Abstract
Background and aims
Increasing global atmospheric nitrogen (N) deposition has a considerable impact on soil respiration. Due to different carbon dioxide (CO2) resources of different components of soil respiration, there may be different mechanisms by which soils respond to N additions. The aims of this study are to investigate the effects of N additions on total soil respiration (RST), rhizospheric respiration (RSR) and heterotrophic respiration (RSH), and to elucidate the potential causal mechanisms.
Methods
An artificial N addition experiment was conducted in an evergreen broad-leaved forest at the western edge of the Sichuan Basin in China. The effects of three N treatment levels (+0, +50, +150 kg N ha−1 yr.−1) were measured, with the N application initiated in April 2013. Trenching method was used for partitioning RST into RST and RSR. The values of RST and its components were measured monthly from May 2015 to April 2016.
Results
The mean annual RST was 1.92 ± 0.18 μmol CO2 m−2 s−1, with RSH and RSR contributing 75 ± 1% and 25 ± 1% to RST, respectively. Nitrogen addition significantly reduced RST and its two components, as well as the contribution of RSR to RST. Microbial biomass carbon (C) and pH in bulk soil decreased significantly after N application. Nitrogen addition had no effect on rhizospheric soil biochemical properties. RSR was significantly positively correlated with root biomass of Castanopsis platyacantha, while RSH was significantly positively correlated with the concentrations of microbial biomass C (MBC) and microbial biomass N (MBN) for bulk soil.
Conclusions
The positive correlation between RSR and root biomass indicates that N application reduced RSR by reducing belowground C allocation and thus C inputs to the rhizosphere. The value of RSH decreased primarily due to a reduction in microbial activity and soil organic matter decomposition in root-free soil after N was added. The presence of plant roots may mitigate the effect of N inputs to the rhizosphere via alteration of root morphology and exudates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Increasing atmospheric nitrogen (N) deposition stemming from anthropogenic activities, such as combustion of fossil fuels and application of mineral N fertilizers in agricultural ecosystems, represents a major contributor and component of global environmental change. Over the past few decades, reactive N produced by human activities has increased by more than 900% and has exceeded the production from all natural terrestrial systems (Galloway et al. 2003). Globally, many ecosystems are experiencing increased inputs of anthropogenic N (Galloway et al. 2008), and 11% of natural vegetation receives N deposition in excess of the critical load threshold of 10 kg N ha−1 yr.−1 (Dentener et al. 2006). Since N is considered a limiting nutrient for most terrestrial ecosystems, increased N deposition is believed to be beneficial for forest ecosystems (Law 2013). In particular, an increase in N deposition may enhance foliar N and chlorophyll content as well as ecosystem water-use efficiency, which in turn may boost photosynthetic rates and net primary production (NPP) (Zhao and Liu 2009; Yan et al. 2014). Over the past 150 years, N deposition has contributed to an increase of 2.0 Pg C yr.−1 in global NPP and approximately 10 Pg C in soil carbon (Devaraju et al. 2016). However, high levels of N deposition have many damaging effects on terrestrial ecosystems, such as significant soil acidification, soil nitrate leaching, and reduction in biodiversity (Bobbink et al. 2010; Law 2013; Ochoa-Hueso et al. 2014). Furthermore, nitrogen deposition strongly influences below-ground carbon (C) cycling (Cusack et al. 2011; Tu et al. 2011a, b).
Soil is the largest C pool in the terrestrial ecosystem and the most heterogeneous component of the biosphere. Batjes (2016) estimated that the global soil organic carbon (SOC) bank (to a 2 m depth) is 2060 Pg C. Soil respiration (RS), the emission of carbon dioxide (CO2) from the soil surface to the atmosphere, is the main pathway of soil-atmosphere C exchange, and its C flux is the second-largest terrestrial C flux after plant photosynthesis (Schlesinger and Andrews 2000; Bond-Lamberty and Thomson 2010). The total global soil CO2 emission has been estimated to be 98 Pg C (Bond-Lamberty and Thomson 2010), and thus minor changes in RS may cause a large change in atmospheric CO2 concentration (Schlesinger and Andrews 2000). Numerous studies have shown that artificial N additions have variable effects on forest RS, including positive (Tu et al. 2013; Comeau et al. 2016), negative (Bowden et al. 2004; Fan et al. 2014), or neutral effects (Allison et al. 2008; Samuelson et al. 2009).
Based on Kuzyakov (2006), there are five main components of total soil respiration (RST): root respiration, rhizomicrobial respiration, microbial decomposition of plant residues, microbial decomposition of soil organic matter (SOM) in rhizospheric soil, and microbial decomposition of SOM in root-free soil. Currently, four principal methods have been used to partition RST: 1) root exclusion –exclude roots by root removal, trenching, or removing aboveground vegetation; 2) physical separation of components – measure the specific rates of CO2 efflux from each soil component after separating soil cores into roots, sieved soil, and litter; 3) isotopic techniques – estimate the contribution of root and SOM decomposition to RST by isotopic labeling using either 14C or 13C; and 4) indirect methods, e.g. mass-balance approach – calculate root respiration as the difference between C input from below- and aboveground litter and RST based on the assumption that the C fluxes into and out of the soil are balanced. For more detailed descriptions about the approaches used to partition RST, the readers are referred to Hanson et al. (2000) and Subke et al. (2006). In many studies, RST has been partitioned into rhizopheric respiration (RSR, CO2 fluxes derived from plant roots and closely associated organisms) and heterotrophic respiration (RSH, SOM-derived CO2) (Scott-Denton et al. 2006; Wang and Yang 2007; Sun et al. 2014). Trenching techniques are commonly used to partition RST into RSR and RSH in forests due to their simplicity and low cost (Wang and Yang 2007). And there is little influence on belowground respiration when measurements begin few months after trenching, although some roots are left in the trenched plot (Bowden et al. 1993). Many previous studies have found that N additions generally reduce both RSR and RSH (Olsson et al. 2005; Fan et al. 2014; Wang et al. 2017a). Because the turnover rate and main controllers of different C pools in soil are very different, the mechanisms to explain the reductions in RSR and RSH after N addition may also be different. For decreases in RSR, decreased C allocation to roots was believed to be the main reason (Janssens et al. 2010; Fan et al. 2014; Wang et al. 2017a); and in some studies, reduced mycorrhizal fungal community size is also considered to be a significant cause (Sun et al. 2014). However, the N-induced decrease in RSH is usually associated with reduced soil microbial community size and the formation of recalcitrant SOM (Janssens et al. 2010; Wang et al. 2017a).
