Abstract
Background and aims
Nutrient availability is a key regulator of soil respiration. Anthropogenic activities have dramatically accelerated the inputs of reactive nitrogen (N) and phosphorous (P) and increased the availability of N and P in soils. However, many previous studies of soil respiration have focused more on the effects of N, and less on the effects of P and its interaction with N. Therefore, the objective here was to examine the roles of N availability, P availability and their interaction in determining soil respiration.
Methods
A manipulative N addition (100 kg N ha−1 year−1), P addition (50 kg P ha−1 year−1) and NP co-addition (100 kg N ha−1 year−1 + 50 kg P ha−1 year−1) experiment was conducted in a subtropical forest in Southwest China. N and P inputs were added monthly beginning in October 2017. The soil respiration rate was measured monthly from March 2018 to February 2019, and soil properties, microbial biomass carbon (MBC), and fine root biomass were measured in July 2018 and January 2019.
Results
The soil respiration rate in the control (without fertilizer added) treatment was 13.5% higher than that in the N treatment but 1.6% and 34.7% lower than those in the P treatment and NP treatment, respectively. Soil respiration was closely associated with soil temperature and soil moisture, and soil temperature played a more important role in regulating soil respiration than did soil moisture. The temperature sensitivity of respiration (Q10 values) in the CT, N, P and NP treatments did not differ significantly. Path analysis showed that nutrient additions changed soil respiration by regulating the MBC and fine root biomass.
Conclusions
Our results highlight the divergent effects of N addition and NP co-addition on soil respiration. Ongoing N and P inputs may increase soil respiration by stimulating soil microbial and plant root activities in the subtropical evergreen broad-leaved forests of Southwest China.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Soil respiration is the second-largest carbon (C) flux behind plant photosynthesis between the atmosphere and terrestrial ecosystems and plays an important role in regulating atmospheric CO2 concentration (Chang et al. 2016; Yao et al. 2019). Anthropogenic activities such as fossil fuel combustion and fertilizer have dramatically accelerated the inputs of reactive N and P into the biosphere. N inputs have doubled and P inputs have quadrupled relative to preindustrial levels (Falkowski 2000; Bracken et al. 2015). The rapid increase in N and P availability may substantially mediate many ecosystem processes and fluxes. Therefore, it is extremely important to understand the role of N and P availability in determining C fluxes, especially soil respiration, under increased N and P inputs.
The effects of N addition on forest soil respiration have been well documented. However, the effects of N addition on soil respiration have been inconsistent in different studies, giving both positive (Chen et al. 2019; Wang et al. 2019a), neutral (Liu et al. 2017) and negative (Mo et al. 2008; Zhou et al. 2018) responses. In a meta-analysis, Janssens et al. (2010) found that N deposition significantly decreased forest soil respiration. Tian et al. (2018) conducted a meta-analysis of soil respiration in China’s forest ecosystems in response to experimental N addition, and the results suggested that soil respiration in temperate forests has a nonlinear response to the N addition gradient, with an increase at low levels of N addition and a decrease at high levels. However, soil respiration consistently decreased with an increase in N addition levels in subtropical and tropical forests, indicating that these variable responses can be attributed to ecosystem types; the form, rate and duration of N addition; and inherent site fertility.
Compared with the effects of N addition on forest soil respiration, only a few studies have focused on the effects of P addition and its interaction with N on forest soil respiration (Zeng and Wang 2015; Zeng et al. 2018), and there has been no consensus on the effects of P addition, alone or in combination with N, on soil respiration in forests. In addition, the majority of those studies were conducted in tropical forests (Cleveland and Townsend 2006; Liu et al. 2012) and temperature forests (Zeng et al. 2018; Kang et al. 2016). However, the response of forest soil respiration to P addition and NP co-addition in subtropical forests has been poorly studied.
Therefore, we conducted a manipulation experiment to examine the effects of N and P additions on soil respiration in a subtropical forest in Southwest China. The widespread and representative zonal forest communities in subtropical China are secondary evergreen broad-leaved forests (Lin et al. 2012; Peng et al. 2018). Thus, we chose a secondary evergreen broad-leaved forest as the study object. In this region, the average atmospheric wet N deposition was approximately 95 kg N ha−1 from 2008 to 2010 (Xu et al. 2013), which is much higher than that in other areas of world. N manipulation experiments in subtropical forests in Southwest China suggested that N addition accelerated soil acidification and significantly suppressed microbial and root activity, and thus soil respiration in a secondary evergreen broad-leaved forest (Peng et al. 2018) and a natural evergreen broad-leaved forest (Zhou et al. 2018). Therefore, we proposed hypothesis 1, that N addition would suppress soil respiration in this forest. The influence of P addition and the interaction of N addition and P addition on soil respiration in this region has not been studied. However, the forest ecosystems in subtropical forests in Southwest China may move towards being limited by P or co-limited by N and P under the background of high N deposition (Tu et al. 2014; Zhou et al. 2017) and phosphorus-poor conditions in subtropical forests soil (Chen et al. 2015). Therefore, we proposed hypothesis 2, that P may be a primary limiting factor of plant and microorganism activities and that P addition, alone or in combination with N, would stimulate soil respiration in this forest. In addition, due to the strong coupling among the C, N and P cycles (Poeplau et al. 2015), we proposed hypothesis 3, that there is a significant interactive effect of N addition and P addition on soil respiration. The main objective of this study was to understand the roles of N and P availability in determining soil respiration in subtropical forests in Southwest China.
