pH (Price 2006, Penn and Camberato 2019). Thus phosphate is thought to be present as discrete particles and we refer to this as the particle hypothesis. The alternative view is that phosphate reaction involves specific adsorption of phosphate ions onto variable charges surfaces followed by diffusive penetration; the adsorption-penetration hypothesis (Barrow 1999). The two hypotheses can be distinguished by observing the effects of the background electrolyte. When measured in a dilute solution of a salt of a monovalent cation there is a large decrease in sorption with increasing pH. As the concentration of salt increases, or if a divalent cation is used, the decrease is much smaller and may be reversed at high pH (Barrow 1984, Barrow and Debnath 2015). According to the adsorption-penetration hypothesis this is the consequence of compression of the double layer as a result of which the negative potential becomes less negative and reaction is increased. The particle hypothesis has no means to explain this. We therefore argue that the commonly accepted effect of pH on availability is based on a false hypothesis.
There are two further reasons for querying the effects of pH on phosphate availability. One derives from the argument that it is desorption not adsorption that controls release of phosphate. Available evidence is that phosphate desorption is least near pH 5 and increases at lower and higher pH (Barrow 2017). Increasing desorption as pH decreased was also observed by Saleque and Kirk (1995). These observations were dismissed by Penn and Camberato (2019) largely because they thought them inconsistent with their theories. In the work reported here we show that the phosphorus supplied by soil to plants, and therefore desorbed, is indeed affected by pH and that there is a minimum near pH 5.
The other reason is that uptake of phosphate from solution by plant roots decreases with increasing pH. It was noted that the rate of phosphate uptake closely matched the proportion of phosphate present as a monovalent H2PO4− ion and this was interpreted as showing that the uptake mechanism was specific to this ion (Vange et al. 1974). Subsequently, in a comprehensive review, Rausch and Bucher (2002) considered several lines of evidence and concluded that the mechanism of phosphate uptake involves H+/Pi co-transport and this explains why it is not favoured at high pH.
It seems that the belief that phosphate availability increases with increasing pH is widely accepted but, as far as we are aware, it has never subjected to experimental test. The limited data that are available come from experiments designed for different purposes. In the work reported here we grew three species of plants at 10 levels of supplied phosphate and at pH(CaCl2) values ranging from 3.99 to 7.26.
Methods
Soil
We used a soil of low phosphorus status and high buffering capacity for P from the Regional Research Station of Bidhan Chandra Krishi Viswavidyalaya at Jhargram in West Bengal, India (22°26′58.99″ N, 86°59′49.23″E). This soil was also used by Barrow et al. (2020). The site was well-drained and not used for cultivation. The average annual rainfall is 1400 mm, 81% of which falls in the monsoon months from June to early October. The average maximum temperature in summer is 37 °C and average minimum in winter is 15 °C. We collected 250 kg of the soil in January 2019, when the soil was dry, to a depth of about 20 cm using a spade. The soil was thoroughly mixed; ground to pass through a 2 mm sieve and stored at 25 °C prior to use. The soil is classified as a coarse loamy Typic Haplustulf in the red and lateritic soil zone. Some of its properties are: Colwell P, 11.36 mg kg−1 (Colwell 1963); Bray P, 3.17 mg kg−1 (Bray and Kurtz 1945); cation exchange capacity, 9.2 cmol (p+) kg−1 (Dewis and Freitas 1984); water holding capacity 28% (Piper 1966); organic C content, 3.1 g kg−1 (Walkley and Black 1934). The effects of pH and period of reaction on P sorption by this soil were described by Barrow et al. (2020).
Design of the experiment
In designing our experiment we followed the advice of Webster (2007). “In experiments with graded treatments do not make multiple comparisons of any kind; instead fit a response curve and analyse the data by regression“. We fitted a “surface” in which observed yield is related to both pH and P level. If we do not wish to compare pairs of observations, nothing is gained by replicating the observations and much is lost for there would be fewer treatments and the surface would be less well defined. When a regression is found to be significant it can be taken that any change in the independent variable will produce a change in the dependent variable – with a confidence that depends on the goodness of fit as reflected in R2.
Preparation of the treatments
We applied our treatments to subsamples of the soil with the aim of adding a band of treated soil to each pot. We initially altered the pH of six 2 kg subsamples of the soil. To each of these six subsamples we added powdered CaCO3 at 4, 7, 10, 15, 20 and 30 mmol kg−1. We then moistened the soil (26%) and incubated at 60 °C for 2 days. The 60 °C temperature was used to accelerate the rate of reaction with lime (Barrow and Cox 1990). The sequence of pH values obtained after treatment was: 3.99 (untreated), 4.55, 5.19, 5.90, 6.40, 6.95 and 7.26. We divided each subsample into 10 lots each weighing 200 g. To each 200 g lot we added solutions of KH2PO4 to give: 0, 20, 50, 100, 200, 300, 400, 600, 800 and 1000 mg P kg−1.