The western edge of the Sichuan Basin, China, which is located near an important industrial-agricultural center, the Chengdu Plain, is a large and complex ecotone with a cloudy and wet climate. Rapid and intense economic development on the Chengdu Plain is responsible for heightened production of reactive N in this region; this N is transported to the western edge of the Sichuan Basin by monsoon winds. Due to orographic lift of warm and moist air on the western edge of the Sichuan Basin, this region experiences considerable rainfall during the summer. Thus, the average atmospheric N wet deposition level in the center of this region is very high, approaching 95 kg N ha−1 yr.−1 (Xu et al. 2013). This is considerably higher than the average annual N deposition in the 2000s in China (21.1 kg N ha−1; Liu et al. 2013). From 1980 to 2010, N deposition in China has significantly enhanced with an average annual increase of 0.41 kg N ha−1 (Liu et al. 2013). In the next few decades, the largest increases in N deposition in the world have been predicted to occur in this region which is presently receiving extremely high levels of ambient N deposition (Dentener et al. 2006). Therefore, it is imperative to conduct N addition experiments in this area to evaluate their environmental impacts. Previous field-scale N addition experiments conducted in several forest plantations in this region revealed that simulated N deposition decreased RST in Betula luminifera (Luo et al. 2010) and Neosinocalamus affinis (Li et al. 2010) plantations, but increased RST in hybrid bamboo (Bambusa pervariabilis × Dendrocala mopsi) (Tu et al. 2011a), Pleioblastus amarus (Tu et al. 2013) and Eucalyptus grandis (Xiang et al. 2014) plantations. It is probable that these dissimilarities in the responses of soil respiration were primarily a function of differences in soil C and N status. In this region, the most widely distributed and representative plant communities are evergreen broad-leaved secondary forests. Furthermore, the soil C and N status of natural forests are significantly different from that of plantations. Thus, we speculate that the responses of natural forests and plantations to N additions may be potentially different. These above studies done in this region revealed that the responses of soil respiration to added N were associated with the responses of microbes and roots. In order to more accurately assess the responses of RSR and RSH to N additions, it is necessary to study root biomass, rhizospheric soil and bulk soil separately because: 1) Roots are the main source of RSR, thus, changes in root biomass directly impact the amount of CO2 derived from plant roots; 2) changes in the rhizospheric and bulk soil may alter microbial microenvironments and thus affect microbial communities in both rhizospheric and bulk soils. Therefore, we conducted a field N addition experiment in a subtropical evergreen broad-leaved secondary forest on the western edge of the Sichuan Basin, China. The aims of this study were to investigate the responses of RST, RSR and RSH to artificial N addition and to determine the potential mechanisms behind these responses.
Materials and methods
Site description
This study was performed in a moist evergreen broad-leaved forest in Wawushan Mountain, situated in Hongya, Sichuan, China (29°32′35″N, 103°15′41″E, 1600 m a.s.l.). The area has a mid-subtropical, monsoon-influenced, humid climate. The annual mean temperature is 10 °C, with the lowest and highest monthly means being −0.9 °C in January and 22.5 °C in July, respectively. The annual precipitation and evapotranspiration are 2323 mm and 467 mm, respectively, thus this area shows a high annual average relative humidity ranging from 85 to 90%. The soil is classified as a Lithic Dystrudepts (according to USDA Soil Taxonomy), overlying granite bedrock. The soil physicochemical properties are summarized in Table 1. Before 1956, the site was representative of the mid-subtropical evergreen broad-leaved biome characterizing the study area, dominated by Castanopsis platyacantha. In 1956, most of the big trees in the primary forest were felled. After that, there was no further disturbance, which allowed the surviving saplings to naturally recover into a mature forest. The average plant density at this site was 725 stems ha−1 and the mean diameter at the breast height (DBH, 1.3 m) was 23.5 cm. The dominant species in this forest is C. platyacantha with mean DBH value of 23.8 cm. Other plant species include the trees Schima sinensis, Lithocarpus hancei and Acanthopanax evodiaefolius var. gracilis, the shrubs Ilex purpurea and Eurya japonica, and the herbaceous species Cyperus rotundus.
Experimental design
In October 2012, nine plots (20 m × 20 m for each plot) were established at intervals of ≥20 m and randomly divided into three N treatments with three replicate plots for each treatment. The three N treatments are as follows: control (ambient N, i.e. +0 kg N ha−1 yr.−1), Low-N (+50 kg N ha−1 yr.−1), and High-N (+150 kg N ha−1 yr.−1). The Low-N and High-N treatments respectively simulate scenarios of N deposition increased by 50 and 150%. Consequently, the annual cumulated doses received by control, Low-N and High-N plots were 95, 145, and 245 kg N ha−1, respectively. Because atmospheric N deposition mainly consists of ammonium (NH4+) and nitrate (NO3−), N was applied in the form of ammonium nitrate (NH4NO3) to simulate increased N deposition. NH4NO3 solution was added to the soil surface monthly using a sprayer beginning in April 2013. The NH4NO3 was weighed, dissolved in 10 L of water, and applied to each plot every month. The control plots received an equivalent volume of water without NH4NO3. Although external water was added to plots while applied N, the additional water in each plot every year is only equivalent to an increase of 0.3 mm (0.013%) in annual precipitation. Therefore, the influence of external water may be negligible.
In March 2014, six 0.5 m × 0.5 m subplots were randomly designated in each plot. One soil respiration sampling polyvinyl chloride (PVC) collar (20 cm inside diameter, 8 cm height) was installed in each subplot. Each PVC collar was inserted into the soil at a 6 cm depth. Three of these subplots in each plot were selected randomly for trenching. On the outside edges of each designated trenched subplot, trenches of 0.5 m depth (below which few roots were found) were dug, and all roots were severed. Each trenched subplot was lined with a double-layered nylon mesh (0.1 mm diameter) extending 10 cm above the ground surface to prevent other roots from re-colonizing the subplot. The trench was then refilled carefully with soil. Next, all aboveground vegetation was removed within these subplots, and this vegetation-free status was maintained throughout the study. The CO2 efflux from the untrenched (control) subplot was considered to be total soil respiration (RST) and that from the trenched subplot was considered to be heterotrophic respiration (RSH). Rhizospheric respiration (RSR) was calculated from the formula RSR = RST – RSH.
Soil respiration measurement
To avoid the influence of residual decomposing roots left in the trenched subplots, the measurements of soil respiration began in May 2015 (14 months after subplots were trenched). A Li-8100 automated soil CO2 efflux system (LI-COR Inc., Lincoln, NE, USA) was used to measure soil respiration monthly for one year. All measurements occurred between 12:00 and 15:00 (local time) on each measurement date. Our previous measurements at the same site showed that the soil CO2 fluxes measured between 12: 00 to 15: 00 approximately equal to the average soil respiration rates of the diel cycle. The average rate of CO2 efflux measured on a sampling day for each plot was assumed to represent the diurnal mean of the plot, and the total CO2 quantity released on that day was multiplied by the number of days in the sampling month to estimate the total CO2 efflux during that month (Fan et al. 2014). The soil CO2 efflux rate was expressed as μmol CO2 m−2 s−1. The soil temperature at a 15 cm depth and moisture within the upper 5 cm of the soil horizon were measured using a soil temperature and moisture probe near the PVC collar at the time when soil respiration was measured.