Materials and methods
Site description
This study was conducted at Bi Feng Gorge, Sichuan Province, China (103°0′25″E, 30°4′ 6″N). This region experiences a subtropical monsoon annual temperature is 17.9 °C. The mean annual precipitation in this region was 1719 mm from 1951 to 2010 (Chen et al. 2017). The soil in this study site is classified as a Ferralsol (according to World Reference Base for Soil Resources 2014), with old alluvial yellow loam and a granular structure, derived from granite, which is typical of soils in subtropical zones (Peng et al. 2019). The depth of the soil is more than 60 cm. The mean concentrations of soil organic C, total N, total P, hydrolytic N (HN), and bioavailable P (AP, PO43−–P) in soils (0–20 cm depth) are 30.78 ± 1.88 g.kg−1, 2.15 ± 0.22 g.kg−1, 0.19 ± 0.02 g.kg−1, 144.21 ± 8.54 mg.kg−1, and 2.63 ± 0.14 mg.kg−1, respectively, and the pH is 4.18 ± 0.05 (September 2017). The plant community at the experimental site is dominated by Schima superba, Symplocos botryantha, Lithocarpus hancei, Eurya fangii, Camellia japonica, Lindera glauca, Humulus scandens, and Pteridium revolutum.
Experimental design
An N and P addition experiment was established with a complete randomized block design in a subtropical forest. Three replicate blocks 20 m apart were established in September 2017. In each block, four treatments were randomly assigned to four plots (5 × 5 m), and each plot was separated by a 2-m-wide buffer zone. With reference to previous studies (Jing et al. 2016; Ren et al. 2016), the following treatments were established: control (CT; without N and P additions), N addition (N, 100 kg N ha−1 year−1), P addition (P, 50 kg P ha−1 year−1), and N and P co-addition (NP, 100 kg N ha−1 year−1 + 50 kg P ha−1 year−1). N was added as NH4NO3, and P was added as NaH2PO4. N and P supplies were applied monthly beginning in October 2017. During each supply application, the supplies were dissolved in 2 L of water and sprayed onto the forest floor using a hand-held sprayer. The control plots received 24 L of water without supply each year (equivalent to an annual precipitation increase of 0.96 mm, which was negligible).
Soil respiration measurement
In September 2017, three polyvinyl chloride rings (10 cm in height, inside diameter of 20 cm) were randomly placed and inserted 6 cm into the soil in each plot (9 rings per treatment). The soil respiration rate was measured monthly from March 2018 to February 2019 using a Li-8100 automated soil CO2 efflux system (LI-COR Inc., Lincoln, NE, USA). All living plants and litter inside the collars was removed 24 h before soil respiration was measured. All measurements occurred in each plot between 10:00 and 16:00 local time. The soil respiration rates were expressed as μmol CO2 m−2 s−1. For each plot, average CO2 efflux rates were calculated from the 3 chamber measurements obtained during a single sampling date. The measured plot average CO2 efflux was used as a replicate (n = 3 for each treatment). When soil respiration was measured, the soil temperature (°C) at a soil depth of 10 cm was measured by vertically inserting a soil thermometer (KT300, Odatime, China) which was adjacent to the PVC collars, and the soil moisture was measured within the upper 5 cm by a volumetric soil hygrometer (TR-6, Shunkeda, China). Before the first measurement, the soil thermometer and the soil hygrometer were calibrated for this soil.
In the month with the highest soil respiration rate (July 2018) and the month with the lowest soil respiration rate (January 2019), soil samples from a depth of 0–20 cm were collected using an auger (20 cm in height, inside diameter of 9 cm). In each plot, 5 soil cores were collected and mixed to form one sample. The roots were separated from the soils by washing them with water. Fine roots with diameters of less than 1 mm were selected using tweezers and Vernier calipers. The fine root samples were oven dried at 80 °C to a constant weight and then weighed to estimate the fine root biomass. At the time fine roots were sampled, three soil cores (0–20 cm) in each plot were also collected, mixed, passed through a 2 mm sieve and separated into two parts. One part was used to measure the microbial biomass carbon (MBC) and dissolved organic carbon (DOC), and the other part was air dried and used to measure the soil HN, AP and pH. The soil MBC was estimated using the chloroform fumigation extraction method (Brookes et al. 1985). In brief, the soil MBC was extracted with 0.5 mol L−1 K2SO4 and agitated on an orbital shaker at 120 rpm for 30 min; the supernatant was then filtered through a 0.45 μm membrane. The filtrate was analysed using a total organic carbon (TOC) analyser (Elementar Vario TOC., Germany), and the differences in extractable C between fumigated and unfumigated soils were divided by 0.45 (Wu et al. 1990). The soil DOC was extracted with distilled water and agitated on an orbital shaker at 120 rpm for 5 h. Then, the DOC was measured with a TOC analyser after filtration through a 0.45 μm membrane. The soil HN and AP in the soil samples were measured using standard protocols (LY/T1228- 2015; LY/T1232- 2015). The soil AP was extracted using 0.72 mol L−1 sodium acetate anhydrous and 0.52 mol L−1 acetic acid and analysed calorimetrically using a spectrophotometer (UV2550, Shimadzu, Japan). The soil pH was determined in a 1:2.5 soil: water solution (w/v).