Cultivating the plants
We used pots with a surface diameter of 115 mm. We first added 700 g of bulk soil. We then added (in sequence) to each pot: the 200 g test soil samples, 50 g of bulk soil, seeds, and a further 50 g of bulk soil. After each addition, the soil surface was levelled. The advantage of this approach was that most of the soil in which plants were grown had not been incubated at high temperature and therefore would not have sustained any adverse effects from this treatment. We grew three species: rice (Oryza sativa. L. IET 4786, mustard (Brassica campestris L. ‘B9’) and lucerne (Medicago sativa L. commercial source). The variety of rice used is recommended for rain fed agriculture and is widely grown on acid to neutral soils in West Bengal. After germination, seven healthy plants were kept for rice, six plants for mustard and ten plants for lucerne in respective pots maintaining equal spaces as far possible. Rice plants were sown during the second week of September, mustard in the first week of November and lucerne in the first week of December, 2019. Seven days after emergence, we added a solution which gave the following additions as basal nutrient (mg nutrient kg−1 soil): Mn 4, Mo 1, Cu 1, B 0.2, Zn 10, K 100, Mg 6, and N 100. Half the N was added as NH4Cl and half as NaNO3. Soils were irrigated each day to maintain 50% of water holding capacity. Plants were grown in the dry season for 30 days in the open air on a rooftop.
The seeds added only negligible amounts of P (Table 1). At the end of the experiment, the aboveground parts of plants were collected, washed with 0.2% solution of phosphate–free detergent (ARIEL powder, Worlee-Chemie India Pvt. Ltd) to remove any waxy or greasy material, then with dilute H2SO4, and finally with distilled water, air dried and then oven dried at 60 °C until a constant weight was reached.
The dried above-ground plant parts were pulverized and the P concentration in the ground leaf was determined by digestion with a mixture of acids (HNO3:H2SO4:HClO4− 10:1:4 by volume). The P content in the extract was analysed using the vanado-molybdo-phosphoric acid method (Singh et al. 1999) using a UV-vis-spectrophotometer to measure the yellow colour.
Aluminium was determined by Atomic Absorption Spectrophotometer (AAS, Perkin Elmer).
Analysis of the data
For each pH treatment and for each plant species, we fitted response curves in the form
where y is the yield; x is the phosphorus applied; and m, c, and d are parameters. The parameter m indicates the maximum yield to which the data trend; the parameter c indicates the slope of the response curve; and the parameter d indicates the P coming from the soil and seed. We could therefore separate the effects of pH on the supply of P from the soil (d) from those on the uptake and utilisation of P (c). The parameter c can be regarded as a measure of the effectiveness of the fertiliser; the larger its value, the smaller the amount of fertiliser needed for a given response.
We wrote a program in QB64, a BASIC language, to fit equations in which the parameters of Eq. (1) were replaced by functions of pH. The program uses the Simplex algorithm to find the values of the parameters that give the smallest value of residual sums of squares. Each cycle of the program involves 300 iterations. We restarted the cycle 200 times giving a total of 60,000 iterations. We compared different functions of pH and chose functions that most efficiently described the data. Thus we fitted surfaces such that plant yield was related to both pH and P level.
Results
For all three species, largest yields were obtained for the pH 5.19 treatment. There were small decreases in yield when the pH was decreased and large decreases in yield when the pH was increased. Even so, the yields for the lowest pH were appreciably greater than for the highest pH (Fig. 1). Our confidence in this and other statements in this section is based on the close fit of the surface to the data. This is evident in Fig. 1 and in the values for the correlation coefficients; these were: rice 0.990, mustard 0.992, and lucerne 0.988 (p < 0.00001).
Effect of the initial pH on the response of three species of plants to 10 levels of phosphate. Response curves for pH 4.55, 5.90, and 6.95 (which were intermediate in value) have been omitted to improve clarity. The curves are not fitted to the data points for each pH; they are lines for each pH on the surface obtained when the data are described by y = m [1 – exp (−c(x + d)] where y is the yield; x is the phosphorus applied; and m, a, and d are functions of pH as indicated in Fig. 2. (Values for R2 are: rice 0.990, mustard 0.992, and lucerne 0.988)
There was a broad similarity in the components of the response across the three species (Fig. 2). For all species: there was a small decline in the fitted value for the maximum sorption with increasing pH; there was a maximum value for the effectiveness for the pH 5.19 treatment with a small decrease at lower pH and a large decrease at higher pH; and for rice and mustard there was a minimum of the d parameter for the pH 5.19 treatment with increases at both higher and lower pH.