Soil properties measurement
Bulk and rhizospheric soil samples were collected in June 2016. Three healthy, average-sized trees (C. platyacantha) with a DBH of 24 cm were selected as target trees for rhizospheric soil collection. Around each target tree, 10 fragments of fine roots with a diameter less than 2 mm were excavated carefully. Bulk soil, defined as the soil weakly adhering to fine roots, was collected by shaking the excavated fine root fragments gently. The soil still adhering to fine roots after gentle shaking was defined as rhizospheric soil and collected by further shaking and soft brushing (Feudis et al. 2017; Xiao et al. 2017). All soil samples were collected from the top 10 cm of the soil horizon. Thirty subsamples collected from each plot were immediately mixed into a single composite sample. Visible roots remaining in the bulk and rhizospheric soil were carefully removed with tweezers, after which all soil samples were ground and sieved through a 2-mm mesh. Both bulk and rhizospheric soil samples were then divided into two parts. One part was used to measure microbial biomass carbon (MBC) and microbial biomass nitrogen (MBN); another part was used to measure ammonium nitrogen (NH4+-N), nitrate nitrogen (NO3−-N) and pH.
To measure soil MBC and MBN concentrations, the 24-h chloroform (CHCl3) fumigation extraction technique and a total C/N analyzer (Shimadzu model TOC-VcPH +TNM-1, Kyoto, Japan) were used. The concentrations of MBC and MBN were calculated as the differences in extractable C/N between fumigated and unfumigated soils, divided by 0.45 (Wu et al. 1990) and 0.54 (Brookes et al. 1985), respectively. Soil NH4+-N and NO3−-N concentrations were measured by colorimetry after being extracted with a 2 M KCl solution. Soil pH was determined using a glass electrode in aqueous extracts (pHH2O) (solid: liquid ratio was 1: 2.5).
Data analysis
All statistical analyses were conducted using IBM SPSS Statistics 20.0 for Windows (IBM Crop., Armonk, New York, USA). Repeated measures ANOVAs (nitrogen treatment and time as the main effects) with Fisher’s LSD tests were used to analyze soil temperature, soil moisture, RST, RSH and RSR. To determine the effects of N addition and soil types (rhizospheric or bulk soil) on soil properties, the concentrations of NH4+ and NO3−, the NH4+/NO3− ratio, MBC, MBN and pH were compared among N treatments and soil types using two-way ANOVA. One-way ANOVA with Fisher’s LSD test was conducted to examine the effects of N addition on soil properties. Homogeneity of variances was tested using Levene’s test before above ANOVAs, and the variances of all the data were homogeneous. Linear regression was used to determine the relationships between RSR and root biomass, MBC and MBN concentrations for rhizospheric soil, as well as between RSH and concentrations of NH4+-N, MBC and MBN for root-free soil. Significant effects were determined at α = 0.05.
To analyze the response of soil respiration to soil temperature, the eq. RS = αeβT (Lloyd and Taylor 1994) was used in this paper. Where RS is the soil respiration rate (μmol CO2 m−2 s−1), T is the temperature at 15 cm below the soil surface (°C), α is the soil respiration rate (μmol CO2 m−2 s−1) when soil temperature is 0 °C, and β is the temperature reaction coefficient. Then the temperature sensitivity (Q10) of soil respiration was calculated as Q10 = e10β.
Results
Soil temperature and moisture
The annual mean soil temperature at 10 cm below the soil surface was 10.9 ± 0.2 °C, with the lowest monthly value (2.8 ± 0.3 °C) in January and the highest monthly value (18.7 ± 0.3 °C) in July in the control plots (Fig. 1a). Due to the fact that precipitation exceeds evapotranspiration in the study area, the annual soil moisture content in N control plots exhibited a mean of 60 cm3 H2O 100 cm−3 soil (Fig. 1b). Although, compared with N control, the annual mean soil moisture increased slightly in Low-N (by 2%) and High-N (by 5%) plots and the difference between N control and High-N treatment reached a significant level (P = 0.014) under repeated measures ANOVAs, one-way ANOVA for each month showed that the N effect on soil moisture was insignificant (P > 0.05). Before starting measuring soil respiration, soil moisture (May, June, July of 2014 and March, May, June of 2015) and temperature (from May 2014 to April 2015) were measured as pre-experiment; the analysis results showed that both N treatments and trenching had no influence on them (P > 0.05, Table 2).
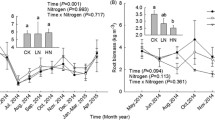
Seasonal variations in soil temperature and soil moisture in a moist evergreen broad-leaved forest in southwestern China (from May 2015 to April 2016). Data presented here are only from untrenched subplots. Plots received three levels of N addition: control (+ 0 kg N ha−1 yr.−1), Low-N (+ 50 kg N ha−1 yr.−1) and High-N (+ 150 kg N ha−1 yr.−1). Monthly applications of NH4NO3 began in April 2013. Values are means ±1 SE. Error bars indicate ±1 SE, n = 3. The results of repeated measures ANOVAs are shown for each parameter. a Soil temperature at 15 cm below surface; b soil moisture in the upper 5 cm of the soil horizon
Effects of N additions on soil respiration
Soil respiration displayed significant seasonal variation (Fig. 2). The annual mean RST in the control plots was 1.92 ± 0.18 μmol CO2 m−2 s−1, with the lowest rate (0.72 ± 0.09 μmol CO2 m−2 s−1) in December and highest rate (3.59 ± 0.51 μmol CO2 m−2 s−1) in July. Repeated measures ANOVAs showed that, compared with the N addition control plots, Low-N did not significantly changed the RST, while High-N decreased the RST by 39%, a significant (P = 0.025) change (Fig. 2a). Nonlinear regression analysis revealed that RST was significantly positively exponentially related to soil temperature (P < 0.0001, R2 > 0.77; Table 2). The Q10 of RST was 3.29, 3.13 and 3.06 in N control, Low-N and High-N plots, respectively (Table 3).