Data analysis
The relationships between the soil respiration rate and soil temperature were fitted with an exponential function model, SR = αeβt (Lloyd and Taylor 1994). In this equation, SR is the soil respiration rate (μmol CO2 m−2 s−1), t is the soil temperature (°C), α is the soil respiration rate (μmol CO2 m−2 s−1) when the soil temperature is 0 °C, and β is the temperature reaction coefficient. To test the effect of nutrient addition on the correlation between soil respiration and soil temperature, we transformed eq. SR = αeβt into eq. Ln SR = Lnα + βt using the natural logarithm transformation. Then, the temperature sensitivity (Q10) of soil respiration was calculated as Q10 = e10β. One-way analysis of variance (ANOVA) with Fisher’s LSD test was used to inspect differences in Lnα and Q10 among all treatments.
The relationships between the soil respiration rate and soil moisture were fitted with a linear regression model, SR = aW + b; exponential function, SR = aebW; and quadratic model, SR = aW2 + bW + c, where W indicates the soil moisture (%). To minimize the effect of soil temperature on the correlation between soil respiration and soil moisture, measured soil respiration was normalized to soil respiration at 15 °C (SR15) using eq. SR15 = SR × eb(15–t) (Falk et al. 2005; Wang et al. 2011). The relationships between the normalized soil respiration rate and soil moisture were fitted with a linear regression model, SR15 = aW + b; exponential function model, SR15 = aebW; and quadratic model, SR15 = aW2 + bW + c. In addition, the relationships of the soil respiration rate with soil temperature and soil moisture were fitted with SR = a + bt + cW, SR = aebt + cW and SR = aebtWc.
A general linear model was used to examine the main effects of N addition, P addition and the interaction of N addition and P addition on the soil respiration rate, temperature, moisture and soil properties during the study period. In addition, the interaction of N addition and P addition on the soil respiration rate was measured by the relative excess risk due to interaction (RERI) and the attributable proportion of interaction (API) (Hallqvist et al. 1996; Andersson et al. 2005). These measures were defined as RERI = RR11 - RR10 - RR01 + 1 and API = RERI / RR11, where RR11 is the ratio of the soil respiration rate in the NP treatment to the CT treatment, RR10 is the ratio of the soil respiration rate in the N treatment to the CT treatment and RR01 is the ratio of the soil respiration rate in the P treatment to the CT treatment. RERI represents the difference between the co-effects of two factors and their individual effects, and AP was intended to capture the proportion of the measured indicators with two factors that was due to the interaction between the two factors. Supposing that there is no interaction, RERI and API are equal to 0 (Andersson et al. 2005; VanderWeele 2013). Therefore, Student’s t test was used to examine the probability that RERI and API were not equal to 0.
After establishing the general linear model, multiple comparisons with Fisher’s least significant difference (LSD) tests were used to test the differences between treatments on the soil respiration rate, temperature, moisture and soil properties during the research period. One-way ANOVA with Fisher’s LSD test was used to inspect differences in the soil properties among all treatments in July 2018 and January 2019 and differences in Ln α, and Q10 among all treatments in the fitted equation of SR against soil temperature. If the homogeneity test of variance was not satisfied, a nonparametric hypothesis test, Dunnett’s T3 test, was used. Path analysis was used to analyse hypothetical pathways that soil properties may explain soil respiration in July 2018 and January 2019. In the path analysis, the data were fit to the model using the maximum likelihood estimation method. The general linear model and multiple comparisons analyses were performed in Minitab17.0 (Minitab Inc., USA). One-way ANOVA was performed in SPSS 18.0 (SPSS Inc., USA), and the path analysis was performed in Amos 21.0. Statistically significant differences were tested at P < 0.05 unless otherwise stated.
Results
The effects of nitrogen and phosphorus additions on soil respiration
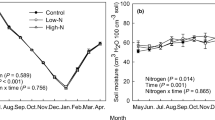
Under the seasonal pattern of soil respiration, the maximum rates of soil respiration occurred in July, and the minimum rates of soil respiration occurred in January (Fig. 1). The main effect of N addition marginally significantly affected soil respiration (P = 0.069, Fig. 1), and the main effect of P addition and the interaction of N addition and P addition had significant effects on soil respiration (P < 0.001 for all, Fig. 1). In addition, the NP treatment and the P treatment increased soil respiration by 34.7% and 1.6%, respectively, but the N treatment decreased soil respiration by 13.5%. Multiple comparisons showed that the NP treatment had the highest average soil respiration rate (3.38 μmol CO2 m−2 s−1, Fig. 1), which was significantly higher than those in the N treatment, P treatment and CT treatment (P < 0.05 for all, Fig. 1). The soil respiration rate in the N treatment was marginally significantly lower than that in the CT treatment (P = 0.073).
Monthly dynamics of the soil respiration rate from March 2018 to February 2019. Values are means of three replicates ± SE. A general linear model was used to determine the main effects of N addition and P addition and the interaction between N and P effects on soil respiration. The P-values of the general linear model are shown in the text. Average values of yearly measures are shown in the histograms (values are means of three replicates ± SE). Different letters denote significant differences (P < 0.05) between treatments as determined by multiple comparisons with Fisher’s LSD test. CT: control, without N and P additions; N: N addition treatment; P: P addition treatment; and NP: N and P co-addition treatment
We used the RERI and API indexes to assess the interaction of N addition and P addition on soil respiration. The results of Student’s t test showed that the RERI values during the study period were 0.463 ± 0.104. The probability of the RERI indexes not being equal to zero during the entire study period was 0.990, which indicated that there was a significant interactive effect between N addition and P addition on soil respiration. Furthermore, the API values during the study period were 34.7% ± 7.9%, and the probability of the API values not being equal to zero was 0.989, which indicated that approximately 35% of the soil respiration in the NP plots originated from the interaction between N addition and P addition.