Effect of pH on functions used to describe the data. Points are values of the parameters obtained for the equation y = m [1 – exp (−c(x + d)] where y is the yield and x is the phosphorus applied which was fitted to the data of Fig. 1 for each pH. Curves illustrate the functions used to describe the surface for which yield is related to both pH and P supplied
The most marked difference between the species was in the values for the parameter d. As the amount of P supplied by the seeds was trivial, this parameter reflects the amount coming from the soil. Before P can be accessed by plant roots, it has to be desorbed. We therefore interpreted this parameter as reflecting the amounts of desorption. The values were in the sequence: mustard > rice > lucerne. Further, the effects of pH on desorption were marked for both rice and mustard but not discernible for lucerne.
Smaller, but nevertheless important, differences existed between the species in the value of the slope term (c). This reflects the ability of the plants to utilise the phosphate supplied. The sequence for this property was: rice > lucerne > mustard. Thus mustard was very good at obtaining the soil phosphate but poor at using it. We found that “broken-stick” functions closely and efficiently described the relationships. Such functions are consistent with different processes controlling the response at high and at low pH.
When plots of plant weight against P concentration fall about a common line, it can be assumed that they have an equivalent source of phosphate (Black and Scott 1956) and that growth is not limited by anything else. Figure 3 shows that this was the case for most of the pH treatments. However, for the most-acid treatments this was not so. For the three lowest pH values for lucerne and for the two lowest pH values for the other species the points fell below the common line. That is, a higher concentration was needed to produce the same yield. This indicates that response was affected by something else.
There was appreciable aluminium present in the plant tissue (Fig. 4). Values were highest for rice and lowest for lucerne. The aluminium concentration decreased with increasing pH, but did not decline to very low levels because roots had access to the bulk soil which was at a low pH. Figure 5 shows that the decrease in yield at low pH was associated with increasing aluminium concentrations. Rice had highest aluminium concentration; lucerne the lowest.
Discussion
Largest yields occurred near pH 5 and lowest at neutral or near neutral pH. Thus there are now five species of plants for which similar results have been observed; the three species used here plus Trifolium subterranean and Zea mays (Barrow 2017). The effects on T. subterranean were dismissed by Penn and Camberato (2019) – arguing that “certain plants prefer acid conditions”. This dismissal is hard to sustain especially with a plant such as lucerne which has a reputation for preferring neutral pH and, as shown here, is sensitive to aluminium. We think that the low effectiveness at higher pH can be attributed to effects on the uptake mechanism by plant roots. This involves H+/Pi co-transport (Rausch and Bucher 2002). As far as is known, this mechanism is common to all plant roots. It would be reasonable to expect the direction of the effect to be the same no matter which plant was used. The magnitude of the effect may however differ between plants as it did for the plants used here.
On the other hand, the smaller depression in yield at low pH can be attributed to aluminium toxicity. Plants differ in their sensitivity to aluminium and soils differ in the extent to which they supply aluminium. It would therefore be reasonable to expect slightly different results with different combinations of plants and soils. We also suspect that if the bulk soil were not as acid, the decrease in growth at low pH may not have been as large.
The form in which nitrogen is supplied is known to have a large effect on the pH of the rhizosphere (Riley and Barber 1971). Here we used an equal mixture of nitrate and ammonium. If we had supplied nitrogen solely as nitrate, we would expect the effects to shift to a lower initial pH, and if we had supplied it solely as ammonium to shift to a higher optimum initial pH.
The other component of the effect of pH on phosphorus response is the effect of pH on desorption. Plots of the desorption term from the fitted response equations against pH were “u-shaped” with a minimum near pH 5. This has not been previously observed when the effect is measured by plant response. It is however consistent with laboratory studies on desorption as summarised by Barrow (2017). The mechanism has not previously been understood. However, it was recently shown that the rate of sorption when plotted against pH also formed a “u-shaped” curve with a minimum near pH 5 (Barrow et al. 2020). That is, sorption was faster at both high and low pH. This was characterised using a mechanistic model in which the rate was assumed to be limited by the rate of diffusive penetration and this was characterised by a diffusion coefficient. Figure 6 shows that values of the desorption term obtained here are well correlated with values of the diffusion coefficient. That is, the rates of desorption and the rates of sorption are similarly affected by pH.