Seasonal variations in total soil respiration, heterotrophic respiration and rhizospheric respiration (from May 2015 to April 2016), in a moist evergreen broad-leaved forest in southwestern China. a Total soil respiration (RST); b heterotrophic respiration (RSH); c rhizospheric respiration (RSR). Histograms on the right side indicate annual mean values. Values are mean ± 1 SE. Error bars indicate ±1 SE, n = 3. The results of repeated measures ANOVAs are shown for each parameter. Different lowercase letters denote significant differences (One-way ANOVA with Fisher’s LSD test, α = 0.05) among treatments
The annual mean CO2 effluxes from RSH and RSR in the control plots were 1.43 ± 0.11 and 0.49 ± 0.06 μmol CO2 m−2 s−1, and reached their maxima in July and August, respectively (Fig. 2 b and c). The rates of RSH and RSR generally decreased with increasing N addition rates. Under Low-N treatment, the decreases in the rates of RSH and RSR were insignificant (P > 0.05). The CO2 effluxes from RSH and RSR in the High-N treatment were significantly (P = 0.027 and P = 0.011, respectively) lower (by 29 and 69%, respectively) than that of the control N treatment.
For the measurement period, the cumulative CO2 flux in the control plots was 7.3 ± 0.7 t C ha−1 (Fig. 3 a). One-way ANOVA revealed that the Low-N addition had no significant effect on cumulative CO2 effluxes, while the High-N treatment significantly reduced cumulative CO2 effluxes (P = 0.015) by 39%. Totally, the cumulative CO2 emission decreased 2.9 t C ha−1 in response to the High-N treatment. The cumulative CO2 effluxes from RSH and RSR in the control plots were 5.5 ± 0.4 and 1.9 ± 0.2 t C ha−1 (Fig. 3 a), with respective contributions of 75 ± 1% and 25 ± 1% to RST (Fig. 3 b). The N treatments decreased the cumulative CO2 fluxes from both RSH and RSR by 1.1–1.6 (20–29%) and 0.6–1.3 (30–69%) t C ha−1, respectively, compared to the N treatment control. However, it significantly increased the proportion of RSH in total respiration by 17% and decreased that of RSR by 49% (P = 0.022).
Cumulative CO2 flux from total soil respiration and different components of soil respiration (a), and the contributions of heterotrophic respiration and rhizospheric respiration to total soil respiration (b). Error bars indicate ±1SE, n = 3. In (a), different lowercase letters denote significant differences (One-way ANOVA with Fisher’s LSD test, α = 0.05) among treatments. In (b) different lowercase letters denote significant differences in the contribution of RSR to RST, different capital letters denote significant differences in the contribution of RSH to RST (One-way ANOVA with Fisher’s LSD test, α = 0.05) among treatments
Effect of N additions on bulk and rhizospheric soil properties
In the present study, the concentrations of NH4+-N and NO3−-N and the NH4+-N/ NO3−-N ratio of bulk soil were much lower than that in rhizospheric soil. Especially, the NH4+-N concentration in rhizospheric soil was around 7 times of that in bulk soil in control plots, which reached 13 and 23 times in Low-N and High-N treatments, respectively (Fig. 4 a). The MBC and MBN levels in bulk soil were also lower than those in rhizospheric soil. However, the pH of bulk soil was significantly higher than that of rhizospheric soil (Fig. 4).
Bulk and rhizospheric soil properties under different nitrogen treatments in a moist evergreen broad-leaved forest in southwestern China. Values are means ±1 SE. Error bars indicate ±1 SE, n = 3. The results of two-way ANOVAs are shown for each parameter. Different capital letters and lowercase letters denote significant differences (One-way ANOVA with Fisher’s LSD test, α = 0.05) among treatments in bulk and rhizospheric soil, respectively
One-way ANOVA revealed that N additions significantly decreased MBC, the NH4+ − NO3− ratio and the NH4+ concentration in bulk soil by 42%–47%, 33%–45 and 38%–49%, respectively (Fig. 4 a, c, d). The High-N treatment was also associated with a significant decrease in the pH of bulk soil samples (Fig. 4 f). However, N additions were not associated with any significant effects on any properties of rhizospheric soil.
Biotic and abiotic controls on soil respiration and its components
Correlation analysis indicated that RSR was significantly positively correlated with root biomass of C. platyacantha (R2 = 0.766, P = 0.002) (Fig. 5 a), had no significant relationship with the concentrations of MBC and MBN for rhizospheric soil (Fig. 5 b, c). However, soil RSH was significantly positively correlated with the concentrations of NH4+-N (R2 = 0.569, P = 0.019), MBC (R2 = 0.592, P = 0.015) and MBN (R2 = 0.601, P = 0.013) for bulk soil (Fig. 5 d − f).
Discussion
Our results indicated that RST in all treatments displayed seasonal variation following that in soil temperature, with the highest and lowest values in July and January, respectively. This is consistent with the findings of several other studies (Bowden et al. 2004; Mo et al. 2008). Using nonlinear regression analysis, we found that RST exhibited a significantly positive exponential relationship with soil temperature (P < 0.0001, R2 > 0.77). This suggests that soil temperature was the dominant controller of soil CO2 emissions. Based on the soil temperature at 15 cm below the surface, the temperature sensitivity of RST (Q10) in the control was 3.3, which is within the range typical of tropical/subtropical forests (1.4–4.6) according to Chen and Tian (2005), but higher than the mean (2.2 ± 0.9) of such forests. In the same region, the Q10 of RST in this forest was lower than that of B. luminifera (3.9) (Luo et al. 2010) and N. affinis (3.7) (Li et al. 2010) plantations and higher than that of hybrid bamboo (2.7) (Tu et al. 2011a, b), P. amarus (2.9) (Tu et al. 2013) and E. grandis (2.3) (Xiang et al. 2014) plantations. The average annual soil CO2 flux in the control was 7.3 ± 0.7 t C ha−1 yr.−1, which is higher than the global mean (6.3 t C ha−1 yr.−1) estimated by Zhao et al. (2017) and the annual mean (3.9–5.9 t C ha−1 yr.−1) in the plantations in this region mentioned above (Luo et al. 2010; Li et al. 2010; Tu et al. 2011a, b, 2013; Xiang et al. 2014), but considerably lower than that in tropical forests (13 t C ha−1 yr.−1) (Zhao et al. 2017). Our results also indicated that N treatments reduced RSH and RSR, which consequently resulted in a decrease in RST. The cumulative CO2 flux in High-N-treated plots decreased by an equivalent of 2.9 t C ha−1 (39%), which was significant compared to the control. Similar results have been reported by previous researchers (Olsson et al. 2005; Janssens et al. 2010; Fan et al. 2014; Sun et al. 2014). In addition, we found that the High-N treatment increased the contribution of RSH to RST by 17% but that of RSR decreased by 49%. Furthermore, we found that N fertilization decreased NH4+ concentration, MBC and pH for bulk soil, but no significant effects on these variables were observed in rhizospheric soil.