The relationship between soil respiration, soil temperature and soil moisture
The soil temperature exhibited a clear seasonal pattern, highest in July 2018 and lowest in January 2019 (Fig. S1a). The soil moisture was stable, ranging from 30.2% to 40.4% (Fig. S1c). The rate of soil respiration was exponentially related to the soil temperature, and the exponential function model explained 59.6–78.5% of the soil respiration variation. Nutrient addition did not significantly change lnα and Q10, which suggested that nutrient addition had no effect on the relationship between soil respiration and soil temperature and the temperature sensitivity, Q10 (Table 1).
The relationships between the soil respiration rate and soil moisture were fitted with a linear regression model, exponential function model and quadratic model. A total of 31.2–47.3%, 32.0–41.6% and 29.8–48.8% of the total variance in soil respiration was explained by these models, respectively (Table S1). However, only 10.4–23.2%, 10.8–16.0% and 8.0–24.7% of the total variance in soil respiration at 15 °C soil temperature was explained by the linear regression model, exponential function model and quadratic model, respectively. This result suggested that soil moisture had less effect than soil temperature on the soil respiration rate (Table S1).
The relationships of the soil respiration rate with soil temperature and soil moisture were fitted with eq. SR = a + bt + cW, eq. SR = aebt + cW and eq. SR = aebtWc. A total of 69.2–77.5%, 69.4–80.2% and 69.5–84.8% of the total variance in soil respiration was explained by these equations, respectively (Table S2), which demonstrated that soil respiration was co-controlled by soil temperature and soil moisture.
The effects of nitrogen and phosphorus additions on soil properties
Multiple comparisons showed that neither the N nor P treatment changed the DOC and pH in July 2018, but the N treatment significantly decreased the DOC and pH, (P < 0.05 for all, Table 2), and the P treatment still did not significantly change the DOC and pH in January 2019. The NP treatment significantly increased the DOC and pH in July 2018 and January 2019 (P < 0.05 for all, Table 2). The N treatment and NP treatment enhanced the HN in July 2018 (P < 0.05 and P > 0.05, respectively, Table 2) and January 2019 (P < 0.05 and P > 0.05, respectively, Table 2), but the P treatment marginally significantly reduced the HN in July 2018 (P = 0.071) and significantly reduced the HN in January 2019 (P < 0.05, Table 2). In July 2018 and January 2019, the P treatment significantly enhanced the AP (P < 0.05 for all, Table 2), and the NP treatment slightly enhanced the AP, but the N treatment significantly reduced the AP (P < 0.05 for all, Table 2).
Similar to DOC and pH, the MBC barely changed in the P addition plots and significantly increased in the NP co-addition plots in July 2018 and January 2019 (P < 0.05 for all, Table 2). However, the MBC in the N addition plot barely changed in July 2018 and significantly decreased in January 2019 (P < 0.05, Table 2). Fine root biomass was slightly changed in the N addition plots and P addition plots, although it significantly increased in the NP co-addition plots in July 2018 and January 2019 (P < 0.05 for all, Table 2).
Pathways determining soil respiration by N and P additions
The path analysis model explained 70% of the soil respiration variation (Fig. 2). Soil temperature was positively correlated with the DOC and fine root biomass, but negatively correlated with HN. Soil moisture was positively correlated with the pH. N addition increased the soil N availability but decreased the soil P availability. However, P addition increased the soil P availability but decreased the soil N availability. The DOC and pH decreased under the N treatment and remained unchanged under the P treatment. However, the interaction of N addition and P addition increased the DOC and pH. In July 2018 and January 2019, the DOC and pH were positively correlated with MBC; 90% of the total variance in MBC was explained by DOC and pH, which may demonstrate that DOC and pH are the main controllers of MBC. The HN, AP and pH was positively correlated with the fine root biomass in July 2018 and January 2019; 71% of the total variance in fine root biomass was explained by soil temperature, HN, AP and pH, which may demonstrate that soil temperature, HN, AP and pH are the controllers of fine root biomass. In July 2018 and January 2019, the MBC and fine root biomass were positively correlated with soil respiration. In addition, the MBC and fine root biomass co-explained 70% of the soil respiration variation, which may demonstrate that the MBC and fine root biomass co-controlled soil respiration. Moreover, regression analysis revealed that DOC was significantly linearly correlated with fine root biomass in July 2018 and January 2019 (R2 adj = 0.650, P = 0.001). However, because fine root biomass may be a confounding variable in this model (soil temperature, N addition, P addition and the interaction of N and P were the basic reasons for DOC change), we removed this likely pathway.
The path analysis model considered the plausible pathways by which soil temperature, soil moisture, N addition, P addition and the interaction of N addition and P addition influence soil respiration in July 2018 and January 2019. Green and red arrows represent significant positive and negative pathways (P < 0.05), respectively; black arrows indicate nonsignificant relationships (P > 0.05). The numbers at arrows are the standardized path coefficients, and the arrow width is proportional to the strength of the relationship. The R2 values on the right of the response variables indicate the proportion of variation explained by the relationships with other variables. Tem (soil temperature); Moi (soil moisture); N addition (the effect of N addition); P addition (the effect of P addition); Interaction of N and P (the interaction of N addition and P addition); DOC (soil dissolved organic carbon); HN (hydrolytic nitrogen); AP (available phosphorus); MBC (microbial biomass carbon); FRB (plant fine root biomass); and SR (soil respiration)
The standardized total effects from the path analysis showed that soil temperature had the most powerful positive effect on soil respiration, the interaction of N addition and P addition had the second most powerful positive effect on soil respiration, and the P addition and soil moisture had a slight positive effect on soil respiration, but N addition had negative effects on soil respiration in July 2018 and January 2019 (Fig. 3).