Plots of desorption as measured in this experiment against the diffusion coefficient for P sorption from Barrow et al. (2020). Desorption is reflected in the value of the parameter d when the equation y = m [1 – exp (−c(x + d)] where y is the yield and x is the phosphorus applied was fitted to the several values for pH as shown in Fig. 1. In the work of Barrow et al. (2020), four levels of phosphate were incubated with soil for periods between one and 30 days and at pH values ranging from 3.01 to 7.95. The mechanistic model of Barrow (1983) closely described the results. In that model, the rate of sorption is determined by the rate of diffusion into the adsorbing particle characterised by the diffusion coefficient
Many plants are observed to acidify the rhizosphere especially when P is limiting. This observation is difficult to explain if phosphate availability increases with increasing pH but is consistent with our results. In many cases organic acids are secreted and these would be expected to sequester aluminium thus decreasing the effects of aluminium toxicity at low pH.
Conclusions
The conventional view is wrong. It is based on a theory that is both wrong and irrelevant. The optimum pH for phosphate availability is not close to 7 but much lower. The optimum pH for growth depends on the interplay between decreasing rate of uptake as pH is increased and increasing aluminium toxicity as it is decreased. For the soil, plants and nitrogen supply used here it was somewhat above 5.
References
Barrow NJ (1983) A mechanistic model for describing the sorption and desorption of phosphate by soil. J Soil Sci 34:733–750
Barrow NJ (1999) The four laws of soil chemistry: the Leeper lecture 1998. Aust J Soil Res 37:787–829
Barrow NJ (2017) The effects of pH on phosphate uptake from the soil. Plant Soil 410:401–410. https://doi.org/10.1007/s11104-016-3008-9
Barrow NJ, Cox VC (1990) A quick and simple method for determining the titration curve and estimating the lime requirement of soil. Aust J Soil Res 28:685–694
Barrow NJ, Debnath A (2015) Effect of phosphate status and pH on sulphate sorption and desorption. Europ J Soil Sc 66:286–297
Barrow NJ, Debnath A, Sen A (2020) Effect of pH and prior treatment with phosphate on the rate of reaction of soils with phosphate. Europ J Soil Sci. https://doi.org/10.1111/ejss.12968
Black CA, Scott CO (1956) Fertiliser evaluation. 1. Fundamental principles. Soil Sci Soc Amer Proc 20:176–179
Bray RH, Kurtz LT (1945) Determination of total, organic and available forms of phosphorus in soils. Soil Sci 59:39–46
Colwell JD (1963) The estimation of the phosphorus fertilizer requirements of wheat in southern New South Wales by soil analysis. Aust J Exp Agric Anim Husb 3:190–197
Dewis J, Freitas F (1984) Physical and chemical methods of soil and water analysis. Oxford and IBH Publishing Company, New Delhi, pp 51–106
Penn CJ, Camberato JJ (2019) A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 9:120–138
Piper CS (1966) Single value physical constants in: soil and plant analysis. Hans Publishers, Bombay, pp 80–111
Price G (ed) (2006) Australian soil fertility manual, 3rd edn. Fertilizer Industry Federation of Australia and CSIRO, Collingwood, p 45
Rausch C, Bucher M (2002) Molecular mechanisms of phosphate transport in plants. Planta 216:23–37
Riley D, Barber SA (1971) Effect of ammonium and nitrate fertilization on phosphorus uptake as related to root-induced pH changes at the root-soil interface. Soil Sci Soc Amer Proc 35:301–306
Saleque MA, Kirk GJD (1995) Root-induced solubilization of phosphate in the rhizosphere of lowland rice. New Phytol 129:325–336
Singh D, Chhonkar PK, Pandey RN (1999) Soil plant water analysis – a methods manual. ICAR, New Delhi
Vange MS, Holmern K, Nissen P (1974) Multiphasic uptake of sulfate by barley roots I. Effects of analogues, phosphate, and pH. Physiol Plant 31:292–301
Walkley AJ, Black IA (1934) Estimation of soil organic carbon by the chromic acid titration method. Soil Sci 37:29–38. https://doi.org/10.1097/00010694-193401000-00003
Webster R (2007) Analysis of variance, inference, multiple comparisons and sampling effects in soil research. Europ J Soil Sci 58:74–82
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Tim S. George
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Barrow, N., Debnath, A. & Sen, A. Measurement of the effects of pH on phosphate availability. Plant Soil 454, 217–224 (2020). https://doi.org/10.1007/s11104-020-04647-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04647-5