Many studies have shown that soil respiration is controlled by certain biotic and abiotic factors, such as soil temperature and moisture (Mande et al. 2015; Liu et al. 2016), plant roots (Mo et al. 2008), and soil microorganisms (Treseder 2008). Therefore, changes in these variables caused by N additions may influence soil respiration. Numerous studies have shown that declines in soil respiration after N additions are correlated with N-induced reductions in fine root biomass and/or in soil microbial activities (Bowden et al. 2004; Mo et al. 2008; Fan et al. 2014). On the contrary, other studies have found that soil respiration increased in N-treated plots as a consequence of increases in root and/or soil microbial biomass (Tu et al. 2011a, b, 2013). Therefore, it is plausible that the individual responses of plant roots and soil microorganisms determine the effects of N additions on soil respiration.
To understand the contribution of plant roots and soil microorganisms in the reductions in soil respiration after N addition, soil respiration was partitioned into RSR and RSH for analysis. Our study revealed that both RSH and RSR decreased with increasing N addition rates. The reduction in both RSH and RSR reached a significant level under High-N treatment but insignificant in Low-N plots, compared to N controls. Many other studies have reported similar results in different ecosystems. For example, Olsson et al. (2005) reported that both RSH and RSR in a boreal forest were significantly lower in N-fertilized plots. Fan et al. (2014) conducted an 8-year field experiment in a subtropical Chinese fir (Cunninghamia lanceolata) forest and found that N application significantly reduced both RSH and RSR. A meta-analysis by Janssens et al. (2010) suggested that in temperate forest ecosystems, RST, RSH and RSR significantly declined by 10%, 15 and 20% after N fertilizer was added. Several potential mechanisms may be used to explain the N-induced declines in soil respiration and its components.
The most plausible explanation is that N addition may reduce below-ground carbon allocation. With the exception of a few ecosystems (e.g. deserts), most terrestrial ecosystems are N-limited (Lebauer and Treseder 2008). Therefore, enrichment of soil N availability can be expected to reduce plant competition for N uptake and the need for trees to allocate C to the nutrient acquisition system (Janssens et al. 2010). This shift in C allocation consequently leads to a decrease in belowground biomass. There is abundant evidence that N fertilization considerably reduces root biomass in many different ecosystems (Wurzburger and Wright 2015; Wang et al. 2017b). Similarly, our previous study conducted at this site found that the root biomass of C. platyacantha in N-treated plots was significantly lower than that in the control plots (Peng et al. 2017). Analysis using a minirhizotron method at this study site indicated that the total root surface area in the top 15 cm of the soil horizon decreased after N additions (Chen et al. 2017a), suggesting that addition of N reduced belowground C allocation. As the most important contributor to RSR, root biomass reductions can directly result in decreases in RSR. This was borne out by the significant positive correlation between RSR and root biomass observed in our study (Fig. 5 a). In addition, a reduction in root biomass may also influence RSR by reducing rhizospheric C input, and this reduced C availability leads to a consequent reduction in rhizospheric microbial activity (Janssens et al. 2010). In a study conducted under controlled conditions, induced rhizospheric C input was considered to be a casual factor for a reduction in root-derived CO2 (Kuzyakov et al. 2002). Although we did not find a significant effect on rhizospheric microbial activity, N additions may decrease the rate of decomposition of SOM in the rhizosphere (Zang et al. 2016), which in turn would be expected to reduce RSR.
The changes in RSH under N treatment indicate a decline in SOM decomposition in root-free soil. Many studies have reported that N fertilization exerts a depressive effect on SOM decomposition (Riggs et al. 2015; Li et al. 2017). Two mechanisms may explain this phenomenon. Firstly, N addition modifies microbial communities. In this study, we observed that MBC in bulk soil significantly decreased (by 42%–47%) in N-treated plots. This tendency was also found with respect to MBN. The decreases in MBC and MBN indicate a decline in soil microbial biomass after N treatment. Numerous previous studies also reported similar findings (Treseder 2008; Mo et al. 2008). Using linear regression analysis, we found that RSH was significantly and positively correlated with MBC and MBN, which explains why the reduction in RSH was associated with a reduction in microbial activity. Secondly, N fertilization may enhance chemical stabilization of SOM by increasing the proportion of recalcitrant C that resists microbial decomposition (Janssens et al. 2010). For example, Riggs et al. (2015) reported that N addition could stabilize SOM and increase its resistance to microbial decomposition. Moran et al. (2005) found that N input promoted the transformation of residue C into more stable SOM. There is evidence that inorganic N can be incorporated into SOM forming recalcitrant compounds (e.g., indoles and pyrroles) (Thorn and Mikita 1992) that are highly resistant to microbial degradation. Therefore, we conclude that the reduction in soil microbial biomass and enhancement in the formation of recalcitrant SOM are the main reasons for the retardation of SOM decomposition. This would consequently result in a decline in RSH after N fertilization.
Several previous studies have reported the differences in soil properties and microbial communities between rhizospheric and bulk soil (Agnelli et al. 2016; Xiao et al. 2017). In this present study, significant differences between rhizospheric and bulk soil were also observed. The concentrations of NH4+, NO3−, MBC and MBN, and the NH4+/NO3− ratio in rhizospheric soil were significantly higher than those in bulk soil, while the opposite pattern was observed for pH. Compared to bulk soil, the rhizosphere has much higher process rates although the rhizospheric volume is very small (<10%) (Kuzyakov and Blagodatskaya 2015). Those processes include decomposition and mineralization of plant litter and SOM, microbial turnover, associated release of dissolved organics, CO2, NH4+ and nutrients, as well as microbial immobilization of N and other nutrients (Kuzyakov and Blagodatskaya 2015). The lower pH in the rhizosphere indicates acidification likely induced by the release of H+ as a consequence of root cation uptake (Agnelli et al. 2016). In general, plant roots are able to affect soil C and nutrient status, as well as microbial communities, by releasing organic acids, ligands, protons (H+) and enzymes into the soil (Cocco et al. 2013; Agnelli et al. 2016). It was estimated that 5–21% of the total C fixed by photosynthesis is transferred to the soil through root exudates (Haichar et al. 2014). Root exudates are important nutrient and C sources for soil microorganisms, thus, microbial communities in the rhizosphere are usually more abundant and diverse than those in bulk soil (Rengel and Marschner 2005; Hartmann et al. 2009). This enhancement in microbial populations and diversity may in turn impact nutrient availability in the rhizosphere (Rengel and Marschner 2005). Especially, the NH4+ concentration in rhizospheric soil was 7 times of that in bulk soil. In most cases, N availability in the rhizosphere increases when plant roots and microbes in the rhizosphere excrete enzymes that promote soil N mineralization (Jackson et al. 2012; Rengel and Marschner 2005). Our previous study conducted at this site showed that root isolation reduced the NH4+ concentration in soil (Chen et al. 2018), suggesting that the presence of roots has a stimulatory effect on soil NH4+ concentration. In addition, root exudates may include nitrification inhibitors that suppress nitrification in the rhizosphere (Subbarao et al. 2009). Therefore, the rhizospheric soil showed a much higher NH4+ concentration than bulk soil.