The path analysis derived the standardized total effects of soil temperature, soil moisture, N addition, P addition and the interaction of N addition and P addition on soil respiration in July 2018 and January 2019. The abbreviations are provided in Fig. 2
Discussion
The relationship between soil respiration, soil temperature and soil moisture
A total of 69.2–77.5%, 69.4–80.2% and 69.5–84.8% of the total variance in soil respiration was explained by eq. SR = a + bt + cW, eq. SR = aebt + cW and eq. SR = aebtWc, respectively (Table S2), which demonstrated that soil respiration was co-controlled by soil temperature and soil moisture. However, 59.6–78.5% of the total variance in soil respiration variation was explained by the exponential function model, SR = aebt (Table 1). Only 10.4–23.2%, 10.8–16.0% and 8.0–24.7% of the total variance in soil respiration at 15 °C soil temperature was explained by eq. SR15 = aW + b, eq. SR15 = aebW, and eq. SR15 = aW2 + bW + c, respectively (Table S1). The standardized total effects of the path analysis also showed that the soil temperature contributed more than the soil moisture in impacting soil respiration in July 2018 and January 2019 (Fig. 3). This finding indicated that soil temperature plays a more important role in regulating soil respiration than does soil moisture. However, Zhang et al. (2001) found that soil respiration was regulated by temperature only, with no effect of soil moisture. Jia et al. (2006) reported that soil moisture was the main driving factor of seasonal variation in soil respiration. These differences may be attributed to annual rainfall. Soil moisture is more important than soil temperature in regulating soil respiration under drought conditions (Holt et al. 1990; Jia et al. 2006).
Q10 values, which indicate the temperature sensitivity of soil respiration, are regarded as an important tool for predicting the soil respiration rate against the background of global warming in the future (Davidson and Janssens 2006; Wang et al. 2019b). No effects of nutrient addition on Q10 values were found (Table 1), which was consistent with the result of Wang et al. (2017) but contrasted with the study of Zhou et al. (2018), which demonstrated a decline in the Q10 of soil respiration from 2.98 to 2.65 after N addition in a nearby natural evergreen broad-leaved forest. This discrepancy is most likely due to differences in soil types and tree species composition. Different tree species compositions may change the stability of soil organic carbon by regulating the quantity and quality of litter and rhizosphere secretion, and different soil types may change the temperature sensitivities of soil microbial respiration by regulating the number and community structure of soil microorganisms.
The effects of nitrogen and phosphorus additions on soil respiration
Interestingly, P addition alone had no effect on soil respiration but had a strong positive interaction with N addition, which not only removed the slight negative effect of N addition on soil respiration but also significantly increased soil respiration. Therefore, we suggest that soil respiration may be co-limited by N and P in this subtropical forest ecosystem. Under P or N addition alone, although the availability of the added element increased, there was still a limitation of the other nutrient on soil respiration. However, when they were co-input, the restrictive effects of P and N would be alleviated at the same time, thus greatly stimulating CO2 emission. This scenario may be related to the soil N and P, especially P, deficiency conditions in our study region (Table 2). Castle et al. (2017) also found that soil microbial respiration was co-limited by N and P at Mendenhall Glacier, Alaska. In addition, path analysis showed that fine root biomass was regulated by HN and AP and that MBC was regulated by DOC (Fig. 2), which may suggest that plants are limited by N and P, and that soil microorganisms are limited by C in this forest. Further research needs to be carried out to support this suggestion.
Soil respiration can be divided into heterotrophic respiration (derived from microbial decomposition of soil organic matter) and autotrophic respiration (originating from plant roots) (Zeng and Wang 2015; Li et al. 2018). Heterotrophic respiration is caused by soil microorganisms, and autotrophic respiration is correlated with fine root biomass (Wang et al. 2017; Peng et al. 2018). Therefore, we assumed that soil respiration may be associated with soil MBC and fine root biomass. In this study, we measured the soil properties in the month with the highest soil respiration rate (July 2018) and the month with the lowest soil respiration rate (January 2019) and established a path analysis between soil properties and the soil respiration rate. The path analysis model suggested that soil respiration was closely associated with MBC and fine root biomass (Fig. 2). The path analysis model explained 70% of the variation in soil respiration (Fig. 2). This result indicated that the nutrient additions changed the soil respiration by regulating the MBC and fine root biomass.
In agreement with hypothesis 1, soil respiration marginally decreased under N addition throughout the study period. The decreased soil respiration may be explained by the unchanged fine root biomass and the decreased MBC. On the one hand, N addition did not affect the fine root biomass in July 2018 and January 2019 (Table 2) and may not have affected autotrophic respiration. Our study found that N addition increased N availability, but reduced P availability and pH (Table 2). This was probably because the higher soil mineral N content under N addition soils stimulated biotic demands for P and resulted in reduced P availability. Furthermore, Lu et al. (2011a, 2011b) suggested that N addition significantly stimulated soil inorganic N leaching by 461%, nitrification by 154% and denitrification by 84%, and resulted in a 3.5% decrease in pH in a meta-analysis. Similar to previous studies(Li et al. 2018; Tu et al. 2013), the fine root biomass was controlled by soil temperature, N availability, P availability and pH in the present study. Therefore, the contrasting effects of increased HN, unchanged soil temperature and decreased AP and pH under N addition (Table 2 and Fig. S1b) may help to maintain relatively stable fine root biomass and autotrophic respiration by changing the availability of nutrients and soil acidity. On the other hand, N addition reduced the MBC in July 2018 and January 2019 (P > 0.05 and P < 0.05, respectively, Table 2) and may have reduced heterotrophic respiration. Our study found that N addition decreased the DOC and pH. In general, root biomass may play an important role in changing the concentration of soil DOC by regulating rhizospheric C inputs (Janssens et al. 2010), which was supported by the positive linear relationship between the fine root biomass and DOC in this study (R2 adj = 0.631, P = 0.001). However, N addition did not affect fine root biomass but reduced DOC in the present study. Zhou et al. (2017) reported that N addition reduced the litter decomposition rate in a nearby natural evergreen broad-leaved forest. Hence, N addition may reduced soil DOC by regulating the litter decomposition rate, not by regulating fine root biomass, in this forest. In this study, the path analysis model showed that DOC and pH are the main controllers of the MBC (Fig. 2), which is in line with previous studies in other forest ecosystems (Wang et al. 2017; Zhalnina et al. 2015). Therefore, the reduced C availability and pH under N addition (Table 2) may lead to a reduction in microbial activity and heterotrophic respiration.
Similar to hypothesis 2, our results showed that soil respiration was unaffected under P addition alone but significantly increased under N and P co-addition (Fig. 1). Jiang et al. (2013) and Potthast et al. (2012) also found that NP co-inputs enhanced soil CO2 emissions. The enhancement in soil respiration after the N and P co-addition in this forest may be attributed to the following three plausible mechanisms. First, on the one hand, increasing soil nutrient availability may lead to reduced vegetation dependence on ectomycorrhizal fungi and increased dependence on plant roots (Franklin et al. 2014), thus increasing fine root biomass. On the other hand, the increased nutrient uptake by plants may increase photosynthesis rates and lead to the allocation of more C to meet the metabolic demands of increased root growth (Yuan and Chen 2012). This was supported by HN in the NP treatment, which was approximately 20% lower than the value in the N treatment, and AP in the NP treatment, which was approximately 20% lower than the value in the P treatment (Table 2). An increase in the fine root biomass under N and P co-addition (Table 2) may stimulate autotrophic respiration. Consistent with our results, Yuan and Chen (2012) also suggested that the fine root biomass was enhanced after NP co-addition in a meta-analysis. Second, plants absorb nitrate and phosphate while simultaneously releasing hydroxide ions, which may enhance the pH. Onipchenko et al. (2012) also found that the pH was slightly enhanced after NP fertilization in an NP co-limited lichen heath and geranium meadow. An enhanced pH following the N and P co-addition (Table 2) relieved the acid stress of fine roots and microorganisms and resulted in increased autotrophic respiration and heterotrophic respiration. Last, significantly enhanced fine root biomass after the N and P co-addition (Table 2) may have increased the C input for microorganisms, and thus MBC and heterotrophic respiration.
In this study, there was a significant interaction between N addition and P addition (Fig. 1), which is consistent with hypothesis 3. The API index indicated that approximately 35% of soil respiration in the NP plots originated from the interaction of N addition and P addition, and the standardized total effects of the path analysis showed that the interaction of N addition and P addition contributed more than the N addition, P addition and soil moisture in impacting soil respiration, although slightly less than soil temperature, in this forest (Fig. 3). However, to date, the majority of studies have focused on the role of soil temperature, soil moisture and N availability in soil respiration, while little attention has been paid to how the P status and interaction of N and P may interact with plant and soil microorganisms in soil respiration. Therefore, we need to consider the effects of P addition and the interaction of N and P and incorporate these factors into a biogeochemical model to accurately predict global C flux in future scenarios of increasing N and P inputs because the interaction of N and P may play an important role in changing soil respiration.
Conclusion
N and P co-addition significantly stimulated soil respiration, but N addition alone marginally decreased CO2 emissions, and P addition alone showed few effects. Given the dramatic increase in N and P inputs, the increased availability of N and P may enhance soil respiration in subtropical forests in China by stimulating soil microbial and plant root activities.