We also tested the biochemical responses of rhizospheric and bulk soil to N additions. Nitrogen addition significantly decreased the pH, MBC and NH4+ concentrations and the NH4+ − NO3− ratio for bulk soil, but no significant effects on rhizospheric soil were noted. Due to the high temperature and moisture, NH4+ in root-free soil may be rapidly transformed to NO3− via nitrification. It was reported that added N could significantly accelerate nitrification (Baldos et al. 2015; Xiao et al. 2015), as the NH4+ is the substrate of nitrification and the suddenly enhanced NH4+ helps to mitigate the fierce competition of NH4+ between nitrobacteria and plant uptake and heterotrophic microorganisms’ immobilization. Then, the excess NO3− could be lost from soil by leaching because of the abundant rainfall in summer and in the forms of NO and N2O through denitrification. Several previous studies have demonstrated that N input does increase NO3− leaching and N-oxide emissions (Corre et al. 2010; Shcherbak et al. 2014; Shrestha et al. 2015). The process NO3− leaching could lead to a loss of metal cations based on the charge balance in soil solutions, weakening their buffering against soil acidification, and finally decreased soil pH (Tian and Niu 2015). In addition, as a result of the decrease of soil microorganisms, less mobilization of organic N occurred. Therefore, the NH4+ concentration in bulk soil decreased,and thus resulted a decline in the ratio of NH4+ to NO3−. However, in rhizosphere, the process of nitrification is suppressed by the nitrification inhibitors included in root exudates (Subbarao et al. 2009), thus NH4+ that is not taken up by plants will accumulate in rhizosphere and/or be immobilized by rhizospheric microbes. Due to the high base amount of NH4+ in rhizosphere, N additions show less effect on the rhizospheric NH4+ concentration. The differences in the responses between rhizospheric and bulk soil may be due to the effects of plant roots which can significantly influence soil properties. The presence of roots may ameliorate the effects of N additions on rhizospheric soil biochemistry, by modifying their morphology (Chen et al. 2017b; Wang et al. 2017b) and exudates to adapt to the altered microenvironment.
Overall, N addition significantly reduced soil RSR and RSH, and consequently decreased RST. The positive correlation between RSR and root biomass of C. platyacantha indicates that N addition reduced RSR by reducing root C allocation and thus depressing C transfer to the rhizosphere. Nitrogen addition diminished RSH by reducing microbial biomass and enhancing chemical stabilization of SOM in root-free soil. Artificial N deposition also reduced the contribution of RSR to RST. To conclude, N addition depressed microorganism populations and reduced pH in bulk soil, however, no significant effects were observed on rhizospheric soil properties. Finally, one limitation that soil moisture in trenched subplots was not measured in the study period should be pointed out. As the lack of soil moisture measurements in trenched subplots, the trenching effect on soil moisture in the study period can not be determined, although the pre-experiment data did not indicate a significant trenching effect on soil moisture.
References
Agnelli A, Massaccesi L, De Feudis M, Cocco S, Courchesne F, Corti G (2016) Holm oak (Quercus ilex L.) rhizosphere affects limestone-derived soil under a multi-centennial forest. Plant Soil 400:297–314
Allison SD, Czimczik CI, Tresedek KK (2008) Microbial activity and soil respiration under nitrogen addition in Alaskan boreal forest. Glob Chang Biol 14:1156–1168
Baldos AP, Corre MD, Veldkamp E (2015) Response of N cycling to nutrient inputs in forest soils across a 1000–3000 m elevation gradient in the Ecuadorian Andes. Ecology 96:749–761
Batjes NH (2016) Harmonized soil property values for broad-scale modelling (WISE30sec) with estimates of global soil carbon stocks. Geoderma 269:61–68
Bobbink R, Hicks K, Galloway J, Spranger T, Alkemade R, Ashmore M, Bustamante M, Cinderby S, Davidson E, Dentener F, Emmett B, Erisman JW, Fenn M, Gilliam F, Nordin A, Pardo L, De Vries W (2010) Global assessment of nitrogen deposition effects on terrestrial plant diversity: a synthesis. Ecol Appl 20:30–59
Bond-Lamberty B, Thomson A (2010) Temperature-associated increases in the global soil respiration record. Nature 464:579–582
Bowden RD, Nadelhoffer KJ, Boone RD, Melillo JM, Garrison JB (1993) Contributions of above ground litter, below ground litter, and root respiration to total soil respiration in a temperate mixed hardwood forest. Can J For Res 23:1402–1407
Bowden RD, Davidson E, Savage K, Arabia C, Steudler P (2004) Chronic N additions reduce total soil respiration and microbial respiration in temperate forest soils at the Harvard Forest. For Ecol Manage 196:43–56
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Chen H, Tian HQ (2005) Does a general temperature-dependent Q10 model of soil respiration exist at biome and global scale? J Integr Plant Biol 47:1288–1302
Chen GT, Peng Y, Zheng J, Li S, Peng TC, Qiu XR, Tu LH (2017a) Effects of short-time nitrogen addition on fine root biomass, lifespan and morphology of Castanopsis platyacantha in a subtropical secondary evergreen broad-leaved forest. Chin J Plant Ecol 41:1041–1050 (in Chinese)
Chen GT, Tu LH, Peng Y, Hu HL, Hu TX, Xu ZF, Liu L, Tang Y (2017b) Effect of nitrogen additions on root morphology and chemistry in a subtropical bamboo forest. Plant Soil 412:441–451
Chen GT, Li S, Peng TC, Qiu XR, Chen YQ, Ma HY, Tu LH (2018) Effect of root isolation and nitrogen addition on soil carbon and nitrogen fractions in a moist evergreen broad-leaved forest. Chin J App Environ Biol 24:1135–1141 (in Chinese)
Cocco S, Agnelli A, Gobran GR, Corti G (2013) Modifications induced by the roots of Erica arborea L. to create a suitable environment in soils developed from alkaline and fine-textured marine sediments. Plant Soil 368:297–313