References
Andersson T, Alfredsson L, Källberg H, Zdravkovic S, Ahlbom A (2005) Calculating measures of biological interaction. Eur J Epidemiol 20:575–579
Bracken MES, Hillebrand H, Borer ET, Seabloom EW, Cebrian J, Cleland EE, Elser JJ, Gruner DS, Harpole WS, Ngai JT, Smith JE (2015) Signatures of nutrient limitation and co-limitation: responses of autotroph internal nutrient concentrations to nitrogen and phosphorus additions. Oikos 124:113–121
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Castle SC, Sullivan BW, Knelman J, Hood E, Nemergut DR, Schmidt SK, Cleveland CC (2017) Nutrient limitation of soil microbial activity during the earliest stages of ecosystem development. Oecologia 185:513–524
Chang SX, Shi Z, Thomas BR (2016) Soil respiration and its temperature sensitivity in agricultural and afforested poplar plantation systems in northern Alberta. Biol Fertil Soils 52:629–641
Chen F, Niklas KJ, Liu Y, Fang X, Wan S, Wang H (2015) Nitrogen and phosphorus additions alter nutrient dynamics but not resorption efficiencies of Chinese fir leaves and twigs differing in age. Tree Physiol 35:1106–1117
Chen LL, Wang D, Ye Q, Liu KP (2017) Characteristic wavelet analysis of precipitation variation in Yaan in recent 50 years. J Southwest China Normal Univ (Nat Sci Ed) 42:25–30 in Chinese, with English abstract
Chen F, Yan G, Xing Y, Zhang J, Wang Q, Wang H, Huang B, Hong Z, Dai G, Zheng X, Liu T (2019) Effects of N addition and precipitation reduction on soil respiration and its components in a temperate forest. Agric For Meteorol 271:336–345
Cleveland CC, Townsend AR (2006) Nutrient additions to a tropical rain Forest drive substantial soil carbon dioxide losses to the atmosphere. P Natl Acad Sci USA 103:10316–10321
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Falk M, Paw UKT, Wharton S, Schroeder M (2005) Is soil respiration a major contributor to the carbon budget within a Pacific northwest old-growth forest? Agric For Meteorol 135:269–283
Falkowski P (2000) The global carbon cycle: a test of our knowledge of earth as a system. Science 290:291–296
Franklin O, Näsholm T, Högberg P, Högberg MN, Sveriges L (2014) Forests trapped in nitrogen limitation – an ecological market perspective on ectomycorrhizal symbiosis. New Phytol 203:657–666
Hallqvist J, Ahlbom A, Diderichsen F, Reuterwall C (1996) How to evaluate interaction between causes: a review of practices in cardiovascular epidemiology. J Intern Med 239:377–382
Holt JA, Hodgen MJ, Lamb D (1990) Soil respiration in the seasonally dry tropics near Townsville, North Queensland. Aust J Soil Res 28:737–745
Janssens IA, Dieleman W, Luyssaert S, Subke J, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G, Papale D, Piao SL, Schulze E, Tang J, Law BE (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322
Jia B, Zhou G, Wang Y, Wang F, Wang X (2006) Effects of temperature and soil water-content on soil respiration of grazed and ungrazed Leymus chinensis steppes, Inner Mongolia. J Arid Environ 67:60–76
Jiang J, Zong N, Song M, Shi P, Ma W, Fu G, Shen Z, Zhang X, Ouyang H (2013) Responses of ecosystem respiration and its components to fertilization in an alpine meadow on the Tibetan plateau. Eur J Soil Biol 56:101–106
Jing X, Yang X, Ren F, Zhou H, Zhu B, He J (2016) Neutral effect of nitrogen addition and negative effect of phosphorus addition on topsoil extracellular enzymatic activities in an alpine grassland ecosystem. Appl Soil Ecol 107:205–213
Kang H, Fahey TJ, Bae K, Fisk M, Sherman RE, Yanai RD, See CR (2016) Response of forest soil respiration to nutrient addition depends on site fertility. Biogeochemistry 127:113–124
Li Y, Sun J, Tian D, Wang J, Ha D, Qu Y, Jing G, Niu S (2018) Soil acid cations induced reduction in soil respiration under nitrogen enrichment and soil acidification. Sci Total Environ 615:1535–1546
Lin D, Lai J, Muller-Landau HC, Mi X, Ma K (2012) Topographic variation in aboveground biomass in a subtropical evergreen broad-leaved forest in China. PLoS One 7(10):e48244
Liu L, Gundersen P, Zhang T, Mo J (2012) Effects of phosphorus addition on soil microbial biomass and community composition in three forest types in tropical China. Soil Biol Biochem 44:31–38
Liu X, Yang Z, Lin C, Giardina CP, Xiong D, Lin W, Chen S, Xu C, Chen G, Xie J, Li Y, Yang Y (2017) Will nitrogen deposition mitigate warming-increased soil respiration in a young subtropical plantation? Agric For Meteorol 246:78–85
Lloyd J, Taylor JA (1994) On the temperature dependence of soil respiration. Funct Ecol 8:315–323
Lu M, Zhou X, Luo Y, Yang Y, Fang C, Chen J, Li B (2011a) Minor stimulation of soil carbon storage by nitrogen addition: a meta-analysis. Agric Ecosyst Environ 140:234–244
Lu M, Yang Y, Luo Y, Fang C, Zhou X, Chen J, Yang X, Li B (2011b) Responses of ecosystem nitrogen cycle to nitrogen addition: a meta-analysis. New Phytol 189:1040–1050
LY/T1228-2015 (2015) Nitrogen determination methods of Forest soils. The State Forestry Administration of the People's Republic of China
LY/T1232-2015 (2015) Phosphorus determination methods of Forest soils. The State Forestry Administration of the People's Republic of China
Mo J, Zhang W, Zhu W, Gundersen P, Fang Y, Li D, Wang H (2008) Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob Chang Biol 14:403–412
Onipchenko VG, Makarov MI, Akhmetzhanova AA, Soudzilovskaia NA, Aibazova FU, Elkanova MK, Stogova AV, Cornelissen JHC (2012) Alpine plant functional group responses to fertiliser addition depend on abiotic regime and community composition. Plant Soil 357:103–115
Peng Y, Chen G, Li S, Hu H, Hu T, Liu L, Tang Y, Tu L (2018) Nitrogen additions reduce rhizospheric and heterotrophic respiration in a subtropical evergreen broad-leaved forest. Plant Soil 431:449–463
Peng Y, Song S, Li Z, Li S, Chen G, Hu H, Xie J, Chen G, Xiao Y, Liu L, Tang Y, Tu L (2019) Influences of nitrogen addition and aboveground litter-input manipulations on soil respiration and biochemical properties in a subtropical forest. Soil Biol Biochem. https://doi.org/10.1016/j.soilbio.2019.107694
Poeplau C, Bolinder MA, Kirchmann H, Kätterer T (2015) Phosphorus fertilisation under nitrogen limitation can deplete soil carbon stocks – evidence from Swedish meta-replicated long-term field experiments. Biogeosci Discuss 12:16527–16551
Potthast K, Hamer U, Makeschin F (2012) In an Ecuadorian pasture soil the growth of Setaria sphacelata, but not of soil microorganisms, is co-limited by N and P. Appl Soil Ecol 62:103–114
Ren F, Yang X, Zhou H, Zhu W, Zhang Z, Chen L, Cao G, He J (2016) Contrasting effects of nitrogen and phosphorus addition on soil respiration in an alpine grassland on the Qinghai-Tibetan plateau. Sci Rep 6:34786
Tian D, Du E, Jiang L, Ma S, Zeng W, Zou A, Feng C, Xu L, Xing A, Wang W, Zheng C, Ji C, Shen H, Fang J (2018) Responses of forest ecosystems to increasing N deposition in China: a critical review. Environ Pollut 243:75–86
Tu L, Hu T, Zhang J, Li X, Hu H, Liu L, Xiao Y (2013) Nitrogen addition stimulates different components of soil respiration in a subtropical bamboo ecosystem. Soil Biol Biochem 58:255–264
Tu L, Chen G, Peng Y, Hu H, Hu T, Zhang J, Li X, Liu L, Tang Y (2014) Soil biochemical responses to nitrogen addition in a bamboo forest. PLoS One 9:e102315
VanderWeele TJ (2013) Reconsidering the denominator of the attributable proportion for interaction. Eur J Epidemiol 28:779–784
Wang Y, Li Q, Wang H, Wen X, Yang F, Ma Z, Liu Y, Sun XYG (2011) Precipitation frequency controls interannual variation of soil respiration by affecting soil moisture in a subtropical forest plantation. Can J For Res 41:1897–1906
Wang Q, Zhang W, Sun T, Chen L, Pang X, Wang Y, Xiao F (2017) N and P fertilization reduced soil autotrophic and heterotrophic respiration in a young Cunninghamia lanceolata forest. Agric For Meteorol 232:66–73
Wang J, Wang G, Fu Y, Chen X, Song X (2019a) Short-term effects of nitrogen deposition on soil respiration components in two alpine coniferous forests, southeastern Tibetan plateau. J Forestry Res 30:1029–1041
Wang Q, Zhao X, Chen L, Yang Q, Chen S, Zhang W (2019b) Global synthesis of temperature sensitivity of soil organic carbon decomposition: latitudinal patterns and mechanisms. Funct Ecol 33:514–523
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction—an automated procedure. Soil Biol Biochem 22:1167–1169
Xu Z, Tu L, Hu T, Schädler M (2013) Implications of greater than average increases in nitrogen deposition on the western edge of the Szechwan Basin. China Environ Pollut 177:201–202
Yao X, Yu K, Wang G, Deng Y, Lai Z, Chen Y, Jiang Y, Liu J (2019) Effects of soil erosion and reforestation on soil respiration, organic carbon and nitrogen stocks in an eroded area of southern China. Sci Total Environ 683:98–108
Yuan ZY, Chen HYH (2012) A global analysis of fine root production as affected by soil nitrogen and phosphorus. Proc R Soc B Biol Sci 279:3796–3802
Zeng W, Wang W (2015) Combination of nitrogen and phosphorus fertilization enhance ecosystem carbon sequestration in a nitrogen-limited temperate plantation of northern China. Forest Ecol Manag 341:59–66
Zeng W, Zhang J, Wang W (2018) Strong root respiration response to nitrogen and phosphorus addition in nitrogen-limited temperate forests. Sci Total Environ 642:646–655
Zhalnina K, Dias R, de Quadros PD, Davis-Richardson A, Camargo FAO, Clark IM, McGrath SP, Hirsch PR, Triplett EW (2015) Soil pH determines microbial diversity and composition in the park grass experiment. Microb Ecol 69:395–406
Zhang X, Xu H, Chen G (2001) Major factors controlling nitrous oxide emission and methane uptake from forest soil. J For Res 12:239–242
Zhou S, Huang C, Han B, Xiao Y, Tang J, Xiang Y, Luo C (2017) Simulated nitrogen deposition significantly suppresses the decomposition of forest litter in a natural evergreen broad-leaved forest in the rainy area of Western China. Plant Soil 420:135–145
Zhou S, Xiang Y, Tie L, Han B, Huang C (2018) Simulated nitrogen deposition significantly reduces soil respiration in an evergreen broadleaf forest in western China. PLoS One 13:e204661
Acknowledgments
This study was supported by the Sci-tech Project of the‘12th Five-year Plan’ of China (2010BACO1A11) and the Crop Breeding Research Project of the ‘12th Five-year Plan’ of Sichuan Province (2011NZ0098-10). We would like to thank Fuzhong Wu, Dr. Shixing Zhou, the editor Dr. Elizabeth M Baggs and the three anonymous reviewers, whose constructive comments have greatly improved the quality of the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Elizabeth M Baggs.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 406 kb)
Rights and permissions
About this article
Cite this article
Wei, S., Tie, L., Liao, J. et al. Nitrogen and phosphorus co-addition stimulates soil respiration in a subtropical evergreen broad-leaved forest. Plant Soil 450, 171–182 (2020). https://doi.org/10.1007/s11104-020-04498-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-020-04498-0