Comeau LP, Hergoualc'h K, Hartill J, Smith J, Verchot LV, Peak D, Salim AM (2016) How do the heterotrophic and the total soil respiration of an oil palm plantation on peat respond to nitrogen fertilizer application? Geoderma 268:41–51
Corre MD, Veldkamp E, Arnold J, Wright SJ (2010) Impact of elevated N input on soil N cycling and losses in old-growth lowland and montane forests in Panama. Ecology 91:1715–1729
Cusack DF, Silver WL, Torn MS, McDowell WH (2011) Effects of nitrogen additions on above- and belowground carbon dynamics in two tropical forests. Biogeochemistry 104:203–225
Dentener F, Drevet J, Lamarque JF, Bey I, Eickhout B, Fiore AM, Hauglustaine D, Horowitz LW, Krol M, Kulshrestha UC, Lawrence M, Galy-Lacaux C, Rast S, Shindell D, Stevenson D, Van Noije T, Atherton C, Bell N, Bergman D, Butler T, Cofala J, Collins B, Doherty R, Ellingsen K, Galloway J, Gauss M, Montanaro V, Müller JF, Pitari G, Rodriguez J, Sanderson M, Solmon F, Strahan S, Schultz M, Sudo K, Szopa S, Wild O (2006) Nitrogen and sulfur deposition on regional and global scales: a multimodel evaluation. Global Biogeochem Cycles 20:16615–16615
Devaraju N, Bala G, Caldeira K, Nemani R (2016) A model based investigation of the relative importance of CO2-fertilization, climate warming, nitrogen deposition and land use change on the global terrestrial carbon uptake in the historical period. Clim Dyn 47:173–190
Fan HB, Wu JP, Liu WF, Yuan YH, Huang RZ, Liao YC, Li YY (2014) Nitrogen deposition promotes ecosystem carbon accumulation by reducing soil carbon emission in a subtropical forest. Plant Soil 379:361–337
Feudis MD, Cardelli V, Massaccesi L, Hofmann D, Berns AE, Bol R, Cocco S, Corti G, Agnelli A (2017) Altitude affects the quality of the water-extractable organic matter (WEOM) from rhizosphere and bulk soil in European beech forests. Geoderma 302:6–13
Galloway JN, Aber JD, Erisman JW, Seitzinger SP, Howarth RW, Cowling EB, Cosby BJ (2003) The nitrogen cascade. Bioscience 53:341–356
Galloway JN, Townsend AR, Erisman JW, Bekunda M, Cai ZC, Freney JR, Martinelli LA, Seitzinger SP, Sutton MA (2008) Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science 320:889–892
Haichar FEZ, Santaella C, Heulin T, Achouak W (2014) Root exudates mediated interactions belowground. Soil Biol Biochem 77:69–80
Hanson PJ, Edwards NT, Garten CT, Andrews JA (2000) Separating root and soil microbial contributions to soil respiration: a review of methods and observations. Biogeochemistry 48:115–146
Hartmann A, Schmid M, van Tuinen D, Berg G (2009) Plant-driven selection of microbes. Plant Soil 321:235–257
Jackson LE, Bowles TM, Hodson AK, Lazcano C (2012) Soil microbial-root and microbial-rhizosphere processes to increase nitrogen availability and retention in agroecosystems. Curr Opin Environ Sustain 4:517–522
Janssens IA, Dieleman W, Luyssaert S, Subke JA, Reichstein M, Ceulemans R, Ciais P, Dolman AJ, Grace J, Matteucci G, Papale D, Piao SL, Schulze ED, Tang J, Law BE (2010) Reduction of forest soil respiration in response to nitrogen deposition. Nat Geosci 3:315–322
Kuzyakov Y (2006) Sources of CO2 efflux from soil and review of partitioning methods. Soil Biol Biochem 38:425–448
Kuzyakov Y, Blagodatskaya E (2015) Microbial hotspots and hot moments in soil: concept & review. Soil Biol Biochem 83:184–199
Kuzyakov Y, Siniakina SV, Ruehlmann J, Domanski G, Stahr K (2002) Effect of nitrogen fertilisation on below-ground carbon allocation in lettuce. J Sci Food Agr 82:1432–1441
Law B (2013) Nitrogen deposition and forest carbon. Nature 496:307–308
Lebauer DS, Treseder KK (2008) Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 89:371–379
Li RH, Tu LH, Hu TX, Zhang J, Lu Y, Liu WT, Luo SH, Xiang YB (2010) Effects of simulated nitrogen deposition on soil respiration in a Neosinocalamus affinis plantation in rainy area of West China. Chin J Appl Ecol 21:1649–1655 (in Chinese)
Li XG, Jia B, Lv JT, Ma QJ, Kuzyakov Y, Li FM (2017) Nitrogen fertilization decreases the decomposition of soil organic matter and plant residues in planted soils. Soil Biol Biochem 112:47–55
Liu XJ, Zhang Y, Han WX, Tang AH, Shen JL, Cui ZL, Vitousek P, Erisman JW, Goulding K, Christie P, Fangmeier A, Zhang FS (2013) Enhanced nitrogen deposition over China. Nature 494:459–462
Liu T, Xu ZZ, Hou YH, Zhou GS (2016) Effects of warming and changing precipitation rates on soil respiration over two years in a desert steppe of northern China. Plant Soil 400:15–27
Lloyd J, Taylor JA (1994) On the temperature dependence of soil respiration. Funct Ecol 8:315–323
Luo SH, Hu TX, Zhang J, Dai HZ, Tu LH, Li RH, Huang LH (2010) Responses of soil respiration to nitrogen fertilization in Betulah luminifera forest, rainy area of West China. J Agro-Environ Sci 29:1834–1839 (in Chinese)
Mande HK, Abdullah AM, Aris AZ, Ainuddin AN (2015) Factors responsible for spatial and temporal variation of soil CO2 efflux in a 50 year recovering tropical forest, peninsular Malaysia. Environ Earth Sci 73:5559–5569
Mo JM, Zhang W, Zhu WX, Gundersen P, Fang YT, Li DJ, Wang H (2008) Nitrogen addition reduces soil respiration in a mature tropical forest in southern China. Glob Chang Biol 14:403–412
Moran KK, Six J, Horwath WR, van Kessel C (2005) Role of mineral-nitrogen in residue decomposition and stable soil organic matter formation. Soil Sci Soc Am J 69:1730–1736
Ochoa-Hueso R, Arróniz-Crespo M, Bowker MA, Maestre FT, Pérez-Corona ME, Theobald MR, Vivanco MG, Manrique E (2014) Biogeochemical indicators of elevated nitrogen deposition in semiarid Mediterranean ecosystems. Environ Monit Assess 186:5831–5842
Olsson P, Linder S, Giesler R, Högberg P (2005) Fertilization of boreal forest reduces both autotrophic and heterotrophic soil respiration. Glob Chang Biol 11:1745–1753
Peng Y, Chen GS, Chen GT, Li S, Peng TC, Qiu XR, Luo J, Yang SS, Hu TX, Hu HL, Xu ZF, Liu L, Tang Y, Tu LH (2017) Soil biochemical responses to nitrogen addition in a secondary evergreen broad-leaved forest ecosystem. Sci Rep 7:2783. https://doi.org/10.1038/s41598-017-03044-w
Rengel Z, Marschner P (2005) Nutrient availability and management in the rhizosphere: exploiting genotypic differences. New Phytol 168:305–312
Riggs CE, Hobbie SE, Bach EM, Hofmockel KS, Kazanski CE (2015) Nitrogen addition changes grassland soil organic matter decomposition. Biogeochemistry 125:203–219
Samuelson L, Mathew R, Stokes T, Feng YC, Aubrey D, Coleman M (2009) Soil and microbial respiration in a loblolly pine plantation in response to seven years of irrigation and fertilization. For Ecol Manage 258:2431–2438
Schlesinger WH, Andrews JA (2000) Soil respiration and the global carbon cycle. Biogeochemistry 48:7–20
Scott-Denton LE, Rosenstiel TN, Monson RK (2006) Differential controls by climate and substrate over the heterotrophic and rhizospheric components of soil respiration. Glob Chang Biol 12:205–216
Shcherbak I, Millar N, Robertson GP (2014) Global metaanalysis of the nonlinear response of soil nitrous oxide (N2O) emissions to fertilizer nitrogen. Proc Natl Acad Sci U S A 111:9199–9204
Shrestha RK, Strahm BD, Sucre EB (2015) Greenhouse gas emissions in response to nitrogen fertilization in managed forest ecosystems. New For 46:167–193
Subbarao GV, Nakahara K, Hurtado MP, Ono H, Moreta DE, Salcedo AF, Yoshihashi AT, Ishikawa T, Ishitani M, Ohnishi-Kameyama M, Yoshida M, Rondon M, Rao IM, Lascano CE, Berry WL, Ito O (2009) Evidence for biological nitrification inhibition in Brachiaria pastures. Proc Natl Acad Sci U S A 106:17302–17307
Subke J, Inglima I, Cotrufo M (2006) Trends and methodological impacts in soil CO2 efflux partitioning: a metaanalytical review. Glob Chang Biol 12:921–943
Sun ZZ, Liu LL, Ma YC, Yin GD, Zhao C, Zhang Y, Piao SL (2014) The effect of nitrogen addition on soil respiration from a nitrogen-limited forest soil. Agric For Meteorol 197:103–110
Thorn KA, Mikita MA (1992) Ammonia fixation by humic substances: a nitrogen-15 and carbon-13 NMR study. Sci Total Environ 113:67–87
Tian DS, Niu SL (2015) A global analysis of soil acidifcation caused by nitrogen addition. Environ Res Lett 10:24019–24028
Treseder KK (2008) Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol Lett 11:1111–1120
Tu LH, Dai HZ, Hu TX, Zhang J, Luo SH (2011a) Effects of simulated nitrogen deposition on soil respiration in a Bambusa pervariabilis × Dendrocala mopsi plantation in rainy area of West China. Chin J Appl Ecol 22:829–839 (in Chinese)
Tu LH, Hu TX, Zhang J, Li RH, Dai HZ, Luo SH (2011b) Short-term simulated nitrogen deposition increases carbon sequestration in a Pleioblastus amarus plantation. Plant Soil 340:383–396
Tu LH, Hu TX, Zhang J, Li XW, Hu HL, Liu L, Xiao YL (2013) Nitrogen addition stimulates different components of soil respiration in a subtropical bamboo ecosystem. Soil Biol Biochem 58:255–264
Wang CK, Yang JY (2007) Rhizospheric and heterotrophic components of soil respiration in six Chinese temperate forests. Glob Chang Biol 13:123–131
Wang QK, Zhang WD, Sun T, Chen LC, Pang XY, Wang YP, Xiao FM (2017a) N and P fertilization reduced soil autotrophic and heterotrophic respiration in a young Cunninghamia lanceolata forest. Agric For Meteorol 232:66–73
Wang GL, Liu F, Xue S (2017b) Nitrogen addition enhanced water uptake by affecting fine root morphology and coarse root anatomy of Chinese pine seedlings. Plant Soil 418:177–189
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of soil microbial biomass C by fumigation-extraction-an automated procedure. Soil Biol Biochem 22:1167–1169
Wurzburger N, Wright SJ (2015) Fine-root responses to fertilization reveal multiple nutrient limitation in a lowland tropical forest. Ecology 96:2137–2146
Xiang YB, Huang CD, Hu TX, Tu LH, Yang WQ, Li RH, Hu C (2014) Response of soil respiration to simulated nitrogen deposition in a Eucalyptus grandis plantation in the Rainy Area of Western China. Sci Silvae Sin 50:21–26 (in Chinese)
Xiao YL, Tu LH, Chen G, Peng Y, Hu HL, Hu TX, Liu L (2015) Soil-nitrogen net mineralization increased after nearly six years of continuous nitrogen additions in a subtropical bamboo ecosystem. J For Res 26:949–956
Xiao SH, You HM, You WB, Liu JS, Cai CT, Wu JQ, Ji ZR, Zhan SH, Hu ZS, Zhang ZR, He DJ (2017) Rhizosphere and bulk soil enzyme activities in a Nothotsuga longibracteata forest in the Tianbaoyan National Nature Reserve, Fujian Province, China. J For Res 28:521–528
Xu ZF, Tu LH, Hu TX, Schädler M (2013) Implications of greater than average increases in nitrogen deposition on the western edge of the Szechwan Basin, China. Environ Pollut 177:201–202
Yan JH, Zhang DQ, Liu JX, Zhou GY (2014) Interactions between CO2 enhancement and N addition on net primary productivity and water-use efficiency in a mesocosm with multiple subtropical tree species. Glob Chang Biol 20:2230–2239
Zang HD, Wang JY, Kuzyakov Y (2016) N fertilization decreases soil organic matter decomposition in the rhizosphere. Appl Soil Ecol 108:47–53
Zhao CZ, Liu Q (2009) Growth and photosynthetic responses of two coniferous species to experimental warming and nitrogen fertilization. Can J For Res 39:1–11
Zhao ZY, Peng CH, Yang Q, Meng FR, Song XZ, Chen ST, Epule TE, Li P, Zhu Q (2017) Model prediction of biome-specific global soil respiration from 1960 to 2012. Earths Future 5:715–729
Acknowledgements
This project was supported by the National Natural Science Foundation of China (No. 31300522).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Feike A. Dijkstra.
Rights and permissions
About this article
Cite this article
Peng, Y., Chen, Gt., Li, S. et al. Nitrogen additions reduce rhizospheric and heterotrophic respiration in a subtropical evergreen broad-leaved forest. Plant Soil 431, 449–463 (2018). https://doi.org/10.1007/s11104-018-3751-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3751-1